Experimental Design and Numerical Optimization of Photochemical Oxidation Removal of Tetracycline from Water Using Fe3O4-Supported Fruit Waste Activated Carbon
Abstract
1. Introduction
2. Materials and Methods
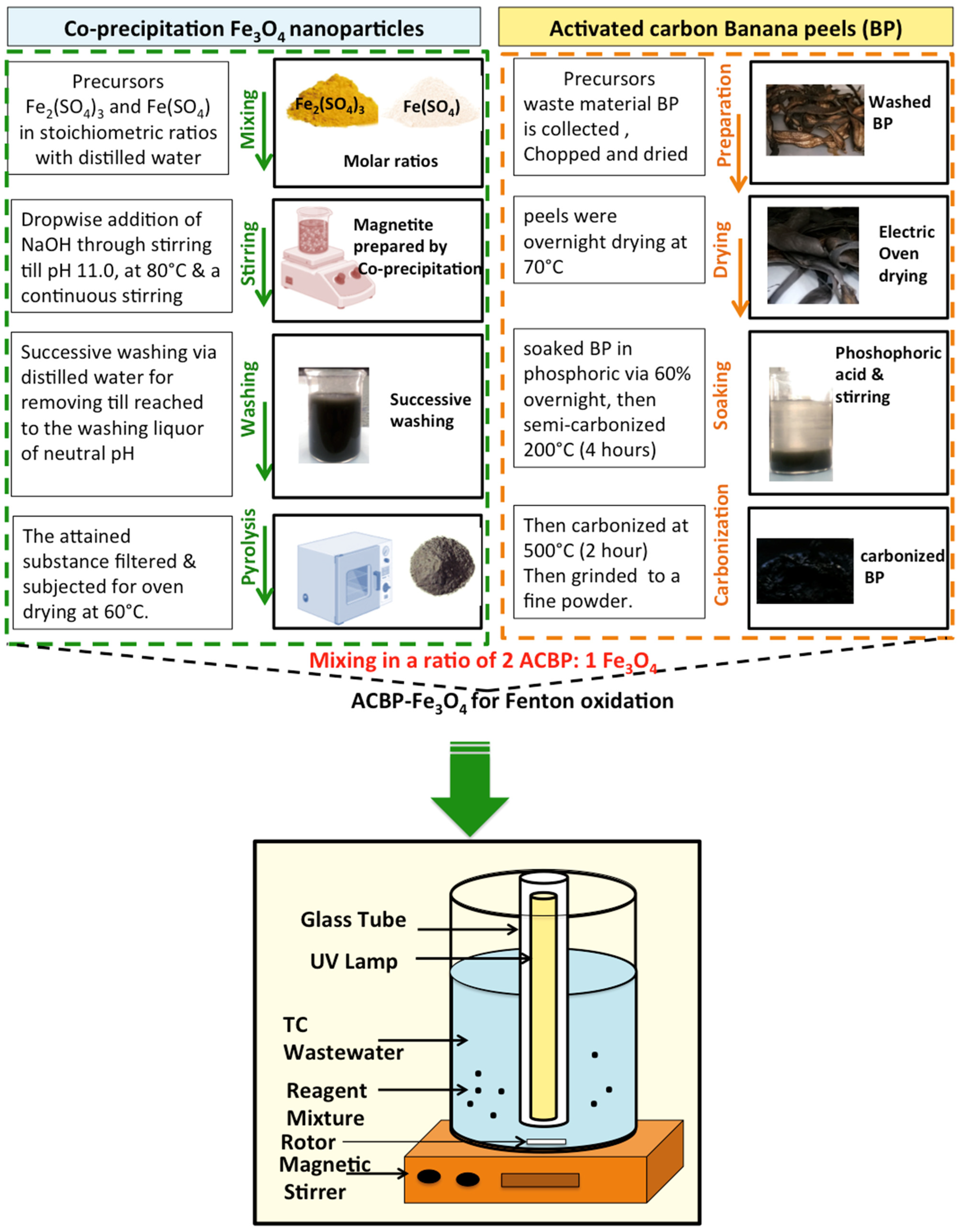
2.1. Synthesis of Photocatalyst
2.2. Characterization of the Photocatalyst
2.3. Methodology
2.4. Analysis
2.5. Box–Behnken Factorial Design
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of ACBP-Fe3O4
3.2. Photochemical Oxidation
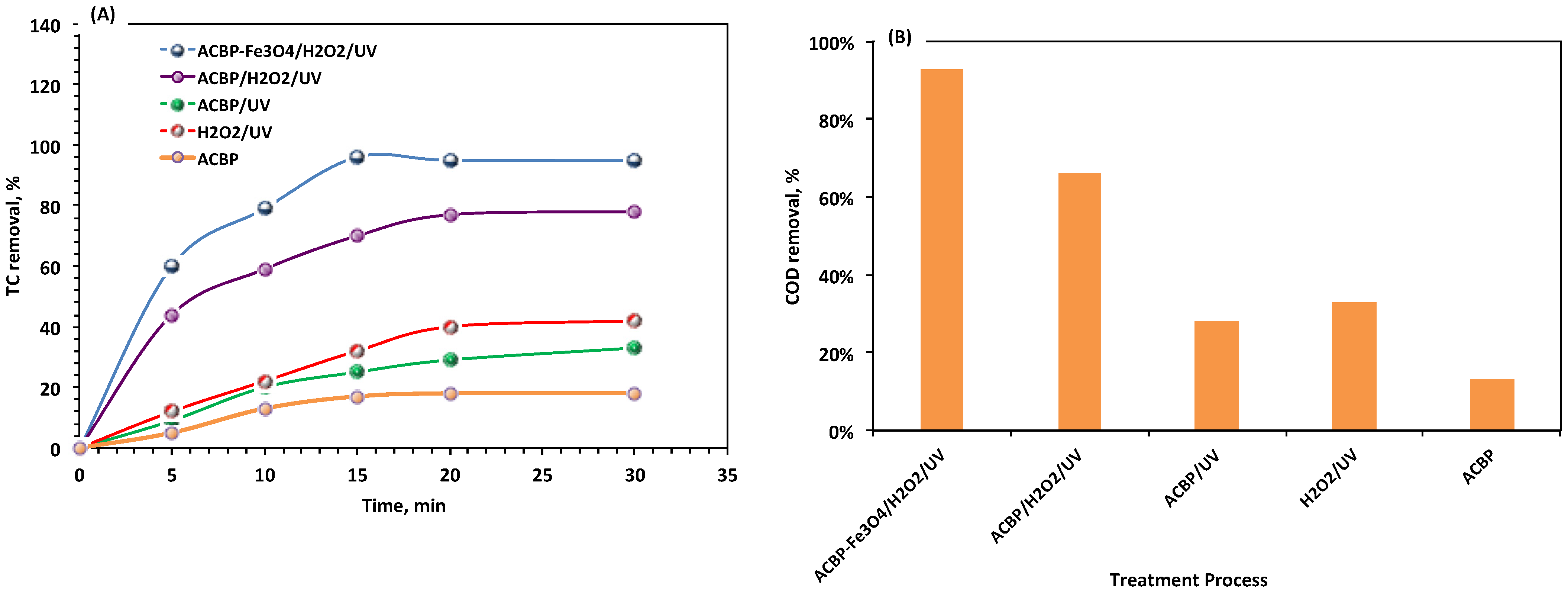
3.2.1. Effect of Oxidation Time and Different Oxidation Systems
3.2.2. Effect of Fenton’s Reagent Parameters
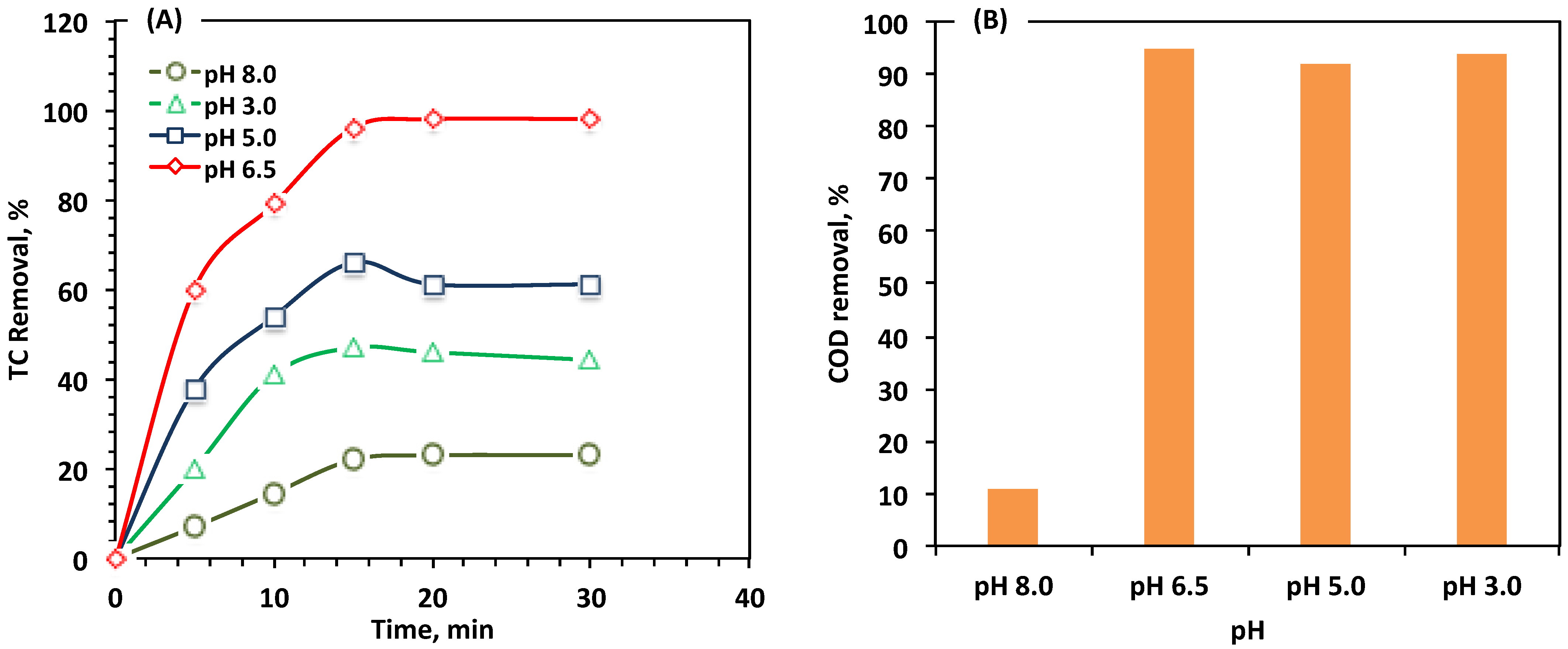
Effect of Reaction pH
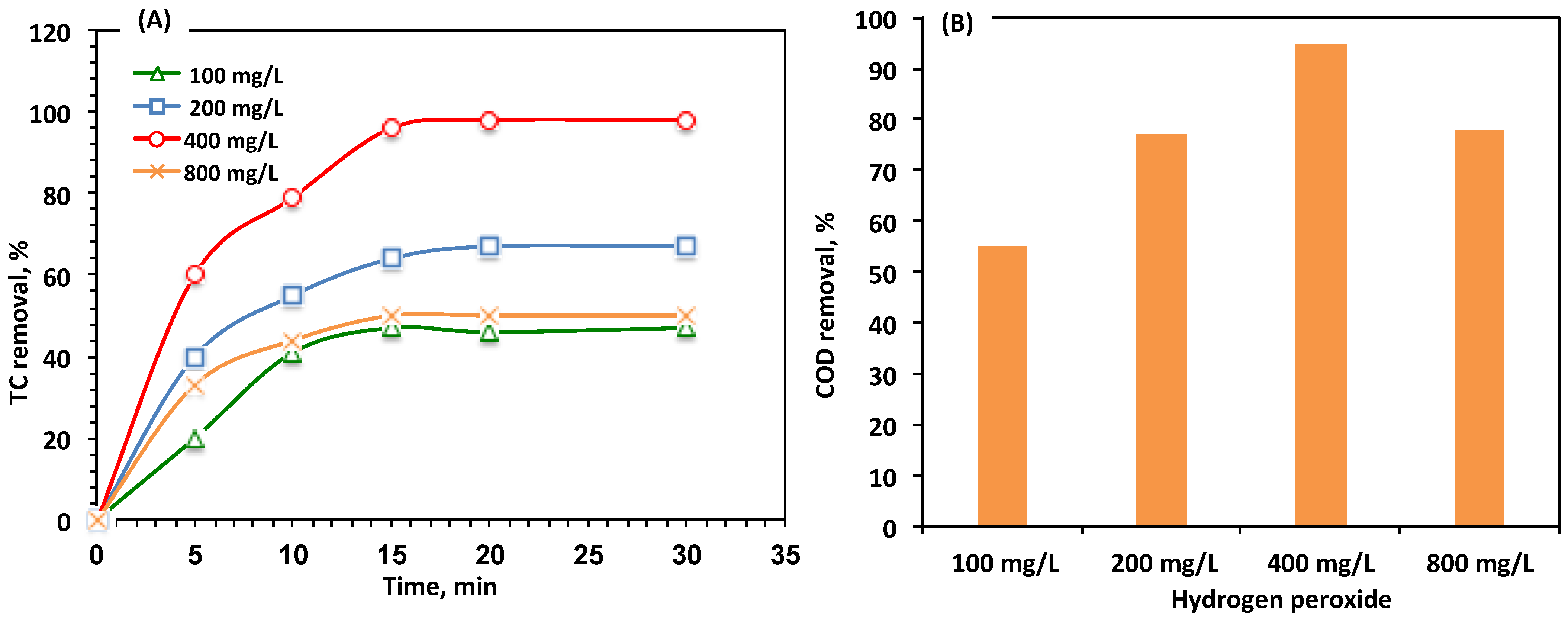
Effect of H2O2 Reagent
Effect of Catalyst “ACBP-Fe3O4” Reagent
3.3. Numerical Optimization
3.3.1. Model Establishment
3.3.2. ANOVA Test
3.3.3. Graphical Illustration
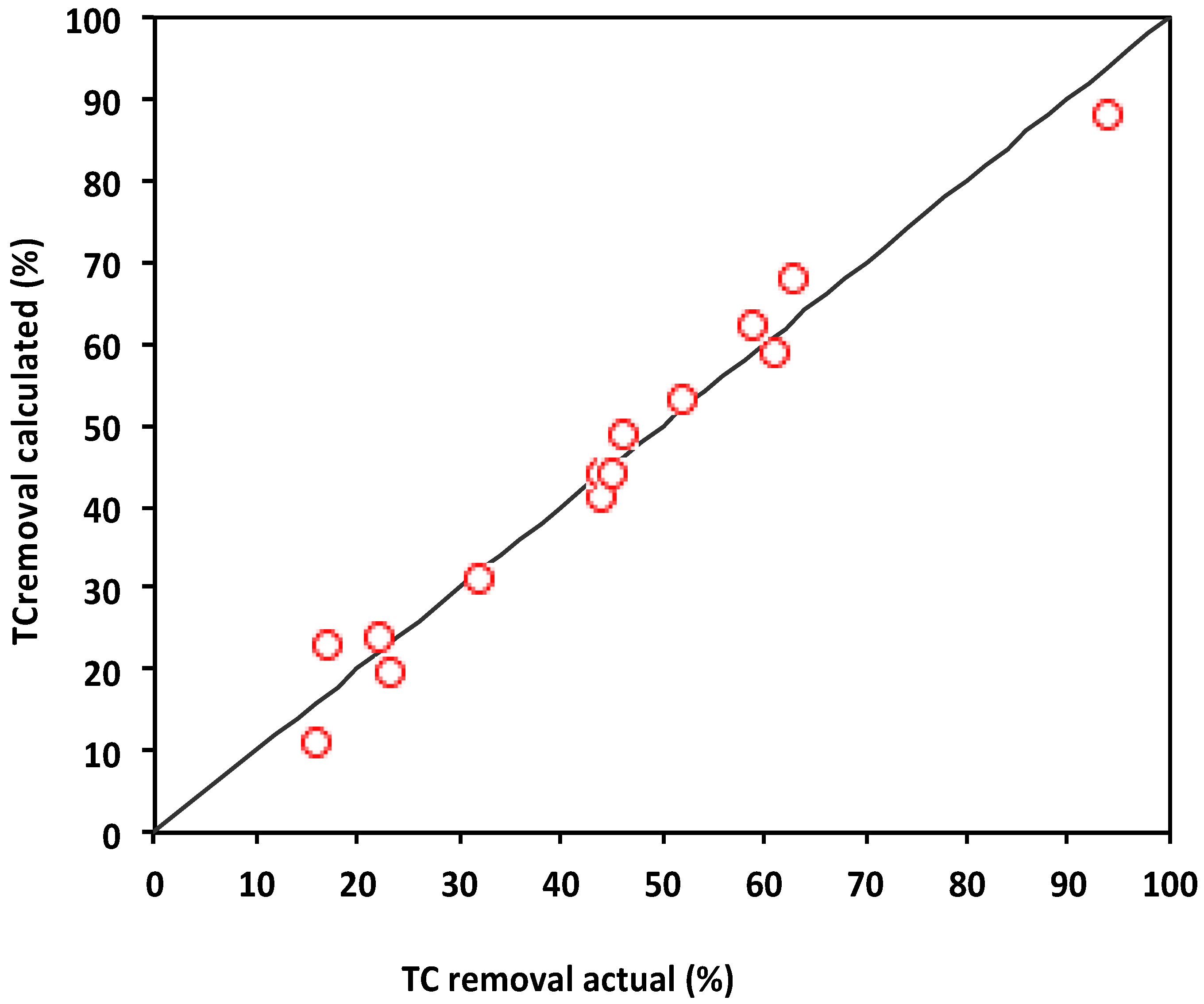
3.3.4. Model Verification
3.4. Study of Temperature on Reaction Kinetics and Thermodynamics
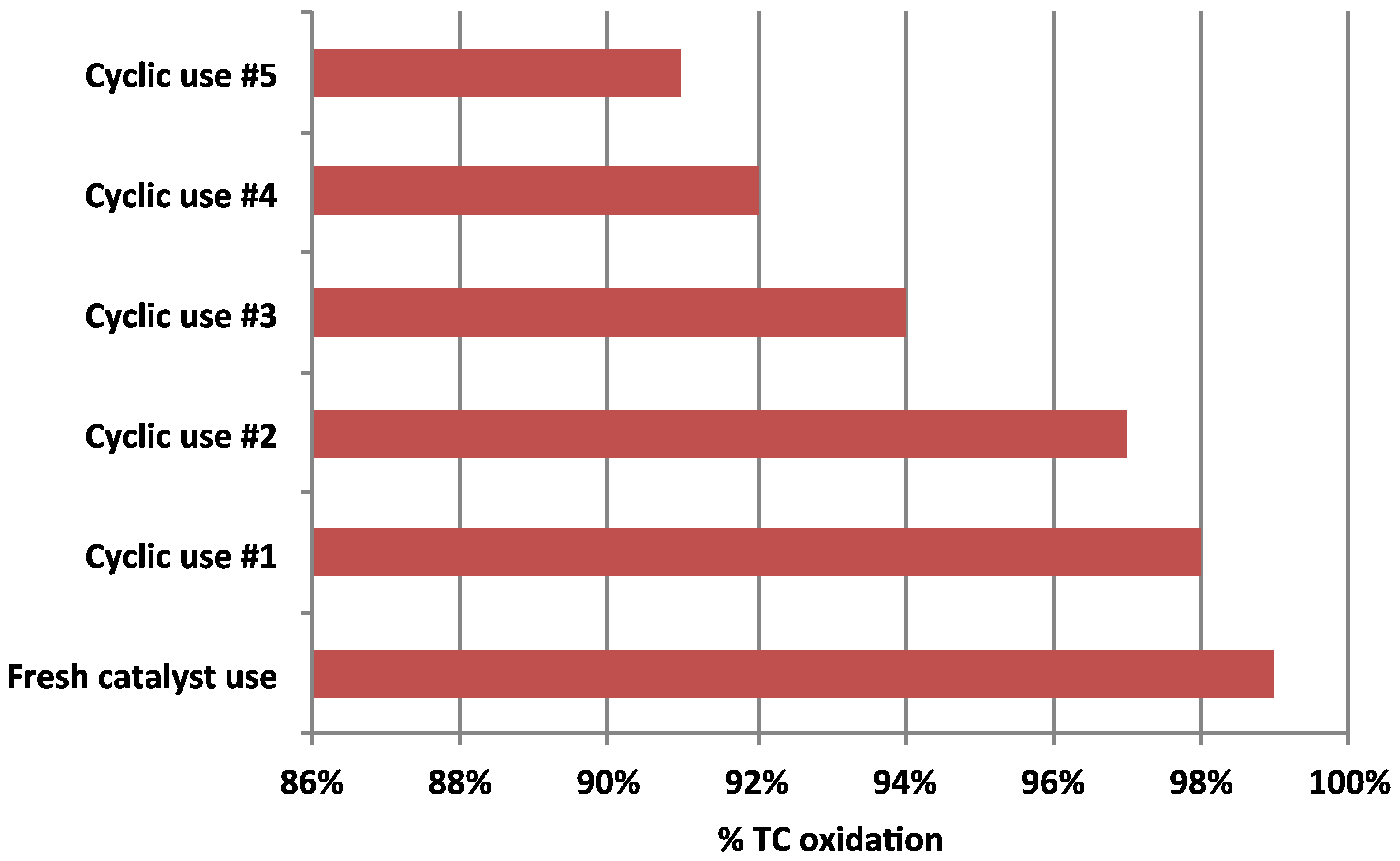
3.5. Catalyst Sustainability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bahar, M.M.; Mahbub, K.R.; Naidu, R.; Megharaj, M. As(V) removal from aqueous solution using a low–cost adsorbent coir pith ash: Equilibrium and kinetic study. Environ. Technol. Innov. 2018, 9, 198–209. [Google Scholar] [CrossRef]
- Basheer, A.A. Chemical chiral pollution: Impact on the society and science and need of the regulations in the 21st century. Chirality 2018, 30, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Basheer, A.A.; Ali, I. Stereoselective uptake and degradation of (±)-o,p-DDD pesticide stereomers in water–sediment system. Chirality 2018, 30, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Bashir, A.; Malik, L.A.; Ahad, S.; Manzoor, T.; Bhat, M.A.; Dar, G.N.; Pandith, A.H. Removal of heavy metal ions from aqueous system by ion–exchange and biosorption methods. Environ. Chem. Lett. 2019, 17, 729–754. [Google Scholar] [CrossRef]
- Beni, A.A.; Esmaeili, A. Biosorption, an efficient method for removing heavy metals from industrial effluents: A review. Environ. Technol. Innovat. 2020, 17, 100503. [Google Scholar] [CrossRef]
- Bhaumik, R.; Mondal, N.K. Optimizing adsorption of fluoride from water by modified banana peel dust using response surface modelling approach. Appl. Water Sci. 2016, 6, 115–135. [Google Scholar] [CrossRef]
- Tony, M.A.; Lin, L.S. Iron recovery form acid mine drain sludge as a Fenton source for municipal wastewater treatment. Int. J. Environ. Anal. Chem. 2020, 102, 1245–1260. [Google Scholar] [CrossRef]
