Study of Biochar with Different Cellulose/Lignin Ratios for Organic Pollutant Removal in Water Through Fenton-like Catalysis Assisted with Adsorption
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Biochar
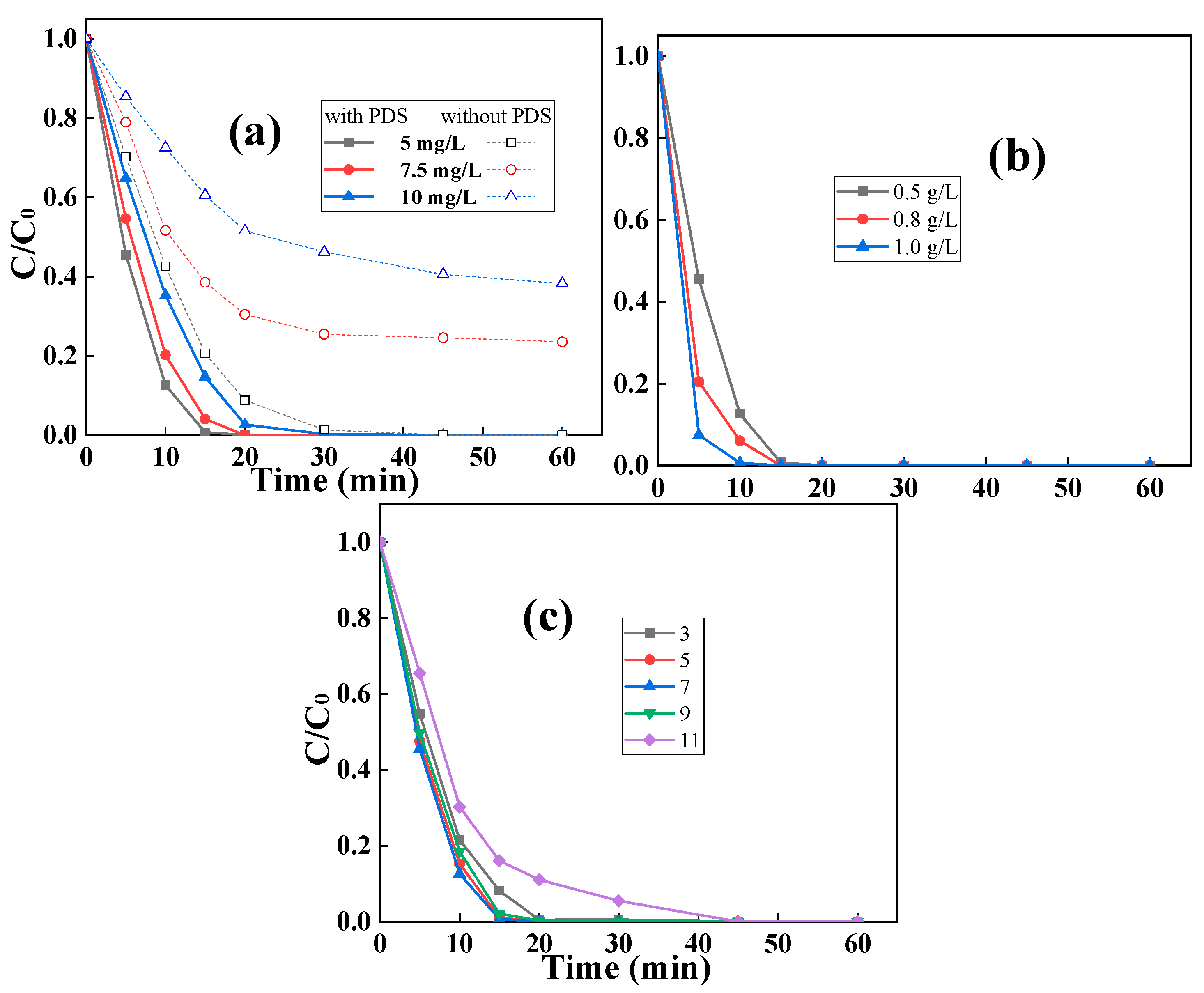
2.2. Removal of RhB by Biochar via Adsorptive/Catalytic Synergy
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Preparation of Cellulose/Lignin-Derived Biochar (CL-Biochar)
3.3. Characterizations
3.4. Removal of RhB in CL-Biochar/PDS Catalytic System
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gan, L.; Xu, L.; Qian, K. Preparation of core-shell structured CoFe2O4 incorporated Ag3PO4 nanocomposites for photocatalytic degradation of organic dyes. Mater. Des. 2016, 109, 354–360. [Google Scholar] [CrossRef]
- Turhan, K.; Durukan, I.; Ozturkcan, S.A.; Turgut, Z. Decolorization of textile basic dye in aqueous solution by ozone. Dye. Pigment. 2012, 92, 897–901. [Google Scholar] [CrossRef]
- Gan, L.; Geng, A.; Xu, L.; Chen, M.; Wang, L.; Liu, J.; Han, S.; Mei, C.; Zhong, Q. The fabrication of bio-renewable and recyclable cellulose based carbon microspheres incorporated by CoFe2O4 and the photocatalytic properties. J. Clean. Prod. 2018, 196, 594–603. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Shi, S.; Pan, Y.; Qin, R.; Zhang, Y. The influence of substituent effects of polyphenols on the removal of Cr(VI): The significance of electron-donating and electron-withdrawing groups. Sep. Purif. Technol. 2025, 364, 132500. [Google Scholar] [CrossRef]
