Genome Mining of the Biocontrol Agent Trichoderma afroharzianum Unearths a Key Gene in the Biosynthesis of Anti-Fungal Volatile Sesquiterpenoids
Abstract
1. Introduction
2. Results and Discussion
2.1. Genome Mining and Bioinformatic Analysis
2.2. Expression Levels of the TaTS Genes upon Foc Induction
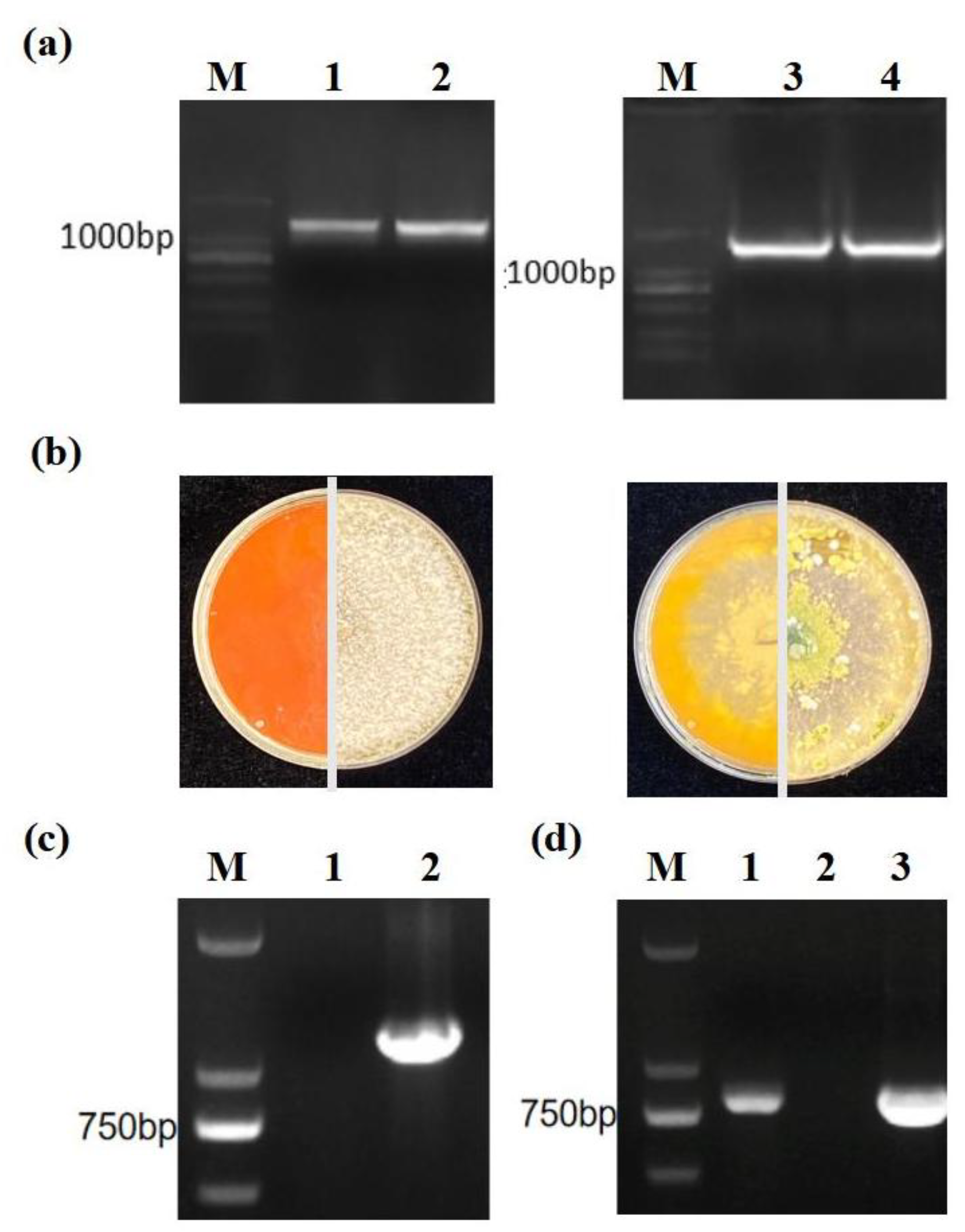
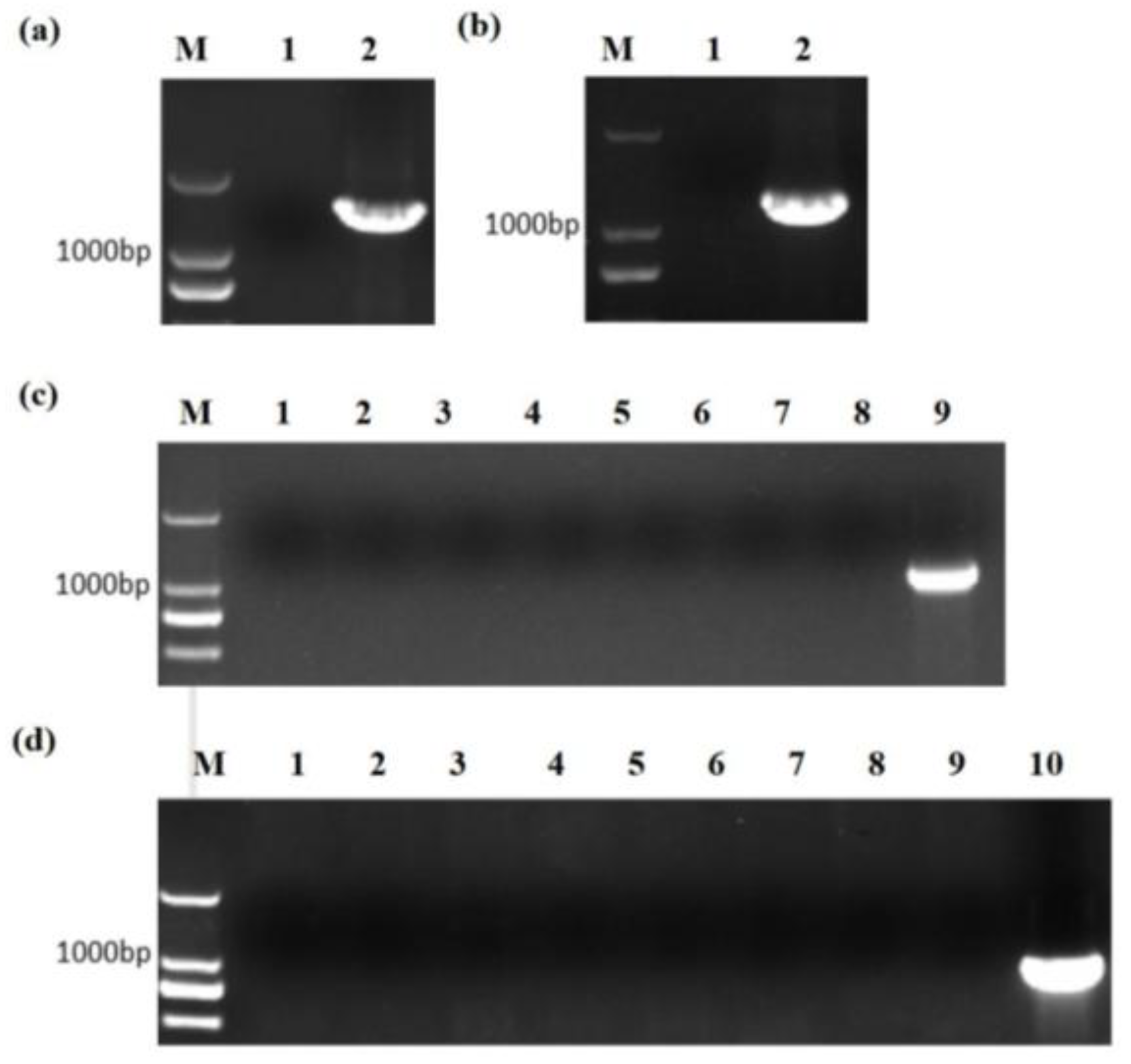
2.3. Creation and Verification of the TaTS Mutants
2.4. Characterization of the TaST Mutants
2.5. VOC Metabolomes of the Chassis and Mutant Strains
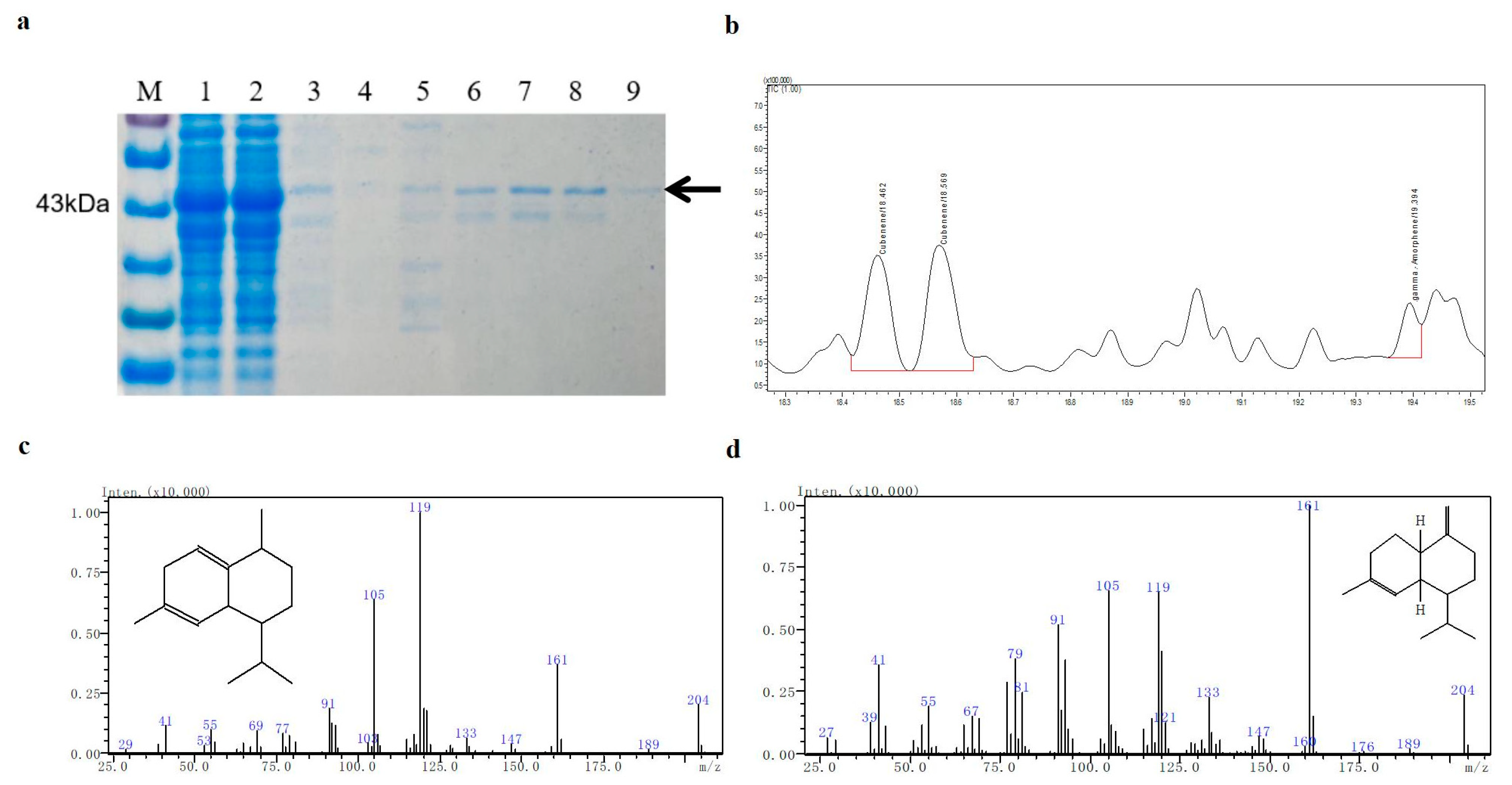
2.6. Production and Purification of the Recombinant TaTS9
2.7. Identification of the Sesquiterpene Products Catalyzed by TaTS9
3. Materials and Methods
3.1. Strains, Plasmids, and Culture Conditions
3.2. Genome Sequencing and Mining
3.3. Expression Induction of the TaTS Genes
3.4. RT-qPCR Analysis of the TaTS Transcript Profiles
3.5. Construction of the TaST Knockout Strains
3.6. GC-MS Analysis of the VOC Metabolomes
3.7. Protein Expression and Purification
3.8. In Vitro Enzyme Assay
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reino, J.L.; Guerrero, R.F.; Hernández-Galán, R.; Collado, I.G. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem. Rev. 2008, 7, 89–123. [Google Scholar] [CrossRef]
- Chen, H.; Mao, L.; Zhao, N.; Xia, C.; Liu, J.; Kubicek, C.P.; Wu, W.; Xu, S.; Zhang, C. Verification of TRI3 acetylation of trichodermol to trichodermin in the plant endophyte Trichoderma taxi. Front. Microbiol. 2021, 12, 731425. [Google Scholar] [CrossRef]
- Saldaña-Mendoza, S.A.; Pacios-Michelena, S.; Palacios-Ponce, A.S.; Chávez-González, M.L.; Aguilar, C.N. Trichoderma as a biological control agent: Mechanisms of action, benefits for crops and development of formulations. World J. Microbiol. Biotechnol. 2023, 39, 269. [Google Scholar] [CrossRef] [PubMed]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “secrets” of a multitalented biocontrol agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef]
