Fabrication of CoP@P, N-CNTs-Deposited Nickel Foam for Energy-Efficient Hydrogen Generation via Electrocatalytic Urea Oxidation
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Fabrication of CoP/NF Electrode
2.3. Fabrication of P, N-CNTs/NF Electrode
2.4. Fabrication of CoP@P, N-CNTs/NF Electrode
2.5. Characterization
2.6. Electrochemical Measurements
3. Results
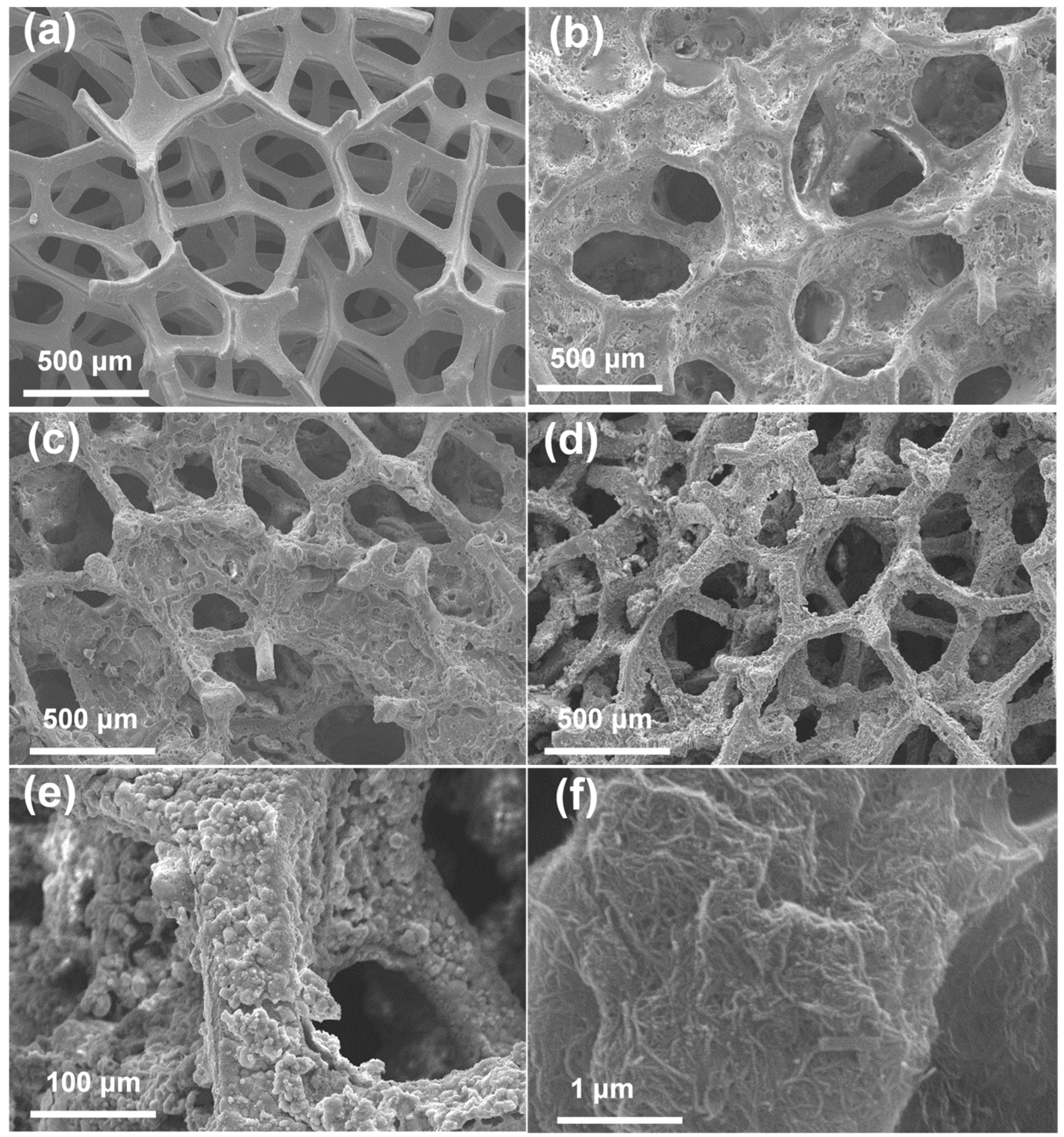
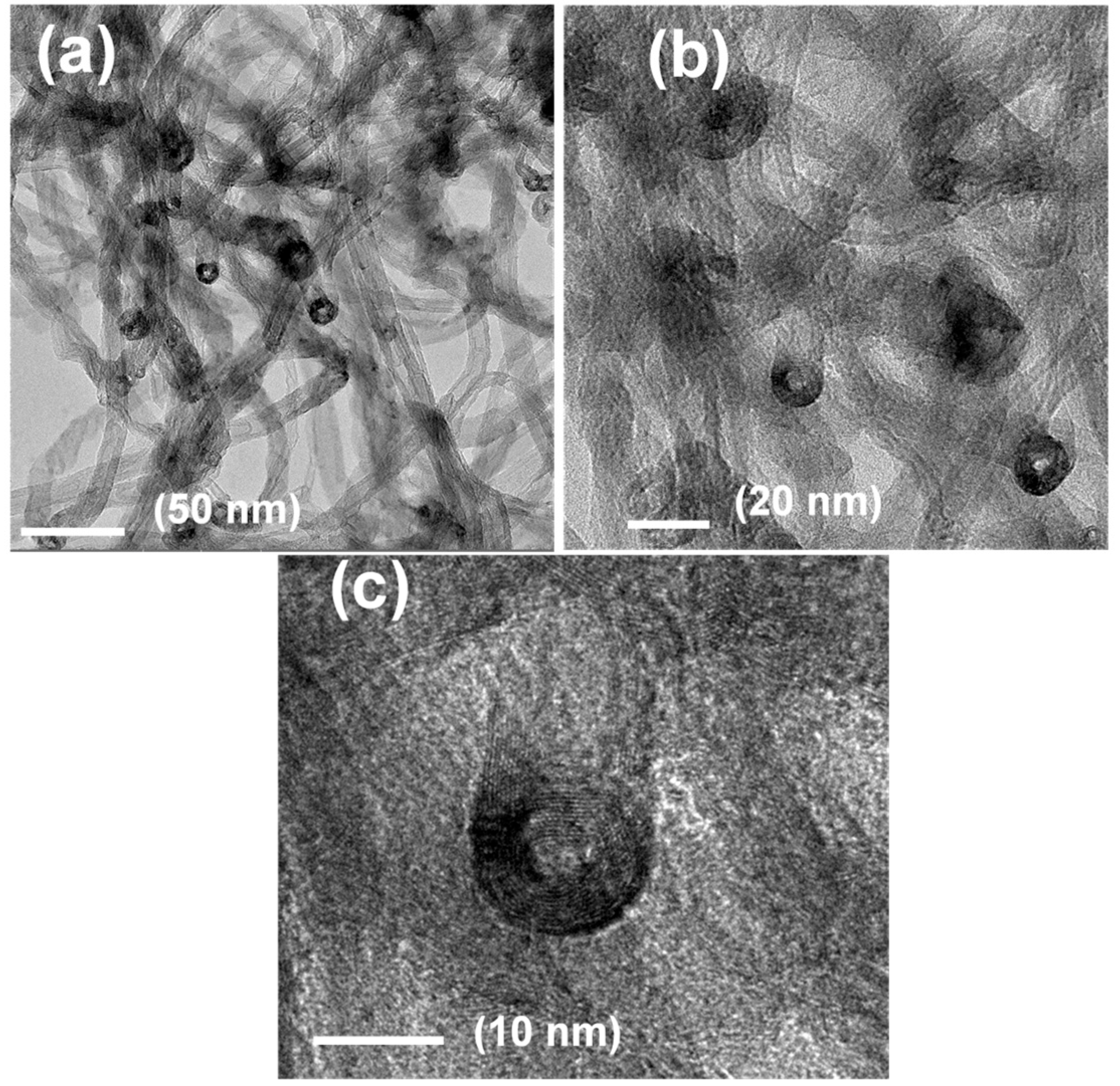
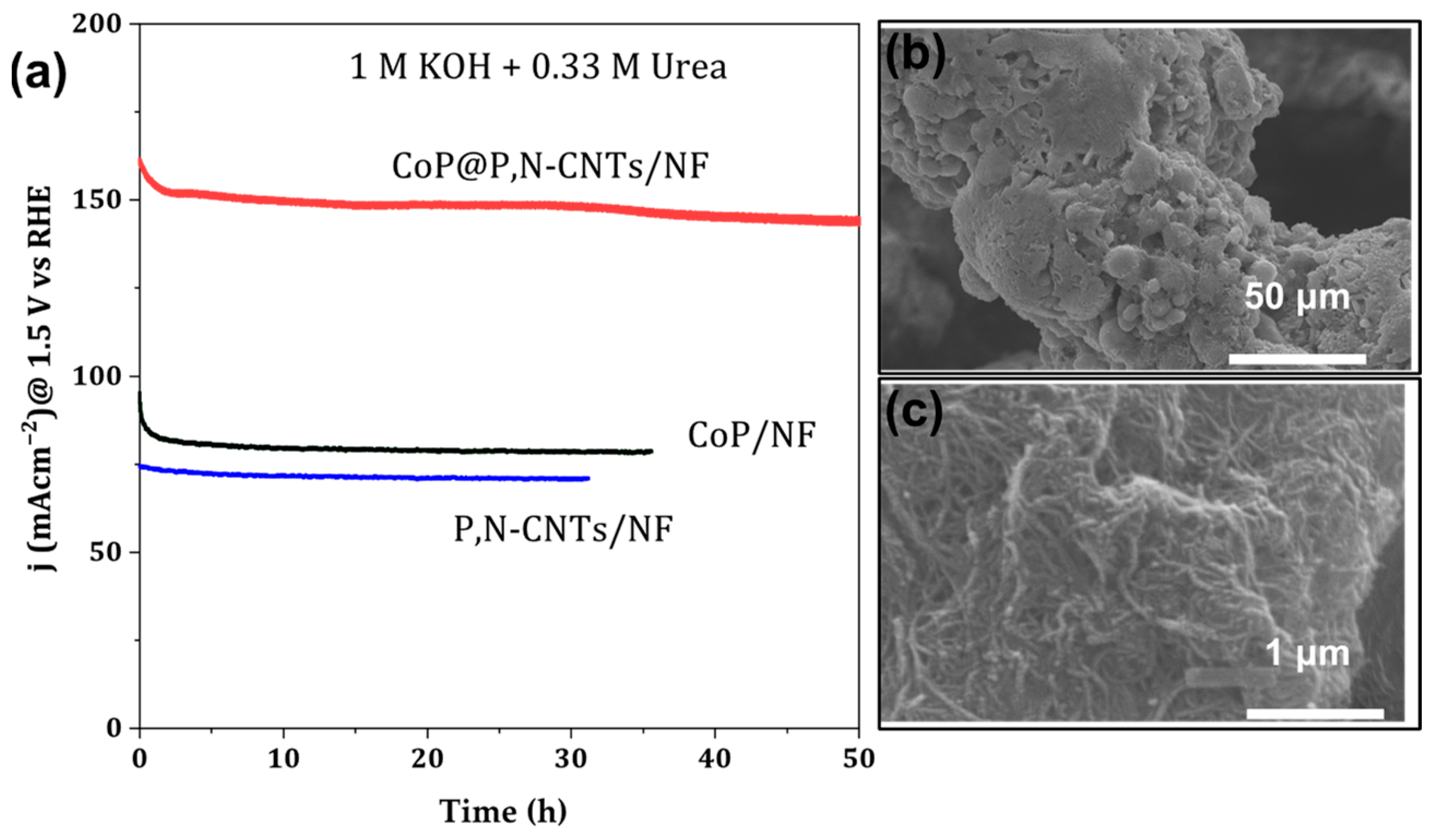
3.1. Morphological Features of CoP@P, N-CNTs/NF
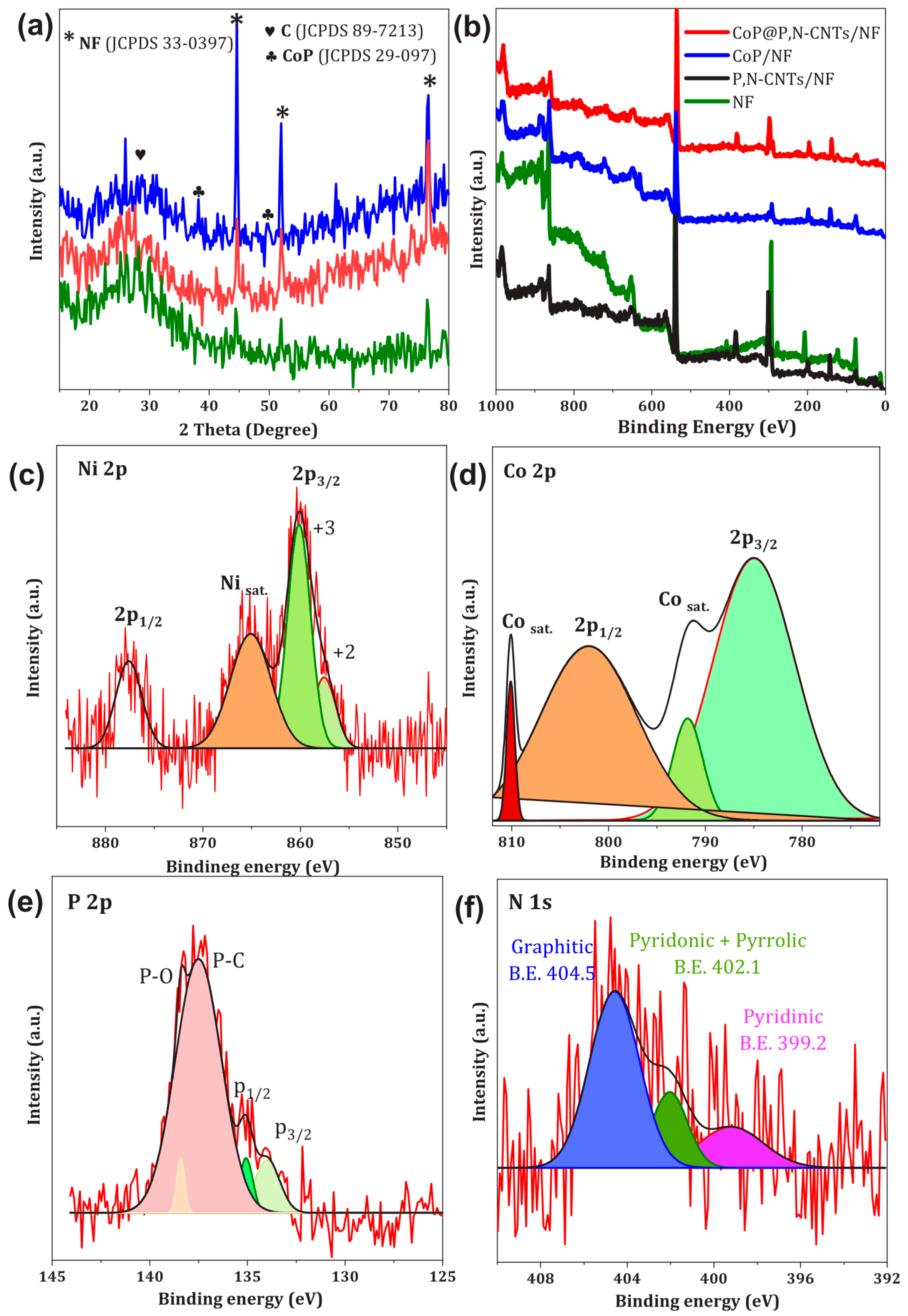
3.2. Crystalline and Chemical Composition Features of Electrodes
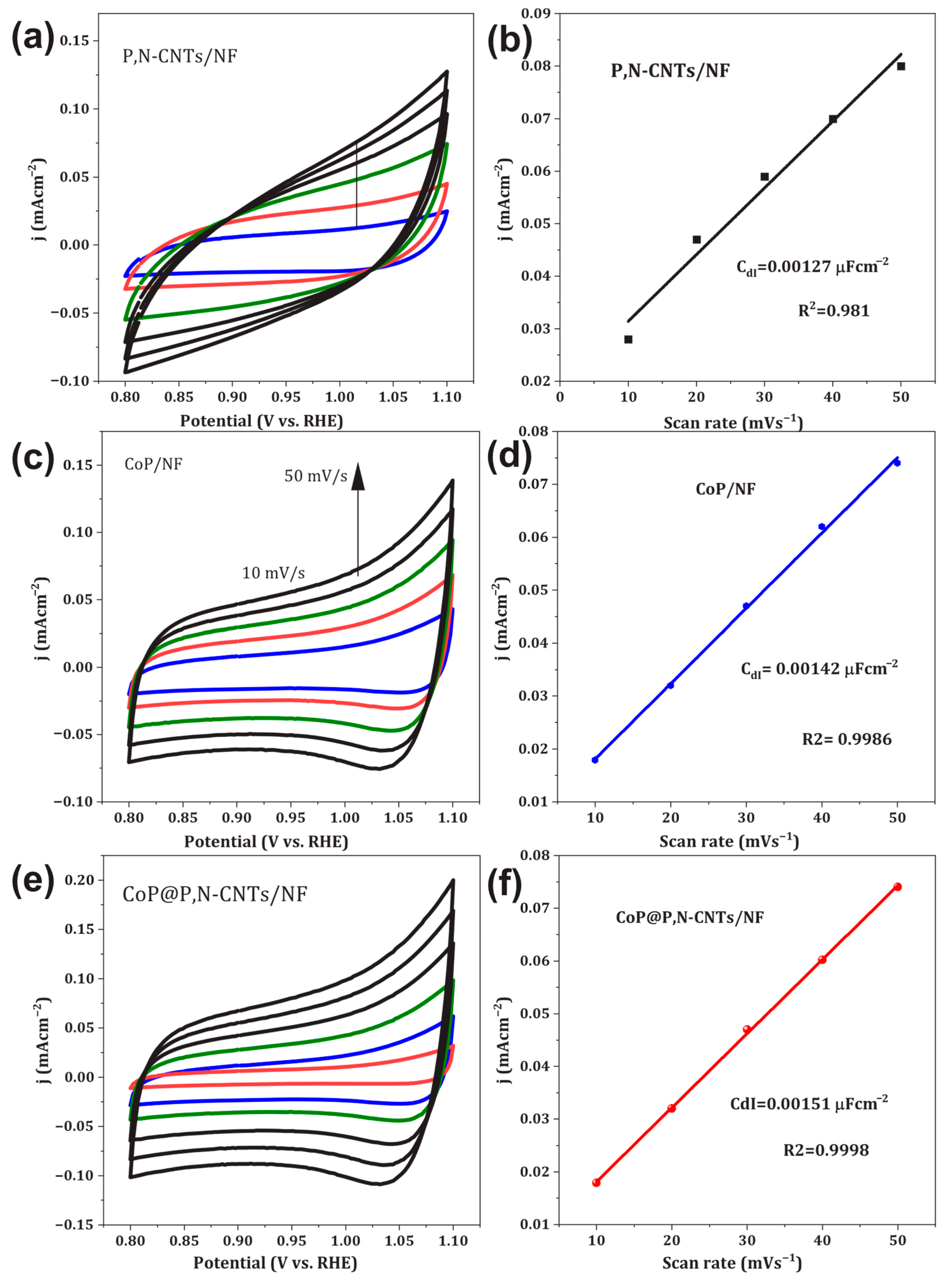
3.3. Electrochemical Properties of Catalysts
3.3.1. OER and UOR Characteristics
3.3.2. Long-Term Stability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Strielkowski, W.; Civín, L.; Tarkhanova, E.; Tvaronavičienė, M.; Petrenko, Y. Renewable Energy in the Sustainable Development of Electrical Power Sector: A Review. Energies 2021, 14, 8240. [Google Scholar] [CrossRef]
- Qazi, A.; Hussain, F.; Rahim, N.A.B.D.; Hardaker, G.; Alghazzawi, D.; Shaban, K.; Haruna, K. Towards Sustainable Energy: A Systematic Review of Renewable Energy Sources, Technologies, and Public Opinions. IEEE Access 2019, 7, 63837–63851. [Google Scholar] [CrossRef]
- Agyekum, E.B.; Nutakor, C.; Agwa, A.M.; Kamel, S.; Kame, S. A Critical Review of Renewable Hydrogen Production Methods: Factors Affecting Their Scale-Up and Its Role in Future Energy Generation. Membranes 2022, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, G.; Sun, Z.; Zhang, Y.; Xu, D. Review of Renewable Energy-Based Hydrogen Production Processes for Sustainable Energy Innovation. Glob. Energy Interconnect. 2020, 2, 436–443. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Kim, H.; Jung, W.C.; Zhou, W.; Shao, Z. Electrochemical Water Splitting: Bridging the Gaps Between Fundamental Research and Industrial Applications. Energy Environ. Mater. 2023, 6, e12441. [Google Scholar] [CrossRef]
- Kumar, S.; Rajan, A.; Rugma, T.P.; Bernaurdshaw, N. A Review on Particulate Photocatalytic Hydrogen Production System: Progress Made in Achieving High Energy Conversion Efficiency and Key Challenges Ahead. Renew. Sustain. Energy Rev. 2021, 152, 111694. [Google Scholar]
- Zhu, B.; Liang, Z.; Zou, R. Designing Advanced Catalysts for Energy Conversion Based on Urea Oxidation Reaction, REVIEW. Small 2020, 16, 1906133. [Google Scholar] [CrossRef]
