Abstract
The global shortage of freshwater resources and the energy crisis have propelled solar-driven interfacial evaporation (SDIE) coupled with photocatalytic technology to become a research focus in efficient and low-carbon water treatment. Graphene-based materials demonstrate unique advantages in SDIE–photocatalysis integrated systems, owing to their broadband light absorption, ultrafast thermal carrier dynamics, tunable electronic structure, and low evaporation enthalpy characteristics. This review systematically investigates the enhancement mechanisms of graphene photothermal conversion on photocatalytic processes, including (1) improving light absorption through surface morphology modulation, defect engineering, and plasmonic material compositing; (2) reducing water evaporation enthalpy via hydrophilic functional group modification and porous structure design; (3) suppressing heat loss through thermal insulation layers and 3D structural optimization; and (4) enhancing water transport efficiency via fluid channel engineering and wettability control. Furthermore, salt resistance strategies and structural optimization significantly improve system practicality and stability. In water treatment applications, graphene-based SDIE systems achieve synergistic “adsorption–catalysis–evaporation” effects, enabling efficient the degradation of organic pollutants, reduction in/fixation of heavy metal ions, and microbial inactivation. However, practical implementation still faces challenges including low steam condensation efficiency, insufficient long-term material durability, and high scaling-up costs. Future research should prioritize enhancing heat and mass transfer in condensation systems, optimizing material environmental adaptability, and developing low-cost manufacturing processes to promote widespread application of graphene-based SDIE–photocatalysis integrated systems.
1. Introduction
The rapid socio-economic development and significant global population growth have exacerbated freshwater shortages and energy crises worldwide, with nearly half of the population facing water stress [1,2,3]. Although the Earth has abundant water resources, only 3% is freshwater, which is unsuitable for direct consumption or use in households and agriculture [4,5]. Therefore, the development of efficient and low-energy-consumption water treatment technologies has become a core challenge in the field of environmental science. Traditional water treatment methods often face bottlenecks such as high energy consumption, reliance on chemical reagents, and secondary pollution. As a result, it is necessary to explore alternative strategies to address global freshwater scarcity and complex water pollution issues [6,7,8].
In pursuit of sustainable development goals, the utilization of clean solar energy has rapidly advanced across various fields in forms such as photochemical synthesis, photovoltaic power generation, photothermal conversion, and photocatalysis [8,9,10,11,12]. As a cutting-edge approach in this field, solar-powered interfacial evaporation (SDIE) represents a novel vapor generation methodology. This technique utilizes photothermal conversion materials operating at gas-liquid interfaces, implementing a spatially confined thermal management paradigm through its distinctive “targeted heating” mechanism. SDIE technology focuses solar energy at the water–air interface, either by heating large volumes of water with high thermal inertia or by heating only a thin layer of water at the interface, which has negligible thermal inertia and, thus, responds very quickly [13]. This technology harnesses renewable solar energy to replace traditional energy sources, obtaining clean water with minimal environmental impact, addressing freshwater shortages, and achieving low-carbon, efficient, and sustainable desalination. It has become a significant research focus in the field of clean water resource acquisition [14].
However, the singular evaporation process, being fundamentally a physical phase-change separation mechanism, exhibits constrained purification efficacy due to its dependence on pollutant vapor pressure characteristics. This process inevitably leads to co-evaporation of contaminants with water vapor. For high-boiling-point, non-volatile organic compounds, while apparent removal can be achieved through interception effects, the enrichment of pollutants in concentrated solutions still poses secondary contamination risks. Furthermore, the concentration polarization layer formed at the evaporation interface accelerates inorganic salt crystallization, inducing scaling on photothermal material surfaces that causes irreversible decay in light absorption efficiency and evaporation rate degradation. In this context, the synergistic integration of interfacial evaporation with photocatalytic processes establishes a novel paradigm for multi-energy field coordination in water treatment, enabling the efficient conversion of photo-thermal-chemical energy coupled with simultaneous pollutant removal. Photocatalytic technology leverages the photoexcitation properties of semiconductor materials to generate high-energy electron-hole pairs that initiate advanced oxidation processes (AOPs) [15]. Following photoexcitation, valence band holes directly oxidize organic compounds or react with water molecules to produce ·OH radicals, while conduction band electrons reduce O2 to form ·O2− species, ultimately mineralizing pollutants into CO2 and H2O. Nevertheless, critical limitations including high charge carrier recombination rates and low energy utilization efficiency persist as key performance constraints. Therefore, it is imperative to engineer an optimized photothermal-photocatalytic synergistic system that overcomes these inherent drawbacks while achieving enhanced evaporation performance and profound contaminant degradation.
Based on the photothermal conversion mechanism, photothermal materials are primarily categorized into four types: plasmonic metal nanoparticles [16,17,18,19], semiconductors [20,21,22], polymers, and carbon-based materials [23,24,25,26,27,28,29]. Among these, carbon-based materials have been widely used across various fields due to their excellent photothermal properties, low cost, and abundant source materials [30,31,32]. Graphene emerges as an exceptional medium for photon-to-thermal energy conversion, exhibiting intrinsic material properties uniquely suited to this energy transformation mechanism. Compared to other two-dimensional materials—such as transition metal dichalcogenides constrained by narrow-band light absorption, MXenes with limited photothermal conversion efficiency, and graphitic carbon nitride suffering from high carrier recombination rates—graphene emerges as an ideal platform for photothermal-photochemical synergistic water treatment, owing to its broadband light absorption, ultrafast hot carrier dynamics, tunable electronic structure, and low evaporation enthalpy [33]. Additionally, as a novel low-dimensional quantum material, graphene provides a unique platform for investigating photoexcited hot carrier dynamics and their energy conversion applications. Its unique electronic structure offers three key advantages: Firstly, the strong electron–electron interactions (with Coulomb interaction strength reaching ~25 eV) facilitate the thermalization process of carriers on an ultrafast timescale (approximately 50 fs), with experiments confirming that over 50% of the energy from photoexcited carriers can be efficiently converted into thermal energy of the electronic system in the initial stage [34,35]. This unique ultrafast thermalization mechanism effectively avoids the phonon scattering energy losses commonly found in traditional semiconductors [36]. Secondly, the two-dimensional Dirac fermion nature of graphene significantly reduces the electron–phonon coupling strength (with a coupling constant λ~0.05), allowing the thermalized carriers to maintain a non-equilibrium distribution for over 1 ps, which is 2–3 orders of magnitude longer than in conventional semiconductor materials. Notably, under continuous wave (CW) excitation, by optimizing the dynamic balance between light absorption and heat dissipation, a stable non-equilibrium state can be achieved where the electron temperature (Te) significantly exceeds the lattice temperature (Tph) (Te ≫ Tph). Thirdly, gate modulation allows for continuous tuning of the Fermi level within the range of 0.3–1.5 eV, enabling precise control over the Schottky barrier height, which provides an important dimension for regulating the selective energy transport of thermal carriers. Leveraging these unique quantum attributes, graphene is increasingly recognized as a prime candidate for modulable thermionic carrier injection with optimized energy conversion efficiency [37]. Despite remarkable advancements in graphene-based SDIE systems, three fundamental challenges persist: (i) insufficient mechanistic understanding of the synergistic interplay between photothermal energy conversion and interfacial fluid dynamics, (ii) unverified durability under complex aqueous matrices containing organic/inorganic contaminants, and (iii) suboptimal economic viability for scalable manufacturing.
Despite significant progress in this field, graphene-based SDIE-coupled photocatalytic systems still face critical challenges: the synergistic optimization mechanisms between photothermal conversion and photocatalytic carrier transport remain ambiguous, long-term stability under complex water matrices requires urgent verification, and the cost-effectiveness ratio of scaled-up fabrication demands further improvement. This paper mainly reviews the optimization of the graphene-based SIED system and the effective application of the graphene-based SIED catalytic system in water treatment in recent years in terms of graphene optimization modification and structural adjustment, and reviews the problems and challenges it still faces in the field of seawater desalination, in order to provide a theoretical reference and technical inspiration for the development of the next generation of efficient solar water treatment systems (Figure 1).
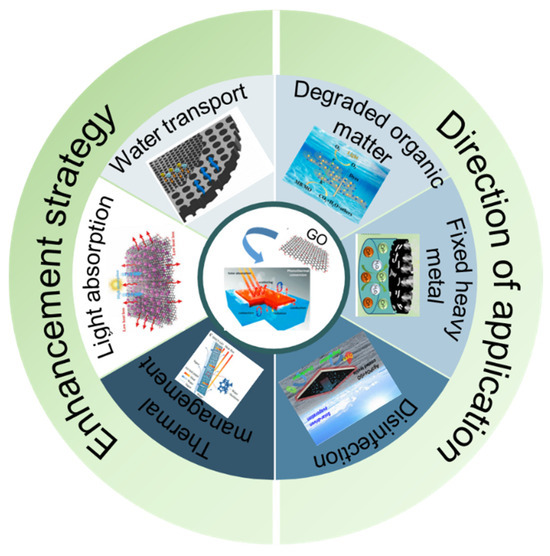
Figure 1.
Schematic of enhancement strategies and application directions for graphene-based SDIE coupled photocatalytic systems [38,39,40,41,42,43].
2. Promotion of Photocatalysis by Photothermal Conversion of Graphene
Graphene is a single-layer, two-dimensional honeycomb lattice structure material composed of carbon atoms arranged in sp2 hybridized orbitals [44]. This material has garnered ubiquitous research attention owing to its array of attractive properties, including excellent tunability [45,46,47], high fluid permeability [48,49], high thermal conductivity [50,51], abundant active sites [52,53], superior carrier mobility at room temperature [54,55,56], good electrical conductivity [57,58,59], extremely high theoretical specific surface area [60,61], excellent environmental compatibility [62], and strong adsorption capacity for both organic and inorganic molecules [63,64,65]. These characteristics collectively demonstrate the immense potential of graphene-based materials.
Graphene features both σ and π bonds, with the π bonds formed by 2p orbitals being responsible for its unique properties. On one hand, compared to the larger energy gap between σ and σ* orbitals, the π bonds, which are generally weaker than σ bonds, allow electrons to be excited from the π to the π* orbitals with relatively low energy input. The delocalized π-electron networks induce bathochromic shifts in optical absorption profiles through conjugation-length-dependent bandgap narrowing. Enhanced π-orbital overlap progressively reduces the HOMO-LUMO energy separation, enabling photon harvesting across visible to near-infrared regimes. This structural configuration supports multi-mode π-π electronic transitions under broad-spectrum solar excitation. Consequently, graphene exhibits strong absorption across a broad solar spectrum. Moreover, when excited electrons relax back to the ground state, they release heat. The large absorptive surface area and efficient photothermal conversion make graphene a representative photothermal material (Figure 2a). Additionally, a pristine monolayer of graphene has a Young’s modulus of about 1.0 TPa, a third-order elastic stiffness of approximately 2.0 TPa, and an intrinsic strength of 130 GPa. Experimental evaluations confirm the composite’s exceptional structural robustness, where graphene integration significantly improves mechanical characteristics through interfacial reinforcement mechanisms. The thermal conduction behavior in graphene arises from lattice vibrational quanta propagation dynamics, manifesting as temperature-dependent transport regimes: Disordered phonon scattering dominates at elevated temperatures, while coherent wave-like phonon transmission prevails in cryogenic conditions. State-of-the-art computational models identify defect configuration, strain field gradients, and morphological parameters as critical factors modulating thermal conductivity via selective phonon mode suppression. Although reported thermal conductivity values exhibit methodological variations, consensus measurements surpass 2000 W·m−1·K−1, substantiating graphene’s unparalleled heat dissipation capacity for advanced thermal regulation systems [66,67].
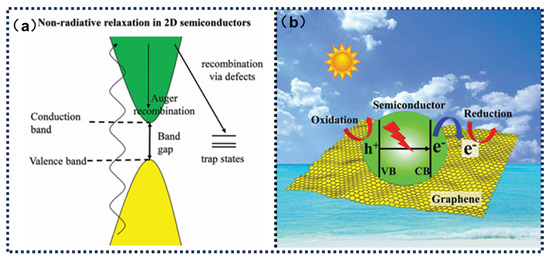
Figure 2.
(a) The photothermal mechanism of graphene. Reprinted with permission from ref. [68]. Copyright 2020 John Wiley and Sons. (b) The photocatalytic mechanism of graphene. Reprinted with permission from ref. [69]. Copyright 2025 John Wiley and Sons.
Meanwhile, the localized temperature rise induced by the photothermal effect exerts multidimensional synergistic enhancements on photocatalytic processes. First, the input of thermal energy reduces the recombination probability of photogenerated carriers by enhancing their kinetic energy to overcome the Schottky barrier at heterojunction interfaces, thereby accelerating the directional separation and migration of electron–hole pairs (Figure 2b). Second, elevated temperature significantly modulates the adsorption–desorption equilibrium of reactant molecules on the catalyst surface, lowering the reaction activation energy and promoting the diffusion mass transfer of intermediate products, which optimizes the kinetic pathways of redox reactions. More importantly, the interfacial coupling effects between graphene and photocatalytic components enable cooperative regulation of energy band structures. Photothermally induced lattice vibrations (alterations in phonon modes) may facilitate the formation of dynamic charge transport channels at heterojunction interfaces, while thermal perturbations partially compensate for the bandgap limitations of semiconductor materials, allowing carrier transitions under lower-energy photon excitation. This photothermal synergy mechanism not only enhances the comprehensive utilization efficiency of solar energy but also provides theoretical foundations for designing high-efficiency photocatalytic systems capable of adapting to ambient temperature variations. Through precise regulation of the microstructure, interfacial properties, and photothermal response behavior of graphene composites, cross-scale optimization of energy transfer and material conversion in photocatalytic reactions can be achieved, paving new pathways for clean energy conversion and environmental remediation technologies.
3. Strategies for Enhancing System of SDIE-Coupled Photocatalytic
3.1. Enhancing Light Absorption
Optical harvesting efficiency serves as a critical determinant of photothermal conversion efficacy in evaporative systems, fundamentally modulating the photonic-to-thermal energy conversion dynamics that govern vapor generation kinetics. Ideal photothermal materials should minimize the reflection and transmission of sunlight across the entire wavelength range to achieve high solar absorption and efficient photothermal conversion [70]. Graphene, with its unique electronic structure, acts as a zero-gap material with metallic-like properties and linear energy dispersion near these points. Nair and colleagues discovered that suspended graphene exhibits a broadband and relatively high light absorption rate of 2.3%. Additionally, the absorption increases linearly with the number of layers, with each additional layer increasing absorption by another 2.3% (Figure 3a) [71]. Paradoxically, the elevated infrared emissivity inherent to graphene architectures constrains their photothermal conversion efficiency, mandating hybrid integration with photon-trapping nanostructures to achieve panchromatic absorption profiles [16]. By modifying graphene-based materials through surface structure design, plasmonic material modification, defect control, and hybrid material design, researchers can develop efficient, stable, and cost-effective photothermal materials, advancing society towards more sustainable and environmentally friendly solutions.
During the interfacial solar evaporation process, the surface of the evaporator acts as the evaporation front, and its surface structure significantly influences the water evaporation behavior. Flat structures have a theoretical limit on energy efficiency due to diffuse reflection and thermal radiation. This limitation can be overcome by increasing surface roughness. Uneven structures provide a unique light-trapping space, allowing incident light to reflect multiple times, thereby significantly enhancing light absorption and photothermal conversion efficiency. Therefore, designing evaporators with high surface roughness is crucial for improving the material’s light absorption properties [70,72,73]. Zhou et al. [74] proposed a novel strategy to modulate the surface morphology of graphene oxide (GO) using polyaniline (PANI) nanocone arrays. By employing electrostatic assembly and in situ polymerization, the GO surface was fully hybridized with the PANI nanocone arrays, forming a highly folded periodic structure (Figure 3b). This structure not only enhanced the material’s light absorption capability but also improved the thermal localization effect, significantly boosting the energy conversion efficiency from sunlight to steam. Additionally, rational vacancy engineering can extend the wavelength range of light absorption, enhance broadband light absorption, alter the optical properties of materials, and increase carrier concentration, thereby improving the material’s light absorption performance. Xiong et al. [75] developed a novel photothermal platform by preparing CoCr2O4 nanocrystals with abundant oxygen vacancies. These oxygen vacancies imparted the nanoparticles with significant localized surface plasmon resonance effects and excellent photothermal properties (Figure 3c).
