CuCl/Ionic Liquid Catalyzed Cascade Transformation of CO2 and Alkyne-1,2-Diols: Synthesis of Keto-Functionalized Cyclic Carbonates
Abstract
1. Introduction
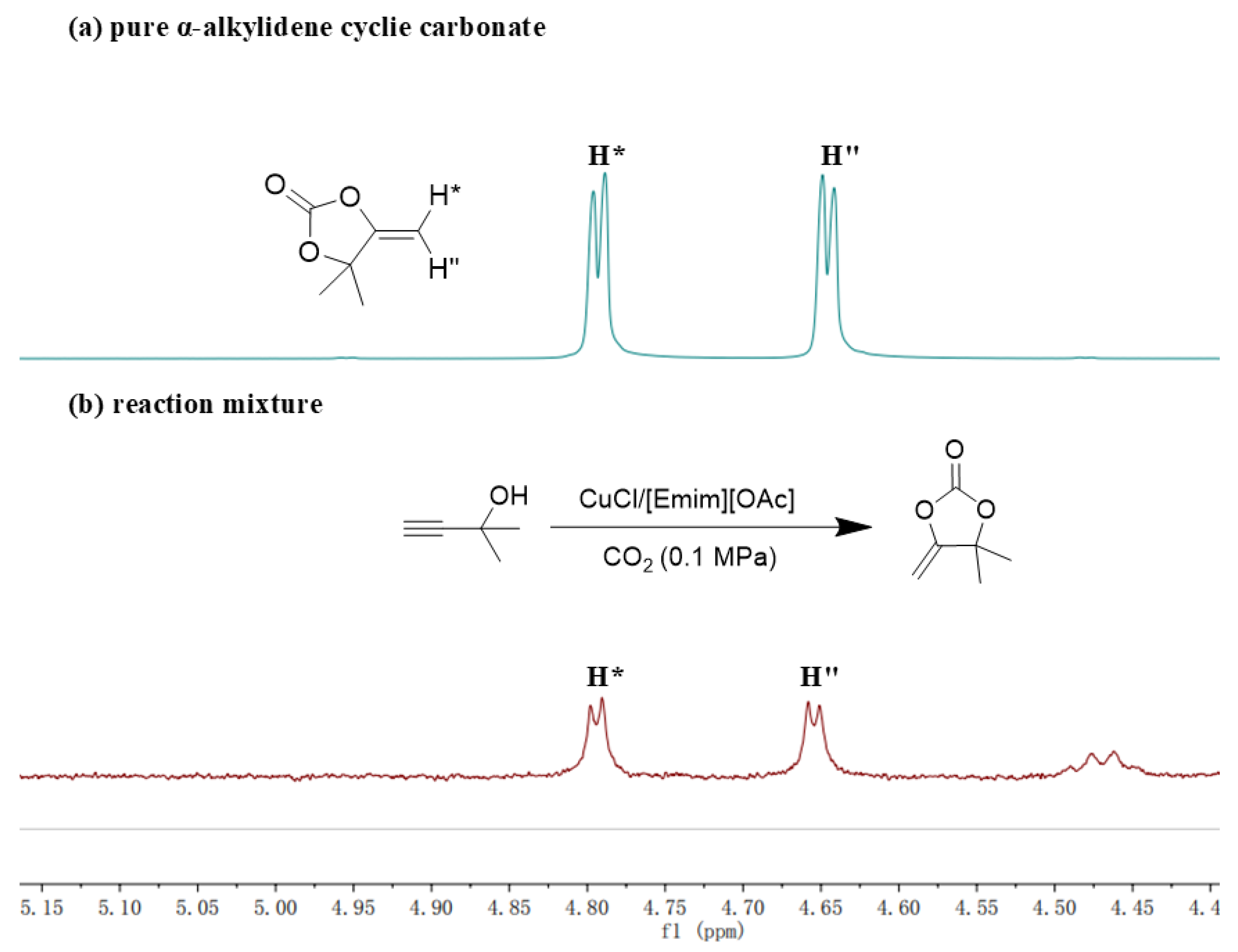
2. Results and Discussion
3. Experiment
3.1. Materials and Methods
3.2. The Synthesis Method for Substrate 1 (1a, 1b, 1c, 1d, 1e)
3.3. Synthesis of Ionic Liquids by Anion-Exchange Resin Method
3.4. The General Procedure for Synthesizing Products 2 (2a, 2b, 2c, 2d, 2e)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, S.; Yu, Z.; Zhang, Y.; Xu, T. Review of non-isothermal processes in CCUS from a geomechanical perspective. Earth-Sci. Rev. 2024, 255, 104848. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Lee, C.-C. Convergence of the world’s energy use. Resour. Energy Econ. 2020, 62, 101199. [Google Scholar] [CrossRef]
- Cao, C.; Zhu, H.; Hou, Z. Advances in Carbon Capture, Utilization and Storage (CCUS). Energies 2024, 17, 4784. [Google Scholar] [CrossRef]
- Mon, M.T.; Tansuchat, R.; Yamaka, W. CCUS Technology and Carbon Emissions: Evidence from the United States. Energies 2024, 17, 1748. [Google Scholar] [CrossRef]
- Zhi, K.; Li, Z.; Wang, B.; Klemeš, J.J.; Guo, L. A review of CO2 utilization and emissions reduction: From the perspective of the chemical engineering. Process Saf. Environ. Prot. 2023, 172, 681–699. [Google Scholar] [CrossRef]
- Ding, Y.; Dong, Y.; Ma, M.; Luo, L.; Wang, X.; Fang, B.; Li, Y.; Liu, L.; Ren, F. CO2 electrocatalytic reduction to ethylene and its application outlook in food science. iScience 2023, 26, 108434. [Google Scholar] [CrossRef]
- Villegas, M.E.; Aredo, V.; Asevedo, K.J.E.; Lourenço, R.V.; Bazito, R.C.; Oliveira, A.L. Commercial Starch Behavior When Impregnated with Food Additives by Moderate Temperature Supercritical CO2 Processing. Starch-Starke 2020, 72, 1900231. [Google Scholar] [CrossRef]
- Dowaidar, M. Synthetic biology of metabolic cycles for Enhanced CO2 capture and Sequestration. Bioorganic Chem. 2024, 153, 107774. [Google Scholar] [CrossRef]
- Li, M.; Abdolmohammadi, S.; Hoseininezhad-Namin, M.S.; Behmagham, F.; Vessally, E. Carboxylative cyclization of propargylic alcohols with carbon dioxide: A facile and Green route to α-methylene cyclic carbonates. J. CO2 Util. 2020, 38, 220–231. [Google Scholar] [CrossRef]
