ZIF-67-Derived Co−N−C Supported Ni Nanoparticles as Efficient Recyclable Catalyst for Hydrogenation of 4-Nitrophenol
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Characterizations of Ni(x)@Co-N-C
2.2. Evaluation of the Catalytic Activity of the Catalyst
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Carbonized ZIF-67 Supported Ni Nanoparticles (Ni@ Co−N−C)
3.3. Characterizations
3.4. Catalytic Reduction of 4-NP to 4-AP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xia, M.; Zhou, J.; Hu, L.; Li, Y. Highly stable and efficient gold nanoparticles films facilitated by thiourea for catalytic reduction of nitrophenol in water. J. Environ. Chem. Eng. 2024, 12, 113004. [Google Scholar] [CrossRef]
- Tharmaraj, V.; Vandarkuzhali, S.A.A.; Karthikeyan, G.; Pachamuthu, M.P. Efficient and recyclable AuNPs/aminoclay nanocomposite catalyst for the reduction of organic dyes. Surf. Interfaces 2022, 32, 102052. [Google Scholar] [CrossRef]
- Gong, L.; Qiu, L.; Xing, X.; Zhu, J.; Lu, M.; Dong, F.; Yu, Y.; Yu, W. Coupling Fe-Co atomic pair to promote the selective reduction of nitroaromatics under mild conditions. Sci. Total Environ. 2024, 912, 169161. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Nitrophenols: 2-Nitrophenol, 4-Nitrophenol; U.S. Public Health Service: Washington, DC, USA, 1992. [Google Scholar]
- Dai, H.; Deng, Z.; Zeng, Y.; Zhang, J.; Yang, Y.; Ma, Q.; Hu, W.; Guo, L.; Li, L.; Wan, S.; et al. Highly sensitive determination of 4-nitrophenol with coumarin-based fluorescent molecularly imprinted poly(ionic liquid). J. Hazard. Mater. 2020, 398, 122854. [Google Scholar] [CrossRef]
- Menezes, O.; Kocaman, K.; Wong, S.; Rios-Valenciana, E.E.; Baker, E.J.; Hatt, J.K.; Zhao, J.; Madeira, C.L.; Krzmarzick, M.J.; Spain, J.C.; et al. Quinone moieties link the microbial respiration of natural organic matter to the chemical reduction of diverse nitroaromatic compounds. Environ. Sci. Technol. 2022, 56, 9387–9397. [Google Scholar]
- Woo, Y.-T.; Lai, D.Y. Aromatic Amino and Nitro-Amino Compounds and Their Halogenated Derivatives, Patty’s Toxicol; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 1–96. [Google Scholar]
- Qin, Y.; Luo, J.; Zhao, Y.; Yao, C.; Li, Y.; An, Q.; Xiao, Z.; Zhai, S. Dual-wastes derived biochar with tailored surface features for highly efficient p-nitrophenol adsorption. J. Clean. Prod. 2022, 353, 131571. [Google Scholar]
- Wang, Q.; Wang, P.; Xu, P.; Li, Y.; Duan, J.; Zhang, G.; Hu, L.; Wang, X.; Zhang, W. Visible-light-driven photo-Fenton reactions using Zn1-1.5xFexS/g-C3N4 photocatalyst: Degradation kinetics and mechanisms analysis. Appl. Catal. B Environ. 2020, 266, 118653. [Google Scholar]
- Li, M.; Wei, D.; Yan, L.; Yang, Q.; Liu, L.; Xu, W.; Du, B.; Wang, Q.; Hou, H. Aerobic biodegradation of p-nitrophenol in a nitrifying sludge bioreactor: System performance, sludge property and microbial community shift. J. Environ. Manag. 2020, 265, 110542. [Google Scholar] [CrossRef]
