Abstract
The electrochemical reduction of carbon dioxide (CO2RR) utilizing intermittent electricity from renewable energy sources represents an emerging and promising approach to achieve carbon neutrality and mitigate the greenhouse effect. This review comprehensively summarizes recent advances in Cu-based metal–organic framework (MOF) electrocatalysts for CO2RR, focusing on their applications in producing C1 and C2+ products. This paper highlights key strategies such as nanostructure manipulation, multi-component tandem catalysis, single-atom alloying, and ligand functionalization to optimize the binding energies of intermediate species and promote selective CO2RR pathways. Numerous examples are presented, showcasing remarkable Faradaic efficiencies and product selectivities achieved through rational catalyst design. Furthermore, the use of MOF-derived materials and composites with other materials, like carbon nanotubes, graphene, and metal oxides, is discussed to enhance conductivity, stability, and selectivity. Despite the significant progress, challenges remain in achieving stable and scalable catalysts with high activity and selectivity towards specific C2+ products. This review underscores the importance of precise control of catalyst composition, structure, and surface properties to tackle these challenges and provides valuable insights for future research directions in developing advanced Cu-based MOF electrocatalysts for practical applications in CO2 conversion technologies.
1. Introduction
Energy plays a crucial role in the development of human society, and fossil fuels have been the dominant global energy source. However, the combustion of fossil fuels results in the emission of a significant amount of carbon dioxide, leading to various environmental problems like the greenhouse effect [1,2]. These issues ultimately affect the sustainable development of human beings. As renewable energy generation becomes more widespread and cheaper, there is a growing interest in using electrocatalytic CO2RR [3]. This method offers multiple benefits such as an easy design, gentle reaction conditions, and simple scalability [4,5]. Additionally, it produces various chemical substances of high economic value (such as methanol, ethanol, and ethylene), while significantly reducing carbon dioxide emissions [6,7,8]. Hence, the electrochemical reduction of carbon dioxide (CO2RR) shows great promise for extensive application in the future.
The process of CO2RR involves three stages: adsorption, activation, and desorption on the catalyst’s surface [9]. A general mechanism of CO2RR involves a complex series of electrochemical reactions that transform carbon dioxide into valuable chemicals and fuels. The process typically commences with the adsorption of CO2 molecules onto the active sites of the catalyst surface, where they are activated and reduced. The key intermediates formed during this process include adsorbed CO2, CO, OCHO, and CHO. For the generation of C2+ products, a crucial step is the coupling of two *CO intermediates to form a *COCO or *OCCO intermediate. This C-C coupling process is facilitated by the appropriate distance and coordination environment of the metal active sites, with Cu-based catalysts exhibiting moderate binding energy for *CO, making them particularly effective for this reaction. The reaction pathways for C2+ product formation can vary, including the *CO dimerization pathway, which involves the direct coupling of two *CO molecules, and the *CO-CHO coupling pathway, which occurs at higher overpotentials. The formation of ethylene, for instance, typically involves the coupling of two *CO molecules to form *OCCO, followed by subsequent hydrogenation steps. Other C2+ products such as ethanol can be formed through different intermediate species and reaction pathways. In addition to the C-C coupling reaction, competitive hydrogen evolution reaction (HER) is also a significant process that needs to be considered during CO2RR. The selectivity towards C2+ products is heavily influenced by the ability of the catalyst to suppress HER and promote C-C coupling. Moreover, the reaction mechanism is highly dependent on the catalyst structure and properties, which can be tailored to optimize the adsorption and activation of CO2 molecules, as well as the stabilization of key intermediates, thereby enhancing the selectivity and activity towards C2+ products.
Although recent progress has been made in the design of catalysts and the understanding of catalytic mechanisms in CO2RR, the following three major problems still exist [10,11,12,13,14]. Firstly, the C = O bond in linear carbon dioxide molecules is very stable, which means that high overpotentials are required to trigger the reaction and it is inefficient [15]. Secondly, CO2RR is usually performed in an aqueous solution, which means that the HER competes with it [16]. Thirdly, the multiple proton coupling and electron transfer steps in the C-C coupling process lead to slow reaction kinetics and low product selectivity [17,18]. Therefore, it is essential to optimize the activity, selectivity, and stability of the catalysts to promote the development of CO2RR.
Cu is the only metal that has been reported to effectively reduce carbon dioxide to multi-carbon products, which can be attributed to the fact that copper crystals have negative *CO adsorption energy, which promotes CO adsorption and C-C coupling [19,20]. However, Cu produces a series of reduction products during the electrocatalytic reduction of CO2, resulting in low selectivity for the target products [21]. In addition, the potential required for the HER and the reduction potential of the target product are similar, resulting in a low overall catalytic activity of CO2 [22]. In order to improve the performance of Cu-based catalysts in CO2RR, nanostructure manipulation, multi-component tandem strategy, and single-atom alloying can be adopted [9,23,24].
Omar M. Yaghi’s research group first proposed the concept of the metal–organic framework (MOF). MOFs are networked, porous crystalline material composed of organic ligands linked to inorganic metal-containing secondary building units (SBUs), which are highly tunable, have high surface area, and are versatile [25,26,27,28]. MOFs can be used either directly as the electrocatalyst-or as the catalyst precursor in CO2RR [22,29]. However, MOF materials also have shortcomings such as limited electrical conductivity and insufficient structural stability [30]. Strategies such as manipulating inorganic metal units, changing organic ligands, constructing MOF-based hybrids, and preparing MOF-derived materials have been proven to be effective in improving the gas adsorption capacity, catalytic capacity, and conductivity of MOFs and their derivative materials [31,32,33,34]. Among many metals, the Cu species has the ability to generate organic compounds such as hydrocarbons, carboxylic acids, and alcohols, and its introduction into the MOF framework can show excellent catalytic performance in CO2RR via synergistic effects [35]. Cu-MOF is a crystalline structure material formed by linking copper ions (Cu2+) to organic ligands. The material has a high surface area and pore structure regulation, providing a large number of active sites and good conditions for chemical reactions. In the process of participating in CO2RR, the coordinated copper ions interact with small molecules, such as CO2 and N2, and can be converted into multiple valence states, such as Cu+, Cu0 or Cu3+, Cu4+ [22]. In addition, Cu-MOF has a tunable pore structure, which can realize molecular screening and catalysis of specific reactants through selective adsorption and separation. The high activity and high selectivity of catalytic characteristics make Cu-MOF and its derivative materials have a wide range of application potentials in organic synthesis, gas separation, gas storage, catalytic conversion, and other fields [36,37].
To this end, the present work aims to offer comprehensive review of the latest advancements in Cu-based MOF electrocatalysts for CO2RR. By highlighting key strategies employed to optimize the catalytic performance, we endeavor to offer valuable insights into the design principles and mechanisms underlying these catalysts. By doing so, we hope to contribute to the ongoing efforts in developing the advanced Cu-based MOF and its derivative electrocatalysts that can pave the way for practical applications in CO2 conversion technologies.
2. Recent Advances in Cu-Based MOF Electrocatalysts
2.1. Overview of CO2RR C1 Products
2.1.1. Carbon Monoxide
Carbon monoxide is a significant industrial raw material and serves as an intermediate in the synthesis of numerous valuable chemicals such as acetic acid, methanol, formaldehyde, and synthetic fuels. The electrocatalytic reduction of carbon dioxide to carbon monoxide offers a sustainable and environmentally friendly pathway for producing these chemicals.
A key factor in modulating the selectivity and activity of catalytic products in the electrochemical carbon dioxide reduction reaction is the optimization of the binding energy between the intermediate and the active site. One effective strategy to achieve this optimization is through the incorporation of transition metals into MOFs. An ion exchange strategy was reported to incorporate Pd into the Cu-based MOF, HKUST-1 [Cu3(BTC)2] (BTC = benzentricarboxylate), resulting in the formation of Cu-Pd paddle-wheel dimers [38]. This modification significantly improved the Faradaic efficiency (FE) for CO production from 28.7% to 84.8% at −0.77 V versus (vs.) the reversible hydrogen electrode (RHE) in CO2-saturated 0.5 M KHCO3 (Figure 1). Specifically, at an applied potential of −0.77 V vs. RHE, the bimetallic catalyst exhibited a maximum FE for CO production, which decreased as the potential became more negative. In contrast, the FE for H2 production increased significantly when the applied potential was below −0.77 V vs. RHE.
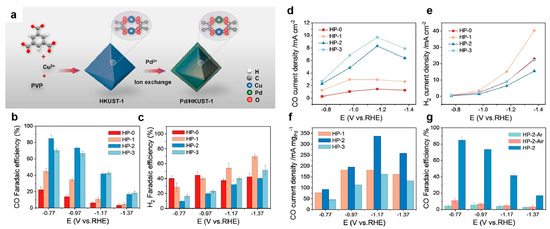
Figure 1.
(a) Synthesis scheme of the Pd/HKUST-1. CO2RR activities of samples: (b) FE for CO, (c) FE for H2, (d) partial current density for CO production, (e) partial current density for H2 production, and (f) Pd mass activity for CO for the prepared catalysts as a function of applied electrochemical potential. (g) FE for CO of HP-2 and its derivatives, obtained by pyrolysis in the air (HP-2-Air) and Ar (HP-2-Ar) atmospheres, as a function of applied electrochemical potential. Reproduced with permission from ref. [38]. Copyright Wiley-VCH GmbH, 2024.
Systematic doping of transition metal ions into MOFs has been explored to further enhance catalytic properties. For example, transition metal ions including nickel (Ni2+), iron (Fe2+), and copper (Cu2+) were systematically doped into the zeolite-imidazolate-framework-8 (ZIF-8) at varying compositional ratios. Notably, Cu doping exhibited remarkable effects on the catalytic properties, with Cu0.5Zn0.5/ZIF-8 achieving a high partial current density of 11.57 mA/cm2 and a maximum FE of 88.5% for CO production at −1.0 V vs. RHE. Density functional theory (DFT) calculations revealed that Cu doping altered the electronic structures of the sp2 carbon sites in the 2-methylimidazolate organic ligand, facilitating COOH* intermediate formation and subsequent CO production [39].
Another example is that an ultrathin 2D triptycene-based MOF, denoted as Cu-HHTT, was designed and synthesized for highly selective CO2RR to CO [40]. The Cu-HHTT nanosheets, featuring CuO4 units, exhibited remarkable performance with an FE of 96.6% towards CO production at a potential of −0.6 V vs. RHE, accompanied by a current density of 18 mA/cm2, which was attributed to the lower energy barrier for CO desorption compared to *CO intermediate hydrogenation, as revealed by DFT calculations. The stability of Cu-HHTT was also confirmed through long-term durability tests, with only a slight decrease in FE(CO) after continuous operation for at least 4 h.
Moreover, the porous nanofibers embedded with Cu clusters through the carbonization of electrospun MOF-545-Cu/PAN (MCP) nanofibers, as presented by Xin et al. [41], exhibited excellent CO2RR performance. The obtained Cu cluster-doped porous nanofibers exhibit high porosity, conductivity, and uniformly dispersed Cu clusters, making them promising catalysts for CO2RR. Among them, MCP-500 demonstrates the best performance with an FE of CO reaching 98% at −0.8 V vs. RHE and maintaining above 95% after 10 h of continuous electrocatalysis. As the voltage varied from −0.6 to −1.0 V vs. RHE, the FE for CO remained consistently high, ranging from over 80% to 98%. The high performance is attributed to the synergistic effect of the layered graphene skeleton and uniformly dispersed Cu clusters, which enhance electron conductivity, mass transfer, and catalytic activity.
2.1.2. Methane
Methane is a clean-burning fuel with a high energy density, making it an attractive product from the electrocatalytic reduction of carbon dioxide, which offers a promising solution for storing renewable energy in the form of chemical bonds. Nevertheless, the HER often competes with methane formation, reducing the overall efficiency of the process. Moreover, designing efficient catalysts that selectively promote methane formation while suppressing the HER remains a substantial challenge.
The higher value of deeply reduced products has led to increased focus on electrocatalytic CO2RR to multi-electron-transfer products, with a promising approach being the design of multi-component tandem catalysts to decouple the complex pathway steps. For instance, the enhancement of CO2 electroreduction to hydrocarbons via tandem electrocatalysis using a Cu@BIF-144(Zn) (BIF = boron imidazolate framework) composite was reported [42]. The Cu nanoparticles (NPs) were incorporated into the boron imidazolate framework BIF-144(Zn) through in situ electrochemical transformation. The functional BH(im)3− ligands on the pore surfaces of BIF-144(Zn) modulate the microenvironment, enabling a synergistic effect between the host and the Cu NPs. Electrochemical results show that as the voltage increased, the FE of CO for the Cu@BIF-144(Zn) tandem catalyst decreased sharply, becoming negligible at higher potentials. Conversely, the FE of CH4 and C2H4 significantly increased with increasing voltage, achieving maximum values of 41.8% for CH4 at −1.6 V vs. RHE and 12.9% for C2H4 at −1.5 V vs. RHE, respectively. The tandem catalyst leverages the CO-rich microenvironment generated by the Zn catalytic center in BIF-144(Zn) to enhance the deep reduction of CO2 on the Cu NPs. The composite exhibits higher current density, lower Tafel slope, and smaller charge transfer resistance compared to BIF-144(Zn) alone, demonstrating the effectiveness of the tandem catalyst design in boosting CO2RR performance.