- Qiu, Z. Transition Metal-Based Electrocatalysts for Alkaline Water Splitting and CO2 Reduction-Dissertation; Ångström Laboratory: Uppsala, Sweden, 2019. [Google Scholar]
- Yang, X.; Sun, X.; Qi, J.; Zhang, J.; Zheng, X.; Zhang, X.; Lei, F.; Sun, X.; Tang, B.; Xie, J. Two-Dimensional Confined Topotactic Transformation to Produce Co-Pi/Co3O4 Hybrid Porous Nanosheets for Promoted Water Oxidation. J. Colloid Interface Sci. 2025, 677, 406–416. [Google Scholar] [CrossRef]
- Chen, J.; Wang, K.; Liu, Z.; Sun, X.; Zhang, X.; Lei, F.; Wan, X.; Xie, J.; Tang, B. Sulfurization-Induced Lattice Disordering in High-Entropy Catalyst for Promoted Bifunctional Electro-Oxidation Behavior. Chem. Eng. J. 2024, 489, 151234. [Google Scholar] [CrossRef]
- Noor, T.; Yaqoob, L.; Iqbal, N. Recent Advances in Electrocatalysis of Oxygen Evolution Reaction Using Noble-Metal, Transition-Metal, and Carbon-Based Materials. ChemElectroChem 2021, 8, 447–483. [Google Scholar] [CrossRef]
- Chang, H.; Liang, Z.; Wang, L.; Wang, C. Research Progress in Improving the Oxygen Evolution Reaction by Adjusting the 3d Electronic Structure of Transition Metal Catalysts. Nanoscale 2022, 14, 5639–5656. [Google Scholar] [CrossRef]
- Omar ben Gubaer, S.; Shaddad, M.N.; Arunachalam, P.; Amer, M.S.; Aladeemy, S.A.; Al-Mayouf, A.M. Enhanced Electrocatalytic Oxygen Redox Reactions of Iron Oxide Nanorod Films by Combining Oxygen Vacancy Formation and Cobalt Doping. RSC Adv. 2023, 13, 33242–33254. [Google Scholar] [CrossRef]
- Shaddad, M.N.; Alharthi, A.I.; Aladeemy, S.A.; Arunachalam, P. Engineering Oxygen Vacancy on Nickel-Doped Iron Oxide Nanorods as Efficient Bifunctional Electrocatalysts for Oxygen Evolution and Urea Oxidation Reaction. J. Taiwan Inst. Chem. Eng. 2024, 105928, in press. [Google Scholar] [CrossRef]
- Aladeemy, S.A.; Arunachalam, P.; Al-Mayouf, A.M.; Sudha, P.N.; Rekha, A.; Vidhya, A.; Hemapriya, J.; Latha, S.; Prasad, P.S.; Pavithra, S.; et al. Engineered CoS/Ni3S2 Heterointerface Catalysts Grown Directly on Carbon Paper as an Efficient Electrocatalyst for Urea Oxidation. Catalysts 2024, 14, 570. [Google Scholar] [CrossRef]
- Aladeemy, S.A.; Arunachalam, P.; Amer, M.S.; Al-Mayouf, A.M. Electrochemically Embedded Heterostructured Ni/NiS Anchored onto Carbon Paper as Bifunctional Electrocatalysts for Urea Oxidation and Hydrogen Evolution Reaction. RSC Adv. 2025, 15, 14–25. [Google Scholar] [CrossRef]
