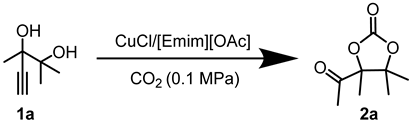
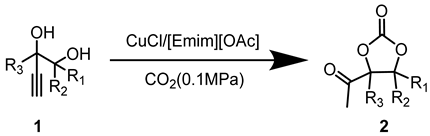
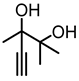
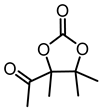
Abstract
The cyclization of propargyl alcohols with CO2 represents a highly significant method for the utilization of CO2. The resulting cyclic carbonates possesses high chemical value and hold great potential for applications in battery electrolytes, polymer precursors, and pharmaceutical intermediates. However, most existing reports on this cyclization have been limited to simple propargyl alcohol substrates that are substituted with inert alkyl, cycloalkyl, and phenyl groups. For functionalized propargyl alcohols, such as alkyne-1,2-diols, only a single report has been documented thus far. In this study, we have developed an innovative catalytic system comprising cost-effective copper salts and environmentally friendly ionic liquids (CuCl/1-ethyl-3-methylimidazolium acetate) for the cyclization of alkyne-1,2-diols with CO2. Compared to the previously reported AgF/bulky monophosphine ligand (BrettPhos) system, our system is free of traditional volatile solvents, phosphine ligands, and additives. Notably, this is the first reported Cu(I)-catalyzed system for this cyclization, offering significant advantages in terms of cost-effectiveness and reduced toxicity compared to silver salts. Moreover, the use of ionic liquids ensures considerable recyclability, further enhancing the sustainability and practicality of this approach.
1. Introduction
Carbon dioxide (CO2) is a major greenhouse gas, contributing to the greenhouse effect, global warming, glacier melting, and sea level rise [1]. In recent years, the situation of global climate has become increasingly severe, as the continuous rise in CO2 emissions which reached a record of 37.4 billion tons in 2023 [2]. Therefore, it is crucial not only to implement energy-saving and emission-reduction measures but also to focus on the capture and utilization of CO2 [3,4]. On the other hand, CO2 is an abundant, renewable, non-toxic, and non-flammable gas that possesses potential applications in various areas, such as chemistry [5], bromatology [6,7], biology [8], and pharmacology [9]. Recent decades have witnessed significant advancements in the catalytic conversion of CO2. There are numerous examples of converting CO2 into fine chemicals such as carbonates [10,11,12], aldehydes [13], ketones [14,15,16], carboxylic acids [17,18,19], alcohols [20,21], amides [22], alkanes [23], quinazolines [24], etc. Among these various pathways of CO2 conversion, the catalytic synthesis of cyclic carbonates is particularly notable. These cyclic carbonate products can be used as electrolyte solutions [25,26], precursors for polycarbonates [27], and other polymers [28].
Nowadays, the synthesis of cyclic carbonates from CO2 can be achieved through various methods, including the cycloaddition of epoxides with CO2 [29,30], the carboxylation of olefins [31], the condensation of 1,2-diols with CO2 [32], the reaction of halohydrins with CO2 [33], and the carboxylative cyclization of propargylic alcohols with CO2 [10]. Particularly, the carboxylative cyclization of propargylic alcohols with CO2 garnered significant attention, due to the corresponding cyclic carbonates, α-alkylene carbonates, are a series of multifunctional molecules that can be further transformed into numerous important skeletons in pesticides [34], medicines [35], polymers [27], etc. In 2007, T. Yamada et al. reported a AgOAc/1,8-Diazabicyclo [5.4.0]undec-7-ene (DBU) system which efficiently catalyzed this cyclization and obtained the carbonates as pure Z-isomers [36]. In 2015, He and his team reported a catalytic system of Ag2CO3/triphenylphosphine that effectively promotes cycloaddition between propargyl alcohol and CO2 under mild temperature and pressure conditions [37]. In 2016, Wang et al. reported a AgOAc/IL catalytic system for this cycloaddition reaction, focusing on how the basicity of ionic liquid affected the catalytic process [38]. In 2017, Yuan and his team reported a catalytic system consisting of AgI/ionic liquid with a silver loading of 1 mol%, which can be recycled 20 times [10]. In 2023, A. Gual et al. reported that a catalytic system combining copper salts with imidazolium-based ionic liquids efficiently synthesized cyclic carbonates with high yields under mild conditions [39].
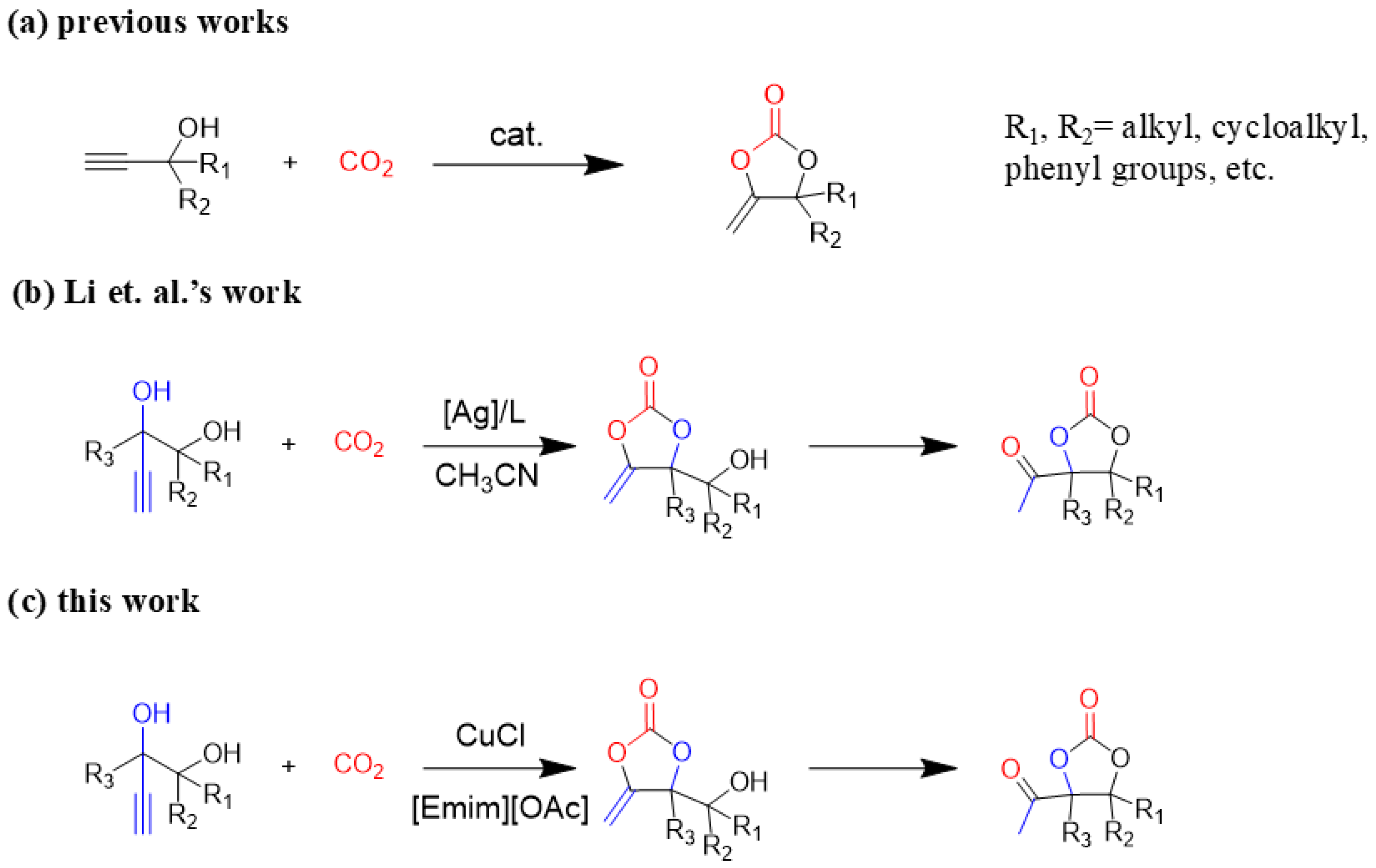
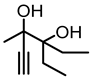
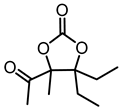
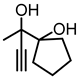
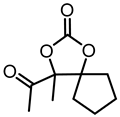
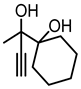
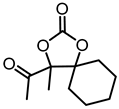
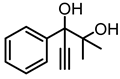
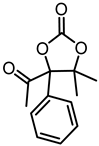
Although great progress has been achieved in the cyclic reaction between propargyl alcohols and CO2, there are still several obstacles remain. For example, most existing reports on this cyclization have been limited to simple propargyl alcohol substrates that are substituted with inert alkyl, cycloalkyl, and phenyl groups [11,12] (Figure 1a). For functionalized propargyl alcohols, such as alkyne-1,2-diols, only a single report has been documented thus far. In 2022, Li et al. reported the cyclization of CO2 and a series of novel alkyne-1,2-diol substrates which features two hydroxyl groups [40] (Figure 1b). After the carboxylative cyclization of alkyne-1,2-diol substrates with CO2, the second hydroxyl group prompts the in situ intramolecular ring-opening of the cyclic carbonates, which produces a series of novel keto-functionalized cyclic carbonates (Figure 1c). Up to now, this work is the only study on the cyclization of CO2 with alkyne-1,2-diol substrates, as well as the in situ intramolecular transformation of the corresponding carbonates. It opens a new area for this cyclization and provided more possibilities for its further applications. Nevertheless, this work employed relatively expensive and sensitive silver salt (AgF) as the catalyst, with complicated phosphine ligands (BrettPhos) as the additives and volatile acetonitrile as the solvents. Therefore, it is urgently necessary to develop more practicable catalytic systems for the cyclization of CO2 with alkyne-1,2-diol substrates. Furthermore, it is also crucial to improve the economy, stability, greenness, and recyclability of the catalytic system.