- Yuan, Y.; Xie, Y.; Zeng, C.; Song, D.; Chaemchuen, B.; Chen, C.; Verpoort, F. A recyclable AgI/OAc− catalytic system for the efficient synthesis of α-alkylidene cyclic carbonates: Carbon dioxide conversion at atmospheric pressure. Green Chem. 2017, 19, 2936–2940. [Google Scholar] [CrossRef]
- Cervantes-Reyes, A.; Farshadfar, K.; Rudolph, M.; Rominger, F.; Schaub, T.; Ariafard, A.; Hashmi, A.S.K. Copper-catalysed synthesis of α-alkylidene cyclic carbonates from propargylic alcohols and CO2. Green Chem. 2021, 23, 889–897. [Google Scholar] [CrossRef]
- Dabral, S.; Bayarmagnai, B.; Hermsen, M.; Schießl, J.; Mormul, V.; Hashmi, A.S.K.; Schaub, T. Silver-Catalyzed Carboxylative Cyclization of Primary Propargyl Alcohols with CO2. Org. Lett. 2019, 21, 1422–1425. [Google Scholar] [CrossRef] [PubMed]
- Huadsai, W.; Westerhausen, M.; Bontemps, S. Alkaline Earth Catalyzed CO2 Hydroboration into Acetal Derivatives Leading to C–S Bond Formation. Organometallics 2023, 42, 2921–2926. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Xu, Y.; Yan, X.; Zhang, S.; Duan, K.; Chen, C.; Yuan, Y.; Verpoort, F. CO2-induced dissolution of ZnO into ionic liquids and its catalytic application for the hydration of propargylic alcohols. Appl. Catal. B-Environ. 2022, 310, 121270. [Google Scholar] [CrossRef]
- Li, D.; Gong, Y.; Du, M.; Bu, C.; Chen, C.; Chaemcheun, B.; Hu, J.; Zhang, Y.; Yuan, Y.; Verpoort, F. CO2-Promoted Hydration of Propargylic Alcohols: Green Synthesis of α-Hydroxy Ketones by an Efficient and Recyclable AgOAc/Ionic Liquid System. ACS Sustain. Chem. Eng. 2020, 8, 8148–8155. [Google Scholar] [CrossRef]
- Yuan, Y.; Hu, J.; Wang, Y.; Huang, L.; Zheng, J.; Zhao, Y.; Chen, Y.; Chen, C.; Verpoort, F. A green and recyclable CuSO4·5H2O/ionic liquid catalytic system for the CO2-promoted hydration of propargyl alcohols: An efficient assembly of α-hydroxy ketones. J. Catal. 2022, 405, 561–570. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Wang, Y.; Li, Y.; He, J.; Wang, Y.; Zhang, L.; Wang, Z.; Qian, Q.; Han, B. Synthesis of Carboxylic Acids via Reaction of Ketones/Aldehydes with CO2 and H2. Organometallics 2023, 42, 2312–2318. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Qian, Q.; Li, Y.; Bediako, B.B.A.; Zhang, J.; Yang, J.; Li, Z.; Han, B. Synthesis of carboxylic acids via the hydrocarboxylation of alcohols with CO2 and H2. Green Chem. 2022, 24, 1973–1977. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Zhang, Y.; Wang, Z.; Xiang, J.; Han, J.; He, J.; Zhang, L.; Wang, Y.; Meng, Q.; et al. Synthesis of Carboxylic Acids from Saccharides, CO2, and H2. ACS Catal. 2023, 13, 8025–8030. [Google Scholar] [CrossRef]
