Interfacial Engineering of S-Scheme WO3/In2S3 Heterojunction for Efficient Solar-Driven CO2 Photoreduction
Abstract
1. Introduction
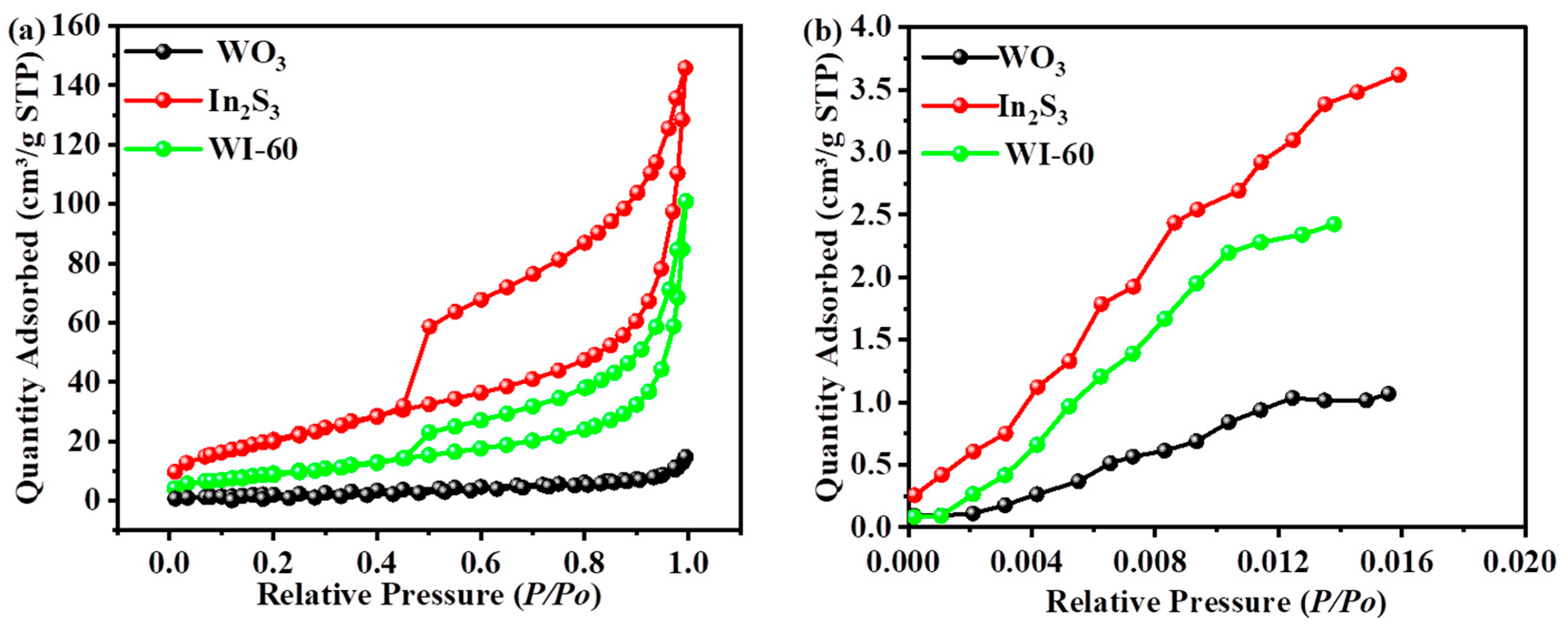
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Catalysts
3.2.1. Fabrication of WO3 Nanosheets (WO3 NSs)
3.2.2. Construction of WO3/In2S3 Heterojunctions
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, Z.; Xing, X.; Qi, Q.; Li, H.; Han, D.; Song, X.; Tang, X.; Ng, H.; Huo, P. Regulation CN reduction of CO2 products selectivity by adjusting the number of V sites and mechanism exploration. Fuel 2025, 388, 134509. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Perumal, S.; Wang, Y.; Ko, H.; Hwang, Y.; Wang, H.; Yang, T.; Zhao, S.; Kim, Y.D.; et al. Enhancing photocatalytic CO2 reduction to butanol by facet-dependent interfacial engineering of CeO2/Cu2O. Appl. Catal. B. Environ. Energy 2025, 368, 125122. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Y.; Wang, X.; Guo, Y.; Ni, C.; Wu, J.; Hao, C. High performance hybrid supercapacitors assembled with multi-cavity nickel cobalt sulfide hollow microspheres as cathode and porous typha-derived carbon as anode. Ind. Crops Prod. 2022, 189, 115863. [Google Scholar] [CrossRef]
- Yang, W.; Gao, M.; Zhang, Y.; Dai, Y.; Peng, W.; Ji, S.; Ji, Y. Self -driven photoelectrochemical sensor based on Z-type perovskite heterojunction for profenofos detection in milk and cabbage. J. Food Compos. Anal. 2024, 136, 106738. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, T.; Li, Y.; Zhang., J.; Du, Y. Construction of core-shell ZnS@In2S3 rhombic dodecahedron Z-scheme heterojunction structure: Enhanced photocatalytic activity and mechanism insight. Chem. Eng. J. 2021, 423, 130138. [Google Scholar] [CrossRef]
- Huang, L.; Mo, S.; Zhao, X.; Zhou, J.; Zhou, X.; Zhang, Y.; Fan, Y.; Xie, Q.; Li, B.; Li, J. Constructing Co and Zn atomic pairs incore-shell Co3S4/NC@ZnS/NC derived from MOF-on-MOF nanostructures for enhanced photocatalytic CO2 reduction to C2H4. Appl. Catal. B Environ. Energy 2024, 352, 124019. [Google Scholar] [CrossRef]
- Zhang, T.; Li, T.; Gao, M.; Lu, W.; Chen, Z.; Ong, W.L.; Wong, A.S.W.; Yang, L.; Kawi, S.; Ho, G.W. Ligand mediated assembly of CdS colloids in 3D porous metal–organic framework derived scaffold with multi-sites heterojunctions for efficient CO2 photoreduction. Adv. Energy Mater. 2024, 14, 2400388. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, Y.; Qian, L.; Yin, Y.; Yuan, Z.; Dai, Y.; Zhang, T.; Yang, D.; Qiu, F. Lamellar Ti3C2 MXene composite decorated with platinum-doped MoS2 nanosheets as electrochemical sensing functional platform for highly sensitive analysis of organophosphorus pesticides. Food. Chem. 2024, 459, 140379. