Synthesis of Black g-C3N4 and Exploration of the Mechanism Underlying the Enhancement of Photocatalytic CO2 Reduction
Abstract
1. Introduction
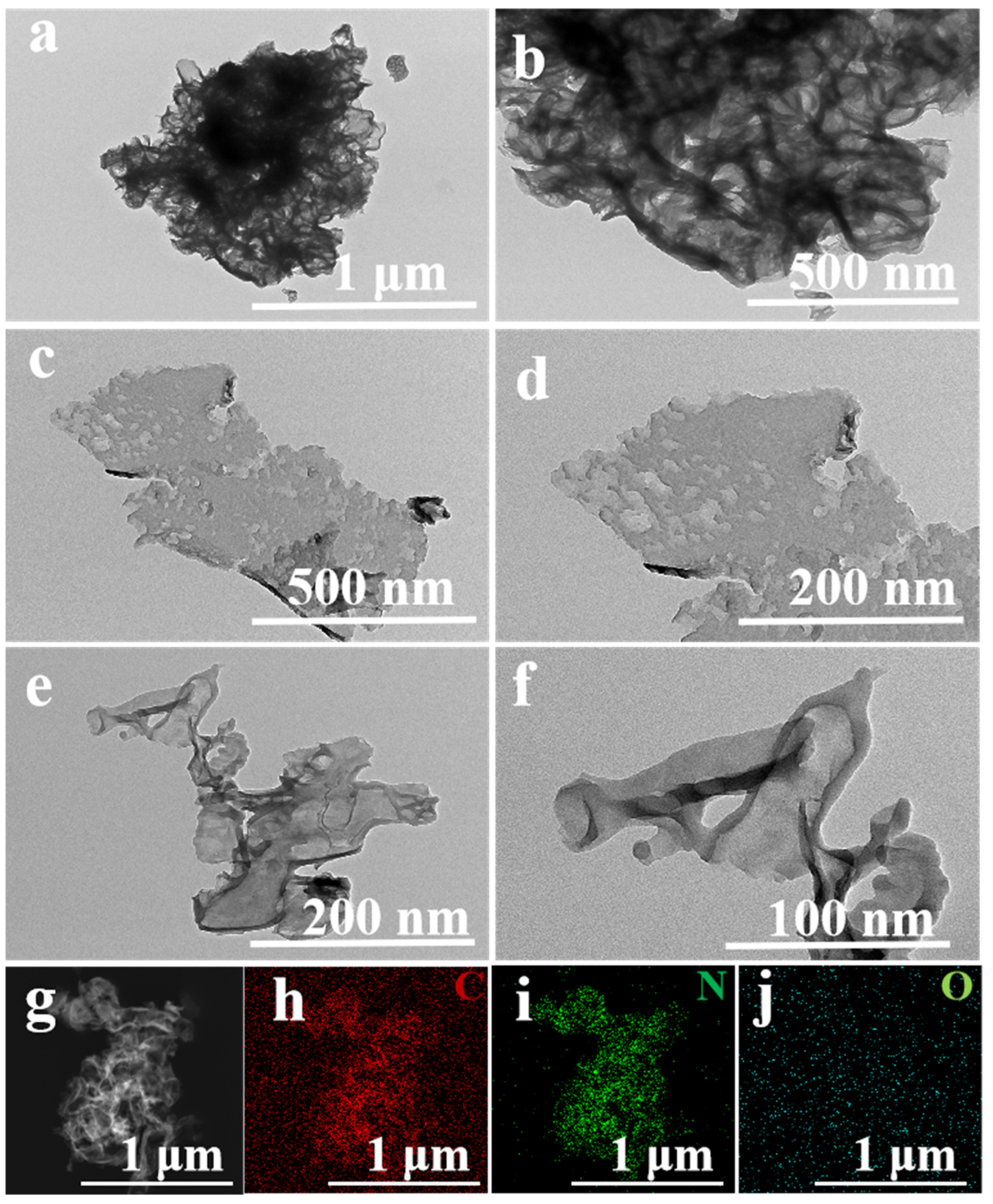
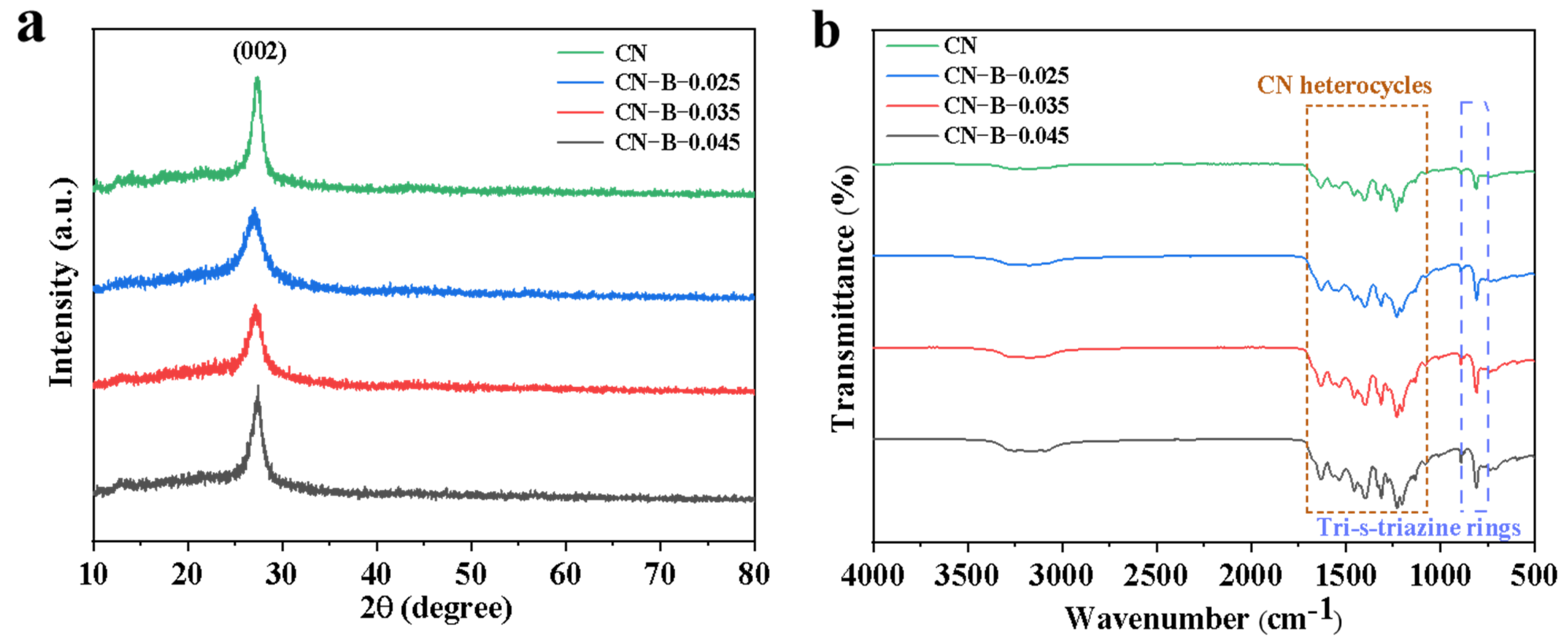
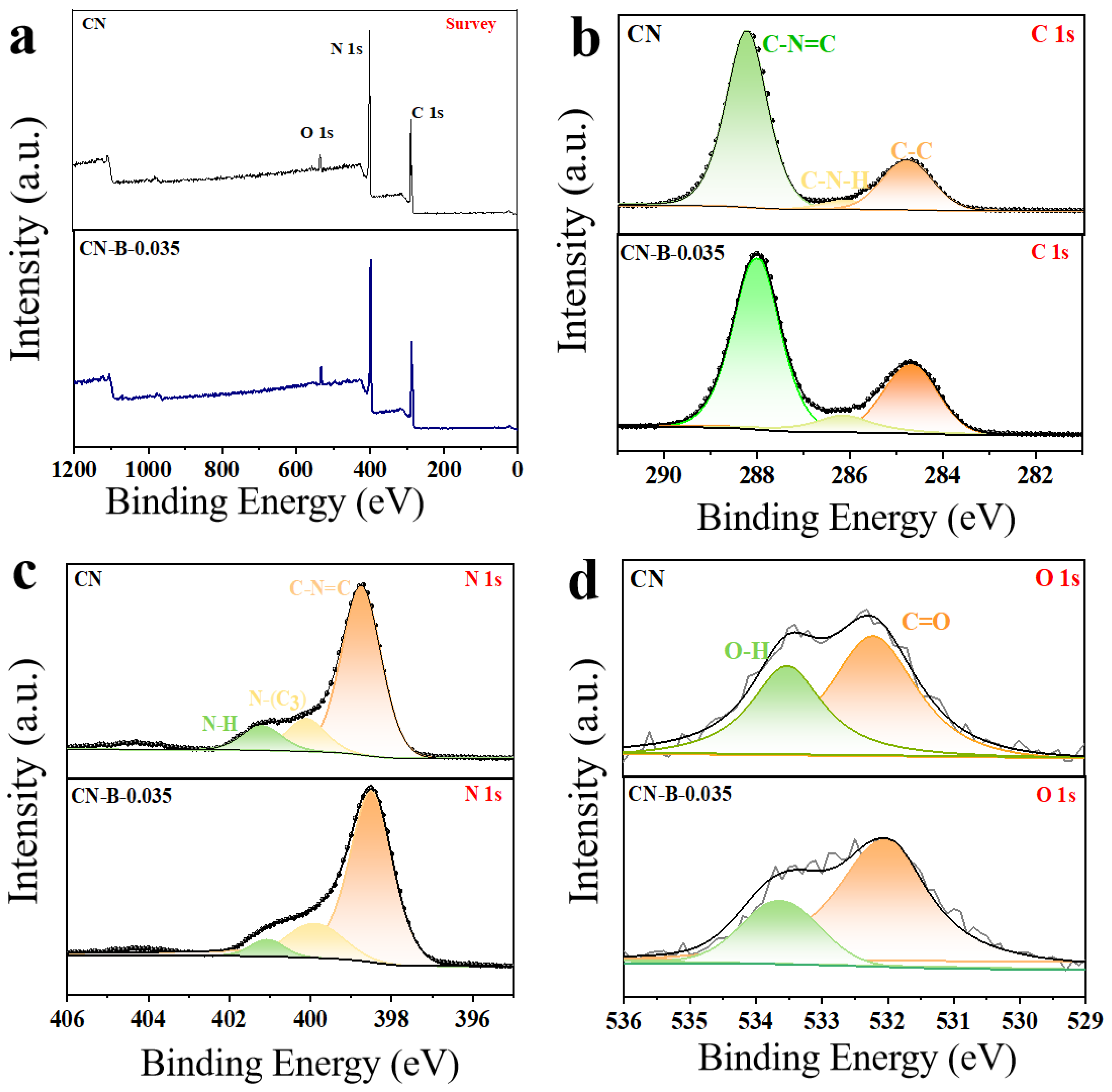
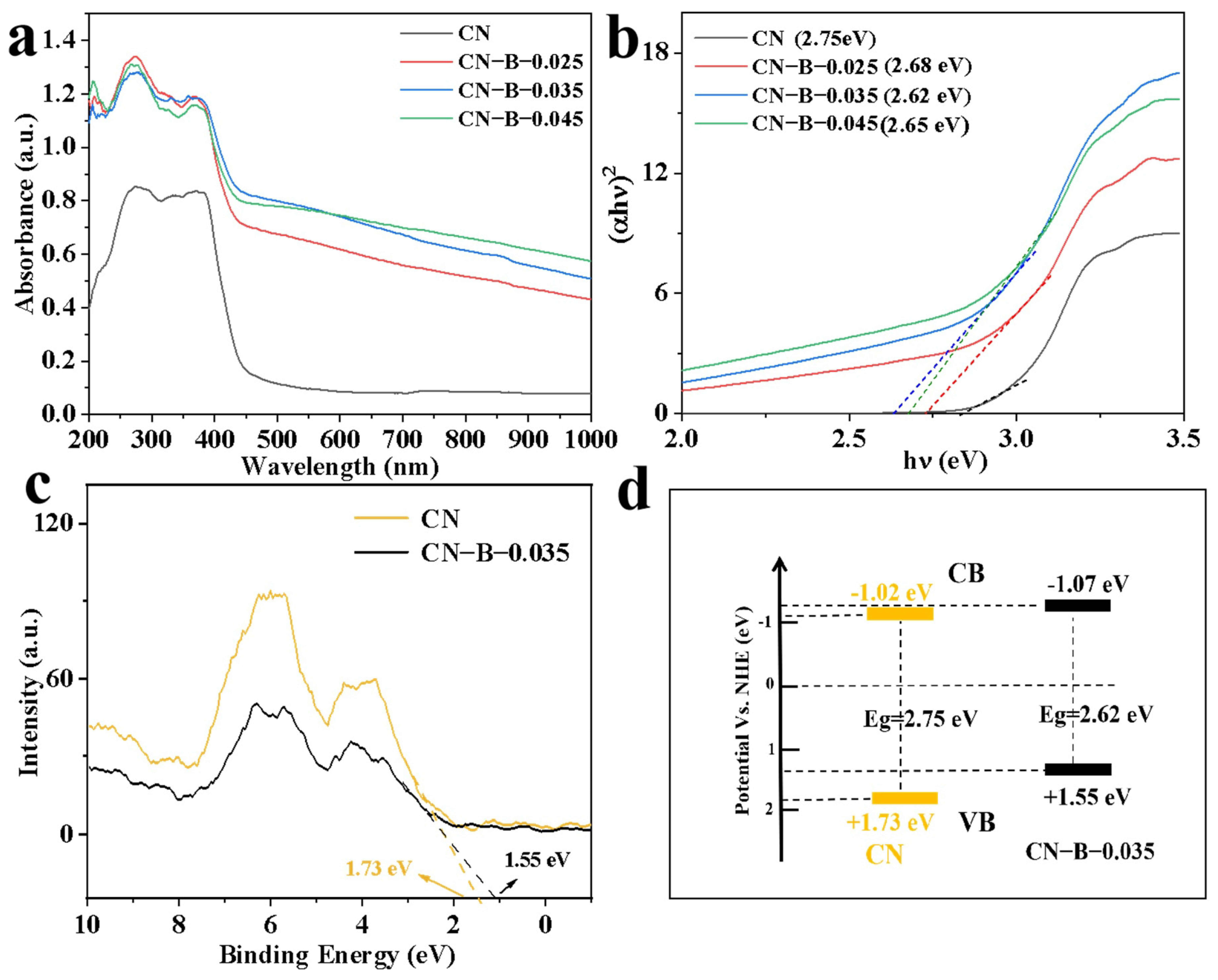
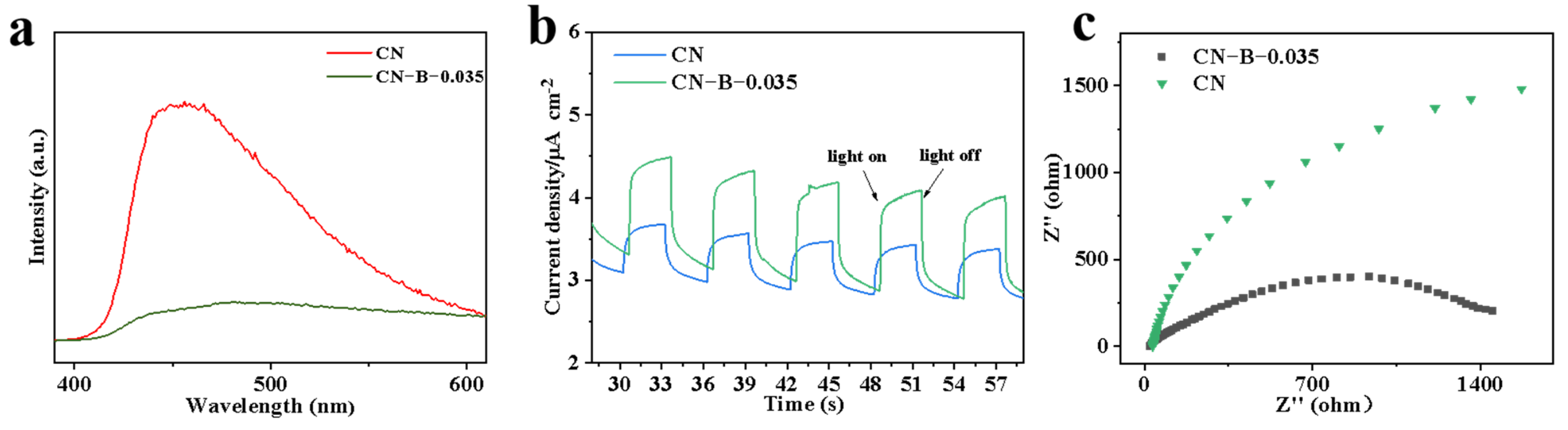
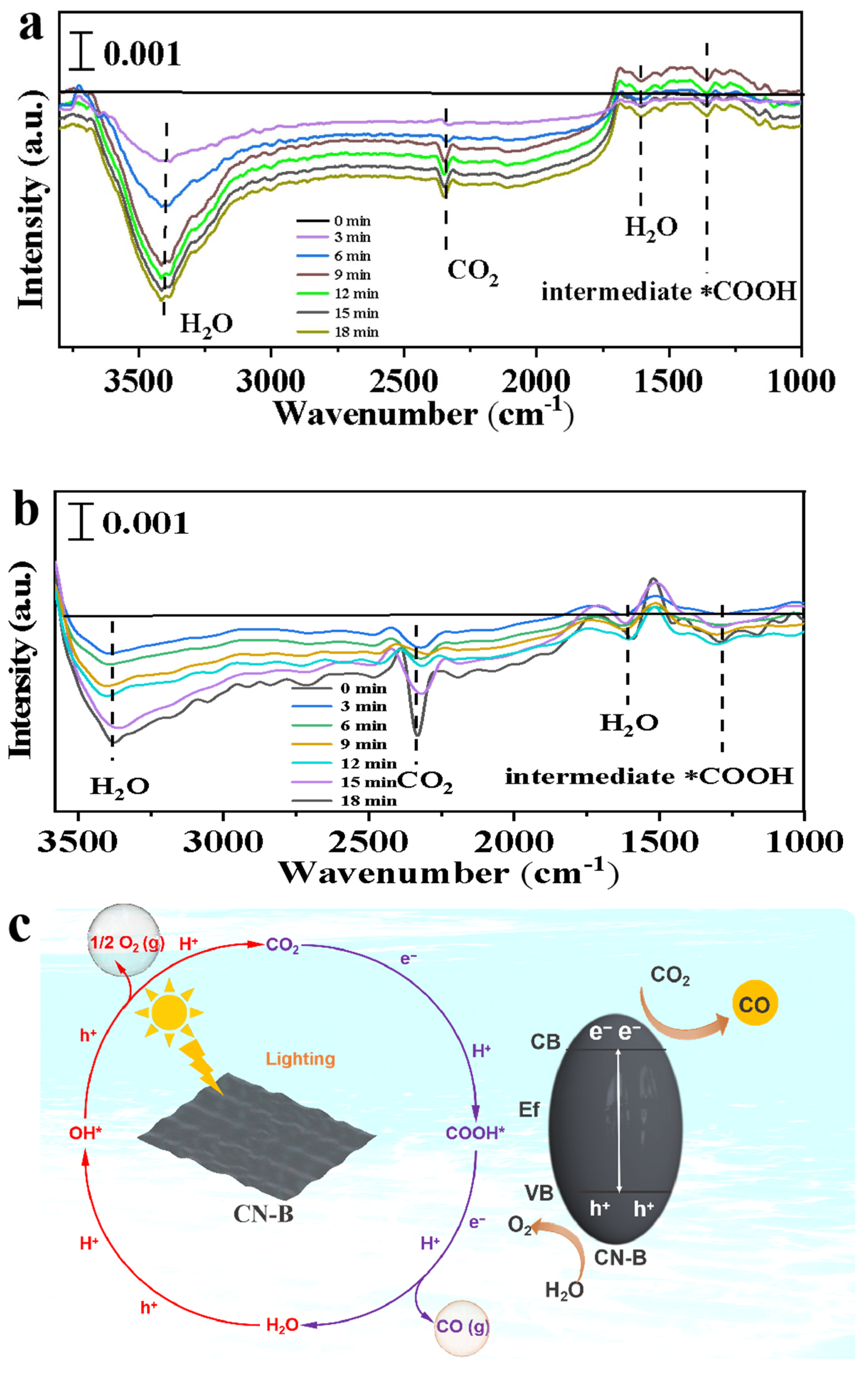
2. Results and Discussion
3. Experimental
3.1. Chemicals and Reagents
3.2. Synthesis and Characterizations
3.3. CO2 Photoreduction Experiments
3.4. Photo-Electrochemical Evaluations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Guan, B.; Wu, X.; Guo, J.; Ma, Z.; Zhang, J.; Huang, Z. Research status, challenges and future prospects of renewable synthetic fuel catalysts for CO2 photocatalytic reduction conversion. Environ. Sci. Pollut. Res. 2023, 30, 11246–11271. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Zhang, Y.; Chen, L.; Wu, F.; Yao, Y.; Wang, W.; Wang, G. A strongly coupled metal/hydroxide heterostructure cascades carbon dioxide and nitrate reduction reactions toward efficient urea electrosynthesis. Angew. Chem. Int. Ed. 2024, 63, e202410105. [Google Scholar]
- Khan, K.Y.; Tang, Y.; Cheng, P.; Song, Y.; Li, X.; Lou, J.; Du, D. Effects of degradable and non-degradable microplastics and oxytetracycline co-exposure on soil N2O and CO2 emissions. Appl. Soil Ecol. 2024, 197, 105331. [Google Scholar]
- Han, C.; Zhang, R.; Ye, Y.; Wang, L.; Ma, Z.; Su, F.; Ye, L. Chainmail co-catalyst of NiO shell-encapsulated Ni for improving photocatalytic CO2 reduction over g-C3N4. J. Mater. Chem. A 2019, 7, 9726–9735. [Google Scholar]
- Fu, J.; Zhu, B.; Jiang, C.; Cheng, B.; You, W.; Yu, J. Hierarchical porous O-doped g-C3N4 with enhanced photocatalytic CO2 reduction activity. Small 2017, 13, 1603938. [Google Scholar]
