Abstract
Developing cost-effective and high-performance non-precious metal-based electrocatalysts for hydrogen evolution reaction is of crucial importance toward sustainable hydrogen energy systems. Herein, we prepare a novel hybrid electrode featuring intermetallic Fe2Mo nanoparticles anchored on the hierarchical nanoporous copper skeleton as robust hydrogen evolution electrocatalyst by simple and scalable alloying and dealloying methods. By virtue of the highly active intermetallic Fe2Mo nanoparticles and unique bicontinuous nanoporous copper skeleton facilitating ion/molecule transportation, nanoporous Fe2Mo/Cu electrode shows excellent hydrogen evolution reaction electrocatalysis, with a low Tafel slope (~71 mV dec−1) to realize ampere-level current density of 1 A cm−2 at a low overpotential of ~200 mV in 1 M KOH electrolyte. Furthermore, nanoporous Fe2Mo/Cu electrode exhibits long−term stability exceeding 400 h to maintain ~250 mA cm−2 at an overpotential of 150 mV. Such outstanding electrocatalytic performance enables the nanoporous Fe2Mo/Cu electrode to be an attractive hydrogen evolution reaction catalyst for water splitting in the hydrogen economy.
1. Introduction
Severe environmental pollution and global warming crisis led by the overconsumption of fossil fuels have underscored significant consensus on the movement towards a reliable and sustainable energy framework [1,2,3,4]. Molecular hydrogen has garnered much attention as a promising alternative for its high gravimetric energy density, recyclability, and carbon-neutral attributes [1,3,5]. Electrochemical water splitting powered by renewable electricity from solar and wind resources provides a benign opportunity for green hydrogen generation and the establishment of a sustainable water cycle [3,6,7,8]. However, its practical application often encounters great challenges, particularly for hydrogen exchange membrane water electrolyzers (HEMWE) and alkaline water electrolyzers (AWE) [3,9,10,11,12]. Although both HEMWE and AWE are more cost-effective and adaptable in less corrosive conditions compared to proton exchange membrane water electrolyzer (PEMWE) [13,14], they suffer from sluggish kinetics of water dissociation to generate reactive hydrogen intermediates [3,15,16,17]. Thus, it is of crucial importance to design catalysts with proper hydrogen bonding energy (HBE) and low water dissociation barrier [3,16,18,19]. Despite being mediated by platinum group metals (PGMs) with nearly neutral HBE [3,16], the alkaline hydrogen evolution reaction (HER) kinetics is two to three orders of magnitude lower compared to acidic counterparts [20,21], resulting from its monofunctional active site unsatisfied suitable adsorption for hydrogen and hydroxyl intermediates [18,20,22]. Thus, it is essential to develop multisite electrocatalysts to accelerate water dissociation and combination of hydrogen intermediate into H2 [3,18,19,23,24,25]. Many efforts have been devoted to exploring multisite electrocatalysts via heterogeneous interface engineering [26,27,28], doping [29,30], and alloying [31,32,33], which indeed show more or less enhanced activities. However, the majority of them suffer from poor inherent working stability, especially catalysts in low-dimension that require immobilization on current collectors using insulative polymer binder, which inevitably hinders electron transfer and reduces long-term durability [16,34,35]. Therefore, it is imperative to construct monolithic self-supported catalysts to eliminate the use of polymer binders, enhance electron transfer, and ensure long-term durability.
Intermetallic compounds with ordered atomic arrangements and well-defined stoichiometric ratios have attracted tremendous attention and exhibit great potential as advanced functional materials in water electrolysis due to their unique properties, including tunable electronic structures, geometrically optimized active sites, and enhanced steric stabilization effects to optimize adsorption of intermediates and enhance stability [36,37,38]. Intermetallic compounds, such as Ni4Mo [39,40,41], Ni3Al [42], Co7Mo6 [37], and CoSn2 [43], have been proven to be efficient alkaline HER catalysts. Fe-and Mo-based alloys have been proven to be efficient in HER electrocatalysis and have been extensively studied in the past time [37,39,40,41,42,44]. However, to the best of our knowledge, no prior study on the HER activity of intermetallic compound Fe2Mo has been studied. Considering the distinct adsorption of H* and OH* on Mo and Fe, Fe2Mo might be an excellent HER electrocatalyst. Apart from the selection of intermetallic compounds with high intrinsic activity, the design of reasonable architecture is also essential in enhancing HER activity, among which multimodal nanoporous architecture is appealing for its multifunctionality in fast electron/mass transfer and prevention of rapid mechanical damage [44,45].
In this work, we report a self-supported hierarchical nanoporous Fe2Mo/Cu electrode with remarkable efficiency in alkaline hydrogen evolution reaction by a facile and scalable alloying and dealloying method, where the intermetallic Fe2Mo nanoparticles are spontaneously self-separated and anchored on bicontinuous Cu ligaments owing to the poor miscibility between Mo atoms and Cu matrix. Owing to the electronegativity difference between Fe and Mo, charge transfer from Fe to Mo takes place in intermetallic Fe2Mo, resulting in distinct adsorption of H* and OH* on Mo and Fe to accelerate water dissociation. Associated with the bicontinuous and bimodal nanoporous copper skeleton that enables rapid electron transfer and mass transportation, the nanoporous Fe2Mo/Cu electrode exhibits outstanding alkaline water electrocatalysis, with a low Tafel slope of ~71 mV dec−1 to reach high current density of 1 A cm−2 at a low overpotential of ~200 mV, outperforming most non-precious metal-based alkaline HER catalysts reported previously. The remarkable electrocatalytic performance not only demonstrates that the alloying/dealloying method is a scalable and economical approach to preparing advanced HER catalysts but also highlights the potential of nanoporous Fe2Mo/Cu electrode as a promising candidate for large-scale hydrogen production.
2. Results and Discussion
2.1. Synthesis and Microstructural Characterization of Nanoporous Fe2Mo/Cu Electrode
Self-supported nanoporous Fe2Mo/Cu electrodes with hierarchical structure are fabricated by a facile and scalable approach involving alloying and dealloying processes. The precursors, denoted as Cu12−x−yFexMoyAl88 (x, y = 0 or 2) (Figure S1), are synthesized precisely controlling the stoichiometric ratios of Cu, Al, and optionally Fe and Mo metals. For instance, Cu8Fe2Mo2Al88 precursor ingots are firstly synthesized by arc-melting pure Cu, Fe, Mo, and Al metals according to the stochiometric ratio of 8:2:2:88 under an argon atmosphere and cooled to room temperature with the assistance of the cooling system. Each alloy ingot needs to be remelted at least five times to ensure compositional homogeneity. Subsequently, alloy ingots are cut into thin sheets and polished to a thickness of approximately 400 μm. After that, the as-prepared thin alloy sheets are dealloyed in N2-saturated 6 M KOH at 70 °C until no bubbles are visible. Nanoporous electrodes are rinsed in ultrapure water (18.2 MΩ) several times to eliminate residue in the nanoporous structure afterward. Cu12Al88, Cu10Fe2Al88, and Cu10Mo2Al88 are prepared using analogous procedures, changing the input ratio of metals.