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-Scheme Heterojunction Photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Khan, K.; Tang, Y.; Cheng, P.; Song, Y.; Li, X.; Lou, J.; Iqbal, B.; Zhao, X.; Hameed, R.; Li, G.; et al. Effects of degradable and non-degradable microplastics and oxytetracycline co-exposure on soil N2O and CO2 emissions. Appl. Soil Ecol. 2024, 197, 105331. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, C.; Wang, S.; Wang, Q.; Reinhard, M.; Zhang, G.; Zhan, F.; Wang, H.; Skoien, D.; Kroll, T.; et al. Identifying a highly efficient molecular photocatalytic CO2 reduction system via descriptor-based high-throughput screening. Nat. Catal. 2025, 8, 126–136. [Google Scholar] [CrossRef]
- Zeng, P.; Liu, H.; Jia, H.; Cai, J.; Deng, X.; Peng, T. In-situ synthesis of single-atom CoN clusters-decorated TiO2 for highly efficient charge separation and CO2 photoreduction. Appl. Catal. B Environ. 2024, 340, 123268. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, T.; Wen, J.; Liu, X. Flower-like Bi2S3–In2S3 heterojunction for efficient solar light induced photoreduction of Cr (VI). Chem 2021, 278, 130422. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, Y.; Ma, C.; Jiang, H.; Gao, M.; Zhang, S.; Liu, W.; Huo, P.; Wang, H.; Wang, L. Multichannel electron transmission and fluorescence resonance energy transfer in In2S3/Au/rGO Composite for CO2 Photoreduction. ACS Appl. Mater. Interfaces 2021, 13, 11755–11764. [Google Scholar] [CrossRef]
- Xu, A.; Zhang, Y.; Fan, H.; Liu, X.; Wang, F.; Qu, X.; Yang, L.; Li, X.; Cao, J.; Wei, M. WO3 Nanosheet/ZnIn2S4 S-Scheme Heterojunctions for Enhanced CO2 Photoreduction. ACS Appl. Nano Mater. 2024, 7, 3488–3498. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X.; Zhang, Z.; Zhang, L.; Liao, J.; Zhang, X.; Ge, C.; Zhou, W. Lead-free perovskite Cs3Bi2Br9 quantum dots (QDs)/ultra-thin W18O49 nanobelts S-scheme heterojunction toward optimized photocatalytic CO2 reduction coupled with toluene oxidation. Chem. Eng. J. 2025, 505, 159635. [Google Scholar] [CrossRef]
- Ebri, G.; Alhashmi, E.; Baghdadi, Y.; Daboczi, M.; Eslava, S.; Hellgardt, K. Simultaneous photocatalytic CO2 reduction and C-C coupling of benzyl alcohol under high pressure and supercritical conditions. Chem. Eng. J. 2025, 505, 158356. [Google Scholar] [CrossRef]
- Gouda, A.; Hannouche, K.; Mohan, A.; Mao, C.; Nikbin, E.; Carrière, A.; Ye, J.; Howe, J.Y.; Sain, M.; Hmadeh, M.; et al. In-situ restructuring of Ni-based metal organic frameworks for photocatalytic CO2 hydrogenation. Nat. Commun. 2025, 16, 695. [Google Scholar] [CrossRef]