Additionally, the modification with plasmonic materials can enable graphene materials to exhibit significant absorption in the visible light range. When plasmonic nanomaterials absorb incident light that matches the collective oscillation frequency of conductive carriers, the carriers are excited, leading to localized surface plasmon resonance (LSPR) [76]. This phenomenon is markedly pronounced in metallic nanostructures, wherein photoexcited carrier–lattice interactions and subsequent anharmonic lattice vibrations induce substantial surface temperature increments [77]. The resultant thermal energy propagates through interfacial thermalization processes, initiating thermal diffusion within adjacent media. Plasmonically active noble metals such as Au and Ag nanoparticles demonstrate particularly strong light-matter interactions across visible wavelengths due to their characteristic localized surface plasmon resonances. Wang et al. [39] developed a solar steam generation material composed of plasmonic TiN nanoparticles and hydrophilic semi-reduced graphene oxide (semi-rGO) (Figure 3d). As shown in Figure 3e, the incorporation of TiN nanoparticles enhanced light absorption and hydrophilicity while reducing heat loss, thanks to the layered structure and strong interfacial interactions of the TiN/semi-rGO composite.
When defects are introduced into a material, they can create new electronic states within the bandgap, known as “defect states” [78]. These defect states can effectively trap photogenerated carriers, reducing recombination rates and, thereby enhancing light absorption efficiency. Additionally, certain types of point defects, such as oxygen vacancies, can reduce the bandgap of the material, making it sensitive to a broader range of the spectrum and enabling broadband light absorption [79]. This is particularly important for photovoltaic materials and photocatalysts, which need to absorb as much energy from the solar spectrum as possible [80]. Allahbakhsh et al. [81] demonstrated that oxygen-containing functional groups and other types of defects in graphene-based nanostructures can act as phonon scattering sites. By trapping electrons in defect states and converting their energy into heat, these defects contribute to localized heat generation. This not only enhances the SDIE of water molecules but also reduces energy dissipation into the bulk water (Figure 3f).
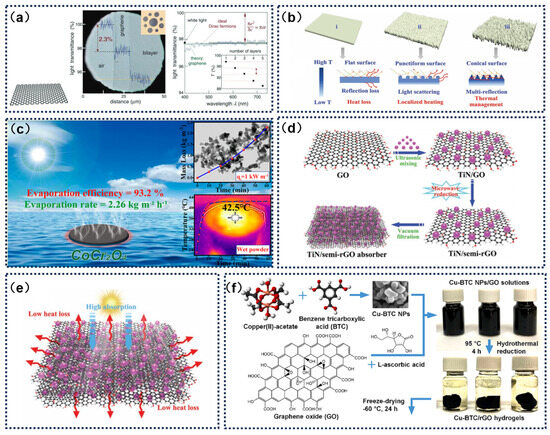
Figure 3.
(a) Light absorption in two-dimensional layers. Reprinted with permission from ref. [71]. Copyright 2019 John Wiley and Sons. (b) Surface-modified PANI/GO composites for solar evaporation. Reprinted with permission from ref. [74]. Copyright 2022 John Wiley and Sons. (c) CoCr2O4 nanocrystals with abundant oxygen vacancies. Reprinted with permission from ref. [75]. Copyright 2023 American Chemical Society. (d) Schematic of the fabrication of plasmonic Tin/semi-RGO nanohybrid solar absorbers. (e) Illustration of the light absorption and heat loss suppression in tin/semi-RGO absorbers. Reprinted with permission from ref. [39]. Copyright 2023 John Wiley and Sons. (f) Fabrication of Cu-BTC/RGO nanocomposite aerogels. Reprinted with permission from ref. [81]. Copyright 2023 Copyright Elsevier.
3.2. Enhancement of Photon Conversion Efficiency
To optimize the photon conversion efficiency of a system, it is essential to synergistically enhance photothermal–photochemical energy transfer pathways and regulate carrier spatial distribution. Common strategies include utilizing wide-bandgap semiconductor heterojunctions, coupling plasmonic metal nanostructures, and implementing defect engineering to suppress carrier recombination and improve full-spectrum photon utilization. Among these, constructing heterojunctions has proven effective for enhancing carrier separation efficiency.
For instance, Wang et al. [82] reported a C3N4/NiIn2S4 heterostructure synthesized via a facile solvothermal method, demonstrating optimized photon utilization efficiency in SDIE and photocatalytic H2O2 production (Figure 4a). The incorporation of NiIn2S4 extended light absorption and enhanced visible-light harvesting, while the heterojunction effectively suppressed photogenerated carrier recombination, enabling efficient charge separation and transport. Under one-sun illumination, the system achieved a solar steam evaporation rate of 3.25 kg·m−2·h−1 with 215.06% efficiency, along with a photocatalytic H2O2 production rate of 1080 μmol·L−1·h−1 under visible light. Gu et al. [83] developed a sunflower-inspired PVA-based hydrogel evaporator featuring vertically aligned microchannels and MXene@TiO2@g-C3N4 heterojunctions to optimize photon utilization for efficient water management (Figure 4b). The vertical microchannels enhanced broadband absorption through multiple light reflections, while the synergistic photothermal effects of polypyrrole (PPy) and MXene enabled an evaporation rate of 3.0 kg·m−2·h−1 and 97.2% energy efficiency under one-sun irradiation. The heterojunction promoted carrier separation via band structure modulation and oxygen vacancy (Ov) generation, extended carrier lifetime, and reduced charge transfer resistance, achieving >94.2% degradation efficiency for high-concentration VOCs under visible light (Figure 4c). Mo et al. [84] designed a bioinspired SDIE system integrating photothermal and photocatalytic functions in TiO2/Ti3C2/C3N4/PVA (TTCP) hydrogel (Figure 4d). The Ti3C2/C3N4 heterojunction broadened light absorption, while vertically aligned microchannels enhanced light trapping through multiple reflections. A Z-scheme heterojunction facilitated carrier separation, and the photothermal effect boosted reactive oxygen species generation (Figure 4e). This system achieved 1.54 kg·m−2·h−1 evaporation rate with 84.1% energy efficiency under one-sun irradiation, along with 69.4–100% removal efficiency for high-concentration VOCs.
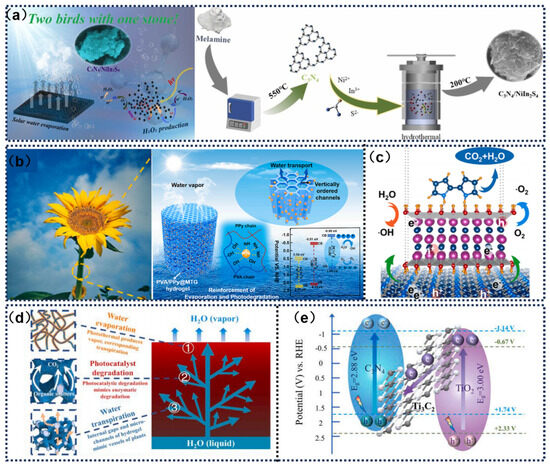
Figure 4.
(a) Schematic diagram of C3N4/NiIn2S4 composite photothermal evaporation system. Reprinted with permission from ref. [82]. Copyright 2025 Elsevier. (b) The PVA/PPy@MTG hydrogel evaporator schematic diagram with vertically ordered channels for integrated solar-driven evaporation and photodegradation. (c) The catalytic degradation mechanism of the MTG-2 heterojunction for contaminant. Reprinted with permission from ref. [83]. Copyright 2025 Elsevier. (d) S Schematic diagram showing the similarities between a plant and the bionic system. (e) Schematic of the Z-scheme heterojunction of C3N4 and TiO2/Ti3C2 hydrogels. Reprinted with permission from ref. [84]. Copyright 2025 Elsevier.
3.3. Reducing the Enthalpy of Water Evaporation
The primary goal of enhancing photothermal conversion efficiency is to provide more energy to increase the solar evaporation rate. Similarly, reducing the energy required for water evaporation can achieve faster evaporation rates under the same energy input conditions. By decreasing the energy demand during the evaporation process, evaporation efficiency can be significantly improved without increasing energy input. This approach allows for the evaporation of more water molecules with limited energy input. Under normal conditions, due to the strong interactions between water molecules (i.e., hydrogen bonds), the enthalpy of water evaporation at 100 °C is approximately 2260 kJ kg−1 [85,86]. Hydrophilic functional groups can effectively dissociate the hydrogen bond network of large water clusters, a mechanism closely related to the solvation effect. Studies have shown that surface polar groups preferentially adsorb adjacent water molecules by forming intermolecular hydrogen bonds, thereby weakening the original cooperative interactions between water molecules [87,88]. In addition to chemical modification strategies, capillary effects have also been shown to significantly reduce the energy required for water phase change When capillary nanochannels are constructed within materials, the resulting ultrathin water films induce dual effects: Firstly, they promote the formation of a three-phase contact line at the edges of nanostructures, and secondly, they generate microdroplets through surface tension, effectively dissociating water molecule aggregates [89]. For example, in graphene-based materials, there is bound water interacting directly with polymer chains, free water within the graphene lattice, and intermediate water between them. The strong interactions between hydrophilic functional groups and bound water weaken the hydrogen bonds of intermediate water molecules, further reducing the enthalpy of vaporization. As an effective strategy to accelerate water evaporation, the proportion of intermediate water can be increased by rationally designing graphene materials with appropriate surface functionalization and structural modulation [90].
Li et al. [91] achieved efficient solar-driven water purification by constructing an MXene/graphene oxide/polyaniline (MGP) plastic composite with a patternable surface (Figure 5a,b). This material utilizes polyaniline to assist in assembling MXene and graphene oxide, forming a structure rich in surface functional groups. When water molecules come into contact with the functional groups on the surface of GO or MXene, the hydrogen bond network between them is rearranged. This new hydrogen bond network is more ordered than that in free water, reducing the number of hydrogen bonds between water molecules. As a result, the cohesive forces between water molecules are weakened, making it easier for them to transition into the gaseous state, thereby lowering the enthalpy of evaporation.
Additionally, the internal structure and channels of porous materials can provide more surface area for water molecules to interact. By optimizing the composition and structure of these materials, the proportion of intermediate-state water can be increased, further reducing energy consumption during the overall evaporation process. For example, through directional assembly methodologies, Xu et al. [92] reported a SDIE based on a reduced graphene oxide/sodium alginate (rGO/SA) hydrogel sponge (RSHS), constructed on a melamine sponge (MS) skeleton through a dipping–drying–crosslinking process (Figure 5c). This design significantly reduces the evaporation enthalpy of water and enhances evaporation efficiency. The porous structure plays a critical role: The interconnected porous network of the MS skeleton provides efficient channels for water transport and steam overflow, while hydrophilic groups in the rGO/SA hydrogel disrupt the equilibrium of water molecule clusters through hydrogen bonding, forming more intermediate water (IW) with lower evaporation enthalpy. LEE et al. [93] explored the impact of Å-scale oxidized functionalized graphene nanopores on water evaporation rates at atomically thin interfaces (Figure 5d). The study found that water molecules near these nanopores exhibited rapid rotational and translational motion, attributed to reduced hydrogen bonding and shorter bond lifetimes between water molecules. Furthermore, the presence of nanopores at the atomic thin-layer interface lowered the free energy barrier for water molecules transitioning from the liquid to the vapor phase, explaining why these nanopores can enhance evaporation flux (Figure 5e–g).
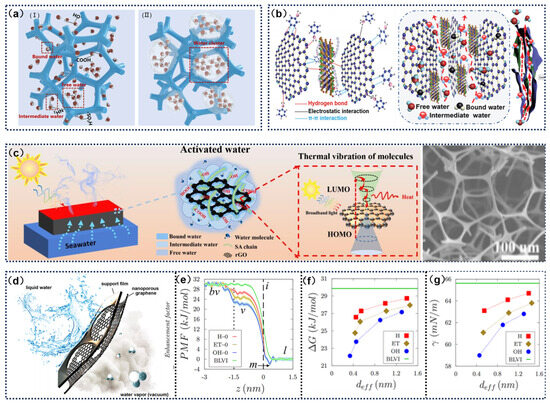
Figure 5.
(a) Mechanism of (I) introducing hydrophilic functional groups and (II) constructing porous materials for enthalpy reduction. (b) Interaction between PANI, GO, and MXENE sheets and the interaction of oxygen-containing groups with water. Reprinted with permission from ref. [91]. Copyright 2021 John Wiley and Son. (c) Schematic diagram of RSHS evaporation system. Reprinted with permission from ref. [92]. Copyright 2023 Elsevier. (d) Schematic of Å-scale oxidized functionalized graphene nanopores. (e) Force curve of evaporating water molecules through nanopores with different regions. (f) Free energy barrier from bulk liquid water to vapor phase. (g) Surface tension at different water vapor interfaces. Reprinted with permission from ref. [93]. Copyright 2022 American Chemical Society.
3.4. Optimization for Environmental Energy Applications
According to the second law of thermodynamics, heat naturally flows from regions of higher temperature to those of lower temperature [94,95]. However, during the evaporation process, the photothermal effect at the surface often results in a surface temperature that is higher than that of the underlying bulk water and surrounding air. Consequently, photothermal materials dissipate energy into the surrounding environment, preventing ideal solar thermal conversion applications. This necessitates the careful design and management of energy distribution and loss. When the evaporation system is passively activated by sunlight, three primary types of heat loss are considered: energy loss to the bulk water through thermal conduction, energy loss to the air through convection, and energy loss to the air through radiation [96,97].
Avoiding direct contact between photothermal materials and bulk water can effectively suppress conductive heat loss. Because water has a relatively high thermal conductivity (0.5–0.7 W m−1 K−1), research on heat conduction in the evaporation process mainly focuses on the energy transfer from photothermal materials to bulk water. Therefore, introducing a low thermal conductivity insulating layer to separate the photothermal materials from the bulk water can also prevent conductive heat loss. The heat flux of conduction to bulk water (Qcon) can be calculated using the following Equation (1):
where K represents the thermal conduction coefficient of the interfacial medium bridging the heated surface and cooler bulk water. The parameter A denotes the effective contact area responsible for thermal energy transfer between the solid material and surrounding water body. Tm indicates the temperature at the photothermally active interface of the material, while Tw corresponds to the thermal condition at the water contact boundary. The variable l quantifies the spatial separation between the heat-generating surface (material interface) and the cooling interface (water contact plane).