Another tandem catalyst example highlights the role of carbon nanotubes (CNTs) in accelerating electron transfer. A tandem catalyst composed of CNTs and R-Cu-TAl with high CO2 capture capability was reported for enhanced CO2-to-CH4 electrochemical conversion [43]. The Cu-based MOFs precursor, Cu-TAl, maintains its stable frameworks after electrochemical activation, ensuring high CO2 adsorption capacity of 134 cm3/g. The incorporation of CNTs accelerates electron transfer and facilitates proton-coupled electron transfer (PCET) reactions during CH4 formation. At −1.56 V vs. RHE, the tandem catalyst R-Cu-TAl-CNTs1 exhibited an FE of 54% for CH4, which was nearly twice that of R-Cu-TAl without CNTs (30%). Furthermore, as the voltage varied from −0.96 to −1.76 V vs. RHE, the FE of CH4/C2H4 ratio reaches 9.5, about 12 times higher than R-Cu-TAl, demonstrating the effectiveness of CNTs in promoting CH4 selectivity. In situ Fourier transform infrared spectroscopy reveals that *CHO and *OCH3 are key intermediates for CH4 formation, while *OCCOH is crucial for C2H4 generation.
Constructing metal and MOF heterogeneous interfaces enhances catalytic properties. A notable example is the work by Sun et al. [44], who reported a Cu@ZIF-8 nanowire (NW) catalyst with a highly exposed Cu and ZIF-8 interface. This unique core−shell structured catalyst exhibited remarkable stability and a high FE of 57.5% towards hydrocarbon production (CH4 and C2H4) at a potential of −0.7 V vs. RHE. The study revealed that the formation of the Cu and ZIF-8 interface optimized the adsorption of reaction intermediates, particularly stabilizing the formation of *CHO, thereby facilitating the efficient preference for hydrocarbons.
The interfacial interactions between MOF-derived catalysts and support materials can also boost CO2RR performance. Notably, Liu et al. [45] demonstrated a highly selective and stable Cu/C catalyst derived from the decomposition/redeposition of a Cu-based MOF for the electrochemical reduction of CO2 to CH4. Their study highlights the crucial role of the electronic metal–support interaction in enhancing the catalytic activity and selectivity. The Cu/C catalyst exhibited a remarkable FE of approximately 55% for CH4 production at −1.4 V vs. RHE for 12.5 h, outperforming many state-of-the-art Cu-based catalysts. DFT calculations elucidated the underlying mechanism, revealing that the interfacial sites between Cu NPs and amorphous carbon support stabilize key intermediates (e.g., COOH* and CHO) more effectively than Cu(111) surfaces, thereby facilitating the formation of CH4.
Structural innovation in MOFs, particularly dimensionality control, has further expanded catalytic possibilities. Building on the success of 2D MOFs for CO2-to-CO conversion, researchers have further explored their potential for multi-carbon product synthesis. An example is the design and synthesis of a triptycene-based two-dimensional vertically conductive MOF (2D-vc-MOF(Cu)) for enhancing the electrocatalytic performance of CO2 reduction to methane [46]. The vertically extended structure of 2D-vc-MOF(Cu) facilitated easy exfoliation and exposure of active sites, leading to a 100% increase in turnover number and a twofold increase in selectivity compared to traditional 2D-c-MOFs. Density functional theory calculations revealed that the vertically extended structure lowers energy barriers, improving reaction kinetics. The results indicated that as the potential shifted from −1.2 to −1.4 V vs. RHE, the FE(CH4) of 2D-vc-MOF(Cu) significantly increased from 20% to 65%, demonstrating a voltage-dependent enhancement in methane selectivity. However, further increasing the potential to −1.6 V vs. RHE led to a decrease in FE(CH4) to 33%, suggesting an optimal voltage range for maximizing methane production.
In addition, encapsulating active sites within carbon layers has proven effective in preventing aggregation and enhancing charge transfer. In order to enhance the performance of catalysts, the in situ carbon-encapsulated Cu/CeO2@C catalyst, reported by Zhang et al. [47], introduced a carbon-encapsulated copper-doped cerium oxide composite (Cu/CeO2@C) derived from MOFs for boosting CO2-to-CH4 electro-conversion (Figure 2). This innovative catalyst achieved a remarkable methane FE of 80.3% and a high partial current density of 138.6 mA/cm2 at −1.5 V vs. RHE. The superior performance of Cu/CeO2@C was attributed to the synergistic effect between the Cu/CeO2 active component and the in situ-formed carbon layer, which not only prevented the agglomeration of metal NPs but also provided robust channels to facilitate charge transfer. Additionally, the carbon encapsulation strengthened the adsorption of CO2 and reaction intermediates on the active sites, ultimately accelerating the reaction kinetics and improving the selectivity towards methane production.
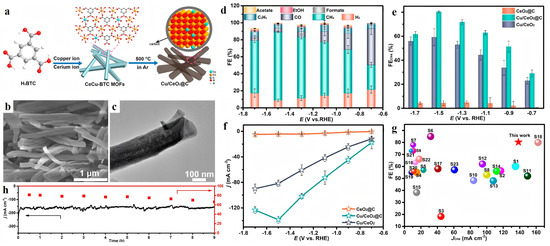
Figure 2.
(a) Schematic illustration for the synthesis of Cu/CeO2@C. (b) SEM micrograph of Cu/CeO2@C. (c) TEM images of Cu/CeO2@C. CO2RR performance for different samples in the flow cell. (d) FEs of all the products for Cu/CeO2@C at varied applied potentials. The comparison of (e) CH4 selectivity and (f) CH4 partial current density of different samples. (g) Comparison of CH4 selectivity and partial current density of Cu/CeO2@C with those of reported electrocatalysts. (h) Long-term stability test of Cu/CeO2@C at −1.5 V vs. RHE for 9 h. Reproduced with permission from ref. [47]. Copyright American Chemical Society, 2023.
To systematically investigate the ligand field effect, three isostructural metal–azolate frameworks (MAFs), namely, Cu-BTP, Cu-BTTri, and Cu-BTT, using pyrazolate-, triazolate-, and tetrazolate-based ligands, were synthesized respectively to tailor the ligand fields of MAFs for achieving highly selective electroreduction of CO2 to hydrocarbons at industrial current densities [48]. Their results revealed that the pyrazolate-based Cu-BTP exhibited remarkable electrocatalytic performance, achieving a current density of 1.25 A/cm2 with an FE of 82% for hydrocarbons (CH4, 60%; C2H4, 22%) in a flow cell. Notably, Cu-BTP demonstrated excellent stability with no obvious degradation over 60 h of continuous operation.
The choice of solvent plays a critical role in the synthesis of monometallic copper-based MOFs and can significantly affect their structure and catalytic performance. Lu et al. reported the synthesis of Cu(I)-5-mercapto-1-methyltetrazole framework (Cu-MMT) nanostructures with different facets through solvent modulation and revealed that the different CO2RR selectivities of these MAF nanostructures arise from their different exposed facets [49]. The use of water as the solvent led to the formation of Cu-MMT nanoribbons with dominant (100) facets, while the use of isopropanol produced Cu-MMT intercrossing nanosheets with (001) facets. These nanostructures exhibited distinct CO2RR selectivities, emphasizing the importance of solvent choice in the synthesis of MOF catalysts. Specifically, Cu-MMT nanoribbons dominantly exposing (100) facets, synthesized using water as the solvent, exhibited a high FE of 55.22% for methane production, indicative of a preference for C1 products. In contrast, Cu-MMT intercrossed nanosheets exposing (001) facets, obtained by using isopropanol, demonstrated a remarkable FE of 73.75% for C2+ products, especially ethylene, with a C2+/C1 product ratio of 3.93.
In addition to structural and solvent-based strategies, doping and alloying provide another avenue to fine-tune catalytic properties. For instance, the incorporation of Ce, which has a large ionic radius, into the Cu lattice, significantly enhanced the electrocatalytic performance and selectivity towards CH4 production, as demonstrated by Chen et al. in their work on atomic cerium-doped CuOx catalysts [50]. Atomic Ce doping induced lattice stretching strain and modulated the local electron densities of surrounding Cu atoms, enhancing the generation of key intermediate *CO and lowering the energy barrier for the conversion of *CO to *CHO. As a result, within a broad potential window ranging from −1.4 to −1.75 V vs. RHE, the FE of CH4 remained above 62%, peaking at 67.4% with a partial current density of 293 mA/cm2 at −1.6 V vs. RHE.
Apart from Ce, atomically doping 3D transition metals such as Co onto Cu can also steer the CO2RR pathway. Sun et al. [51] reported a useful strategy for fabricating atomically dispersed Co-Cu alloy through the electrochemical reconstruction of a trace-Co-doped Cu MOF, which exhibited remarkable CO2RR activity and CH4 selectivity. The Co-Cu alloy catalyst achieved an FE of 60 ± 1% for methane with a corresponding partial current density of 303 ± 5 mA/cm2, outperforming many previously reported Cu-based catalysts. Operando X-ray absorption spectroscopy and attenuated-total-reflection surface-enhanced infrared spectroscopy unveiled that the dispersed Co atoms favored CO protonation via enhancing surface water activation and suppressed C-C coupling by reducing CO coverage, thereby leading to high CH4 selectivity.
The valence state of copper ions has also been identified as a critical factor in CO2RR performance. For example, a novel c-MOF, copper-pyromellitic dianhydride-2-methylbenzimidazole (Cu-PD-2-MBI) was developed by incorporation of electron-withdrawing 2-methylbenzimidazole (2-MBI) into the copper-pyromellitic dianhydride (Cu-PD) interlayer for efficient CO2 reduction to methane [52]. Specifically, the methane FE of Cu-PD-2-MBI reached 73.7% at −1.3 V vs. RHE, accompanied by a partial current density of −428.3 mA/cm2. As the potential shifted from −1.1 to −1.3 V vs. RHE, the FE(CH4) gradually increased, while the FE(H2) decreased, indicating that Cu-PD-2-MBI effectively suppressed the HER at more negative potentials. Conversely, at potentials more negative than −1.3 V vs. RHE, the FE(CH4) decreased due to increased hydrogen liberation, highlighting the optimal voltage range for methane production.
Precise control of electronic states through ligand functionalization enables tailored product selectivity. A strategy to modulate the electronic states of Cu incorporated in MOFs was reported for controllable CH4/C2H4 selectivity in CO2 electroreduction [53]. By functionalizing UiO-66 MOFs with different ligands and metal nodes, the electronic states of Cu sites are tuned, leading to varied product selectivity. Specifically, Cu/UiO-66-H(Ce) achieves a high FE of 58% for methane, while Cu/UiO-66-F(Ce) favors C2H4 production with an FE of 44%. In situ Raman and X-ray absorption spectroscopy revealed that the selectivity switch is attributed to the modulation of Cu’s surface electronic structure by ligand functionalization, which alters the adsorption and activation of CO2RR intermediates.
2.1.3. Formic Acid/Formates
Formic acid and its salts (formates) are versatile chemicals with applications in various industries such as agriculture and leather tanning and as hydrogen storage materials. The electrocatalytic reduction of carbon dioxide to formic acid provides a sustainable route for its production.
Zou et al. introduced a novel BiCu bimetallic organic framework composite catalyst, SU-101-Cu@2.5C, for efficient electrochemical CO2 reduction to formate [54]. The catalyst was synthesized via in situ modification with multi-walled carbon CNTs (MWCNTs) at room temperature, utilizing ellagic acid as the linker. SU-101-Cu@2.5C exhibited remarkable performance, with a small onset potential of −0.46 V vs. RHE, partial current densities exceeding 100 mA/cm2, and a significantly improved FE close to 100% for formate at −0.86 V vs. RHE, as the voltage increased from −0.76 to −0.96 V vs. RHE. Notably, the FE remained above 80% within a wide potential window of −0.86 to −1.06 V vs. RHE, indicating a robust selectivity for formate production over a range of voltages. Density functional theory calculations revealed that Bi served as the active center for CO2 reduction, while the introduction of Cu optimized the overall electronic structure, enhancing catalytic efficiency. The incorporation of MWCNTs significantly facilitated rapid electron transfer, contributing to the excellent electrocatalytic performance. After 30 h of continuous catalysis, SU-101-Cu@2.5C maintained good efficiency and stability, demonstrating its potential for large-scale applications (Figure 3).