- Yang, R.; Li, Q.; Ma, Z.; Liu, S.; Tian, D.; Li, D.; Jiang, D. Improving charge separation in poly (heptazine imide) via synergy of covalent S-scheme heterojunction and Schottky junction for efficient photocatalytic CO2 reduction. Chem. Eng. J. 2025, 506, 160043. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Chen, L.; Naa, O.; Zhou, C. Facile preparation of sugarcane bagasse-derived carbon supported MoS2 nanosheets for hydrogen evolution reaction. Ind. Crops Prod. 2021, 172, 114064. [Google Scholar] [CrossRef]
- Chen, Q.; Jiang, J.; Wu, Y.; Fang, J.; Lu, N.; Wang, G.; Mao, J. Enhancing local CO2 availability via amorphous Bi2O3 enable efficient and stable photocatalytic C2H6 production. Chem. Eng. J. 2025, 504, 158854. [Google Scholar] [CrossRef]
- Zhang, H.; Yohannes, A.; Zhao, H.; Li, Z.; Xiao, Y.; Cheng, X.; Wang, H.; Li, Z.; Siahrostami, S.; Kibria, M.G.; et al. Photocatalytic asymmetric C-C coupling for CO2 reduction on dynamically reconstructed Ruδ+-O/Ru0-O sites. Nat. Commun. 2025, 16, 534. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, W.; Deng, Y.; Zhang, Z.; Wang, H.; Wu, Y. Directed Inward Migration of S-Vacancy in Bi2S3 QDs for Selective Photocatalytic CO2 to CH3OH. Nat. Commun. 2025, 12, e2406925. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Ding, D.; Shi, Y.; Wang, K.; Wang, Q.; Chen, X.; Shen, H.; Zhang, T.; Yang, Y.; Xia, J.; et al. Hydrogen-Bonded organic framework Containing stacked Cu2+ for photocatalytic reduction of CO2 to C2H4. Chem. Eng. J. 2025, 503, 158501. [Google Scholar] [CrossRef]
- Sun, Y.; Lai, K.; Shi, X.; Li, N.; Gao, Y.; Ge, L. Regulating metal cation Cu vacancies on ZnIn2S4/Cu1.81S to achieve high selectivity for the photocatalytic reduction of CO2 to CH4. Appl. Catal. B Environ. Energy 2025, 365, 124907. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, H.; Yao, B.; Wang, Y. Hollow core-shell B-g-C3N4-x@Bi2S3/In2S3 dual S-scheme heterojunction photothermal nanoreactor: Boosting photothermal catalytic activity in confined space. Chem. Eng. J. 2024, 484, 149399. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, Y.; Shi, J.; Zhang, W.; Zhang, X.; Huang, X.; Zou, X.; Li, Z.; Wei, R. Facile synthesis of Au@Ag core-shell nanorod with bimetallic synergistic effect for SERS detection of thiabendazole in fruit juice. Food Chem. 2022, 370, 131276. [Google Scholar] [CrossRef]
- Gong, E.; Ali, S.; Hiragond, C.B.; Kim, H.S.; Powar, N.S.; Kim, D.; Kim, H.; In, S.-I. Solar fuels: Research and development strategies to accelerate photocatalytic CO2 conversion into hydrocarbon fuels. Energy Environ. Sci. 2022, 15, 880–937. [Google Scholar] [CrossRef]
- Chen, X.; Cao, Y.; Li, F.; Tian, Y.; Song, H. Enzyme-assisted microbial electrosynthesis of Poly(3-hydroxybutyrate) via CO2 bio-reduction by engineered Ralstonia eutropha. ACS Catal. 2018, 8, 4429–4437. [Google Scholar] [CrossRef]