Strategic engineering of absorber surface topography and dimensional hierarchy enables the precise regulation of thermal and molecular flux in solar-powered interfacial distillation systems. Gong et al. [98] fabricated an integrated carbon-based layered system via the controlled synthesis of orientation-engineered graphene architectures on graphite fibrous substrates, examining how multiscale graphene structural features (from micron-level patterning to nanoscale texturing) influence energy and mass transfer dynamics at absorber–liquid interfaces. The study revealed that, compared to smooth surfaces, nanostructured surfaces exhibited a higher density and smaller diameter of bubble distribution (Figure 6a–c). These characteristics significantly enhanced heat transfer efficiency through micro-convection and transient conduction mechanisms, thereby improving heat transfer and water evaporation efficiency. Additionally, constructing the macroscopic structure of the evaporator with insulating layers can reduce conductive heat loss. Liang et al. [99] developed a solar steam generation system based on reduced rGO foam, utilizing an injection control technique (ICT) to regulate the capillary water state and optimize evaporation efficiency (Figure 6d). The rGO foam exhibits a full-spectrum high absorption rate and low thermal conductivity, effectively converting solar energy into thermal energy while minimizing heat dissipation. This design rapidly raises the surface temperature to 88.7 °C under illumination and maintains stability. By balancing the water supply rate and evaporation rate through ICT, the system prevents blockage of micrometer-sized pore channels, maximizes the evaporation area, and reduces heat absorption by excess water, thereby achieving thermal localization. Han et al. [100] demonstrated a graphene G@ZIF nano-hybrid structure using metal–organic framework (MOF) isolation. This material leveraged the synergistic effects of an ultrathin and insulating ZIF layer and the G@ZIF interfacial nano-cavities to enhance light absorption and concentrate heat. Under artificial solar illumination, G@ZIF composites reach a thermal peak of 120 °C under ambient conditions and exhibit a near-uniform (98%) photon-to-thermal energy conversion efficiency. The molecular screening porosity of the ZIF framework helps small molecules to selectively enter the thermal interface embedded in graphene, thus enabling guided heat flux propagation (Figure 6e–i).
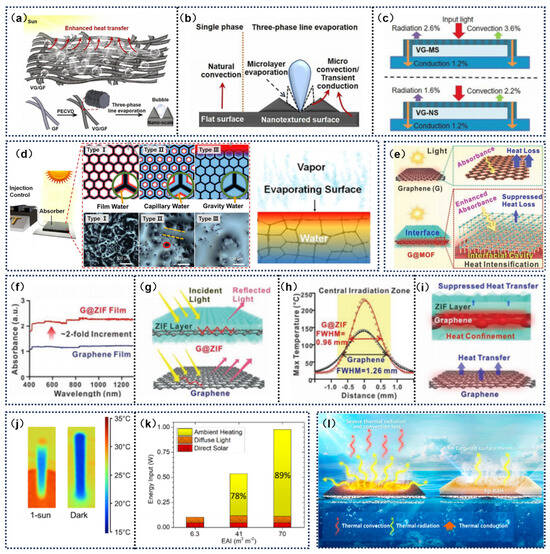
Figure 6.
(a) Schematic illustration of bubble formation on a nanotextured surface during SDIE. (b) Mechanism of enhanced heat transfer through nanostructuring. (c) Heat transfer diagram of microstructured and nanostructured absorbers during evaporation. Reprinted with permission from ref. [98]. Copyright 2022 Elsevie; (d) Schematic of the formation process of a composite material made from hollow melamine foam and rGO. Reprinted with permission from ref. [99]. Copyright 2019 John Wiley and Sons. (e) Diagram of G@MOF, which enhances light absorption and suppresses heat loss, thereby concentrating thermal energy at the confined interface. (f) UV-Vis-NIR absorption of graphene and g@ZIF membranes. (g) Enhanced light absorption of g@ZIF. (h) Spatial temperature distribution in the central irradiated area under laser illumination at maximum equilibrium surface temperature. (i) Thermal localization and suppression of heat transfer in G@ZIF. Reprinted with permission from ref. [100]. Copyright 2021 John Wiley and Sons. (j) 3D thermal images of stems under light and dark conditions. (k) Contributions of direct solar energy, diffuse light, and ambient heating to energy input. Reprinted with permission from ref. [101]. Copyright 2021 American Chemical Society. (l) Schematic of hp RAH photothermal films. Reprinted with permission from ref. [102]. Copyright 2021 American Chemical Society.
In solar-driven water evaporation, the generated steam can potentially carry away some heat energy through thermal convection. Additionally, if the heated water does not evaporate promptly, it may transfer its energy to cooler water through convection. The core of SDIE is the separation of the heat source from the bulk water, but the heated water might still transfer heat to cooler water through free flow and diffusion. Regulating the direction of water transport and the water flux can help reduce the transfer of heat from high-temperature water to low-temperature water via convection (2).
In this thermodynamic model, h is the thermal convection coefficient. Based on the coupling mechanism of multiple physical fields, the surface heat dissipation can be reduced by selecting functional materials with radiation suppression characteristics, and the directional regulation of heat-mass transfer can be achieved by optimizing the structure of the evaporation system driven by phase transformation to reduce heat loss. This cross-scale thermal control strategy achieves accurate control of the thermodynamic balance of the evaporation process by reducing the radiation/conduction loss coefficient at the material level and enhancing the convective heat transfer efficiency at the system level.
Finnerty et al. [101] constructed three-dimensional graphene oxide (3D GO) rods, which, due to their unique 3D structure, can more effectively compress the boundary layer thickness (Figure 6j). This reduces the distance that water molecules need to diffuse and enhances the evaporation driving force. Additionally, the longer average flow path length means that the boundary layer remains thin and continues to develop over a larger surface area of the 3D structure, making the 3D GO rods more responsive to increases in external convection speed. The 3D GO rods not only excel in light absorption and heat localization but also show great potential in promoting thermal convection, accelerating water evaporation, and optimizing the overall efficiency of the system (Figure 6k).
Since photothermal materials absorb light energy and convert it into heat, the temperature of the photothermal layer is generally higher than that of the surrounding environment. As a result, these materials release energy to their surroundings through radiation. The heat flux of radiation Qrad can be calculated using the Stefan–Boltzmann law (3) as follows:
where ε represents the radiant efficiency of a photothermally active surface, and σ represents the universal radiant coefficient in the Stefan–Boltzmann law. Te reflects the ambient thermal distribution. The analysis shows that effective thermal radiation management can be achieved by optimizing the emissivity parameters of the energy harvesting surface, minimizing the radiation interface size and maintaining the thermal difference between the functional surface and the surrounding medium.
Meng et al. [102] developed a hierarchical porous radiative absorption hydrogel membrane (hp-RAH), which significantly enhances solar evaporation efficiency by optimizing thermal radiation management in the photothermal layer (Figure 6l). This hydrogel membrane is coated on the evaporator surface, and the hp-RAH exhibits high absorption in the mid-infrared band. It captures the thermal radiation energy released from the photothermal layer and reuses it for water heating while blocking convective heat loss. Combined with its hierarchical porous structure, the system reduces the originally dissipated thermal radiation and convective losses from 6.6% to 0.39%, achieving effective thermal localization.
3.5. Water Transport Regulation
Adequate and continuous water supply is considered one of the key factors for achieving sustained evaporation in solar evaporators. However, the stacking or agglomeration of graphene nanosheets can lead to inefficient water transfer and vapor escape during the evaporation process. Therefore, ensuring good wettability, designing effective water transport channels, and regulating pumping mechanisms can not only reduce the accumulation of concentrated salts during evaporation but also adjust the heat distribution between the evaporator and the surrounding environment. This can create temperature gradients or cool evaporative surfaces, effectively enhancing the evaporation performance of the system [103,104].
A suitable pore size in micro/nanoporous structures facilitates rapid water transport within graphene driven by capillary action, ensuring efficient evaporation under solar irradiation [105]. Lee et al. [106] developed a nanoporous capillary membrane based on assemblies of highly porous graphene oxide (HGO) nanosheets, which features nanopores and nanochannels to ensure high capillary water flow through low-friction nanochannels. The study found that nanopores formed by highly oxidized sp3 regions and an increased proportion of graphitic regions favor the water transport process and enhance water stability. Additionally, the presence of large nanopores with a broad size distribution (1–40 nanometers) leads to faster evaporation rates and a larger vapor transport surface area (Figure 7a). Lim et al. [38] introduced a layered chitosan aerogel embedded with graphene nanoplatelets (GnP), featuring distinct density variations between its inner and outer sections. The differing densities in these regions result in specialized water transport properties: The low-density, large-pore area allows for swift movement and evaporation of water molecules, whereas the high-density, small-pore region boosts the material’s structural integrity and minimizes surface energy, thus enhancing its ability to retain water (Figure 7b,c). This design effectively combines the advantages of both density variations, optimizing the aerogel’s performance in water management and mechanical strength.
The continuous water-transport capability of graphene-based evaporators largely depends on their wettability. For photothermal interfaces, liquid water tends to spread and form a thin film on hydrophilic surfaces, while accumulating as thicker droplets on hydrophobic surfaces [107]. Previous studies have shown that due to the inherent non-polar carbon structure and rough surface of GO, GO composite aerogels typically exhibit some degree of hydrophobicity, with water contact angles ranging from 120° to 160°. Wettability can be adjusted by incorporating oxygen-containing functional groups or highly absorbent polymers to enhance water transport. Xu et al. [108] developed three-dimensional, hierarchically porous N-doped lignosulfonate/GO aerogels (NLGA) through a single-step solvothermal assembly protocol. By employing ethylenediamine-mediated nitrogen doping strategy and utilizing lignosulfonate’s inherent hydrophilic functionalities as a multifunctional crosslinking agent bearing abundant hydrophilic moieties, this synthesis simultaneously achieved pore architecture engineering and surface chemistry optimization. The coordinated lignosulfonate–graphene interactions synergistically enhanced both surface wettability and hydraulic permeability through controlled capillary networks (Figure 7d).
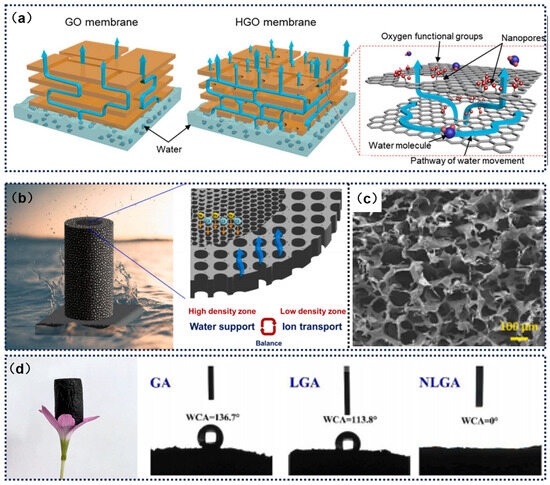
Figure 7.
(a) High evaporation rate and accelerated water transport rate in HGO membranes. Reprinted with permission from ref. [106]. Copyright 2022 Elsevier. (b) Schematic of a 3D laminated chitosan/graphene nanoskeleton evaporator, which provides a balance between water transport and ion transfer. (c) Field emission scanning electron microscope (Fe-SEM) image of LCG. Reprinted with permission from ref. [38]. Copyright 2024 Elsevier. (d) Photograph of NLGA. Reprinted with permission from ref. [108]. Copyright 2023 Elsevier.
3.6. Desalination Strategies
During SDIE operation, convection mass transfer drives salt accumulation at the photothermal interface. When the thermodynamic supersaturation threshold is reached, the crystal precipitation induces a basting phenomenon, which reduces the optical collection efficiency and induces microfluidic channel obstruction, thus limiting the photothermal conversion and disrupting the liquid phase transport. Therefore, salt mitigation strategies are crucial for enhancing the operational lifespan and long-term stability of SDIE systems. While salt fouling can be addressed by rinsing and soaking the photothermal surface after evaporation cycles, this increases costs and may damage the photothermal surface [109]. To tackle this issue, researchers have developed various effective strategies to control salt fouling, which can be broadly categorized as follows: First, achieving salt resistance by regulating water/ion transfer within the photothermal evaporator; secondly, controlling salt precipitation at specific locations on the photothermal evaporator; and finally, implementing dynamic self-regulation of desalination through photothermal evaporators with specific morphologies.
Firstly, by optimizing the hydraulic transport and ion diffusion paths within the evaporator, the excessive accumulation of salt ions on the photothermal surface can be effectively suppressed. Zhang et al. [110] designed a salt-resistant Fe3+@GOM heterogeneous evaporator, utilizing a Janus structure to achieve directional water transport through capillary force differences at the hydrophilic–hydrophobic interface (Figure 8a). Combined with a graphene oxide membrane, Fe3+ cations further enhance the Donnan effect, effectively repelling multivalent salt ions and reducing advection-driven salt deposition from the source, thereby improving salt resistance. Secondly, controlled salt precipitation strategies guide salt crystallization to non-critical areas through structural design, thus preventing the photothermal surface from being covered by salt layers (Figure 8b). Zhou et al. [111] developed an innovative 3D Graded Graphene Helical Sponge (GGS Sponge) using an environmentally friendly fabrication process. This material demonstrates unique advantages in achieving zero liquid discharge (ZLD) desalination of high-concentration brine. The study shows that its helical structure significantly enhances energy capture efficiency during the photothermal conversion process, while the graded network system creates a synergistic effect by optimizing radial water transport paths and directional salt crystallization mechanisms. This structural coupling endows the material with multiple functional properties: It maintains a stable evaporation rate under solar radiation, efficiently collects salt, achieves water purification to ZLD standards, and exhibits excellent anti-fouling performance and structural durability. Additionally, the dynamic self-regulating morphology desalination strategy leverages the responsive characteristics of smart materials to actively disrupt salt crystal adhesion through interfacial deformation or changes in physical properties (Figure 8c). Liu et al. [112] introduced a water/light dual-responsive actuator composed of double-twisted hollow hydrogel fibers loaded with reduced graphene oxide (RGO@HHF). The double-twisted RGO@HHF can elongate to absorb water and contract to evaporate seawater under light stimulation due to differences in thermal expansion coefficients. During the actuation process, mechanical stress causes salt crystals to detach, and the accumulated salt dissolves back into the seawater (Figure 8d,e).
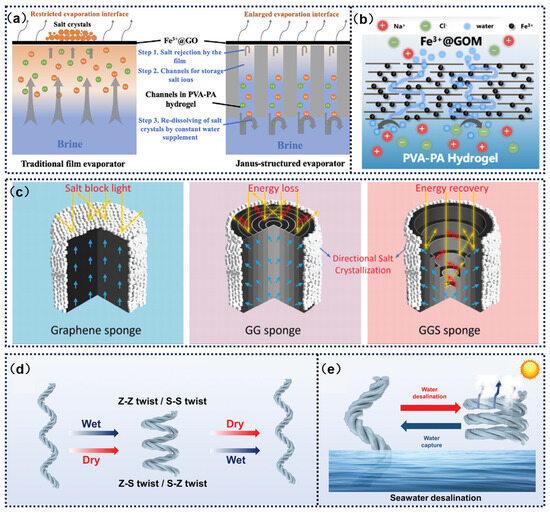
Figure 8.
(a) Mechanism of effective salt resistance in FHJE. (b) Illustration of effective salt resistance mechanism via the Donnan effect with Fe3+@GOM. Reprinted with permission from ref. [110]. Copyright 2023 American Chemical Society. (c) Salt crystallization on the surface of graphene sponge. Reprinted with permission from ref. [110,111]. Copyright 2024 American Chemical Society. (d) Schematic of the responsive behavior of RGO@HHF. (e) Application of RGO@HHF in seawater desalination. Reprinted with permission from ref. [110,112]. Copyright 2023 John Wiley and Sons.
4. Structural Design of Graphene-Based SDIE-Coupled Photocatalytic Systems
The concepts introduced above highlight the development of effective evaporation systems from the perspective of graphene material modification. In addition, researchers have also achieved significant improvements in evaporation efficiency by designing and enhancing the structure of evaporators [96,113]. These designs can be categorized into three types. (1) Single-Layer Structures: These are primarily membrane or thin sheet structures and represent the most basic design for SDIE. They typically consist of a single functional layer. (2) Double-Layer and Multi-Layer Structures: These designs separate the water molecule channel layer from the thermal insulation layer. Through functional stratification, they optimize light absorption, water transport, and thermal insulation properties. (3) 3D Structures: By employing three-dimensional designs, these structures increase the evaporation area, optimize light absorption paths and water transport channels, and effectively reduce heat loss from the device.