- Philip, B.; Behiry, S.I.; Salem, M.Z.M.; Amer, M.A.; El-Samra, I.A.; Abdelkhalek, A.; Heflish, A. Trichoderma afroharzianum TRI07 metabolites inhibit Alternaria alternata growth and induce tomato defense-related enzymes. Sci. Rep. 2024, 14, 1874. [Google Scholar] [CrossRef] [PubMed]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- Ramírez-Valdespino, C.A.; Casas-Flores, S.; Olmedo-Monfl, V. Trichoderma as a model to study efector-like molecules. Front. Microbiol. 2019, 10, 1030. [Google Scholar] [CrossRef]
- Macías-Rodríguez, L.; Contreras-Cornejo, H.A.; Adame-Garnica, S.G.; Del-Val, E.; Larsen, J. The interactions of Trichoderma at multiple trophic levels: Inter-kingdom communication. Microbiol. Res. 2020, 240, 126552. [Google Scholar] [CrossRef]
- Ajith, P.; Lakshmidevi, N. Effect of volatile and non-volatile compounds from Trichoderma spp. against Colletotrichum capsici incitant of anthracnose on bell peppers. Nat. Sci. 2010, 8, 265–269. [Google Scholar] [CrossRef]
- Jiang, C.; Lv, G.; Tu, Y.; Cheng, X.; Duan, Y.; Zeng, B.; He, B. Applications of CRISPR/Cas9 in the synthesis of secondary metabolites in filamentous fungi. Front. Microbiol. 2021, 12, 638096. [Google Scholar] [CrossRef]
- Bai, B.; Liu, C.; Zhang, C.; He, X.; Wang, H.; Peng, W.; Zheng, C. Trichoderma species from plant and soil: An excellent resource for biosynthesis of terpenoids with versatile bioactivities. J. Adv. Res. 2023, 49, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, L.; Monti, M.M.; Mele, F.; Russo, A.; Pedata, P.A.; Ruocco, M. Volatile organic compound (VOC) profiles of different Trichoderma species and their potential application. J. Fungi 2022, 8, 989. [Google Scholar] [CrossRef] [PubMed]
- Elsherbiny, E.A.; Amin, B.H.; Aleem, B.; Kingsley, K.L.; Bennett, J.W. Trichoderma volatile organic compounds as a biofumigation tool against late blight pathogen Phytophthora infestans in postharvest potato tubers. J. Agric. Food Chem. 2020, 68, 8163–8171. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Guo, L.; Jin, B.; Zhu, S.; Mei, X.; Wu, J.; Liu, T.; He, X. Inhibitory mechanism of 6-pentyl-2H-pyran-2-one secreted by Trichoderma atroviride T2 against Cylindrocarpon destructans. Pestic. Biochem. Physiol. 2020, 170, 104683. [Google Scholar] [CrossRef]
- Intana, W.; Kheawleng, S.; Sunpapao, A. Trichoderma asperellum T76-14 released volatile organic compounds against postharvest fruit rot in muskmelons (Cucumis melo) caused by Fusarium incarnatum. J. Fungi. 2021, 7, 46. [Google Scholar] [CrossRef]