- Lu, N.; Jiang, X.; Zhu, Y.; Yu, L.; Du, S.; Huang, J.; Zhang, Z. Single-Atom-Layer Metallization of Plasmonic Semiconductor Surface for Selectively Enhancing IR-Driven Photocatalytic Reduction of CO2 into CH4. Adv. Mater. 2024, 37, e2413931. [Google Scholar] [CrossRef]
- Cai, W.; Qian, Z.; Hu, C.; Zheng, W.; Luo, L.; Zhao, Y. Systematic investigation of MoS2-metal sulfides [Metal = In, Sn, Cu, Cd] heterostructure via metal-sulfur bond for photocatalytic CO2 reduction. Chem. Eng. J. 2024, 479, 147718. [Google Scholar] [CrossRef]
- Wang, K.; Hu, Y.; Liu, X.; Li, J.; Liu, B. Visible-light-driven CO2 photoreduction over atomically strained indium sites in ambient air. Nat. Commun. 2025, 16, 2094. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chang, X.; Shi, Y.; Zhao, X. Synergistic effects of Cu7S4@Cu2O core-shell photocatalyst and S-scheme heterojunction in photocatalytic CO2 reduction. J. Alloys Compd. 2025, 1010, 177572. [Google Scholar] [CrossRef]
- Song, M.; Song, X.; Liu, X.; Zhou, W.; Huo, P. Enhancing photocatalytic CO2 reduction activity of ZnIn2S4/MOF-808 microsphere with S-scheme heterojunction by in situ synthesis method. Chin. J. Catal. 2023, 51, 180–192. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Shi, T.; Liang, Q.; Chen, Z. Hierarchical hollow-microsphere cadmium sulfide-carbon dots composites with enhancing charge transfer efficiency for photocatalytic CO2 reduction. J. Alloys Compd. 2023, 936, 168286. [Google Scholar] [CrossRef]
- Xiong, R.; Sun, Y.; Li, J.; Chen, K.; Liu, F.; Xiao, Y.; Cheng, B.; Lei, S. MgCr2O4/MgIn2S4 Spinel/Spinel S-Scheme Heterojunction: A Robust Catalyst for Photothermal-Assisted Photocatalytic CO2 Reduction. Inorg. Chem. 2024, 63, 19309–19321. [Google Scholar] [CrossRef]
- Yan, C.; Xu, M.; Li, J.; Wang, H.; Huo, P. Fabricated ZnIn2S4/Cu2S S-scheme heterojunction with snowflake structure for boosting photocatalytic CO2 reduction in gas–solid reactor. Fuel 2024, 378, 132921. [Google Scholar] [CrossRef]
- Cai, J.; Li, X.; Su, B.; Guo, B.; Lin, X.; Xing, W.; Lu, X.F.; Wang, S. Rational design and fabrication of S-scheme NiTiO3/CdS heterostructures for photocatalytic CO2 reduction. J. Mater. Sci. Technol. 2025, 234, 82–89. [Google Scholar] [CrossRef]
- Chai, Y.; Kong, Y.; Lin, M.; Lin, W.; Shen, J.; Long, J.; Yuan, R.; Dai, W.; Wang, X.; Zhang, Z. Metal to non-metal sites of metallic sulfides switching products from CO to CH4 for photocatalytic CO2 reduction. Nat. Commun. 2023, 14, 6168. [Google Scholar] [CrossRef]
- Nasir, M.; Sheng, B.; Zhao, Y.; Ye, H.; Song, J.; Li, J.; Wang, P.; Wang, T.; Wang, X.; Huang, Z.; et al. An integrated photocatalytic redox architecture for simultaneous overall conversion of CO2 and H2O toward CH4 and H2O2. Sci. Bull. 2024, 70, 373–382. [Google Scholar] [CrossRef]