4.1. Monolayer Structure
The monolayer structure serves as the foundational configuration for graphene-based SDIE systems. Its core design principle is to achieve the synergistic coupling of photothermal conversion, water transport, and vapor release through a single functional layer, thereby simplifying system complexity and reducing fabrication costs [114,115]. Wo et al. [116] developed an integrated novel wood evaporator (DBW-GC) using ultrasound-assisted deposition. This evaporator is composed of defined camphor wood, reduced graphene oxide, and partially reduced CuO/Cu2O nanoparticles. The synergistic effect of the nanocomposite framework and heterojunction facilitates uniform distribution and sufficient interaction with water molecules, resulting in excellent structural and photothermal properties. Under one sun illumination, the energy conversion efficiency reached 83.39%, achieving an advanced pure water evaporation rate of 2.10 kg·m−2·h−1. Experimental and simulation tests further confirmed the strong hydration capability of the wood evaporator, which reduces the equivalent evaporation enthalpy (Figure 9a). Zang et al. [117] designed a nanofiber hydrogel-reduced graphene oxide (NHrG) composite membrane that integrates the porous hydrophilic network of electrospun hydrogel nanofibers with the efficient photothermal conversion properties of reduced rGO (Figure 9e). Specifically, the open-pore structure of the hydrogel accelerates water transport and vapor escape, while the broad-spectrum light absorption of rGO achieves a remarkable solar energy conversion efficiency of up to 95.4%.
4.2. Multilayer Structures
While single-layer structures offer significant advantages in terms of fabrication processes and water transport efficiency, their ability to localize heat is limited. Some heat is lost through conduction and convection, resulting in theoretical evaporation efficiencies typically below 80%. Additionally, prolonged operation can lead to pore blockage due to salt crystallization. In contrast, multilayer structures can enhance photothermal conversion efficiency by incorporating additional functional intermediate layers, such as insulation, light scattering, or reflective layers, which reduce radiative and convective heat losses. These structures are also better suited for high-salinity environments [118,119]. Sun et al. [120] designed a composite membrane made of graphene oxide (GO) and polytetrafluoroethylene (PTFE), consisting of a hydrophilic GO layer (153–210 nm) supported by a hydrophobic PTFE membrane (Figure 9g). The high thermal conductivity of the GO layer significantly reduces thermal boundary layer resistance, enhancing the temperature gradient. However, the Kelvin effect at the evaporation interface leads to a decrease in vapor pressure, partially offsetting the temperature gradient gain. Conversely, at the condensation interface, the Kelvin effect increases local vapor pressure, becoming the main factor for flux enhancement. Experimental and model validation showed that the flux enhancement at the evaporation interface with the GO layer was more significant (17.8–45.5% compared to 12.4–16.4% at the condensation interface). Wei et al. [121] proposed a hydrophilic composite graphene material combined with CuO, fabricated using a direct laser-induced graphene synthesis method on a polyimide film coated with CuCl2. The enhanced hydrophilicity and layered structure provide rapid capillary action, allowing the assembled laser-induced lattice evaporator to achieve an evaporation rate of 2.54 kg m−2 h−1 under one sun illumination, with an efficiency of 91.1%. It also demonstrated excellent desalination performance at an evaporation rate of 1 kg m−2 h−1.
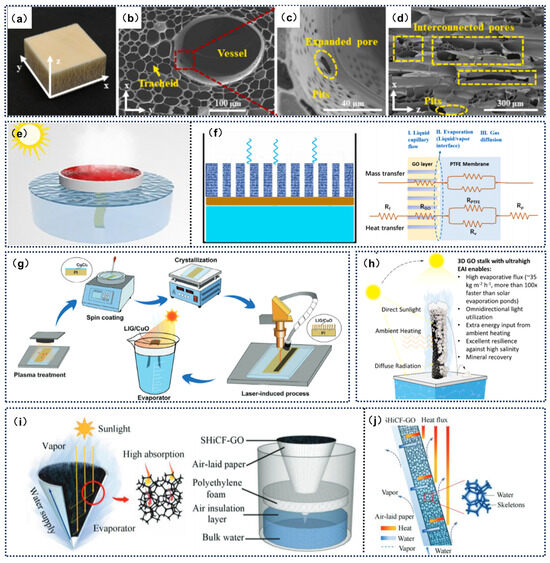
Figure 9.
(a–d) DBW-GC micro-nano structures. Reprinted with permission from ref. [116]. Copyright 2025 Elsevier; (e) Schematic diagram for the NHrG membrane. Reprinted with permission from ref. [117]. Copyright 2021 Elsevier. (f) Schematic diagram of the evaporation test for the GO/PTFE membrane. Reprinted with permission from ref. [120]. Copyright 2022 Elsevier. (g) Process for fabricating laser-induced graphene/CuO composites using a continuous-wave CO laser on CuCl-coated PI films with different concentrations. Reprinted with permission from ref. [121]. Copyright 2024 John Wiley and Sons. (h) Performance comparison between the graphene oxide straw and other 3D evaporators. Reprinted with permission from ref. [101]. Copyright 2021 American Chemical Society. (i) Schematic diagram illustrating the solar evaporation and light absorption principles of the 3D inverted conical solar evaporator. (j) Schematic diagram of the synergistic regulation of thermal-mass water transport and vapor diffusion. Reprinted with permission from ref. [40]. Copyright 2023 John Wiley and Sons.
4.3. 3D Structures
In recent years, the study of 3D evaporators has garnered significant attention from researchers. Compared to 2D structures, 3D systems exhibit notable advantages in photothermal conversion efficiency, water transport dynamics, thermal localization control, and environmental adaptability. By optimizing spatial topology, 3D structures overcome the light absorption limitations of 2D planar structures. Their multi-level micro-nano morphologies (such as arrayed cones, gradient channels, or fractal networks) effectively suppress Fresnel reflection and enhance light capture capabilities. This is particularly beneficial under conditions of low solar incidence angles or diffuse light, where multiple internal reflections and scattering effects enable efficient broadband absorption (250–2500 nm) [122,123,124]. In contrast, 2D planar structures are limited by single-pass reflection absorption and surface flatness, making their photothermal conversion efficiency more susceptible to variations in incident angle and spectral distribution. Furthermore, 3D systems achieve spatial decoupling of water transport paths and evaporation interfaces by constructing vertically interconnected porous channels and lateral capillary force gradients. This design maintains rapid water replenishment while significantly reducing thermal convection losses caused by excessive heating of the bulk water. In comparison, 2D structures, due to the direct contact between the evaporation interface and the water body, are prone to thermal energy loss through heat conduction and natural convection to inactive areas during the evaporation process. This leads to the failure of thermal localization and a decrease in energy utilization efficiency.
Finnerty et al. [101] developed a three-dimensional graphene oxide (3D GO) stem, which confines the capillary core nearby and achieves an evaporation flux of 34.7 kg m−2 h−1 under one sun condition (1 kW/m2). This represents nearly a 20-fold enhancement compared to a two-dimensional GO evaporator. The system also features omnidirectional sunlight utilization and maintains high evaporation flux in dark conditions by more effectively utilizing ambient heating. The evaporation rate is significantly increased by introducing wind, and the system demonstrates scalability and resistance to fouling in the evaporated liquid (Figure 9h). Lv et al. [40] developed a 3D hierarchical inverted cone solar evaporator, composed of a 3D copper foam skeleton cone decorated with micro/nanostructures and functionalized with graphene oxide (Figure 9i). This structure can be fabricated through a simple and scalable dip-coating process, followed by drying at room temperature. The proposed configuration enables efficient solar energy absorption, continuous liquid film diffusion and transport, enhanced localized SDIE, and rapid vapor diffusion through the pores. Notably, the 3D conical evaporator achieves thermal localization at the skeleton interface, allowing evaporation to occur along the entire structure with unobstructed rapid diffusion of liquid and vapor (Figure 9j).
5. Application of Graphene-Based SDIE-Coupled Photocatalytic Systems in Water Treatment
Graphene-based SDIE systems demonstrate significant advantages in water treatment. The core mechanism integrates the synergistic effects of photothermal–adsorption–catalysis and a multi-stage targeted removal mechanism for pollutants. The high specific surface area of graphene and its rich oxygen-containing functional groups endow it with excellent physical adsorption capabilities, enabling the efficient enrichment of heavy metal ions and organic pollutants. Simultaneously, its broad-spectrum absorption properties convert solar energy into heat, driving the generation of photogenerated carriers and activating the catalytic sites, which decompose pollutants via free radical oxidation or direct electron transfer pathways. Additionally, the vapor flow produced by evaporation can carry volatile pollutants to the condensation interface for selective separation. This adsorption–catalysis–evaporation synergy driven by solar energy provides a green solution for the efficient purification and resource recovery of complex wastewater.
5.1. Photocatalytic Degradation of Organic Pollutants
Graphene-based SDIE achieves the highly efficient utilization of light energy and promotes carrier separation in water treatment applications. The high conductivity and ultra-large specific surface area of graphene accelerate the transport of photogenerated electrons while suppressing electron–hole recombination, simultaneously enhancing interfacial adsorption capacity for pollutants. During the interfacial evaporation process, while solar energy is converted into thermal energy to drive water evaporation, the strongly oxidative reactive species generated on the photocatalyst surface can directionally attack organic pollutant molecules. Through reactions such as bond breaking and ring opening, these species gradually mineralize pollutants into CO2 and H2O [125]. Furthermore, the localized thermal effect formed at the evaporation interface accelerates reaction kinetics, while the porous structure of graphene enriches pollutants through adsorption effects, further enhancing mass transfer efficiency. The conjugated π structure of graphene can also adsorb and enrich pollutants through π–π stacking, enhancing the efficiency of interfacial reactions. Its wide-spectrum response characteristic further expands the utilization rate of visible light.
Ma et al. [41] designed a novel reduced graphene oxide/FeOOH (RGO/FeOOH) asymmetric evaporator and demonstrated its excellent photocatalytic degradation ability for organic pollutants in water (Figure 10a). Yan et al. [70] developed a graphene oxide-polypyrrole (GR/PPY) composite aerogel that innovatively realized the dual functions of wastewater purification and freshwater regeneration. The nanocomposite material, prepared by a stepwise hydrothermal-reduction–freeze-drying method, effectively suppressed the stacking of graphene sheets through π–π conjugation between components, constructing a cross-scale network structure with micron-scale fluid channels and nano active sites. This unique configuration significantly enhanced the adsorption–catalysis synergistic effect for organic pollutants by increasing the surface π–electron cloud density. The experimental data showed that the aerogel achieved degradation rates of 96.4%, 92.5%, and 82.6% for typical organic pollutants like Rhodamine B (RHB), phenol, and ciprofloxacin (CIP), respectively, confirming its multifunctional potential in water treatment applications (Figure 10b,c). Li et al. [126] developed a reduced graphene oxide–palladium (rGO–Pd) composite evaporator, which innovatively realized a synergy between SDIE and catalytic reactions. The system exhibited an enhanced apparent utilization rate of solar thermal energy by coupling the evaporation and catalytic energy conversion pathways. The experimental results indicated that thanks to the self-supply of reaction heat, this evaporator achieved a record-breaking water production rate of 12.7 L·m−2·h−1 under saline conditions, far superior to that of existing integrated SDIE systems. Its multi-porous rGO–Pd composite structure created efficient mass transfer channels, combining photothermal effects and rapid material transport, which improved the catalytic activity of Pd to 125.07 min−1—an increase of 275% compared to dark-state conditions. This integrated SDIE system simultaneously enabled freshwater preparation, salt crystallization, and catalytic product recovery, achieving the full-resource utilization of aqueous media (Figure 10d). Huang et al. [127] successfully constructed a multifunctional nanocomposite system based on polyvinylidene fluoride (PVDF)/graphene–BaTiO3 through β-phase self-assembly technology. This material integrated solar evaporation, piezoelectric power generation, pollutant degradation, and self-cleaning functions. Experiments confirmed that, due to the piezoelectric–photonic synergistic coupling effect, the material system effectively suppressed the recombination of photogenerated carriers. Under dynamic conditions, it achieved a 93% degradation efficiency for dyes while also enhancing membrane anti-fouling by 200%. This piezoelectric-catalysis and photocatalysis cross-scale energy synergy mechanism provides a new technological paradigm for the development of smart water treatment systems (Figure 10e).
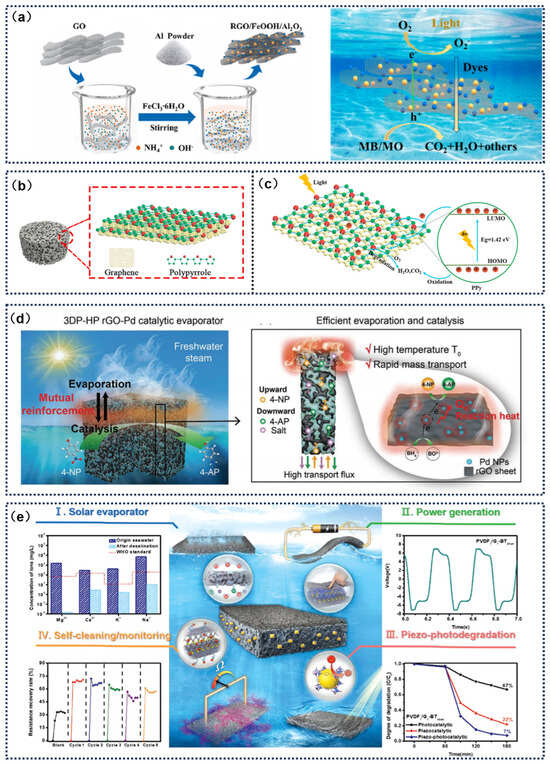
Figure 10.
(a) Synthesis process and degradation mechanism of RGO/FeOOH. Reprinted with permission from ref. [41]. Copyright 2023 Elsevier. (b) Schematic diagram of GR/PPY aerogel. (c) Photocatalytic mechanism of GR/PPY. Reprinted with permission from ref. [70]. Copyright 2022 Elsevier. (d) Schematic diagram of interfacial evaporation and catalytic mechanism in the RGO-PD catalytic evaporator. Reprinted with permission from ref. [126]. Copyright 2023 John Wiley and Sons. (e) Four mechanisms of the PVDF/graphene-BaTiO3 composite membrane. Reprinted with permission from ref. [127]. Copyright 2023 John Wiley and Sons.
5.2. Reduction in and Fixation of Heavy Metal Ions
Leveraging graphene’s efficient photothermal conversion and synergistic reduction-adsorption mechanisms enables the reduction in and immobilization of heavy metal ions in water. With its high specific surface area and abundant surface functional groups, graphene-based SDIE effectively adsorbs and enriches heavy metal ions through electrostatic interactions, complexation, or ion exchange, significantly increasing their localized concentration. Under solar-driven conditions, the heterojunction structure formed between graphene and semiconductors facilitates the directional migration of photogenerated electrons to pollutant interfaces via Fermi-level alignment. This reduces higher-valent heavy metal ions to less toxic or easily precipitable lower-valent forms, while photogenerated holes participate in oxidation reactions to avoid electron–hole recombination. The localized high-temperature environment generated during interfacial evaporation not only accelerates reduction reaction kinetics but also induces the formation of stable coordination compounds or mineral phases between heavy metal ions and material surface functional groups, achieving long-term immobilization [128,129]. Additionally, evaporation-induced salt crystallization effects synergistically promote heavy metal ion co-precipitation, and graphene’s hierarchical porous structure provides physical confinement spaces for immobilized products, preventing secondary release.