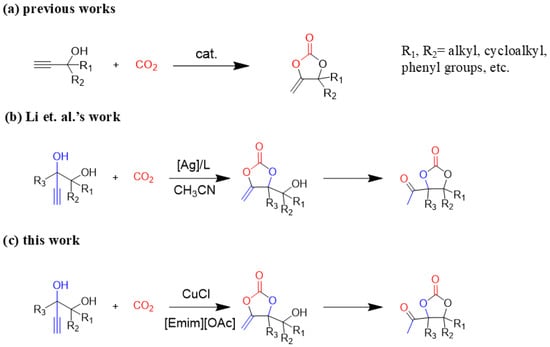
Figure 1.
(a) The reaction processes of previous works [11,12]. (b) The reaction processes of Li et al.’s work [40]. (c) The reaction processes of this work.
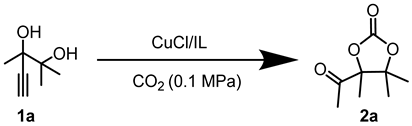
Ionic liquids (ILs) are composed of cations and anions, exhibiting high stability and nonvolatility, which remains liquid at or near room temperature. Therefore, they are considered as the model green solvents. Particularly, ILs are highly tunable, which have the potential to be applied as the catalyst and solvent simultaneously [41]. Herein, an effective protocol for the cyclization of CO2 with alkyne-1,2-diol substrates were established. The employed catalytic system consisted of economical and low-toxic copper(I) salt (CuCl) and the green IL (1-ethyl-3-methylimidazolium acetate, [Emim][OAc]). It is worth mentioning that some green metrics were calculated between the catalytic system and Li et al.’s catalytic system [40] (see the Supporting Information for full details), which suggests the green nature of this study. Particularly, this is the first reported Cu-catalyzed system for the target reaction. Moreover, this system is free of ligands and volatile solvents.
2. Results and Discussion
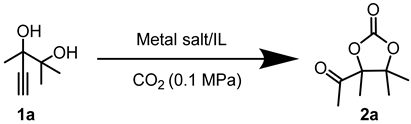
In this segment, 2,3-dimethyl-4-pentyn-2,3-diol (1a) was selected as the initial substrate to assess the efficiency of the catalytic system. Initially, a screening process was conducted for the metal salt component within the catalytic system. As shown in Table 1, no product was formed in the absence of catalysts. (Table 1, entry 1). When [Emim][OAc] was added, only a trace amount of product was detected (Table 1, entry 2), indicating the reactivity was relatively low under the catalysis of the single ionic liquid component. Thereafter, the IL [Emim][OAc] was combined with various metal salts such as CuCl, CuO, Cu2O, CuI, CuS, CuBr2, CuSO4, CuCl2, Cu2(OH)3Cl, AgOAc, Ag2CO3, ZnO, Co(OAc)2·4H2O, and Ni(OAc)2·4H2O for the catalysis of target reactions. It was observed that only the introduction of Cu and Ag salts displayed catalytic activity (Table 1, entries 3–13). Among them, CuCl demonstrated the highest yield of the desired products. Particularly, the efficiency of silver salts was relatively poor (Table 1, entries 13,14), probably owing to the low stability of silver salts in this reaction. When ZnO, Co(OAc)2·4H2O, and Ni(OAc)2·4H2O were used as catalysts, no product was observed (Table 1, entries 14–16). This may be attributed to the weak interaction between the carbon-carbon triple bonds and Zn, Co, or Ni metal salts. Consequently, CuCl exhibited the best catalytic activity and was selected as the optimal metal salt for subsequent investigations.

Table 1.
Screening of metal salts a.
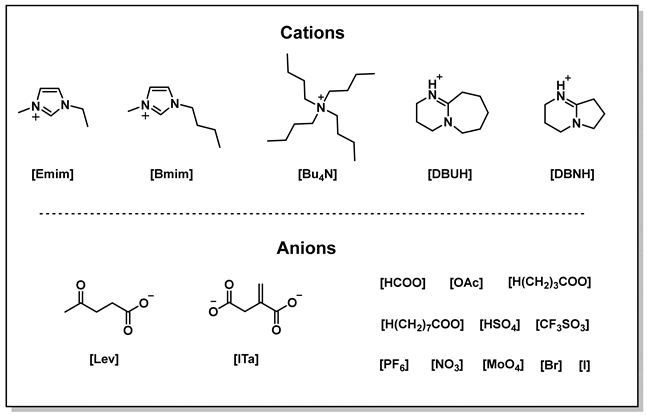
After determining CuCl as the optimal metal salt, a series of control experiments for the IL component were conducted. Firstly, it was observed that when CuCl was the sole component added to the reaction, no product was detected (Table 2, entry 1), indicating that CuCl was not sufficient to catalyze the model reaction individually. When CuCl was combined with the IL of [Emim][OAc], the desired products were obtained (Table 2, entry 2). Subsequently, a screening process was carried out for the ILs with the metal salt fixed as CuCl. The anions in ILs were first adjusted. The results indicated that the acidic or neutral anions, such as [CF3SO3], [HSO4], [NO3], [Br] and [I], were not effective for the target reactions (Table 2, entries 3–7). On the contrary, basic anions, specifically those bearing carboxylic groups, demonstrate considerable activity (Table 2, entries 2, 8–12). Particularly, [Emim][OAc] produced the desired products in the highest yield. Regarding the superior activation of [Emim][OAc] in this system, previous studies [38] indicated that the basicity of ILs significantly impact this type of reaction. A too strong basicity would reduce product yield due to the side reaction. Meanwhile, a too weak basicity was not able to effectively catalyze the target reactions. Therefore, the suitable basicity of [OAc] might be one reason for its high activity. Subsequently, a range of cations in the ILs were assessed. It was discovered that [DBUH][OAc], [Bmim][OAc], and [Bu4N][OAc] also exhibited catalytic activity for the reaction (Table 2, entries 13–15). Additionally, some other basic ILs presented certain catalytic activity (Table 2, entries 16,17). Consequently, [Emim][OAc] was determined to be the IL that provided the optimal catalytic performance. In summary, under the given reaction conditions, the CuCl/[Emim][OAc] catalytic system demonstrated the best catalytic performance for this model reaction.

Table 2.
Screening of ILs a.