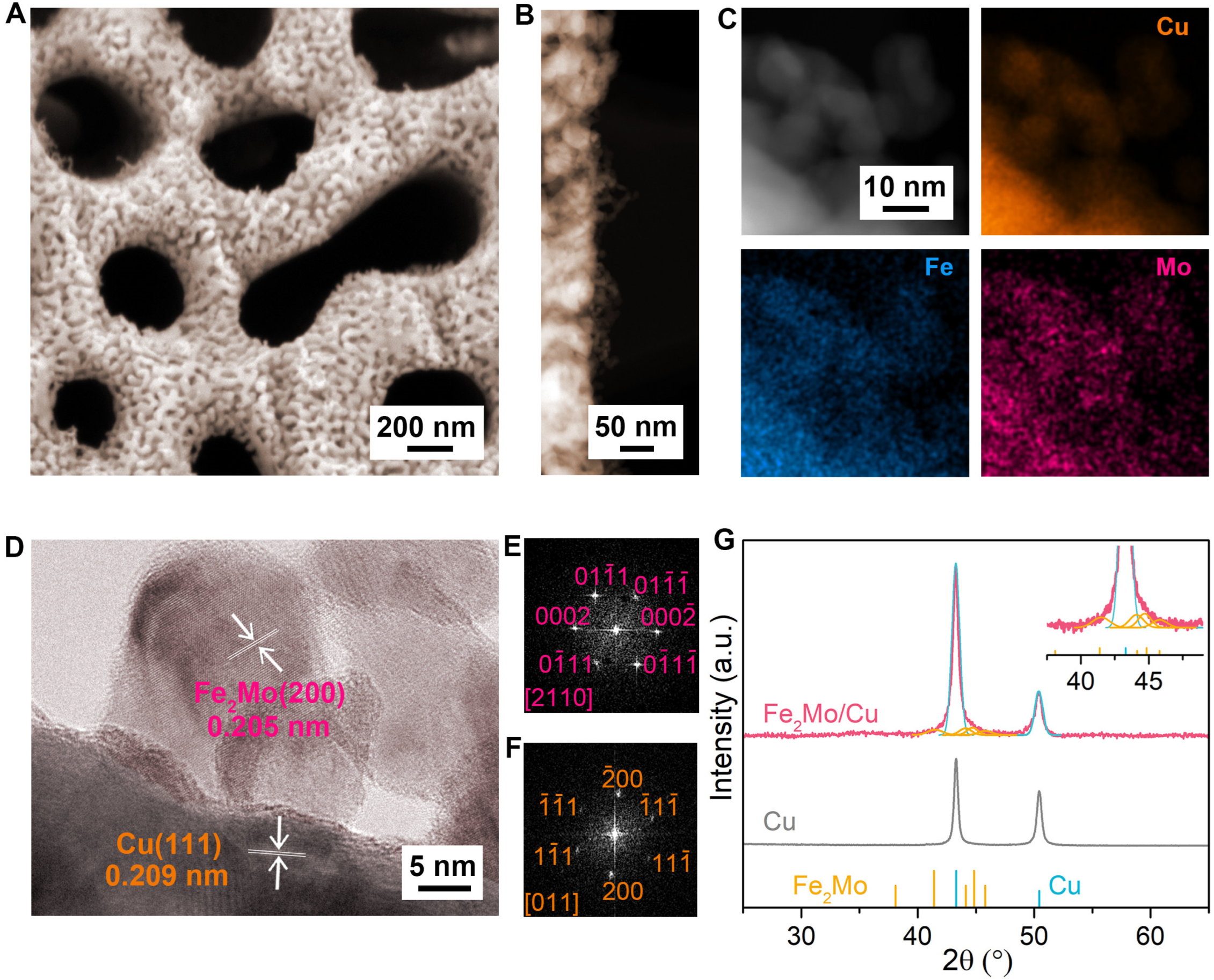
The precursor alloys (Cu12Al88, Cu10Fe2Al88, Cu10Mo2Al88, and Cu8Fe2Mo2Al88) consist of immiscible multiphase components, primarily with α-Al phase and Al2Cu intermetallic compounds. Depending on the specific composition, additional intermetallic phases such as Fe2Mo, Al5Mo, and Al7Cu2Fe may also be incorporated (Figures S2 and S3) [46,47]. The fabrication process involves a critical chemical dealloying step in a 6 M KOH solution, where a two-stage corrosion process takes place [41,44]. Initially, the α-Al phase undergoes rapid dissolution to form large channels, accompanied by the dissolution of Al5Mo and Al7Cu2Fe precipitates. Subsequently, a selective etching process occurs, where the less-noble Al component is preferentially removed from the Al2Cu phase to generate small nanopores. This controlled dealloying process results in the formation of a hierarchical nanoporous skeleton with ~300 nm large channels and ~20 nm small nanopores, respectively, as depicted in Figure 1A. Driven by the inherent miscibility between intermetallic Fe2Mo nanoparticles and the Cu matrix [44], ultrasmall intermetallic Fe2Mo nanoparticles with diameter of ~10 nm are spontaneously separated and uniformly anchored on Cu ligaments (Figure 1B), which is verified by evident Mo element aggregation on nanoparticles in the scanning transmission electron microscope (STEM) X-ray energy dispersive spectroscopy (EDS) elemental mapping (Figure 1C). Notably, the integrated hybrid electrode preserves the intrinsic geometric characteristics of the bicontinuous nanoporous Cu structure. The unique structure is further attested by a typical high-resolution transmission electron microscope (HRTEM) image (Figure 1D) and their corresponding fast Fourier transform (FFT) patterns (Figure 1E,F), which display an intimate metallic interface between nanoparticles and matrix. Such well-integrated architecture facilitates efficient electron transport and ensures good structural stability. The interplanar spacings of 0.209 nm and 0.205 nm are ascribed to the lattice planes of face-centered cubic (fcc) Cu(111) and hexagonal Fe2Mo(200) planes, respectively. The X-ray diffraction (XRD) patterns of nanoporous Fe2Mo/Cu electrode reveal two distinct sets of diffraction patterns: the evident diffraction peaks at 2θ = ~43.3°, ~50.4° assigned to (111) and (200) planes of face-centered cubic Cu (JCPDS 04-0836) and the other diffraction peaks at 2θ = ~41.4°, ~44.1°, ~44.8°, ~45.8° belonging to (103), (200), (112), (201) planes of hexagonal intermetallic Fe2Mo (JCPDS 06-0622) (Figure 1G) [48], in sharp contrast with the XRD patterns of nanoporous Cu, Mo/Cu, Fe/Cu electrodes, in which no corresponding peaks are observed due to either too little or too small nanoparticles on the Cu substrates (Figures S4–S8).
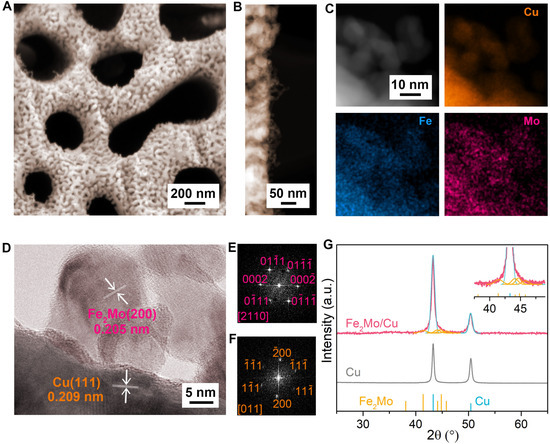
Figure 1.
Microstructure of nanoporous Fe2Mo/Cu electrode. (A) SEM image of nanoporous Fe2Mo/Cu electrode with a bicontinuous nanoporous architecture. (B) High-resolution TEM image of Fe2Mo/Cu electrode featuring numerous in situ integrated intermetallic Fe2Mo nanoparticles anchored on nanoporous Cu ligaments. (C) STEM image of nanoporous Fe2Mo/Cu electrode and the corresponding EDS elemental mappings of Cu (orange), Fe (blue), Mo (pink). (D) HRTEM image of nanoporous Fe2Mo/Cu electrode, where Fe2Mo nanoparticles are in situ integrated on Cu ligaments. (E,F) FFT patterns of Fe2Mo (top) and Cu (bottom) in the selected areas in (D). (G) XRD patterns of nanoporous Fe2Mo/Cu and bare Cu electrodes. The line patterns show reference cards 06-0622 for hexagonal Fe2Mo and 04-0836 for face-centered cubic Cu according to JCPDS, respectively. Inset: magnified XRD patterns of nanoporous Fe2Mo/Cu electrode.
2.2. Electrochemical Properties of Nanoporous Fe2Mo/Cu Electrode
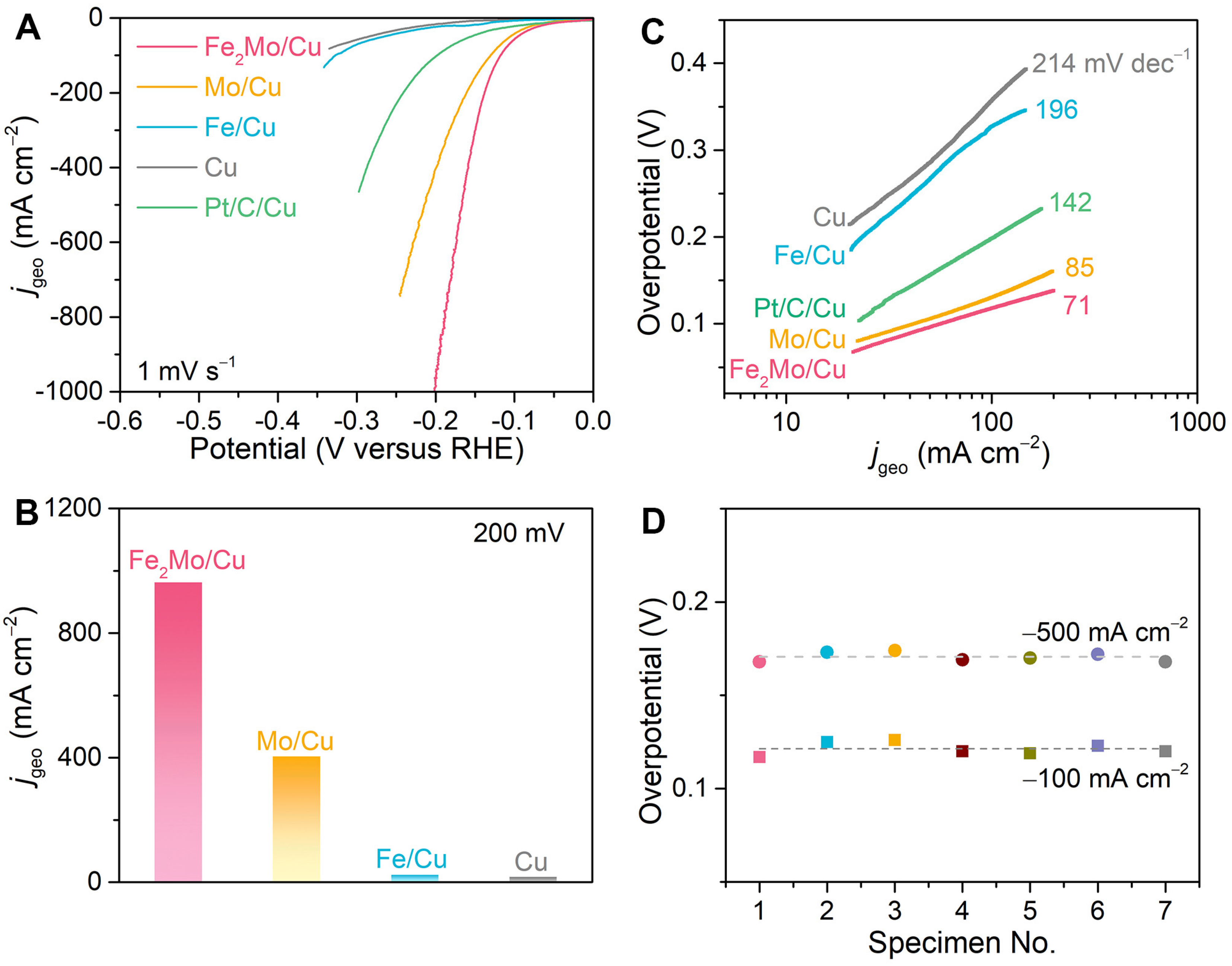
A classical three-electrode system is constructed to comprehensively evaluate the alkaline hydrogen evolution reaction performance of the fabricated nanoporous electrocatalysts. This system comprises a graphite rod serving as the counter electrode, an Ag/AgCl electrode functioning as the reference electrode, and as-prepared nanoporous electrocatalysts as the working electrode. All potentials are calibrated to RHE and iR-corrected according to the equations, ERHE = EAg/AgCl + 1.016 V, and ERHE = EAg/AgCl + 1.016 V − iR, respectively (R represents the intrinsic resistance of electrode and electrolyte and is determined by electrochemical impedance spectroscopy analysis at 100 mV with a frequency range from 10 mHz to 100 kHz. The intercept on the real axis of the Nyquist plot at the high frequencies represents the R-value.) (Figure S9). In Figure 2A, a typical HER polarization curve with the scan rate of 1 mV s−1 of the nanoporous Fe2Mo/Cu electrode is compared with those of nanoporous Mo/Cu, Fe/Cu, and bare Cu electrodes, as well as that of commercially