- Elsayed, S.A.; El-Sayed, I.E.T.; Abdel-Bary, H.M.; Tony, M.A. Chitosan impregnated with magnetite as a versatile photocatalytic nanocomposite for Synozol Red KHL dye elimination from aqueous effluent. Int. J. Environ. Anal. Chem. 2023, 104, 7871–7897. [Google Scholar]
- Tony, M.A.; Mansour, S.A. Microwave-assisted catalytic oxidation of methomyl pesticide by Cu/Cu2O/CuO hybrid nanoparticles as a Fenton-like source. Int. J. Environ. Sci. Technol. 2020, 17, 161–174. [Google Scholar]
- Tony, M.A.; Zhao, Y.Q.; El-Sherbiny, M.F. Fenton and Fenton-like AOPs for alum sludge conditioning: Effectiveness comparison with different Fe2+ and Fe3+ salts. Chem. Eng. Commun. 2011, 198, 442–452. [Google Scholar]
- Deng, D.; Lin, L.S. Continuous sulfidogenic wastewater treatment with iron sulfide sludge oxidation and recycle. Water Res. 2017, 114, 210–217. [Google Scholar] [PubMed]
- Pan, W.; Zhang, G.; Zheng, T.; Wang, P. Degradation of p-nitrophenol using CuO/Al2O3 as a Fenton-like catalyst under microwave irradiation. RSC Adv. 2015, 5, 27043–27051. [Google Scholar]
- Laureni, M.; Weissbrodt, D.G.; Szivak, I.; Robin, O.; Nielsen, J.; Morgenroth, E.; Joss, A. Activity and growth of anammox biomass on aerobically pre-treated municipal wastewater. Water Res. 2015, 80, 325–336. [Google Scholar]
- Villegas-Guzmana, P.; Giannakis, S.; Rtimi, S.; Grandjeanc, D.; Bensimonc, M.; Alencastro, I.; Torres-Palma, R.; Pulgarin, C. A green solar photo-Fenton process for the elimination of bacteria and micropollutants in municipal wastewater treatment using mineral iron and natural organic acids. Appl. Catal. B Environ. 2017, 219, 538–549. [Google Scholar]
- Van der Zaag, P.J.; Ruigrok, J.J.; Noordermeer, A.; Van Delden, A. The effect of intragranular domain walls in MgMnZn-ferrite. J. Appl. Phys. 1993, 74, 4085. [Google Scholar]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar]
- Thiam, A.; Salazar, R.; Brillas, E.; Sirés, I. In-situ dosage of Fe2+ catalyst using natural pyrite for thiamphenicol mineralization by photoelectro-Fenton process. J. Environ. Manag. 2020, 270, 110835. [Google Scholar]
- Poormand, H.; Leili, M.; Khazaei, M. Adsorption of methylene blue from aqueous solutions using water treatment sludge modified with sodium alginate as a low cost adsorbent. Water Sci. Technol. 2017, 75, 281–295. [Google Scholar]
- Saleem, M.; Fang, L.; Ruan, H.B.; Wu, F.; Huang, Q.L.; Xu, C.L.; Kong, C.Y. Effect of zinc acetate concentration on the structural and optical properties of ZnO thin films deposited by sol-gel method. Int. J. Phys. Sci. 2012, 7, 2971–2979. [Google Scholar]