- Bhosale, G.S.; Vaidya, P.D.; Joshi, J.B.; Patil, R.N. Analysis of reaction kinetics of the ozonation of phenolic compounds and assessment of the role of mass transfer in the overall rate. Ind. Eng. Chem. Res. 2023, 62, 8181–8190. [Google Scholar]
- Fadillah, G.; Saleh, T.A.; Wahyuningsih, S. Enhanced electrochemical degradation of 4-nitrophenol molecules using novel Ti/TiO2-NiO electrodes. J. Mol. Liq. 2019, 289, 111108. [Google Scholar] [CrossRef]
- Shi, G.-M.; Li, S.-T.; Shi, F.-N.; Shi, X.-F.; Lv, S.-H.; Cheng, X.-B. A facile strategy for synthesis of Ni@C(N) nanocapsules with enhanced catalytic activity for 4-nitrophenol reduction. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 170–179. [Google Scholar]
- Wang, Z.; Zhang, H.; Chen, L.; Miao, S.; Wu, S.; Hao, X.; Zhang, W.; Jia, M. Interfacial synergy of PtPd nanoparticles dispersed on amine-modified ZrSBA-15 in catalytic dehydrogenation of ammonia borane and reduction of p-nitrophenol. J. Phys. Chem. C 2018, 122, 12975–12983. [Google Scholar]
- Zhang, W.; Tan, F.; Wang, W.; Qiu, X.; Qiao, X.; Chen, J. Facile, template-free synthesis of silver nanodendrites with high catalytic activity for the reduction of p-nitrophenol. J. Hazard. Mater. 2012, 217–218, 36–42. [Google Scholar]
- Wang, J.; Zhang, X.B.; Wang, Z.L.; Wang, L.; Xing, W.; Liu, X. One-step and rapid synthesis of “clean” and monodisperse dendritic Pt nanoparticles and their high performance toward methanol oxidation and p-nitrophenol reduction. Nanoscale 2012, 4, 1549–1552. [Google Scholar]
- Tian, M.; Long, Y.; Xu, D.; Wei, S.; Dong, Z. Hollow mesoporous silica nanotubes modified with palladium nanoparticles for environmental catalytic applications. J. Colloid Interface Sci. 2018, 521, 132–140. [Google Scholar] [CrossRef]
- Goyal, A.; Bansal, S.; Singhal, S. Facile reduction of nitrophenols: Comparative catalytic efficiency of MFe2O4 (M = Ni, Cu, Zn) nano ferrites. Int. J. Hydrogen Energy 2014, 39, 4895–4908. [Google Scholar]
- Song, J.M.; Zhang, S.S.; Yu, S.H. Multifunctional Co0.85Se-Fe3O4 nanocomposites: Controlled synthesis and their enhanced performances for efficient hydrogenation of p-nitrophenol and adsorbents. Small 2014, 10, 717–724. [Google Scholar] [PubMed]
- Ni, S.; Yang, L.; Qu, H.; Zhu, X.; Xu, Z.; Yuan, M.; Xing, H.; Wang, L.; Yu, J.; Liu, H. Tailoring the structure and energy level over transition-metal doped MoS2 towards enhancing 4-nitrophenol reduction reaction. J. Environ. Chem. Eng. 2021, 9, 105101. [Google Scholar]
- Zhou, L.; We, M.; Wu, Q.; Wu, D. Fabrication and catalytic activity of FeNi@Ni nanocables for the reduction of p-nitrophenol. Dalton Trans. 2014, 43, 7924–7929. [Google Scholar]
- Liu, X.; Wang, X.; Yuan, X.; Dong, W.; Huang, F. Rational composition and structural design of in situ grown nickel-based electrocatalysts for efficient water electrolysis. J. Mater. Chem. A 2016, 4, 167–172. [Google Scholar] [CrossRef]