available Pt/C catalysts immobilized on nanoporous Cu current collector using Nafion as a polymer binder (Pt/C/Cu). Although all electrodes share the common feature of hierarchical nanoporous architectures, the nanoporous Fe2Mo/Cu electrode exhibits outstanding alkaline electrocatalysis compared to other nanoporous electrodes as well as Pt/C/Cu. The superior electrocatalytic performance of nanoporous Fe2Mo/Cu electrode is demonstrated by its remarkably low overpotentials. Specifically, the nanoporous Fe2Mo/Cu electrode needs low overpotentials of ~118 and ~167 mV to reach the current densities of 100 and 500 mA cm−2, respectively, which are much smaller than those of nanoporous Mo/Cu (~131 and ~214 mV), nanoporous Fe/Cu (~328 and ~401 mV) and nanoporous Cu (~358 and ~485 mV) electrodes. The exceptional performance becomes even more sharply obvious at higher current densities, as evidenced by the capacity of nanoporous Fe2Mo/Cu electrode to deliver a current density of ~962 mA cm−2 at an overpotential of 200 mV, which is significantly higher than those of nanoporous Mo/Cu (~404 mA cm−2), nanoporous Fe/Cu (~23 mA cm−2) as well as nanoporous Cu (~17 mA cm−2) electrodes as shown in Figure 2B. Despite commercially available Pt/C is regarded as the benchmark electrocatalyst [3,16], it shows subdued performance originating from the limitations of single-site Pt in multistep electrocatalytic processes of alkaline HER [22,23], as well as insulative polymer binder hindering electron transfer and obscuring active sites [4,34]. Associated with the dual-interconnective nanoporous structure, nanoporous Fe2Mo/Cu electrodes expose sufficient active sites and greatly improve mass transportation and electron transfer. Furthermore, the Tafel slopes (Figure 2C) are calculated to characterize the HER kinetics. By fitting the linear part of Tafel plot, the Tafel slope of nanoporous Fe2Mo/Cu electrode is determined to be ~71 mV dec−1, smaller than those of nanoporous Mo/Cu (~85 mV dec−1), Fe/Cu (~196 mV dec−1), Cu (~214 mV dec−1) as well as Pt/C/Cu (~142 mV dec−1) electrodes. The Tafel slope of Fe2Mo/Cu is in the range of 40–120 mV dec−1, suggesting the electrode follows the Volmer–Heyrovsky reaction mechanism during the HER process [3,16]. To demonstrate reproducibility, seven nanoporous Fe2Mo/Cu electrodes are produced using the same dealloying procedure. As clearly illustrated in Figure 2D, they exhibit approximately ~121 mV and ~170 mV at the current densities of −100 and −500 mA cm−2, respectively, which confirms the robustness and scalability of the alloying and dealloying fabrication method, highlighting its potential for large-scale industrial applications in hydrogen production.
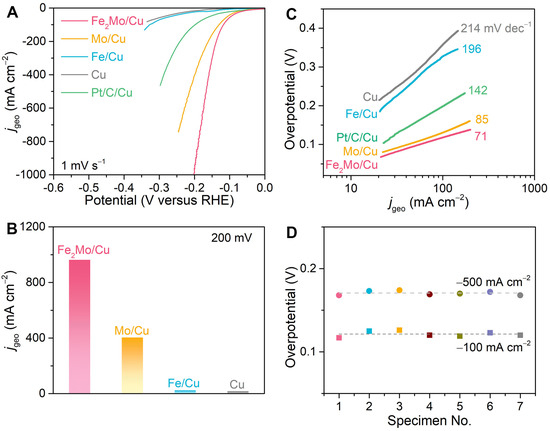
Figure 2.
Electrochemical properties of nanoporous electrocatalysts. (A) Alkaline, HER polarization curves for nanoporous Fe2Mo/Cu, Mo/Cu, Fe/Cu, and Cu electrodes, comparing with commercially available Pt/C catalysts immobilized on nanoporous Cu current collector (Pt/C/Cu) in Ar-saturated 1 M KOH electrolyte. Scan rate: 1 mV s−1. (B) Comparison of geometric current densities at the overpotential of 200 mV for nanoporous Fe2Mo/Cu, Mo/Cu, Fe/Cu and bare Cu electrodes. (C) Comparison of the Tafel slopes of different catalysts according to the HER polarization curves in panels (A). (D) Overpotentials at current densities of −100 and −500 mA cm−2 for seven nanoporous Fe2Mo/Cu electrodes prepared by the same alloying and dealloying procedure. The different colors represent different specimen.
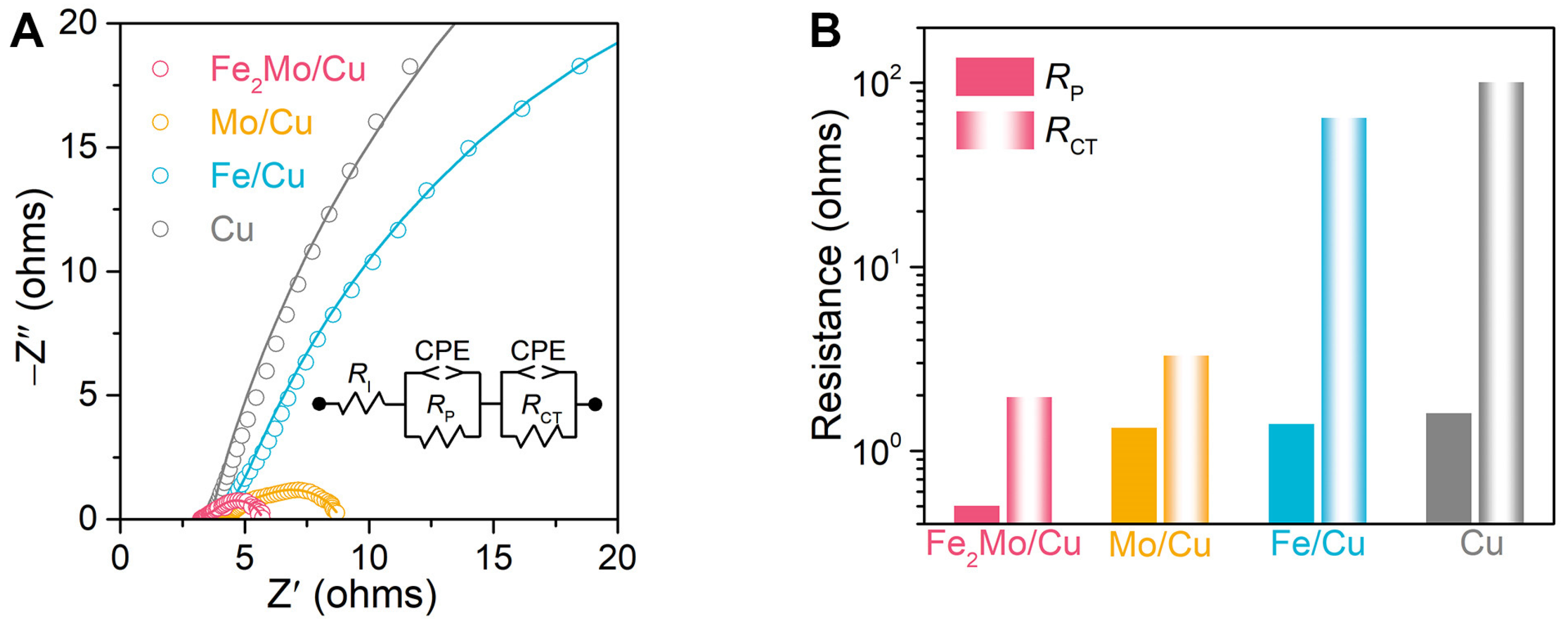
The electrochemical impedance spectroscopy (EIS) analysis is further conducted to obtain deep insights into the electrochemical behavior as well as charge transfer and reaction kinetics of these nanoporous electrodes. EIS analysis is performed at an overpotential of 100 mV with a frequency range from 10 mHz to 100 kHz. As depicted in the Nyquist plots in Figure 3A for nanoporous Fe2Mo/Cu, Mo/Cu, Fe/Cu, and bare Cu electrodes, their EIS spectra display two characteristic semicircles with distinct diameters in the middle- to low-frequency range corresponding to distinct electrochemical processes. The intercept on the real axis at the high frequencies represents the intrinsic resistance (RI) of the electrode and electrolyte [41,44]. The first semicircle in the middle-frequency range corresponds to the pore resistance (RP) associated with mass transport kinetics, while the second semicircle in the low-frequency range reflects the transfer resistance (RCT) in parallel with their CPEs (inset of Figure 3A) [41,44]. Nanoporous Fe2Mo electrode exhibits smallest RP and RCT values as low as ~0.50 and ~1.96 Ω, respectively, indicating the superior mass transportation and reaction kinetics, significantly lower than those of nanoporous Mo/Cu (~1.33 Ω, ~3.29 Ω), Fe/Cu (~1.40 Ω, ~64.6 Ω) as well as bare Cu (~1.6 Ω, ~101 Ω) electrodes (Figure 3B).