- Al-Sulmi, W.A.; Ghanem, M.A.; Aladeemy, S.A.; Al-Mayouf, A.M.; Alotaibi, N.H. Chemical Deposition from a Liquid Crystal Template: A Highly Active Mesoporous Nickel Phosphate Electrocatalyst for Hydrogen Green Production via Urea. Arab. J. Chem. 2023, 16, 105266. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, J.; Li, J.; Wu, Q. Urea Electrooxidation: Current Development and Understanding of Ni-Based Catalysts. ChemElectroChem 2020, 7, 3211–3228. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Pan, T.; Zhu, Y.; Pang, H. Ni-Based Electrocatalysts for Urea Oxidation Reaction: Mechanism, Catalyst Design Strategies and Future Perspectives. Sci. China Mater. 2025, 68, 317–340. [Google Scholar] [CrossRef]
- Wang, G.; Chen, J.; Li, Y.; Jia, J.; Cai, P.; Wen, Z. Energy-Efficient Electrolytic Hydrogen Production Assisted by Coupling Urea Oxidation with a PH-Gradient Concentration Cell. Chem. Commun. 2018, 54, 2603–2606. [Google Scholar] [CrossRef]
- Aladeemy, S.A.; Al-Mayouf, A.M.; Shaddad, M.N.; Amer, M.S.; Almutairi, N.K.; Ghanem, M.A.; Alotaibi, N.H.; Arunachalam, P. Electrooxidation of Urea in Alkaline Solution Using Nickel Hydroxide Activated Carbon Paper Electrodeposited from DMSO Solution. Catalysts 2021, 11, 102. [Google Scholar] [CrossRef]
- Hao, M.; Chen, J.; Chen, J.; Wang, K.; Wang, J.; Lei, F.; Hao, P.; Sun, X.; Xie, J.; Tang, B. Lattice-Disordered High-Entropy Metal Hydroxide Nanosheets as Efficient Precatalysts for Bifunctional Electro-Oxidation. J. Colloid Interface Sci. 2023, 642, 41–52. [Google Scholar] [CrossRef]
- Sun, W.; Li, J.; Gao, W.; Kang, L.; Lei, F.; Xie, J. Recent Advances in the Pre-Oxidation Process in Electrocatalytic Urea Oxidation Reactions. Chem. Commun. 2022, 58, 2430–2442. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Kang, L.; Wei, Z.; Lou, S.; Lei, F.; Hao, P.; Xie, J.; Tang, B. A Self-Sacrificial Templated Route to Fabricate CuFe Prussian Blue Analogue/Cu(OH)2 Nanoarray as an Efficient Pre-Catalyst for Ultrastable Bifunctional Electro-Oxidation. Chem. Eng. J. 2021, 422, 130139. [Google Scholar] [CrossRef]
- Wei, Z.; Sun, W.; Liu, S.; Qi, J.; Kang, L.; Li, J.; Lou, S.; Xie, J.; Tang, B.; Xie, Y. Lanthanum-Doped α-Ni(OH)2 1D-2D-3D Hierarchical Nanostructures for Robust Bifunctional Electro-Oxidation. Particuology 2021, 57, 104–111. [Google Scholar] [CrossRef]
- Xie, J.; Gao, L.; Cao, S.; Liu, W.; Lei, F.; Hao, P.; Xia, X.; Tang, B. Copper-Incorporated Hierarchical Wire-on-Sheet α-Ni(OH)2 Nanoarrays as Robust Trifunctional Catalysts for Synergistic Hydrogen Generation and Urea Oxidation. J. Mater. Chem. A 2019, 7, 13577–13584. [Google Scholar] [CrossRef]