- Srivastava, V.C.; Swamy, M.M.; Mall, I.D.; Prasad, B.; Mishra, I.M. Adsorptive removal of phenol by bagasse fly ash and activated carbon: Equilibrium, kinetics and thermodynamics. Colloids Surf. A 2006, 272, 89–104. [Google Scholar]
- Tiwari, E.M.; Shukla, S.P.; Dhiman, N.; Mohan, D.; Kisku, G.C.; Roy, S. An Efficient Removal of Disperse Dye from Wastewater Using Zeolite. J. Hazard. Toxic Radioact. Waste 2017, 21, 04017017. [Google Scholar] [CrossRef]
- Tong, D.S.; Liu, M.; Lin, C.X.; Yu, W.H.; Zhi, P.X.; Zhou, C.H. Transformation of alunite residuals into layered double hydroxides and oxides for adsorption of acid red G dye. Appl. Clay Sci. 2012, 70, 1–7. [Google Scholar]
- Manera, C.A.; Tonello, P.A.; Perondi, D.; Godinho, M. Adsorption of leather dyes on activated carbon from leather shaving wastes: Kinetics, equilibrium and thermodynamics studies. Environ. Technol. 2019, 40, 2756–2768. [Google Scholar] [CrossRef]
- Doherty, L.; Zhao, Y.Q.; Zhao, X.H.; Wang, W. Nutrient and organics removal from swine slurry with simultaneous electricity generation in an alum sludge-based constructed wetland incorporating microbial fuel cell technology. Chem. Eng. J. 2015, 266, 74–81. [Google Scholar]
- El-Mekkawi, D.M.; Ibrahim, F.A.; Selim, M.M. Removal of methylene blue from water using zeolites prepared from Egyptian kaolins collected from different sources. J. Environ. Chem. Eng. 2016, 4, 1417–1422. [Google Scholar]
- Fungaro, D.A.; Silva, M. Utilization of water treatment plant sludge and coal fly ash in brick manufacturing. Amer. J. Environ. Prot. 2014, 2, 83–88. [Google Scholar]
- Geng, Y.; Zhang, J.; Zhou, J.; Le, J. Study on adsorption of methylene blue by a novel composite material of TiO2 and alum sludge. RSC Adv. 2018, 8, 32799–32807. [Google Scholar] [PubMed]
- Gomez, S.; Lerici, L.; Saux, C.; Perez, A.L.; Brondino, C.D.; Peirella, L.; Pizzio, L. Fe/ZSM-11 as a novel and efficient photocatalyst to degrade Dichlorvos on water solutions. Appl. Cata 2017, 202, 580–586. [Google Scholar] [CrossRef]
- Jangkorn, S.; Kuhakaew, S.; Theantanoo, S.; Klinla-or, H.; Sriwiriyarat, T. Evaluation of reusing alum sludge for the coagulation of industrial wastewater containing mixed anionic surfactants. J. Environ. Sci. 2011, 23, 587–594. [Google Scholar] [CrossRef]
- Guo, Y.; Xue, Q.; Zhang, H.; Wang, N.; Chang, S.; Wang, H.; Pang, H.; Chen, H. Treatment of real benzene dye intermediates wastewater by the Fenton method: Characteristics and multi-response optimization. RSC Adv. 2018, 8, 80–90. [Google Scholar]
- Bolobajev, J.; Katte, E.; Viisimaa, M.; Go, A.; Trapido, M.; Tenno, T.; Dulova, N. Reuse of ferric sludge as an iron source for the Fenton-based process in wastewater treatment. Chem. Eng. J. 2014, 255, 8–13. [Google Scholar] [CrossRef]