- Witoon, T.; Numpilai, T.; Nijpanich, S.; Chanlek, N.; Kidkhunthod, P.; Kidkhunthod, C.K.; Ng, K.H.; Vo, D.N.; Ittisanronnachai, S.; Wattanakit, C.; et al. Enhanced CO2 hydrogenation to higher alcohols over K-Co promoted In2O3 catalysts. Chem. Eng. J. 2022, 431, 133211. [Google Scholar] [CrossRef]
- Yang, H.; Wei, Z.; Zhang, J.; Dang, Y.; Li, S.; Bu, X.; Zhou, Z.; Gong, C.; Wang, H.; Li, J.; et al. Tuning the selectivity of CO2 hydrogenation to alcohols by crystal structure engineering. Chem 2024, 10, 2245–2265. [Google Scholar] [CrossRef]
- Skarżyńska, A.; Gil, W.; Trzeciak, A.M. Pd/PVP- Catalyzed Formylation of Amines and Nitroarenes to N-Formamides with CO2 and Dimethylamine Borane: The Influence of CO2 on the Formation of Azoxyarenes. ChemCatChem 2024, 16, e202401260. [Google Scholar] [CrossRef]
- Xing, S.; Lv, P.; Yuan, H.; Yang, L.; Wang, Z.; Yuan, Z.; Chen, Y. Insight into forced hydrogen re-arrangement and altered reaction pathways in a protocol for CO2 catalytic processing of oleic acid into C8–C15 alkanes. Green Chem. 2017, 19, 4157–4168. [Google Scholar] [CrossRef]
- Rajanna, D.; Srinivasappa, P.M.; Sudhakaran, A.; Biradar, A.; Samal, A.K.; Bembalge, O.B.; Jadhav, A.H. Recent Advances in the Fixation of CO2 into Quinazoline and Benzimidazole. Energy Fuels 2024, 38, 8481–8515. [Google Scholar] [CrossRef]
- Huang, Q.; Weng, J.; Ouyang, D.; Chen, M.; Wang, X.; Wang, J. Comparative studies on the combustion characteristics of electrolytes and carbonate mixed solvents with flame retardant additives under low pressures. Case Stud. Therm. Eng. 2023, 43, 102810. [Google Scholar] [CrossRef]
- Huang, X.-H.; Chung, Y.-H.; Guo, G.-S.; Shu, C.-M. Investigating the thermal stability and explosion characteristics of electrolytes composed of different ratios of carbonate organic solvents. J. Loss Prev. Process Ind. 2025, 94, 105509. [Google Scholar] [CrossRef]
- Morodo, R.; Dumas, D.M.; Zhang, J.; Lui, K.H.; Hurst, P.J.; Bosio, R.; Campos, L.M.; Park, N.H.; Waymouth, R.M.; Hedrick, J.L. Ring-Opening Polymerization of Cyclic Esters and Carbonates with (Thio)urea/Cyclopropenimine Organocatalytic Systems. ACS Macro Lett. 2024, 13, 181–188. [Google Scholar] [CrossRef]