- Xie, J.; Qu, H.; Lei, F.; Peng, X.; Liu, W.; Gao, L.; Hao, P.; Cui, G.; Tang, B. Partially Amorphous Nickel-Iron Layered Double Hydroxide Nanosheet Arrays for Robust Bifunctional Electrocatalysis. J. Mater. Chem. A 2018, 6, 16121–16129. [Google Scholar] [CrossRef]
- Xie, J.; Liu, W.; Lei, F.; Zhang, X.; Qu, H.; Gao, L.; Hao, P.; Tang, B.; Xie, Y. Iron-Incorporated α-Ni(OH)2 Hierarchical Nanosheet Arrays for Electrocatalytic Urea Oxidation. Chem.–A Eur. J. 2018, 24, 18408–18412. [Google Scholar] [CrossRef]
- Hopsort, G.; Carmo, D.P.D.; Latapie, L.; Loubière, K.; Serrano, K.G.; Tzedakis, T. Progress toward a Better Understanding of the Urea Oxidation by Electromediation of Ni(III)/Ni(II) System in Alkaline Media. Electrochim. Acta 2023, 442, 141898. [Google Scholar] [CrossRef]
- Boggs, B.K.; King, R.L.; Botte, G.G. Urea Electrolysis: Direct Hydrogen Production from Urine. Chem. Commun. 2009, 2009, 4859–4861. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, C.; Wang, Y.; Wang, K. Advanced Nickel-Based Catalysts for Urea Oxidation Reaction: Challenges and Developments. Catalysts 2022, 12, 337. [Google Scholar] [CrossRef]
- Asnavandi, M. Nanostructured Metallic Catalysts for Electrochemical Energy Conversion-Dissertation. Ph.D. Thesis, University of New South Wales, Sydney, Australia, 2017. [Google Scholar]
- Singh, R.K.; Schechter, A. Electrochemical Investigation of Urea Oxidation Reaction on β Ni(OH)2 and Ni/Ni(OH)2. Electrochim. Acta 2018, 278, 405–411. [Google Scholar] [CrossRef]
- Wei, S.; Wang, X.; Wang, J.; Sun, X.; Cui, L.; Yang, W.; Zheng, Y.; Liu, J. CoS2 Nanoneedle Array on Ti Mesh: A Stable and Efficient Bifunctional Electrocatalyst for Urea-Assisted Electrolytic Hydrogen Production. Electrochim. Acta 2017, 246, 776–782. [Google Scholar] [CrossRef]
- Yan, Y.; Xia, B.Y.; Zhao, B.; Wang, X. A Review on Noble-Metal-Free Bifunctional Heterogeneous Catalysts for Overall Electrochemical Water Splitting. J. Mater. Chem. A 2016, 4, 17587–17603. [Google Scholar] [CrossRef]
- Cheng, Y.; Liao, F.; Dong, H.; Wei, H.; Geng, H.; Shao, M. Engineering CoN/Ni(OH)2 Heterostructures with Improved Intrinsic Interfacial Charge Transfer toward Simultaneous Hydrogen Generation and Urea-Rich Wastewater Purification. J. Power Sources 2020, 480, 229151. [Google Scholar] [CrossRef]