Fan et al. [43] innovatively converted waste polyethylene terephthalate (PET) into bismuth-based metal–organic framework (Bi-MOF) microcrystals and built a solar-driven system that simultaneously reduced hexavalent chromium and produced freshwater. The Bi-MOF/graphene composite evaporator demonstrated broad-spectrum light absorption (380–2500 nm), 92.3% photothermal conversion efficiency, and a 0.12° water contact angle. When coupled with a photo-Fenton catalytic system, the localized micro-effect generated during the interface evaporation process significantly enhanced hydrogen peroxide activation, achieving a hexavalent chromium reduction efficiency of 91.3%, with a reaction rate constant of 0.0548 min−1—178% higher than that of traditional non-evaporation systems. This solar-driven microenvironment regulation mechanism provides a new technological coupling path for heavy metal pollution control and water resource regeneration (Figure 11a). Tian et al. [130] innovatively combined Ag-MnO2 catalytic nanostructures with nitrogen-doped graphene aerogels, creating a three-dimensional cylindrical evaporator (SMNG) with both ultra-high evaporation performance and efficient water treatment capability. Under light irradiation, this device achieved an evaporation rate of 6.46 kg·m−2·h−1 with a 90.25% photothermal conversion efficiency. For seawater desalination applications, the SMNG exhibited an evaporation performance of 6.15 kg·m−2·h−1 and 99.9% ion removal efficiency. In outdoor tests, its evaporation performance was improved to 8.57 kg·m−2·h−1, confirming its practical feasibility. This design not only optimized evaporation efficiency and energy conversion performance but also significantly enhanced wastewater treatment ability through catalytic properties, achieving over 99.9% purification efficiency for organic dyes, heavy metal ions, formaldehyde, and E. coli (Figure 11b). Dong et al. [131] prepared a porous hydrophilic/hydrophobic cellulose graphene aerogel (CGA) for application in solar-driven water evaporation systems to achieve seawater desalination and heavy metal wastewater purification. By leveraging the synergistic effects of interfacial evaporation and adsorption, the CGA utilizes oxygen-containing functional groups to form hydrogen bonds and chelating bonds with heavy metal ions (e.g., Ni2+, Cu2+, Pb2+, Cd2+), effectively reducing the concentration of heavy metals in wastewater to trace levels and significantly decreasing the wastewater volume (Figure 11c,d).
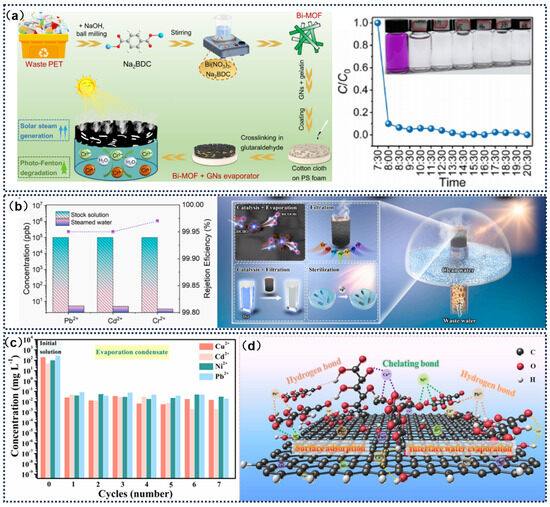
Figure 11.
(a) Schematic diagram of BI-MOF/GNS evaporator preparation for ISS and photo-Fenton Cr(VI) reduction, and photo-Fenton reduction efficiency of Cr(VI). Reprinted with permission from ref. [43]. Copyright 2024 Elsevier. (b) Effect of SMNG system on heavy metal concentrations. Reprinted with permission from ref. [130]. Copyright 2023 Elsevier. (c) The influence of different cycles on the concentrations of Ni2+, Cu2+, Pb2+ and Cd2+ in CGA. (d) Mechanism diagram of CGA-8 purification of heavy metal wastewater. Reprinted with permission from ref. [131]. Copyright 2022 Elsevier.
5.3. Microbial Inactivation and Anti-Biofouling
Graphene-based SDIE technology demonstrates significant application potential in water treatment, particularly showcasing unique advantages in microbial inactivation. Leveraging graphene’s exceptional light absorption and thermal conduction properties, this technology achieves highly efficient water evaporation processes through solar energy conversion. During interfacial evaporation, graphene materials efficiently convert solar energy into thermal energy, creating localized high-temperature zones at the water-air interface that enable rapid inactivation of aquatic microorganisms. The high specific surface area and abundant surface functional groups of graphene facilitate the adsorption and enrichment of microorganisms in water, further enhancing inactivation efficiency. Moreover, graphene-based materials generate reactive oxygen species (ROS) such as hydroxyl radicals (·OH) and superoxide anions (O2−) through photocatalytic activity [132,133]. These reactive substances can damage microbial cell membranes and DNA structures, substantially improving inactivation performance. By optimizing the structural design and surface chemistry of graphene-based materials, the highly efficient inactivation of diverse pathogenic microorganisms—including bacteria, viruses, and protozoa—can be achieved.
Lu et al. [134] developed a seawater desalination composite membrane material with enhanced antimicrobial properties. The study evenly dispersed GO nanosheets within a polymer matrix, utilizing magnetic field-controlled solvent evaporation dynamics to prepare vertically oriented GO nanocomposite films. This unique orientation significantly enhanced the interface effects at the edges of the nanosheets, fundamentally improving the material’s antibacterial properties. This nanocomposite membrane is not only suitable for seawater desalination but also exhibits multifunctional potential in environmental engineering, such as serving as high-performance sensors, catalytic electrode materials, and efficient adsorption media (Figure 12a). Noureen et al. [42] developed a functionalized coating fabric based on an Ag3PO4-reduced graphene oxide (Ag3PO4-rGO) nanocomposite system. The material was loaded onto a cotton substrate to form a solar-driven floating water purification device. This fabric achieved an evaporation rate of 1.31 kg·m−2·h−1 by converting solar heat to enhance interface temperature, while integrating the photocatalytic degradation of organic dyes and microbial inactivation. Moreover, they proposed an innovative photothermal aerogel design by constructing a three-dimensional porous system with an Ag3VO4-rGO composite structure, which incorporated solar steam generation, pollutant photocatalysis, and seawater desalination functions, forming a highly efficient water treatment system (Figure 12b) [135]. Lin et al. [136] constructed a polyacrylamide-reduced graphene oxide composite hydrogel system based on radiation reduction technology, which simultaneously loaded silver nanoparticles to form a porous ArG–Ag composite hydrogel. This material integrated rapid water transport channels, localized thermal effects, and low evaporation enthalpy properties, achieving an evaporation rate of 3.089 kg·m−2·h−1 under standard illumination, with energy conversion efficiency exceeding 122.7%. Its structural design provided both mechanical strength and salt tolerance, effectively regulating ion migration for water purification. The system demonstrated a 99% inhibition rate against E. coli after 24 h and exhibited long-term antifungal properties, significantly enhancing anti-biofouling performance. This research innovatively integrated high-efficiency evaporation, salt regulation, broad-spectrum antibacterial, fungal inhibition, and catalytic functions into a single system, offering a new multifunctional solution for marine environmental applications (Figure 12c,d).
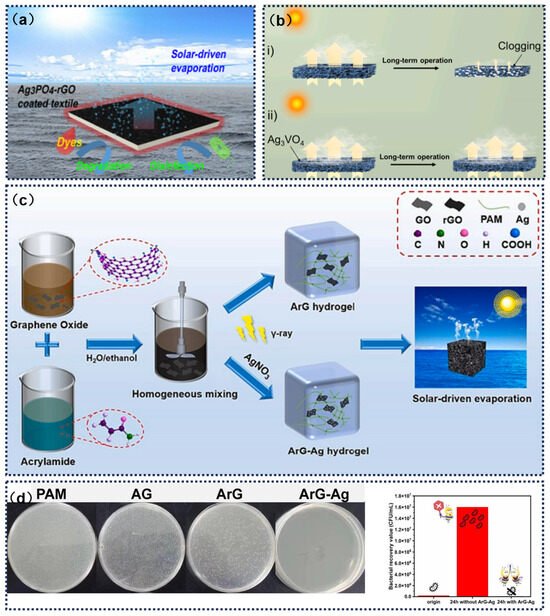
Figure 12.
(a) Antibacterial performance of Ag3PO4-rGO against E. coli. Reprinted with permission from ref. [42]. Copyright 2020 American Chemical Society. (b) Photothermal membrane: (i) current issues (clogging/biofouling) and (ii) photothermal membrane with anti-clogging/anti-biofouling functionalities. Reprinted with permission from ref. [135]. Copyright 2024 Elsevier. (c) Synthesis process of ArG–Ag. (d) Antibacterial behavior of ArG–Ag toward E. coli. Reprinted with permission from ref. [136]. Copyright 2025 Elsevier.
6. Design and Scale-Up of Graphene-Based Evaporation–Catalytic Experimental Systems
In the previous section, we have systematically analyzed photothermal catalytic materials and conducted a comparative assessment of their performance and characteristics (Table 1). However, the ultimate objective of photothermal–photocatalytic systems is to achieve large-scale freshwater collection and water purification through efficient air–water interface evaporation. This necessitates the development of high-performance evaporators and well-designed purification–collection devices to provide an environmentally friendly and cost-effective sustainable solution for global freshwater scarcity. Laboratory-scale graphene-based photothermal evaporation–photocatalytic systems are typically composed of multiple functional layers: The photothermal conversion layer employs high-surface-area materials with nano-engineered structures to localize solar-to-thermal energy conversion; the water transport layer utilizes hydrophilic porous substrates to enable continuous water supply through capillary action while inhibiting salt crystallization; and the thermal insulation layer incorporates low-thermal-conductivity materials to minimize heat conduction losses and maintain elevated temperatures at the evaporation interface. Integrated photocatalytic components degrade organic pollutants through photogenerated carriers, achieving simultaneous evaporation and water purification. During operation, the photothermal layer generates localized thermal gradients under solar irradiation, driving the rapid vaporization of interfacial water molecules. The generated vapor is subsequently condensed and collected as freshwater, while photocatalytic reactions decompose contaminants either at the evaporation interface or in adjacent regions, forming a high-efficiency energy–water co-treatment system.

Table 1.
Comparison of graphene-based solar driven interfacial evaporation coupled photocatalytic systems.
Although experimental systems demonstrate remarkable performance under controlled laboratory conditions, they face limitations in scale and material stability assessment. Moreover, laboratory environments may inadequately simulate real-world challenges such as complex water quality or fluctuating illumination conditions. In contrast, pilot-scale devices provide reliable transitional solutions for industrial applications through engineering optimizations. Li et al. [137] developed a three-dimensional interconnected porous carbon foam (3D IPCF) fabricated via gas-assisted expansion and perforation processes, achieving an exceptional evaporation rate of 10.9 kg m−2 h−1 under 1 sun irradiation with natural convection. The study emphasized that the material’s interconnected pore architecture and millimeter-scale pore size significantly enhanced vapor diffusion efficiency, with theoretical modeling and experiments confirming the critical role of pore morphology. In scaled-up trials, a large-scale device comprising 20 independent 3D IPCF units demonstrated continuous outdoor operation for 13 h, accumulating an evaporation yield of 42.0 kg m−2. The system maintained stable daytime and nighttime evaporation rates of 2.3–6.1 kg m−2 h−1 and 1.6–2.1 kg m−2 h−1 respectively, showcasing all-weather high-throughput capability. Furthermore, the material exhibited excellent performance in treating dye wastewater and heavy metal-ion contaminated water, achieving environmental discharge standards with good cyclic stability. Zhang et al. [138] engineered a vertically aligned graphene sheet membrane (VA-GSM) through anti-freezing-assisted freeze casting, where its through-channel structure dramatically improved water transport efficiency. The system attained evaporation rates of 1.62 kg m−2 h−1 and 6.25 kg m−2 h−1 under 1 sun and 4 sun irradiance, respectively, corresponding to solar-thermal conversion efficiencies of 86.5% and 94.2%. Scalability was confirmed through the successful fabrication of 16 cm × 16 cm large-area samples by adjusting mold dimensions (Figure 13d). When applied to Bohai seawater treatment under 4 sun conditions, the VA-GSM achieved continuous clean water production at 6.22 kg m−2 h−1 with ion rejection rates exceeding 99%, meeting international drinking water standards. Remarkably, the membrane maintained stable evaporation performance in strongly acidic (0.2–8 mol mL−1 H+) and alkaline (0.2–8 mol mL−1 OH−) environments, while reducing heavy metal-ion concentrations below 0.01 mg kg−1, demonstrating broad-spectrum wastewater treatment capabilities for complex effluents.
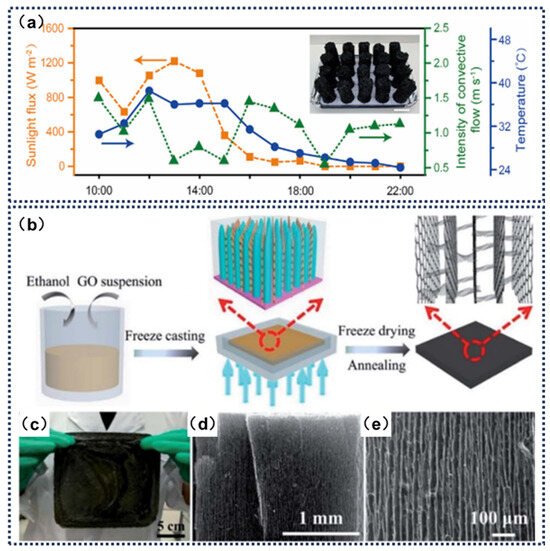
Figure 13.
(a) Outdoor evaporation of large equipment settings. Outdoor conditions for evaporation: incident sunlight flux, intensity of natural convective flow, and ambient temperature over time. Reprinted with permission from ref. [137]. Copyright 2025 Elsevier. (b) Manufacturing process and characterization diagram of VA-GSM. (c) VA-GSM monocoque photo with a size of 16 cm × 16 cm. (d,e) SEM images of VA-GSM at different magnifications. Reprinted with permission from ref. [138] Copyright 2017 American Chemical Society.
7. Challenges and Future Perspectives
7.1. Low Steam Condensation Efficiency Affecting Subsequent Freshwater Collection
Although graphene-based materials have demonstrated excellent photothermal conversion and steam generation performance in SDIE, the low efficiency of steam condensation remains a critical bottleneck limiting the actual yield of freshwater. Current research often focuses on thermodynamic optimization at the evaporation end, neglecting the dynamic regulation of latent heat release during the steam phase change and the enhancement of heat and mass transfer at the condensation end, which restricts the overall energy utilization efficiency of the system. On one hand, the spatial separation design between the evaporation system and the condensation module leads to significant heat dissipation during steam diffusion and transport due to heat exchange with the surrounding medium and turbulence disturbances, resulting in reduced latent heat recovery rates. On the other hand, traditional condenser surfaces lack the ability for directional adsorption of microscale steam molecules and rapid liquid drainage, causing the formation of insulating vapor films or liquid retention layers at the condensation interface, which severely hinders the improvement of phase change heat transfer coefficients. Additionally, the mismatch between the high-temperature steam (typically 60–90 °C) produced by graphene-based evaporation systems and the insufficient temperature difference for conventional condensation further reduces the condensation driving force. This issue is particularly pronounced in humid and hot climates or under high ambient temperature conditions, where freshwater collection efficiency may decrease by more than 40%.
7.2. Stability of Solar Evaporators in Long-Term Practical Operation
The stability of graphene-based materials in solar-driven interfacial evaporators during long-term practical operation remains a core challenge for large-scale application. Firstly, material degradation under complex conditions is a prominent issue: Graphene nanosheets are prone to photo-oxidation or structural wrinkling during continuous photothermal cycling, leading to the attenuation of surface plasmon resonance effects and the disruption of thermal conduction networks. Hydrophilic polymer substrates (such as cellulose and polyacrylamide) are susceptible to hydrolysis or crosslinking failure under high temperature, high salinity, or UV radiation, causing the collapse of porous structures and blockage of water transport channels. Secondly, salt crystallization and pollutant deposition are significant problems, especially in desalination scenarios. Supersaturated salt precipitation near the evaporation interface can cover photothermal active sites, while the adsorption of impurities like oil and microorganisms alters surface wettability, resulting in a gradual decline in evaporation efficiency. Additionally, insufficient mechanical stability limits device lifespan; for example, structural stress from wave scouring or ice crystal expansion may compromise the integrity of the three-dimensional porous framework, and weak interfacial bonding between graphene and the substrate can lead to interlayer delamination under thermal stress.
