Green Innovation: Multifunctional Zinc Oxide Nanoparticles Synthesized Using Quercus robur for Photocatalytic Performance, Environmental, and Antimicrobial Applications
Abstract
1. Introduction
2. Results
2.1. Characterisation
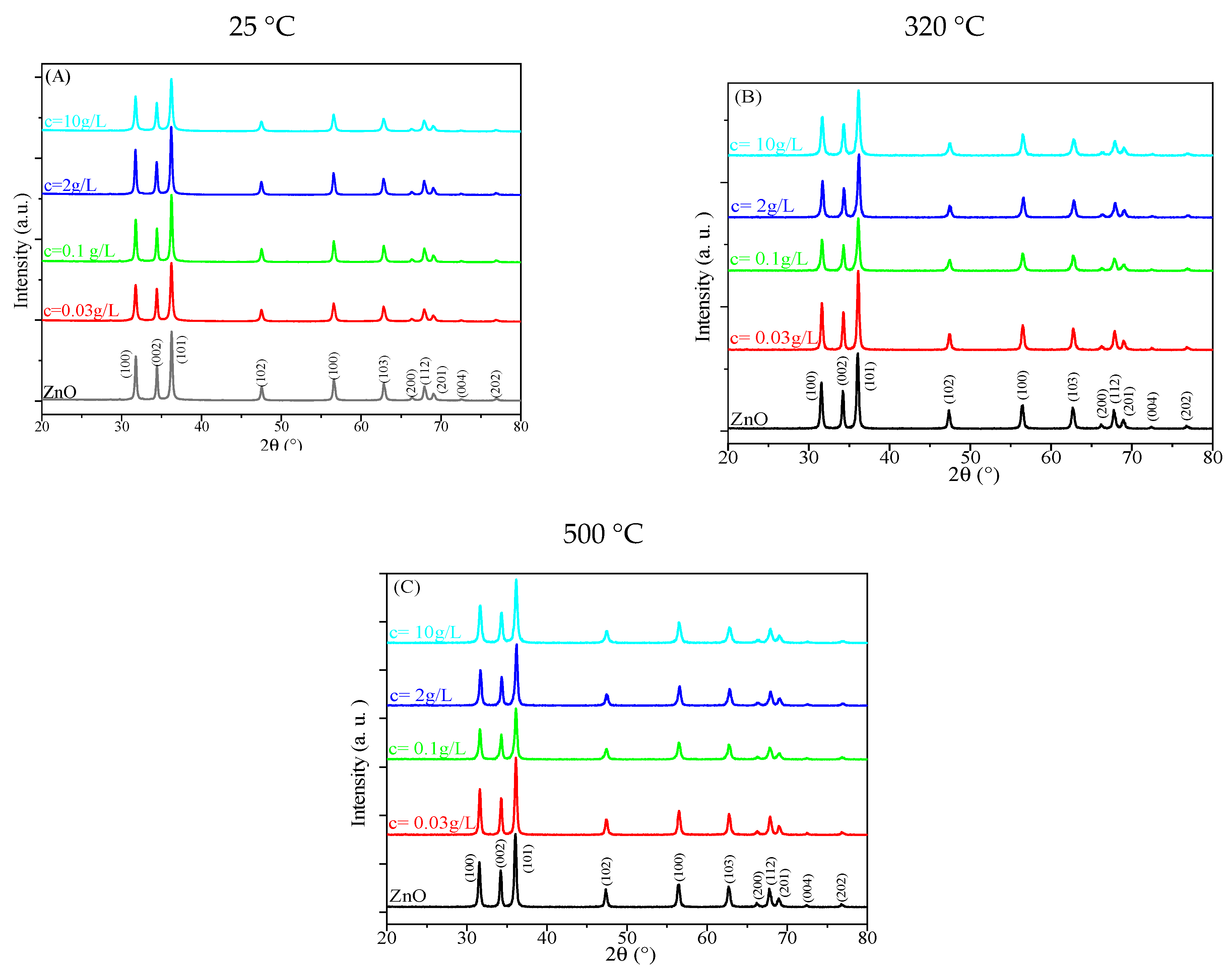
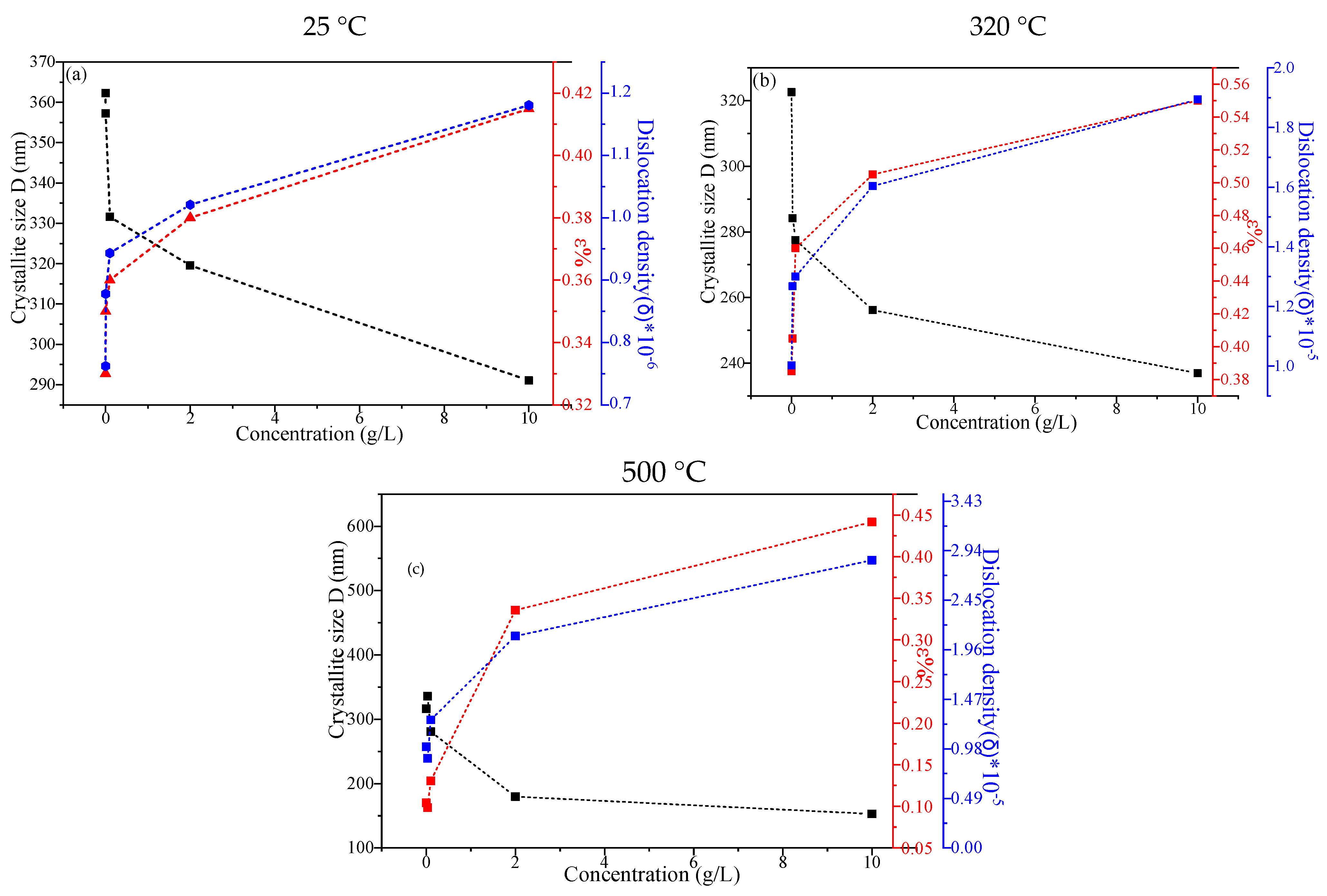
2.1.1. X-Ray Diffraction
- D denotes the mean crystallite size;
- β represents the full width at half-maximum (FWHM) of the XRD peak, quantified in radians;
- θ represents the Bragg angle, defined as half the angle formed between the incident X-ray beam and the dispersed X-ray beam.
2.1.2. Scanning Electron Microscopy (SEM)
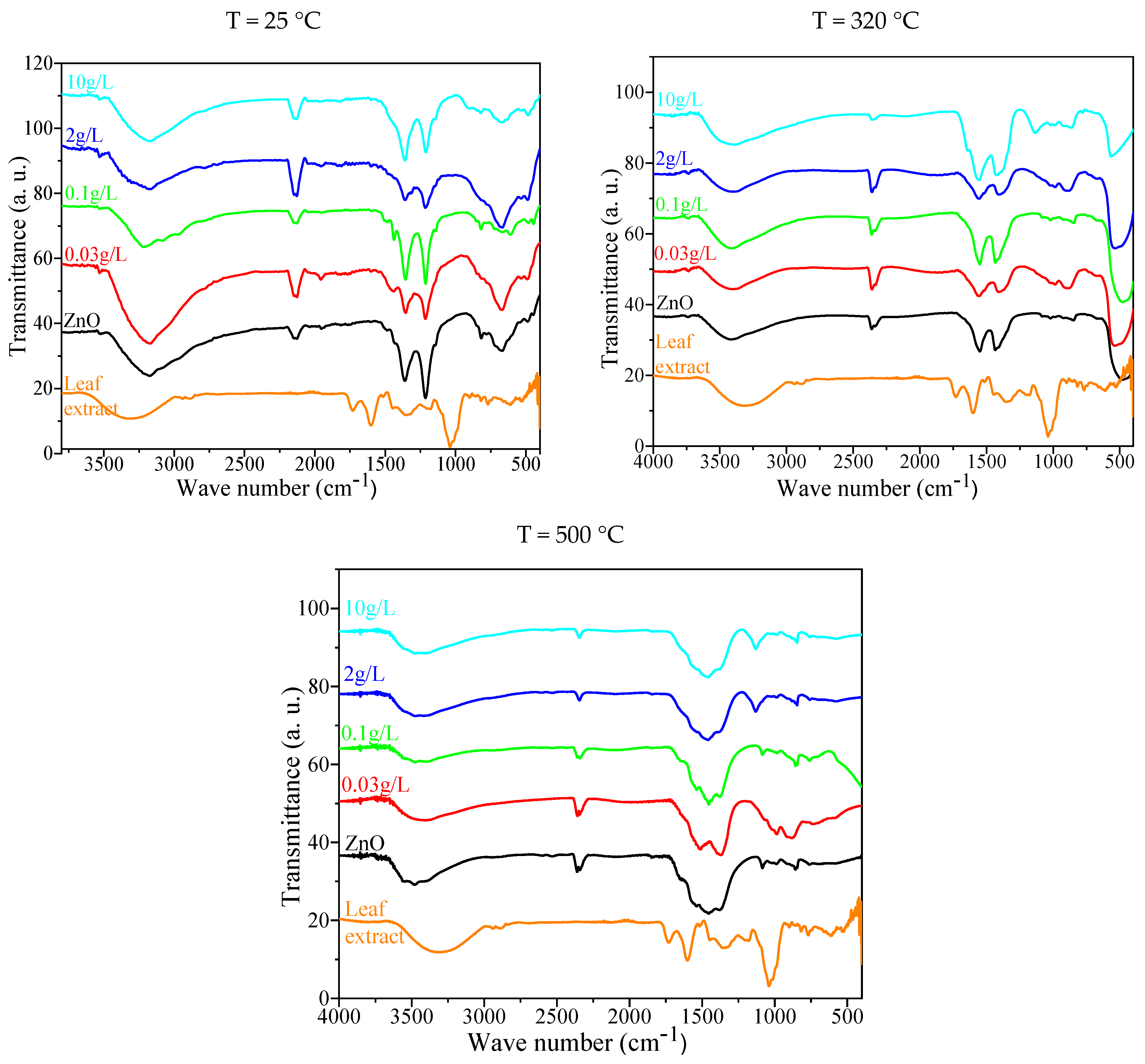
2.1.3. Fourier Transformation Infrared Spectroscopy (FTIR)
2.1.4. UV–Vis Spectroscopy
2.2. Photocatalysis
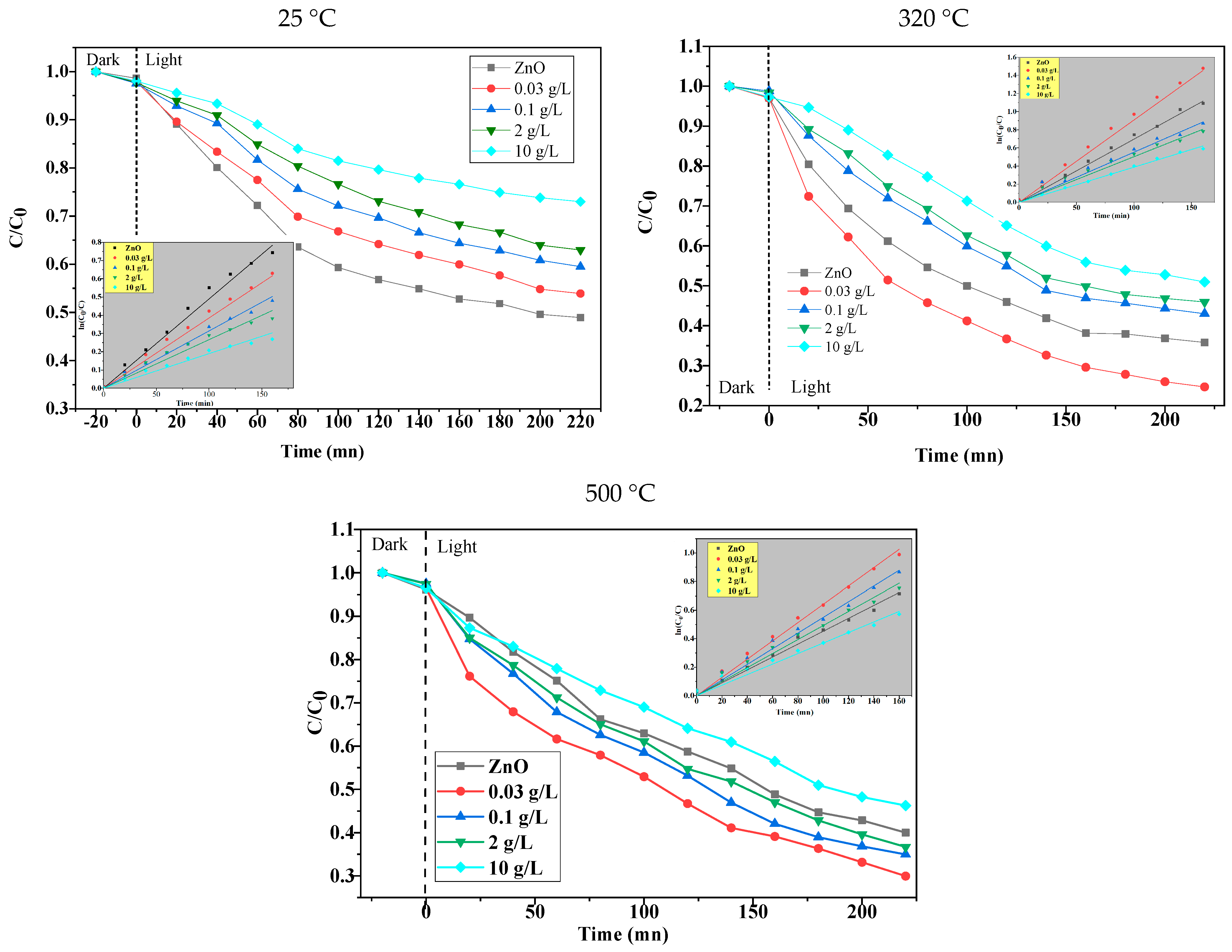
2.2.1. Photocatalytic Activity
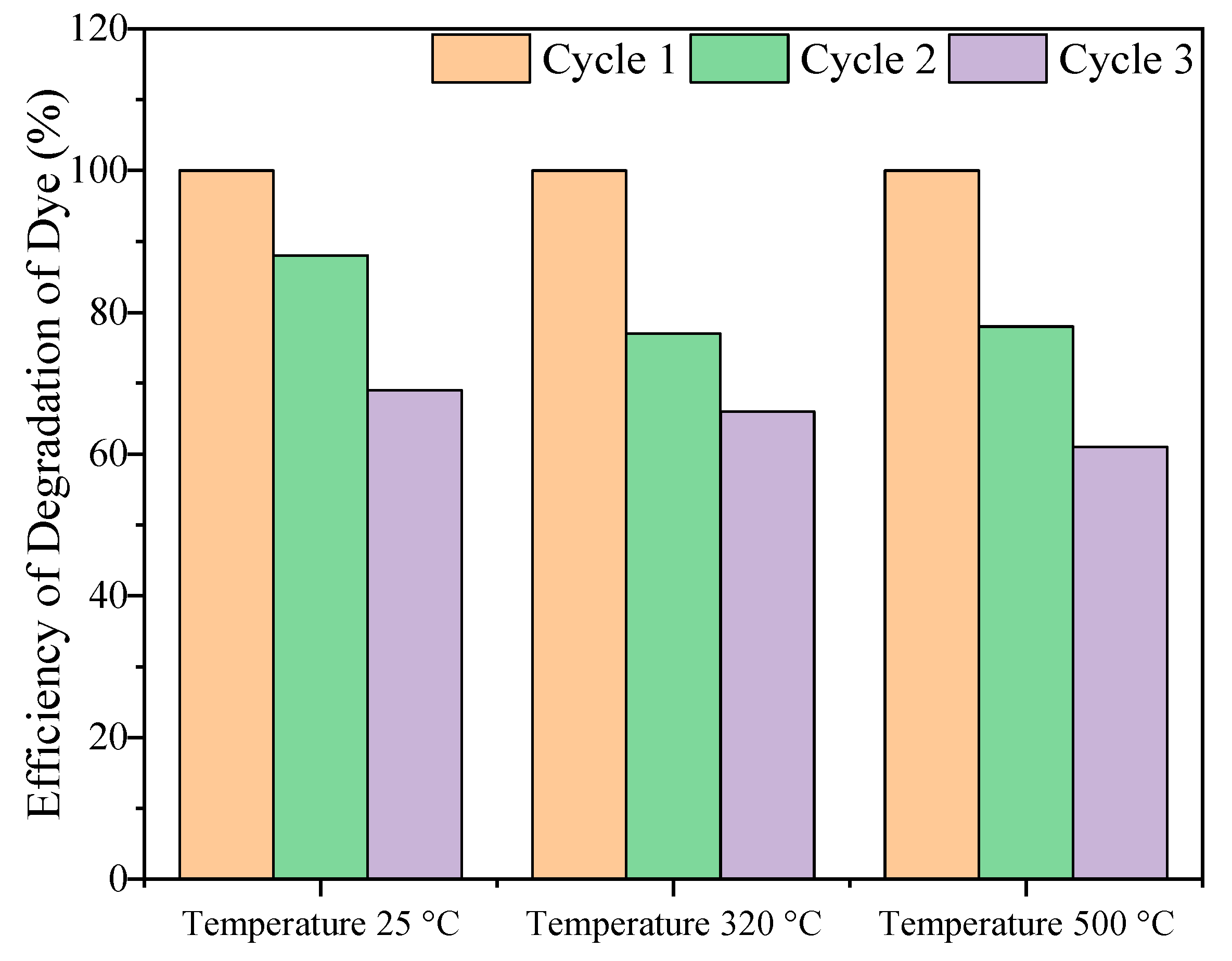
2.2.2. Photocatalytic Cycling Test for Methylene Blue Degradation Under UV Light