- Vidhya, M.S.; Ravi, G.; Yuvakkumar, R.; Velauthapillai, D.; Thambidurai, M.; Dang, C.; Saravanakumar, B. Nickel-Cobalt Hydroxide: A Positive Electrode for Supercapacitor Applications. RSC Adv. 2020, 10, 19410–19418. [Google Scholar] [CrossRef]
- Aladeemy, S.A.; AlRijraji, T.R.; Amer, M.S.; Arunachalam, P.; Al-Mayouf, A.M. Electrooxidation of Ethylene Glycol Coupled with Hydrogen Production on Porous NiO/Ni@NF Nanosheet Electrocatalysts. Catal. Sci. Technol. 2025, 15, 2571–2583. [Google Scholar] [CrossRef]
- Xu, X.; Wang, T.; Su, L.; Zhang, Y.; Dong, L.; Miao, X. In Situ Synthesis of Superhydrophilic Amorphous NiFe Prussian Blue Analogues for the Oxygen Evolution Reaction at a High Current Density. ACS Sustain. Chem. Eng. 2021, 9, 5693–5704. [Google Scholar] [CrossRef]
- Guo, F.; Cao, D.; Du, M.; Ye, K.; Wang, G.; Zhang, W.; Gao, Y.; Cheng, K. Enhancement of Direct Urea-Hydrogen Peroxide Fuel Cell Performance by Three-Dimensional Porous Nickel-Cobalt Anode. J. Power Sources 2016, 307, 697–704. [Google Scholar] [CrossRef]
- Peng, L.; Shen, J.; Zheng, X.; Xiang, R.; Deng, M.; Mao, Z.; Feng, Z.; Zhang, L.; Li, L.; Wei, Z. Rationally Design of Monometallic NiO-Ni3S2/NF Heteronanosheets as Bifunctional Electrocatalysts for Overall Water Splitting. J. Catal. 2019, 369, 345–351. [Google Scholar] [CrossRef]
- Yu, H.; Quan, T.; Mei, S.; Kochovski, Z.; Huang, W.; Meng, H.; Lu, Y. Prompt Electrodeposition of Ni Nanodots on Ni Foam to Construct a High-Performance Water-Splitting Electrode: Efficient, Scalable, and Recyclable. Nano-Micro Lett. 2019, 11, 41. [Google Scholar] [CrossRef]
- Babar, P.; Patil, K.; Lee, D.M.; Karade, V.; Gour, K.; Pawar, S.; Kim, J.H. Cost-Effective and Efficient Water and Urea Oxidation Catalysis Using Nickel-Iron Oxyhydroxide Nanosheets Synthesized by an Ultrafast Method. J. Colloid Interface Sci. 2021, 584, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Hu, Q.T.; Wang, G.; Zhang, W.D.; Liu, J.; Li, T.; Gu, Z.G. NiCo Layered Double Hydroxide/Hydroxide Nanosheet Heterostructures for Highly Efficient Electro-Oxidation of Urea. Int. J. Hydrogen Energy 2020, 45, 19206–19213. [Google Scholar] [CrossRef]
- Wang, Y.-T.; He, X.-F.; Chen, X.-M.; Zhang, Y.; Li, F.-T.; Zhou, Y.; Meng, C. Laser Synthesis of Cobalt-Doped Ni3S4-NiS/Ni as High-Efficiency Supercapacitor Electrode and Urea Oxidation Electrocatalyst. Appl. Surf. Sci. 2022, 596, 153600. [Google Scholar] [CrossRef]
- Justyna, Ł.; Lieder, M. Nickel-Based Catalysts for Electrolytic Decomposition of Ammonia towards Hydrogen Production. Adv. Colloid Interface Sci. 2023, 319, 102963. [Google Scholar]