- Hodge, P.; Kamau, S.D.; Ben-Haida, A.; Williams R., T. Cyclo-depolymerizations of polycarbonates in solution: Use of the macrocyclic oligomers obtained in entropically-driven ring-opening polymerizations and copolymerizations to give carbonate–carbonate and carbonate–carboxylate ester copolymers. React. Funct. Polym. 2012, 72, 868–877. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, K.; Yang, B.; Sun, Z.; Zhao, Y. Synergistic catalysis of dual-sites promoted cycloaddition of CO2 with epoxides. Fuel 2025, 381, 133305. [Google Scholar] [CrossRef]
- Tappe, N.A.; Reich, R.M.; D’elia, V.; Kühn, F.E. Current advances in the catalytic conversion of carbon dioxide by molecular catalysts: An update. Dalton Trans. 2018, 47, 13281–13313. [Google Scholar] [CrossRef]
- Zhang, S.; Xia, Z.; Zou, Y.; Cao, F.; Liu, Y.; Ma, Y.; Qu, Y. Interfacial Frustrated Lewis Pairs of CeO2 Activate CO2 for Selective Tandem Transformation of Olefins and CO2 into Cyclic Carbonates. J. Am. Chem. Soc. 2019, 141, 11353–11357. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Wang, Y.; Yan, Z.; Hou, J.; Xu, G.; Shi, L. One-step DMC synthesis from CO2 under catalysis of ionic liquids Pj. L.repared with 1,2-propylene glycol. Catal. Today 2023, 418, 114052. [Google Scholar] [CrossRef]
- Han, F.; Li, H.; Zhuang, H.; Hou, Q.; Yang, Q.; Zhang, B.; Miao, C. Direct synthesis of cyclic carbonates from olefins and CO2: Single- or multi-component catalytic systems via epoxide or halohydrin intermediate. J. CO2 Util. 2021, 53, 101742. [Google Scholar] [CrossRef]
- Kobayashi, T.; Hotta, H.; Miyake, T.; Nomura, M.; Horikoshi, R.; Yamamoto, K. Discovery of flometoquin, a novel quinoline insecticide. J. Pestic. Sci. 2023, 48, 168–174. [Google Scholar] [CrossRef]
- Min, S.; Kim, T.; Jeong, T.; Yang, J.; Oh, Y.; Moon, K.; Rakshit, A.; Kim, I.S. Synthesis of 2-Formyl Carbazoles via Tandem Reaction of Indolyl Nitrones with 2-Methylidene Cyclic Carbonate. Org. Lett. 2023, 25, 4298–4302. [Google Scholar] [CrossRef]
- Yamada, W.; Sugawara, Y.; Cheng, H.M.; Ikeno, T.; Yamada, T. Silver-Catalyzed Incorporation of Carbon Dioxide into Propargylic Alcohols. Eur. J. Org. Chem. 2007, 2007, 2604–2607. [Google Scholar] [CrossRef]