- Surface Degradation: Prolonged exposure to reactive species or harsh conditions may lead to surface degradation of ZnO, such as oxidation, reduction, or the formation of defects that diminish its active sites;
- Structural Changes: High temperatures or repeated cycles of catalysis can induce morphological or structural changes in ZnO, such as sintering or particle agglomeration, reducing the surface area available for catalysis;
- Contamination or Fouling: The active surface of ZnO may become contaminated by impurities, byproducts, or undesired species, leading to a reduction in catalytic efficiency over time;
- Leaching of Active Sites: In liquid-phase catalysis, ZnO may experience leaching, where catalytic components are gradually dissolved into the reaction medium, leading to performance decay;
- Formation of Inactive Phases: Chemical interactions between ZnO and the reaction medium could result in the formation of secondary, less active, or inactive phases, altering the material’s catalytic behavior;
- Photo-Induced Changes: For photocatalytic applications, prolonged exposure to UV or visible light can induce photocorrosion or electron–hole recombination, which can degrade the material over time.
2.2.3. Effect of Catalyst Dosage
2.2.4. Effect of Reaction pH
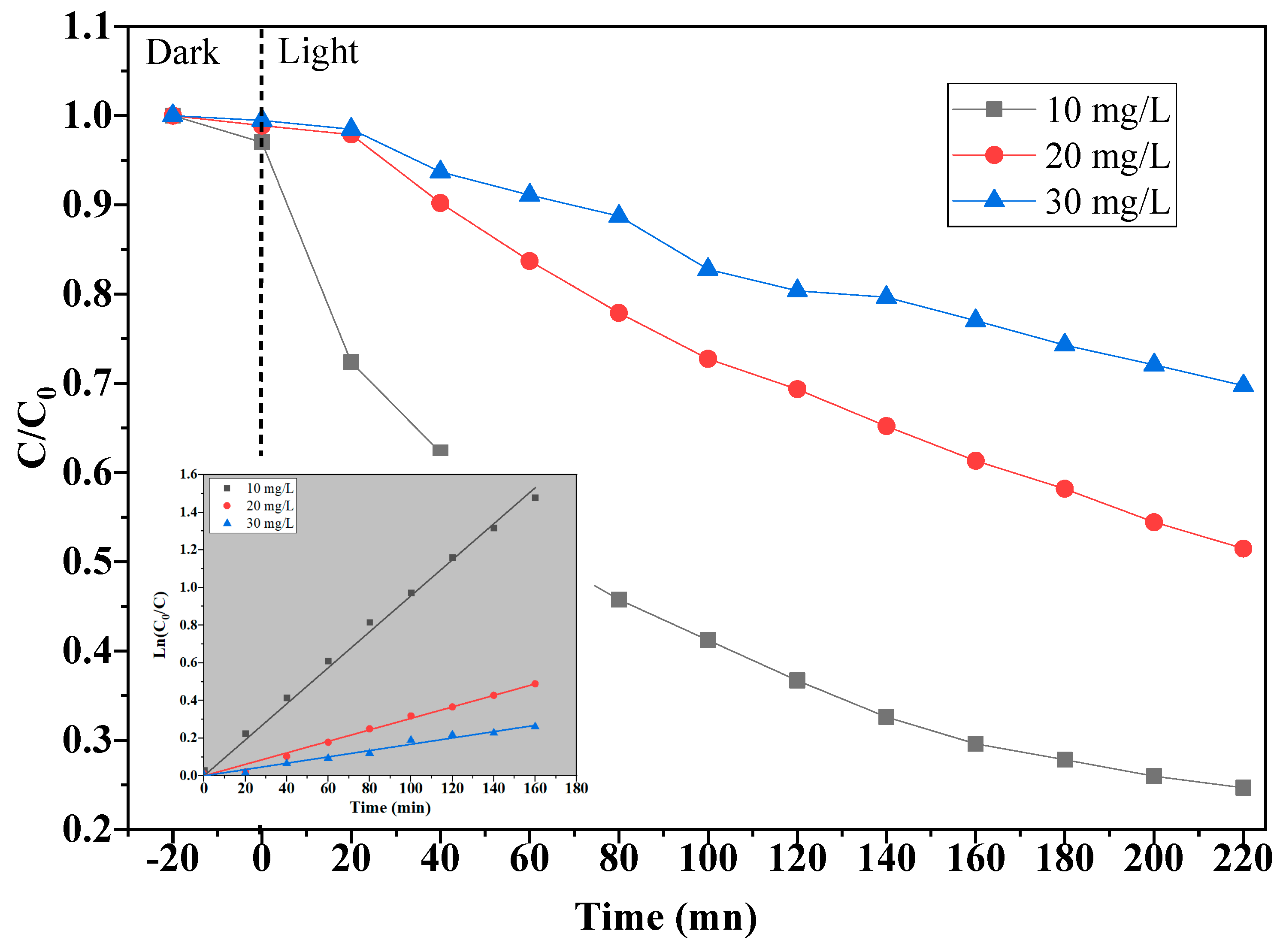
2.2.5. Effect of Initial Concentrations of MB
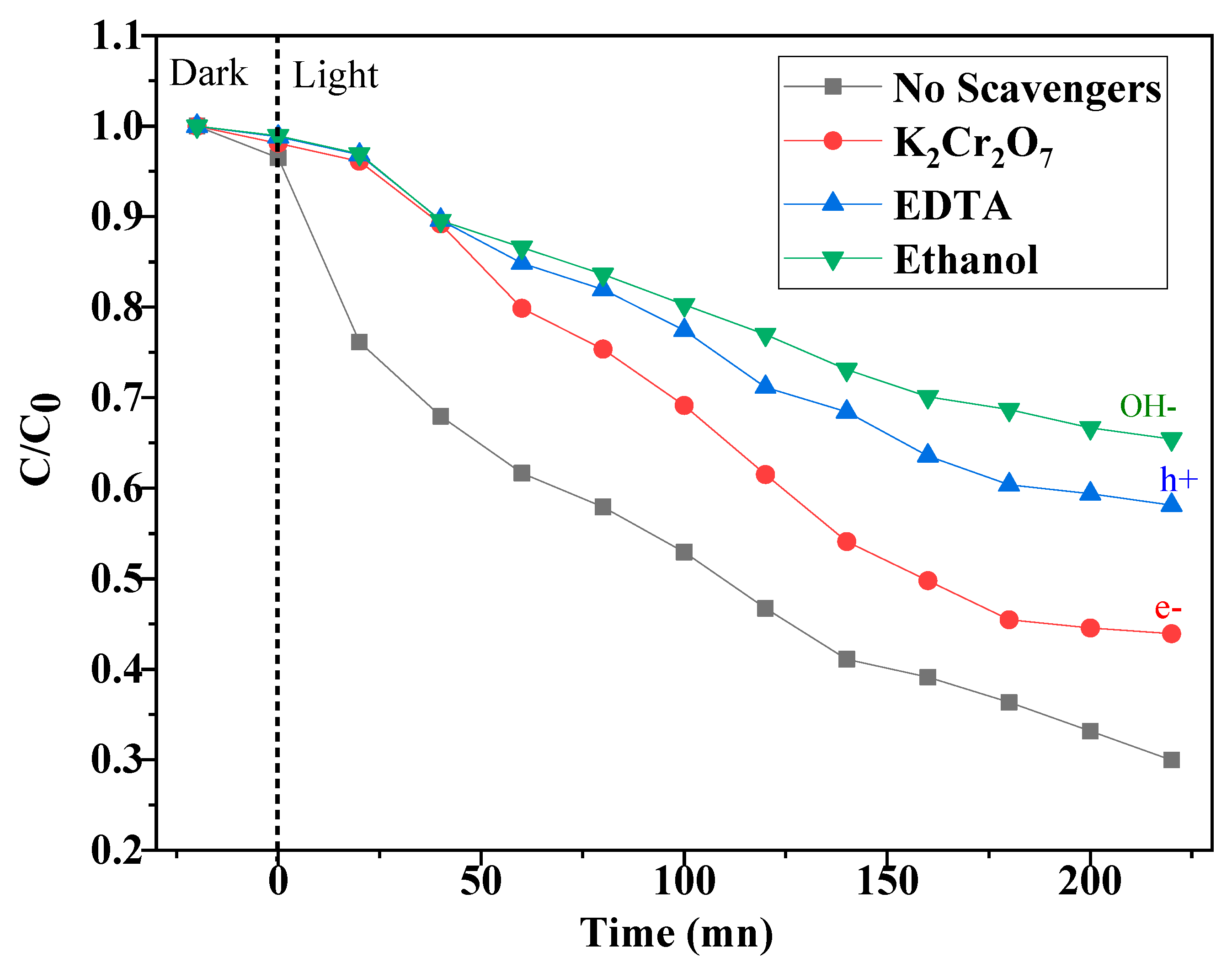
2.2.6. Effect of Scavengers
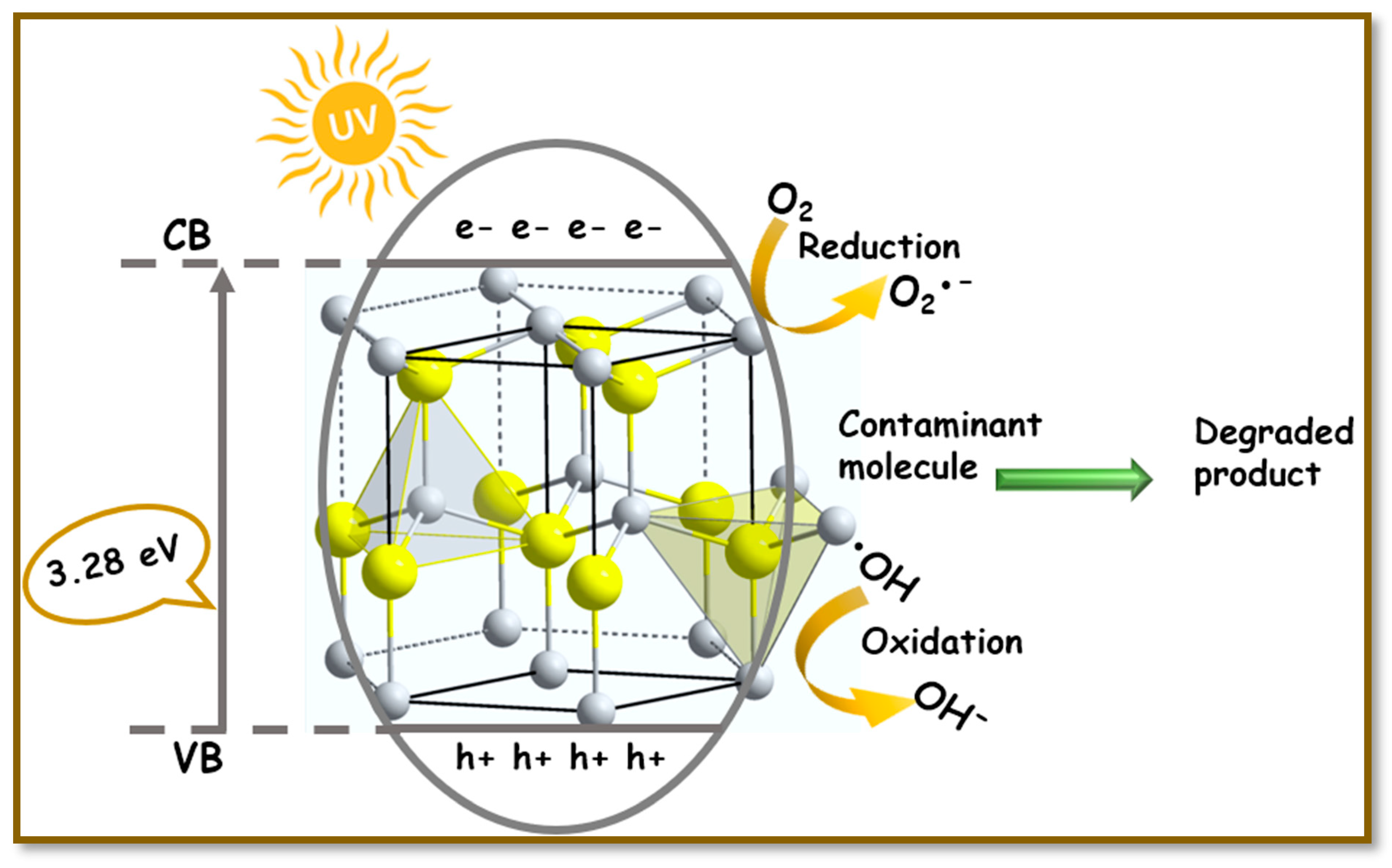
2.3. Photocatalytic Mechanism
2.4. Comparison of the Photocatalytic Efficacy of ZnO Nanoparticles Synthetized by Different Methods
2.4.1. Antibacterial Activity
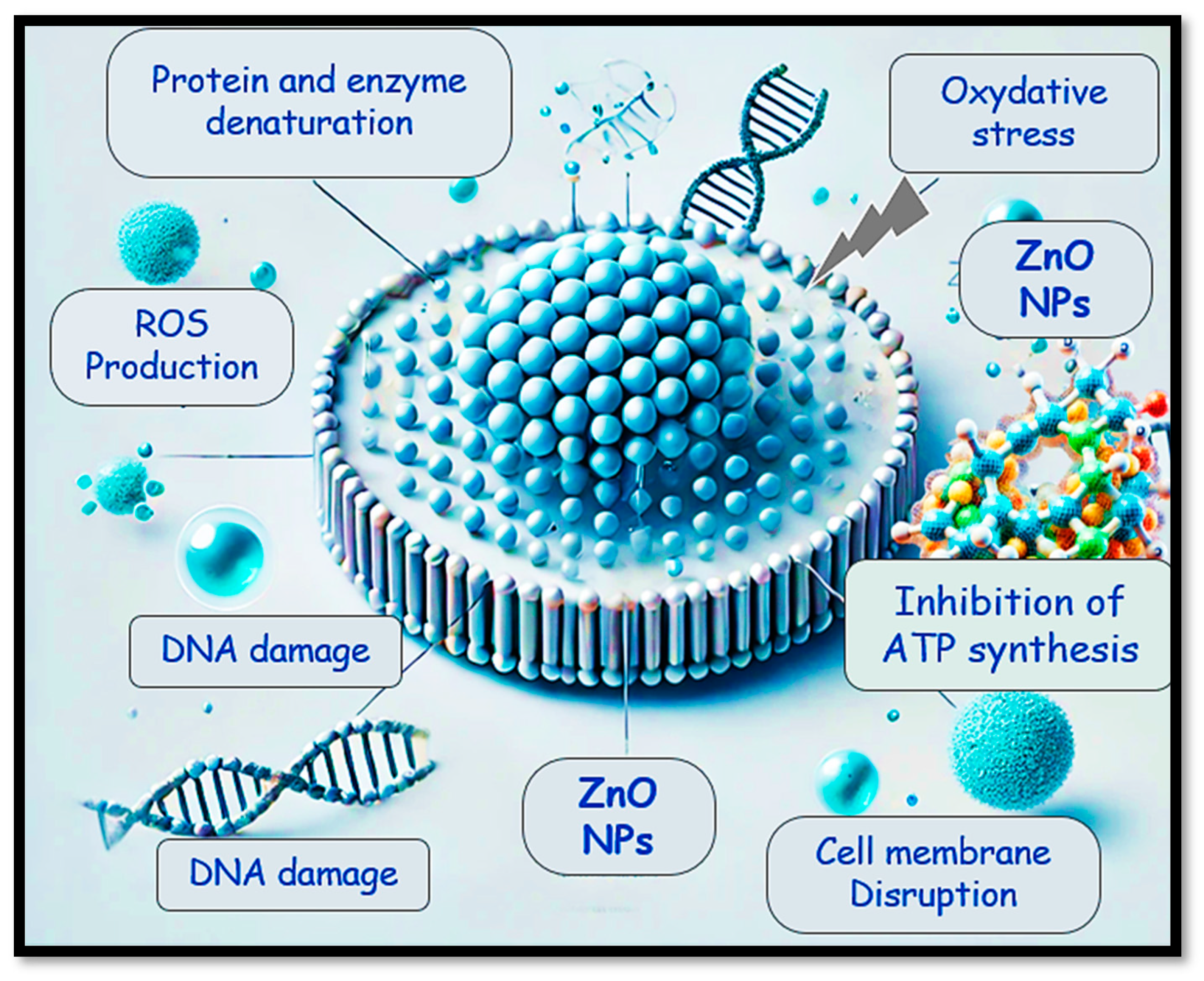
2.4.2. Antibacterial Mechanism
3. Materials and Methods
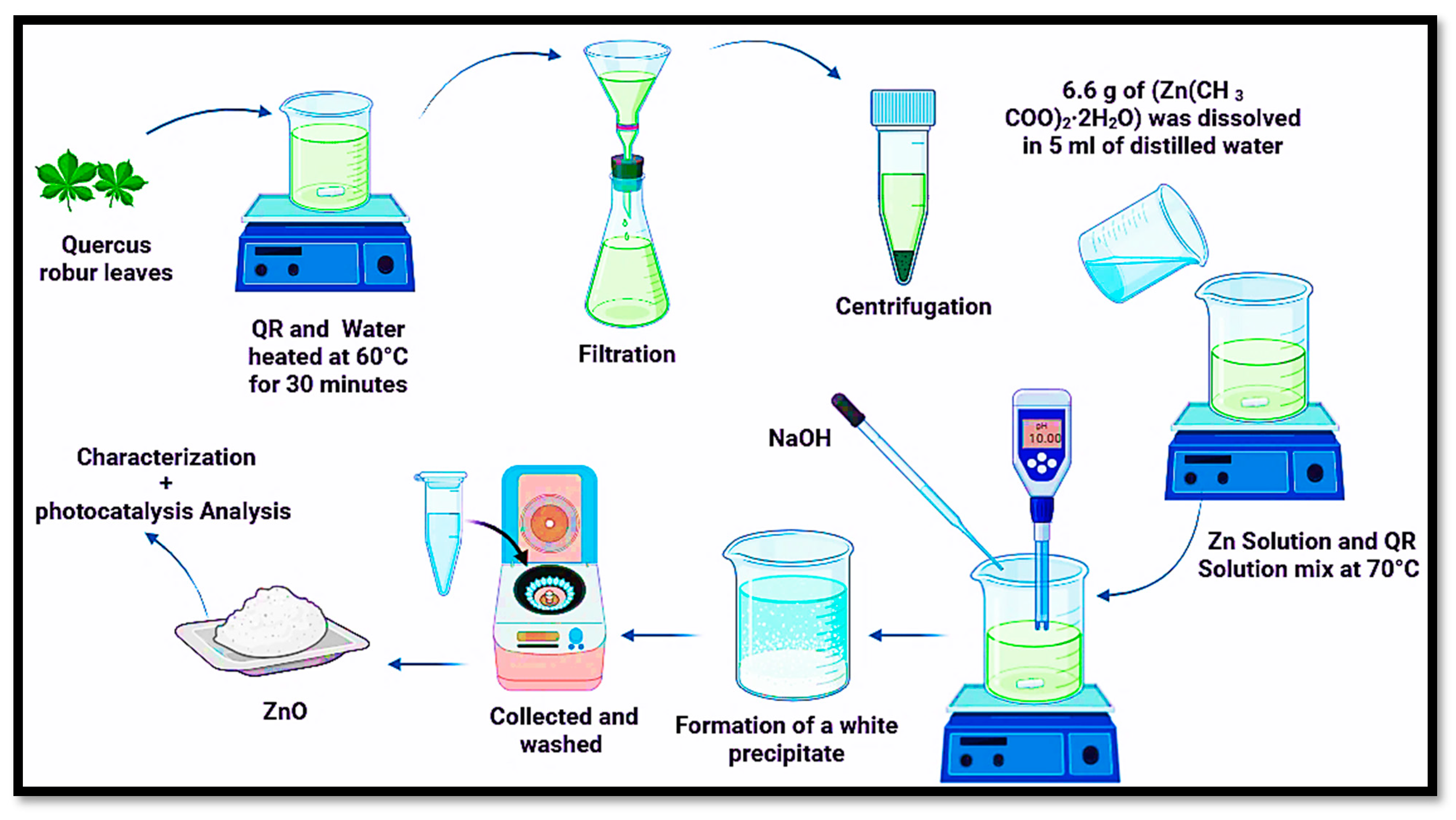
3.1. Preparation of Leaf Extract
3.2. Green Synthesis of ZnO NPs
3.3. Photocatalytic Tests