- Hirpara, D.G.; Gajera, H.P. Intracellular metabolomics and microRNAomics unveil new insight into the regulatory network for potential biocontrol mechanism of stress-tolerant Trichofusants interacting with phytopathogen Sclerotium rolfsii Sacc. J. Cell. Physiol. 2023, 238, 1288–1307. [Google Scholar] [CrossRef]
- Kumari, N.; Srividhya, S. Chapter 24—Secondary metabolites and lytic tool box of Trichoderma and their role in plant health. In Molecular Aspects of Plant Beneficial Microbes in Agriculture; Sharma, V., Salwan, R., Al-Ani, L.K.T., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 24, pp. 305–320. [Google Scholar]
- Li, W.; Fan, J.; Liao, G.; Yin, W.B.; Li, S.M. Precursor supply increases the accumulation of 4-hydroxy-6-(4-hydroxyphenyl)-α-pyrone after NRPS-PKS gene expression. J. Nat. Prod. 2021, 84, 2380–2384. [Google Scholar] [CrossRef]
- Li, W.C.; Lee, C.Y.; Lan, W.H.; Woo, T.T.; Liu, H.C.; Yeh, H.Y.; Chang, H.Y.; Chuang, Y.C.; Chen, C.Y.; Chuang, C.N.; et al. Trichoderma reesei Rad51 tolerates mismatches in hybrid meiosis with diverse genome sequences. Proc. Natl. Acad. Sci. USA 2021, 118, e2007192118. [Google Scholar] [CrossRef]
- Murai, K.; Lauterbach, L.; Teramoto, K.; Quan, Z.; Barra, L.; Yamamoto, T.; Nonaka, K.; Shiomi, K.; Nishiyama, M.; Kuzuyama, T.; et al. An unusual skeletal rearrangement in the biosynthesis of the sesquiterpene trichobrasilenol from Trichoderma. Angew. Chem. Int. Ed. 2019, 58, 15046–15050. [Google Scholar] [CrossRef]
- Yang, W.; Tian, S.; Du, Y.F.; Zeng, X.L.; Liang, J.J.; Lan, W.J.; Li, H. Genome mining of the marine-derived fungus Trichoderma erinaceum F1-1 unearths bergamotene-type sesquiterpenoids. J. Nat. Prod. 2024, 87, 2746–2756. [Google Scholar] [CrossRef]
- Chen, J.S.; Huang, Y.Y.; Xiang, J.; Guo, Q.H.; Li, S.G.; Gu, J.G. Carbon source metabolism of Trichoderma afroharzianum with high yield of antifungaI volatile organic compounds. Sci. Agric. Sin. 2020, 53, 4601–4612. [Google Scholar] [CrossRef]
- Olumakaiye, R.; Corre, C.; Alberti, F. Identification of a terpene synthase arsenal using long-read sequencing and genome assembly of Aspergillus wentii. BMC Genom. 2024, 25, 1141. [Google Scholar] [CrossRef]