- Gan, L.; Xu, L.; Pan, Z.; Jiang, F.; Shang, S. Alginic acid/graphene oxide hydrogel film coated functional cotton fabric for controlled release of matrine and oxymatrine. RSC Adv. 2016, 6, 76420–76425. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, Y.; Puyang, C.; Tang, S.; Guo, H. Periodate activation by plasma coupled with Fe N C for contaminant removal: Machine learning assisted catalyst optimization and electron shuttle mechanism. J. Colloid Interface Sci. 2025, 685, 975–987. [Google Scholar] [CrossRef]
- Liu, Z.; Li, X.; Guo, H.; Zhang, Z.; Wang, Y.; Pang, P.; Gao, Y.; Guo, H. Enhancing the synergistic effect by introducing Cu(II)-PMS into the underwater bubble plasma treatment process for efficient degradation of emerging contaminants in water. Chem. Eng. J. 2025, 507, 160480. [Google Scholar] [CrossRef]
- Veksha, A.; McLaughlin, H.; Layzell, D.B.; Hill, J.M. Pyrolysis of wood to biochar: Increasing yield while maintaining microporosity. Bioresour. Technol. 2014, 153, 173–179. [Google Scholar] [CrossRef]
- Lyu, H.; Zhang, Q.; Shen, B. Application of biochar and its composites in catalysis. Chemosphere 2020, 240, 124842. [Google Scholar] [CrossRef]
- Qiu, B.; Shao, Q.; Shi, J.; Yang, C.; Chu, H. Application of biochar for the adsorption of organic pollutants from wastewater: Modification strategies, mechanisms and challenges. Sep. Purif. Technol. 2022, 300, 121925. [Google Scholar] [CrossRef]
- Chen, S.; Ling, Z.; Zhang, X.; Kim, Y.S.; Xu, F. Towards a multi-scale understanding of dilute hydrochloric acid and mild 1-ethyl-3-methylimidazolium acetate pretreatment for improving enzymatic hydrolysis of poplar wood. Ind. Crop. Prod. 2018, 114, 123–131. [Google Scholar] [CrossRef]