- Song, Q.-W.; Chen, W.-Q.; Ma, R.; Yu, A.; Li, Q.-Y.; Chang, Y.; He, L.-N. Back Cover: Bifunctional Silver(I) Complex-Catalyzed CO2 Conversion at Ambient Conditions: Synthesis of α-Methylene Cyclic Carbonates and Derivatives (ChemSusChem 5/2015). ChemSusChem 2015, 8, 910. [Google Scholar] [CrossRef]
- Chen, K.; Shi, G.; Dao, R.; Mei, K.; Zhou, X.; Li, H.; Wang, C. Tuning the basicity of ionic liquids for efficient synthesis of alkylidene carbonates from CO2 at atmospheric pressure. Chem. Commun. 2016, 52, 7830–7833. [Google Scholar] [CrossRef]
- Miró, R.; Lara-Rimbau, F.X.; Vicente, I.; Gual, A. CO2 Transformations in Ionic Liquids: In Situ NHC-Cu Formation for Carbonate and Carbamate Production. ACS Sustain. Chem. Eng. 2023, 11, 17600–17606. [Google Scholar] [CrossRef]
- Li, X.; Villar-Yanez, A.; Ngassam Tounzoua, C.; Benet-Buchholz, J.; Grignard, B.; Bo, C.; Detrembleur, C.; Kleij A., W. Cascade Transformation of Carbon Dioxide and Alkyne-1,n-diols into Densely Substituted Cyclic Carbonates. ACS Catal. 2022, 12, 2854–2860. [Google Scholar] [CrossRef]
- Chen, X.; Zhong, Q.; Ma, X.; Li, S.; Sun, W.; Liu, C. Synthesis of benzothiazoles catalyzed by [Bmim]PF6 ionic liquid in solvent-free condition. J. Catal. 2024, 429, 115274. [Google Scholar] [CrossRef]
- Hu, J.; Ma, J.; Lu, L.; Qian, Q.; Zhang, Z.; Xie, C.; Han, B. Synthesis of Asymmetrical Organic Carbonates using CO2 as a Feedstock in AgCl/Ionic Liquid System at Ambient Conditions. ChemSusChem 2017, 10, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Dong, S.; Zhang, Z.; Wang, L.; Zhang, J. Insights into the synergistic influence of [Emim][OAc] and AgOAc for the hydration of propargylic alcohols to α-hydroxy ketones in the presence of CO2. Catal. Sci. Technol. 2021, 11, 5641–5649. [Google Scholar] [CrossRef]
- Qiu, J.; Zhao, Y.; Li, Z.; Wang, H.; Fan, H.; Wang, J. Efficient Ionic-Liquid-Promoted Chemical Fixation of CO2 into α-Alkylidene Cyclic Carbonates. ChemSusChem 2017, 10, 1120–1127. [Google Scholar] [CrossRef]
- Arayachukiat, S.; Kongtes, C.; Barthel, A.; Vummaleti, S.V.C.; Poater, A.; Wannakao, S.; Cavallo, L.; D’elia, V. Ascorbic Acid as a Bifunctional Hydrogen Bond Donor for the Synthesis of Cyclic Carbonates from CO2 under Ambient Conditions. ACS Sustain. Chem. Eng. 2017, 5, 6392–6397. [Google Scholar] [CrossRef]