- Patil, S.J.; Chodankar, N.R.; Hwang, S.K.; Rama Raju, G.S.; Huh, Y.S.; Han, Y.K. Fluorine Engineered Self-Supported Ultrathin 2D Nickel Hydroxide Nanosheets as Highly Robust and Stable Bifunctional Electrocatalysts for Oxygen Evolution and Urea Oxidation Reactions. Small 2022, 18, 2103326. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhao, J.; Guan, J.; Wu, M.; Li, G.R. Ni3S2 in Situ Grown on Ni Foam Coupled with Nitrogen-Doped Carbon Nanotubes as an Efficient Electrocatalyst for the Hydrogen Evolution Reaction in Alkaline Solution. ACS Omega 2019, 4, 20244–20251. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Chen, S.; Li, J.; Wu, H.; Zhang, C.; Zhang, D.; Chen, X.; Gao, Y.; He, F.; Hui, L.; et al. Two-Dimensional Carbon Graphdiyne: Advances in Fundamental and Application Research. ACS Nano 2023, 17, 14309–14346. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Ghanem, M.A.; El-Zatahry, A.A.; Al-Deyab, S.S. Nickel Oxide/Nitrogen Doped Carbon Nanofibers Catalyst for Methanol Oxidation in Alkaline Media. Electrochim. Acta 2014, 137, 774–780. [Google Scholar] [CrossRef]
- Karuppiah, C.; Venkatesh, K.; Arunachalam, P.; Ramaraj, S.K.; Al-Mayouf, A.M.; Yang, C.C. Optimization of S-Dopant on N, S Co-Doped Graphene/CNT-Fe3C Nanocomposite Electrode for Non-Enzymatic H2O2 Sensor. Mater. Lett. 2021, 285, 129001. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, T.; Sumboja, A.; Zang, W.; Xie, J.; Gao, D.; Pennycook, S.J.; Liu, Z.; Guan, C.; Wang, J. Integrated Hierarchical Carbon Flake Arrays with Hollow P-Doped CoSe2 Nanoclusters as an Advanced Bifunctional Catalyst for Zn–Air Batteries. Adv. Funct. Mater. 2018, 28, 1–9. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Al Haj, Y.; Alajami, M.; Alawadhi, H.; Barakat, N.A.M. Ni-Cd Carbon Nanofibers as an Effective Catalyst for Urea Fuel Cell. J. Environ. Chem. Eng. 2018, 6, 332–337. [Google Scholar] [CrossRef]
- Yan, L.; Sun, Y.; Hu, E.; Ning, J.; Zhong, Y.; Zhang, Z.; Hu, Y. Facile In-Situ Growth of Ni2P/Fe2P Nanohybrids on Ni Foam for Highly Efficient Urea Electrolysis. J. Colloid Interface Sci. 2019, 541, 279–286. [Google Scholar] [CrossRef]
- Qian, G.; Chen, J.; Luo, L.; Zhang, H.; Chen, W.; Gao, Z.; Yin, S.; Tsiakaras, P. Novel Bifunctional V2O3Nanosheets Coupled with N-Doped-Carbon Encapsulated Ni Heterostructure for Enhanced Electrocatalytic Oxidation of Urea-Rich Wastewater. ACS Appl. Mater. Interfaces 2020, 12, 38061–38069. [Google Scholar] [CrossRef] [PubMed]