- Pintor, A.M.; Vilar, V.J.; Boaventura, R.A. Decontamination of cork wastewaters by solar-photo-Fenton process using cork bleaching wastewater as H2O2 source. Sol. Energy 2011, 85, 579–587. [Google Scholar] [CrossRef]
- Tony, M.A.; El-Gendy, N.S.; Hussien, M.; Ahmed, A.A.S.; Xin, J.; Lu, X.; El-Sayed, I.E.T. Nano-Magnetic Sugarcane Bagasse Cellulosic Composite as a Sustainable Photocatalyst for Textile Industrial Effluent Remediation. Catalyst 2024, 14, 354. [Google Scholar] [CrossRef]
- Bounab, L.; Iglesias, O.; González-Romero, E.; Pazos, M.; Sanroman, M. Effective heterogeneous electro-Fenton process of m-cresol with iron loaded actived carbon. RSC Adv. 2015, 5, 31049–31056. [Google Scholar] [CrossRef]
- Nabwey, H.A.; Tony, M.A.; Nour, M.M. Acetylcellulose recovery from waste residual for attenuating reactive dye from aquaculture waste as a fascinating synergistic ecology effect. Processes 2023, 11, 2701. [Google Scholar] [CrossRef]
- Xu, H.; Li, M.; Miao, J.; Zou, L. Fenton Reagent Oxidation and Decolorizing Reaction Kinetics of Reactive Red SBE. Energy Procedia 2012, 16, 58–64. [Google Scholar] [CrossRef][Green Version]
- Argun, M.E.; Karatas, E. Application of Fenton process for decolorization of reactive black 5 from synthetic wastewater: Kinetics and thermodynamics. Environ. Prog. Sus. Ener. 2011, 30, 540–548. [Google Scholar]
- Dubber, D.; Gray, N.F. Replacement of chemical oxygen demand (COD) with total organic carbon (TOC) for monitoring wastewater treatment performance to minimize disposal of toxic analytical waste. J. Environ. Sci. Health 2010, A45, 1595–1600. [Google Scholar] [CrossRef]
- Aziz, J.A.; Tebbutt, T.Y. Significance of COD, BOD and TOC correlations in kinetic–models of biological oxication. Water Res. 1980, 14, 319–324. [Google Scholar]
- Hassan, E.A.; Tony, M.A.; Nabwey, H.A.; Awad, M.M. Potential of the Biomass Waste Originating from Saccharum officinarum as a Fenton Precursor for the Efficient Oxidation of Azo Dye from an Aqueous Stream. Processes 2023, 11, 1394. [Google Scholar] [CrossRef]
- Nabwey, H.A.; Tony, M.A. Distinct pathway of multiferroic silver-decorated zinc ferrite nanocatalyst performance for Acinate insecticide oxidation. Sci. Rep. 2024, 14, 27078. [Google Scholar]
- Yuste-Córdoba, F.J.; Pérez-Salguero, C.; Santiago-Codosero, T.; Godoy-Cancho, B. Improvement of the treatment of cork boiling wastewater by solar photo-Fenton process. Results Eng. 2024, 22, 102252. [Google Scholar]