- Fan, J.; Wei, P.L.; Li, Y.; Zhang, S.; Ren, Z.; Li, W.; Yin, W.B. Developing filamentous fungal chassis for natural product production. Bioresour. Technol. 2025, 415, 131703. [Google Scholar] [CrossRef]
- Liu, H.; Wang, G.; Li, W.; Liu, X.; Li, E.; Yin, W.B. A highly efficient genetic system for the identification of a harzianum B biosynthetic gene cluster in Trichoderma hypoxylon. Microbiology 2018, 164, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Weisskopf, L.; Schulz, S.; Garbeva, P. Microbial volatile organic compounds in intra-kingdom and inter-kingdom interactions. Nat. Rev. Microbiol. 2021, 19, 391–404. [Google Scholar] [CrossRef]
- Crutcher, F.K.; Parich, A.; Schuhmacher, R.; Mukherjee, P.K.; Zeilinger, S.; Kenerley, C.M. A putative terpene cyclase, vir4, is responsible for the biosynthesis of volatile terpene compounds in the biocontrol fungus Trichoderma virens. Fungal Genet. Biol. 2013, 56, 67–77. [Google Scholar] [CrossRef]
- Chen, C.; Yao, G.; Wang, F.; Bao, S.; Wan, X.; Han, P.; Wang, K.; Song, T.; Jiang, H. Identification of a (+)-cubenene synthase from filamentous fungi Acremonium chrysogenum. Biochem. Biophys. Res. Commun. 2023, 677, 119–125. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, W.; Li, H.; Mao, J.; Guo, C.; Ding, R.; Wang, Y.; Fang, L.; Chen, Z.; Yang, G. Analysis of volatile compounds in pears by HS-SPME-GC×GC-TOFMS. Molecules 2019, 24, 1795. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Orban, A.; Shukal, S.; Birk, F.; Too, H.P.; Rühl, M. Agrocybe aegerita serves as a gateway for identifying sesquiterpene biosynthetic enzymes in higher fungi. ACS Chem. Biol. 2020, 15, 1268–1277. [Google Scholar] [CrossRef]
- Li, G.; Liang, H.; Gao, R.; Qin, L.; Xu, P.; Huang, M.; Zong, M.H.; Cao, Y.; Lou, W.Y. Yeast metabolism adaptation for efficient terpenoids synthesis via isopentenol utilization. Nat. Commun. 2024, 15, 9844. [Google Scholar] [CrossRef]
- Li, N.; Alfiky, A.; Wang, W.; Islam, M.; Nourollahi, K.; Liu, X.; Kang, S. Volatile compound-mediated recognition and inhibition between Trichoderma biocontrol agents and Fusarium oxysporum. Front. Microbiol. 2018, 9, 2614. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [PubMed]