- Wang, K.; Fu, J.; Zheng, Y. Insights into photocatalytic CO2 reduction on C3N4: Strategy of simultaneous B, K co-doping and enhancement by N vacancies. Appl. Catal. B Environ. 2019, 254, 270–282. [Google Scholar]
- Liu, Z.; Wang, L.; Liu, P.; Zhao, K.; Ye, S.; Liang, G. Rapid ultrasensitive and non-enzyme electrochemiluminescence detection of hydrogen peroxide in food based on the ssDNA/g-C3N4 nanosheets hybrid. Food Chem. 2021, 357, 129753. [Google Scholar]
- Truc NT, T.; Bach, L.G.; Hanh, N.T.; Pham, T.D.; Le Chi NT, P.; Tran, D.T.; Nguyen, M.V. The superior photocatalytic activity of Nb doped TiO2/g-C3N4 direct Z-scheme system for efficient conversion of CO2 into valuable fuels. J. Colloid Interface Sci. 2019, 540, 1–8. [Google Scholar]
- Zhang, X.; Wang, L.; Du, Q.; Wang, Z.; Ma, S.; Yu, M. Photocatalytic CO2 reduction over B4C/C3N4 with internal electric field under visible light irradiation. J. Colloid Interface Sci. 2016, 464, 89–95. [Google Scholar]
- Gu, W.; Lu, D.; Kondamareddy, K.K.; Li, J.; Cheng, P.; Ho, W.; Wang, Z. Efficient photocatalytic decomposition of NO and mechanism insight enabled by NaBH4-reduced N (ligancy-3)-vacancy-rich-graphitic carbon nitride. Mater. Today Phys. 2024, 46, 101487. [Google Scholar]
- Talapaneni, S.N.; Singh, G.; Kim, I.Y.; AlBahily, K.; Al-Muhtaseb AA, H.; Karakoti, A.S.; Vinu, A. Nanostructured carbon nitrides for CO2 capture and conversion. Adv. Mater. 2020, 32, 1904635. [Google Scholar] [CrossRef] [PubMed]
- Kong, T.; Jiang, Y.; Xiong, Y. Photocatalytic CO2 conversion: What can we learn from conventional COx hydrogenation. Chem. Soc. Rev. 2020, 49, 6579–6591. [Google Scholar] [CrossRef]
- Wang, Q.; Fang, Z.; Zhang, W.; Zhang, D. High-efficiency g-C3N4 based photocatalysts for CO2 reduction: Modification methods. Adv. Fiber Mater. 2022, 4, 342–360. [Google Scholar] [CrossRef]