- Wang, P.; Li, D.; Wang, L.; Guo, S.; Zhao, Y.; Shang, H.; Wang, D.; Zhang, B. Ultrafine CoNi alloy nanoparticles anchored on surface-roughened halloysite nanotubes for highly efficient catalytic hydrogenation of 4-nitrophenol. Chem. Eng. J. 2024, 495, 153631. [Google Scholar] [CrossRef]
- Guo, Y.; Dai, M.; Zhu, Z.; Chen, Y.; He, H.; Qin, T. Chitosan modified Cu2O nanoparticles with high catalytic activity for p-nitrophenol reduction. Appl. Surf. Sci. 2019, 480, 601–610. [Google Scholar]
- Wang, J.; Wu, Z.; Li, T.; Ye, J.; Shen, L.; She, Z.; Liu, F. Catalytic PVDF membrane for continuous reduction and separation of p-nitrophenol and methylene blue in emulsified oil solution. Chem. Eng. J. 2018, 334, 579–586. [Google Scholar]
- Nguyen, C.; Lee, S.; Chung, Y.G.; Chiang, W.-H.; Wu, K.C.-W. Synergistic effect of metal-organic framework-derived boron and nitrogen heteroatom-doped three-dimensional porous carbons for precious-metal-free catalytic reduction of nitroarenes. Appl. Catal. B Environ. 2019, 257, 117888. [Google Scholar]
- Zhou, M.; Wei, X.; Zhang, X.; Gao, X.; Wang, X.; Wu, W.D.; Selomulya, C.; Wu, Z. Uniform mesoporous carbon hollow microspheres imparted with surface-enriched gold nanoparticles enable fast flow adsorption and catalytic reduction of nitrophenols. J. Colloid Interface Sci. 2019, 537, 112–122. [Google Scholar]
- Zou, X.; Ying, E.; Dong, S. Seed-mediated synthesis of branched gold nanoparticles with the assistance of citrate and their surface-enhanced Raman scattering properties. Nanotechnology 2006, 17, 4758. [Google Scholar] [CrossRef]
- Kim, K.-S.; Demberelnyamba, D.; Lee, H. Size-selective synthesis of gold and platinum nanoparticles using novel thiol-functionalized ionic liquids. Langmuir 2004, 20, 556–560. [Google Scholar] [PubMed]
- Bhadra, B.N.; Vinu, A.; Serre, C.; Jhung, S.H. MOF-derived carbonaceous materials enriched with nitrogen: Preparation and applications in adsorption and catalysis. Mater. Today 2019, 25, 88–111. [Google Scholar]
- Zhang, H.; Nai, J.; Yu, L.; Lou, X.W. Metal-organic-framework-based materials as platforms for renewable energy and environmental applications. Joule 2017, 1, 77–107. [Google Scholar]
- Xue, Y.; Zheng, S.; Xue, H.; Pang, H. Metal–organic framework composites and their electrochemical applications. J. Mater. Chem. A 2019, 7, 7301–7327. [Google Scholar]
- Marpaung, F.; Kim, M.; Khan, J.H.; Konstantinov, K.; Yamauchi, Y.; Hossain, M.S.A.; Na, J.; Kim, J. Metal-organic framework (MOF)-derived nanoporous carbon materials. Chem. Asian J. 2019, 14, 1331–1343. [Google Scholar] [PubMed]
- Rocca, J.D.; Liu, D.; Lin, W. Nanoscale metal–organic frameworks for biomedical imaging and drug delivery. Acc. Chem. Res. 2011, 44, 957–968. [Google Scholar] [PubMed]
- Shen, K.; Chen, X.; Chen, J.; Li, Y. Development of MOF-derived carbon-based nanomaterials for efficient catalysis. ACS Catal. 2016, 6, 5887–5903. [Google Scholar]