7.3. Adaptive Limitations in Complex Water Bodies
In terms of adaptability to complex water bodies, the graphene-based SDIE–photocatalytic coupling system still faces multiple technical bottlenecks: the salt crystallization behavior caused by ion enrichment in high-salinity water bodies easily leads to the blockage of the porous structure of the evaporation layer, significantly reducing the steam mass transfer efficiency and light penetration rate; low-concentration and highly toxic emerging pollutants such as perfluorinated compounds, antibiotic resistance genes, and nanoplastics are difficult to achieve efficient targeted degradation of due to the competition for interfacial adsorption sites and insufficient selective oxidation by free radicals; when multiple pollutants coexist, heavy metal ions and dissolved organic matter (DOM) may form antagonistic effects that inhibit photocatalytic kinetics through electron transfer competition, active site shielding, or free radical quenching effects; and in addition, the non-uniform deposition of suspended particles and colloidal substances at heterogeneous interfaces will aggravate light scattering loss and mass transfer resistance, leading to a significant deterioration in the long-term operational stability and anti-pollution ability of the system, which restricts its universal application in non-ideal water environments such as industrial wastewater and seawater desalination.
8. Conclusions
This paper systematically reviews the research progress of graphene-based materials in SDIE-coupled photocatalytic technology and their application potential in water treatment. Leveraging its broadband light absorption, ultrafast hot carrier dynamics, tunable electronic structure, and low evaporation enthalpy, graphene has emerged as a core material for photothermal–photochemical synergistic water treatment systems. Strategies such as surface morphology modulation, plasmonic material integration, and defect engineering have significantly enhanced light absorption efficiency. Concurrently, hydrophilic functional group modification, porous structure design, and thermal localization optimization effectively reduce water evaporation enthalpy while suppressing energy losses. Furthermore, a 3D structural design, salt-resistance strategies, and fluid channel engineering have improved system evaporation performance and environmental adaptability. Studies demonstrate that graphene-based SDIE systems efficiently degrade organic pollutants, immobilize heavy metal ions, and inactivate microorganisms, offering a green solution for the advanced purification and resource recovery of complex wastewater. However, challenges such as low steam condensation efficiency, insufficient long-term material stability, high costs in scalable manufacturing, and limited adaptability to complex water matrices remain bottlenecks for practical implementation. Future research should focus on enhancing heat-mass transfer in condensation systems, optimizing material environmental tolerance, developing low-cost fabrication processes, and exploring multi-pollutant synergistic removal mechanisms. These advancements will propel graphene-based SDIE technology from laboratory research to large-scale applications, ultimately contributing to global sustainable water resource management.
Author Contributions
Writing—original draft preparation, Y.Z.; writing—review and editing, H.W.; visualization, supervision, J.Z.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No data was used for the research described in the article.
Conflicts of Interest
Author Jisheng Zhang was employed by the company Jiangsu Agrochem Laboratory Co., Ltd., Changzhou, China. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Chen, A.; Liang, H.; Chen, T.; Yang, W.; Ding, C. Influence of long-term irrigation with treated papermaking wastewater on soil ecosystem of a full-scale managed reed wetland. J. Soils Sediments 2015, 16, 1352–1359. [Google Scholar] [CrossRef]
- Magni, M.; Jones, E.R.; Bierkens, M.F.P.; van Vliet, M.T.H. Global energy consumption of water treatment technologies. Water Res. 2025, 277, 123245. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Qiu, J.; Xu, X.; Wan, R.; Yao, M.; Wang, H.; Zhou, Z.; Xu, J. Solar driven kaolin-based hydrogels for efficient interfacial evaporation and heavy metal ion adsorption from wastewater. Sep. Purif. Technol. 2025, 354, 129243. [Google Scholar] [CrossRef]
- Jiang, Y.; Tang, Y.; Li, H. A review of trends in the use of sewage irrigation technology from the livestock and poultry breeding industries for farmlands. Irrig. Sci. 2022, 40, 297–308. [Google Scholar] [CrossRef]
- Kama, R.; Liu, Y.; Aidara, M.; Kpalari, D.F.; Song, J.; Diatta, S.; Sulemana, H.; Li, H.; Li, Z. Plant-Soil Feedback Combined with Straw Incorporation Under Maize/Soybean Intercropping Increases Heavy Metals Migration in Soil-Plant System and Soil HMRG Abundance Under Livestock Wastewater Irrigation. J. Soil Sci. Plant Nutr. 2024, 24, 7090–7104. [Google Scholar] [CrossRef]
- Yu, J.; Wang, C.; Liu, S.; Yang, S.; Du, X.; Liu, S.; Shao, C.; Kong, H.; Wang, B.; Wu, T.; et al. Solid waste-derived solar desalination devices: Enhanced efficiency in water vapor generation and diffusion. Chem. Eng. J. 2024, 485, 149870. [Google Scholar] [CrossRef]
- Giwa, A.; Yusuf, A.; Dindi, A.; Balogun, H.A. Polygeneration in desalination by photovoltaic thermal systems: A comprehensive review. Renew. Sustain. Energy Rev. 2020, 130, 109946. [Google Scholar] [CrossRef]
- Ali, A.; Tufa, R.A.; Macedonio, F.; Curcio, E.; Drioli, E. Membrane technology in renewable-energy-driven desalination. Renew. Sustain. Energy Rev. 2018, 81, 1–21. [Google Scholar] [CrossRef]
- Richards, B.S.; Howard, I.A. Luminescent solar concentrators for building integrated photovoltaics: Opportunities and challenges. Energy Environ. Sci. 2023, 16, 3214–3239. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Zhang, J.; Han, T.; Hu, X.; Lee, M.M.S.; Wang, D.; Tang, B.Z. A Facile Strategy of Boosting Photothermal Conversion Efficiency through State Transformation for Cancer Therapy. Adv. Mater. 2021, 33, e2105999. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Z.P.; Wu, X.; Wang, W.J.; Yang, C.; Xu, G.J.; Wu, C.A.; Bao, E.C. Open-Field Agrivoltaic System Impacts on Photothermal Environment and Light Environment Simulation Analysis in Eastern China. Agronomy 2023, 13, 1820. [Google Scholar] [CrossRef]
- Bei, X.R.; Yu, X.J.; Li, D.Q.; Sun, Q.L.; Yu, Y.H.; Wang, Y.Q.; Okonkwo, C.E.; Zhou, C.S. Heat source replacement strategy using catalytic infrared: A future for energy saving drying of fruits and vegetables. J. Food Sci. 2023, 88, 4827–4839. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Xu, J.; Tian, S.; Shi, K.; Gu, T.; Zhao, J.; Li, X.; Zhou, Z.; Tijing, L.; Shon, H.K. Hydrodynamic solar-driven interfacial evaporation—Gone with the flow. Water Res. 2024, 266, 122432. [Google Scholar] [CrossRef]
- Li, L.; Sun, X.S.; Miao, J.; Wang, H.N.; Song, Y.C.; Tang, D.W. Gradient engineering in interfacial evaporation for water, energy, and mineral harvesting. Energy Environ. Sci. 2025, 18, 1176–1190. [Google Scholar] [CrossRef]
- Bi, X.Y.; Li, L.B.; Luo, L.J.; Liu, X.H.; Li, J.M.; You, T.Y. A ratiometric fluorescence aptasensor based on photoinduced electron transfer from CdTe QDs to WS2 NTs for the sensitive detection of zearalenone in cereal crops. Food Chem. 2022, 385, 132657. [Google Scholar] [CrossRef]
- Wang, J.; Cao, X.; Cui, X.; Wang, H.; Zhang, H.; Wang, K.; Li, X.; Li, Z.; Zhou, Y. Recent Advances of Green Electricity Generation: Potential in Solar Interfacial Evaporation System. Adv. Mater. 2024, 36, e2311151. [Google Scholar] [CrossRef]
- Cai, M.; Li, C.; An, X.; Zhong, B.; Zhou, Y.; Feng, K.; Wang, S.; Zhang, C.; Xiao, M.; Wu, Z.; et al. Supra-Photothermal CO2 Methanation over Greenhouse-Like Plasmonic Superstructures of Ultrasmall Cobalt Nanoparticles. Adv. Mater. 2024, 36, e2308859. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Xiang, H.; Yang, D.; Lin, K.T.; Wu, Y.; Zhou, R.; Gu, Z.; Yan, L.; Zhao, Y.; Tan, W. Plasmonic AuPt@CuS Heterostructure with Enhanced Synergistic Efficacy for Radiophotothermal Therapy. J. Am. Chem. Soc. 2021, 143, 16113–16127. [Google Scholar] [CrossRef]
- Wan, X.; Li, Y.; Chen, Y.; Ma, J.; Liu, Y.A.; Zhao, E.D.; Gu, Y.; Zhao, Y.; Cui, Y.; Li, R.; et al. A nonmetallic plasmonic catalyst for photothermal CO2 flow conversion with high activity, selectivity and durability. Nat. Commun. 2024, 15, 1273. [Google Scholar] [CrossRef]
- He, S.S.; Jiang, Y.Y.; Li, J.C.; Pu, K.Y. Semiconducting Polycomplex Nanoparticles for Photothermal Ferrotherapy of Cancer. Angew. Chem. Int. Ed. 2020, 59, 10633–10638. [Google Scholar] [CrossRef]
- Sun, L.; Li, Z.; Su, R.; Wang, Y.; Li, Z.; Du, B.; Sun, Y.; Guan, P.; Besenbacher, F.; Yu, M. Phase-Transition Induced Conversion into a Photothermal Material: Quasi-Metallic WO2.9 Nanorods for Solar Water Evaporation and Anticancer Photothermal Therapy. Angew. Chem. Int. Ed. Engl. 2018, 57, 10666–10671. [Google Scholar] [CrossRef]
- Yang, M.Q.; Tan, C.F.; Lu, W.; Zeng, K.; Ho, G.W. Spectrum Tailored Defective 2D Semiconductor Nanosheets Aerogel for Full-Spectrum-Driven Photothermal Water Evaporation and Photochemical Degradation. Adv. Funct. Mater. 2020, 30, 2004460. [Google Scholar] [CrossRef]
- Hu, A.; Zhao, Y.; Hu, Q.; Chen, C.; Lu, X.; Cui, S.; Liu, B. Highly efficient solar steam evaporation via elastic polymer covalent organic frameworks monolith. Nat. Commun. 2024, 15, 9484. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Huang, J.X.; Chiao, Y.H.; Chang, C.M.; Hung, W.S.; Lue, S.J.; Wang, C.F.; Hu, C.C.; Lee, K.R.; Pan, H.H.; et al. Tailoring of a Piezo-Photo-Thermal Solar Evaporator for Simultaneous Steam and Power Generation. Adv. Funct. Mater. 2021, 31, 2010422. [Google Scholar] [CrossRef]
- Qi, D.; Liu, Y.; Liu, Y.; Liu, Z.; Luo, Y.; Xu, H.; Zhou, X.; Zhang, J.; Yang, H.; Wang, W.; et al. Polymeric Membranes with Selective Solution-Diffusion for Intercepting Volatile Organic Compounds during Solar-Driven Water Remediation. Adv. Mater. 2020, 32, e2004401. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wang, F.; Yang, X.; Ning, B.; Harp, M.G.; Culp, S.H.; Hu, S.; Huang, P.; Nie, L.; Chen, J.; et al. Gold Nanoparticle Coated Carbon Nanotube Ring with Enhanced Raman Scattering and Photothermal Conversion Property for Theranostic Applications. J. Am. Chem. Soc. 2016, 138, 7005–7015. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Y.; Zhang, X.; Lv, B.; Xu, Y.; Fan, X. Co-enhancing volatile organic compound degradation and steam generation in solar interfacial evaporation by integrating with electro-Fenton. Water Res. 2025, 277, 123348. [Google Scholar] [CrossRef]
- Xia, Q.; Wang, C.; Xu, N.; Yang, J.; Gao, G.; Ding, J. A Floating Integrated Solar Micro-Evaporator for Self-Cleaning Desalination and Organic Degradation. Adv. Funct. Mater. 2023, 33, 2214769. [Google Scholar] [CrossRef]
- Huang, L.; Liu, R.; Yang, J.; Shuai, Q.; Yuliarto, B.; Kaneti, Y.V.; Yamauchi, Y. Nanoarchitectured porous organic polymers and their environmental applications for removal of toxic metal ions. Chem. Eng. J. 2021, 408, 127991. [Google Scholar] [CrossRef]
- Jing, Z.; Ding, J.; Zhang, T.; Yang, D.; Qiu, F.; Chen, Q.; Xu, J. Flexible, versatility and superhydrophobic biomass carbon aerogels derived from corn bracts for efficient oil/water separation. Food Bioprod. Process. 2019, 115, 134–142. [Google Scholar] [CrossRef]
- Fang, S.; Huang, W.; Wu, J.; Han, J.; Wang, L.; Wang, Y. Separation and Purification of Recombinant beta-Glucosidase with Hydrophobicity and Thermally Responsive Property from Cell Lysis Solution by Foam Separation and Further Purification. J. Agric. Food Chem. 2023, 71, 3362–3372. [Google Scholar] [CrossRef]
- Shoaib, M.; Li, H.; Khan, I.M.; Hassan, M.M.; Zareef, M.; Niazi, S.; Chen, Q. Emerging MXenes-based aptasensors: A paradigm shift in food safety detection. Trends Food Sci. Technol. 2024, 151, 104635. [Google Scholar] [CrossRef]
- Li, W.T.; Zhang, X.A.; Shi, Y.Q.; Hu, X.T.; Wang, X.; Liang, N.N.; Shen, T.T.; Zou, X.B.; Shi, J.Y. A dual-modal biosensor coupling cooperative catalysis strategy for sensitive detection of AFB1 in agri-products. Food Chem. 2023, 426, 136553. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Wang, W.; Wei, J.; Chen, X.; Chen, Q.; Chen, X.; Oyama, M. Novel dual-emissive fluorescent immunoassay for synchronous monitoring of okadaic acid and saxitoxin in shellfish. Food Chem. 2022, 368, 130856. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, P.; Yin, L.; Zuo, M.; Chen, Q.; El-Seedi, H.R.; Zou, X. Determination of lead in food by surface-enhanced Raman spectroscopy with aptamer regulating gold nanoparticles reduction. Food Control 2022, 132, 108498. [Google Scholar] [CrossRef]