- Li, Y.; Fang, Y.; Cao, Z.; Li, N.; Chen, D.; Xu, Q.; Lu, J. Construction of g-C3N4/PDI@ MOF heterojunctions for the highly efficient visible light-driven degradation of pharmaceutical and phenolic micropollutants. Appl. Catal. B Environ. 2019, 250, 50–162. [Google Scholar] [CrossRef]
- Wu, M.; He, X.; Jing, B.; Wang, T.; Wang, C.; Qin, Y.; An, T. Novel carbon and defects co-modified g-C3N4 for highly efficient photocatalytic degradation of bisphenol A under visible light. J. Hazard. Mater. 2020, 384, 121323. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Han, D.; Hu, B.; Liang, J.; Tang, X.; Zhu, Z.; Huo, P. Insights into enhanced photocatalytic CO2 reduction on carbon nitride: A strategy of simultaneous O, S co-do. J. Environ. Chem. Eng. 2025, 13, 116121. [Google Scholar] [CrossRef]
- Liu, G.; Dong, G.; Zeng, Y.; Wang, C. The photocatalytic performance and active sites of g-C3N4 effected by the coordination doping of Fe (III). Chin. J. Catal. 2020, 41, 1564–1572. [Google Scholar] [CrossRef]
- Hu, X.; Lu, P.; Pan, R.; Li, Y.; Bai, J.; He, Y.; Zhang, C.; Fu, M. Metal-ion-assisted construction of cyano group defects in g-C3N4 to simultaneously degrade wastewater and produce hydrogen. Chem. Eng. J. 2021, 423, 130278. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, X.; Li, W.; Sun, H.; Jia, S.; Zhou, Y.; Yan, Y. Harnessing photo-to-thermal conversion in sulfur-vulcanized Mxene for high-efficiency solar-to-carbon-fuel synthesis. Adv. Funct. Mater. 2024, 34, 2400121. [Google Scholar] [CrossRef]
- Zhang, S.M.; He, Z.H.; Shi, J.J.; Wang, S.W.; Liu, J.; Wang, K.; Liu, Z.T. Photothermal-driven CO2 reduction over Nd2O3/TiO2 heterojunction catalysts in aqueous medium. Mol. Catal. 2024, 559, 114078. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Y.; Zhang, X.; Pang, X.; Yang, Z. Bio-inspired self-assembled bacteriochlorin nanoparticles for superior visualization and photothermal ablation of tumors. Biomed. Pharmacother. 2023, 165, 115014. [Google Scholar]