- Tony, M.A.; Lin, L.S. Attenuation of organics contamination in polymers processing effluent using iron-based sludge. Environ. Technol. 2020, 43, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lia, B.; Wang, X.; Zhao, G.; Hu, B.; Lu, Z.; Wena, T.; Chend, J.; Wang, X. Recent developments of two-dimensional graphene-based composites in visible-light photocatalysis for eliminating persistent organic pollutants from wastewater. Chem. Eng. J. 2020, 390, 124642. [Google Scholar]
- Shende, T.P.; Bhanvase, B.A.; Rathod, A.P.; Pinjari, D.V.; Sonawane, S.H. Sonochemical synthesis of Graphene-Ce-TiO2 and Graphene-Fe-TiO2 ternary hybrid photocatalyst nanocomposite and its application in degradation of crystal violet dye. Ultrason. Sonochem. 2018, 41, 582–589. [Google Scholar]
- Bosio, G.N.; García Einschlag, F.S.; Carlos, L.; Mártire, D.O. Recent Advances in the Development of Novel Iron–Copper Bimetallic Photo Fenton Catalysts. Catalysts 2023, 13, 159. [Google Scholar] [CrossRef]
- Lai, Y.J.; Lee, D.J. Solid Mediator Z–Scheme Heterojunction Photocatalysis for Pollutant Oxidation in Water: Principles and Synthesis Perspectives. J. Taiwan Inst. Chem. Eng. 2021, 125, 88–114. [Google Scholar]
- Zolfaghari, G.; Esmaili-Sari, A.; Anbia, M.; Younesi, H.; Ghasemian, M.B. A zinc oxide-coated nanoporous carbon adsorbent for lead removal from water: Optimization, equilibrium modeling, and kinetics studies. Int. J. Environ. Sci. Technol. 2013, 10, 325–340. [Google Scholar]
- Joshi, S.; Garg, V.K.; Kataria, N.; Kadirvelu, K. Applications of Fe3O4@ AC nanoparticles for dye removal from simulated wastewater. Chemosphere 2019, 236, 124280. [Google Scholar]
- Zhang, M.H.; Dong, H.; Zhao, L.; Wang, D.-X.; Meng, D. A Review on Fenton Process for OrganicWastewater Treatment Based on Optimization Perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar]
- Abdollahzadeh, H.; Fazlzadeh, M.; Afshin, S.; Arfaeinia, H.; Feizizadeh, A.; Poureshgh, Y.; Rashtbari, Y. Efficiency of activated carbon prepared from scrap tires magnetized by Fe3O4 nanoparticles: Characterisation and its application for removal of reactive blue19 from aquatic solutions. Int. J. Environ. Anal. Chem. 2020, 102, 1911–1925. [Google Scholar] [CrossRef]
- Tony, M.A.; Ali, I.A. Mechanistic implications of redox cycles solar reactions of recyclable layered double hydroxides nanoparticles for remazol brilliant abatement. Int. J. Environ. Sci. Technol. 2021, 19, 9843–9860. [Google Scholar] [CrossRef]
- Maroudas, A.; Pandis, P.K.; Chatzopoulou, A.; Davellas, L.-R.; Sourkouni, G.; Argirusis, C. Synergetic decolorization of azo dyes using ultrasounds, photocatalysis and photo-fenton reaction. Ultrason. Sonochem. 2021, 71, 105367. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Nan, Z. Formation of octahedron-shaped ZnFe2O4/SiO2 with yolk–shell structure. J. Phys. Chem. Solids 2020, 141, 109410. [Google Scholar] [CrossRef]