- Bresso, E.; Leroux, V.; Urban, M.; Hammond-Kosack, K.E.; Maigret, B.; Martins, N.F. Structure-based virtual screening of hypothetical inhibitors of the enzyme longiborneol synthase-a potential target to reduce Fusarium head blight disease. J. Mol. Model. 2016, 22, 163. [Google Scholar] [CrossRef] [PubMed]
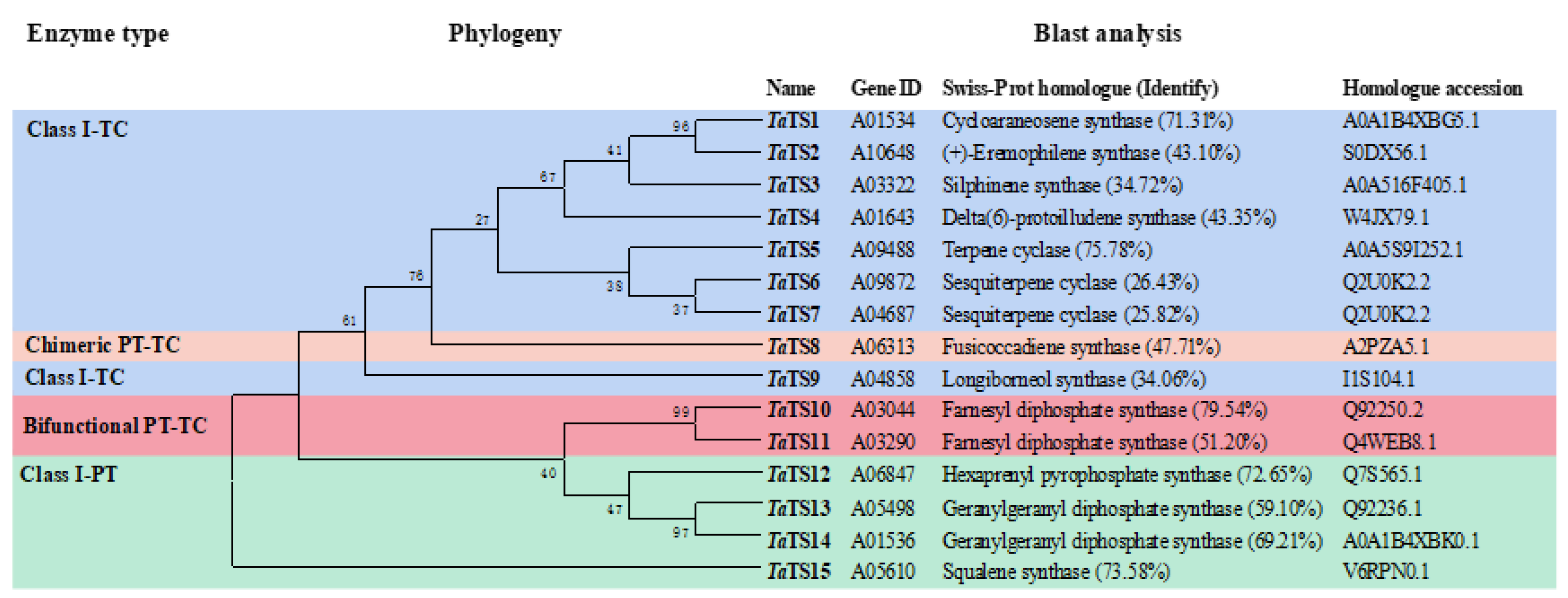



| No. | Gene ID | EC | Enzyme | Product |
|---|---|---|---|---|
| 1 | A03930 | 2.3.1.9 | Acetyl-CoA acetyltransferase | MVA pathway to produce IPP |
| 2 | A05584 | 2.3.1.9 | Acetyl-CoA acetyltransferase | |
| 3 | A02030 | 2.3.3.10 | Hydroxymethylglutaryl-CoA synthase | |
| 4 | A07965 | 1.1.1.34 | Hydroxymethylglutaryl-CoA reductase | |
| 5 | A02844 | 2.7.1.36 | Mevalonate kinase | |
| 6 | A03938 | 2.7.4.2 | Phosphomevalonate kinase | |
| 7 | A10372 | 4.1.1.33 | Diphosphomevalonate decarboxylase | |
| 8 | A03978 | 5.3.3.2 | Isopentenyl diphosphate delta-isomerase | DMAPP (C5) |
| 9 | A03044 | 2.5.1.1 | Geranyl diphosphate synthase | GPP (C10) |
| 10 | A03290 | 2.5.1.10 | Farnesyl diphosphate synthase | FPP (C15) |
| 11 | A01536 | 2.5.1.29 | Geranylgeranyl diphosphate synthase | GGPP (C20) |
| 12 | A05498 | 2.5.1.29 | Geranylgeranyl diphosphate synthase | GGPP (C20) |
| 13 | A06847 | 2.5.1.83 | Hexaprenyl pyrophosphate synthase | HexPP (C20) |
| 14 | A05610 | 2.5.1.21 | Farnesyl-diphosphate farnesyltransferase | FFPP (C30) |
| 15 | A01534 | 4.2.3.- | Cycloaraneosene synthase | Terpenoid skeleton |
| 16 | A01643 | 4.2.3.- | Delta(6)-protoilludene synthase | |
| 17 | A03322 | 4.2.3.- | Silphinene synthase | |
| 18 | A04687 | 4.2.3.- | Sesquiterpene cyclase | |
| 19 | A04858 | 4.2.3.- | Longiborneol synthase | |
| 20 | A06313 | 4.2.3.- | Fusicoccadiene synthase | |
| 21 | A09488 | 4.2.3.- | Terpene cyclase | |
| 22 | A09872 | 4.2.3.- | Sesquiterpene cyclase | |
| 23 | A10648 | 4.2.3.- | (+)-Eremophilene synthase | |
| 24 | A01608 | 2.5.1.87 | Polyprenyl diphosphate synthase | Side-chain-decorated terpenoids |
| 25 | A02633 | 2.5.1.87 | Dehydrodolichyl diphosphate synthase | |
| 26 | A02814 | 2.5.1.75 | tRNA dimethylallyl transferase | |
| 27 | A02831 | 3.4.24.84 | STE24 endopeptidase | |
| 28 | A02842 | 2.5.1.58/59 | Farnesyl/geranylgeranyl transferase | |