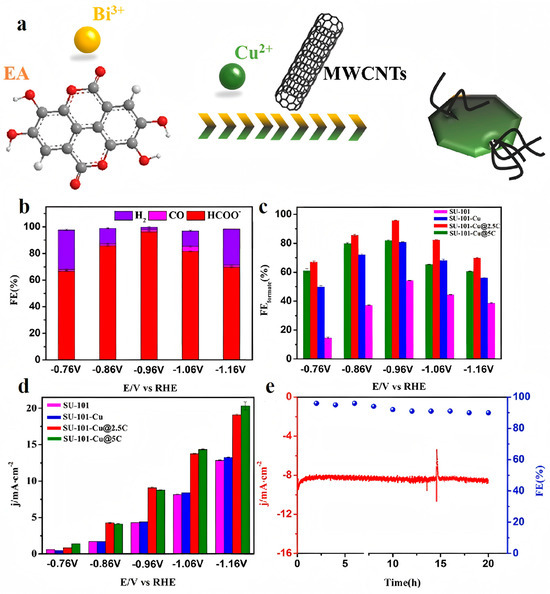
Figure 3.
(a) Schematic diagram of the synthesis process of SU-101-Cu@xC. (b) FEs of formate, CO, and H2 for SU-101-Cu@2.5C. (c) FEformate and (d) current density at different applied potentials for SU-101, SU-101-Cu, and SU-101-Cu@xC. (e) Long-term durability of SU-101-Cu@2.5C at −0.96 V vs. RHE for 20 h. Reproduced with permission from ref. [54]. Copyright Elsevier B.V., 2023.
Similarly, Huang et al. reported an MIL-68(In)/CuO heterostructure catalyst that exhibited remarkable CO2RR performance towards formate production [55]. By introducing enriched microcrystalline CuO(111) onto the surface of MIL-68(In), the catalyst achieved a high FE of 89.7% for formate at a low potential of −0.7 V vs. RHE in a flow cell. This exceptional performance was attributed to the formation of In-Cu dual catalytic active sites, which facilitated the formation of the key intermediate *HCOO− for 180 h at 2.7 V vs. RHE in a membrane electrode assembly (MEA) cell. Electrochemical investigations, both ex situ and in situ, indicated that the incorporation of CuO enhances the generation rate of the *HCOO− intermediate during carbon dioxide reduction and suppresses the competing HER.
In addition to compositional modifications, the structural design of bimetallic copper-based MOFs also plays a crucial role in determining their catalytic performance. Liu et al. demonstrated a controlled one-step synthesis of Cu@Bi1/2 catalyst through a solvothermal method, achieving a high FE of 89.2% for formic acid production during CO2RR [56]. The one-step synthesis of Cu@Bi1/2 resulted in a more uniform distribution of MOFs on the copper foam, providing more active sites compared to the step-by-step synthesis of Cu@Bi. The improved catalytic performance was attributed to the optimized structure and enhanced CO2 adsorption capacity of Cu@Bi1/2.
Addressing the persistent challenge of HER competition, Lee et al. drew inspiration from enzymatic systems to develop PPF-100, a 2D porphyrinic MOF with saddle-distorted ligands and Cu paddle-wheel nodes, which aimed to mimic the active centers and tunable second coordination spheres of natural enzymes [57]. This work successfully imparted hydrophobicity and remarkable water/chemical stability to PPF-100, leading to the preparation of the PPF-100 nanozyme. Notably, the nanozyme exhibited complete suppression of HER, achieving high FEs for CO and HCOOH production. The study thus addresses the critical issue of undesirable competing hydrogen evolution, enhancing the selectivity of CO2 reduction through an innovative material design that mimics natural enzymatic functions.
2.2. Overview of CO2RR C2+ Products
The importance of C2+ products, such as ethanol, ethylene, and other higher carbon chain compounds, derived from carbon dioxide reduction lies in their versatile applications and higher energy densities compared to C1 products like methane. Ethanol, for instance, is a widely used biofuel that can be blended with gasoline, reducing dependence on fossil fuels and lowering greenhouse gas emissions. Ethylene, on the other hand, is a fundamental building block in the petrochemical industry, used to produce a range of plastics, fibers, and other chemicals. However, the production of C2+ products from carbon dioxide reduction faces several difficulties and challenges. Firstly, the formation of C2+ products typically requires more complex catalytic pathways involving multiple electron and proton transfers, which makes the process kinetically slower and thermodynamically less favorable than the formation of C1 products. This necessitates the development of highly active and selective catalysts that can efficiently promote the coupling of carbon dioxide molecules to form higher carbon chains. Secondly, the selectivity towards specific C2+ products is often low, with a mixture of different carbon chain lengths and functional groups being produced. This complicates the downstream separation and purification processes, increasing the overall cost and complexity of the production process. Lastly, the stability and durability of catalysts for C2+ production remain a significant challenge. The harsh electrochemical environment and the reactive nature of the intermediates involved can lead to catalyst deactivation and loss of activity over time. Therefore, ongoing research is needed to develop more robust and stable catalysts that can withstand the demanding conditions of carbon dioxide reduction to C2+ products. To overcome the aforementioned challenges, extensive research has been conducted for C2+ products, such as ethylene and ethanol, leading to significant progress.
2.2.1. Ethylene
He et al. reported a highly selective CO2RR to C2H4 using a dual-site Cu(II) porphyrin framework coupled with Cu2O NPs via a synergetic-tandem strategy [58]. This innovative catalyst, denoted as CuPOF-Bpy/Cu2O@CNT, exhibited remarkable performance with the FE of C2H4 increased dramatically from −1.0 to −1.4 V vs. RHE, reaching a peak FE of 71.0% at −1.1 V vs. RHE. Notably, the FE of C2H4 exceeded 40% across the entire potential range (Figure 4). The key to this outstanding performance lies in the synergistic effect between the dual active sites (CuPor and CuBpy) in the Cu(II) porphyrin framework and the Cu2O NPs. The CuPor and CuBpy sites facilitated the reduction of CO2 to *CO intermediates, which were subsequently transferred to the neighboring Cu2O sites for C-C coupling to form C2H4. This tandem mechanism significantly lowered the energy barrier for C-C coupling, thus enhancing the selectivity and productivity of C2H4. Furthermore, the incorporation of Cu2O NPs on the carbon CNT scaffold improved the electronic conductivity and stability of the catalyst, contributing to its long-term durability during CO2RR.
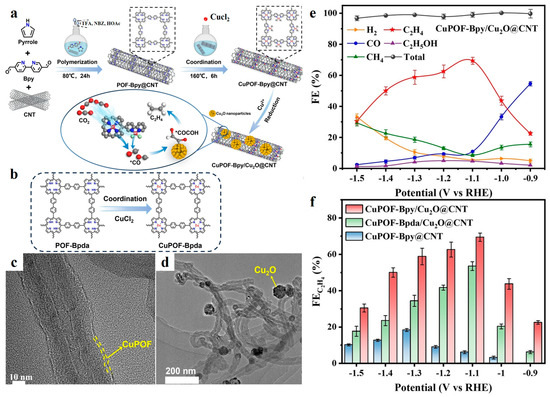
Figure 4.
(a) Schematic representation of the preparation of CuPOF-Bpy/Cu2O@CNT and the pathway for locally producing high concentration of *CO to facilitate the C2H4 production. (b) Molecule structure of CuPOF-Bpda. (c) HR-TEM image of CuPOF-Bpy@CNT. (d) TEM image of CuPOF-Bpy/Cu2O@CNT. (e) FE of CuPOF-Bpy/Cu2O@CNT. (f) FEC2H4 of CuPOF-Bpy/Cu2O@CNT. Reproduced with permission from ref. [58]. Copyright Wiley-VCH GmbH, 2024.
Moreover, oxide-derived copper has garnered considerable interest due to its remarkable selectivity towards C2+ products in CO2RR. To delve deeper into the impact of metal dopants, there is a pressing need for CuOx precursors incorporating novel dopants. Jang et al. [59] demonstrated that aluminum doping induced the reconstruction of CuO into nanoflake structures during the CO2RR process. This reconstruction significantly improved the hydrophobicity and electrochemically active surface area (ECSA) of the catalyst, leading to remarkable enhancements in both activity and selectivity towards C2+ products. Specifically, the CuO_Al catalyst exhibited a high FE of 68.4% and a large partial current density of 478.7 mA/cm2 towards C2+ products at −1.08 V vs. RHE in a neutral 1 M KHCO3 electrolyte. These outstanding performances were attributed to the increased local CO2 concentration facilitated by the high hydrophobicity and the abundant active sites provided by the high ECSA. Furthermore, the presence of partially oxidized Cu species (Cuδ+) in the reconstructed CuO_Al catalyst was found to promote the C-C coupling process, contributing to the enhanced selectivity towards C2+ products. The stability of the CuO_Al catalyst was also confirmed through long-term electrolysis experiments, showing no decay in FE for ethylene over 5 h.
A surface modification strategy for Cu2O octahedrons using an MOF of CuBTC was presented to enhance the efficiency and stability of CO2 electroreduction to C2+ chemicals [60]. The Cu2O@CuBTC heterostructure was synthesized via an in situ transformation method, where CuBTC acts as a protective layer to maintain the active Cu+ species under reductive conditions. The CuBTC shell prevented the reduction of Cu+ to Cu0, thereby maintaining the active sites for C-C coupling. Additionally, the CuBTC shell enhanced the hydrophobicity and ECSA of the catalyst, contributing to its high performance and stability.
Similarly, the electrocatalytic performance of 2D MOFs can be further tuned through post-synthetic structural modifications. As shown Zheng et al., the electrocatalytic selectivity can be inverted by bending the local structure of 2D coordination polymers [61]. Post-synthetic modification was utilized to introduce an amino group onto the triazolate ligand in Cu(I)-based coordination networks, transforming the shape of the two-dimensional layer from planar to wavy. This structural modulation inversed the electrocatalytic selectivity from the HER (selectivity 80%) to CO2RR (selectivity 76%), with a significant increase in C2H4 selectivity up to 52%. Computational simulations revealed that the wavy structure facilitated attractive hydrogen-bonding interactions between the amino groups and the key reaction intermediates of CO2RR, lowering the energy barrier for ethylene formation. Concurrently, steric hindrance between the amino groups and the HER intermediates inhibited the competing HER process.
The recent research by Yan et al. [62] has demonstrated the remarkable potential of combining a single-site MOF with copper foil to create a tandem catalyst for highly selective CO2RR to C2H4. The Cu-MOF-CF (CF = Cu foil) catalyst, comprising a porous Cu-MOF anchored on Cu foil, exhibited enhanced electrochemical performance due to several key factors. Firstly, the single-site Cu-MOF supplied abundant CO intermediates to the Cu foil surface, facilitating the carbon–carbon coupling process and thus boosting the selectivity towards C2H4. Notably, the Cu-MOF-CF catalyst achieved an FE of 48.6% for C2H4 production at −1.11 V vs. RHE, significantly surpassing that of bare Cu foil (22.4% at −1.16 V vs. RHE). Furthermore, the partial current density of C2H4 for Cu-MOF-CF was over four times higher than that of Cu foil. This exceptional performance is attributed to the improved surface microenvironment of the Cu foil, enriched active sites, and the enlarged active surface area contributed by the porous Cu-MOF. The in situ hydrothermal synthesis method employed ensured close cooperation between the Cu-MOF and Cu foil, further enhancing the electrocatalytic activity and stability. Additionally, DFT calculations revealed that the Cu(200) facets of Cu foil, favored for C2H4 formation, were enriched on the Cu-MOF-CF surface, while the Cu(111) facets, conducive to methane production, were almost completely suppressed.
Notably, well-defined copper-based tandem catalysts with an optimal Cu coordination environment are highly sought after for the electrochemical CO2RR, as their unique geometric and electronic properties are instrumental in elucidating structure–property relationships. For instance, a tandem catalyst, Ag@BIF-104NSs(Cu), where Ag NPs are anchored in atomic proximity to Cu sites via coordination with abundant carboxylic functional ligands on the ultrathin BIF nanosheets was synthesized [18]. This unique design exhibited remarkable CO2 electroreduction performance, achieving an FE of 21.43% for ethylene production, which is almost sixfold higher than that of the pristine BIF-104NSs(Cu) catalyst (3.82%). The improved performance was attributed to the synergistic effect between Ag and Cu sites, where Ag efficiently reduced CO2 to *CO intermediates, which subsequently migrated to Cu sites for C-C coupling and further formation of C2H4. DFT calculations further confirmed that the Ag sites lowered the free energy of *COOH and *CO intermediates, facilitating the generation of abundant *CO near the Cu sites, thereby promoting the C-C coupling step.