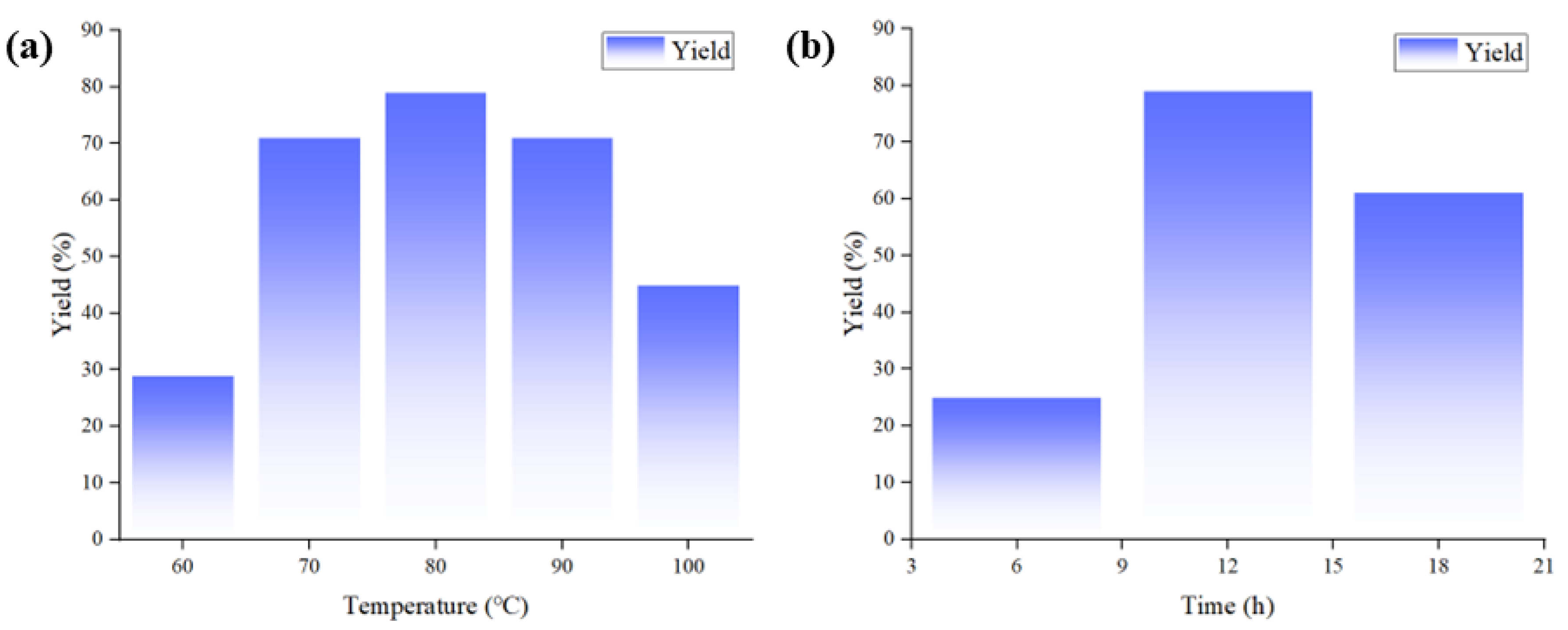
After CuCl and [Emim][OAc] were determined as the optimal catalytic system, the optimization of reaction conditions was performed. The results were detailed in Table 3. Firstly, the reactions were conducted for 12 h with the temperature gradually increased from 60 °C to 100 °C. It was observed that the yields increased steadily from 60 °C to 80 °C but decreased from 80 °C to 100 °C (Table 3, entries 1–6 and Figure 2a). The rising yields from 60 °C to 80 °C could be attributed to the better catalytic activity and reaction activity of Cu salts at higher temperatures, whereas the decrease in the yields from 80 °C to 100 °C might be attributed to the volatilization of substrate 1a under higher temperatures. Therefore, the reaction temperature was fixed as 80 °C. Afterwards, the reaction times of 6 h and 18 h were performed (Table 3, entries 7, 8). The results indicated that the optimal yield was obtained at 12 h (Figure 2b). This might be due to prolonged reaction time leading to further conversion of product 2a, resulting in decreased yield. Finally, the catalyst loading was screened. When the amounts of ILs gradually increased from 0.5 to 1 equiv., the yields reached the highest value. However, more amounts of ILs would lead to the significant decrease in the yields (Table 3, entries 4, 9–11). Kinetics curves for 1 and 3 equiv. of ILs were performed (Table S1 and Figure S1), which indicated that the reaction rate of 3 equiv. of IL was constantly slower than 1 equiv. of IL, probably due to the increased amount of IL reduced the concentration of substrate and Cu catalyst and limited their contact with CO2. After establishing that 1 equiv. of IL was optimal, the amount of CuCl was also tuned. The result indicated that 10 mol% of CuCl exhibited the best catalytic performance (Table 3, entries 4,12–14). Consequently, the final reaction conditions were determined as CuCl (10 mol%), [Emim][OAc] (1 equiv.), 80 °C, 12 h.

Table 3.
Screening of the reaction condition a.
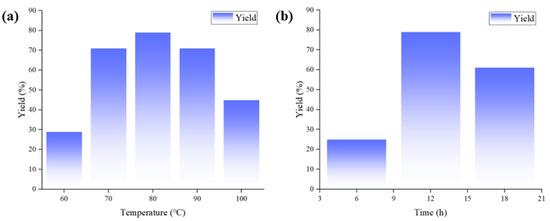
Figure 2.
(a) The relationship between reaction temperature and yield. (b) The relationship between reaction time and yield.
After determining the optimal reaction conditions, the substrate scope of the CuCl/[Emim][OAc] catalytic system was further explored under these conditions, as shown in Table 4. To our delight, this catalytic system exhibited considerable activity for different alkyne-1,2-diols with alkyl, cycloalkyl, and phenyl groups (Table 4, 1a–e). Particularly, Reactions for 1d and 1e required 1 MPa of CO2 (Table 4, 1d, 1e). This might be attributed to the significant steric hindrance caused by the six-membered phenyl or alkyl ring at the R3 position. Furthermore, the recyclability of the CuCl/[Emim][OAc] system was investigated. Once the reaction finished, the system was extracted following the standard procedures and the bottom layer was dried under vacuum to obtain the recycled catalytic system. This system was then employed directly in the next round with 1a. A yield of 63 % was obtained, which was slightly lower than that of the fresh catalyst (79 %). This result demonstrated the considerable recyclability of the CuCl/[Emim][OAc] system.

Table 4.
Screening of the substrates a.