- Zhang, F.; Chen, L.; Yang, H.; Peng, Y.; Luo, X.; Ahmad, A.; Ramzan, N.; Xu, Y.; Shi, Y. Ultrafine Co nanoislands grafted on tailored interpenetrating N-doped carbon nanoleaves: An efficient bifunctional electrocatalyst for rechargeable Zn-air batteries. Chem. Eng. J. 2022, 431, 133734. [Google Scholar] [CrossRef]
- Mohamed, A.M.; Abo El Naga, A.O.; Zaki, T.; Hassan, H.B.; Allam, N.K. Bimetallic Co–W–S chalcogenides confined in N, S-codoped porous carbon matrix derived from metal–organic frameworks for highly stable electrochemical supercapacitors. ACS Appl. Energy Mater. 2020, 3, 8064–8074. [Google Scholar]
- Yusran, Y.; Xu, D.; Fang, Q.; Zhang, D.; Qiu, S. MOF-derived Co@NC nanocatalyst for catalytic reduction of 4-nitrophenol to 4-aminophenol. Microporous Mesoporous Mater. 2017, 241, 346–354. [Google Scholar] [CrossRef]
- Liu, W.; Duan, W.; Zhang, Q.; Gong, X.; Tian, J. Novel bimetallic MOF derived N-doped carbon supported Ru nanoparticles for efficient reduction of nitro aromatic compounds and rhodamine B. New J. Chem. 2022, 46, 17004–17015. [Google Scholar]
- Li, Y.; Hu, Z.; Sheng, M.; Gan, C.; Hu, H.; Sun, B.; Wang, X.; Jiang, H. ZIF-67 template-assisted porous carbon based RuCo synergistic effect for efficient NH3BH3 hydrolysis & 4-nitrophenol reduction: Effect of morphology and pore structure adjustment. Int. J. Hydrogen Energy 2023, 48, 36795–36809. [Google Scholar]
- Munnik, P.; De Jongh, P.E.; De Jong, K.P. Recent developments in the synthesis of supported Catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar]
- Cao, Y.; Mao, S.; Li, M.; Chen, Y.; Wang, Y. Metal/porous carbon composites for heterogeneous catalysis: Old catalysts with improved performance promoted by N-Doping. ACS Catal. 2017, 7, 8090–8112. [Google Scholar]
- Deng, J.; Li, M.; Wang, Y. Biomass-derived carbon: Synthesis and applications in energy storage and conversion. Green Chem. 2016, 18, 4824–4854. [Google Scholar]
- Lu, Y.; Zhan, W.; He, Y.; Wang, Y.; Kong, X.; Kuang, Q.; Xie, Z.; Zheng, L. MOF-templated synthesis of porous Co3O4 concave nanocubes with high specific surface area and their gas sensing properties. ACS Appl. Mater. Interfaces 2014, 6, 4186–4195. [Google Scholar] [PubMed]
- Nam, K.M.; Shim, J.H.; Ki, H.; Choi, S.I.; Lee, G.; Jang, J.K.; Jo, Y.; Jung, M.H.; Song, H.; Park, J.T. Single-crystalline hollow face-centered-cubic cobalt nanoparticles from solid face-centered-cubic cobalt oxide nanoparticles. Angew. Chem. Int. Ed. 2008, 47, 9504–9508. [Google Scholar]
- Pan, W.; Zheng, J.; Du, H.; Zhang, B.; Xu, J.; Zhang, M. Facile synthesis of MOF-derived one-dimensional nitrogen-doped carbon/Ni composites and their application as catalysts and protein adsorbents. ChemistrySelect 2022, 7, e202104619. [Google Scholar]
- Li, Z.H.; Shao, M.F.; Zhou, L.; Yang, Q.H.; Zhang, C.; Wei, M.; Evans, D.G.; Duan, X. Carbon-based electrocatalyst derived from bimetallic metal-organic framework arrays for high performance oxygen reduction. Nano Energy 2016, 25, 100–109. [Google Scholar]