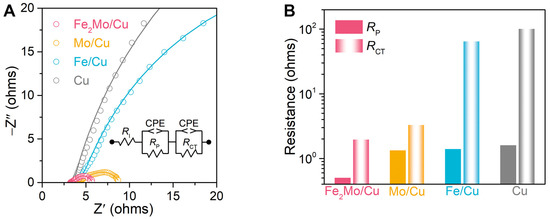
Figure 3.
Electrochemical impedance spectroscopy (EIS) analysis of nanoporous electrocatalysts. (A) EIS spectra of nanoporous Fe2Mo/Cu, Mo/Cu, Fe/Cu, and Cu electrodes (B) Comparison of pore resistance (RP) and charge transfer resistance (RCT) for nanoporous Fe2Mo/Cu (pink), Mo/Cu (orange), Fe/Cu (blue) and bare Cu (gray) electrodes according to EIS analysis. The columns with solid colors and gradient colors represent RP and RCT, respectively.
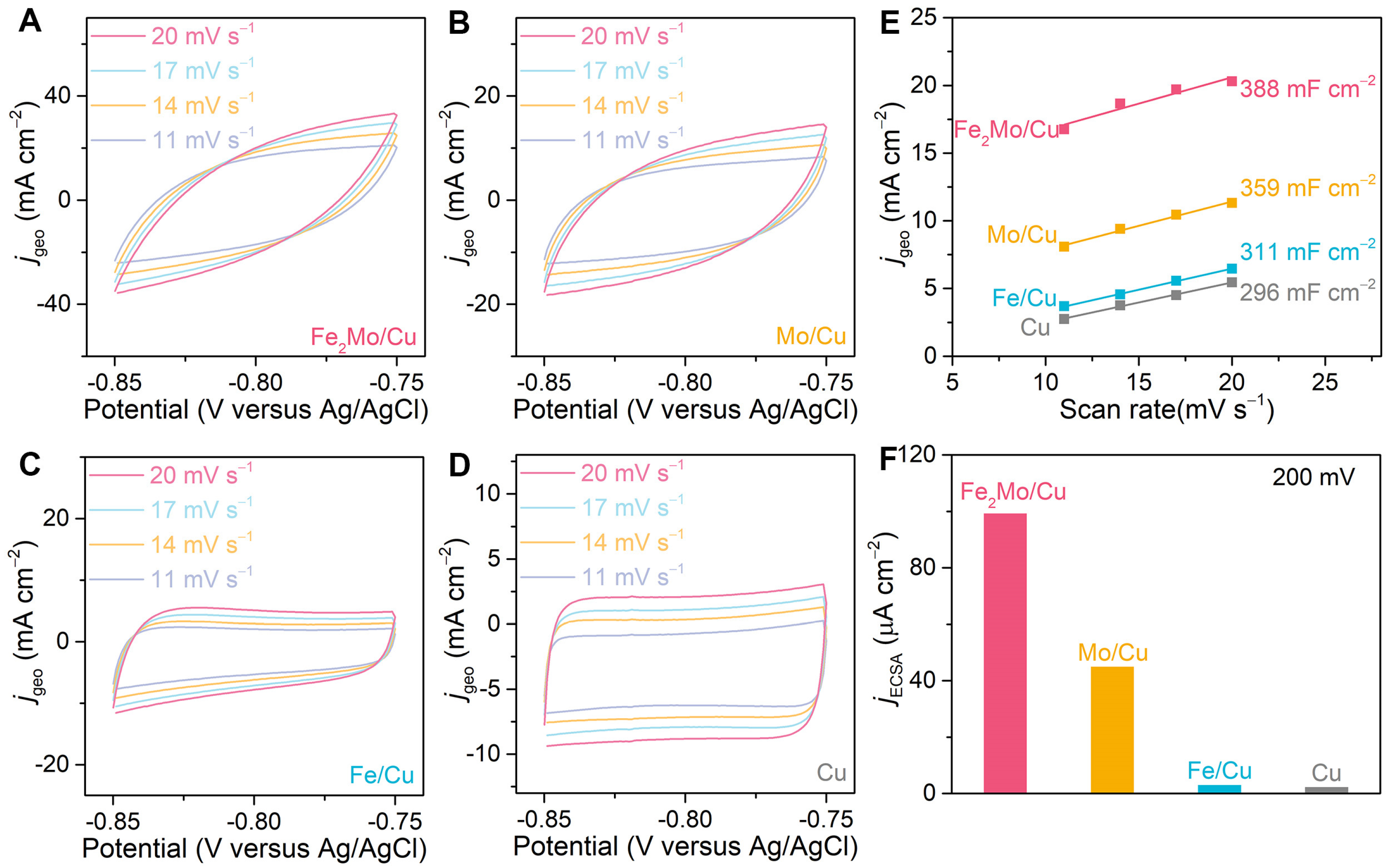
Specific activities are systematically calculated by normalizing the current densities to the electrochemical surface area (ECSA) to quantitatively evaluate the enhancement of HER intrinsic activity of nanoporous electrodes and to evaluate the enhancement of HER intrinsic activity of nanoporous electrodes. First, electrochemical surface areas of nanoporous Fe2Mo/Cu, Mo/Cu, Fe/Cu and bare Cu electrodes are assessed by their double-layer capacitances (Cdl) in a non-Faradaic voltage window ranging from −0.85 V to −0.75 V (vs. Ag/AgCl) by cycling voltammetry method at scan rates of 20, 17, 14 and 11 mV s−1 in 1 M KOH (Figure 4A–D). The double-layer capacitances are derived from the linear relationship between the current density differences at −0.8 V (vs. Ag/AgCl) and the corresponding scan rates, as illustrated in Figure 4E. In view of their similar hierarchical nanoporous architectures, the Cdl values of nanoporous Fe2Mo/Cu (388 mF cm−2), nanoporous Mo/Cu (359 mF cm−2), nanoporous Fe/Cu electrodes (311 mF cm−2) are only 1.31-, 1.20-, and 1.05-fold that of nanoporous bare Cu (296 mF cm−2) electrode (Figure 4E). The ECSA is calculated based on the following formula: AECSA = Cdl/Cs (Cs refers to the specific capacitance of the electrocatalyst, usually Cs = 40 μF cm−2). The ECSAs of nanoporous Fe2Mo/Cu, Mo/Cu, Fe/Cu, and bare Cu electrodes are calculated to be 9700, 8975, 7775, and 7400 cmECSA2 per geometric square centimeter, respectively. However, this relatively modest increase in active surface area alone cannot account for the substantial improvement in geometric current density observed during HER, thereby highlighting the pivotal role of the intermetallic Fe2Mo clusters in enhancing electrocatalytic performance. The intrinsic activity of Fe2Mo/Cu electrode is calculated to be ~99.2 μA cmECSA−2, ~2.20-, ~32.85- and ~43.89-fold those of Mo/Cu (~45.0 μA cmECSA−2), Fe/Cu (~3.02 μA cmECSA−2) and bare Cu (~2.26 μA cmECSA−2) at the overpotential of 200 mV according to the formula: js = jgeo/AECSA (js and jgeo refer to specific current density and geometric current density, respectively), respectively (Figure 4F). This is because H* and OH* intermediates prefer to adsorb on Mo and Fe atoms of intermetallic Fe2Mo, respectively, hence accelerating water dissociation. Simultaneously, the electrons transfer from less electronegative Fe to more electronegative Mo, which optimizes the electronic local environment and adjusts adsorption energy for intermediates, thus promoting combine of H* into H2.
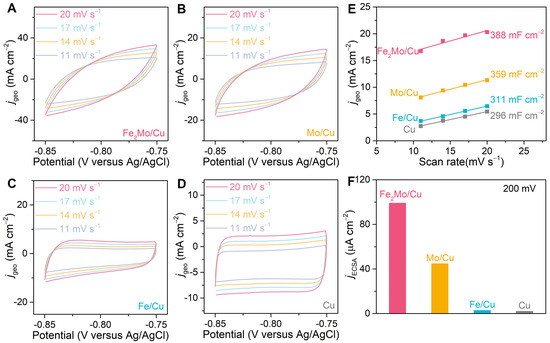
Figure 4.
Electrochemical double-layer capacitance measurements. (A–D) CV curves in the potential windows ranging from −0.85 V to −0.75 V (vs. Ag/AgCl) for nanoporous Fe2Mo/Cu (A), Mo/Cu (B), Fe/Cu (C), and Cu (D) electrodes at scan rates of 20, 17, 14 and 11 mV s−1 in 1 M KOH. (E) Double-layer capacitances (Cdl) are calculated by the differences in current densities at −0.8 V (vs. Ag/AgCl) for the nanoporous electrodes as a linear function of scan rates. (F) Specific activities of nanoporous Fe2Mo/Cu, Mo/Cu, Fe/Cu, and Cu at the overpotential of 200 mV.