3.4. Antimicrobial Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palai, P.; Muduli, S.; Priyadarshini, B.; Sahoo, T.R. A Facile Green Synthesis of ZnO Nanoparticles and Its Adsorptive Removal of Congo Red Dye from Aqueous Solution. Mater. Today Proc. 2021, 38, 2445–2451. [Google Scholar] [CrossRef]
- Kaliraj, L.; Ahn, J.C.; Rupa, E.J.; Abid, S.; Lu, J.; Yang, D.C. Synthesis of Panos Extract Mediated ZnO Nano-Flowers as Photocatalyst for Industrial Dye Degradation by UV Illumination. J. Photochem. Photobiol. B Biol. 2019, 199, 111588. [Google Scholar] [CrossRef]
- Toumi, S.; Lekmine, S.; Touzout, N.; Moussa, H.; Elboughdiri, N.; Boudraa, R.; Benslama, O.; Kebir, M.; Danish, S.; Zhang, J. Harnessing Deep Learning for Real-Time Water Quality Assessment: A Sustainable Solution. Water 2024, 16, 3380. [Google Scholar] [CrossRef]
- Chung, K.T.; Fulk, G.E.; Andrews, A.W. Mutagenicity Testing of Some Commonly Used Dyes. Appl. Environ. Microbiol. 1981, 42, 641–648. [Google Scholar] [CrossRef]