- Wu, R.; Wang, D.P.; Rui, X.; Liu, B.; Zhou, K.; Law, A.W.K.; Yan, Q.; Wei, J.; Chen, Z. In-situ formation of hollow hybrids composed of cobalt sulfides embedded within porous carbon polyhedra/carbon nanotubes for high-performance lithium-ion batteries. Adv. Mater. 2015, 27, 3038–3044. [Google Scholar]
- Liu, B.; Liu, L.R.; Liu, X.J.; Liu, M.J.; Xiao, Y.S. Variation of crystal structure in nickel nanoparticles filled in carbon nanotubes. Mater. Sci. Technol. 2009, 28, 1345–1348. [Google Scholar]
- Li, X.; Guan, B.Y.; Gao, S.; Lou, X.W. A general dual-templating approach to biomass-derived hierarchically porous heteroatom-doped carbon materials for enhanced electrocatalytic oxygen reduction. Energy Environ. Sci. 2019, 12, 648–655. [Google Scholar]
- Chen, H.; Sun, F.; Wang, J.; Li, W.; Qiao, W.; Ling, L.; Long, D. Nitrogen doping effects on the physical and chemical properties of mesoporous carbons. J. Phys. Chem. C 2013, 117, 8318–8328. [Google Scholar]
- Arrigo, R.; Schuster, M.E.; Xie, Z.; Yi, Y.; Wowsnick, G.; Sun, L.L.; Friedrich, M.; Kast, P.; Hävecker, M.; Knop-Gericke, A.; et al. Nature of the N-Pd interaction in nitrogen-doped carbon nanotube catalysts. ACS Catal. 2015, 5, 2740–2753. [Google Scholar]
- Wang, Z.; Ang, J.M.; Liu, J.; Daphne Ma, X.Y.; Kong, J.H.; Zhang, Y.F.; Yan, T.; Lu, X. FeNi alloys encapsulated in N-doped CNTs-tangled porous carbon fibers as highly efficient and durable bifunctional oxygen electrocatalyst for rechargeable zinc-air battery. Appl. Catal. B Environ. 2019, 263, 118344. [Google Scholar]
- Zhang, M.; Uchaker, E.; Hu, S.; Zhang, Q.; Wang, T.; Cao, G.; Li, J. CoO–carbon nanofiber networks prepared by electrospinning as binder-free anode materials for lithium-ion batteries with enhanced properties. Nanoscale 2013, 5, 12342–12349. [Google Scholar] [PubMed]
- Xie, W.F.; Song, Y.K.; Li, S.J.; Li, J.B.; Yang, Y.S.; Liu, W.; Shao, M.; Wei, M. Single-atomic-Co electrocatalysts with self-supported architecture toward oxygen-involved reaction. Adv. Funct. Mater. 2019, 29, 1906477. [Google Scholar]
- Fu, K.; Wang, Y.; Mao, L.; Yang, X.X.; Jin, J.; Yang, S.; Li, G. Strongly coupled Co, N co-doped carbon nanotubes/graphene-like carbon nanosheets as efficient oxygen reduction electrocatalysts for primary zinc-air battery. Chem. Eng. J. 2018, 351, 94–102. [Google Scholar]
- Gopi, S.; Selvamani, V.; Yun, K. MoS2 decoration followed by P inclusion over Ni-Co bimetallic metal–organic framework-derived heterostructures for water splitting. Inorg. Chem. 2021, 60, 10772–10780. [Google Scholar]
- Jugnet, Y.; Duc, T.M. Structure electronique des oxydes de cobalt CoO et Co3O4. J. Phys. Chem. Solids 1979, 40, 29–37. [Google Scholar]
- Hüfner, S.; Wertheim, G.K. X-ray photoelectron band structure of some transition-metal compounds. Phys. Rev. B 1973, 8, 4857. [Google Scholar]