- Channei, D.; Thammaacheep, P.; Jannoey, P. Utilizing banana peel in conjunction with TiO2 photocatalyst for the efficient decolorization of malachite green. Chem. Phys. Impact. 2024, 8, 100629. [Google Scholar] [CrossRef]
- Eskandari, P.; Farhadian, M.; Solaimany Nazar, A.R.; Goshadrou, A. Cyanide adsorption on activated carbon impregnated with ZnO, Fe2O3, TiO2 nanometal oxides: A comparative study. Int. J. Environ. Sci. Technol. 2021, 18, 297–316. [Google Scholar] [CrossRef]
- Hami, H.K.; Abbas, R.F.; Eltayef, E.M.; Mahdi, N.I. Applications of aluminum oxide and nano aluminum oxide as adsorbents: Review. Samarra J. Pure Appl. Sci. 2021, 2, 19–32. [Google Scholar] [CrossRef]
- Serna-Jimenez, J.A.; Luna-Lama, F.; Caballero, A.; Martín, M.A.; Chica, A.F.; Siles, J.A. Valorisation of banana peel waste as a precursor material for different renewable energy systems. Biomass Bioenerg. 2021, 155, 106279. [Google Scholar] [CrossRef]
- Abdel-Khalek, A.; Hamed, A.; Hasheesh, W. The Potential Use of Orange and Banana Peels to Minimize the Toxicological Effects of Silver Nanoparticles in Oreochromis Niloticus. Bull. Environ. Contam. Toxicol. 2022, 108, 985–994. [Google Scholar]
- Ozmen, M.; Can, K.; Arslan, G.; Tor, A.; Cengeloglu, Y.; Ersoz, M. Adsorption of Cu (II) from aqueous solution by using modified Fe3O4 magnetic nanoparticles. Desalination 2010, 254, 162–169. [Google Scholar] [CrossRef]
- Singh, C.; Rubina Chaudhary, R.; Gandhi, K. Preliminary study on optimization of pH, oxidant and catalyst dose for high COD content: Solar parabolic trough collector. Iran J. Environ. Health Sci. Eng. 2013, 10, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lopez, C.; Martin-Pascual, J.; Martınez-Toledo, M.V.; Gonzalez-Lopez JHontoria, E.; Poyatos, J.M. Effect of the operative variables on the treatment of wastewater polluted with Phthalo Blue by H2O2/UV process. Water Air Soil Poll. 2013, 224, 1725. [Google Scholar] [CrossRef]
- Fu, X.; Clark, L.A.; Zeltner, W.A.; Anderson, M.A. Effects of reaction temperature and water vapor content on the heterogeneous photocatalytic oxidation of ethylene. J. Photochem. Photobiol. A Chem. 1996, 97, 181–186. [Google Scholar] [CrossRef]
- Soares, E.T.; Lansarin, M.A.; Moro, C.C. A study of process variables for the photocatalytic degradation of Rhodamine B. Braz. J. Chem. Eng. 2007, 24, 29–36. [Google Scholar]
- Buthiyappan, A.; Raman, A.; Daud, W. Development of an advanced chemical oxidation wastewater treatment system for the batik industry in Malaysia. RSC Adv. 2016, 30, 25222–25241. [Google Scholar] [CrossRef]