The coordination mode of Cu sites has been identified as a critical factor in CO2RR selectivity. For instance, isomeric Cu(I) triazolate frameworks (MAF-2Fa and MAF-2Fb) with similar thermal/chemical stabilities and different coordination modes have been researched [63]. MAF-2Fa, featuring a monotypic planar dinuclear Cu(I) coordination mode, achieved remarkably high selectivity for C2H4 (53%) and C2+ products (70%), maintaining stable performance over a wide potential window (−1.1 to −1.5 V vs. RHE), which was attributed to the favorable coordination environment of the dinuclear Cu(I) sites, facilitating C-C coupling and suppressed competing HER. In contrast, MAF-2Fb, with multiple Cu(I) coordination modes, displayed lower C2+/C1 product selectivity and decomposed during CO2RR, leading to deteriorated performance (Figure 5).
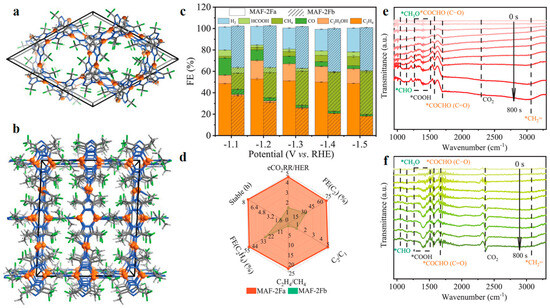
Figure 5.
(a) Crystal structures of MAF-2Fa. (b) Crystal structures of MAF-2Fb. (c) FEs of MAF-2Fa and MAF-2Fb. (d) Comparison of typical performances at −1.2 V vs. RHE. (e) ATR-FTIR spectra of MAF-2Fa. (f) ATR-FTIR spectra of MAF-2Fb. Reproduced with permission from ref. [63]. Copyright Wiley-VCH GmbH, 2024.
The core−shell structure exhibits significant advantages in the CO2RR process by providing enhanced stability, improved electron transfer efficiency, and increased selectivity towards desirable products through synergistic effects between the core and shell materials. For example, a core−shell structured catalyst, Cu2O@Cu-TCPP(M) (M = Co, Fe, Ni, TCPP = tetrakis(4-carboxyphenyl)porphyrin), was introduced for boosting ethylene production in neutral electrolyte during CO2 electroreduction [64]. The Cu2O nanocubes were encapsulated with a thin layer of metalloporphyrin frameworks, which act as a CO generator, protective sheath, and porous platform to enrich reactive species. It was observed that as the voltage decreased from −0.2 to −1.6 V vs. RHE, the FEs of C2H4 and C2+ products progressively increased, reaching maxima of 54 ± 2% and 69 ± 4% at 500 mA/cm2 in 1 M KCl, respectively. Comprehensive characterizations revealed that the high CO2 adsorption capacity, high CO concentration yielded by metalloporphyrins, high local pH, and highly dispersed Cu crystallites exposed (200) facets contribute to the enhanced C-C coupling process. In situ infrared spectroscopy and Raman measurements confirmed the role of tandem mechanism and local microenvironment in promoting C2+ production. The core−shell structure also preserved the Cu2O crystalline state and valence, resulting in prolonged stability (Figure 6).
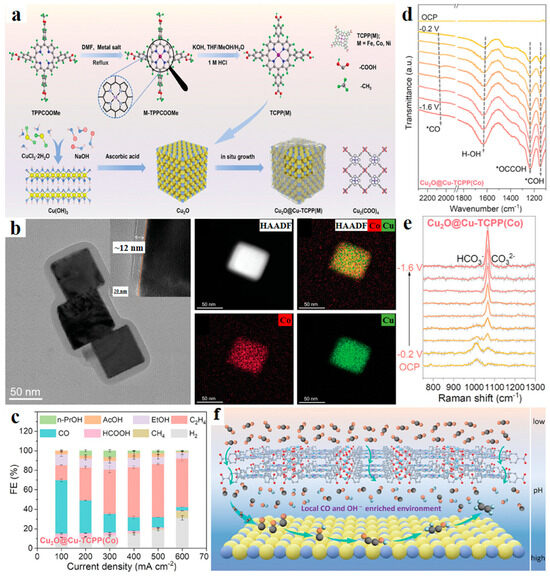
Figure 6.
(a) Synthesis scheme for Cu2O@Cu-TCPP(M) (M = Fe, Co, and Ni). (b) HR-TEM images and TEM-EDX elemental mapping images of Cu2O@Cu-TCPP (Co). (c) FE of different reduction products under various current densities for Cu2O@Cu-TCPP(Co). (d) In situ ATR-SEIRAS spectra taken on Cu2O@Cu-TCPP(Co). (e) Operando Raman spectra recorded for measuring local pH on Cu2O@Cu-TCPP(Co). (f) Schematic illustration of the Cu-TCPP(M) (M = Co, Fe) overlayer on Cu2O to boost local CO and OH− concentrations at the interface. Reproduced with permission from ref. [64]. Copyright Wiley-VCH GmbH, 2024.
Moreover, a core−shell structured HKUST-1@Cu2O nanocomposite decorated with polytetrafluoroethylene (PTFE) was synthesized to achieve highly efficient and selective CO2RR towards ethylene [65]. The HKUST-1 MOF provided a high specific surface area and abundant active sites, contributing to enhanced CO2 adsorption and catalytic activity. Notably, the hydrophobic interface constructed by PTFE effectively inhibited the occurrence of competing HER, leading to a significant improvement in the FE for hydrocarbon fuels, especially C2H4. Specifically, the HKUST-1@Cu2O/PTFE-1 catalyst exhibited a remarkable FE of 67.41% for hydrocarbon fuels and 46.08% for ethylene at −1.0 V vs. RHE, surpassing many previously reported Cu-based catalysts. Furthermore, the stability of the catalyst was verified by long-term durability tests, showing negligible current decay and maintained ethylene FE over 12 h.
It was reported that there was a remarkable enhancement in the selectivity and stability of CO2RR to C2H4 using Cu nanocubes coated with a nitrogen-doped carbon shell (Cu-cube/CN) [66]. This catalyst, derived in situ from a Cu-based MOF through electrochemical reduction, exhibited a high FE of 49.6% for ethylene production at −1.15 V vs. RHE, significantly surpassing that of the pristine Cu foil electrode. Specially, at a voltage of −0.7 V vs. RHE, the main products from the MOF/Cu foil electrode are CO and H2. As the voltage increases, the selectivity for C2H4 gradually rises, reaching a peak FE of 49.6% at −1.15 V vs. RHE. Beyond this optimal voltage, the selectivity for C2H4 begins to decline, indicating that a moderate voltage is crucial for promoting the dimerization of *CO intermediates and enhancing ethylene generation. Utilizing in situ ATR-SEIRAS along with DFT calculations, the CN coating, owing to its electron-donating capacity, strengthens the adsorption of *CO. This led to an increase in *CO coverage and a reduction in the reaction free energy for *CO dimerization and subsequently promoted C-C coupling and the generation of ethylene. Moreover, with a nitrogen-doped carbon (CN) coating, a CuxOy/CN electrocatalyst derived from a nitrogen-containing Cu-based MOF was presented for enhanced CO2 reduction to C2H4 [67]. The CuxOy/CN catalyst exhibited excellent ethylene selectivity with an FE of 44% at 500 mA/cm2 and a partial current density of 220 mA/cm2, significantly outperforming commercial CuO catalysts. The CN coating stabilized Cu+ species and *CO intermediates, promoting C-C coupling and enhancing ethylene selectivity and stability. Operando Raman spectroscopy confirmed the stabilizing effect of the CN coating on Cu+ species. In situ ATR-SEIRAS and DFT calculations revealed that the CN coating increases *CO adsorption energy and coverage, facilitating CO-CO coupling. The CuxOy/CN catalyst also demonstrated robust stability, running for at least 20 h without significant performance degradation.
The confinement effect plays a pivotal role in CO2RR processes by localizing reactants and intermediates within the pores of Cu-based MOFs, thereby promoting efficient mass transfer and enhancing the interaction between the catalyst and the reactants, leading to improved catalytic activity and selectivity for the desired products. As demonstrated by Heng et al. [68], confinement effects enhanced the loading and dispersion of active sites. In the research, the nanocage confinement of MOFs effectively prevented the aggregation of copper dual-atom sites during high-temperature carbonization, leading to a record copper loading of 21.2 wt% in nitrogen-doped porous carbons (Figure 7). Increasing the voltage from −1.6 to −1.4 V vs. RHE led to a gradual enhancement in the partial current density for C2H4 production. This high loading, in turn, facilitated the C-C coupling process and promoted the formation of C2H4 with an FE of 52% and a current density of 180 mA/cm2 at −1.4 V vs. RHE in neutral electrolyte. Similarly, the confinement of CuI sites within a partially electro-reduced 2D conductive Cu-MOF film has been demonstrated to boost the CO2RR performance towards C2+ products [69]. The FE of C2+ products first increased and then decreased within the potential range from −0.77 to −1.77 V vs. RHE. At −1.17 V vs. RHE, the maximum FE of 72.6% (C2H4: 42.3%, C2H5OH: 30.3%) with a high current density of over 300 mA/cm2 was achieved, demonstrating the optimal voltage for C2+ production. This enhanced performance was attributed to the stable CuI sites promoting C-C coupling, leading to enhanced C2+ product formation. The increase in voltage initially promoted the *OCCOH coupling, favoring C2+ product formation, while further increasing the voltage shifted the selectivity towards H2 production, indicating that the voltage plays a crucial role in tuning the reduction pathways and enhancing the selectivity of desired products.
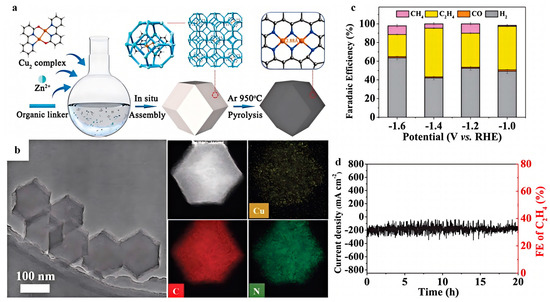
Figure 7.
(a) Schematic illustration of the synthesis of NPCMAF-4-Cu2. (b) STEM and elemental mapping images of NPCMAF-4-Cu2-21 showing the homogenous distribution of all four elements of Cu, C, and N. (c) FEs of different reduced products by NPCMAF-4-Cu2-21 as catalyst at different potentials. (d) Durability test of NPCMAF-4-Cu2-21 at −1.4 V vs. RHE. Reproduced with permission from ref. [68]. Copyright Wiley-VCH GmbH, 2024.
The reconstruction of many MOF materials is an inevitable process under reduction conditions, underscoring the importance of leveraging this restructuring for the rational design of high-performance catalysts tailored for the production of C2+ products. The a-HKUST-1 catalyst was prepared via a solvent process and exhibited a pill-like morphology. During CO2RR, the a-HKUST-1 was electrochemically reconstructed into a Cu catalyst (aHD-Cu) with low-coordinated sites. The aHD-Cu catalyst demonstrated high CO2-to-C2H4 conversion performance, achieving an FE of 56% for C2H4 and 80% for C2+ products at −150 mA/cm2 in alkaline electrolyte. In situ Raman spectroscopy revealed that the aHD-Cu surface had a higher coverage of *CO intermediates, facilitating the C-C coupling step and hydrocarbon production. The aHD-Cu catalyst also showed long-term stability and a higher ECSA compared to commercial Cu NPs [70].
Morphology and grain boundary engineering has also been found to be effective in improving CO2RR performance [71,72]. For instance, Kim et al. [73] reported the synthesis of grain boundary-rich copper nanocatalysts derived from MOF NPs. These catalysts exhibited remarkable performance in terms of both activity and selectivity towards C2+ products. Specifically, the copper oxide nanocrystals catalyst prepared by calcining Cu-MOF NPs at an optimal temperature (n-MDC-250) demonstrated a high FE of 63.1% towards ethylene and 80.9% towards total C2+ products at −1.01 V vs. RHE in neutral electrolytes. As the applied voltage increased, the FE for C2H4 and C2+ products gradually decreased, while the FE for H2 production increased significantly. Specifically, at higher voltages, the partial current density towards C2+ products was limited due to mass transport issues, highlighting the optimal voltage range for maximizing C2H4 and C2+ selectivity. Moreover, the catalyst showed prolonged stability for up to 10 h and achieved a high partial current density of −255 mA/cm2 towards C2+ products in an alkaline flow cell system. The enhanced performance was attributed to the high density of grain boundaries in the copper oxide NPs, which facilitated the adsorption of CO intermediates and promoted C-C coupling, leading to improved ethylene and C2+ product selectivity.
Wang et al. demonstrated that the synthesis of CuTrz (HTrz = 1H,1,2,4-triazole) MOF nanostructures with different sizes could be achieved by modulating the solvent conditions, leading to variations in their catalytic performance towards CO2RR [74]. Their findings revealed that smaller CuTrz nanostructures, enriched with grain boundaries and defects, exhibited superior CO2RR performance compared to larger counterparts. As demonstrated in the study, the FE of C2H4 and C2+ products significantly varies with the voltage. At lower voltages (e.g., −0.9 V vs. RHE), the CuTrz nanostructures, especially CuTrz-109nm, exhibit enhanced FE towards liquid alcohols such as ethanol (C2H5OH) and n-propanol (n-C3H7OH). As the voltage is increased to −1.15 V vs. RHE, CuTrz-109nm achieves the highest FE for C2H4 at 55.4% and for C2+ products at 81.8%. This enhanced performance was attributed to the increased surface area, improved CO2 adsorption, and facilitated C-C coupling process enabled by the small size and abundant defects.