- Wertheim, G.K.; Hüfner, S. X-ray photoemission band structure of some transition-metal oxides. Phys. Rev. Lett. 1972, 28, 1028. [Google Scholar]
- Top, T.; Yurderi, M.; Bulut, A.; Karakoyun, N.; Ceylan, E.; Zahmakıran, M. Preparation of carbon supported NiCo alloy nanoparticles and evaluation of their catalytic activity in the methanolysis of ammonia borane. Fuel 2023, 341, 127700. [Google Scholar]
- Li, Y.; Yan, X.; Hu, X.; Feng, R.; Zhou, M. Trace pyrolyzed ZIF-67 loaded activated carbon pellets for enhanced adsorption and catalytic degradation of Rhodamine B in water. Chem. Eng. J. 2019, 375, 122003. [Google Scholar]
- Wang, L.; Liu, J.; Tian, C.; Zhao, W.; Li, P.; Liu, W.; Song, L.; Liu, Y.; Wang, C.-A.; Xie, Z. MOF-derived CoNi nanoalloy particles encapsulated in nitrogen-doped carbon as superdurable bifunctional oxygen electrocatalyst. Nanomaterials 2013, 13, 715. [Google Scholar]
- Patra, A.K.; Dutta, A.; Bhaumik, A. Cu nanorods and nanospheres and their excellent catalytic activity in chemoselective reduction of nitrobenzenes. Catal. Commun. 2010, 11, 651–655. [Google Scholar]
- El-Tantawy, A.I.; Elsaeed, S.M.; Neiber, R.R.; Eisa, W.H.; Aleem, A.A.H.A.; El-Hamalawy, A.A.; Maize, M.S. Silver nanoparticles-based thioureidophosphonate composites: Synthesis approach and their exploitation in 4-nitrophenol reduction. Surf. Interfaces 2023, 40, 103006. [Google Scholar]
- Liu, G.; Li, X.; Ganesan, P.; Popov, B.N. Development of non-precious metal oxygen reduction catalysts for PEM fuel cells based on N-doped ordered porous carbon. Appl. Catal. B Environ. 2009, 93, 156–165. [Google Scholar]
- Wang, J.; Zhu, H.; Yu, D.; Chen, J.; Chen, J.; Zhang, M.; Wang, L.; Du, M. Engineering the composition and structure of bimetallic Au–Cu alloy nanoparticles in carbon nanofibers: Self-supported electrode materials for electrocatalytic water splitting. ACS Appl. Mater. Interfaces 2017, 9, 19756–19765. [Google Scholar] [PubMed]
- Xu, Y.; Yin, S.; Li, C.; Deng, K.; Xue, H.; Li, X.; Wang, H.; Wang, L. Low-ruthenium-content NiRu nanoalloys encapsulated in nitrogen-doped carbon as highly efficient and pH-universal electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A 2018, 6, 1376–1381. [Google Scholar]
- Gu, S.; Wunder, S.; Lu, Y.; Ballauff, M.; Fenger, R.; Rademann, K.; Jaquet, B.; Zaccone, A. Kinetic analysis of the catalytic reduction of 4-nitrophenol by metallic nanoparticles. J. Phys. Chem. C 2014, 118, 18618–18625. [Google Scholar]
- Kong, X.; Zhu, H.; Chen, C.L.; Huang, G.; Chen, Q. Insights into the reduction of 4-nitrophenol to 4-aminophenol on catalysts. Chem. Phys. Lett. 2017, 684, 148–152. [Google Scholar]
- Deng, J.-P.; Shih, W.-C.; Mou, C.-Y. Electron transfer-induced hydrogenation of anthracene catalyzed by gold and silver nanoparticles. J. Phys. Chem. C 2007, 111, 9723–9728. [Google Scholar]
- Nørskov, J.K.; Bligaard, T.; Rossmeisl, J.; Christensen, C.H. Towards the computational design of solid catalysts. Nat. Chem. 2009, 1, 37–46. [Google Scholar]