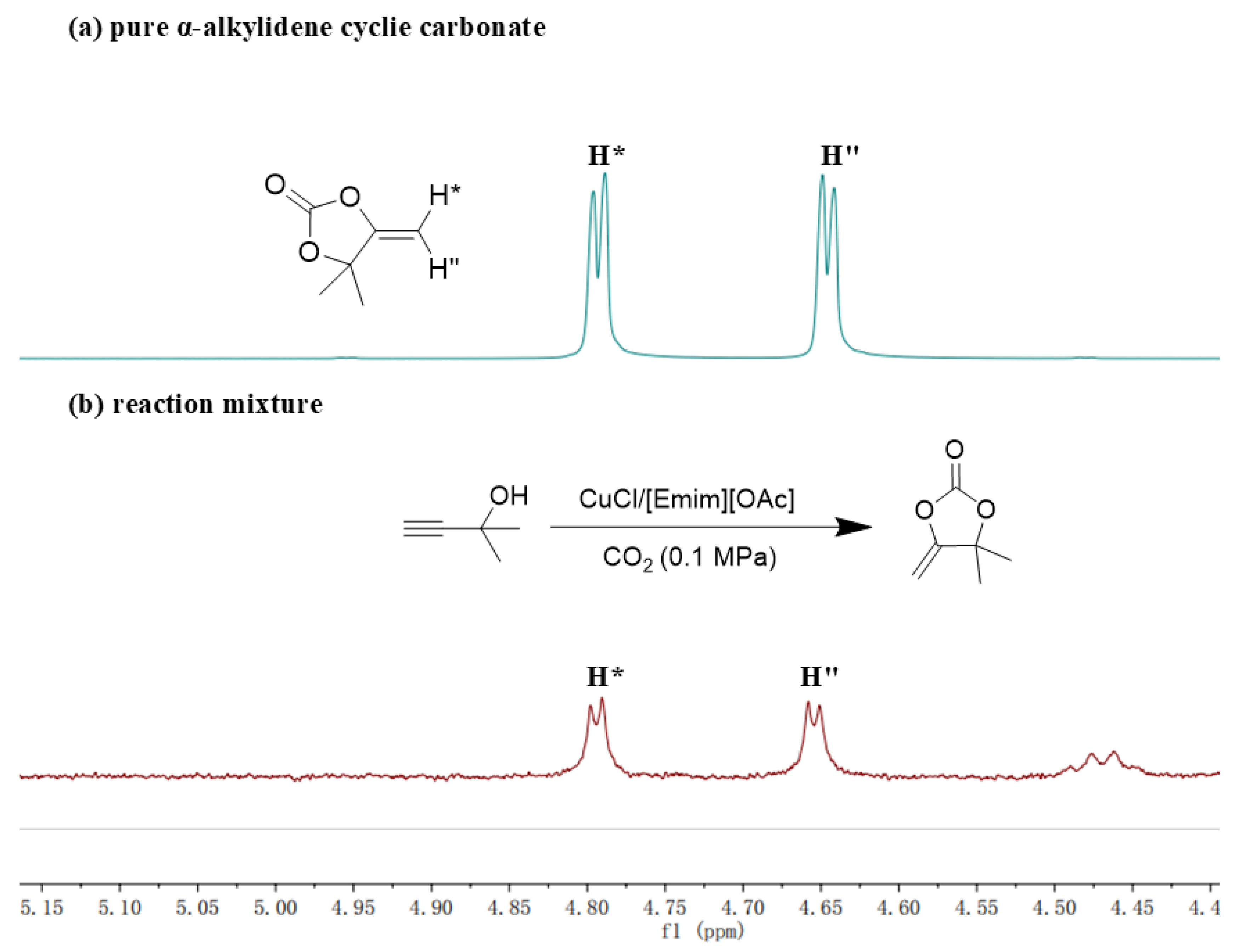
Following the aforementioned exploration, further investigation into the catalytic mechanism of the CuCl/[Emim][OAc] system was conducted. In the previous study, Li et al.’s reported that the reaction of CO2 and alkyne-1,2-diol catalyzed by their AgF/BrettPhos system could be divided into two steps [40]. Firstly, the cyclization of CO2 and alkyne-1,2-diols to generate α-alkylidene cyclic carbonates; Secondly, the in situ alcoholysis of the α-alkylidene cyclic carbonates to obtain the keto-functionalized cyclic carbonates. In order to investigate whether the CuCl/[Emim][OAc] system followed this stepwise mechanism, 2-methyl-3-butyn-2-ol was selected as the model substrate to replace 1a in the reaction process of Table 4. Upon reaction completion, the reaction mixture was analyzed directly by 1H NMR. As hypothesized, the characteristic peaks ”H*” and ”H”” of α-alkylidene cyclic carbonates (Figure 3a,b) were observed in the 1H NMR spectrum, indicating that the CuCl/[Emim][OAc] system effectively catalyzed the first step of cyclization. This model reaction would not come to the second alcoholysis step automatically, due to 2-methyl-3-butyn-2-ol contains only one hydroxyl group. This was beneficial for us to confirm the occurrence of the cyclization step. For the second alcoholysis step, it had been reported that [Bmim][OAc] could effectively catalyze the alcoholysis reactions between the α-alkylidene cyclic carbonates and alcohols [42]. In conclusion, the reaction of CO2 and alkyne-1,2-diol catalyzed by the CuCl/[Emim][OAc] system followed the stepwise mechanism, namely the first cyclization to generate α-alkylidene cyclic carbonates and the in situ alcoholysis to obtain the keto-functionalized cyclic carbonates.
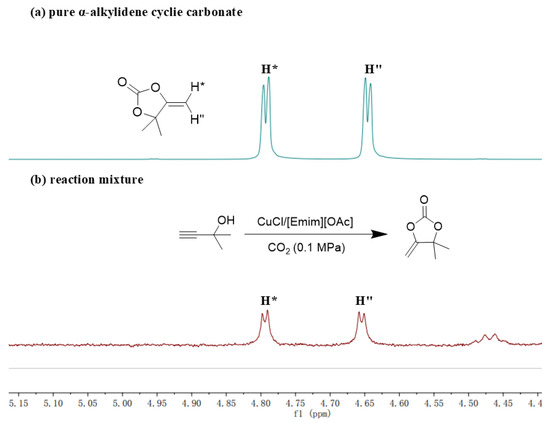
Figure 3.
(a) 1H NMR for pure α-alkylidene cyclic carbonate. (b) 1H NMR for reaction mixture.
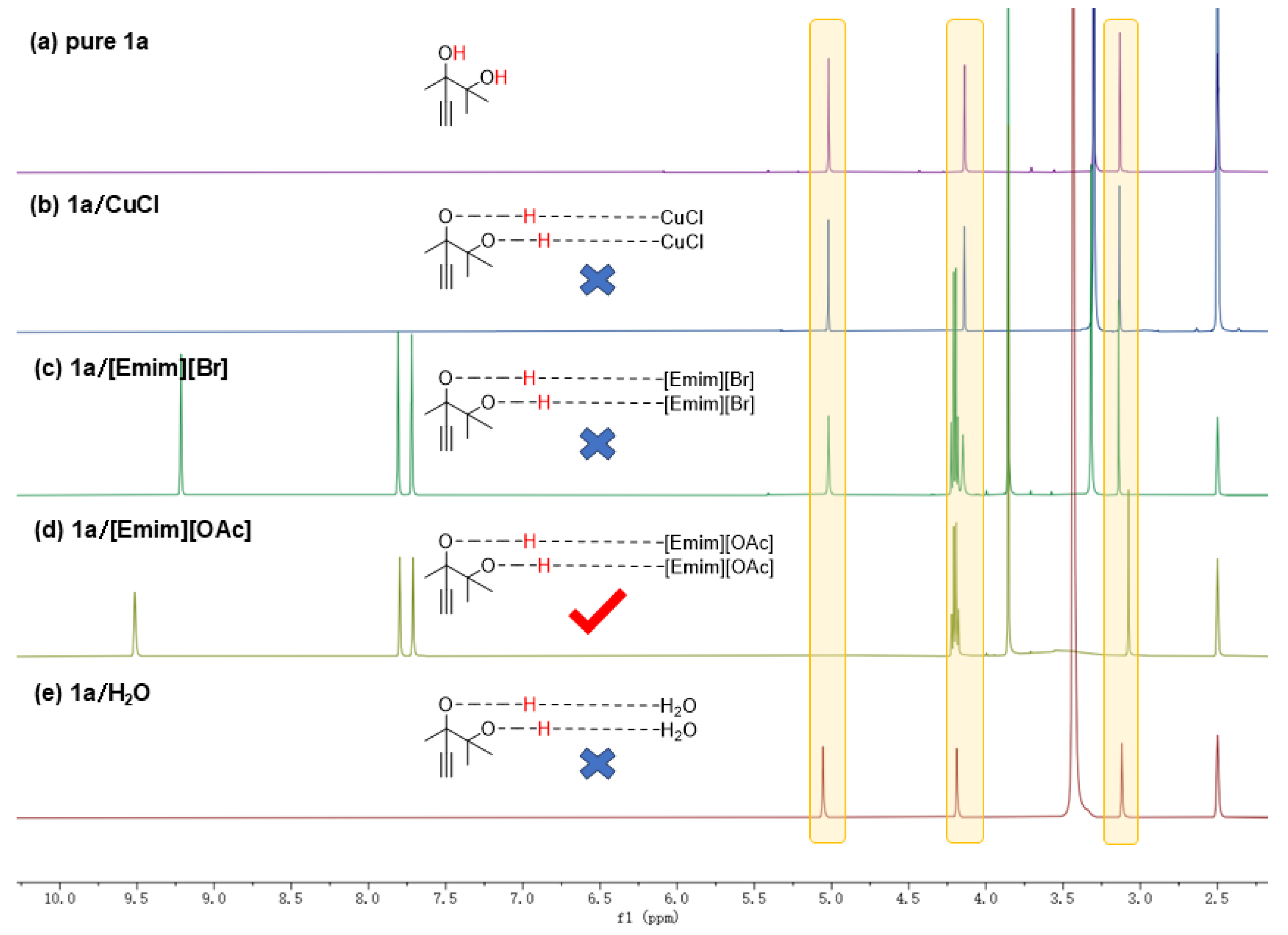
After determining the stepwise reaction process of cyclization and alcoholysis, we further explored the activation of the hydroxyl and alkynyl groups in alkyne-1,2-diol, which was considered as the crucial step for the first cyclization step [10]. As illustrated in Figure 4, the 1H NMR spectra of pure 1a, 1a with CuCl, 1a with [Emim][Br], 1a with [Emim][OAc], and 1a with H2O were tested in DMSO-d6 under identical conditions. In the 1H NMR spectrum of pure 1a, two sharp and distinct peaks were observed at δ = 4.14 ppm and δ = 5.02 ppm, representing that the hydroxyl groups in 1a were not activated. In Figure 4b,c,e the hydroxyl peaks remained sharp and clear, indicating that neither CuCl, [Emim][Br] nor H2O activated the hydroxyl group. However, in Figure 4d, these two peaks became broad and shifted, representing the hydroxyl groups were successfully activated. Moreover, the activation of hydroxyl groups in 1a/[Emim][OAc] can also be observed in their FTIR spectrums (Figure S2). Comparing Figure 4c with Figure 4a,b,e, it could be observed that the position of the alkyne carbon had changed from δ = 3.14 ppm to δ = 3.07 ppm, representing that the [OAc] group in [Emim][OAc] could also activate the alkynyl groups. In conclusion, it is the acetate anions in [Emim][OAc] that accomplish the activation of hydroxyl and alkynyl groups in the substrates.