- Hou, B.; Shi, Z.; Kong, D.; Chen, Z.; Yang, K.; Ming, X.; Wang, X. Scalable porous Al foil/reduced graphene oxide/Mn3O4 composites for efficient fresh water generation. Mater. Today Energy 2020, 15, 100371. [Google Scholar]
- Sun, Z.; Fischer JM, T.A.; Li, Q.; Hu, J.; Tang, Q.; Wang, H.; Wang, L. Enhanced CO2 photocatalytic reduction on alkali-decorated graphitic carbon nitride. Appl. Catal. B Environ. 2017, 216, 146–155. [Google Scholar]
- Wang, Y.; Yang, W.; Chen, X.; Wang, J.; Zhu, Y. Photocatalytic activity enhancement of core-shell structure g-C3N4@TiO2 via controlled ultrathin g-C3N4 layer. Appl. Catal. B Environ. 2018, 220, 337–347. [Google Scholar]
- Guo, F.; Sun, H.; Huang, X.; Shi, W.; Yan, C. Fabrication of TiO2/high-crystalline g-C3N4 composite with enhanced visible-light photocatalytic performance for tetracycline degradation. J. Chem. Technol. Biotechnol. 2020, 95, 2684–2693. [Google Scholar] [CrossRef]
- Tan, X.; Jiang, K.; Zhai, S.; Zhou, J.; Wang, J.; Cadien, K.; Li, Z. X ray spectromicroscopy investigation of heterogeneous sodiation in hard carbon nanosheets with vertically oriented (002) Planes. Small 2021, 17, 2102109. [Google Scholar]
- Guo, F.; Wang, L.; Sun, H.; Li, M.; Shi, W. High-efficiency photocatalytic water splitting by a N-doped porous g-C3N4 nanosheet polymer photocatalyst derived from urea and N, N-dimethylformamide. Inorg. Chem. Front. 2020, 7, 1770–1779. [Google Scholar]
- Ji, Y.; Larsen, D.H.; Huang, Y.; Heuvelink, E.; Marcelis, L.F. Gradually increasing light intensity during the growth period increases dry weight production compared to constant or gradually decreasing light intensity in lettuce. Sci. Hortic. 2023, 311, 111807. [Google Scholar]