| 29 | A05170 | 2.1.1.100 | S-isoprenylcysteine O-methyltransferase | |
| 30 | A06568 | 1.8.3.5/6 | Prenylcysteine oxidase/farnesylcysteine lyase | |
| 31 | A06978 | 2.5.1.58 | Farnesyl transferase | |
| 32 | A07199 | 1.13.11.59 | Torulene dioxygenase | |
| 33 | A08871 | 3.4.22.- | Prenyl protein peptidase | |
| 34 | A09359 | 1.2.1.82 | Beta-apo-4′-carotenal oxygenase | |
| 35 | A03646 | 1.14.14.17 | Squalene monooxygenase |
| Time | Inhibitory Rate (%) | |||||
|---|---|---|---|---|---|---|
| ΔTalig4::neo | ΔTalig4::neoΔTaTS3::hph | ΔTalig4::neoΔTaTS4::hph | ΔTalig4::neoΔTaTS8::hph-5 | ΔTalig4::neoΔTaTS9::hph-3 | ΔTalig4::neoΔTaTS9::hph-130 | |
| Day 3 | 18.56 | 14.90 ** | 17.21 | 14.70 ** | 14.63 ** | 17.83 |
| Day 4 | 17.16 | 14.45 ** | 17.96 | 10.60 *** | 13.78 *** | 18.17 |
| Day 5 | 22.37 | 15.33 ** | 18.88 * | 13.14 *** | 19.40 * | 19.08 ** |
| No. | Compound | Retention Time (min) | Formula | Molecular Weight | Absolute Amount | ||
|---|---|---|---|---|---|---|---|
| ΔTalig4::neo | ΔTalig4::neoΔTaTS8::hph | ΔTalig4::neoΔTaTS9::hph | |||||
| 1 | 1-Pentanol | 3.146 | C5H12O | 88.15 | 1.07 × 108 | 2.68 × 107 | 2.68 × 107 |
| 2 | 2-Ethyl-1-hexanol | 10.781 | C8H18O | 130.23 | 3.35 × 107 | 2.07 × 106 | 2.59 × 106 |
| 3 | Phenylethyl alcohol | 12.723 | C8H10O | 122.16 | 3.47 × 107 | 8.68 × 106 | 1.09 × 107 |
| 4 | 3-Octanol, acetate | 12.950 | C10H20O2 | 172.26 | 2.01 × 105 | * | 1.01 × 105 |
| 5 | 2-Heptenoic acid, 4-nitrophenyl ester | 13.159 | C13H15NO4 | 249.26 | 7.80 × 105 | 5.04 × 104 | 2.08 × 105 |
| 6 | β-Cubenene | 18.511 | C15H24 | 204.35 | 6.58 × 106 | 1.95 × 105 | * |
| 7 | β-Cubenene | 18.620 | C15H24 | 204.35 | 7.54 × 106 | 1.64 × 106 | * |
| 8 | cis-α-Bergamotene | 18.741 | C15H24 | 204.35 | 3.70 × 105 | * | * |
| 9 | Bicyclo[5.2.0]nonane, 4-methylene-2,8,8-trimethyl-2-vinyl- | 18.849 | C15H24 | 204.35 | 7.57 × 105 | * | * |
| 10 | Cyclohexane, 1,2-diethenyl-4-(1-methylethylidene)-, cis- | 18.958 | C13H20 | 176.33 | * | 1.88 × 106 | 1.69 × 106 |
| 11 | 1,3a-Ethano-3aH-indene, 1,2,3,6,7,7a-hexahydro-2,2,4,7a-tetramethyl-, [1R-(1.alpha.,3a.alpha.,7a.alpha.)]- | 18.969 | C15H24 | 204.35 | 1.13 × 106 | * | 2.30 × 106 |
| 12 | (1S,4aR,7R)-1,4a-Dimethyl-7-(prop-1-en-2-yl)-1,2,3,4,4a,5,6,7-octahydronaphthalene | 19.041 | C15H24 | 204.35 | 7.92 × 105 | 9.25 × 104 | * |