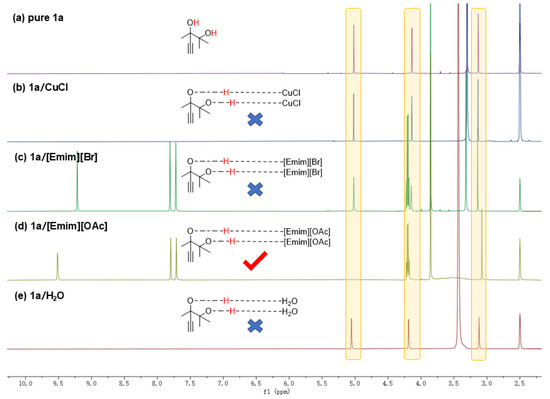
Figure 4.
Reaction conditions: 1a, 2 mmol, 80 °C, 12 h (a) 1H NMR for only 1a. (b) 1H NMR for 1a and CuCl. (c) 1H NMR for 1a and [Emim][Br]. (d) 1H NMR for 1a and [Emim][OAc]. (e) 1H NMR for 1a and H2O.
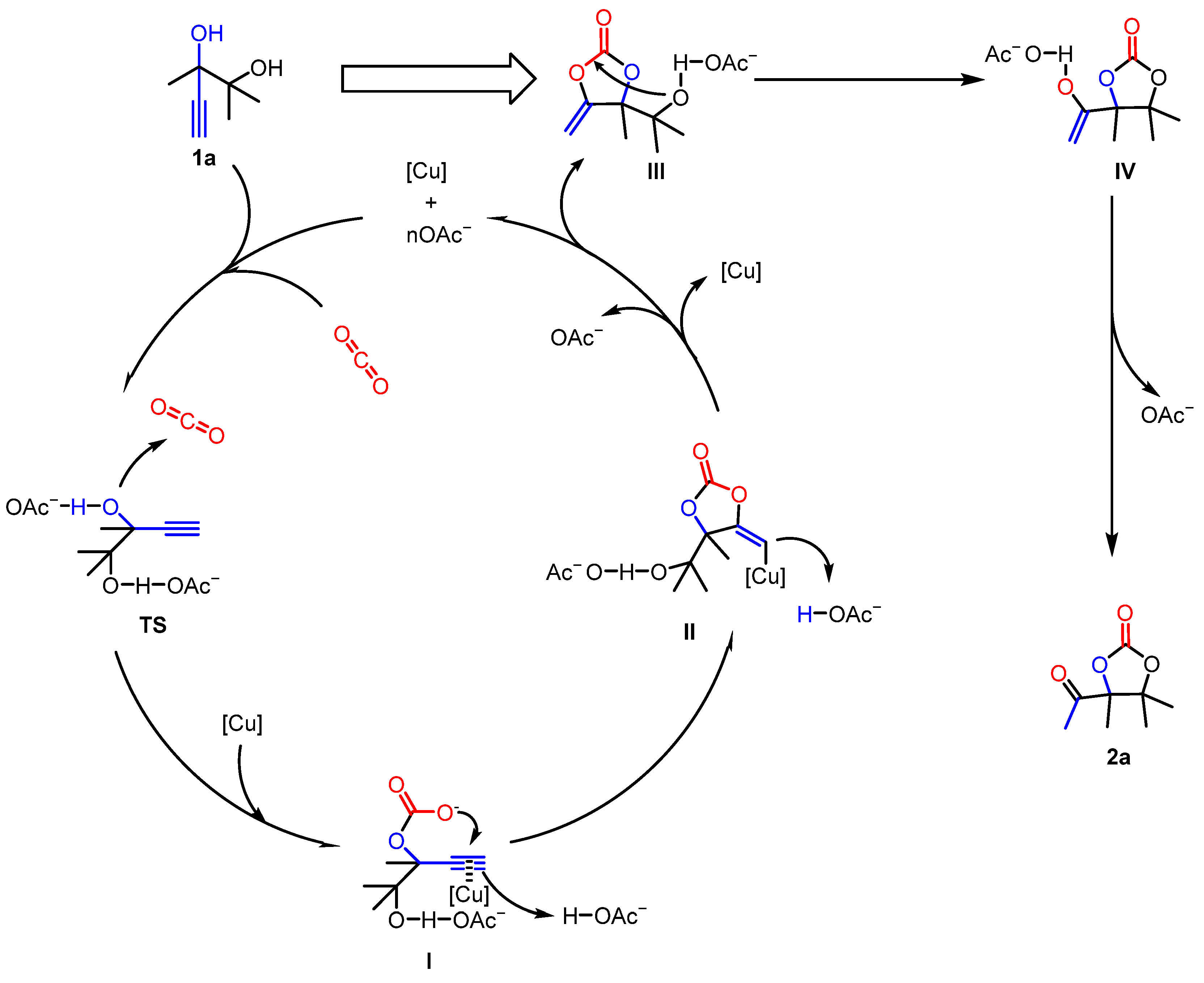
Based on the above investigations and previous studies including computational [38,40,43,44,45,46] and experimental works [47], we proposed a possible reaction mechanism for the reaction process using 2,3-dimethyl-4-pentyne-2,3-diol (1a) as an example (Figure 5). Initially, the acetate ion activates the hydroxyl group in the substrate (TS). Subsequently, as reported [11,39,44,48], the hydroxyl group launches a nucleophilic attack on the CO2, forming intermediate I. Concurrently, [Cu] and the acetate ion jointly activate the alkynyl group, enabling the carbon atom of the alkynyl group to bond with the oxygen atom, resulting in intermediate II. Then, based on previous studies [40,43,46,48], intermediate II releases the acetate ion and [Cu] to transform into α-alkylidene cyclic carbonate III. The first cyclization step is finished. Afterwards, the hydroxyl group in carbonate III was activated by the acetate ion. A nucleophilic attack from the hydroxyl oxygen to the carbon center occurs, leading to the rearrangement of the cyclic carbonate to form intermediate IV. Finally, intermediate IV loses the acetate ion and undergoes keto-enol tautomerism to yield the target product 2a.