- Shi, Y.; Li, L.; Sun, H.; Xu, Z.; Cai, Y.; Shi, W.; Du, X. Engineering ultrathin oxygen-doped g-C3N4 nanosheet for boosted photoredox catalytic activity based on a facile thermal gas-shocking exfoliation effect. Sep. Purif. Technol. 2022, 292, 121038. [Google Scholar]
- Shi, W.; Shu, K.; Sun, H.; Ren, H.; Li, M.; Chen, F.; Guo, F. Dual enhancement of capturing photogenerated electrons by loading CoP nanoparticles on N-deficient graphitic carbon nitride for efficient photocatalytic degradation of tetracycline under visible light. Sep. Purif. Technol. 2020, 246, 116930. [Google Scholar]
- Lin, J.; Tian, W.; Zhang, H.; Duan, X.; Sun, H.; Wang, S. Graphitic carbon nitride-based Z-scheme structure for photocatalytic CO2 reduction. Energy Fuels 2020, 35, 7–24. [Google Scholar] [CrossRef]
- Zeng, D.; Ong, W.J.; Chen, Y.; Tee, S.Y.; Chua, C.S.; Peng, D.L.; Han, M.Y. Co2P nanorods as an efficient cocatalyst decorated porous g-C3N4 nanosheets for photocatalytic hydrogen production under visible light irradiation. Part. Part. Syst. Charact. 2018, 35, 1700251. [Google Scholar] [CrossRef]
- Shi, W.; Yang, S.; Sun, H.; Wang, J.; Lin, X.; Guo, F.; Shi, J. Carbon dots anchored high-crystalline g-C3N4 as a metal-free composite photocatalyst for boosted photocatalytic degradation of tetracycline under visible light. J. Mater. Sci. 2021, 56, 2226–2240. [Google Scholar] [CrossRef]
- Yu, M.; Liang, H.; Zhan, R.; Xu, L.; Niu, J. Sm-doped g-C3N4/Ti3C2 MXene heterojunction for visible-light photocatalytic degradation of ciprofloxacin. Chin. Chem. Lett. 2021, 32, 2155–2158. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Q.; Li, X.; Ji, H.; Shen, Z. Two-dimensional g-C3N4 nanosheets-based photo-catalysts for typical sustainable processes. Chin. Chem. Lett. 2023, 34, 108306. [Google Scholar] [CrossRef]