An innovative pyrazolate-stabilized asymmetric Ni-Cu hybrid site MOF catalyst, designated as Cu1Ni-BDP(BDP = 1,4-benzenedipyrazolate), demonstrated remarkable enhancements in CO2RR performance [75]. This catalyst exhibited a high FE of 52.7% for ethylene with a total current density of 0.53 A/cm2 at −1.3 V vs. RHE in 1.0 M KOH aqueous solution, surpassing the majority of prevailing Cu-based catalysts. The exceptional stability of Cu1Ni-BDP was highlighted by its minimal reduction in FE of only 4.5% for ethylene after 25 h of continuous CO2 electrolysis at a constant current density of 500 mA/cm2. The underlying mechanism was elucidated through operando infrared spectroscopy and DFT calculations, revealing that the asymmetric Ni/Cu sites in Cu1Ni-BDP effectively lowered the energy barrier for the rate-limiting step in the transition from CO2 to C2H4, thereby enhancing C-C coupling and promoting ethylene selectivity.
Deng et al. reported a strategy to synthesize a Cu(I)-BTC framework enriched with free carboxyl groups via a novel “reduction-cleavage-recrystallization” method [76]. This Cu(I)-BTC catalyst exhibited superior catalytic activity and ethylene selectivity compared to its Cu(II)-BTC counterpart. Specifically, at a voltage of −1.6 V vs. RHE, the optimized Cu(I)-BTC catalyst (HC-HKUST-3) exhibits a remarkable FE(C2H4) of 57%, outperforming the Cu(II)-BTC catalyst (HKUST-1) with an FE of only 22%. As the voltage is varied, the FE for C2H4 consistently surpasses that of methane, with a peak FE(C2H4) of 49% and a minimum FE(CH4) of 6.5% observed within the potential window of −1.4 to −1.8 V vs. RHE for HC-HKUST-1. This trend underscores the voltage-dependent modulation of product selectivity, where lower hydrogen evolution and enhanced C-C coupling are favored at optimal voltages, leading to improved C2H4 production. The stability of the optimized Cu(I)-BTC catalyst was also remarkable, maintaining nearly unchanged FE for ethylene about 38 h of continuous operation.
Addressing the challenge of acidic CO2RR environments, Yu et al. developed a Cu-btca (btca = benzotriazole-5-carboxylic acid) MOF catalyst acting as a stable catalyst for the CO2RR in an acidic environment [77]. The resultant Cu-btca MOF network achieves FEs of 51.2% and 81.9% for ethylene and multicarbon products, respectively, at a current density of 300 mA/cm2 in a strong acidic electrolyte. The porous network structure of the Cu-btca MOF enables efficient mass transport and limits the reduction of protons, thus suppressing the competing HER.
Modulating the spin density of active sites is an effective approach to improve the selectivity of CO2 electroreduction catalysts. For example, a novel strategy for enhancing CO2-to-C2H4 conversion by regulating the spin density of active sites using TEMPOL molecules in HKUST-1-derived electrocatalysts was presented [78]. TEMPOL molecules were adsorbed onto the Cu(II) sites of HKUST-1, inducing spin−spin interactions and forming asymmetric spin-ordered/disordered sites. The resultant TEMPOL@HKUST-1 catalyst exhibited a twofold increase in ethylene selectivity compared to pristine HKUST-1 in CO2 electroreduction. In situ ATR-SEIRAS spectra revealed that the asymmetric spin configuration significantly reduced the kinetic barrier for *CO intermediate dimerization. Furthermore, applying a magnetic field further enhanced the ethylene selectivity to over 50% by polarizing the electron spins.
Defect engineering plays a crucial role in enhancing the performance of CO2RR processes by optimizing the electronic structure, increasing active site density, and promoting efficient charge transfer, thereby improving the catalytic activity and selectivity towards valuable products. Bohan et al. constructed defect-engineered Cu-based MOFs with missing linkers (UC-Cu-BTEC, where BTEC = 1,2,4,5-benzenetetracarboxylic) through a facile solvothermal method followed by thermal annealing [79]. The FE for C2+ generation on UC-Cu-BTEC attained 77.2%, with the FE for C2H4 being 50.1% and the FE for C2H5OH being 27.1% and remaining over 70% across a broad potential range of 400 mV vs. RHE, markedly surpassing that of pristine MOFs. In a flow cell under neutral conditions, the C2+ partial current density measured −153.5 mA/cm2.
2.2.2. Ethanol
The incorporation of Pd into copper-based MOFs has been shown to stabilize key intermediates and promote subsequent PCET, enhancing the selectivity towards alcohol products. Xie et al. successfully synthesized Pd-Cu@CuPz2 (Pz = Pyrazole) catalysts with Cu-Pd dual sites bridged by Cu nodes of CuPz2 for the electroreduction of CO2 [80]. The work showcased a 1.4%PdÀCu@CuPz2 catalyst comprising CuOx and PdO dual nano-clusters embedded in a CuPz2 MOF, achieving an exceptional FE of 81.9% for C2+ and a remarkable FE of 47.5% for alcohol under optimal conditions. As the potential increases, the FE for C2+ products gradually decreases, likely due to limited CO2 mass transfer and enhanced HER competitiveness. For instance, in the flow-cell system with 1 M KOH electrolyte at −0.69 V vs. RHE, the FE for C2+ products remains over 50% but with a higher current density of 109.5 mA/cm2. Experimental and DFT calculations demonstrated that the enhanced interfacial electron transfer on the Cu-Pd dual sites facilitated the adsorption of *CO intermediate and CO-CO dimerization. The oxophilicity of Pd can protect the C-O bond, stabilizing the key intermediate *CH2CHO and promoting subsequent PCET, thus enhancing the selectivity towards ethanol and accelerating the reductive conversion of CO2 to C2+.
A bimetallic tandem catalytic system is an efficient approach for promoting CO2RR to ethanol, yet achieving high selectivity for ethanol remains challenging due to the insufficiency of biphasic boundaries in most bimetallic catalysts. Su et al. [81] reported the synthesis of CuAg bimetallic catalysts derived from an Ag-anchored Cu-based MOF (NH2-Cu-BDC, where BDC = 1,4-benzenedicarboxylic acid). These CuAgx@NC catalysts exhibited superior selectivity towards C2+ products, particularly ethanol, during CO2RR. Specifically, the CuAg5@NC catalyst exhibited a volcano-like trend, where the FE for ethanol production peaked at 51.8% at −1.0 V vs. RHE, while the total FE for C2+ products reached a maximum of 82.6% at −1.2 V vs. RHE. Conversely, the FE for byproduct H2 decreased as the potential became more negative, reaching a minimum of 12.1% at −1.2 V vs. RHE. The high performance of CuAg5@NC can be attributed to the abundant Cu-Ag biphasic boundaries, which facilitated the migration of CO generated at Ag sites to Cu sites, enhancing CO coverage and promoting C-C coupling. Moreover, in situ Raman spectroscopy and DFT calculations revealed that the introduction of Ag reduced the energy barrier for C-C coupling and increased the thermodynamic advantage of the ethanol generation pathway.
Moreover, an innovative approach in designing an MOF with heterodimetal dual sites, specifically CuSn-HAB (HAB = hexaiminobenzene), was introduced for the highly efficient electroreduction of CO2 to C2H5OH [82]. At a voltage of −0.57 V vs. RHE, the CuSn-HAB catalyst, featuring heterometallic Sn-Cu dual sites, exhibited an impressive FE of 56(2)% for ethanol production, accompanied by a high current density of 68 mA/cm2. Notably, as the voltage became more negative, the FE for ethanol gradually decreased, with a concurrent increase in the formation of formate, suggesting that the competition between ethanol and formate pathways is voltage-dependent. This voltage-driven selectivity modulation underscores the crucial role of applied potential in dictating the product distribution during CO2RR processes. In addition, no significant degradation was observed over a continuous 35 h operation at the specified current density. This remarkable performance can be attributed to the unique Sn-Cu heterodimetal dual sites within the MOF, which facilitate the asymmetric C-C coupling reaction, a key step in the ethanol formation pathway. The heterometallic dual sites were proved to be more thermodynamically favorable for the asymmetric C-C coupling between *CO and *OCH2, leading to the formation of the key intermediate CO-OCH2, which is favorable for yielding ethanol product.
By electrochemically reducing a 3D microporous Cu-based MOF, the catalyst achieved an FE of 82.5% for ethanol at −1.0 V vs. RHE, with an effective current density of 8.66 mA/cm2. The multi-site synergism of Cu LNCCs (low-nuclearity cluster catalysts) enhanced the C-C coupling effect, leading to the high selectivity [83]. However, as the voltage deviated from this optimum value, the FE for ethanol decreased, accompanied by increases in the formation of byproducts such as formate and methanol. For instance, at a lower potential of −0.8 V vs. RHE, the FE for ethanol dropped to 7.2%, while the FE for formate surged to 38.9%. Density functional theory calculations and operando ATR-SEIRAS confirmed the reaction path and mechanism. The LNCCs originated from unstable metal coordination centers and were uniformly dispersed on the MOF support, benefiting from the MOF’s pore space limitation. The high selectivity for ethanol was attributed to Cu LNCCs rather than Cu SACs or open metal sites, indicating the crucial role of catalytic sites in the reaction pathway.
Another example is that Zhang et al. demonstrated a self-polarization triggered multiple polar unit strategy for electrochemical reduction of CO2 to ethanol with high selectivity [84]. The catalyst composed of Cu2O NPs encapsulated with bimetallic NiCu-based MOF nanorod arrays grown on Cu foam (Cu2O@MOF/CF) exhibited remarkable FEethanol of 44.3% with an energy efficiency of 27% at a low working potential. This outstanding performance was attributed to the intense internal electric field derived from the asymmetric electron distribution at the Cu2O-MOF interface, which facilitated the C-C coupling and stabilized the reaction intermediates. The doping of Ni into Cu2O not only modulated the electronic structure but also promoted the adsorption of *CO, thereby assisting in the formation of ethanol. Furthermore, the porous structure of the MOF provided abundant active sites and facilitated mass transport, contributing to the high catalytic activity and selectivity.
Asymmetric coordination environments in 2D MOFs have also emerged as a powerful strategy to enhance CO2RR performance. Another research study by Wang et al. [85] has shed light on the remarkable performance of asymmetric Cu-N1O3 sites in a novel 2D MOF, termed BIT-119, for electrocatalytic CO2RR. BIT-119, constructed via a bottom-up assembly approach, exhibited asymmetric N/O mixed coordination around the Cu centers, leading to a redistribution of the local electron structure. This structural feature profoundly impacted the adsorption strength of key intermediates during CO2RR. Specifically, the FE for C2H4 is ~33.0%, and the FE for C2H5OH is ~42.0%. The asymmetric Cu-N1O3 sites in BIT-119 were capable of coupling atop-type on Cu sites and bridge-type on Cu-N sites for adsorbed *C1 species, as evidenced by in situ spectroscopic analyses. This unique adsorption mode facilitated the PCET process and subsequent C-C coupling, resulting in the formation of C2 products.
2.2.3. Other C2+ Products
Lewis acid−base pairs enhance the catalytic performance during CO2RR by facilitating the targeted chemisorption and activation of CO2 molecules. The integration of Lewis acid−base pairs into MOFs represents another innovative strategy. Liu et al. [86] reported a significant advancement by employing an electron-beam irradiation strategy to rapidly synthesize a Cu-based MOF, Cu(PTC)(PTC = pyridine-2,4,6-tricarboxylic acid), with well-defined Lewis pairs (Cu-Npyridinic) (Figure 8). This material exhibited remarkable performance in CO2RR, achieving a total FE of 70.0% for C2+ at −0.88 V vs. RHE. Beyond this voltage, ethylene becomes the predominant C2+ product. In contrast, the Cu(BTC) catalyst displays a higher hydrogen evolution rate across the voltage range, leading to increased methane production and lower C2+ selectivity, with a maximum FE for C2+ of 41.4% at −0.88 V vs. RHE. The superior catalytic performance of Cu(PTC) can be attributed to its Lewis acid−base pairs, which facilitate the targeted chemisorption and activation of CO2 molecules, leading to enhanced C-C coupling and subsequent formation of high-value C2+ products such as ethylene and ethanol.