- Duan, Y.; Li, X.; Gao, S.; Yu, M.; Fu, L.; Zhang, Y.; Sun, H.; Pan, Y. Lignosulfonate modified zero-valent iron enhanced activation of peroxymonosulfate for sulfamethazine removal: Performance and mechanism. Chem. Eng. J. 2024, 504, 159054. [Google Scholar] [CrossRef]
- Geng, A.; Xu, L.; Gan, L.; Mei, C.; Wang, L.; Fang, X.; Li, M.; Pan, M.; Han, S.; Cui, J. Using wood flour waste to produce biochar as the support to enhance the visible-light photocatalytic performance of BiOBr for organic and inorganic contaminants removal. Chemosphere 2020, 250, 126291. [Google Scholar] [CrossRef]
- Shi, J.; Dai, B.; Fang, X.; Xu, L.; Wu, Y.; Lu, H.; Cui, J.; Han, S.; Gan, L. Waste preserved wood derived biochar catalyst for promoted peroxymonosulfate activation towards bisphenol A degradation with low metal ion release: The insight into the mechanisms. Sci. Total. Environ. 2022, 813, 152673. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhu, Y.; Niu, Q.; Zeng, G.; Lai, C.; Liu, S.; Huang, D.; Qin, L.; Liu, X.; Li, B.; et al. New notion of biochar: A review on the mechanism of biochar applications in advannced oxidation processes. Chem. Eng. J. 2021, 416, 129027. [Google Scholar] [CrossRef]
- Zhao, C.; Ge, L.; Wang, R.; Chu, H.; Mai, L.; Zha, W.; Wang, Y.; Xu, C. Effects of cellulose addition on the physicochemical properties, pore structure and iodine adsorption of lignin-based biochar. Fuel 2023, 352, 129061. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Wu, Y.; Zheng, M. A comparison of biochars from lignin, cellulose and wood as the sorbent to an aromatic pollutant. J. Hazard. Mater. 2014, 280, 450–457. [Google Scholar] [CrossRef]
- Chen, C.; Sun, K.; Huang, C.; Yang, M.; Fan, M.; Wang, A.; Zhang, G.; Li, B.; Jiang, J.; Xu, W.; et al. Investigation on the mechanism of structural reconstruction of biochars derived from lignin and cellulose during graphitization under high temperature. Biochar 2023, 5, 1–14. [Google Scholar] [CrossRef]