- LaKind, J.S.; Anthony, L.G.; Goodman, M. Review of Reviews on Exposures to Synthetic Organic Chemicals and Children’s Neurodevelopment: Methodological and Interpretation Challenges. J. Toxicol. Environ. Health Part B 2017, 20, 390–422. [Google Scholar] [CrossRef]
- Ming, J.; Ni, S.-Q.; Guo, Z.; Wang, Z.-B.; Xie, L. Photocatalytic Material–Microorganism Hybrid Systems in Water Decontamination. Trends Biotechnol. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Soto-Robles, C.A.; Luque, P.A.; Gómez-Gutiérrez, C.M.; Nava, O.; Vilchis-Nestor, A.R.; Lugo-Medina, E.; Ranjithkumar, R.; Castro-Beltrán, A. Study on the Effect of the Concentration of Hibiscus Sabdariffa Extract on the Green Synthesis of ZnO Nanoparticles. Results Phys. 2019, 15, 102807. [Google Scholar] [CrossRef]
- Tahraoui, H.; Belhadj, A.-E.; Amrane, A.; Toumi, S.; Jaouadi, B.; Zhang, J. Maximizing Diclofenac Bioremoval Efficiency Using Chlorella vulgaris Strain H1 and Chlorella sorokiniana Strain H2: Unveiling the Impact of Acetic Acid on Microalgae. J. Taiwan Inst. Chem. Eng. 2024, 165, 105783. [Google Scholar] [CrossRef]
- Smara, M.; Khalladi, R.; Moulai-Mostefa, N.; Madi, K.; Mansour, D.; Lekmine, S.; Benslama, O.; Tahraoui, H.; Zhang, J.; Amrane, A. Efficiency of Hydrogen Peroxide and Fenton Reagent for Polycyclic Aromatic Hydrocarbon Degradation in Contaminated Soil: Insights from Experimental and Predictive Modeling. Processes 2024, 12, 621. [Google Scholar] [CrossRef]
- Nedjhioui, M.; Nasrallah, N.; Kebir, M.; Tahraoui, H.; Bouallouche, R.; Assadi, A.A.; Amrane, A.; Jaouadi, B.; Zhang, J.; Mouni, L. Designing an Efficient Surfactant–Polymer–Oil–Electrolyte System: A Multi-Objective Optimization Study. Processes 2023, 11, 1314. [Google Scholar] [CrossRef]
- Kebir, M.; Benramdhan, I.