- Kurapati, R.; Kostarelos, K.; Prato, M.; Bianco, A. Biomedical Uses for 2D Materials Beyond Graphene: Current Advances and Challenges Ahead. Adv. Mater. 2016, 28, 6052–6074. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Lei, H.; Kan, A.K.; Xie, H.Q.; Yu, W. Photothermal applications based on graphene and its derivatives: A state-of-the-art review. Energy 2021, 216, 119262. [Google Scholar] [CrossRef]
- Lim, H.W.; Seung Lee, H.; Joon Lee, S. Laminated chitosan/graphene nanoplatelets aerogel for 3D interfacial solar desalination with harnessing wind energy. Chem. Eng. J. 2024, 480, 148197. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Zhang, Q.; Wang, C.; Huang, S.; Liu, Y.; Yu, T.; Yang, R.; Chen, G.; Chaker, M.; et al. Stable, Cost-Effective TiN-Based Plasmonic Nanocomposites with over 99% Solar Steam Generation Efficiency. Adv. Funct. Mater. 2023, 33, 2212301. [Google Scholar] [CrossRef]
- Lv, F.; Miao, J.; Hu, J.; Orejon, D. 3D Solar Evaporation Enhancement by Superhydrophilic Copper Foam Inverted Cone and Graphene Oxide Functionalization Synergistic Cooperation. Small 2023, 19, e2208137. [Google Scholar] [CrossRef]
- Ma, J.; Guo, Z.; Han, X.; Guo, K.; Li, H.; Fang, P.; Wang, X.; Xin, J. Reduced graphene oxide/FeOOH-based asymmetric evaporator for the simultaneous generation of clean water and electrical power. Carbon 2023, 201, 318–327. [Google Scholar] [CrossRef]
- Fan, Z.; Liu, J.; Liu, H.; Liu, L.; She, Y.; Wen, X.; Wang, H.; Hu, G.; Niu, R.; Gong, J. Synergism of solar-driven interfacial evaporation and photo-Fenton Cr(VI) reduction by sustainable Bi-MOF-based evaporator from waste polyester. J. Energy Chem. 2024, 94, 527–540. [Google Scholar] [CrossRef]
- Noureen, L.; Xie, Z.; Gao, Y.; Li, M.; Hussain, M.; Wang, K.; Zhang, L.; Zhu, J. Multifunctional Ag3PO4-rGO-Coated Textiles for Clean Water Production by Solar-Driven Evaporation, Photocatalysis, and Disinfection. ACS Appl. Mater. Interfaces 2020, 12, 6343–6350. [Google Scholar] [CrossRef]
- Sharma, A.S.; Ali, S.; Sabarinathan, D.; Murugavelu, M.; Li, H.; Chen, Q. Recent progress on graphene quantum dots-based fluorescence sensors for food safety and quality assessment applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5765–5801. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Zhang, D.; Tan, W.; Shi, J.; Li, Z.; Liu, H.; Yu, Y.; Yang, L.; Wang, X.; et al. A fluorescence resonance energy transfer probe based on functionalized graphene oxide and upconversion nanoparticles for sensitive and rapid detection of zearalenone. LWT 2021, 147, 111541. [Google Scholar] [CrossRef]
- Rong, J.; Zhou, Z.; Wang, Y.; Han, J.; Li, C.; Zhang, W.; Ni, L. Immobilization of Horseradish Peroxidase on Multi-Armed Magnetic Graphene Oxide Composite: Improvement of Loading Amount and Catalytic Activity. Food Technol. Biotechnol. 2019, 57, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, S.; Sun, L.; Kong, D.; Sheng, J.; Wang, K.; Dong, C. A Green, Simple, and Rapid Detection for Amaranth in Candy Samples Based on the Fluorescence Quenching of Nitrogen-Doped Graphene Quantum Dots. Food Anal. Methods 2019, 12, 1658–1665. [Google Scholar] [CrossRef]
- Ren, H.Y.; Tang, M.; Guan, B.L.; Wang, K.X.; Yang, J.W.; Wang, F.F.; Wang, M.Z.; Shan, J.Y.; Chen, Z.L.; Wei, D.; et al. Hierarchical Graphene Foam for Efficient Omnidirectional Solar-Thermal Energy Conversion. Adv. Mater. 2017, 29, 1702590. [Google Scholar] [CrossRef]
- Wei, N.; Peng, X.S.; Xu, Z.P. Understanding Water Permeation in Graphene Oxide Membranes. Acs Appl. Mater. Interfaces 2014, 6, 5877–5883. [Google Scholar] [CrossRef]
- Chen, S.S.; Wu, Q.Z.; Mishra, C.; Kang, J.Y.; Zhang, H.J.; Cho, K.J.; Cai, W.W.; Balandin, A.A.; Ruoff, R.S. Thermal conductivity of isotopically modified graphene. Nat. Mater. 2012, 11, 203–207. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.Z.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Duan, N.; Qiu, Y.; Li, J.; Wang, Z. Colorimetric aptasensor for the detection of Salmonella enterica serovar typhimurium using ZnFe2O4-reduced graphene oxide nanostructures as an effective peroxidase mimetics. Int. J. Food Microbiol. 2017, 261, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Tang, Z.R.; Xu, Y.J. Multifunctional graphene-based composite photocatalysts oriented by multifaced roles of graphene in photocatalysis. Chin. J. Catal. 2022, 43, 708–730. [Google Scholar] [CrossRef]
- Luo, L.; Liu, X.; Ma, S.; Li, L.; You, T. Quantification of zearalenone in mildewing cereal crops using an innovative photoelectrochemical aptamer sensing strategy based on ZnO-NGQDs composites. Food Chem. 2020, 322, 126778. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Ma, S.; Li, L.; Liu, X.; Zhang, J.; Li, X.; Liu, D.; You, T. Monitoring zearalenone in corn flour utilizing novel self-enhanced electrochemiluminescence aptasensor based on NGQDs-NH2-Ru@SiO2 luminophore. Food Chem. 2019, 292, 98–105. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, Y.; Zou, X. Rapid determination of cadmium in rice using an all-solid RGO-enhanced light addressable potentiometric sensor. Food Chem. 2018, 261, 1–7. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, W.; Shi, J.; Zou, X.; Li, Y.; Haroon Elrasheid, T.; Huang, X.; Li, Z.; Zhai, X.; Hu, X. Electrodeposition of gold nanoparticles and reduced graphene oxide on an electrode for fast and sensitive determination of methylmercury in fish. Food Chem. 2017, 237, 423–430. [Google Scholar] [CrossRef]
- Zhou, W.; Li, C.; Sun, C.; Yang, X. Simultaneously determination of trace Cd2+ and Pb2+ based on L-cysteine/graphene modified glassy carbon electrode. Food Chem. 2016, 192, 351–357. [Google Scholar] [CrossRef]
- Zhang, J.; Lei, Y.; Lin, Z.; Xie, P.; Lu, H.; Xu, J. A novel approach to recovery of lithium element and production of holey graphene based on the lithiated graphite of spent lithium ion batteries. Chem. Eng. J. 2022, 436, 135011. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, D.S.; Moradi, H.; Chang, Y.Y.; Yang, J.K. Highly porous biobased graphene-like carbon adsorbent for dye removal: Preparation, adsorption mechanisms and optimization. J. Environ. Chem. Eng. 2023, 11, 109278. [Google Scholar] [CrossRef]
- Cao, L.Y.; Wang, C.W.; Huang, Y.X. Structure optimization of graphene aerogel-based composites and applications in batteries and supercapacitors. Chem. Eng. J. 2023, 454, 140094. [Google Scholar] [CrossRef]
- Han, J.; Fang, S.; He, X.; Wang, L.; Li, C.; Wu, J.; Cai, Y.; Wang, Y. Combination of aqueous two-phase flotation and inverse transition cycling: Strategies for separation and purification of recombinant beta-glucosidase from cell lysis solution. Food Chem. 2022, 373, 131543. [Google Scholar] [CrossRef]
- Duan, N.; Gong, W.; Wu, S.; Wang, Z. Selection and Application of ssDNA Aptamers against Clenbuterol Hydrochloride Based on ssDNA Library Immobilized SELEX. J. Agric. Food Chem. 2017, 65, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ma, Y.; Zhou, R.; He, Y.; Wu, Y.; Yi, Y.; Zhu, G. Highly sensitive electrochemical detection of paraoxon ethyl in water and fruit samples based on defect-engineered graphene nanoribbons modified electrode. J. Food Meas. Charact. 2022, 16, 2596–2603. [Google Scholar] [CrossRef]
- Chao, Y.; Pang, J.; Bai, Y.; Wu, P.; Luo, J.; He, J.; Jin, Y.; Li, X.; Xiong, J.; Li, H.; et al. Graphene-like BN@SiO2 nanocomposites as efficient sorbents for solid-phase extraction of Rhodamine B and Rhodamine 6G from food samples. Food Chem. 2020, 320, 126666. [Google Scholar] [CrossRef]
- Zhu, Y.W.; Murali, S.; Cai, W.W.; Li, X.S.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Han, B.; Zhang, Y.L.; Chen, Q.D.; Sun, H.B. Carbon-Based Photothermal Actuators. Adv. Funct. Mater. 2018, 28, 1802235. [Google Scholar] [CrossRef]
- Xie, Z.; Duo, Y.; Lin, Z.; Fan, T.; Xing, C.; Yu, L.; Wang, R.; Qiu, M.; Zhang, Y.; Zhao, Y.; et al. The Rise of 2D Photothermal Materials beyond Graphene for Clean Water Production. Adv. Sci. 2020, 7, 1902236. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Xie, J. Graphene in Photocatalysis: A Review. Small 2016, 12, 6640–6696. [Google Scholar] [CrossRef]
- Yan, S.W.; Song, H.J.; Li, Y.; Yang, J.; Jia, X.H.; Wang, S.Z.; Yang, X.F. Integrated reduced graphene oxide/polypyrrole hybrid aerogels for simultaneous photocatalytic decontamination and water evaporation. Appl. Catal. B-Environ. Energy 2022, 301, 120820. [Google Scholar] [CrossRef]
- Li, Q.; Lu, J.; Gupta, P.; Qiu, M. Engineering Optical Absorption in Graphene and Other 2D Materials: Advances and Applications. Adv. Opt. Mater. 2019, 7, 1900595. [Google Scholar] [CrossRef]
- Zeng, L.; Deng, D.X.; Zhu, L.Y.; Zhang, Z.K.; Gu, X.; Wang, H.M.; Jiang, Y.J. Multi-scale CuS-rGO pyramidal photothermal structure for highly efficient solar-driven water evaporation and thermoelectric power generation. Nano Energy 2024, 125, 109531. [Google Scholar] [CrossRef]
- Ma, H.D.; Yu, L.J.; Li, Z.Z.; Chen, J.L.; Meng, J.G.; Song, Q.W.; Liu, Y.M.; Wang, Y.Z.; Wu, Q.; Miao, M.H.; et al. A Lotus Seedpods-Inspired Interfacial Solar Steam Generator with Outstanding Salt Tolerance and Mechanical Properties for Efficient and Stable Seawater Desalination. Small 2023, 19, 2304877. [Google Scholar] [CrossRef]
- Zhao, X.; Meng, X.; Zou, H.; Wang, Z.; Du, Y.; Shao, Y.; Qi, J.; Qiu, J. Topographic Manipulation of Graphene Oxide by Polyaniline Nanocone Arrays Enables High-Performance Solar-Driven Water Evaporation. Adv. Funct. Mater. 2022, 33, 2209207. [Google Scholar] [CrossRef]
- Xiong, R.; Zhong, L.; Song, Y.; Xu, J.; Xiao, Y.; Cheng, B.; Lei, S. CoCr2O4 Nanoparticles with Abundant Oxygen Vacancies: A New Photothermal Platform for Efficient Solar Evaporation. ACS Mater. Lett. 2023, 5, 1992–2001. [Google Scholar] [CrossRef]
- Li, G.Q.; Wang, Q.; Wang, J.; Ye, J.Y.; Zhou, W.W.; Xu, J.; Zhuo, S.; Chen, W.F.; Liu, Y. Carbon-supported nano tungsten bronze aerogels with synergistically enhanced photothermal conversion performance: Fabrication and application in solar evaporation. Carbon 2022, 195, 263–271. [Google Scholar] [CrossRef]
- Meng, X.Y.; Yang, J.H.; Ramakrishna, S.; Sun, Y.M.; Dai, Y.Q. Gradient-aligned Au/graphene meshes with confined heat at multiple levels for solar evaporation and anti-gravity catalytic conversion. J. Mater. Chem. A 2020, 8, 16570–16581. [Google Scholar] [CrossRef]
- Wang, X.L.; Lyu, Q.; Tong, T.Z.; Sun, K.; Lin, L.C.; Tang, C.Y.Y.; Yang, F.L.; Guiver, M.D.; Quan, X.; Dong, Y.C. Robust ultrathin nanoporous MOF membrane with intra-crystalline defects for fast water transport. Nat. Commun. 2022, 13, 266. [Google Scholar] [CrossRef]
- Ren, L.T.; Yang, X.N.; Sun, X.; Yuan, Y.P. Synchronizing Efficient Purification of VOCs in Durable Solar Water Evaporation over a Highly Stable Cu/W18O49@Graphene Material. Nano Lett. 2023, 24, 715–723. [Google Scholar] [CrossRef]
- Ji, M.Y.J.; Kim, J.H.; Jeon, H.Y.; Han, S.H.; Lee, D.H.; Lee, Y.I. High-performance interfacial water evaporation of black TiO2-xwith high-concentration bulk oxygen vacancies. Chem. Eng. J. 2024, 483, 149435. [Google Scholar] [CrossRef]
- Allahbakhsh, A.; Jarrahi, Z.; Farzi, G.; Shavandi, A. Solar-powered and antibacterial water purification via Cu-BTC-embedded reduced graphene oxide nanocomposite aerogels. Chem. Eng. J. 2023, 467, 143472. [Google Scholar] [CrossRef]
- Wang, A.; Liang, H.; Chen, F.; Tian, X.; Yin, S.; Jing, S.; Tsiakaras, P. Facile synthesis of C3N4/NiIn2S4 heterostructure with novel solar steam evaporation efficiency and photocatalytic H2O2 production performance. Appl. Catal. B Environ. 2022, 310, 121336. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, Y.; Xiao, P.; Fan, Q.; Guan, J.; Liang, K.; Chen, T. Sunflower-inspired hydrogel evaporator with mutual reinforcement of evaporation and photodegradation for integrated water management. Chem. Eng. J. 2024, 490, 151550. [Google Scholar] [CrossRef]
- Mo, H.; Wang, Y. A bionic solar-driven interfacial evaporation system with a photothermal-photocatalytic hydrogel for VOC removal during solar distillation. Water Res. 2022, 226, 119276. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Jiang, Y.; Zou, X.Q.; Xing, L.; Liu, W.X.; Huang, Z.H.; Feng, Y.W.; Wang, J.K. Biomass-Based Ferric Tannate Hydrogel with a Photothermal Conversion Function for Solar Water Evaporation. Acs Appl. Polym. Mater. 2023, 5, 9574–9584. [Google Scholar] [CrossRef]
- Dao, V.-D.; Vu, N.H.; Thi Dang, H.-L.; Yun, S. Recent advances and challenges for water evaporation-induced electricity toward applications. Nano Energy 2021, 85, 105979. [Google Scholar] [CrossRef]
- Lu, Y.; Fan, D.; Shen, Z.; Zhang, H.; Xu, H.; Yang, X. Design and performance boost of a MOF-functionalized-wood solar evaporator through tuning the hydrogen-bonding interactions. Nano Energy 2022, 95, 107016. [Google Scholar] [CrossRef]