- Qiu, J.; Zhao, Y.; Wang, H.; Cui, G.; Wang, J. AgX@carbon (X = Br and I) as robust and efficient catalysts for the reaction of propargylic alcohols and CO2 to carbonates under ambient conditions. RSC Adv. 2016, 6, 54020–54026. [Google Scholar] [CrossRef]
- Li, W.; Cheng, W.; Yang, X.; Su, Q.; Dong, L.; Zhang, P.; Yi, Y.; Li, B.; Zhang, S. Synthesis of Cyclic Carbonate Catalyzed by DBU Derived Basic Ionic Liquids. Chin. J. Chem. 2018, 36, 293–298. [Google Scholar] [CrossRef]
- Zhou, Z.-H.; Zhang, X.; Huang, Y.-F.; Chen, K.-H.; He, L.-N. Synthesis of α-hydroxy ketones by copper(I)-catalyzed hydration of propargylic alcohols: CO2 as a cocatalyst under atmospheric pressure. Chin. J. Catal. 2019, 40, 1345–1351. [Google Scholar] [CrossRef]
- Mezzetta, A.; Becherini, S.; Pretti, C.; Monni, G.; Casu, V.; Chiappe, C.; Guazzelli, L. Insights into the levulinate-based ionic liquid class: Synthesis, cellulose dissolution evaluation and ecotoxicity assessment. New J. Chem. 2019, 43, 13010–13019. [Google Scholar] [CrossRef]
- Bragato, N.; Perosa, A.; Selva, M.; Fiorani, G.; Calmanti, R. Molybdate ionic liquids as halide-free catalysts for CO2 fixation into epoxides. Green Chem. 2023, 25, 4849–4860. [Google Scholar] [CrossRef]



 | |||
|---|---|---|---|
| Entry. | Metal Salt | IL | Yield (%) b |
| 1 | / | / | / |
| 2 | / | [Emim][OAc] | 3 |
| 3 | CuCl | [Emim][OAc] | 79 |
| 4 | CuO | [Emim][OAc] | 23 |
| 5 | Cu2O | [Emim][OAc] | 35 |
| 6 | CuI | [Emim][OAc] | 53 |
| 7 | CuS | [Emim][OAc] | / |
| 8 | CuBr2 | [Emim][OAc] | 53 |
| 9 | CuSO4 | [Emim][OAc] | 30 |
| 10 | CuCl2 | [Emim][OAc] | 36 |
| 11 | Cu2(OH)3Cl | [Emim][OAc] | 58 |
| 12 | AgOAc | [Emim][OAc] | 18 |
| 13 | Ag2CO3 | [Emim][OAc] | 20 |
| 14 | ZnO | [Emim][OAc] | / |
| 15 | Co(OAc)2·4H2O | [Emim][OAc] | / |
| 16 | Ni(OAc)2·4H2O | [Emim][OAc] | / |
 | |||
|---|---|---|---|
 | |||
| Entry | Metal Salt | IL | Yield (%) b |
| 1 | CuCl | / | / |
| 2 | CuCl | [Emim][OAc] | 79 |
| 3 | CuCl | [Emim][CF3SO3] | / |
| 4 | CuCl | [Emim][HSO4] | / |
| 5 | CuCl | [Emim][NO3] | / |
| 6 | CuCl | [Emim][Br] | / |
| 7 | CuCl | [Emim][I] | / |
| 8 | CuCl | [Emim][Lev] | 32 c |
| 9 | CuCl | [Emim][PF6] | 11 |
| 10 | CuCl | [Emim][HCOO] | 29 |
| 11 | CuCl | [Emim][H(CH2)3COO] | 52 |
| 12 | CuCl | [Emim][H(CH2)7COO] | 53 |
| 13 | CuCl | [DBUH][OAc] | 12 |
| 14 | CuCl | [Bmim][OAc] | 37 |
| 15 | CuCl | [Bu4N][OAc] | 63 |
| 16 | CuCl | [Bu4N]2[MoO4] | 12 |
| 17 | CuCl | [DBUH]2[ITA] | 7 |
 | |||||