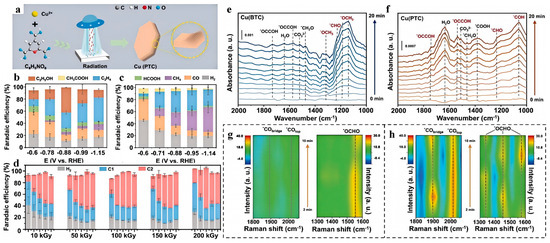
Figure 8.
(a) A schematic illustration of the synthesis process of MOFs. (b) FE of Cu(PTC). (c) FE of Cu(BTC). (d) The FE of Cu(PTC) under different irradiation doses. (e) In situ ATR-FTIR spectra of Cu(BTC). (f) In situ ATR-FTIR spectra of Cu(PTC). (g) In situ Raman spectra of Cu(BTC). (h) In situ Raman spectra of Cu(PTC). Reproduced with permission from ref. [86]. Copyright Wiley-VCH GmbH, 2024.
Liu et al. [87] reported a notable example by decorating Cu2O NPs on two-dimensional (2D) Cu-BDC MOFs, resulting in a heterostructured catalyst with abundant heterogeneous interfaces. The Cu2O@Cu-BDC catalyst exhibited exceptional performance, achieving a high FE of 72.1% for C2+ products in an H-type cell and 58.2% in a flow cell. The remarkable performance of Cu2O@Cu-BDC can be attributed to several factors. Firstly, the 2D Cu-MOFs served as an effective support to stabilize Cu+ sites, which are known to be highly active for CO2RR but prone to reduction under reaction conditions. Secondly, the heterogeneous interfaces formed between Cu2O and Cu-BDC optimized the adsorption of key intermediates (*CHO, *COH, and *CO), facilitating C-C coupling and thus promoting the formation of C2+ products. This was evidenced by the in situ ATR-FTIRS analysis, which revealed the stabilization of these intermediates on Cu2O@Cu-BDC during the CO2RR process. Furthermore, the catalyst demonstrated exceptional stability, maintaining high FE of around 70% for C2+ over a 6 h stability test in the H-type cell. This stability is likely due to the strong interaction between Cu2O NPs and Cu-BDC nanosheets, which prevents the aggregation and deactivation of active sites.
Zou et al. [88] developed nitrogen-doped carbon confined Cu-Ag bimetallic catalysts (Cu-Ag/NC) via an MOF-mediated synthesis method. The unique structure of Cu-Ag NPs encapsulated within a porous nitrogen-doped carbon octahedral shell exhibits remarkable activity and selectivity towards high-order products. As demonstrated in their work, the Cu-Ag/NC catalyst achieved a total FE of 55.3% for CH4, C2H4, C2H5OH, and CH3COOH at a relatively low overpotential of −1.2 V vs. RHE, accompanied by a high current density of 5.3 mA/cm2. This superior performance surpasses that of monometallic Cu/NC and non-confined Cu-Ag catalysts, underscoring the importance of the synergistic effect between the bimetallic active sites and the nitrogen-doped carbon support. The theoretical investigations further revealed that the Cu-Ag interface facilitates the migration of *CO intermediates, promoting C-C coupling through an interesting CHO-CO pathway with a significantly lower energy barrier. Moreover, the porous nitrogen-doped carbon shell enhanced the adsorption of CO2 and enriches the local concentration of intermediates, thereby boosting the mass transport and overall efficiency of the CO2RR process.
Further refining the coordination environment of Cu sites, Yang et al. recently demonstrated a novel approach to synthesize Cu-based MOFs with less-coordinated atomic copper dimers via a liquid-phase strategy [89]. The Cu-TDC (TDC = 2,5-thiophenedicarboxylic acid) MOF synthesized exhibited a high current density of 0.9 A/cm2 and an FE of 71% towards C2+ products, surpassing many previously reported copper-based catalysts. The presence of atomic copper dimers in Cu-TDC, characterized by a coordination number of 2.8 ± 0.1, facilitated the stable adsorption of two *CO intermediates on each site, promoting C-C coupling and subsequently leading to the formation of C2+ products such as ethylene and ethanol. Furthermore, in situ spectroscopic techniques and DFT calculations revealed that the less-coordinated copper dimers in Cu-TDC possessed a stronger binding energy for *CO compared to traditional copper monomers, thereby enhancing the C2+ product selectivity.
Ligand functionalization has proven effective in optimizing catalytic sites. Mao et al. synthesized an artificial enzyme MIL-53(Cu) with an optimal Cu-Cu distance for CO2 conversion by using a density functional theory method [90]. By substituting the ligands in MIL-53(Cu) with functional groups ranging from electron-donating NH2 to electron-withdrawing NO2, they could significantly alter the Cu-Cu distance and charge of Cu atoms, thereby modulating the adsorption strength of CO2. Notably, the COOH-ligand-decorated MIL-53(Cu) exhibited an optimal Cu-Cu distance that facilitated C-C coupling, leading to remarkable catalytic activity and selectivity for the reduction of CO2 to C2H5OH. The DFT calculations predicted a limiting potential of only 0.47 eV for this process, significantly lower than that of benchmark Cu catalysts. Experimentally, the synthesized COOH-ligand-decorated MIL-53(Cu) catalyst achieved an FE of 55.5% for C2+ products at −1.19 V vs. RHE.
Moreover, the CO2 reduction performance of Cu-based MOF catalysts in recent years is also summarized in Table 1.

Table 1.
Performance comparison table of Cu-based metal−organic framework electrocatalysts.
3. Conclusions and Perspectives
This paper presents a comprehensive review of recent advances in Cu-based MOF electrocatalysts for CO2RR. It highlights the potential of Cu-based MOFs and their derivatives due to their high surface area, tunable pore structures, and diverse compositions, which facilitate enhanced catalytic performance. It encompasses the latest research findings on Cu-based MOF electrocatalysts for producing C1 and C2+ products. Key strategies discussed include nanostructure manipulation, multi-component tandem catalysis, single-atom alloying, and ligand functionalization to optimize the binding energies of intermediate species and promote selective CO2RR pathways. This paper also delves into the use of MOF-derived materials and composites with other materials such as carbon nanotubes, graphene, and metal oxides to enhance conductivity, stability, and selectivity. The remarkable progress made in developing Cu-based MOF electrocatalysts, with several examples showcasing high FEs and product selectivities, is emphasized. However, challenges remain in achieving stable and scalable catalysts with high activity and selectivity towards specific C2+ products. This review underscores the importance of rational catalyst design through precise control of composition, structure, and surface properties to tackle these challenges and advance the field of CO2RR and provides valuable insights for future research directions in developing advanced Cu-based MOF electrocatalysts for practical applications in CO2 conversion technologies.
Developing more robust MOF structures that can withstand the harsh reaction conditions is essential for long-term operation. The density of active sites within Cu-MOFs plays a pivotal role in their catalytic performance. While Cu-MOFs offer a high surface area and tunable porosity, the actual number of active sites can be limited. Increasing the density of active sites through rational design or post-synthetic modifications is necessary to further boost their activity and selectivity.
Moreover, understanding the reaction mechanism and intermediates formed during the CO2RR on Cu-MOFs remains an ongoing challenge. Advanced in situ characterization techniques, such as operando X-ray absorption spectroscopy and infrared spectroscopy, are required to provide insights into the dynamic changes occurring on the catalyst surface during the reaction. Scalability is also a critical factor for the commercialization of Cu-MOFs. The synthesis of Cu-MOFs often involves complex and costly procedures, making it difficult to scale up production. Developing more scalable and cost-effective synthesis methods is essential for their widespread application.
Furthermore, the selectivity of Cu-MOFs towards specific products, such as hydrocarbons or alcohols, needs to be further improved. While some Cu-MOFs have demonstrated high selectivity for certain products, optimizing their structures and composition to target specific products remains a challenge. Looking ahead, the integration of computational modeling with experimental studies holds promise for the rational design of Cu-MOF-based catalysts. By understanding the structure−property relationships and predicting the catalytic performance of different MOF architectures, researchers can design more efficient and selective catalysts.
Additionally, the exploration of bimetallic and multi-metallic MOFs offers exciting opportunities to enhance the catalytic properties through synergistic effects. Combining Cu with other metals can lead to the formation of unique active sites with optimized binding energies for CO2 and intermediates, thereby improving the activity and selectivity.
In conclusion, while Cu-based MOFs and their derivatives have shown promising results in electrochemical CO2RR, addressing the challenges related to conductivity, stability, active site density, reaction mechanism, scalability, selectivity, and integrating computational modeling will be crucial for their widespread application and commercialization. Future research efforts should focus on developing more robust, conductive, and selective Cu-MOF-based catalysts through innovative design strategies and advanced characterization techniques.
Author Contributions
Conceptualization, H.G. and A.D.; methodology, H.G. and T.Y.; resources, A.D. and Z.W.; data curation, H.G., Y.G. and W.N.; writing—original draft preparation, T.Y., H.G. and W.N.; writing—review, and editing, H.G. and A.D.; visualization, H.G. and T.Y.; supervision, A.D. and Z.W.; project administration, H.G. and A.D.; funding acquisition, H.G. and A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Postdoctoral Science Foundation of China (2020M670801), the Fundamental Research Funds for the Central Universities (Grant No. 3132023197), Open Fund of State Key Laboratory of Catalysis in DICP CAS (N-19-07), The Ministry of Education’s Industry-University-Research Collaborative Education Project (Grant No. 240224070201), Experimental Education Reform Project of Dalian Maritime University (Grant No. syq2024), and 2024 Basic scientific Research Projects of colleges and universities of Liaoning Province Education Department (Grant NOs. LJ212410151017 and LJ212410151018).