- Wu, X.; Lu, Y.; Ren, X.H.; Wu, P.; Chu, D.W.; Yang, X.F.; Xu, H.L. Interfacial Solar Evaporation: From Fundamental Research to Applications. Adv. Mater. 2024, 36, 2313090. [Google Scholar] [CrossRef]
- Guo, Y.; de Vasconcelos, L.S.; Manohar, N.; Geng, J.; Johnston, K.P.; Yu, G. Highly Elastic Interconnected Porous Hydrogels through Self-Assembled Templating for Solar Water Purification. Angew. Chem. Int. Ed. Engl. 2022, 61, e202114074. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, Y.; Zhu, B.; Sha, Z.; Zhu, H.; Wu, Z.; Nawaz, F.; Wei, Y.; Luo, L.; Que, W. Low vaporization enthalpy hydrogels for highly efficient solar-driven interfacial evaporation. Desalination 2023, 568, 116999. [Google Scholar] [CrossRef]
- Li, X.P.; Li, X.; Li, H.; Zhao, Y.; Wu, J.; Yan, S.; Yu, Z.Z. Reshapable MXene/Graphene Oxide/Polyaniline Plastic Hybrids with Patternable Surfaces for Highly Efficient Solar-Driven Water Purification. Adv. Funct. Mater. 2021, 32, 2110636. [Google Scholar] [CrossRef]
- Li, W.; Tian, X.; Li, X.; Han, S.; Li, C.; Zhai, X.-Z.; Kang, Y.; Yu, Z.-Z. Ultrahigh solar steam generation rate of a vertically aligned reduced graphene oxide foam realized by dynamic compression. J. Mater. Chem. A 2021, 9, 14859–14867. [Google Scholar] [CrossRef]
- Lee, W.C.; Ronghe, A.; Villalobos, L.F.; Huang, S.; Dakhchoune, M.; Mensi, M.; Hsu, K.J.; Ayappa, K.G.; Agrawal, K.V. Enhanced Water Evaporation from A-Scale Graphene Nanopores. ACS Nano 2022, 16, 15382–15396. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Burhan, M.; Shahzad, M.W.; Ybyraiymkul, D.; Akhtar, F.H.; Li, Y.; Ng, K.C. A zero liquid discharge system integrating multi-effect distillation and evaporative crystallization for desalination brine treatment. Desalination 2021, 502, 114928. [Google Scholar] [CrossRef]
- Chen, Y.F.; Chen, H.; Dai, Y.T.; Xiao, J.L.; Qiu, F.X.; Zhang, T. Honeycomb porous regenerated cellulose aerogel films with enhanced thermal dissipation for agricultural mulch application. Food Bioprod. Process. 2024, 147, 418–427. [Google Scholar] [CrossRef]
- Wang, J.; Kong, Y.; Liu, Z.; Wang, H. Solar-driven interfacial evaporation: Design and application progress of structural evaporators and functional distillers. Nano Energy 2023, 108, 108115. [Google Scholar] [CrossRef]
- Ni, G.; Li, G.; Boriskina, S.V.; Li, H.X.; Yang, W.L.; Zhang, T.J.; Chen, G. Steam generation under one sun enabled by a floating structure with thermal concentration. Nat. Energy 2016, 1, 16126. [Google Scholar] [CrossRef]
- Gong, B.; Yang, H.; Wu, S.; Tian, Y.; Yan, J.; Cen, K.; Bo, Z.; Ostrikov, K. Nanotexturing-enhanced heat transfer and interfacial evaporation for energy-efficient solar-thermal water desalination. Int. J. Heat Mass Transf. 2022, 186, 122462. [Google Scholar] [CrossRef]
- Meng, S.; Zhao, X.; Tang, C.-Y.; Yu, P.; Bao, R.-Y.; Liu, Z.-Y.; Yang, M.-B.; Yang, W. A bridge-arched and layer-structured hollow melamine foam/reduced graphene oxide composite with an enlarged evaporation area and superior thermal insulation for high-performance solar steam generation. J. Mater. Chem. A 2020, 8, 2701–2711. [Google Scholar] [CrossRef]
- Han, X.; Besteiro, L.V.; Koh, C.S.L.; Lee, H.K.; Phang, I.Y.; Phan-Quang, G.C.; Ng, J.Y.; Sim, H.Y.F.; Lay, C.L.; Govorov, A.; et al. Intensifying Heat Using MOF-Isolated Graphene for Solar-Driven Seawater Desalination at 98% Solar-to-Thermal Efficiency. Adv. Funct. Mater. 2021, 31, 2008904. [Google Scholar] [CrossRef]
- Finnerty, C.T.K.; Menon, A.K.; Conway, K.M.; Lee, D.; Nelson, M.; Urban, J.J.; Sedlak, D.; Mi, B. Interfacial Solar Evaporation by a 3D Graphene Oxide Stalk for Highly Concentrated Brine Treatment. Env. Sci. Technol. 2021, 55, 15435–15445. [Google Scholar] [CrossRef]
- Shi, L.; Wang, Y.; Zhang, L.; Wang, P. Rational design of a bi-layered reduced graphene oxide film on polystyrene foam for solar-driven interfacial water evaporation. J. Mater. Chem. A 2017, 5, 16212–16219. [Google Scholar] [CrossRef]
- Song, H.M.; Liu, Y.H.; Liu, Z.J.; Singer, M.H.; Li, C.Y.; Cheney, A.R.; Ji, D.X.; Zhou, L.; Zhang, N.; Zeng, X.; et al. Cold Vapor Generation beyond the Input Solar Energy Limit. Adv. Sci. 2018, 5, 1800222. [Google Scholar] [CrossRef]
- Wang, Y.D.; Wu, X.; Yang, X.F.; Owens, G.; Xu, H.L. Reversing heat conduction loss: Extracting energy from bulk water to enhance solar steam generation. Nano Energy 2020, 78, 105269. [Google Scholar] [CrossRef]
- Kashyap, V.K.; Ghasemi, H.G. Solar heat localization: Concept and emerging applications. J. Mater. Chem. A 2020, 8, 7035–7065. [Google Scholar] [CrossRef]
- Lee, K.H.; Kang, D.J.; Eom, W.; Lee, H.; Han, T.H. Holey graphene oxide membranes containing both nanopores and nanochannels for highly efficient harvesting of water evaporation energy. Chem. Eng. J. 2022, 430, 132759. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, L.; Tao, S.; Li, R.; Wang, Y. Recent research advances in efficient solar-driven interfacial evaporation. Chem. Eng. J. 2024, 489, 151157. [Google Scholar] [CrossRef]
- Xu, H.; Lu, Y.; Jiang, F.; Zhang, J.; Ge, Y.; Li, Z. 3D porous N-doped lignosulfonate/graphene oxide aerogel for efficient solar steam generation and desalination. Int. J. Biol. Macromol. 2023, 233, 123469. [Google Scholar] [CrossRef]
- Liu, X.H.; Mishra, D.D.; Wang, X.B.; Peng, H.Y.; Hu, C.Q. Towards highly efficient solar-driven interfacial evaporation for desalination. J. Mater. Chem. A 2020, 8, 17907–17937. [Google Scholar] [CrossRef]
- Zhang, H.; Du, Y.; Jing, D.; Yang, L.; Ji, J.; Li, X. Integrated Janus Evaporator with an Enhanced Donnan Effect and Thermal Localization for Salt-Tolerant Solar Desalination and Thermal-to-Electricity Generation. ACS Appl. Mater. Interfaces 2023, 15, 49892–49901. [Google Scholar] [CrossRef]
- Zhao, D.; Ding, M.; Lin, T.; Duan, Z.; Wei, R.; Feng, P.; Yu, J.; Liu, C.Y.; Li, C. Gradient Graphene Spiral Sponges for Efficient Solar Evaporation and Zero Liquid Discharge Desalination with Directional Salt Crystallization. Adv. Sci. 2024, 11, e2400310. [Google Scholar] [CrossRef]
- Liu, H.; Luo, H.; Huang, J.; Chen, Z.; Yu, Z.; Lai, Y. Programmable Water/Light Dual-Responsive Hollow Hydrogel Fiber Actuator for Efficient Desalination with Anti-Salt Accumulation. Adv. Funct. Mater. 2023, 33, 2302038. [Google Scholar] [CrossRef]
- Sun, Y.; Tan, X.; Yuan, X.; Li, J. Solar-driven interfacial evaporation: Research advances in structural design. Chem. Eng. J. 2024, 495, 153316. [Google Scholar] [CrossRef]
- Zhou, J.G.; Sun, Z.L.; Chen, M.Q.; Wang, J.T.; Qiao, W.M.; Long, D.H.; Ling, L.C. Macroscopic and Mechanically Robust Hollow Carbon Spheres with Superior Oil Adsorption and Light-to-Heat Evaporation Properties. Adv. Funct. Mater. 2016, 26, 5368–5375. [Google Scholar] [CrossRef]
- Li, R.X.; Wu, M.R.; Ma, H.J.; Zhu, Y.Q.; Zhang, H.Y.; Chen, Q.M.; Zhang, C.H.; Wei, Y. A Single Component, Single Layer Flexile Foam Evaporator with the Higher Efficiency for Water Generation. Adv. Mater. 2024, 36, 2402016. [Google Scholar] [CrossRef]
- Wo, Z.; Sun, X.; Sun, H.; Su, Y.; Xie, Y.; Yang, N.; Zhang, X. All-in-one design of wood evaporator with highly-efficient salt resistance for sustainable solar desalination and contaminated water purification. Chem. Eng. J. 2025, 507, 160715. [Google Scholar] [CrossRef]
- Du, H.; Ge, C.; Xu, D.; Qian, Y.; Chen, Z.; Gao, C.; Song, B.; Shen, Z.; Chen, J.; Liu, K.; et al. Multifunctional woven fabric for integrated solar-driven water generation and personal thermal management. Cellulose 2023, 30, 9207–9220. [Google Scholar] [CrossRef]
- Sun, Z.Z.; Han, C.A.L.; Gao, S.W.; Li, Z.X.; Jing, M.X.; Yu, H.P.; Wang, Z.A.K. Achieving efficient power generation by designing bioinspired and multi-layered interfacial evaporator. Nat. Commun. 2022, 13, 5077. [Google Scholar] [CrossRef]
- Meng, F.L.; Gao, M.M.; Ding, T.P.; Yilmaz, G.; Ong, W.L.; Ho, G.W. Modular Deformable Steam Electricity Cogeneration System with Photothermal, Water, and Electrochemical Tunable Multilayers. Adv. Funct. Mater. 2020, 30, 2002867. [Google Scholar] [CrossRef]
- Sun, N.; Li, J.; Ren, J.; Xu, Z.; Sun, H.; Du, Z.; Zhao, H.; Ettelatie, R.; Cheng, F. Insights into the enhanced flux of graphene oxide composite membrane in direct contact membrane distillation: The different role at evaporation and condensation interfaces. Water Res. 2022, 212, 118091. [Google Scholar] [CrossRef]
- Wei, Y.; Li, W.; Zhang, S.; Yu, J.; Tang, Y.; Wu, J.; Yu, S. Laser-Induced Porous Graphene/CuO Composite for Efficient Interfacial Solar Steam Generation. Adv. Funct. Mater. 2024, 34, 2401149. [Google Scholar] [CrossRef]
- Li, W.C.; Zheng, Z.H.; Qian, Z.Q.; Liu, H.; Wang, X.D. Phase Change Material Boosting Electricity Output and Freshwater Production through Hierarchical-Structured 3D Solar Evaporator. Adv. Funct. Mater. 2024, 34, 2316504. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Zhong, Q.Y.; Huang, Q.; Hu, M.; He, F.; Li, Y.X.; Wang, Z.X. Surface Engineering of 3D Solar Evaporator for Uncompromising Water Evaporation and Salt Production Toward High Concentration Brine. Adv. Funct. Mater. 2024, 34, 2408554. [Google Scholar] [CrossRef]
- Eskafi, A.F.; De Finnda, C.; Garcia, C.A.; Mi, B.X. Mineral Scaling in 3D Interfacial Solar Evaporators-A Challenge for Brine Treatment and Lithium Recovery. Environ. Sci. Technol. 2025, 59, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Payanthoth, N.S.; Mut, N.N.N.; Samanta, P.; Li, G.L.; Jung, J.H. A review of biodegradation and formation of biodegradable microplastics in soil and freshwater environments. Appl. Biol. Chem. 2024, 67, 110. [Google Scholar] [CrossRef]
- Li, D.; Liang, Z.; Yang, H.; Zhang, M.; Cao, K.; Zhao, B.; Wang, Y.; Peng, M.; Sun, Y.; Jiang, L. Mutual Reinforcement of Evaporation and Catalysis for Efficient Freshwater–Salt–Chemical Production. Adv. Funct. Mater. 2023, 33, 2300353. [Google Scholar] [CrossRef]
- Huang, T.H.; Tian, X.Y.; Chen, Y.Y.; Widakdo, J.; Austria, H.F.M.; Setiawan, O.; Subrahmanya, T.M.; Hung, W.S.; Wang, D.M.; Chang, C.Y.; et al. Multifunctional Phra Phrom-like Graphene-Based Membrane for Environmental Remediation and Resources Regeneration. Adv. Funct. Mater. 2023, 34, 2308321. [Google Scholar] [CrossRef]
- Han, Z.X.; Wang, N.; Zhang, H.L.; Yang, X.Y. Heavy metal contamination and risk assessment of human exposure near an e-waste processing site. Acta Agric. Scand. Sect. B Soil Plant Sci. 2017, 67, 119–125. [Google Scholar] [CrossRef]
- Jiang, C.L.; Wang, X.H.; Hou, B.X.; Hao, C.; Li, X.; Wu, J.B. Construction of a Lignosulfonate-Lysine Hydrogel for the Adsorption of Heavy Metal Ions. J. Agric. Food Chem. 2020, 68, 3050–3060. [Google Scholar] [CrossRef]
- Tian, Y.; Du, C.; Yong, S.; Zhou, X.; Zhou, C.; Yang, S. Catalysis-involved 3D N-doped graphene aerogel achieves a superior solar water purification rate and efficiency. Chem. Eng. J. 2023, 453, 139793. [Google Scholar] [CrossRef]
- Dong, S.; Zhao, Y.; Yang, J.; Li, W.; Luo, W.; Li, S.; Liu, X.; Guo, H.; Yu, C.; Sun, J.; et al. Solar water recycling of carbonaceous aerogel in open and colsed systems for seawater desalination and wastewater purification. Chem. Eng. J. 2022, 431, 133824. [Google Scholar] [CrossRef]
- Cui, H.Y.; Zhou, H.; Lin, L. The specific antibacterial effect of the Salvia oil nanoliposomes against Staphylococcus aureus biofiims on milk container. Food Control 2016, 61, 92–98. [Google Scholar] [CrossRef]
- Hu, W.; Li, C.Z.; Dai, J.M.; Cui, H.Y.; Lin, L. Antibacterial activity and mechanism of Litsea cubeba essential oil against methicillin-resistant Staphylococcus aureus (MRSA). Ind. Crops Prod. 2019, 130, 34–41. [Google Scholar] [CrossRef]
- Lu, X.; Feng, X.; Zhang, X.; Chukwu, M.N.; Osuji, C.O.; Elimelech, M. Fabrication of a Desalination Membrane with Enhanced Microbial Resistance through Vertical Alignment of Graphene Oxide. Environ. Sci. Technol. Lett. 2018, 5, 614–620. [Google Scholar] [CrossRef]
- Noureen, L.; Wang, Q.; Ismail, P.M.; Alomar, M.; Arshad, N.; Irshad, M.S.; Xu, Q.; Wang, X. Multifunctional aerogel with antibiofouling properties for efficient solar steam generation and seawater desalination. Nano Today 2024, 54, 102130. [Google Scholar] [CrossRef]
- Lin, L.; Wang, W.; Li, D.; Xu, S.; Sun, Y.; Li, L.; Fan, K.; Xing, C.; Zhang, L.; Li, J. Multifunctional graphene/Ag hydrogel with antimicrobial and catalytic properties for efficient solar-driven desalination and wastewater purification. Chem. Eng. J. 2023, 478, 147249. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Lin, Z.; Xu, N.; Li, X.; Liang, J.; Zhao, W.; Lin, R.; Zhu, B.; Liu, G.; et al. Over 10 kg m−2 h−1 Evaporation Rate Enabled by a 3D Interconnected Porous Carbon Foam. Joule 2020, 4, 928–937. [Google Scholar] [CrossRef]
- Zhang, P.; Li, J.; Lv, L.; Zhao, Y.; Qu, L. Vertically Aligned Graphene Sheets Membrane for Highly Efficient Solar Thermal Generation of Clean Water. ACS Nano 2017, 11, 5087–5093. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).