- Qin, J.; Chen, J.; Salisbury, J.B. Photon transferred TL signals from potassium feldspars and their effects on post-IR IRSL measurements. J. Lumin. 2015, 160, 1–8. [Google Scholar] [CrossRef]
- Liu, G.; Yan, S.; Shi, L.; Yao, L. The improvement of photocatalysis H2 evolution over g-C3N4 with Na and cyano-group co-modification. Front. Chem. 2019, 7, 639. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Zhang, L.; Xu, J.; Liu, Y.; Jiang, D.; Du, P. Nitrogen photofixation on holey g-C3N4 nanosheets with carbon vacancies under visible-light irradiation. Chin. Chem. Lett. 2020, 31, 792–796. [Google Scholar] [CrossRef]
- Shi, Y.; Li, L.; Xu, Z.; Sun, H.; Guo, F.; Shi, W. One-step simple green method to prepare carbon-doped graphitic carbon nitride nanosheets for boosting visible-light photocatalytic degradation of tetracycline. J. Chem. Technol. Biotechnol. 2021, 96, 3122–3133. [Google Scholar] [CrossRef]
- Wang, Y.; Shang, X.; Shen, J.; Zhang, Z.; Wang, D.; Lin, J.; Li, C. Direct and indirect Z-scheme heterostructure-coupled photosystem enabling cooperation of CO2 reduction and H2O oxidation. Nat. Commun. 2020, 11, 3043. [Google Scholar] [CrossRef]
- Wang, Y.; He, F.; Chen, L.; Shang, J.; Wang, J.; Wang, S.; Sun, H. Acidification and bubble template derived porous g-C3N4 for efficient photodegradation and hydrogen evolution. Chin. Chem. Lett. 2020, 31, 2668–2672. [Google Scholar]
- Shen, W.; Qi, Q.; Hu, B.; Zhu, Z.; Huo, P.; Jiang, J.; Tang, X. Studying bimetals copper-indium for enhancing PCN photocatalytic CO2 reduction activity and selectivity mechanism. J. Ind. Eng. Chem. 2024, 145, 384–394. [Google Scholar]