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
Author Zhen Wang was employed by the company Hangzhou Dahua Apparatus Manufacture Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Liu, Q.; Wu, L.; Jackstell, R.; Beller, M. Using Carbon Dioxide as a Building Block in Organic Synthesis. Nat. Commun. 2015, 6, 15. [Google Scholar] [CrossRef]
- Duma, Z.G.; Moma, J.; Langmi, H.W.; Louis, B.; Parkhomenko, K.; Musyoka, N.M. Towards High CO2 Conversions Using Cu/Zn Catalysts Supported on Aluminum Fumarate Metal-Organic Framework for Methanol Synthesis. Catalysts 2022, 12, 1104. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.B.; Norskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef]
- Chang, F.; Xiao, M.; Miao, R.; Liu, Y.; Ren, M.; Jia, Z.; Han, D.; Yuan, Y.; Bai, Z.; Yang, L. Copper-Based Catalysts for Electrochemical Carbon Dioxide Reduction to Multicarbon Products. Electrochem. Energy Rev. 2022, 5, 4. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, Y.; Ma, L.; Zhu, G.; Wang, Y.; Xue, X.; Chen, R.; Yang, S.; Jin, Z. Progress and Perspective of Electrocatalytic CO2 Reduction for Renewable Carbonaceous Fuels and Chemicals. Adv. Sci. 2018, 5, 24. [Google Scholar] [CrossRef]
- Zhong, H.; Ghorbani-Asl, M.; Ly, K.H.; Zhang, J.; Ge, J.; Wang, M.; Liao, Z.; Makarov, D.; Zschech, E.; Brunner, E.; et al. Synergistic electroreduction of carbon dioxide to carbon monoxide on bimetallic layered conjugated metal-organic frameworks. Nat. Commun. 2020, 11, 1409. [Google Scholar] [CrossRef]
- Hernandez, S.; Farkhondehfal, M.A.; Sastre, F.; Makkee, M.; Saracco, G.; Russo, N. Syngas production from electrochemical reduction of CO2: Current status and prospective implementation. Green Chem. 2017, 19, 2326–2346. [Google Scholar] [CrossRef]
- Adegoke, K.A.; Maxakato, N.W. Electrocatalytic CO2 conversion on metal-organic frameworks derivative electrocatalysts. J. CO2 Util. 2023, 69, 34. [Google Scholar] [CrossRef]
- Xie, H.; Wang, T.; Liang, J.; Li, Q.; Sun, S. Cu-based nanocatalysts for electrochemical reduction of CO2. Nano Today 2018, 21, 41–54. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Zhou, Y.; Zhao, Y.; Liu, C.J. Recent progresses in the size and structure control of MOF supported noble metal catalysts. Catal. Today 2016, 263, 61–68. [Google Scholar] [CrossRef]
- Kong, C.; Jiang, G.; Sheng, Y.; Liu, Y.; Gao, F.; Liu, F.; Duan, X. Progress on Cu-based metal-organic frameworks for high-efficiency electrochemical CO2 conversion. Chem. Eng. J. 2023, 460, 141803. [Google Scholar] [CrossRef]
- Wang, C.; Lv, Z.; Yang, W.; Feng, X.; Wang, B. A rational design of functional porous frameworks for electrocatalytic CO2 reduction reaction. Chem. Soc. Rev. 2023, 52, 1382–1427. [Google Scholar] [CrossRef] [PubMed]
- Han, G.H.; Bang, J.; Park, G.; Choe, S.; Jang, Y.J.; Jang, H.W.; Kim, S.Y.; Ahn, S.H. Recent Advances in Electrochemical, Photochemical, and Photoelectrochemical Reduction of CO2 to C2+ Products. Small 2023, 19, e2205765. [Google Scholar] [CrossRef]
- Qu, J.; Cao, X.; Gao, L.; Li, J.; Li, L.; Xie, Y.; Zhao, Y.; Zhang, J.; Wu, M.; Liu, H. Electrochemical Carbon Dioxide Reduction to Ethylene: From Mechanistic Understanding to Catalyst Surface Engineering. Nano-Micro Lett. 2023, 15, 178. [Google Scholar] [CrossRef]
- Aran-Ais, R.M.; Gao, D.; Roldan Cuenya, B. Structure- and Electrolyte-Sensitivity in CO2 Electroreduction. Acc. Chem. Res. 2018, 51, 2906–2917. [Google Scholar] [CrossRef]
- Goyal, A.; Marcandalli, G.; Mints, V.A.; Koper, M.T.M. Competition between CO2 Reduction and Hydrogen Evolution on a Gold Electrode under Well-Defined Mass Transport Conditions. J. Am. Chem. Soc. 2020, 142, 4154–4161. [Google Scholar] [CrossRef] [PubMed]
- Kortlever, R.; Shen, J.; Schouten, K.J.P.; Calle-Vallejo, F.; Koper, M.T.M. Catalysts and Reaction Pathways for the Electrochemical Reduction of Carbon Dioxide. J. Phys. Chem. Lett. 2015, 6, 4073–4082. [Google Scholar] [CrossRef]
- Shao, P.; Zhang, H.-X.; Hong, Q.-L.; Yi, L.; Li, Q.-H.; Zhang, J. Enhancing CO2 Electroreduction to Ethylene via Copper-Silver Tandem Catalyst in Boron-Imidazolate Framework Nanosheet. Adv. Energy Mater. 2023, 13, 2300088. [Google Scholar] [CrossRef]
- Zhong, D.; Zhao, Z.-J.; Zhao, Q.; Cheng, D.; Liu, B.; Zhang, G.; Deng, W.; Dong, H.; Zhang, L.; Li, J.; et al. Coupling of Cu(100) and (110) Facets Promotes Carbon Dioxide Conversion to Hydrocarbons and Alcohols. Angew. Chem. Int. Ed. 2021, 60, 4879–4885. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, Z.; Zhong, L.; Hsu, C.-S.; Xu, X.; Li, Y.; Zhao, S.; Chen, S.; Yu, J.; Chen, S.; et al. Product-Specific Active Site Motifs of Cu for Electrochemical CO2 Reduction. Chem 2021, 7, 406–420. [Google Scholar] [CrossRef]
- Jin, H.; Xiong, L.; Zhang, X.; Lian, Y.; Chen, S.; Lu, Y.; Deng, Z.; Peng, Y. Cu-Based Catalyst Derived from Nitrogen-Containing Metal Organic Frameworks for Electroreduction of CO2. Acta Phys. Chim. Sin. 2021, 37, 11. [Google Scholar] [CrossRef]
- Kang, X.; Fu, G.; Fu, X.-Z.; Luo, J.-L. Copper-based metal-organic frameworks for electrochemical reduction of CO2. Chin. Chem. Lett. 2023, 34, 107757. [Google Scholar] [CrossRef]
- Xiong, W.; Si, D.; Yi, J.; Huang, Y.; Li, H.; Cao, R. Morphology and composition dependence of multicomponent Cu-based nanoreactor for tandem electrocatalysis CO2 reduction. Appl. Catal. B-Environ. 2022, 314, 121498. [Google Scholar] [CrossRef]
- Zheng, T.; Liu, C.; Guo, C.; Zhang, M.; Li, X.; Jiang, Q.; Xue, W.; Li, H.; Li, A.; Pao, C.-W.; et al. Copper-catalysed exclusive CO2 to pure formic acid conversion via single-atom alloying. Nat. Nanotechnol. 2021, 16, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Howarth, A.J.; Peters, A.W.; Vermeulen, N.A.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Best Practices for the Synthesis, Activation, and Characterization of Metal-Organic Frameworks. Chem. Mater. 2017, 29, 26–39. [Google Scholar] [CrossRef]
- Dae-Hyun, N.; Bushuyev, O.S.; Li, J.; De Luna, P.; Seifitokaldani, A.; Cao-Thang, D.; de Arquer, F.P.G.; Wang, Y.; Liang, Z.; Proppe, A.H.; et al. Metal-Organic Frameworks Mediate Cu Coordination for Selective CO2 Electroreduction. J. Am. Chem. Soc. 2018, 140, 11378–11386. [Google Scholar] [CrossRef]
- Xu, X.; Sun, H.; Jiang, S.P.; Shao, Z. Modulating metal-organic frameworks for catalyzing acidic oxygen evolution for proton exchange membrane water electrolysis. Susmat 2021, 1, 460–481. [Google Scholar] [CrossRef]
- Zheng, W.; Lee, L.Y.S. Metal-Organic Frameworks for Electrocatalysis: Catalyst or Precatalyst? Acs Energy Lett. 2021, 6, 2838–2843. [Google Scholar] [CrossRef]
- Shao, P.; Yi, L.; Chen, S.; Zhou, T.; Zhang, J. Metal-organic frameworks for electrochemical reduction of carbon dioxide: The role of metal centers. J. Energy Chem. 2020, 40, 156–170. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.; Wan, J.; Yu, C. MOF-on-MOF hybrids: Synthesis and applications. Coord. Chem. Rev. 2021, 432, 23. [Google Scholar] [CrossRef]
- Gebremariam, S.K.; Dumee, L.F.; Llewellyn, P.L.; AlWahedi, Y.F.; Karanikolos, G.N. Metal-organic framework hybrid adsorbents for carbon capture-A review. J. Environ. Chem. Eng. 2023, 11, 30. [Google Scholar] [CrossRef]
- Nwosu, U.; Siahrostami, S. Copper-based metal-organic frameworks for CO2 reduction: Selectivity trends, design paradigms, and perspectives. Catal. Sci. Technol. 2023, 13, 3740–3761. [Google Scholar] [CrossRef]
- Chen, R.; Cheng, L.; Liu, J.; Wang, Y.; Ge, W.; Xiao, C.; Jiang, H.; Li, Y.; Li, C. Toward High-Performance CO2-to-C2 Electroreduction via Linker Tuning on MOF-Derived Catalysts. Small 2022, 18, 2200720. [Google Scholar] [CrossRef]
- Jana, A.; Snyder, S.W.; Crumlin, E.J.; Qian, J. Integrated carbon capture and conversion: A review on C2+ product mechanisms and mechanism-guided strategies. Front. Chem. 2023, 11, 11. [Google Scholar] [CrossRef]
- Marcos, F.C.F.; Costa, M.J.F.; Catuzo, G.L.; de Moraes, D.A.; de Oliveira Junior, M.; Mastelaro, V.R.; Assaf, J.M.; Giudici, R.; Assaf, E.M. Supported Cu catalysts on UiO-66 toward enhanced methanol selectivity by CO2 hydrogenation: Effect of Cu loading. J. Catal. 2023, 427, 9. [Google Scholar] [CrossRef]
- Kim, D.; Kim, I.J.; Kwon, H.T.; Paeng, K.; Lee, H. CuBTC Metal-Organic Framework Decorated with FeBTC Nanoparticles with Enhance Water Stability for Environmental Remediation Applications. Acs Omega 2023, 8, 14900–14906. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; Ding, P.; Huang, J.; Dierolf, M.; Kelly, S.D.; Qiu, X.; Chen, Y.; Hussain, M.Z.; Li, W.; et al. Engineering a Cu-Pd Paddle-Wheel Metal-Organic Framework for Selective CO2 Electroreduction. Angew. Chem. Int. Ed. Engl. 2024, 63, e202414600. [Google Scholar] [CrossRef]
- Cho, J.H.; Lee, C.; Hong, S.H.; Jang, H.Y.; Back, S.; Seo, M.-g.; Lee, M.; Min, H.-K.; Choi, Y.; Jang, Y.J.; et al. Transition Metal Ion Doping on ZIF-8 Enhances the Electrochemical CO2 Reduction Reaction. Adv. Mater. 2023, 35, e2208224. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Zhu, H.; Huang, J.; Liao, P.; Chen, X. Ultrathin two-dimensional triptycence-based metal-organic framework for highly selective CO2 electroreduction to CO. Chin. Chem. Lett. 2023, 34, 107134. [Google Scholar] [CrossRef]
- Xin, Z.; Yuan, Z.; Liu, J.; Wang, X.; Shen, K.; Chen, Y.; Lan, Y.-Q. Cu cluster embedded porous nanofibers for high-performance CO2 electroreduction. Chin. Chem. Lett. 2023, 34, 107458. [Google Scholar] [CrossRef]
- Shao, P.; Wan, Y.-M.; Yi, L.; Chen, S.; Zhang, H.-X.; Zhang, J. Enhancing Electroreduction CO2 to Hydrocarbons via Tandem Electrocatalysis by Incorporation Cu NPs in Boron Imidazolate Frameworks. Small 2024, 20, 8. [Google Scholar] [CrossRef]
- Cui, S.; Yu, C.; Tan, X.; Li, W.; Zhang, Y.; Qiu, J. A tandem catalyst with high CO2 capture capability to achieve a promoted CO2-to-CH4 electrochemical conversion. Chem. Eng. J. 2023, 470, 144083. [Google Scholar] [CrossRef]
- Sun, B.; Hu, H.; Liu, H.; Guan, J.; Song, K.; Shi, C.; Cheng, H. Highly-exposed copper and ZIF-8 interface enables synthesis of hydrocarbons by electrocatalytic reduction of CO2. J. Colloid Interface Sci. 2024, 661, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Trinh, Q.T.; Wang, H.; Wu, S.; Arce-Ramos, J.M.; Sullivan, M.B.; Kraft, M.; Ager, J.W.W.; Zhang, J.; Xu, R. Selective and Stable CO2 Electroreduction to CH4 via Electronic Metal-Support Interaction upon Decomposition/Redeposition of MOF. Small 2023, 19, e2301379. [Google Scholar] [CrossRef]
- Lv, J.; Li, W.; Li, J.; Zhu, Z.; Dong, A.; Lv, H.; Li, P.; Wang, B. A Triptycene-Based 2D MOF with Vertically Extended Structure for Improving the Electrocatalytic Performance of CO2 to Methane. Angew. Chem. Int. Ed. 2023, 62, e202217958. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.-Y.; Sun, W.Y. In Situ Carbon-Encapsulated Copper-Doped Cerium Oxide Derived from MOFs for Boosting CO2-to-CH4 Electro-Conversion. Acs Catal. 2023, 13, 1545–1553. [Google Scholar] [CrossRef]
- Xue, H.; Huang, J.-R.; Wang, Z.-S.; Zhao, Z.-H.; Shi, W.; Liao, P.-Q.; Chen, X.-M. Tailoring ligand fields of metal-azolate frameworks for highly selective electroreduction of CO2 to hydrocarbons at industrial current density. Chin. J. Catal. 2023, 53, 102–108. [Google Scholar] [CrossRef]