| 13 | 1H-Cyclopenta[1,3]cyclopropa[1,2]benzene, octahydro-7-methyl-3-methylene-4- (1-methylethyl)-, 3aS-(3a.alpha.,3b.beta.,4.beta.,7 | 19.370 | C15H24 | 204.35 | * | 1.89 × 105 | * |
| 14 | γ-Amorphene | 19.380 | C15H24 | 204.35 | 2.72 × 105 | * | * |
| 15 | (1R,4R,5S)-1,8-Dimethyl-4-(prop-1-en-2-yl)spiro[4.5]dec-7-ene | 19.434 | C15H24 | 204.35 | 1.03 × 106 | 6.84 × 101 | * |
| 16 | (1R,5S)-1,8-Dimethyl-4-(propan-2-ylidene)spiro[4.5]dec-7-ene | 20.017 | C15H24 | 204.35 | 7.97 × 105 | 2.84 × 105 | * |
| 17 | Bicyclo[2.2.1]heptane, 2-(1,1-dimethyl-2-propenyl)- | 20.320 | C12H20 | 164.29 | 2.02 × 105 | 1.98 × 105 | * |
| 18 | cis-Calamenene | 20.340 | C15H22 | 202.33 | 3.84 × 105 | 7.89 × 101 | * |
| 19 | Tricyclo[6.3.0.0(1,5)]undec-2-en-4-one, 5,9-dimethyl- | 20.590 | C13H18O | 190.28 | 1.29 × 105 | * | 1.23 × 105 |
| 20 | Biphenylene, 1,2,3,6,7,8,8a,8b-octahydro-4,5-dimethyl- | 21.290 | C14H20 | 188.31 | 1.13 × 105 | 6.81 × 104 | 2.52 × 105 |
| 21 | 1H-Benzocyclohepten-7-ol, 2,3,4,4a,5,6,7,8-octahydro-1,1,4a,7-tetramethyl-, cis- | 22.305 | C15H26O | 222.37 | 5.09 × 105 | 2.56 × 105 | 6.28 × 105 |
| 22 | β-Ocimene | 22.421 | C10H16 | 136.26 | 3.31 × 105 | 1.99 × 105 | |
| 23 | α-Bisabolol | 22.454 | C15H26O | 222.37 | 1.67 × 105 | 5.05 × 104 | * |
| 24 | Spiro[4.5]dec-6-en-8-one, 1,7-dimethyl-4-(1-methylethyl)- | 22.635 | C15H24O | 220.35 | 6.10 × 105 | 9.60 × 104 | * |
| 25 | Di-epi-1,10-cubenol | 23.477 | C15H26O | 222.37 | 4.96 × 105 | 3.23 × 104 | * |
| 26 | Tricyclo[5.4.0.0(2,8)]undec-9-ene, 2,6,6,9-tetramethyl-, (1R,2S,7R,8R)- | 23.670 | C15H24 | 204.35 | 1.03 × 105 | 2.84 × 104 | * |
| 27 | Hinesol | 24.433 | C15H26O | 222.37 | 2.68 × 105 | 1.27 × 105 | * |
| 28 | Hexadecanoic acid, methyl ester | 25.256 | C17H34O2 | 270.50 | 2.97 × 105 | * | * |
| 29 | n-Hexadecanoic acid | 25.621 | C16H32O2 | 256.42 | 3.87 × 106 | * | * |
| 30 | Hexadecanoic acid, ethyl ester | 25.974 | C18H36O2 | 284.50 | 1.16 × 105 | * | * |
| 31 | Bicyclo[9.3.1]pentadeca-3,7-dien-12-ol, 4,8,12,15,15-pentamethyl-, [1R-(1R*,3E,7E,11R*,12R*)]- | 26.150 | C20H34O | 290.50 | 2.86 × 106 | 8.27 × 104 | 3.78 × 105 |