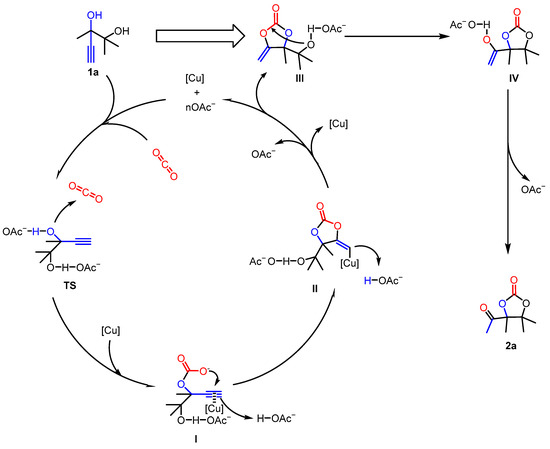
Figure 5.
Proposed catalytic mechanism of the CuCl/[Emim][OAc] system.
3. Experiment
3.1. Materials and Methods
Unless otherwise specified, 3-hydroxy-3-methyl-2-butanone, various metal salts, and multiple ionic liquids ([Emim][OAc], [Emim][CF3SO3], [Emim][HSO4], [Emim][NO3], [Emim][Br], [Emim][I], [Emim][PF6]) used in analytical purity which were sourced from Aladdin (Shanghai, China), Sigma-Aldrich (Shanghai, China), TCI (Tokyo, Japan), Macklin (Shanghai, China), Alfa (Shanghai, China), Energy Chemical (Shanghai, China), and were used without further treatment. The α-hydroxy ketones (3-ethyl-3-hydroxypentan-2-one, 1-(1-hydroxycyclopentyl)ethan-1-one, 1-(1-hydroxycyclohexyl) ethan-1-one, 2-hydroxy-2-methyl-1-phenylpropan-1-one) were synthesized according to the literature [15]. Ionic liquids ([DBUH][OAc], [DBUH]2 [ITA]) were synthesized according to the literature [49]. Ionic liquids ([Emim][HCOO], [Emim][H(CH2)3COO], [Emim][H(CH2)7COO], [Bmim][OAc], [Bu4N][OAc], [Bu4N]2 [MoO4]) were synthesized according to the procedures in Section 3.3.
Common organic solvents such as CH2Cl2, CHCl3, (CH3CH2)2O, CH3CH2OH, CH3(CH2)4CH3, and CH3COOC2H5 in analytical purity were purchased from China National Pharmaceutical Group Chemical Reagent Co., Ltd. The CO2 (99.999%) was supplied by Wuhan Xiangyun Industrial and Trade Co., Ltd. Silica gel and plates for column chromatography were purchased from Qingdao Marine Chemical Factory and Yantai Jiangyou Silica Gel Development Co., Ltd. The anion-exchange resin (model D201) was supplied by Zhejiang Zhengguang Industrial Co., Ltd. The nuclear magnetic resonance (NMR) spectra were obtained using a Bruker AVANCE III HD (Ascend 500 MHz) spectrometer (Germany). For 1H NMR, tetramethylsilane (TMS, 0 ppm) was used as the reference standard, while for 13C NMR, the chemical shifts in deuterated chloroform (CDCl3, 77.0 ppm) and deuterated dimethyl sulfoxide (DMSO-d6, 39.9 ppm) served as reference standards. Chemical shifts were reported in parts per million (ppm), and coupling constants (J) are given in Hertz (Hz). Multiplicity abbreviations: s = singlet, d = doublet, t = triplet, q = quartet, and m = multiplet.
3.2. The Synthesis Method for Substrate 1 (1a, 1b, 1c, 1d, 1e)
The synthesis method used in this study, exemplified by the synthesis of 1a. Under an argon atmosphere, 40 mL of tetrahydrofuran (THF) was added to a three-necked flask. Subsequently, 5 mmol of 3-hydroxy-3-methyl-2-butanone was introduced, and the mixture was cooled to 0 °C using an ice-water bath. Once cooled, 30 mL of ethynylmagnesium bromide solution (0.5 M in THF) was slowly added dropwise using a syringe while stirring. After its complete addition, the mixture was stirred continuously at room temperature for 16 h. When the reaction was complete, the mixture was quenched by adding a saturated ammonium chloride (NH4Cl) aqueous solution, and stirred for an additional 2 h. The mixture was then extracted with ethyl acetate (3 × 50 mL), and the organic phase was dried, filtered, and concentrated. Finally, the crude product was purified by column chromatography (hexane:ethyl acetate = 3:1) to obtain the target compound 1a.
3.3. Synthesis of Ionic Liquids by Anion-Exchange Resin Method
The synthesis method used in this study, exemplified by the synthesis of [Emim][HCOO], was based on previously reported literature [49,50]. First, 20 mmol of [Emim][Br] was added to a 100 mL round-bottom flask, followed by the addition of 20 mL of anhydrous ethanol. After [Emim][Br] was completely dissolved, 20 cm3 of the basic anion exchange resin was added, and the mixture was stirred at room temperature for 6 h. Upon completion of the reaction, [Emim][Br] was converted to [Emim][OH] in the system. The mixture was then filtered through a Buchner funnel, and the filtrate was collected. The partial ethanol solvent was removed using a rotary evaporator. Next, HCOOH was added dropwise to the [Emim][OH] solution with continuous stirring. The pH of the solution was monitored throughout the process. The addition was stopped once the pH of the mixture matched that of the standard [Emim][HCOO] solution. Subsequently, the mixture was then stirred at room temperature for an additional 6 h. After the reaction was completed, the solvent of the reaction mixture was removed under vacuum, yielding the target ionic liquid.
3.4. The General Procedure for Synthesizing Products 2 (2a, 2b, 2c, 2d, 2e)
Taking the reaction of 1a with CO2 to produce 2a under the catalysis of CuCl and [Emim][OAc] as an example. Amounts of 2 mmol of 2,3-dimethyl-4-pentyne-2,3-diol, 0.2 mmol of CuCl, and 2 mmol of [Emim][OAc] were added into a 15 mL sealed Schlenk flask. Subsequently, the atmosphere in the system was rapidly replaced three times with high-purity CO2 to remove other impurity gases from the reaction tube. The reaction mixture was then placed in an oil bath at 80 °C to react under 1 bar of CO2 12 h. After the reaction was completed, the gas valve was closed. Once the reaction tube had cooled to room temperature, extracting the mixture with ether (3 × 10 mL), then the ether phase was concentrated. Finally, the crude product was purified by column chromatography (hexane:ethyl acetate = 4:1) to obtain the target compound 2a.
4. Conclusions
In summary, this study has designed a green catalytic system based on CuCl/[Emim][OAc] that efficiently converts alkyne-1,2-diols into keto-functionalized cyclic carbonates under mild and atmospheric pressure conditions. This is the first reported Cu(I) catalytic system for the reaction to date. Moreover, it is free of volatile solvents, ligands, and additives, which provides a green, straightforward, and effective way for synthesizing functionalized cyclic carbonates.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal15030279/s1, Figure S1: kinetics curve of conversion rate and time.; Table S1: the relationship of conversion rate and time; Figure S2: FTIR of 1a spectra combined with different catalysts; [40].