|---|---|---|---|---|---|
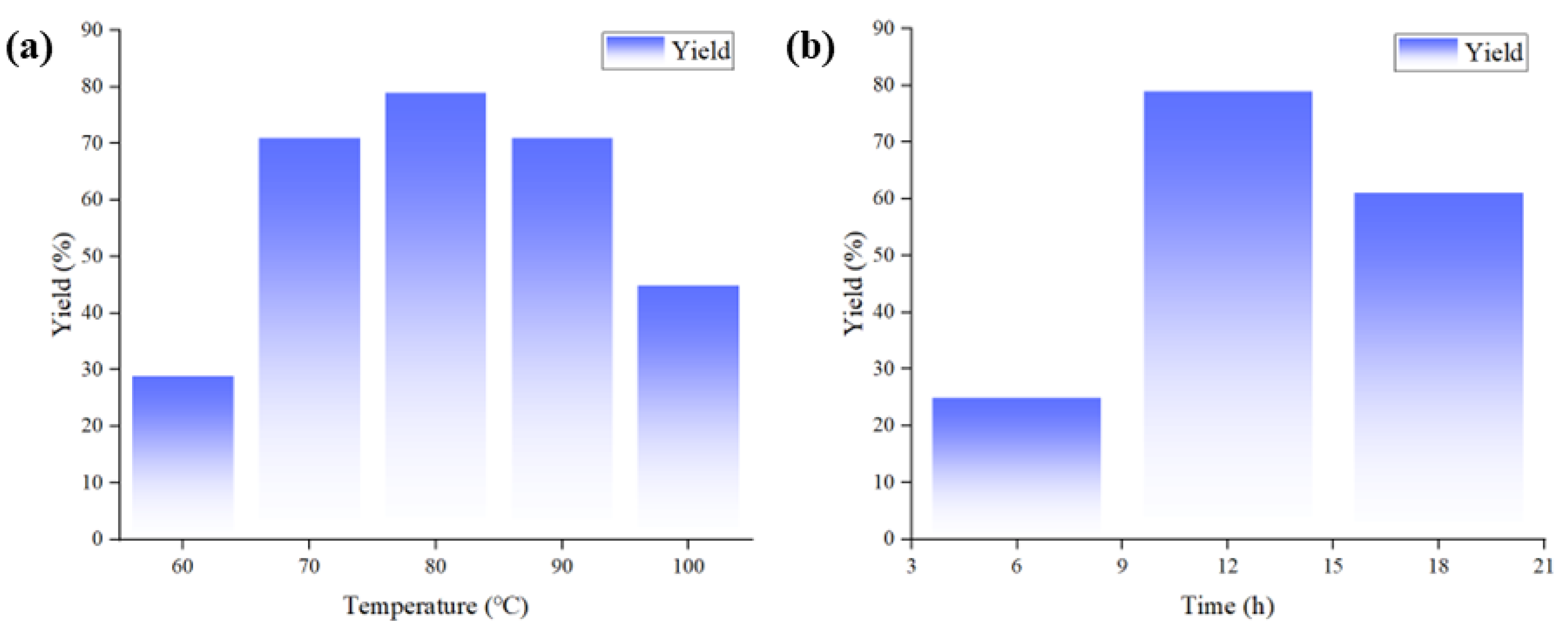
| Entry | CuCl (mol%) c | [Emim][OAc] (equiv.) c | Temperature (°C) | Time (h) | Yield (%) b |
| 1 | 10 | 1 | 60 | 12 | 29 |
| 2 | 10 | 1 | 70 | 12 | 71 |
| 3 | 10 | 1 | 75 | 12 | 75 |
| 4 | 10 | 1 | 80 | 12 | 79 |
| 5 | 10 | 1 | 90 | 12 | 71 |
| 6 | 10 | 1 | 100 | 12 | 45 |
| 7 | 10 | 1 | 80 | 6 | 25 |
| 8 | 10 | 1 | 80 | 18 | 61 |
| 9 | 10 | 0.5 | 80 | 12 | 57 |
| 10 | 10 | 2 | 80 | 12 | 53 |
| 11 | 10 | 3 | 80 | 12 | 36 |
| 12 | 15 | 1 | 80 | 12 | 68 |
| 13 | 30 | 1 | 80 | 12 | 61 |
| 14 | 5 | 1 | 80 | 12 | 64 |
 | ||||
|---|---|---|---|---|
| Substrate | Product | Yield (%) b | ||
| 1a | 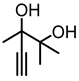 | 2a | 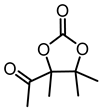 | 79 (63) c [69] e |
| 1b | 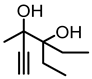 | 2b |  | 34 [17] e |
| 1c | 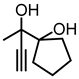 | 2c | 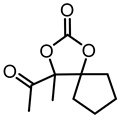 | 73 [59] e |
| 1d |  | 2d | 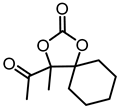 | 56 d [44] e |
| 1e | 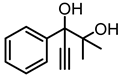 | 2e | 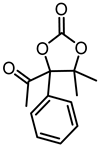 | 10 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, D.; Wang, C.; Liu, J.; Cao, D.; Guo, K.; Wang, Y.; Yuan, Y.; Verpoort, F. CuCl/Ionic Liquid Catalyzed Cascade Transformation of CO2 and Alkyne-1,2-Diols: Synthesis of Keto-Functionalized Cyclic Carbonates. Catalysts 2025, 15, 279. https://doi.org/10.3390/catal15030279
Chai D, Wang C, Liu J, Cao D, Guo K, Wang Y, Yuan Y, Verpoort F. CuCl/Ionic Liquid Catalyzed Cascade Transformation of CO2 and Alkyne-1,2-Diols: Synthesis of Keto-Functionalized Cyclic Carbonates. Catalysts. 2025; 15(3):279. https://doi.org/10.3390/catal15030279
Chicago/Turabian StyleChai, Duozhen, Chongli Wang, Jinzhen Liu, Dongfeng Cao, Kaixuan Guo, Yuankun Wang, Ye Yuan, and Francis Verpoort. 2025. "CuCl/Ionic Liquid Catalyzed Cascade Transformation of CO2 and Alkyne-1,2-Diols: Synthesis of Keto-Functionalized Cyclic Carbonates" Catalysts 15, no. 3: 279. https://doi.org/10.3390/catal15030279
APA StyleChai, D., Wang, C., Liu, J., Cao, D., Guo, K., Wang, Y., Yuan, Y., & Verpoort, F. (2025). CuCl/Ionic Liquid Catalyzed Cascade Transformation of CO2 and Alkyne-1,2-Diols: Synthesis of Keto-Functionalized Cyclic Carbonates. Catalysts, 15(3), 279. https://doi.org/10.3390/catal15030279