- Sun, X.; Shi, Y.; Lu, J.; Shi, W.; Guo, F. Template-free self-assembly of three-dimensional porous graphitic carbon nitride nanovesicles with size-dependent photocatalytic activity for hydrogen evolution. Appl. Surf. Sci. 2022, 606, 154841. [Google Scholar] [CrossRef]
- Shi, W.; Cao, L.; Shi, Y.; Chen, Z.; Cai, Y.; Guo, F.; Du, X. Environmentally friendly supermolecule self-assembly preparation of S-doped hollow porous tubular g-C3N4 for boosted photocatalytic H2 production. Ceram. Int. 2023, 49, 11989–11998. [Google Scholar]
- Zhang, Z.; Gao, Y.; Li, P.; Qu, B.; Mu, Z.; Liu, Y.; Jing, L. Highly sensitive fluorescence detection of chloride ion in aqueous solution with Ag-modified porous g-C3N4 nanosheets. Chin. Chem. Lett. 2020, 31, 2725–2729. [Google Scholar]
- Guo, F.; Li, L.; Shi, Y.; Shi, W.; Yang, X. Synthesis of N-deficient g-C3N4/epoxy composite coating for enhanced photocatalytic corrosion resistance and water purification. J. Mater. Sci. 2023, 58, 4223–4239. [Google Scholar]
- Yu, X.; Tang, X.; Luo, H.; Mao, Y. Construction of biochar assisted S-scheme of CeO2/g-C3N4 with enhanced photoreduction CO2 to CO activity and selectivity. Mater. Res. Bull. 2025, 181, 113085. [Google Scholar]
- Zhu, Z.; Ye, J.; Tang, X.; Chen, Z.; Yang, J.; Huo, P.; Crittenden, J. Vacancy-rich CoSx@LDH@Co-NC catalytic membrane for antibiotic degradation with mechanistic insights. Environ. Sci. Technol. 2023, 57, 16131–16140. [Google Scholar]
- Zhu, Z.; Xing, X.; Qi, Q.; Shen, W.; Wu, H.; Li, D.; Huo, P. Fabrication of graphene modified CeO2/g-C3N4 heterostructures for photocatalytic degradation of organic pollutants. Chin. J. Struct. Chem. 2023, 42, 100194. [Google Scholar] [CrossRef]
- Zhu, Z.; Xing, X.; Qi, Q.; Li, H.; Han, D.; Song, X.; Huo, P. Regulation CN reduction of CO2 products selectivity by adjusting the number of V sites and mechanism exploration. Fuel 2025, 388, 134509. [Google Scholar]