- Lu, P.; Lv, J.; Chen, Y.; Ma, Y.; Wang, Y.; Lyu, W.; Yu, J.; Zhou, J.; Yin, J.; Xiong, Y.; et al. Steering the Selectivity of Carbon Dioxide Electroreduction from Single-Carbon to Multicarbon Products on Metal-Organic Frameworks via Facet Engineering. Nano Lett. 2024, 24, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, A.; Wei, D.; Huang, F.; Ma, J.; He, H.; Xu, J. Atomic cerium-doped CuOx catalysts for efficient electrocatalytic CO2 reduction to CH4. Chin. Chem. Lett. 2025, 36, 110175. [Google Scholar] [CrossRef]
- Sun, H.; Lin, L.; Hua, W.; Xie, X.; Mu, Q.; Feng, K.; Zhong, J.; Lyu, F.; Deng, Z.; Peng, Y. Atomically dispersed Co-Cu alloy reconstructed from metal-organic framework to promote electrochemical CO2 methanation. Nano Res. 2023, 16, 3680–3686. [Google Scholar] [CrossRef]
- Jing, Z.; Su, W.; Fan, Y. Increasing electrochemical carbon dioxide reduction to methane via a novel copper-based conductive metal organic framework. J. Colloid Interface Sci. 2025, 678, 251–260. [Google Scholar] [CrossRef]
- Sun, M.; Cheng, J.; Anzai, A.; Kobayashi, H.; Yamauchi, M. Modulating Electronic States of Cu in Metal-Organic Frameworks for Emerging Controllable CH4/C2H4 Selectivity in CO2 Electroreduction. Adv. Sci. 2024, 11, 2404931. [Google Scholar] [CrossRef]
- Zou, Y.-H.; Wang, X.; Ning, F.; Yi, J.; Liu, Y. Implanting MWCNTs in BiCu-MOFs to enhance electrocatalytic CO2 reduction to formate. Sep. Purif. Technol. 2023, 317, 11. [Google Scholar] [CrossRef]
- Huang, H.; Yue, K.; Liu, C.; Zhan, K.; Dong, H.; Yan, Y. CuO (111) Microcrystalline Evoked Indium-Organic Framework for Efficient Electroreduction of CO2 to Formate. Small 2024, 20, 2400441. [Google Scholar] [CrossRef]
- Liu, S.; Song, L.; Liu, R.; Li, L.; Yang, D.; Yuan, S.; Dai, X. Controlled One-Step Synthesis of MOF-on-MOF Cocatalysts for Two-Channel Electrocatalytic Conversion of CO2 to Formic Acid. Small 2023, 19, 2304808. [Google Scholar] [CrossRef]
- Lee, J.; Choi, H.; Mun, J.; Jin, E.; Lee, S.; Nam, J.; Umer, M.; Cho, J.; Lee, G.; Kwon, Y.; et al. Nanozyme Based on Porphyrinic Metal-Organic Framework for Electrocatalytic CO2 Reduction. Small Struct. 2023, 4, 2200087. [Google Scholar] [CrossRef]
- He, Q.; Li, H.; Hu, Z.; Lei, L.; Wang, D.; Li, T.-T. Highly Selective CO2 Electroreduction to C2H4 Using a Dual-Sites Cu(II) Porphyrin Framework Coupled with Cu2O Nanoparticles via a Synergetic-Tandem Strategy. Angew. Chem. Int. Ed. 2024, 63, e202407090. [Google Scholar] [CrossRef]
- Jang, J.; Lee, K.; Shin, H.; Lee, H.S.; Lee, B.-H.; Jeong, J.; Kim, J.; Hwang, W.; Park, S.; Bootharaju, M.S.; et al. Distinct reconstruction of aluminum-doped oxide-derived copper enhances the selectivity of C2+ products in CO2 electroreduction. J. Mater. Chem. A 2023, 11, 19066–19073. [Google Scholar] [CrossRef]
- Zhou, Z.; Liang, S.; Xiao, J.; Zhang, T.; Li, M.; Xie, W.; Wang, Q. Surface modification of Cu2O with stabilized Cu+ for highly efficient and stable CO2 electroreduction to C2+ chemicals. J. Energy Chem. 2023, 84, 277–285. [Google Scholar] [CrossRef]
- Zheng, K.; Hu, D.-Y.; Zhang, X.-W.; Xiao, X.-X.; Liang, Z.-J.; Wu, J.-X.; Lin, D.-Y.; Zhuo, L.-L.; Yi, H.; Gong, L.; et al. Bending two-dimensional Cu(i)-based coordination networks to inverse electrocatalytic HER/CO2RR selectivity. J. Mater. Chem. A 2024, 12, 16396–16402. [Google Scholar] [CrossRef]
- Yan, T.; Wang, P.; Sun, W.-Y. Single-Site Metal-Organic Framework and Copper Foil Tandem Catalyst for Highly Selective CO2 Electroreduction to C2H4. Small 2023, 19, e2206070. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Hu, D.-Y.; Wang, C.; Liang, Z.-J.; Zhang, X.-W.; Xiao, X.-X.; Wu, J.-X.; Zhuo, L.-L.; Lin, D.-Y.; Zhou, D.-D.; et al. Isomeric Cu(I) Azolate Frameworks Showing Contrasting Electrocatalytic CO2 Reduction Selectivities and Stabilities. Small 2024, 21, 2408510. [Google Scholar] [CrossRef]
- Ma, M.; Xiong, L.; Dong, Y.; Bai, Q.; Hua, W.; Zheng, Z.; Lyu, F.; Lian, Y.; Wei, Z.; Yuan, H.; et al. Metalloporphyrin Frameworks to Encapsulate Copper Oxides for Boosting Ethylene Production in Neutral Electrolyte. Adv. Funct. Mater. 2024, 34, 2315667. [Google Scholar] [CrossRef]
- Wen, Y.; Cheng, W.-H.; Wang, Y.-R.; Shen, F.-C.; Lan, Y.-Q. Tailoring the Hydrophobic Interface of Core-Shell HKUST-1@Cu2O Nanocomposites for Efficiently Selective CO2 Electroreduction. Small 2024, 20, e2307467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, Y. Enhanced CO2 Electroreduction to ethylene on Cu nanocube coated with nitrogen-doped carbon shell in-situ electro-derived from metal-organic framework. Chem. Eng. J. 2024, 499. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Y. Stabilizing *CO intermediate on nitrogen-doped carbon-coated CuxOy derived from metal-organic framework for enhanced electrochemical CO2-to-ethylene. J. Mater. Chem. A 2024, 13, 2902–2910. [Google Scholar] [CrossRef]
- Heng, J.-M.; Zhu, H.-L.; Zhao, Z.-H.; Liao, P.-Q.; Chen, X.-M. Fabrication of Ultrahigh-Loading Dual Copper Sites in Nitrogen-Doped Porous Carbons Boosting Electroreduction of CO2 to C2H4 Under Neutral Conditions. Adv. Mater. 2024, 37, e2415101. [Google Scholar] [CrossRef]
- Chang, B.; Zhang, F.; Min, Z.; Wang, N.; Xia, Q.; Fan, J.; Fan, M.; Wang, J. Confined CuI sites in partially electro-reduced 2D conductive Cu-MOF film for boosting CO2 electrocatalysis to C2 products. Chem. Eng. J. 2024, 496, 153993. [Google Scholar] [CrossRef]
- Wen, C.F.; Yang, S.; He, J.J.; Niu, Q.; Liu, P.F.; Yang, H.G. Anionic Metal-Organic Framework Derived Cu Catalyst for Selective CO2 Electroreduction to Hydrocarbons. Small 2024, 20, e2405051. [Google Scholar] [CrossRef]
- Xu, X.; Zhong, Y.; Wajrak, M.; Bhatelia, T.; Jiang, S.P.; Shao, Z. Grain boundary engineering: An emerging pathway toward efficient electrocatalysis. Infomat 2024, 6, e12608. [Google Scholar] [CrossRef]
- Sun, D.; Xu, X.; Qin, Y.; Jiang, S.P.; Shao, Z. Rational Design of Ag-Based Catalysts for the Electrochemical CO2 Reduction to CO: A Review. Chemsuschem 2020, 13, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Shin, D.; Park, J.; Jung, J.-Y.; Song, H. Grain Boundary-Rich Copper Nanocatalysts Generated from Metal-Organic Framework Nanoparticles for CO2-to-C2+ Electroconversion. Adv. Sci. 2023, 10, 11. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Song, Y.; Geng, S.; Peng, Z.; Yu, J.; Liu, F.; Wang, Y.; Xi, S.; Zhang, Z.; et al. Simultaneous Defect and Size Control of Metal-Organic Framework Nanostructures for Highly Efficient Carbon Dioxide Electroreduction to Multicarbon Products. Acs Mater. Lett. 2023, 5, 2121–2130. [Google Scholar] [CrossRef]
- Huang, L.; Liu, Z.; Gao, G.; Chen, C.; Xue, Y.; Zhao, J.; Lei, Q.; Jin, M.; Zhu, C.; Han, Y.; et al. Enhanced CO2 Electroreduction Selectivity toward Ethylene on Pyrazolate-Stabilized Asymmetric Ni-Cu Hybrid Sites. J. Am. Chem. Soc. 2023, 145, 26444–26451. [Google Scholar] [CrossRef]
- Deng, J.; Qiu, L.; Xin, M.; He, W.; Zhao, W.; Dong, J.; Xu, G. Boosting Electrochemical CO2 Reduction on Copper-Based Metal-Organic Frameworks via Valence and Coordination Environment Modulation. Small 2024, 20, e2311060. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xiao, J.; Guo, L.; Xie, Z.; Wang, K.; Wang, Y.; Hao, F.; Ma, Y.; Zhou, J.; Lu, P.; et al. In Situ Phase Transformation-Enabled Metal-Organic Frameworks for Efficient CO2 Electroreduction to Multicarbon Products in Strong Acidic Media. ACS Nano 2024, 18, 33602–33613. [Google Scholar] [CrossRef]
- Yin, B.; Wang, C.; Xie, S.; Gu, J.; Sheng, H.; Wang, D.-X.; Yao, J.; Zhang, C. Regulating Spin Density using Tempol Molecules for Enhanced CO2-to-Ethylene Conversion by HKUST-1 Framework Derived Electrocatalysts. Angew. Chem. Int. Ed. 2024, 63, e202405873. [Google Scholar] [CrossRef]
- Bohan, A.; Jin, X.; Wang, M.; Wang, Y.; Chen, W.; Wei, Z.; Du, Z.; Liu, X.; Wang, Y.; Zhang, L. Steering CO2 electroreduction toward C2+ products by defect-engineered coordination microenvironments of Cu-MOFs. Chem. Eng. J. 2024, 500, 157076. [Google Scholar] [CrossRef]
- Xie, G.; Guo, W.; Fang, Z.; Duan, Z.; Lang, X.; Liu, D.; Mei, G.; Zhai, Y.; Sun, X.; Lu, X. Dual-Metal Sites Drive Tandem Electrocatalytic CO2 to C2+ Products. Angew. Chem. Int. Ed. 2024, 63, e202412568. [Google Scholar] [CrossRef]
- Su, W.; Guo, W.; Fan, Y. CuAg bimetallic catalysts derived from an Ag-anchored Cu-based metal-organic framework for CO2 electroreduction to ethanol. Chem. Eng. J. 2023, 477, 147204. [Google Scholar] [CrossRef]
- Zhao, Z.-H.; Huang, J.-R.; Liao, P.-Q.; Chen, X.-M. Highly Efficient Electroreduction of CO2 to Ethanol via Asymmetric C-C Coupling by a Metal-Organic Framework with Heterodimetal Dual Sites. J. Am. Chem. Soc. 2023, 145, 26783–26790. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Huang, D.; Huang, R.-K.; He, X.-L.; Luo, Y.; Xiang, Y.-L.; Jiang, L.-b.; Dong, M.; Li, S.; Zhang, Z.; et al. Metal-Organic Framework Supported Low-Nuclearity Cluster Catalysts for Highly Selective Carbon Dioxide Electroreduction to Ethanol. Angew. Chem. Int. Ed. 2024, 63, e202409270. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Wang, X.; Feng, Y.; Zhang, H.; Zhang, G. Self-Polarization Triggered Multiple Polar Units Toward Electrochemical Reduction of CO2 to Ethanol with High Selectivity. Angew. Chem. Int. Ed. 2023, 62, e202302241. [Google Scholar] [CrossRef]
- Wang, C.; Lv, Z.; Liu, Y.; Dai, L.; Liu, R.; Sun, C.; Liu, W.; Feng, X.; Yang, W.; Wang, B. Asymmetric Cu-N1O3 Sites Coupling Atop-type and Bridge-type Adsorbed *C1 for Electrocatalytic CO2-to-C2 Conversion. Angew. Chem. Int. Ed. 2024, 63, e202411216. [Google Scholar] [CrossRef]
- Liu, W.; Tang, B.; Huang, K.; Zhang, Z.; Wang, Z.; An, G.; Zhang, M.; Wang, K.; Fu, S.; Guo, H.; et al. Radiation-Synthesized Metal-Organic Frameworks with Ligand-Induced Lewis Pairs for Selective CO2 Electroreduction. Small 2024, 20, e2408688. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, M.; Ye, J.; Liu, L.; Li, L.; Li, Y.; Huang, X. Highly Selective CO2 Electroreduction to C2+Products over Cu2O-Decorated 2D Metal-Organic Frameworks with Rich Heterogeneous Interfaces. Nano Lett. 2023, 23, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Li, A.; Ma, C.; Gao, Z.; Zhou, B.; Zhu, L.; Huang, Z. Nitrogen-doped carbon confined Cu-Ag bimetals for efficient electroreduction of CO2 to high-order products. Chem. Eng. J. 2023, 468, 143606. [Google Scholar] [CrossRef]
- Yang, K.; Sun, Y.; Chen, S.; Li, M.; Zheng, M.; Ma, L.; Fan, W.; Zheng, Y.; Li, Q.; Duan, J. Less-Coordinated Atomic Copper-Dimer Boosted Carbon-Carbon Coupling During Electrochemical CO2 Reduction. Small 2023, 19, 9. [Google Scholar] [CrossRef]
- Mao, X.; Gong, W.; Fu, Y.; Li, J.; Wang, X.; O’Mullane, A.P.; Xiong, Y.; Du, A. Computational Design and Experimental Validation of Enzyme Mimicking Cu-Based Metal-Organic Frameworks for the Reduction of CO2 into C2 Products: C-C Coupling Promoted by Ligand Modulation and the Optimal Cu-Cu Distance. J. Am. Chem. Soc. 2023, 145, 21442–21453. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).