Author Contributions
D.C. (Duozhen Chai): conceptualization, methodology, data curation, visualization, formal analysis; C.W.: conceptualization, methodology, data curation, visualization, formal analysis; J.L.: investigation, methodology, data curation; D.C. (Dongfeng Cao): formal analysis, software, writing—original draft, project administration; K.G.: investigation, methodology; Y.W.: investigation, methodology; Y.Y.: formal analysis, project administration, resources, editing, writing—review and editing, supervision; F.V.: formal analysis, software, writing—review and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
The project was supported by the National Natural Science Foundation of China (No. 22102127), the National Key R&D Program of China (2024YFF0508403), National Natural Science Foundation of China (Grant No.52273080, 12302481).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lu, S.; Yu, Z.; Zhang, Y.; Xu, T. Review of non-isothermal processes in CCUS from a geomechanical perspective. Earth-Sci. Rev. 2024, 255, 104848. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Lee, C.-C. Convergence of the world’s energy use. Resour. Energy Econ. 2020, 62, 101199. [Google Scholar] [CrossRef]
- Cao, C.; Zhu, H.; Hou, Z. Advances in Carbon Capture, Utilization and Storage (CCUS). Energies 2024, 17, 4784. [Google Scholar] [CrossRef]
- Mon, M.T.; Tansuchat, R.; Yamaka, W. CCUS Technology and Carbon Emissions: Evidence from the United States. Energies 2024, 17, 1748. [Google Scholar] [CrossRef]
- Zhi, K.; Li, Z.; Wang, B.; Klemeš, J.J.; Guo, L. A review of CO2 utilization and emissions reduction: From the perspective of the chemical engineering. Process Saf. Environ. Prot. 2023, 172, 681–699. [Google Scholar] [CrossRef]
- Ding, Y.; Dong, Y.; Ma, M.; Luo, L.; Wang, X.; Fang, B.; Li, Y.; Liu, L.; Ren, F. CO2 electrocatalytic reduction to ethylene and its application outlook in food science. iScience 2023, 26, 108434. [Google Scholar] [CrossRef]
- Villegas, M.E.; Aredo, V.; Asevedo, K.J.E.; Lourenço, R.V.; Bazito, R.C.; Oliveira, A.L. Commercial Starch Behavior When Impregnated with Food Additives by Moderate Temperature Supercritical CO2 Processing. Starch-Starke 2020, 72, 1900231. [Google Scholar] [CrossRef]
- Dowaidar, M. Synthetic biology of metabolic cycles for Enhanced CO2 capture and Sequestration. Bioorganic Chem. 2024, 153, 107774. [Google Scholar] [CrossRef]
- Li, M.; Abdolmohammadi, S.; Hoseininezhad-Namin, M.S.; Behmagham, F.; Vessally, E. Carboxylative cyclization of propargylic alcohols with carbon dioxide: A facile and Green route to α-methylene cyclic carbonates. J. CO2 Util. 2020, 38, 220–231. [Google Scholar] [CrossRef]
- Yuan, Y.; Xie, Y.; Zeng, C.; Song, D.; Chaemchuen, B.; Chen, C.; Verpoort, F. A recyclable AgI/OAc− catalytic system for the efficient synthesis of α-alkylidene cyclic carbonates: Carbon dioxide conversion at atmospheric pressure. Green Chem. 2017, 19, 2936–2940. [Google Scholar] [CrossRef]
- Cervantes-Reyes, A.; Farshadfar, K.; Rudolph, M.; Rominger, F.; Schaub, T.; Ariafard, A.; Hashmi, A.S.K. Copper-catalysed synthesis of α-alkylidene cyclic carbonates from propargylic alcohols and CO2. Green Chem. 2021, 23, 889–897. [Google Scholar] [CrossRef]
- Dabral, S.; Bayarmagnai, B.; Hermsen, M.; Schießl, J.; Mormul, V.; Hashmi, A.S.K.; Schaub, T. Silver-Catalyzed Carboxylative Cyclization of Primary Propargyl Alcohols with CO2. Org. Lett. 2019, 21, 1422–1425. [Google Scholar] [CrossRef] [PubMed]
- Huadsai, W.; Westerhausen, M.; Bontemps, S. Alkaline Earth Catalyzed CO2 Hydroboration into Acetal Derivatives Leading to C–S Bond Formation. Organometallics 2023, 42, 2921–2926. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Xu, Y.; Yan, X.; Zhang, S.; Duan, K.; Chen, C.; Yuan, Y.; Verpoort, F. CO2-induced dissolution of ZnO into ionic liquids and its catalytic application for the hydration of propargylic alcohols. Appl. Catal. B-Environ. 2022, 310, 121270. [Google Scholar] [CrossRef]
- Li, D.; Gong, Y.; Du, M.; Bu, C.; Chen, C.; Chaemcheun, B.; Hu, J.; Zhang, Y.; Yuan, Y.; Verpoort, F. CO2-Promoted Hydration of Propargylic Alcohols: Green Synthesis of α-Hydroxy Ketones by an Efficient and Recyclable AgOAc/Ionic Liquid System. ACS Sustain. Chem. Eng. 2020, 8, 8148–8155. [Google Scholar] [CrossRef]
- Yuan, Y.; Hu, J.; Wang, Y.; Huang, L.; Zheng, J.; Zhao, Y.; Chen, Y.; Chen, C.; Verpoort, F. A green and recyclable CuSO4·5H2O/ionic liquid catalytic system for the CO2-promoted hydration of propargyl alcohols: An efficient assembly of α-hydroxy ketones. J. Catal. 2022, 405, 561–570. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Wang, Y.; Li, Y.; He, J.; Wang, Y.; Zhang, L.; Wang, Z.; Qian, Q.; Han, B. Synthesis of Carboxylic Acids via Reaction of Ketones/Aldehydes with CO2 and H2. Organometallics 2023, 42, 2312–2318. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Qian, Q.; Li, Y.; Bediako, B.B.A.; Zhang, J.; Yang, J.; Li, Z.; Han, B. Synthesis of carboxylic acids via the hydrocarboxylation of alcohols with CO2 and H2. Green Chem. 2022, 24, 1973–1977. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Zhang, Y.; Wang, Z.; Xiang, J.; Han, J.; He, J.; Zhang, L.; Wang, Y.; Meng, Q.; et al. Synthesis of Carboxylic Acids from Saccharides, CO2, and H2. ACS Catal. 2023, 13, 8025–8030. [Google Scholar] [CrossRef]
- Witoon, T.; Numpilai, T.; Nijpanich, S.; Chanlek, N.; Kidkhunthod, P.; Kidkhunthod, C.K.; Ng, K.H.; Vo, D.N.; Ittisanronnachai, S.; Wattanakit, C.; et al. Enhanced CO2 hydrogenation to higher alcohols over K-Co promoted In2O3 catalysts. Chem. Eng. J. 2022, 431, 133211. [Google Scholar] [CrossRef]
- Yang, H.; Wei, Z.; Zhang, J.; Dang, Y.; Li, S.; Bu, X.; Zhou, Z.; Gong, C.; Wang, H.; Li, J.; et al. Tuning the selectivity of CO2 hydrogenation to alcohols by crystal structure engineering. Chem 2024, 10, 2245–2265. [Google Scholar] [CrossRef]
- Skarżyńska, A.; Gil, W.; Trzeciak, A.M. Pd/PVP- Catalyzed Formylation of Amines and Nitroarenes to N-Formamides with CO2 and Dimethylamine Borane: The Influence of CO2 on the Formation of Azoxyarenes. ChemCatChem 2024, 16, e202401260. [Google Scholar] [CrossRef]
- Xing, S.; Lv, P.; Yuan, H.; Yang, L.; Wang, Z.; Yuan, Z.; Chen, Y. Insight into forced hydrogen re-arrangement and altered reaction pathways in a protocol for CO2 catalytic processing of oleic acid into C8–C15 alkanes. Green Chem. 2017, 19, 4157–4168. [Google Scholar] [CrossRef]
- Rajanna, D.; Srinivasappa, P.M.; Sudhakaran, A.; Biradar, A.; Samal, A.K.; Bembalge, O.B.; Jadhav, A.H. Recent Advances in the Fixation of CO2 into Quinazoline and Benzimidazole. Energy Fuels 2024, 38, 8481–8515. [Google Scholar] [CrossRef]