- Xing, Y.; Wang, X.; Hao, S.; Zhang, X.; Wang, X.; Ma, W.; Xu, X. Recent advances in the improvement of g-C3N4 based photocatalytic materials. Chin. Chem. Lett. 2021, 32, 13–20. [Google Scholar]
- Zhu, X.; Yang, J.; Zhu, X.; Yuan, J.; Zhou, M.; She, X.; Xu, H. Exploring deep effects of atomic vacancies on activating CO2 photoreduction via rationally designing indium oxide photocatalysts. Chem. Eng. J. 2021, 422, 129888. [Google Scholar]
- Qi, Q.; Shen, W.; Cai, M.; Cai, J.; Hu, B.; Han, D.; Zhu, Z.; Huo, P. Construction of Cu-Modified g-C3N4 nanosheets for photoinduced CO2 reduction to CO and selectivity mechanism insight. ACS Appl. Nano Mater. 2024, 7, 24788–24797. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, S.; Zhang, J.; Chen, X.; Zou, Y.; Chen, Q.; Yan, Y.; Li, P. Synthesis of Black g-C3N4 and Exploration of the Mechanism Underlying the Enhancement of Photocatalytic CO2 Reduction. Catalysts 2025, 15, 349. https://doi.org/10.3390/catal15040349
Lv S, Zhang J, Chen X, Zou Y, Chen Q, Yan Y, Li P. Synthesis of Black g-C3N4 and Exploration of the Mechanism Underlying the Enhancement of Photocatalytic CO2 Reduction. Catalysts. 2025; 15(4):349. https://doi.org/10.3390/catal15040349
Chicago/Turabian StyleLv, Shaokun, Jun Zhang, Xiaoke Chen, Yue Zou, Qiuli Chen, Yongsheng Yan, and Pengxin Li. 2025. "Synthesis of Black g-C3N4 and Exploration of the Mechanism Underlying the Enhancement of Photocatalytic CO2 Reduction" Catalysts 15, no. 4: 349. https://doi.org/10.3390/catal15040349
APA StyleLv, S., Zhang, J., Chen, X., Zou, Y., Chen, Q., Yan, Y., & Li, P. (2025). Synthesis of Black g-C3N4 and Exploration of the Mechanism Underlying the Enhancement of Photocatalytic CO2 Reduction. Catalysts, 15(4), 349. https://doi.org/10.3390/catal15040349