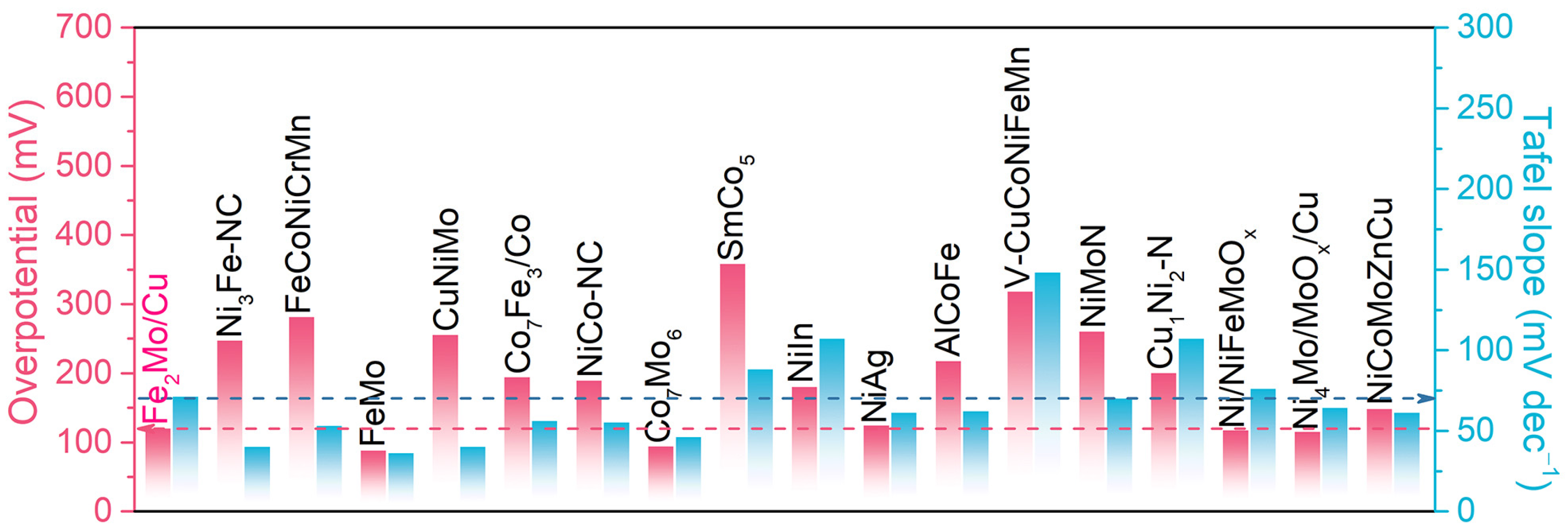
When compared with state-of-the-art non-precious transition-metal-based HER catalysts reported recently, the nanoporous Fe2Mo/Cu electrodes demonstrate exceptional performance metrics in terms of both Tafel slope and overpotential at 100 mA cm−2 (Figure 5 and Table S1) [4,16,17,37,42,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66]. This comprehensive comparison highlights that nanoporous Fe2Mo/Cu electrodes are impressive HER electrocatalysts able to replace noble metal-based catalysts for alkaline hydrogen evolution reactions. The synergistic combination of hierarchical nanoporous architecture, optimized electronic structure, and uniformly distributed active sites contributes to the outstanding electrochemical performance, making this material a promising candidate for practical water electrolysis applications.
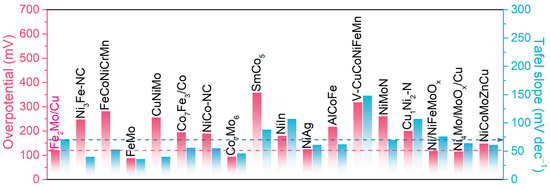
Figure 5.
The overpotential at the geometric current density of 100 mA cm−2 and the Tafel slope for nanoporous Fe2Mo/Cu electrode, compared with the values of representative HER catalysts reported previously.
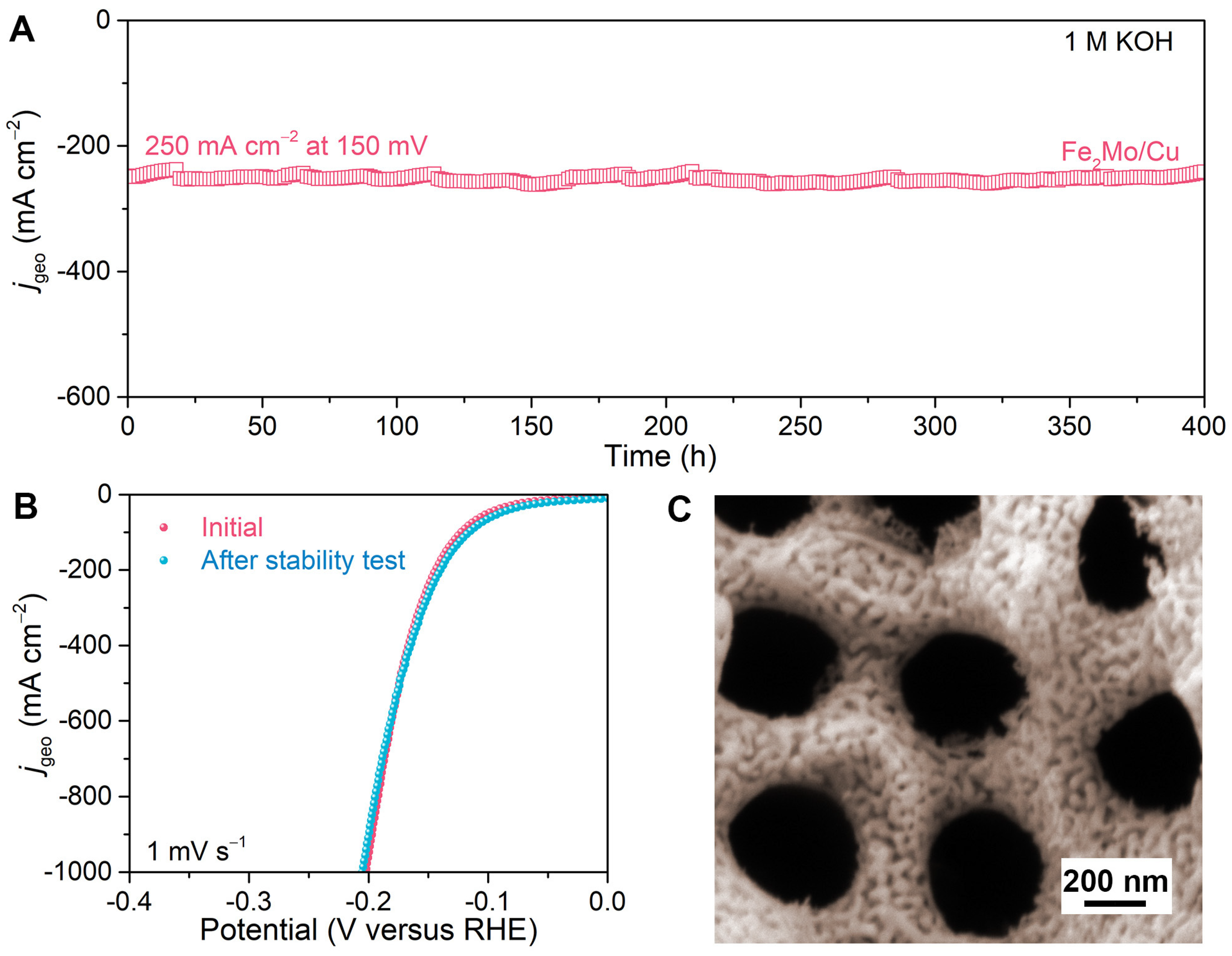
To demonstrate the practical application, the long-term durability of nanoporous Fe2Mo/Cu electrode is assessed for more than 400 h at −150 mV versus RHE with the current density of approximately 250 mA cm−2 in 1 M KOH (Figure 6A). There is no evident change in the current density during the test except for the inevitable minor fluctuation caused by the periodic depletion and replenishment of electrolytes [44]. After the long-term stability test, the nanoporous Fe2Mo/Cu electrode maintains a polarization curve nearly identical to the initial one (Figure 6B) and preserves its original hierarchical nanoporous architecture with continuously distributed Fe2Mo nanoparticles on Cu ligaments (Figure 6C). The ICP-OES analysis of the post-test electrolyte reveals that the ion concentrations of Mo and Al are all below the detection limit, and a trace amount of Cu (0.0020 mg L−1) and Fe (0.0146 mg L−1) are detected, indicating no Mo and Al but minimal Cu and Fe of the electrode dissolute during the long-term stability, demonstrating excellent stability of Fe2Mo/Cu electrode. (Table S2). This exceptional durability is attributed to the robust structural integrity of the intermetallic Fe2Mo nanoparticles and multifunctional nanoporous copper scaffold, which collectively mitigate structural degradation during prolonged electrolysis. These impressive electrochemical properties indicate that the nanoporous Fe2Mo/Cu electrode is one of the most attractive electrocatalysts for alkaline HER.
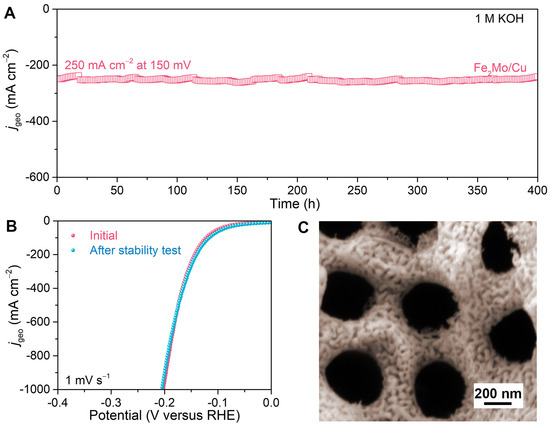
Figure 6.
Long-term stability performance of nanoporous Fe2Mo/Cu electrode for alkaline HER. (A) The durability of nanoporous Fe2Mo/Cu electrode at the overpotential of 150 mV for 400 h. (B) HER polarization curves of nanoporous Fe2Mo/Cu electrode before and after stability test for 400 h. (C) Representative SEM image of nanoporous Fe2Mo/Cu electrode after the durability test for 400 h.