- Huang, Q.; Weng, J.; Ouyang, D.; Chen, M.; Wang, X.; Wang, J. Comparative studies on the combustion characteristics of electrolytes and carbonate mixed solvents with flame retardant additives under low pressures. Case Stud. Therm. Eng. 2023, 43, 102810. [Google Scholar] [CrossRef]
- Huang, X.-H.; Chung, Y.-H.; Guo, G.-S.; Shu, C.-M. Investigating the thermal stability and explosion characteristics of electrolytes composed of different ratios of carbonate organic solvents. J. Loss Prev. Process Ind. 2025, 94, 105509. [Google Scholar] [CrossRef]
- Morodo, R.; Dumas, D.M.; Zhang, J.; Lui, K.H.; Hurst, P.J.; Bosio, R.; Campos, L.M.; Park, N.H.; Waymouth, R.M.; Hedrick, J.L. Ring-Opening Polymerization of Cyclic Esters and Carbonates with (Thio)urea/Cyclopropenimine Organocatalytic Systems. ACS Macro Lett. 2024, 13, 181–188. [Google Scholar] [CrossRef]
- Hodge, P.; Kamau, S.D.; Ben-Haida, A.; Williams R., T. Cyclo-depolymerizations of polycarbonates in solution: Use of the macrocyclic oligomers obtained in entropically-driven ring-opening polymerizations and copolymerizations to give carbonate–carbonate and carbonate–carboxylate ester copolymers. React. Funct. Polym. 2012, 72, 868–877. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, K.; Yang, B.; Sun, Z.; Zhao, Y. Synergistic catalysis of dual-sites promoted cycloaddition of CO2 with epoxides. Fuel 2025, 381, 133305. [Google Scholar] [CrossRef]
- Tappe, N.A.; Reich, R.M.; D’elia, V.; Kühn, F.E. Current advances in the catalytic conversion of carbon dioxide by molecular catalysts: An update. Dalton Trans. 2018, 47, 13281–13313. [Google Scholar] [CrossRef]
- Zhang, S.; Xia, Z.; Zou, Y.; Cao, F.; Liu, Y.; Ma, Y.; Qu, Y. Interfacial Frustrated Lewis Pairs of CeO2 Activate CO2 for Selective Tandem Transformation of Olefins and CO2 into Cyclic Carbonates. J. Am. Chem. Soc. 2019, 141, 11353–11357. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Wang, Y.; Yan, Z.; Hou, J.; Xu, G.; Shi, L. One-step DMC synthesis from CO2 under catalysis of ionic liquids Pj. L.repared with 1,2-propylene glycol. Catal. Today 2023, 418, 114052. [Google Scholar] [CrossRef]
- Han, F.; Li, H.; Zhuang, H.; Hou, Q.; Yang, Q.; Zhang, B.; Miao, C. Direct synthesis of cyclic carbonates from olefins and CO2: Single- or multi-component catalytic systems via epoxide or halohydrin intermediate. J. CO2 Util. 2021, 53, 101742. [Google Scholar] [CrossRef]
- Kobayashi, T.; Hotta, H.; Miyake, T.; Nomura, M.; Horikoshi, R.; Yamamoto, K. Discovery of flometoquin, a novel quinoline insecticide. J. Pestic. Sci. 2023, 48, 168–174. [Google Scholar] [CrossRef]
- Min, S.; Kim, T.; Jeong, T.; Yang, J.; Oh, Y.; Moon, K.; Rakshit, A.; Kim, I.S. Synthesis of 2-Formyl Carbazoles via Tandem Reaction of Indolyl Nitrones with 2-Methylidene Cyclic Carbonate. Org. Lett. 2023, 25, 4298–4302. [Google Scholar] [CrossRef]
- Yamada, W.; Sugawara, Y.; Cheng, H.M.; Ikeno, T.; Yamada, T. Silver-Catalyzed Incorporation of Carbon Dioxide into Propargylic Alcohols. Eur. J. Org. Chem. 2007, 2007, 2604–2607. [Google Scholar] [CrossRef]
- Song, Q.-W.; Chen, W.-Q.; Ma, R.; Yu, A.; Li, Q.-Y.; Chang, Y.; He, L.-N. Back Cover: Bifunctional Silver(I) Complex-Catalyzed CO2 Conversion at Ambient Conditions: Synthesis of α-Methylene Cyclic Carbonates and Derivatives (ChemSusChem 5/2015). ChemSusChem 2015, 8, 910. [Google Scholar] [CrossRef]
- Chen, K.; Shi, G.; Dao, R.; Mei, K.; Zhou, X.; Li, H.; Wang, C. Tuning the basicity of ionic liquids for efficient synthesis of alkylidene carbonates from CO2 at atmospheric pressure. Chem. Commun. 2016, 52, 7830–7833. [Google Scholar] [CrossRef]
- Miró, R.; Lara-Rimbau, F.X.; Vicente, I.; Gual, A. CO2 Transformations in Ionic Liquids: In Situ NHC-Cu Formation for Carbonate and Carbamate Production. ACS Sustain. Chem. Eng. 2023, 11, 17600–17606. [Google Scholar] [CrossRef]
- Li, X.; Villar-Yanez, A.; Ngassam Tounzoua, C.; Benet-Buchholz, J.; Grignard, B.; Bo, C.; Detrembleur, C.; Kleij A., W. Cascade Transformation of Carbon Dioxide and Alkyne-1,n-diols into Densely Substituted Cyclic Carbonates. ACS Catal. 2022, 12, 2854–2860. [Google Scholar] [CrossRef]
- Chen, X.; Zhong, Q.; Ma, X.; Li, S.; Sun, W.; Liu, C. Synthesis of benzothiazoles catalyzed by [Bmim]PF6 ionic liquid in solvent-free condition. J. Catal. 2024, 429, 115274. [Google Scholar] [CrossRef]
- Hu, J.; Ma, J.; Lu, L.; Qian, Q.; Zhang, Z.; Xie, C.; Han, B. Synthesis of Asymmetrical Organic Carbonates using CO2 as a Feedstock in AgCl/Ionic Liquid System at Ambient Conditions. ChemSusChem 2017, 10, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Dong, S.; Zhang, Z.; Wang, L.; Zhang, J. Insights into the synergistic influence of [Emim][OAc] and AgOAc for the hydration of propargylic alcohols to α-hydroxy ketones in the presence of CO2. Catal. Sci. Technol. 2021, 11, 5641–5649. [Google Scholar] [CrossRef]
- Qiu, J.; Zhao, Y.; Li, Z.; Wang, H.; Fan, H.; Wang, J. Efficient Ionic-Liquid-Promoted Chemical Fixation of CO2 into α-Alkylidene Cyclic Carbonates. ChemSusChem 2017, 10, 1120–1127. [Google Scholar] [CrossRef]
- Arayachukiat, S.; Kongtes, C.; Barthel, A.; Vummaleti, S.V.C.; Poater, A.; Wannakao, S.; Cavallo, L.; D’elia, V. Ascorbic Acid as a Bifunctional Hydrogen Bond Donor for the Synthesis of Cyclic Carbonates from CO2 under Ambient Conditions. ACS Sustain. Chem. Eng. 2017, 5, 6392–6397. [Google Scholar] [CrossRef]
- Qiu, J.; Zhao, Y.; Wang, H.; Cui, G.; Wang, J. AgX@carbon (X = Br and I) as robust and efficient catalysts for the reaction of propargylic alcohols and CO2 to carbonates under ambient conditions. RSC Adv. 2016, 6, 54020–54026. [Google Scholar] [CrossRef]
- Li, W.; Cheng, W.; Yang, X.; Su, Q.; Dong, L.; Zhang, P.; Yi, Y.; Li, B.; Zhang, S. Synthesis of Cyclic Carbonate Catalyzed by DBU Derived Basic Ionic Liquids. Chin. J. Chem. 2018, 36, 293–298. [Google Scholar] [CrossRef]
- Zhou, Z.-H.; Zhang, X.; Huang, Y.-F.; Chen, K.-H.; He, L.-N. Synthesis of α-hydroxy ketones by copper(I)-catalyzed hydration of propargylic alcohols: CO2 as a cocatalyst under atmospheric pressure. Chin. J. Catal. 2019, 40, 1345–1351. [Google Scholar] [CrossRef]
- Mezzetta, A.; Becherini, S.; Pretti, C.; Monni, G.; Casu, V.; Chiappe, C.; Guazzelli, L. Insights into the levulinate-based ionic liquid class: Synthesis, cellulose dissolution evaluation and ecotoxicity assessment. New J. Chem. 2019, 43, 13010–13019. [Google Scholar] [CrossRef]
- Bragato, N.; Perosa, A.; Selva, M.; Fiorani, G.; Calmanti, R. Molybdate ionic liquids as halide-free catalysts for CO2 fixation into epoxides. Green Chem. 2023, 25, 4849–4860. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).