- Lédé, J. Cellulose pyrolysis kinetics: An historical review on the existence and role of intermediate active cellulose. J. Anal. Appl. Pyrolysis 2012, 94, 17–32. [Google Scholar] [CrossRef]
- Jiang, G.; Nowakowski, D.J.; Bridgwater, A.V. Effect of the Temperature on the Composition of Lignin Pyrolysis Products. Energy Fuels 2010, 24, 4470–4475. [Google Scholar] [CrossRef]
- Xu, L.; Ye, Z.; Pan, Y.; Zhang, Y.; Gong, H.; Mei, X.; Qiao, W.; Gan, L. Effect of lignocellulosic biomass composition on the performance of biochar for the activation of peroxymonosulfate to degrade diclofenac. Sep. Purif. Technol. 2023, 311, 123312. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.; Dai, Z.; Zhang, A.-Y.; Yin, J.; Peng, S.; Liang, H. Recycling chestnut shell for superior peroxymonosulfate activation in contaminants degradation via the synergistic radical/non-radical mechanisms. J. Hazard. Mater. 2022, 430, 128471. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Liu, X.; Xiong, W.; Wang, H.; Ji, X.; Kong, F.; Yang, G.; Xu, J. Converting amorphous kraft lignin to hollow carbon shell frameworks as electrode materials for lithium-ion batteries and supercapacitors. Ind. Crop. Prod. 2021, 174, 114184. [Google Scholar] [CrossRef]
- Yoo, S.; Kelley, S.S.; Tilotta, D.C.; Park, S. Structural Characterization of Loblolly Pine Derived Biochar by X-ray Diffraction and Electron Energy Loss Spectroscopy. ACS Sustain. Chem. Eng. 2018, 6, 2621–2629. [Google Scholar] [CrossRef]