- Lan, B.Y.; Nigmatullin, R.; Puma, G.L. Ozonation kinetics of cork- processing water in a bubble column reactor. Water Res. 2008, 42, 2473–2482. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.S.; Mosteo, R.; Maldonado, M.I.; Malato, S.; Peres, J.A. Solar photochemical treatment of winery wastewater in a CPC reactor. J. Agric. Food Chem. 2009, 57, 11242–11248. [Google Scholar] [CrossRef]
- Ho, Y.S.; Ng, J.C.Y.; Mckay, G. Kinetics of pollutant sorption by biosorbents: Review. Seperation Purif. Method 2000, 29, 189–232. [Google Scholar]
- Ahmadi, M.; Behin, J.; Mahnam, A.R. Kinetics and thermodynamics of peroxydisulfate oxidation of Reactive Yellow 84. J. Saudi Chem. Soci. 2016, 20, 644–650. [Google Scholar]
- Caballero, A.; Hern’an, L.; Morales, J. Limitations of disordered carbons obtained from biomass as anodes for real lithium-ion batteries. ChemSusChem 2011, 4, 658–663. [Google Scholar]
- Muthukannan, V.; Praveen, K.; Natesan, B. Fabrication and characterization of magnetite/reduced graphene oxide composite incurred from iron ore tailings for high performance application. Mater. Chem. Phys. 2015, 162, 400–407. [Google Scholar]
- Hilder, M.; Winther-Jensen, O.; Winther-Jensen, B.; MacFarlane, D.R. Graphene/zinc nano-composites by electrochemical co-deposition. Phys. Chem. Chem. Phys. 2012, 14, 14034–14040. [Google Scholar] [PubMed]






| Element | C | N | S | Cr | Fe | Ni | Cu | Zn | Total |
| Weight % | 10.56 | 0.55 | 0.04 | 0.06 | 87.96 | 0.26 | 0.42 | 0.15 | 100 |
| Variable | Symbols | Range and Levels | ||||
|---|---|---|---|---|---|---|
| Natural | Coded | −1 | 0 | 1 | ||
| H2O2 (mg/L) | x1 | X1 | 350 | 400 | 450 | |
| ACBP-Fe3O4 (mg/L) | x2 | X2 | 35 | 40 | 45 | |
| pH | x3 | X3 | 6.0 | 6.5 | 7.0 | |
| Run No. | Codified Variables | Natural Variables | Response (%TC Removal) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | x1 | x2 | x3 | Experimental | Predicted | |||
| 1 | −1 | −1 | 0 | 35 | 350 | 6.5 | 61 | 59 | ||
| 2 | −1 | 1 | 0 | 35 | 450 | 6.5 | 94 | 88 | ||
| 3 | 1 | −1 | 0 | 45 | 350 | 6.5 | 17 | 23 | ||
| 4 | 1 | 1 | 0 | 45 | 450 | 6.5 | 22 | 24 | ||
| 5 | 0 | −1 | −1 | 40 | 350 | 6.0 | 32 | 31 | ||
| 6 | 0 | −1 | 1 | 40 | 350 | 7.0 | 44 | 42 | ||
| 7 | 0 | 1 | −1 | 40 | 450 | 6.0 | 46 | 49 | ||
| 8 | 0 | 1 | 1 | 40 | 450 | 7.0 | 52 | 53 | ||
| 9 | −1 | 0 | −1 | 35 | 400 | 6.0 | 59 | 62 | ||
| 10 | 1 | 0 | −1 | 45 | 400 | 6.0 | 16 | 12 | ||
| 11 | −1 | 0 | 1 | 35 | 400 | 7.0 | 63 | 68 | ||
| 12 | 1 | 0 | 1 | 45 | 400 | 7.0 | 23 | 20 | ||
| 13 | 0 | 0 | 0 | 40 | 400 | 6.5 | 44 | 44 | ||
| 14 | 0 | 0 | 0 | 40 | 400 | 6.5 | 44 | 44 | ||
| 15 | 0 | 0 | 0 | 40 | 400 | 6.5 | 45 | 44 | ||