- Kareem, A.; Jose, E.; Thenmozhi, K.; Senthilkumar, S. In Situ Growth of Trimetallic FeCoNi Selenide Nanosheets on Nickel Foam for Electrocatalytic Water Splitting. ACS Appl. Nano Mater. 2025, 8, 8424–8432. [Google Scholar] [CrossRef]
- Leuaa, P.; Farzin, Y.A.; Iqbal, S.; Chatzichristodoulou, C. Cobalt Oxide (CoOx) Coated Ni Foam Anodes for High Temperature (150 °C) and Pressure (45 Bar) Alkaline Electrolysis. J. Power Sources 2025, 625, 235625. [Google Scholar] [CrossRef]
- Mu, J.; Xu, J.; Zhou, C.; Wang, Q.; Wang, X.G.; Liu, Z.Y.; Zhao, X.J.; Yang, E.C. Self-Supported Oxygen and Molybdenum Dual-Doped Cobalt Phosphide Hierarchical Nanomaterials as Superior Bifunctional Electrocatalysts for Overall Water Splitting. ChemElectroChem 2021, 8, 103–111. [Google Scholar] [CrossRef]
- Yao, C.; Xu, J.; Zhu, Y.; Zhang, R.; Shen, Y.; Xie, A. Porous CoP@N/P Co-Doped Carbon/CNTs Nanocubes: In-Situ Autocatalytic Synthesis and Excellent Performance as the Anode for Lithium-Ion Batteries. Appl. Surf. Sci. 2020, 513, 145777. [Google Scholar] [CrossRef]
- Ehsan, M.A.; Ullah, Z.; Nazar, M.F.; Younas, M.; Suliman, M. One Step Fabrication of Nanostructured Nickel Thin Films on Porous Nickel Foam for Drastic Electrocatalytic Oxygen Evolution. Int. J. Hydrogen Energy 2023, 48, 15784–15795. [Google Scholar] [CrossRef]
- Luo, K.-H.; Cheng, C.-K.; Lin, J.-Y.; Huang, C.-H.; Yeh, T.-K.; Hsieh, C.-K. Highly-Porous Hierarchically Microstructure of Graphene-Decorated Nickel Foam Supported Two-Dimensional Quadrilateral Shapes of Cobalt Sulfide Nanosheets as Efficient Electrode for Methanol Oxidation. Surf. Coat. Technol. 2020, 393, 125850. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Wang, X.L.; Ma, Y.; Li, Y.; Yang, X.; Li, Y. Multi-Electron Transfer Mechanism and Band Structures of CNTs in MOF-Derived CNTs/Co Hybrids for Enhanced Electromagnetic Wave Absorption Property. Mater. Today Commun. 2024, 39, 108936. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, X.; Wei, X.; Yu, R.; Shui, J. Porous CNTs/Co Composite Derived from Zeolitic Imidazolate Framework: A Lightweight, Ultrathin, and Highly Efficient Electromagnetic Wave Absorber. ACS Appl. Mater. Interfaces 2016, 8, 34686–34698. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Ye, K.; Du, M.; Huang, X.; Cheng, K.; Wang, G.; Cao, D. Electrochemical Impedance Analysis of Urea Electro-Oxidation Mechanism on Nickel Catalyst in Alkaline Medium. Electrochim. Acta 2016, 210, 474–482. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Duan, Y.; Li, J.; Wang, H.; Yang, X.; Gong, M. Electrochemical Urea Oxidation in Different Environment: From Mechanism to Devices. ChemCatChem 2022, 14, e202101906. [Google Scholar] [CrossRef]