3. Materials and Methods
3.1. Preparation of Nanoporous Electrocatalysts
The nanoporous Fe2Mo/Cu electrodes were fabricated through a well-controlled alloying-dealloying synthesis route. Initially, Cu8Fe2Mo2Al88 precursor alloy ingots were prepared by arc melting high-purity Cu, Fe, Mo, and Al metals (purity > 99.9%) under an argon protective atmosphere, with the atomic ratio precisely maintained at 8:2:2:88. The resulting ingots were then mechanically processed into thin sheets with a uniform thickness of approximately 400 μm through sequential cutting and polishing procedures.
The dealloying process was carried out by immersing the alloy sheets in a 6 M KOH solution saturated with N2 gas, maintained at a constant temperature of 70 °C in a water bath. The dealloying process proceeded until no bubbles were visible. Nanoporous electrodes were rinsed in ultrapure water (18.2 MΩ) several times to eliminate residue.
For comparative studies, three additional alloy counterparts (Cu12Al88, Cu10Fe2Al88, and Cu10Mo2Al88) were synthesized following the identical fabrication protocol, with only the initial metal composition being systematically varied.
To prepare Pt/C catalyst inks, commercially available Pt/C (20 wt%, Johnson Matthey, London, United Kingdom) was mixed with a 0.05 wt%Nafion (Sigma Aldrich, St. Louis, Missouri, USA) solution, 20% isopropanol and 80% water. The mixture was subjected to vigorous sonication to ensure thorough dispersion. Subsequently, 100 μL Pt/C inks were drop cast onto nanoporous Cu electrodes, achieving a mass loading of 2 mg cm−2. These Pt/C/Cu electrodes were then used for subsequent electrochemical measurements.
3.2. Physicochemical Characterization
X-ray diffraction (XRD) measurements were conducted to characterize the chemical phase compositions of precursor alloys and nanoporous electrocatalysts on a Rigaku Smartlab diffractometer, which employed monochromated Cu Kα radiation and operated at a power of 9 kW. An X-ray energy-dispersive spectroscopy (EDS, Tokyo, Japan) integrated with a field-emission scanning electron microscope (JSM-7900 F, JEOL Ltd. Tokyo, Japan) operated at 5 kV was utilized to characterize the microstructure and chemical composition features of nanoporous electrocatalysts. High-resolution transmission electron microscopy (HRTEM, Tokyo, Japan) images were acquired using a field-emission transmission electron microscope (JEOL JEM-2100F, Tokyo, Japan) at an accelerating voltage of 200 keV equipped with EDS. Additionally, the metal ion concentrations were detected using inductively coupled plasma optical emission spectrometry (ICP-OES) analysis (Thermo electron, Massachusetts, USA).
3.3. Electrochemical Measurements
The classic three-electrode system was constructed for electrochemical assessments with a graphite rod, an Ag/AgCl electrode, and a self-supported nanoporous catalyst as counter, reference, and working electrode, respectively. The HER electrocatalytic performance was conducted in an Ar-saturated 1 M KOH aqueous electrolyte with polarization curves recorded at a scan rate of 1 mV s−1 at 25 °C. Potentials were calibrated to a reversible hydrogen electrode (RHE) and iR- compensated using the following equations: ERHE = EAg/AgCl + 1.016 V, and ERHE = EAg/AgCl + 1.016 V − iR. Electrochemical impedance spectroscopy (EIS) was performed at an overpotential of 100 mV, covering a frequency range from 10 mHz to 100 kHz. Cyclic voltammogram (CV) measurements were collected within the non-Faradic voltage window from −0.85 to −0.75 V (vs. Ag/AgCl) for all nanoporous electrodes at various scan rates to determine their double-layer capacitance (Cdl) and evaluate the electrochemically active surface areas (ECSAs). The HER long-term durability of nanoporous Fe2Mo/Cu electrode was operated at the current density of ~250 mA cm−2 at −150 mV versus RHE in 1 M KOH exceeding 400 h.
4. Conclusions
In summary, we have developed a novel hybrid Fe2Mo/Cu electrode composed of self-separated intermetallic Fe2Mo nanoparticles anchored on a bicontinuous nanoporous Cu electrode as an efficient HER catalyst. The synergistic combination of highly active intermetallic Fe2Mo and the unique hierarchical nanoporous skeleton to facilitate rapid electron transfer and mass transportation endows the Fe2Mo/Cu electrode with exceptional HER performance of a low Tafel slope of 71 mV dec−1 to realize a high current density of 1 A cm−2 at a low overpotential of ~200 mV. Furthermore, the nanoporous Fe2Mo/Cu electrode shows impressive stability exceeding 400 h, maintaining ~250 mA cm−2 at an overpotential of 150 mV in 1 M KOH. The combination of high efficiency, stability, and cost-effectiveness positions the nanoporous Fe2Mo/Cu electrode as a highly promising candidate for large-scale hydrogen production in the hydrogen economy.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/catal15030278/s1, Figure S1: EDS elemental characterizations of precursor alloys. EDS spectra of precursor alloys of Cu8Fe2Mo2Al88 (a), Cu10Mo2Al88 (b), Cu10Fe2Al88 (c), Cu12Al88 (d) alloys.; Figure S2: XRD characterizations of precursor alloys. The line patterns show reference cards 04-0787 for α-Al (purple line), 25-0012 for Al2Cu (green line), 44-1102 for Al5Mo (brown line), 25-1121 for Al7Cu2Fe (navy line) and 06-0622 for Fe2Mo (pink line) according to JCPDS.; Figure S3: EDS elemental characterizations of nanoporous electrodes. EDS spectra of nanoporous Fe2Mo/Cu (a), Mo/Cu (b), Fe/Cu (c), Cu (d) electrodes.; Figure S4: XRD patterns of nanoporous Mo/Cu, Fe/Cu, Cu electrodes. The line patterns show reference card 04-0836 for fcc Cu according to JCPDS.; Figure S5: Typical cross-sectional SEM image of nanoporous Cu electrode.; Figure S6: Typical cross-sectional SEM image of nanoporous Fe/Cu electrode.; Figure S7: Typical cross-sectional SEM image of nanoporous Mo/Cu electrode.; Figure S8: Typical cross-sectional SEM image of nanoporous Fe2Mo/Cu electrode.; Figure S9: Calibration of the Ag/AgCl reference electrode. The current–potential curve was obtained using Pt wire as the working electrode in H2-purged 1 M KOH electrolyte to calibrate the Ag/AgCl electrode with respect to RHE in 1 M KOH. ERHE = EAg/AgCl + 1.016.; Table S1: Comparisons of the HER performance of nanoporous Fe2Mo/Cu electrode in 1 M KOH electrolyte with representative electrocatalysts reported previously. Herein, η−10, η−100, η−200 and η−500 represent the overpotentials to achieve current densities of −10, −100, −200 and −500 mA cm−2, respectively.; Table S2: Ion concentrations of Cu, Fe, Mo and Al elements in tested electrolyte after the HER durability test of nanoporous Fe2Mo/Cu electrode for 400 h at an overpotential of 150 mV.
Author Contributions
Conceptualization, X.-Y.L., H.S. and Q.J.; methodology, H.S., X.-Y.L. and Z.-L.Z.; software, Z.-L.Z. and Y.L.; validation, Z.-L.Z., Y.L. and S.-P.Z.; formal analysis, H.S. and S.-P.Z.; investigation, Z.-L.Z., Y.L. and S.-P.Z.; data curation, Z.-L.Z., H.S. and Y.W.; writing—original draft preparation, Z.-L.Z. and H.S.; writing—review and editing, X.-Y.L. and Q.J.; funding acquisition, X.-Y.L., Q.J. and H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (No. 52271217, 52201217, 52130101), China Postdoctoral Support Program for Innovation Talents (BX20220129), China Postdoctoral Science Foundation (2022M711290), Chang Jiang Scholar Program of China (Q2016064), the Program for JLU Science and Technology Innovative Research Team (JLUSTIRT, 2017TD-09), the Fundamental Research Funds for the Central Universities.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2017, 16, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Chatenet, M.; Pollet, B.G.; Dekel, D.R.; Dionigi, F.; Deseure, J.; Millet, P.; Braatz, R.D.; Bazant, M.Z.; Eikerling, M.; Staffell, I.; et al. Water electrolysis: From textbook knowledge to the latest scientific strategies and industrial developments. Chem. Soc. Rev. 2022, 51, 4583–4762. [Google Scholar]