| Source | Degree of Freedom (DF) | Sum of Squares (SS) | Mean Squares (MS) | Fisher (F-Value) | Probability (p-Value) |
|---|---|---|---|---|---|
| Model | 9 | 5850.817 | 650.0907 | 18.58287 | 0.002485 |
| Linear | 3 | 5505.25 | 5505.25 | 157.367772 | 0.15941 |
| Square | 3 | 199.025641 | 199.025641 | 5.689156 | 1.761538 |
| Interaction | 3 | 135.9359 | 135.9359 | 3.885733 | 1.115889 |
| Error | 5 | 174.9167 | 34.98333 | ||
| Total | 14 | 6025.733 | |||
| R2: 97% | |||||
| T, °C | Zero-Order Reaction Kinetics Model | First-Order Reaction Kinetics Model | Second-Order Reaction Kinetics Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ko, min−1 | R2 | t1/2, min | k1, min−1 | R2 | t1/2, min | k2, L mg−1 min−1 | R2 | t1/2, min | |||
| 28 | 0.464(±0.121) | 83 | 10.7758 | 0.047(±0.015) | 0.97 | 3.3804 | 0.241(±0.064) | 0.82 | 0.01085 | ||
| 40 | 0.344(±0.117) | 74 | 14.5348 | 0.081(±0.017) | 0.92 | 8.5556 | 0.016(±0.002) | 0.91 | 0.16351 | ||
| 50 | 0.284(±0.102) | 72 | 17.6056 | 0.064(±0.013) | 0.91 | 10.8281 | 0.008(±0.002) | 0.84 | 0.32703 | ||
| 60 | 0.708(±0.091) | 70 | 7.0621 | 0.047(±0.012) | 0.87 | 14.7446 | 0.005(±0.002) | 0.71 | 0.52326 | ||
| Temperature, °C | Ln k1 | Ea, kJ mol−1 | ∆ G′, kJ mol−1 | ∆H′, kJ mol−1 | ∆S′, J mol−1 |
|---|---|---|---|---|---|
| 28 | −1.58 | 34.3368 (±0.56) | 76.914 | 31.85 | −151.17 |
| 40 | −2.51 | 83.32 | 31.73 | −164.82 | |
| 50 | −2.74 | 86.70 | 31.65 | −170.43 | |
| 60 | −3.06 | 90.33 | 31.56 | −176.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nour, M.M.; Tony, M.A.; Nabwey, H.A.; Shaaban, S.M. Experimental Design and Numerical Optimization of Photochemical Oxidation Removal of Tetracycline from Water Using Fe3O4-Supported Fruit Waste Activated Carbon. Catalysts 2025, 15, 351. https://doi.org/10.3390/catal15040351
Nour MM, Tony MA, Nabwey HA, Shaaban SM. Experimental Design and Numerical Optimization of Photochemical Oxidation Removal of Tetracycline from Water Using Fe3O4-Supported Fruit Waste Activated Carbon. Catalysts. 2025; 15(4):351. https://doi.org/10.3390/catal15040351
Chicago/Turabian StyleNour, Manasik M., Maha A. Tony, Hossam A. Nabwey, and Shaaban M. Shaaban. 2025. "Experimental Design and Numerical Optimization of Photochemical Oxidation Removal of Tetracycline from Water Using Fe3O4-Supported Fruit Waste Activated Carbon" Catalysts 15, no. 4: 351. https://doi.org/10.3390/catal15040351
APA StyleNour, M. M., Tony, M. A., Nabwey, H. A., & Shaaban, S. M. (2025). Experimental Design and Numerical Optimization of Photochemical Oxidation Removal of Tetracycline from Water Using Fe3O4-Supported Fruit Waste Activated Carbon. Catalysts, 15(4), 351. https://doi.org/10.3390/catal15040351