- Shi, J.; Dai, B.; Shen, X.; Xu, L.; Zhang, Y.; Gan, L. Wood induced preparation of Fe3C decorated biochar for peroxymonosulfate activation towards bisphenol a degradation with low ion leaching. J. Environ. Manag. 2023, 340, 117978. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Oh, S.; Park, Y.-K. Overview of biochar production from preservative-treated wood with detailed analysis of biochar characteristics, heavy metals behaviors, and their ecotoxicity. J. Hazard. Mater. 2020, 384, 121356. [Google Scholar] [CrossRef]
- Tan, X.-F.; Zhu, S.-S.; Wang, R.-P.; Chen, Y.-D.; Show, P.-L.; Zhang, F.-F.; Ho, S.-H. Role of biochar surface characteristics in the adsorption of aromatic compounds: Pore structure and functional groups. Chin. Chem. Lett. 2021, 32, 2939–2946. [Google Scholar] [CrossRef]
- Ganguly, P.; Sarkhel, R.; Das, P. Synthesis of pyrolyzed biochar and its application for dye removal: Batch, kinetic and isotherm with linear and non-linear mathematical analysis. Surfaces Interfaces 2020, 20, 100616. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Johir, M.A.H.; Sornalingam, K. Single and competitive sorption properties and mechanism of functionalized biochar for removing sulfonamide antibiotics from water. Chem. Eng. J. 2017, 311, 348–358. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Liu, B.; Wu, Q.; Luo, H.; Zhao, Q.; Si, Q.; Sseguya, F.; Ren, N. Edge-nitrogenated biochar for efficient peroxydisulfate activation: An electron transfer mechanism. Water Res. 2019, 160, 405–414. [Google Scholar] [CrossRef]
- Ouyang, F.; Liu, Y.; Chen, J.; Tang, C.; Wang, A.; Lu, Y.; Yuan, Y. Study on Preparation of Rabbit Manure Biochar and Activation of Peroxymonosulfate for Rhodamine B Degradation. Water 2023, 15, 2015. [Google Scholar] [CrossRef]