- Sun, S.; Zhou, X.; Cong, B.; Hong, W.; Chen, G. Tailoring the d-Band centers endows (NixFe1–x)2P nanosheets with efficient oxygen evolution catalysis. ACS Catal. 2020, 10, 9086–9097. [Google Scholar] [CrossRef]
- Jiang, Z.; Xie, J.; Jiang, D.; Jing, J.; Qin, H. Facile route fabrication of nano-Ni core mesoporous silica shell particles with high catalytic activity towards 4-nitrophenol reduction. CrystEngComm 2012, 14, 4601–4611. [Google Scholar] [CrossRef]
- Larson, R.A.; Weber, E.J. Reaction Mechanisms in Environmental Organic Chemistry; Routledge: London, UK, 2018. [Google Scholar]
- Qian, G.; Chen, J.; Yu, T.; Luo, L.; Yin, S. N-doped graphene-decorated NiCo alloy coupled with mesoporous NiCoMoO nano-sheet heterojunction for enhanced water electrolysis activity at high current density. Nano-Micro Lett. 2021, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Yang, H.; Jiang, Y.; Sun, Z.; Li, X.; Yang, J.; Wang, H.; Zou, R.; Jiang, W.; Qiu, P.; et al. Modulating the electronic structure of FeCo nanoparticles in N-Doped mesoporous carbon for efficient oxygen reduction reaction. Adv. Sci. 2022, 9, 2200394. [Google Scholar]
- Yang, Y.; Ren, Y.; Sun, C.; Hao, S. Facile route fabrication of nickel based mesoporous carbons with high catalytic performance towards 4-nitrophenol reduction. Green Chem. 2014, 16, 2273–2280. [Google Scholar] [CrossRef]
- Xia, J.; He, G.; Zhang, L.; Sun, X.; Wang, X. Hydrogenation of nitrophenols catalyzed by carbon black-supported nickel nanoparticles under mild conditions. Appl. Catal. B Environ. 2016, 180, 408–415. [Google Scholar] [CrossRef]
- Krebsz, M.; Kótai, L.; Sajó, I.E.; Váczi, T.; Pasinszki, T. Carbon Microsphere-Supported Metallic Nickel Nanoparticles as Novel Heterogeneous Catalysts and Their Application for the Reduction of Nitrophenol. Molecules 2021, 26, 5680. [Google Scholar] [CrossRef]
- Ji, Z.; Shen, X.; Zhu, G.; Zhou, H.; Yuan, A. Reduced graphene oxide/nickel nanocomposites: Facile synthesis, magnetic and catalytic properties. J. Mater. Chem. 2012, 22, 3471–3477. [Google Scholar] [CrossRef]
- Bin Jiang, D.; Liu, X.; Yuan, Y.; Feng, L.; Ji, J.; Wang, J.; Losic, D.; Yao, H.-C.; Zhang, Y.X. Biotemplated top-down assembly of hybrid Ni nanoparticles/N doping carbon on diatomite for enhanced catalytic reduction of 4-nitrophenol. Chem. Eng. J. 2020, 383, 123156. [Google Scholar]
- Kansal, S.; Singh, P.; Biswas, S.; Chowdhury, A.; Mandal, D.; Priya, S.; Singh, T.; Chandra, A. Superior-catalytic performance of Ni–Co layered double hydroxide nanosheets for the reduction of p-nitrophenol. Int. J. Hydrogen Energy 2022, 48, 21372–21382. [Google Scholar]
- Fang, J.; Huang, Z.; Zhao, S.; Chen, Z.; Huang, W.; Liang, Z.; Qiu, Y. Smaller ZIF-67-Ni derived composites supported on halloysites as superior one-dimensional bimetallic catalyst for synergistic reduction of nitroaromatics. Colloids Surfaces A: Physicochem. Eng. Asp. 2024, 703, 135279. [Google Scholar] [CrossRef]