-K.; Nasrallah, N.; Tahraoui, H.; Bait, N.; Benaissa, H.; Ameraoui, R.; Zhang, J.; Assadi, A.A.; Mouni, L. Surface Response Modeling of Homogeneous Photo Fenton Fe (III) and Fe (II) Complex for Sunlight Degradation and Mineralization of Food Dye. Catal. Commun. 2023, 183, 106780. [Google Scholar] [CrossRef]
- Mechati, S.; Zamouche, M.; Tahraoui, H.; Filali, O.; Mazouz, S.; Bouledjemer, I.N.E.; Toumi, S.; Triki, Z.; Amrane, A.; Kebir, M. Modeling and Optimization of Hybrid Fenton and Ultrasound Process for Crystal Violet Degradation Using AI Techniques. Water 2023, 15, 4274. [Google Scholar] [CrossRef]
- Moussa, H.; Hamid, S.; Mameri, A.; Lekmine, S.; Tahraoui, H.; Kebir, M.; Touzout, N.; Dahmoune, F.; Ola, M.S.; Zhang, J. From Green Chemistry to Healthy Environments: Silver Nanoparticles as a Dual Antioxidant and Antibacterial Agents for Advancing Biomedicine and Sustainable Wastewater Treatment. Bioengineering 2024, 11, 1205. [Google Scholar] [CrossRef] [PubMed]