- Zhu, B.; Yu, Y.; Ding, Y.; Ge, S. Iron-modified granular sludge biochar-based catalysts for improved Rhodamine B degradation by activating peroxymonosulfate. Biomass-Convers. Biorefinery 2022, 14, 27755–27765. [Google Scholar] [CrossRef]
- Cao, Z.; Xu, J.; Tan, W.; Tang, H.; Li, H.; Li, Y. Enhanced degradation of Rhodamine B through activating peroxydisulfate by humic acid-Fe incorporated biochar: Kinetics and mechanism studies. J. Mol. Liq. 2024, 396, 123962. [Google Scholar] [CrossRef]
- Deng, S.; Yang, M.; An, Q.; Li, Z.; Zhao, B.; Ran, B. Efficient rhodamine B dye degradation by red mud-grapefruit peel biochar catalysts activated persulfate in water. Environ. Sci. Pollut. Res. 2023, 30, 119034–119049. [Google Scholar] [CrossRef]
- Mustafa, F.S.; Aziz, K.H.H. Heterogeneous catalytic activation of persulfate for the removal of rhodamine B and diclofenac pollutants from water using iron-impregnated biochar derived from the waste of black seed pomace. Process. Saf. Environ. Prot. 2022, 170, 436–448. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, L.; Pan, Y.; Qin, R.; Wang, W.; Zhou, M.; Zhang, Y. Detection methodologies and mechanisms of reactive oxygen species generated in Fenton/Fenton-like processes. Sep. Purif. Technol. 2024, 355, 129578. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, W.; Li, Y.; Zhu, D.; Guo, H. Enhanced degradation of sulfamethoxazole in water using non-thermal plasma-activated persulfate: Effects of heat and radical synergies. J. Environ. Chem. Eng. 2025, 13, 115416. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chen, Y.-D.; Li, R.; Zhang, C.; Ge, Y.; Cao, G.; Ma, M.; Duan, X.; Wang, S.; Ren, N.