- Urbańczyk, E.; Sowa, M.; Simka, W. Urea Removal from Aqueous Solutions—A Review. J. Appl. Electrochem. 2016, 46, 1011–1029. [Google Scholar] [CrossRef]
- Xu, S.; Ruan, X.; Ganesan, M.; Wu, J.; Ravi, S.K.; Cui, X. Transition Metal-Based Catalysts for Urea Oxidation Reaction (UOR): Catalyst Design Strategies, Applications, and Future Perspectives. Adv. Funct. Mater. 2024, 34, 2313309. [Google Scholar] [CrossRef]
- Gautam, J.; Lee, S.; Park, S. Cutting-Edge Optimization Strategies and In Situ Characterization Techniques for Urea Oxidation Reaction Catalysts: A Comprehensive Review. Adv. Energy Mater. 2025, 15, 2406047. [Google Scholar] [CrossRef]

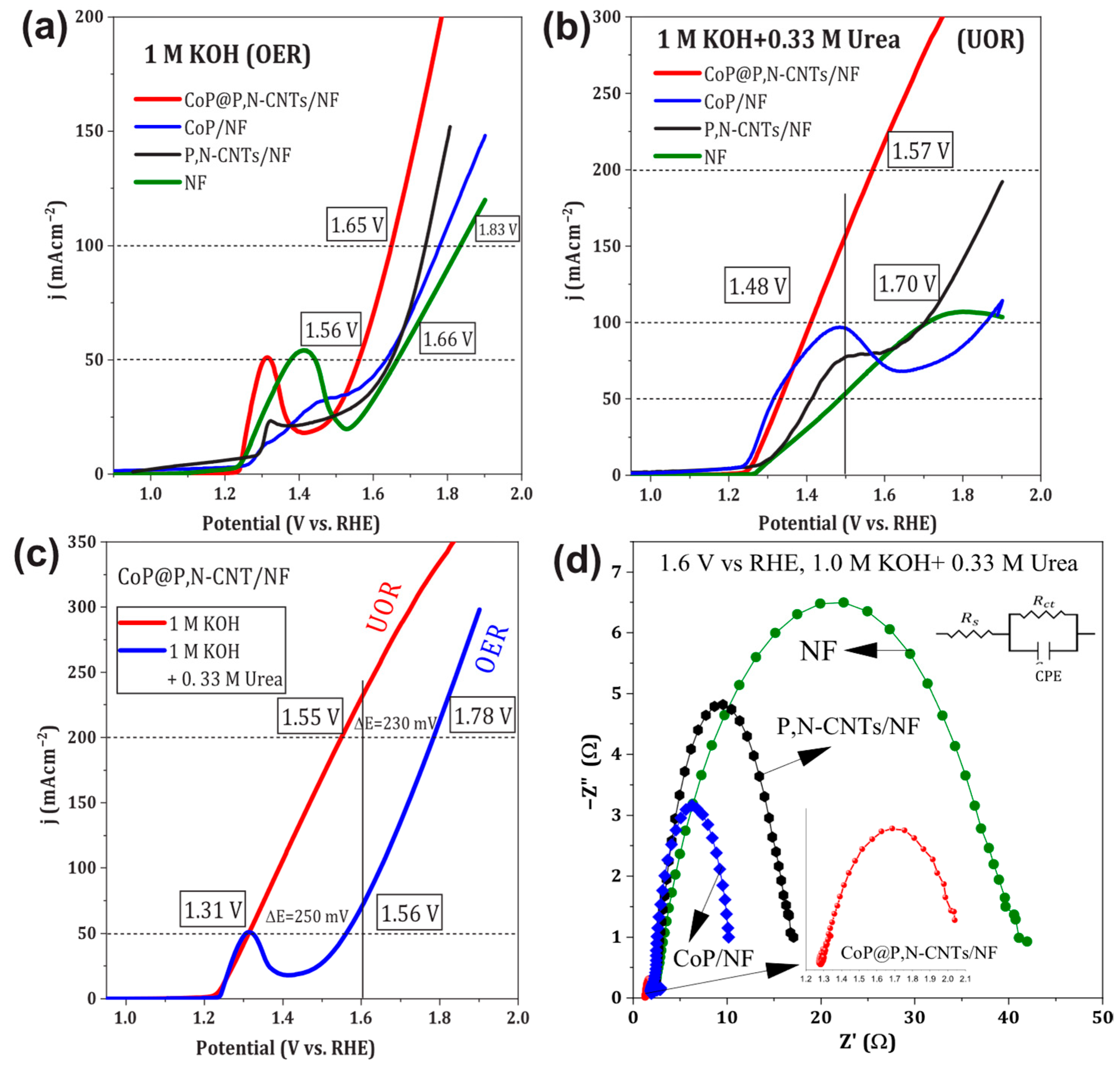
| Electrodes | OER | UOR | |||
| 50 mA/cm2 | 100 mA/cm2 | 50 mA/cm2 | 100 mA/cm2 | 200 mA/cm2 | |
| NF | 1.66 V | 1.83 V | 1.48 V | 1.71 V | ---------- |
| CoP/NF | 1.63 V | 1.78 V | 1.31 V | 1.86 V | ---------- |
| P, N-CNTs/NF | 1.65 V | 1.74 V | 1.41 V | 1.70 V | ---------- |
| CoP@P,N-CNTs/NF | 1.56 V | 1.65 V | 1.33 V | 1.48 V | 1.57 V |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssef, H.M.; Shaddad, M.N.; Aladeemy, S.A.; Aldawsari, A.M. Fabrication of CoP@P, N-CNTs-Deposited Nickel Foam for Energy-Efficient Hydrogen Generation via Electrocatalytic Urea Oxidation. Catalysts 2025, 15, 652. https://doi.org/10.3390/catal15070652
Youssef HM, Shaddad MN, Aladeemy SA, Aldawsari AM. Fabrication of CoP@P, N-CNTs-Deposited Nickel Foam for Energy-Efficient Hydrogen Generation via Electrocatalytic Urea Oxidation. Catalysts. 2025; 15(7):652. https://doi.org/10.3390/catal15070652
Chicago/Turabian StyleYoussef, Hany M., Maged N. Shaddad, Saba A. Aladeemy, and Abdullah M. Aldawsari. 2025. "Fabrication of CoP@P, N-CNTs-Deposited Nickel Foam for Energy-Efficient Hydrogen Generation via Electrocatalytic Urea Oxidation" Catalysts 15, no. 7: 652. https://doi.org/10.3390/catal15070652
APA StyleYoussef, H. M., Shaddad, M. N., Aladeemy, S. A., & Aldawsari, A. M. (2025). Fabrication of CoP@P, N-CNTs-Deposited Nickel Foam for Energy-Efficient Hydrogen Generation via Electrocatalytic Urea Oxidation. Catalysts, 15(7), 652. https://doi.org/10.3390/catal15070652