- He, X.; Zhang, M.; Jin, Z.; Zheng, J.; Xu, J.; Yin, X. Highly active CoNi nanoparticles confined in N-doped carbon microtubes for efficient catalytic performance. Dalton Trans. 2022, 51, 16681. [Google Scholar]
- Xie, S.; Xiao, Y.; Lu, N.; Zhang, M. Formation of one-dimensional hierarchical MoO2@C–Ni/CoNi hybrids as highly efficient catalysts and protein adsorbents. CrystEngComm 2023, 25, 4427–4434. [Google Scholar] [CrossRef]
- Lin, Y.; Dong, X.; Zhao, L. Hollow N-ZIFs@NiCo-LDH as highly efficient catalysts for 4-nitrophenol and dyes. Appl. Organomet. Chem. 2020, 34, e5814. [Google Scholar] [CrossRef]




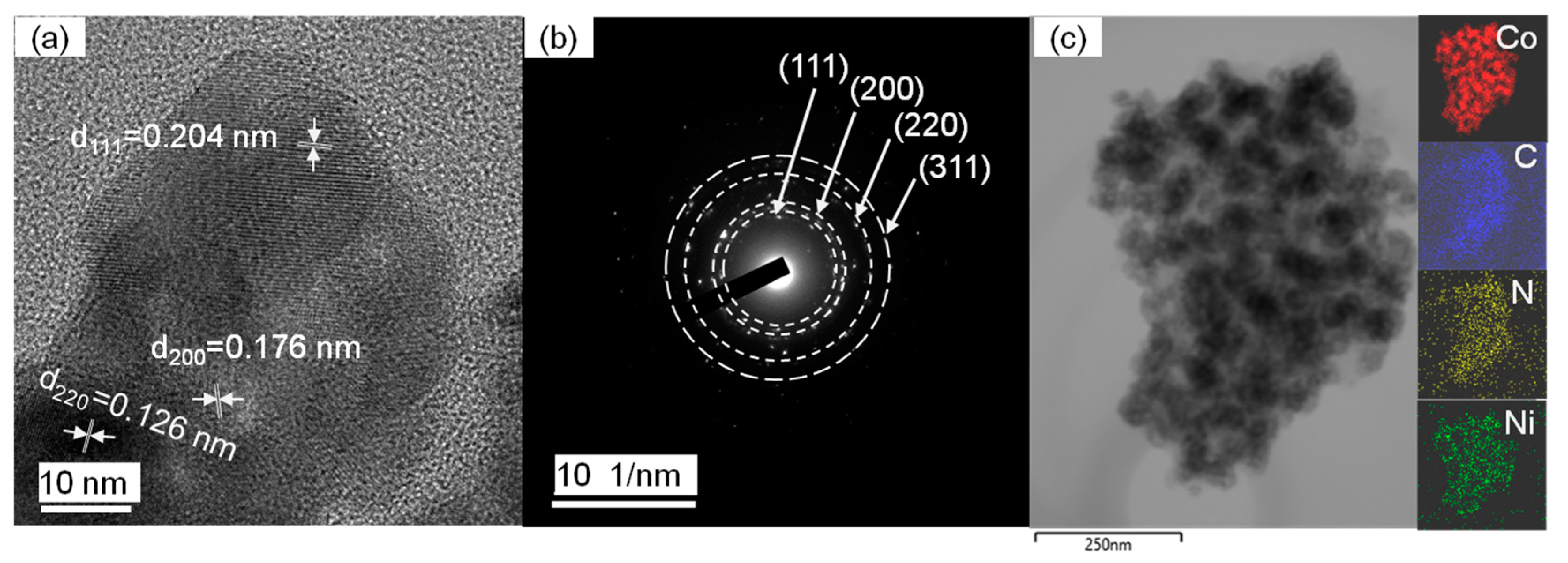

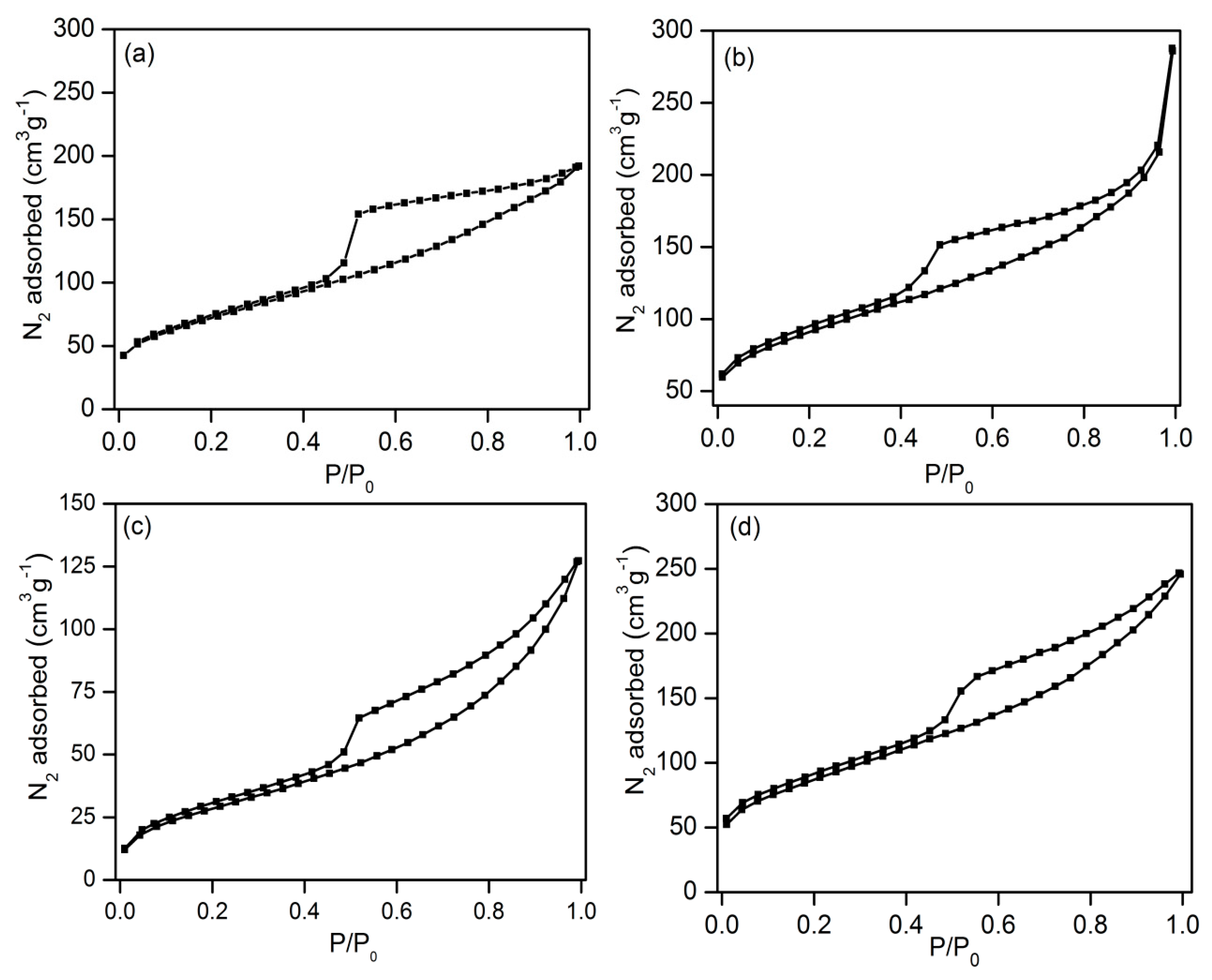
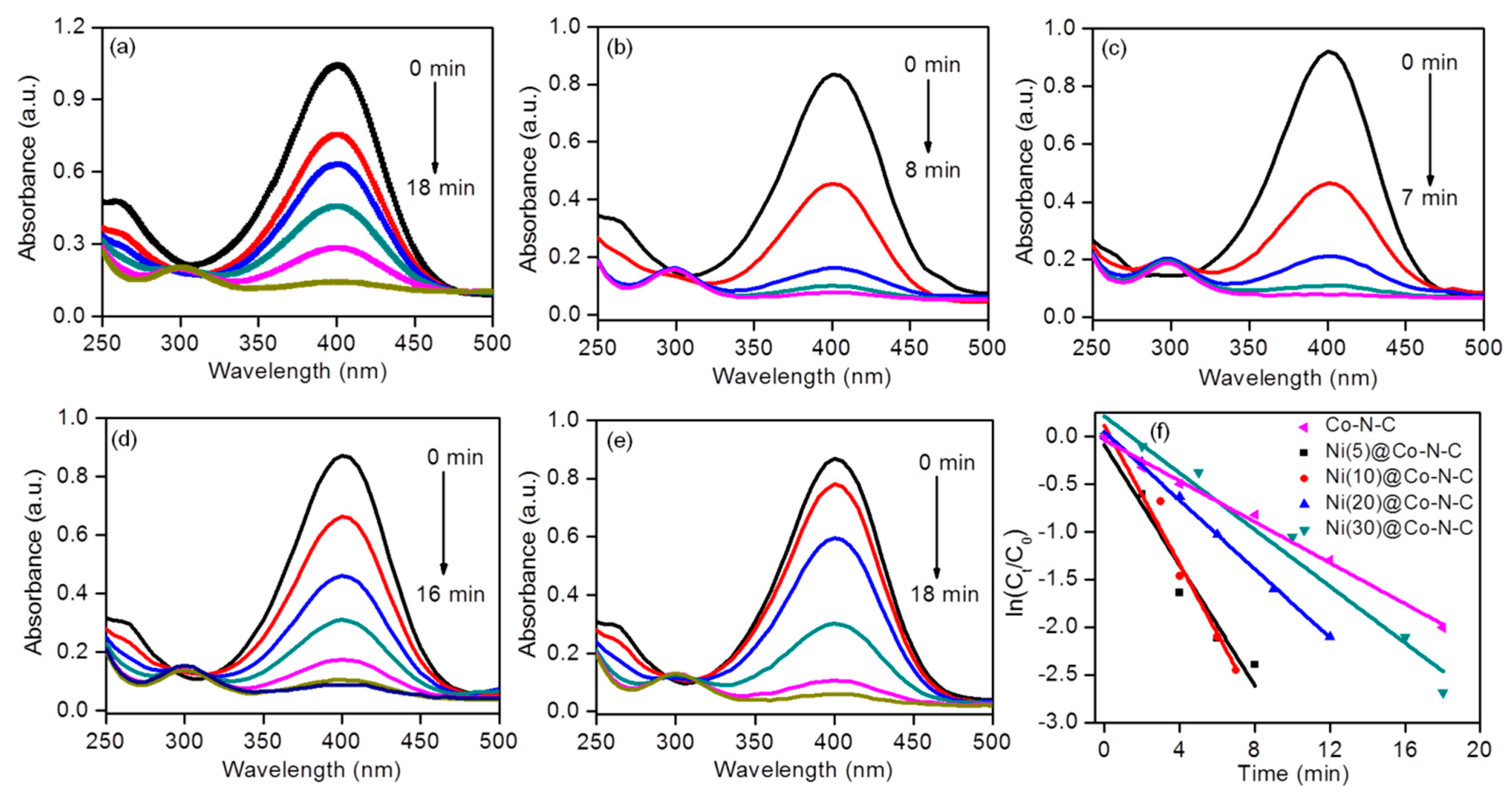

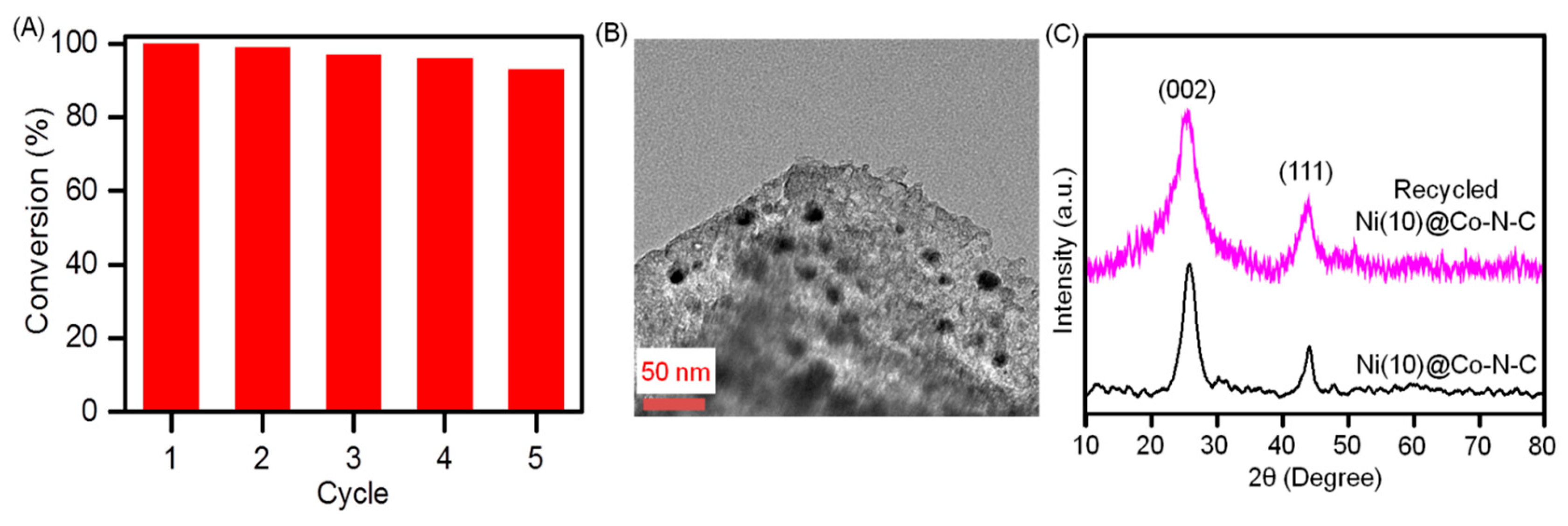
| Sample | Surface Area (m2g−1) | Pore Volume (cm3g−1) | Pore Size (nm) | d111 a (nm) | a0 b (nm) | Metal Loading (wt.%) c | |
|---|---|---|---|---|---|---|---|
| Co | Ni | ||||||
| Co−N−C | 100 | 0.14 | 7.8 | 0.206 | 0.357 | 31.0 | - |
| Ni(5)@Co−N−C | 259 | 0.44 | 4.1 | 0.204 | 0.355 | 29.7 | 2.9 |
| Ni(10)@Co−N−C | 316 | 0.38 | 4.1 | 0.205 | 0.355 | 27.1 | 5.6 |
| Ni(20)@Co−N−C | 312 | 0.29 | 4.1 | 0.205 | 0.355 | 26.1 | 11.1 |
| Ni(30)@Co−N−C | 109 | 0.19 | 4.1 | 0.205 | 0.355 | 24.2 | 16.8 |
| Catalyst | t a (min) | kapp b (s−1) | κ c (s−1gcat.−1) | κ d (s−1gmetal−1) | TOF (Molecules gmetal−1s−1) |
|---|---|---|---|---|---|
| Co−N−C | 18 | 1.7× 10−3 | 9 | 27.4 | 6.96 × 1018 |
| Ni(5)@Co−N−C | 8 | 5.2 × 10−3 | 26 | 79.7 | 1.56 × 1019 |
| Ni(10)@Co−N−C | 7 | 6.1 × 10−3 | 31 | 93.2 | 1.78 × 1019 |
| Ni(20)@Co−N−C | 16 | 3.0 × 10−3 | 15 | 40.3 | 6.75 × 1018 |
| Ni(30)@Co−N−C | 18 | 2.5 × 10−3 | 13 | 30.5 | 5.69 × 1018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deka, J.R.; Saikia, D.; Lin, J.-C.; Chen, W.-Y.; Kao, H.-M.; Yang, Y.-C. ZIF-67-Derived Co−N−C Supported Ni Nanoparticles as Efficient Recyclable Catalyst for Hydrogenation of 4-Nitrophenol. Catalysts 2025, 15, 343. https://doi.org/10.3390/catal15040343
Deka JR, Saikia D, Lin J-C, Chen W-Y, Kao H-M, Yang Y-C. ZIF-67-Derived Co−N−C Supported Ni Nanoparticles as Efficient Recyclable Catalyst for Hydrogenation of 4-Nitrophenol. Catalysts. 2025; 15(4):343. https://doi.org/10.3390/catal15040343
Chicago/Turabian StyleDeka, Juti Rani, Diganta Saikia, Jia-Cheng Lin, Wan-Yu Chen, Hsien-Ming Kao, and Yung-Chin Yang. 2025. "ZIF-67-Derived Co−N−C Supported Ni Nanoparticles as Efficient Recyclable Catalyst for Hydrogenation of 4-Nitrophenol" Catalysts 15, no. 4: 343. https://doi.org/10.3390/catal15040343
APA StyleDeka, J. R., Saikia, D., Lin, J.-C., Chen, W.-Y., Kao, H.-M., & Yang, Y.-C. (2025). ZIF-67-Derived Co−N−C Supported Ni Nanoparticles as Efficient Recyclable Catalyst for Hydrogenation of 4-Nitrophenol. Catalysts, 15(4), 343. https://doi.org/10.3390/catal15040343