- Wang, J.; Gao, Y.; Kong, H.; Kim, J.; Choi, S.; Ciucci, F.; Hao, Y.; Yang, S.H.; Shao, Z.P.; Lim, J. Non-Precious-Metal catalysts for alkaline water electrolysis: Operando characterizations, theoretical calculations, and recent advances. Chem. Soc. Rev. 2020, 49, 9154–9196. [Google Scholar] [CrossRef]
- Guan, D.Q.; Wang, B.W.; Zhang, J.G.; Shi, R.; Jiao, K.; Li, L.C.; Wang, Y.; Xie, B.; Zhang, Q.W.; Yu, J.; et al. Hydrogen society: From present to future. Energy Environ. Sci. 2023, 16, 4926–4943. [Google Scholar] [CrossRef]
- Markovic, N.M. Interfacing electrochemistry. Nat. Mater. 2013, 12, 101–102. [Google Scholar] [CrossRef]
- Cook, T.R.; Dogutan, D.K.; Reece, S.Y.; Surendranath, Y.; Teets, T.S.; Nocera, D.G. Solar energy supply and storage for the legacy and nonlegacy worlds. Chem. Rev. 2010, 110, 6474–6502. [Google Scholar] [CrossRef]
- Glenk, G.; Reichelstein, S. Economics of converting renewable power to hydrogen. Nat. Energy 2019, 4, 216–222. [Google Scholar] [CrossRef]
- Yang, Y.X.; Li, P.; Zheng, X.B.; Sun, W.P.; Dou, S.X.; Ma, T.Y.; Pan, H.G. Anion-exchange membrane water electrolyzers and fuel cells. Chem. Soc. Rev. 2022, 51, 9620–9693. [Google Scholar] [CrossRef]
- Abbasi, R.; Setzler, B.P.; Lin, S.S.; Wang, J.H.; Zhao, Y.; Xu, H.; Pivovar, B.; Tian, B.Y.; Chen, X.; Wu, G.; et al. A roadmap to low-cost hydrogen with hydroxide exchange membrane electrolyzers. Adv. Mater. 2019, 31, 1805876. [Google Scholar] [CrossRef]
- Li, D.G.; Park, E.J.; Zhu, W.L.; Shi, Q.R.; Zhou, Y.; Tian, H.Y.; Lin, Y.H.; Serov, A.; Zulevi, B.; Baca, E.D.; et al. Highly quaternized polystyrene ionomers for high performance anion exchange membrane water electrolysers. Nat. Energy 2020, 5, 378–385. [Google Scholar] [CrossRef]
- Wang, N.; Song, S.Z.; Wu, W.T.; Deng, Z.F.; Tang, C. Bridging laboratory electrocatalysts with industrially relevant alkaline water electrolyzers. Adv. Energy Mater. 2024, 14, 2303451. [Google Scholar] [CrossRef]
- Lagadec, M.F.; Grimaud, A. Water electrolysers with closed and open electrochemical systems. Nat. Mater. 2020, 19, 1140–1150. [Google Scholar] [CrossRef]
- Wang, Y.; Pang, Y.H.; Xu, H.; Martinez, A.; Chen, K.S. PEM fuel cell and electrolysis cell technologies and hydrogen infrastructure development—A review. Energy Environ. Sci. 2022, 15, 2288–2328. [Google Scholar] [CrossRef]
- Jiao, S.L.; Fu, X.W.; Wang, S.Y.; Zhao, Y. Perfecting electrocatalysts via imperfections: Towards the large-scale deployment of water electrocatalysis technology. Energy Environ. Sci. 2021, 14, 1722–1770. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Duan, Y.; Feng, X.Y.; Yu, X.X.; Gao, M.R.; Yu, S.H. Clean and affordable hydrogen fuel from alkaline water splitting: Past, recent progress, and future prospects. Adv. Mater. 2021, 33, 2007100. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, L.S.; Zhao, P.X.; Lee, L.Y.S.; Wong, K.Y. Recent advances in electrocatalytic hydrogen evolution using nanoparticles. Chem. Rev. 2020, 120, 851–918. [Google Scholar] [CrossRef]
- Cui, W.G.; Gao, F.; Na, G.Q.; Wang, X.Q.; Li, Z.L.; Yang, Y.X.; Niu, Z.Q.; Qu, Y.Q.; Wang, D.S.; Pan, H.G. Insights into the pH effect on hydrogen electrocatalysis. Chem. Soc. Rev. 2024, 53, 10253–10311. [Google Scholar] [CrossRef]
- Strmcnik, D.; Lopes, P.P.; Genorio, B.; Stamenkovic, V.R.; Markovic, N.M. Design principles for hydrogen evolution reaction catalyst materials. Nano Energy 2016, 29, 29–36. [Google Scholar] [CrossRef]
- Ledezma-Yanez, I.; Wallace, W.D.Z.; Sebastián-Pascual, P.; Climent, V.; Feliu, J.M.; Koper, M.T.M. Interfacial water reorganization as a pH-dependent descriptor of the hydrogen evolution rate on platinum electrodes. Nat. Energy 2017, 2, 17031. [Google Scholar] [CrossRef]
- Tian, X.Y.; Zhao, P.C.; Sheng, W.C. Hydrogen evolution and oxidation: Mechanistic studies and material advances. Adv. Mater. 2019, 31, 1808066. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Chorkendorff, I. Considerations for the scaling-up of water splitting catalysts. Nat. Energy 2019, 4, 430–433. [Google Scholar] [CrossRef]
- Zhang, B.X.; Wang, J.M.; Liu, G.M.; Weiss, C.M.; Liu, D.Q.; Chen, Y.P.; Xia, L.X.; Zhou, P.; Gao, M.X.; Liu, Y.F.; et al. A strongly coupled Ru-CrOx cluster-cluster heterostructure for efficient alkaline hydrogen electrocatalysis. Nat. Catal. 2024, 7, 441–451. [Google Scholar] [CrossRef]
- Oener, S.Z.; Foster, M.J.; Boettcher, S.W. Accelerating water dissociation in bipolar membranes and for electrocatalysis. Science 2022, 369, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wei, W.R.; Ding, S.Q.; Wu, L.; Qin, H.Y.; Yuan, X.X. A Multi-site synergistic effect in high-entropy alloy for efficient hydrogen evolution. Adv. Funct. Mater. 2025, 35, 2414554. [Google Scholar] [CrossRef]
- Subbaraman, R.; Tripkovic, D.; Strmcnik, D.; Chang, K.C.; Uchimura, M.; Paulikas, A.P.; Stamenkovic, V.; Markovic, N.M. Enhancing hydrogen evolution activity in water splitting by tailoring Li+-Ni(OH)2-Pt interfaces. Science 2011, 334, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.S.; Dutta, S.; Hong, Y.R.; Okello, O.F.N.; Im, H.; Ahn, S.; Choi, S.Y.; Han, J.W.; Ryu, S.; Lee, I.S. Harmonious heterointerfaces formed on 2D-Pt nanodendrites by facet-respective stepwise metal deposition for enhanced hydrogen evolution reaction. Angew. Chem. Int. Ed. 2023, 62, e202307816. [Google Scholar] [CrossRef]
- Xu, X.M.; Zhong, Y.J.; Wajrak, M.; Bhatelia, T.; Jiang, S.P.; Shao, Z.P. Grain boundary engineering: An emerging pathway toward efficient electrocatalysis. InfoMat 2024, 6, e12608. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Klingenhof, M.; Gao, C.L.; Koketsu, T.; Weiser, G.; Pi, Y.C.; Liu, S.H.; Sui, L.J.; Hou, J.R.; Li, J.Y.; et al. Facilitating alkaline hydrogen evolution reaction on the hetero-interfaced Ru/RuO2 through Pt single atoms doping. Nat. Commun. 2024, 15, 1447. [Google Scholar] [CrossRef]
- Cheng, X.Y.; Mao, C.H.; Tian, J.Y.; Xia, M.Q.; Yang, L.J.; Wang, X.Z.; Wu, Q.; Hu, Z. Correlation between heteroatom coordination and hydrogen evolution for single-site Pt on carbon-based nanocages. Angew. Chem. Int. Ed. 2024, 63, e202401304. [Google Scholar] [CrossRef]
- Liu, K.; Yang, H.; Jiang, Y.L.; Liu, Z.J.; Zhang, S.M.; Zhang, Z.X.; Qiao, Z.; Lu, Y.M.; Cheng, T.; Terasaki, O.; et al. Coherent hexagonal platinum skin on nickel nanocrystals for enhanced hydrogen evolution activity. Nat. Commun. 2023, 14, 2424. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.W.; Wang, J.P.; Guo, L.F.; Li, H.D.; Xiao, W.P.; Xu, G.R.; Chen, D.H.; Li, C.X.; Du, Y.M.; Ding, H.; et al. Microwave-assisted PtRu alloying on defective tungsten oxide: A pathway to improved hydroxyl dynamics for highly-efficient hydrogen evolution reaction. Adv. Energy Mater. 2024, 14, 2402372. [Google Scholar] [CrossRef]
- Xu, X.M.; Shao, Z.P.; Jiang, S.P. High-entropy materials for water electrolysis. Energy Technol. 2022, 10, 2200573. [Google Scholar] [CrossRef]
- Sun, H.M.; Yan, Z.H.; Liu, F.M.; Xu, W.C.; Cheng, F.Y.; Chen, J. Self-supported transition-metal-based electrocatalysts for hydrogen and oxygen evolution. Adv. Mater. 2020, 32, 1806326. [Google Scholar] [CrossRef]
- Xiong, H.; Zhuang, R.; Cheng, B.; Liu, D.K.; Du, Y.X.; Wang, H.Y.; Liu, Y.; Xu, F.; Wang, H.Q. Self-supported metallic alkaline hydrogen evolution electrocatalysts tolerant for ampere-level current densities. Adv. Energy Mater. 2025, 15, 2404077. [Google Scholar] [CrossRef]
- Yan, Y.C.; Du, J.S.S.; Gilroy, K.D.; Yang, D.R.; Xia, Y.N.; Zhang, H. Intermetallic nanocrystals: Syntheses and catalytic applications. Adv. Mater. 2017, 29, 1605997. [Google Scholar] [CrossRef]
- Song, R.R.; Han, J.H.; Okugawa, M.; Belosludov, R.; Wada, T.; Jiang, J.; Wei, D.X.; Kudo, A.; Tian, Y.; Chen, M.W.; et al. Ultrafine nanoporous intermetallic catalysts by high-temperature liquid metal dealloying for electrochemical hydrogen production. Nat. Commun. 2022, 13, 5157. [Google Scholar] [CrossRef]