| 32 | Bicyclo[9.3.1]pentadeca-3,7-dien-12-ol, 4,8,12,15,15-pentamethyl-, [1R-(1R*,3E,7E,11R*,12R*)]- | 26.353 | C20H34O | 290.50 | 2.93 × 105 | 4.19 × 104 | 2.21 × 105 |
| 33 | (S,E)-8,12,15,15-Tetramethyl-4-methylenebicyclo[9.3.1]pentadeca-7,11-diene | 26.555 | C20H32 | 272.50 | 4.86 × 105 | 1.52 × 105 | * |
| 34 | 1-Octadecanol | 26.879 | C18H38O | 270.50 | 2.76 × 105 | * | * |
| 35 | Panaxene | 26.935 | C15H24 | 204.35 | 1.24 × 105 | 1.24 × 105 | * |
| 36 | Methyl stearate | 27.293 | C19H38O2 | 298.50 | 1.69 × 105 | * | * |
| 37 | Bicyclo[9.3.1]pentadeca-3,7-dien-12-ol, 4,8,12,15,15-pentamethyl-, [1R-(1R*,3E,7E,11R*,12R*)]- | 29.062 | C20H34O | 290.50 | 2.12 × 106 | 2.58 × 104 | * |
| 38 | Hexanedioic acid, bis(2-ethylhexyl) ester | 30.678 | C22H42O4 | 370.60 | 6.34 × 105 | 6.71 × 104 | 6.31 × 104 |
| 39 | Cholesteryl formate | 36.302 | C28H46O2 | 414.70 | 5.56 × 105 | * | * |
| 40 | Cholesta-2,4-diene | 37.471 | C27H44 | 368.60 | 5.17 × 105 | * | * |
| 41 | Cholest-5-en-3-ol (3.beta.)-, nonanoate | 37.909 | C36H62O2 | 526.90 | 1.74 × 106 | * | * |
| Total | 1.88 × 108 | 4.34 × 107 | 5.29 × 107 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Ma, R.; Huang, Y.; Cui, Y.; Zhou, Q.; Gu, J. Genome Mining of the Biocontrol Agent Trichoderma afroharzianum Unearths a Key Gene in the Biosynthesis of Anti-Fungal Volatile Sesquiterpenoids. Catalysts 2025, 15, 341. https://doi.org/10.3390/catal15040341
Zhang F, Ma R, Huang Y, Cui Y, Zhou Q, Gu J. Genome Mining of the Biocontrol Agent Trichoderma afroharzianum Unearths a Key Gene in the Biosynthesis of Anti-Fungal Volatile Sesquiterpenoids. Catalysts. 2025; 15(4):341. https://doi.org/10.3390/catal15040341
Chicago/Turabian StyleZhang, Fang, Rui Ma, Yuyang Huang, Yang Cui, Qiong Zhou, and Jingang Gu. 2025. "Genome Mining of the Biocontrol Agent Trichoderma afroharzianum Unearths a Key Gene in the Biosynthesis of Anti-Fungal Volatile Sesquiterpenoids" Catalysts 15, no. 4: 341. https://doi.org/10.3390/catal15040341
APA StyleZhang, F., Ma, R., Huang, Y., Cui, Y., Zhou, Q., & Gu, J. (2025). Genome Mining of the Biocontrol Agent Trichoderma afroharzianum Unearths a Key Gene in the Biosynthesis of Anti-Fungal Volatile Sesquiterpenoids. Catalysts, 15(4), 341. https://doi.org/10.3390/catal15040341