-Q. N-doped graphitic biochars from C-phycocyanin extracted Spirulina residue for catalytic persulfate activation toward nonradical disinfection and organic oxidation. Water Res. 2019, 159, 77–86. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Z.; Huang, F.; Liu, Y.; Feng, L.; Jiang, J.; Zhang, L.; Qi, F.; Liu, C. Carbonized polyaniline activated peroxymonosulfate (PMS) for phenol degradation: Role of PMS adsorption and singlet oxygen generation. Appl. Catal. B Environ. 2021, 286, 119921. [Google Scholar] [CrossRef]
- Kang, S.; Hwang, J. CoMn2O4 embedded hollow activated carbon nanofibers as a novel peroxymonosulfate activator. Chem. Eng. J. 2021, 406, 127158. [Google Scholar] [CrossRef]
- Zhang, Q.; He, D.; Li, X.; Feng, W.; Lyu, C.; Zhang, Y. Mechanism and performance of singlet oxygen dominated peroxymonosulfate activation on CoOOH nanoparticles for 2,4-dichlorophenol degradation in water. J. Hazard. Mater. 2020, 384, 121350. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Gan, L.; Wang, L.; Gong, H.; Xu, L.; Wu, Y.; Lu, H.; Han, S.; Cui, J.; Xia, C. Enhanced degradation of bisphenol A by mixed ZIF derived CoZn oxide encapsulated N-doped carbon via peroxymonosulfate activation: The importance of N doping amount. J. Hazard. Mater. 2021, 419, 126363. [Google Scholar] [CrossRef] [PubMed]
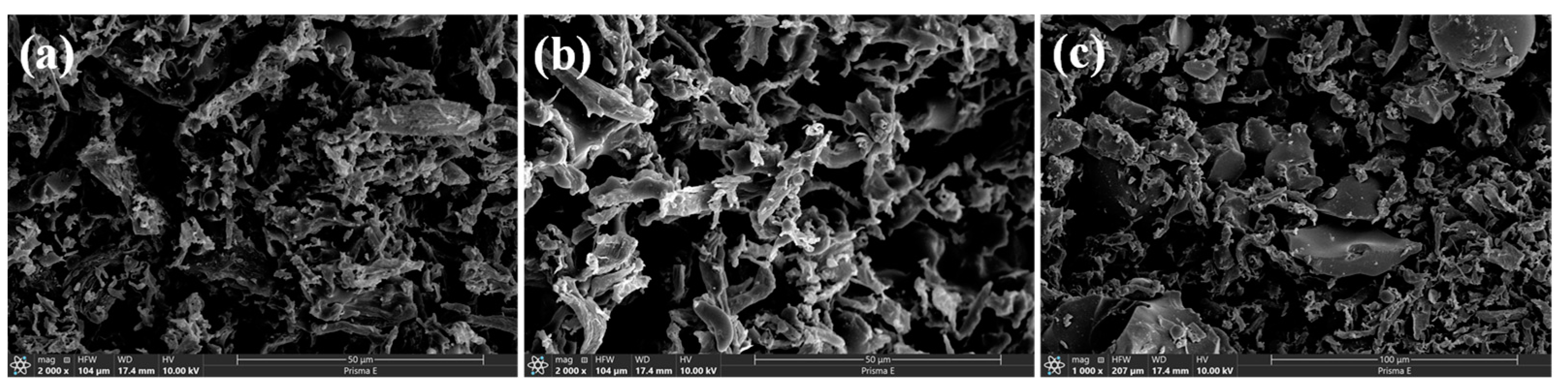

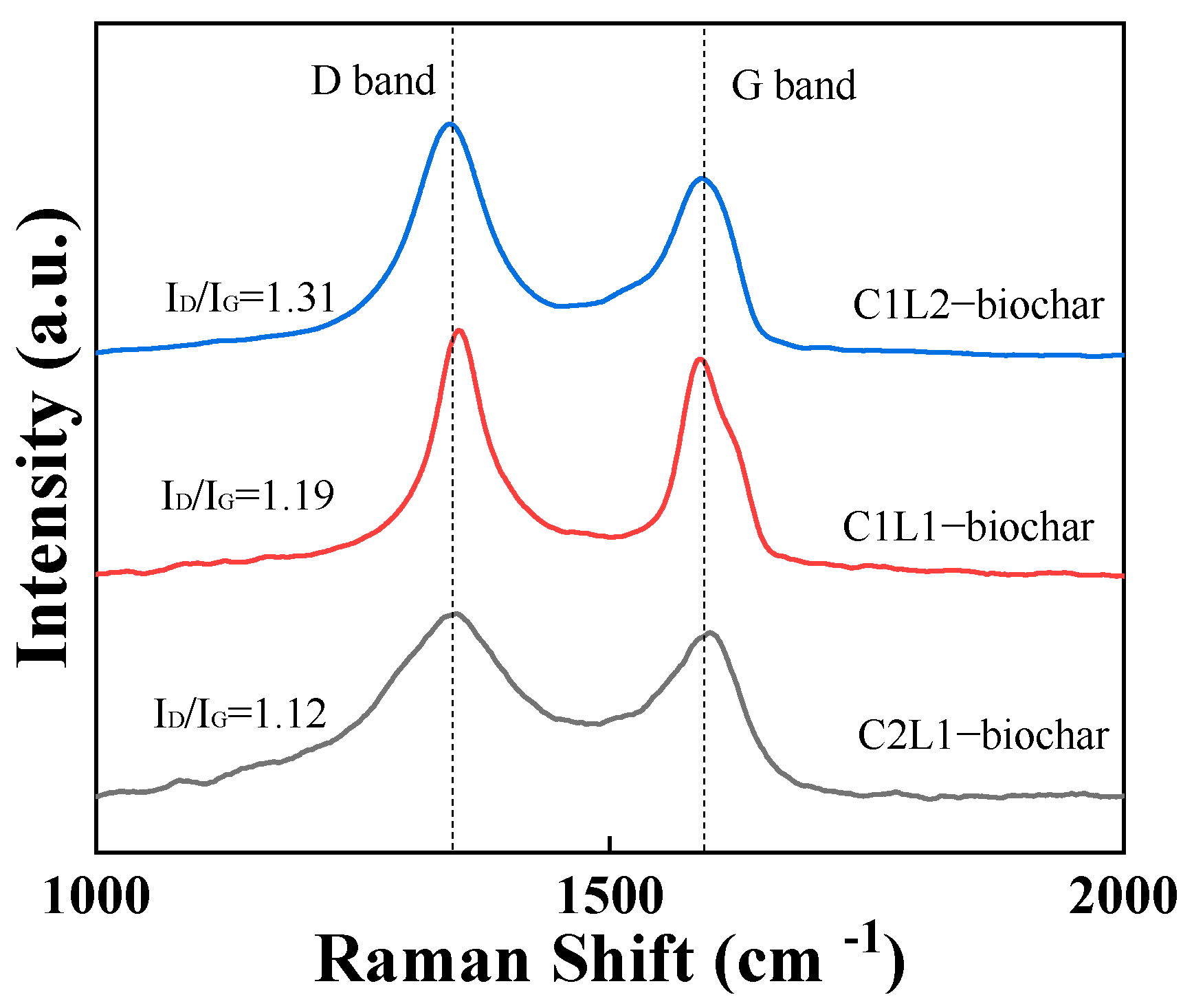
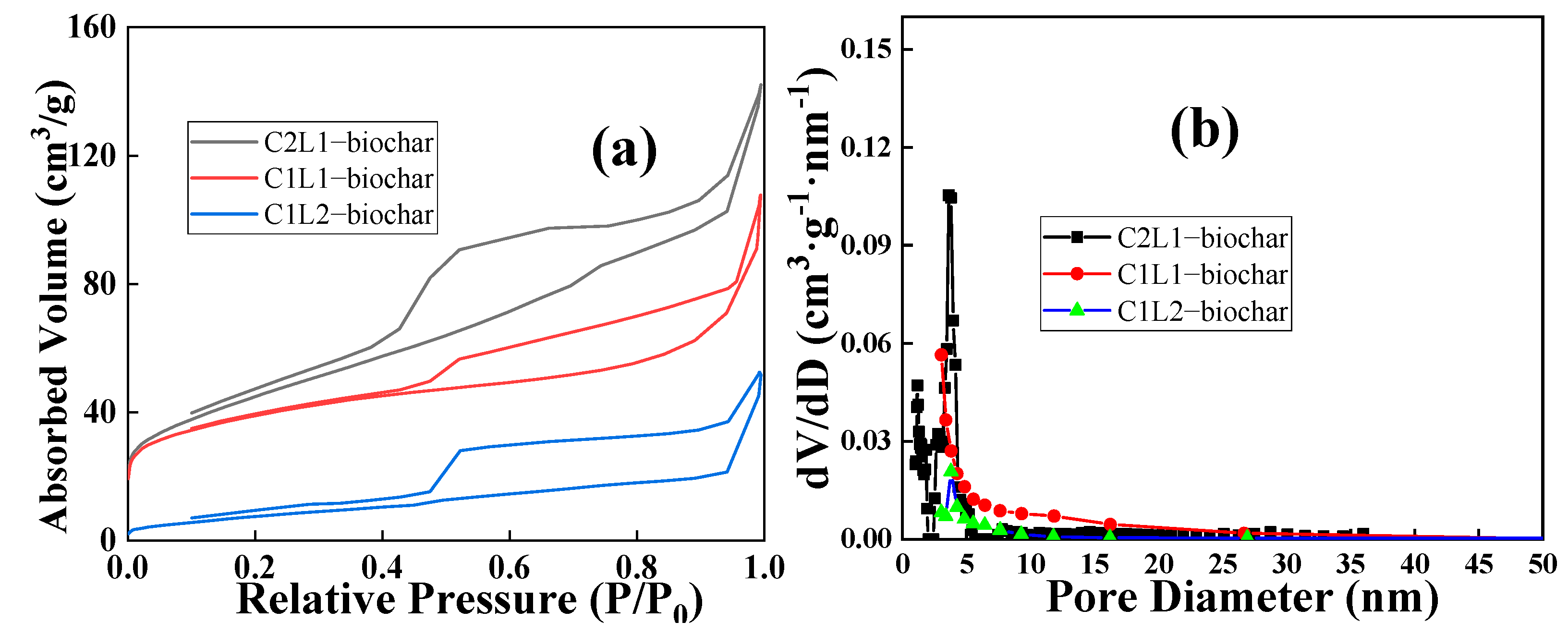
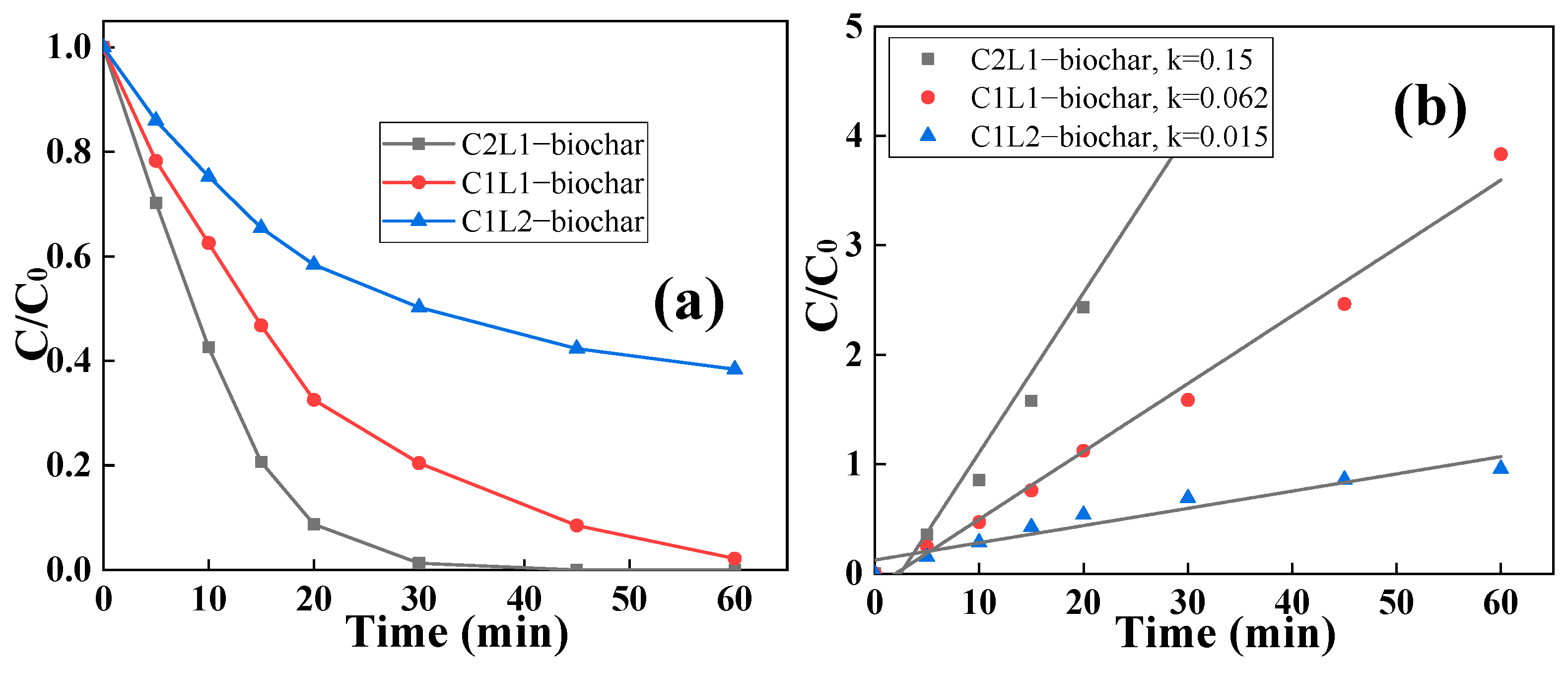




| Sample | Biochar Yield (wt%) | C (wt%) | O (wt%) |
|---|---|---|---|
| C2L1-biochar | 23.6 | 76.2 | 23.8 |
| ClL1-biochar | 26.1 | 76.8 | 23.2 |
| C1L2-biochar | 28.8 | 78.5 | 21.5 |