- Vertepov, A.; Fedorova, A.; Batkin, A.; Knotko, A.; Maslakov, K.; Doljenko, V.; Vasiliev, A.; Kapustin, G.; Shatalova, T.; Sorokina, N.; et al. CO2 hydrogenation to methanol on CuO-ZnO/SiO2 and CuO-ZnO/CeO2− SiO2 catalysts synthesized with β-Cyclodextrin template. Catalysts 2023, 13, 1231. [Google Scholar] [CrossRef]
- Zhang, F.; Xiong, J.; Yu, X.; Wang, L.; Wu, T.; Yu, Z.; Tang, M.; Liu, H.; Chao, Y.; Zhu, W. Coral reef-like CdS/g-C3N5 heterojunction with enhanced CO2 adsorption for efficient photocatalytic CO2 reduction. Catalysts 2025, 15, 94. [Google Scholar] [CrossRef]
- Vattikuti, S.; Sudhani, H.; Habila, M.; Rosaiah, P.; Shim, J. SnO2 quantum dot-decorated g-C3N4 ultrathin nanosheets: A dual-function photocatalyst for pollutant degradation and hydrogen evolution. Catalysts 2024, 14, 824. [Google Scholar] [CrossRef]
- Pei, J.; Li, H.; Yu, D.; Zhang, D. g-C3N4-based heterojunction for enhanced photocatalytic performance: A review of fabrications, applications, and perspectives. Catalysts 2024, 14, 825. [Google Scholar] [CrossRef]
- Fang, J.; Wang, M.; Yang, X.; Sun, Q.; Yu, L. Design and preparation of ZnIn2S4/g-C3N4 Z-scheme heterojunction for enhanced photocatalytic CO2 reduction. Catalysts 2025, 15, 95. [Google Scholar] [CrossRef]
- Wang, X.; Yao, X.; Bai, H.; Zhang, Z. Oxygen vacancy-rich 2D GO/BiOCl composite materials for enhanced photocatalytic performance and semiconductor energy band theory research. Environ. Res. 2022, 212, 113442. [Google Scholar] [CrossRef]
- Wu, Q.; Liang, J.; Huang, Y.; Cao, R. Thermo-, electro-, and photocatalytic CO2 conversion to value-added products over porous metal/covalent organic frameworks. Acc. Chem. Res. 2022, 55, 2978–2997. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, J.; Yin, Q.; Zhang, C.; Zeng, Y.; Xu, S.; Liang, Q.; Zhou, M.; Li, Z. Reinforcing the efficiency of photocatalytic CO2 conversion with H2O vapor through the integration of photothermal Cu/C@Bi/C supported on 3D g-C3N4 under full-spectrum solar irradiation. J. Energy Chem. 2024, 12, 113008. [Google Scholar] [CrossRef]
- Sun, J.; Zhai, N.; Miao, J.; Sun, H. Can Green Finance Effectively Promote the Carbon Emission Reduction in “Local-Neighborhood” Areas? Empirical Evidence from China. Environ. Sci. Pollut. 2022, 12, 10. [Google Scholar]
- Wu, Q.; Zhong, Y.; Chen, R.; Ling, G.; Wang, X. Cu-Ag-C@Ni3S4 with core shell structure and rose derived carbon electrode materials: An environmentally friendly supercapacitor with high energy and power density. Food Chem. 2024, 222, 119676. [Google Scholar] [CrossRef]
- Chen, J.; Jia, Y.; Zhao, H.; Wu, D.; Du, Y. Ternary Heterojunction ZnS-ZnIn2S4-In2S3 Efficiently Catalyzes Triethylamine to Enhance Electrochemiluminescence Imaging Signals. Adv. Funct. Mater. 2024, 35, 2420714. [Google Scholar] [CrossRef]