- Kim, H.Y.; Joo, S.H. Recent advances in nanostructured intermetallic electrocatalysts for renewable energy conversion reactions. J. Mater. Chem. A 2020, 8, 8195–8217. [Google Scholar] [CrossRef]
- Wang, Y.; Arandiyan, H.; Mofarah, S.S.; Shen, X.J.; Bartlett, S.A.; Koshy, P.; Sorrell, C.C.; Sun, H.Y.; Pozo-Gonzalo, C.; Dastafkan, K.; et al. Stacking fault-enriched MoNi4/MoO2 enables high-performance hydrogen evolution. Adv. Mater. 2024, 36, 2402156. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.; Liu, P.; Liao, Z.Q.; Liu, S.H.; Zhuang, X.D.; Chen, M.W.; Zschech, E.; Feng, X.L. Efficient hydrogen production on MoNi4 electrocatalysts with fast water dissociation kinetics. Nat. Commun. 2017, 8, 15437. [Google Scholar] [CrossRef]
- Shi, H.; Dai, T.Y.; Sun, X.Y.; Zhou, Z.L.; Zeng, S.P.; Wang, T.H.; Han, G.F.; Wen, Z.; Fang, Q.R.; Lang, X.Y.; et al. Dual-intermetallic heterostructure on hierarchical nanoporous metal for highly efficient alkaline hydrogen electrocatalysis. Adv. Mater. 2024, 36, 2406711. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Yang, T.; Sun, L.G.; Zhao, Y.L.; Li, W.P.; Luan, J.H.; Lyu, F.C.; Zhang, L.C.; Kruzic, J.J.; Kai, J.J.; et al. A novel multinary intermetallic as an active electrocatalyst for hydrogen evolution. Adv. Mater. 2020, 32, 2000385. [Google Scholar] [CrossRef] [PubMed]
- Menezes, P.W.; Panda, C.; Garai, S.; Walter, C.; Guiet, A.; Driess, M. Structurally ordered intermetallic cobalt stannide nanocrystals for high-performance electrocatalytic overall water-splitting. Angew. Chem. Int. Ed. 2018, 57, 15237–15242. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhou, Y.T.; Yao, R.Q.; Wan, W.B.; Ge, X.; Zhang, W.; Wen, Z.; Lang, X.Y.; Zheng, W.T.; Jiang, Q. Spontaneously separated intermetallic Co3Mo from nanoporous copper as versatile electrocatalysts for highly efficient water splitting. Nat. Commun. 2020, 11, 2940. [Google Scholar] [CrossRef]
- Li, W.X.; Liu, Y.; Azam, A.; Liu, Y.C.; Yang, J.; Wang, D.Y.; Sorrell, C.C.; Zhao, C.; Li, S. Unlocking efficiency: Minimizing energy loss in electrocatalysts for water splitting. Adv. Mater. 2024, 36, 2404658. [Google Scholar] [CrossRef]
- Du, Z.M.; Guo, C.P.; Li, C.R.; Zhang, W.J. Thermodynamic description of the Al-Mo and Al-Fe-Mo systems. J. Phase Equilib. Diffus. 2009, 30, 487–501. [Google Scholar] [CrossRef]
- Guillermet, A.F. The Fe-Mo (iron-molybdenum) system. Bull. Alloy Phase Diagr. 1982, 3, 359–367. [Google Scholar] [CrossRef]
- Yan, J.Y.; Liu, P.; Li, J.W.; Huang, H.; Tong, S.F.; Song, W.B. A bioinspired Fe/Mo bimetallic nitride catalyst for efficient electrochemical ammonia synthesis and Zn-nitrate battery. Chem. Eng. J. 2024, 498, 155108. [Google Scholar] [CrossRef]
- Li, S.B.; Hou, Y.Y.; Feng, G.; Li, Q.C.; Zhai, H.; Hua, Q.F.; Hu, R.M.; Xu, M.; Zhang, C.X.; Huang, Z.Q.; et al. High-entropy alloy nanoflower array electrodes with optimizable reaction pathways for low-voltage hydrogen production at industrial-grade current density. Adv. Mater. 2025, 37, 2416200. [Google Scholar] [CrossRef]
- Li, T.F.; Luo, G.; Liu, K.H.; Li, X.; Sun, D.M.; Xu, L.; Li, Y.F.; Tang, Y.W. Encapsulation of Ni3Fe nanoparticles in N-doped carbon nanotube–grafted carbon nanofibers as high-efficiency hydrogen evolution electrocatalysts. Adv. Funct. Mater. 2018, 28, 1805828. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Mao, J.N.; Zhou, H.; Xing, L.L.; Qian, S.S.; Yuan, J.L.; Mei, B.B.; Wei, Z.X.; Zhao, S.L.; Tang, Y.H.; et al. Coordination shell dependent activity of CuCo diatomic catalysts for oxygen reduction, oxygen evolution, and hydrogen evolution reaction. Adv. Funct. Mater. 2024, 34, 2311664. [Google Scholar] [CrossRef]
- Zhu, H.; Sun, S.H.; Hao, J.C.; Zhuang, Z.C.; Zhang, S.G.; Wang, T.D.; Kang, Q.; Lu, S.L.; Wang, X.F.; Lai, F.L.; et al. A high-entropy atomic environment converts inactive to active sites for electrocatalysis. Energy Environ. Sci. 2023, 16, 619–628. [Google Scholar] [CrossRef]
- Lyu, F.C.; Zeng, S.S.; Jia, Z.; Ma, F.X.; Sun, L.G.; Cheng, L.Z.; Pan, J.; Bao, Y.; Mao, Z.Y.; Bu, Y.; et al. Two-dimensional mineral hydrogel-derived single atoms-anchored heterostructures for ultrastable hydrogen evolution. Nat. Commun. 2022, 13, 6249. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, H.Y.; Zhang, L.F.; Xu, X.N.; Cheng, H.L.; Yan, C.L.; Qian, T. Evolution of grain boundaries promoted hydrogen production for industrial-grade current density. Adv. Mater. 2024, 36, 2313156. [Google Scholar] [CrossRef]
- Tan, X.Y.; Geng, S.Z.; Ji, Y.J.; Shao, Q.; Zhu, T.; Wang, P.T.; Li, Y.Y.; Huang, X.Q. Closest packing polymorphism interfaced metastable transition metal for efficient hydrogen evolution. Adv. Mater. 2020, 32, 2002857. [Google Scholar] [CrossRef]
- Wang, X.Y.; Xu, X.Q.; Nie, Y.; Wang, R.H.; Zou, J.L. Electronic-state modulation of metallic Co-assisted Co7Fe3 alloy heterostructure for highly efficient and stable overall water splitting. Adv. Sci. 2023, 10, 2301961. [Google Scholar] [CrossRef]
- Kumar, A.; Bui, V.Q.; Lee, J.S.; Wang, L.L.; Jadhav, A.R.; Liu, X.H.; Shao, X.D.; Liu, Y.; Yu, J.M.; Hwang, Y.; et al. Moving beyond bimetallic-alloy to single-atom dimer atomic-interface for all-pH hydrogen evolution. Nat. Commun. 2021, 12, 6766. [Google Scholar] [CrossRef]
- Chen, Z.L.; Mebs, S.; Mondal, I.; Yang, H.Y.; Dau, H.; Kang, Z.H.; Haumann, M.; Ghosh, S.; Cen, W.L.; Driess, M.; et al. Hydrogen-induced disproportionation of samarium-cobalt intermetallics enabling promoted hydrogen evolution reaction activity and durability in alkaline media. Adv. Funct. Mater. 2024, 34, 2402699. [Google Scholar] [CrossRef]
- Mondal, S.; Dutta, S.; Mal, S.; Pati, S.K.; Bhattacharyya, S. Lattice mismatch guided nickel-indium heterogeneous alloy electrocatalysts for promoting the alkaline hydrogen evolution. Angew. Chem. Int. Ed. 2023, 62, e202301269. [Google Scholar] [CrossRef]
- Majee, R.; Kumar, A.; Das, T.; Chakraborty, S.; Bhattacharyya, S. Tweaking nickel with minimal silver in a heterogeneous alloy of decahedral geometry to deliver platinum-like hydrogen evolution activity. Angew. Chem. Int. Ed. 2020, 59, 2881–2889. [Google Scholar] [CrossRef]
- Manivelan, N.; Piao, J.J.; Kim, J.; Lee, S.; Kim, Y.; Soundharrajan, V.; Son, M.K.; Humayun, A.; Ganji, M.D.; Ko, H.; et al. Unveiling the aluminum doping effects of in-situ transmogrified dual-LDH heterostructure and its fermi-level alignment to water splitting potentials. Adv. Energy Mater. 2025, 15, 2403889. [Google Scholar] [CrossRef]
- Sivanantham, A.; Lee, H.; Hwang, S.W.; Lee, H.U.; Cho, S.B.; Ahn, B.; Cho, I.S. Complementary functions of vanadium in boosting electrocatalytic activity of CuCoNiFeMn high-entropy alloy for water splitting. Adv. Funct. Mater. 2023, 33, 2301153. [Google Scholar] [CrossRef]
- Li, Y.; Wei, X.F.; Chen, L.S.; Shi, J.L.; He, M.Y. Nickel-molybdenum nitride nanoplate electrocatalysts for concurrent electrolytic hydrogen and formate productions. Nat. Commun. 2019, 10, 5335. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Xu, L.; Huang, F.Z.; Qu, L.B.; Li, J.T.; Owusu, K.A.; Liu, Z.A.; Lin, Z.F.; Xiang, B.H.; Liu, X.; et al. Copper–nickel nitride nanosheets as efficient bifunctional catalysts for hydrazine-assisted electrolytic hydrogen production. Adv. Energy Mater. 2019, 9, 1900390. [Google Scholar] [CrossRef]
- Li, Y.K.; Zhang, G.; Lu, W.T.; Cao, F.F. Amorphous Ni–Fe–Mo suboxides coupled with Ni network as porous nanoplate array on nickel foam: A highly efficient and durable bifunctional electrode for overall water splitting. Adv. Sci. 2020, 7, 1902034. [Google Scholar] [CrossRef]
- An, Y.M.; Long, X.; Ma, M.; Hu, J.; Lin, H.; Zhou, D.; Xing, Z.; Huang, B.L.; Yang, S.H. One-step controllable synthesis of catalytic Ni4Mo/MoOx/Cu nanointerfaces for highly efficient water reduction. Adv. Energy Mater. 2019, 9, 1901454. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).