| Sample | Specific Surface Area (m2/g) | Pore Volume (cm3∙g−1) |
|---|---|---|
| C2L1-biochar | 162.1 | 0.18 |
| ClL1-biochar | 138.6 | 0.11 |
| C1L2-biochar | 30.4 | 0.07 |
| Sample | Dosage (g/L) | Persulfate | RhB Concentration (g/L) | Removal Time (min) | Ref. |
|---|---|---|---|---|---|
| C2L1-biochar | 0.5 | PDS | 0.01 | 20 | This study |
| Rabbit Manure Biochar | 0.6 | PMS | 0.05 | 60 | [31] |
| Fe-sludge biochar | 0.2 | PMS | 0.01 | 40 | [32] |
| Fe-corn straw biochar | 0.5 | PDS | 0.1 | 15 | [33] |
| Red mud-biochar | 2.0 | PDS | 0.05 | 60 | [34] |
| Fe- black seed biochar | 1.5 | PDS | 0.02 | 10 | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Xu, W.; Gan, L. Study of Biochar with Different Cellulose/Lignin Ratios for Organic Pollutant Removal in Water Through Fenton-like Catalysis Assisted with Adsorption. Catalysts 2025, 15, 327. https://doi.org/10.3390/catal15040327
Yu X, Xu W, Gan L. Study of Biochar with Different Cellulose/Lignin Ratios for Organic Pollutant Removal in Water Through Fenton-like Catalysis Assisted with Adsorption. Catalysts. 2025; 15(4):327. https://doi.org/10.3390/catal15040327
Chicago/Turabian StyleYu, Xinyan, Wanting Xu, and Lu Gan. 2025. "Study of Biochar with Different Cellulose/Lignin Ratios for Organic Pollutant Removal in Water Through Fenton-like Catalysis Assisted with Adsorption" Catalysts 15, no. 4: 327. https://doi.org/10.3390/catal15040327
APA StyleYu, X., Xu, W., & Gan, L. (2025). Study of Biochar with Different Cellulose/Lignin Ratios for Organic Pollutant Removal in Water Through Fenton-like Catalysis Assisted with Adsorption. Catalysts, 15(4), 327. https://doi.org/10.3390/catal15040327




_Xu.png)