- Hanye, C.; Shengjie, G.; Guocheng, H.; Qiaoshan, C.; Yanxin, G. Built-in electric field mediated S-scheme charge migration in COF/In2S3 heterojunction for boosting H2O2 photosynthesis and sterilization. Appl. Catal. B Environ. 2024, 343, 123545. [Google Scholar]








| Sample | Light Source | Sacrificial Agent | Evolution Rate μmol/g (4 h) | Ref. |
|---|---|---|---|---|
| MoS2/In2S3 | 300 W Xe-lamp (Beijing Zhongjiao Jinyuan CEL-HXF300, Beijing, China) | Without | CO: 40.36 | [31] |
| Atomic strain In2S3 | 300 W Xe-lamp (Chengdu Lihang PLS-SXE300D), AM 1.5 G filter, Chengdu, China | Without | CO: 20.64 | [32] |
| Cu7S4@Cu2O | 300 W Xe Arc lamp (Beijing Bofeilai, Beijing, China) | Without | CO: 24.76 | [33] |
| ZnIn2S4/MOF-808 | OFR Xe-lamp (Beijing Education Au-light, Beijing, China) | Without | CO: 32.84 | [34] |
| CDs/CdS | 300 W Xe-lamp, AM 1.5 G filter. (Xi’an TopTION Instrument Co., Ltd., Xi’an, China) | Triethanolamine | CO: 48.16 | [35] |
| MgCr2O4/MgIn2S4 | 300 W Xe-lamp (Chengdu Lihang PLS-SXE 300+, Chengdu, China) | Without | CO: 32.12 | [36] |
| ZnIn2S4/Cu2S | 300 W Xe-lamp (Beijing China Education Au-light, Beijing, China) | Without | CO: 13.35/CH4: 34.81 | [37] |
| NiTiO3/CdS | 300 W Xe-lamp (Beijing China Education Au-light, Beijing, China) | Triethanolamine | CO: 20.8 | [38] |
| CuInSnS4 | 300 W Xe-lamp, 420 nm cut-off wavelength filter (Beijing China Education Au-light, Beijing, China) | Without | CH4: 23.32 | [39] |
| WO3/In2S3 | 300W Xe-lamp (Beijing China Education Au-light, Beijing, China) | Without | CO: 55.2 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xu, A.; Lang, J.; Zuo, B.; Yu, Z.; Cui, K.; Li, X.; Zhang, K.; Li, X.; Wei, M.; et al. Interfacial Engineering of S-Scheme WO3/In2S3 Heterojunction for Efficient Solar-Driven CO2 Photoreduction. Catalysts 2025, 15, 460. https://doi.org/10.3390/catal15050460
Wang Y, Xu A, Lang J, Zuo B, Yu Z, Cui K, Li X, Zhang K, Li X, Wei M, et al. Interfacial Engineering of S-Scheme WO3/In2S3 Heterojunction for Efficient Solar-Driven CO2 Photoreduction. Catalysts. 2025; 15(5):460. https://doi.org/10.3390/catal15050460
Chicago/Turabian StyleWang, Yameng, Ao Xu, Jihui Lang, Bin Zuo, Zihan Yu, Keyu Cui, Xuefei Li, Kewei Zhang, Xin Li, Maobin Wei, and et al. 2025. "Interfacial Engineering of S-Scheme WO3/In2S3 Heterojunction for Efficient Solar-Driven CO2 Photoreduction" Catalysts 15, no. 5: 460. https://doi.org/10.3390/catal15050460
APA StyleWang, Y., Xu, A., Lang, J., Zuo, B., Yu, Z., Cui, K., Li, X., Zhang, K., Li, X., Wei, M., & Cao, J. (2025). Interfacial Engineering of S-Scheme WO3/In2S3 Heterojunction for Efficient Solar-Driven CO2 Photoreduction. Catalysts, 15(5), 460. https://doi.org/10.3390/catal15050460