- Tahraoui, H.; Toumi, S.; Boudoukhani, M.; Touzout, N.; Sid, A.N.E.H.; Amrane, A.; Belhadj, A.-E.; Hadjadj, M.; Laichi, Y.; Aboumustapha, M. Evaluating the Effectiveness of Coagulation–Flocculation Treatment Using Aluminum Sulfate on a Polluted Surface Water Source: A Year-Long Study. Water 2024, 16, 400. [Google Scholar] [CrossRef]
- Touahri, S.; Halimi, O.; Zaabat, M.; Mammeri, S.; Boudine, B.; Sebais, M.; Tahraoui, H.; Zhang, J.; Amrane, A. Exploring the Influence of Sr Concentration on the Structural and Catalytic Properties of CuO/SrSO4 Nanocomposites for Organic Dye Degradation. J. Phys. Chem. Solids 2024, 195, 112299. [Google Scholar] [CrossRef]
- Golmohammadi, M.; Honarmand, M.; Ghanbari, S. A Green Approach to Synthesis of ZnO Nanoparticles Using Jujube Fruit Extract and Their Application in Photocatalytic Degradation of Organic Dyes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 117961. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.; Fayyaz, A.; Ahmed, E.; Arooj, M.; Ali, S.; Sarfraz, W.; Abbas, M.; Idrees, A.; Shahzadi, M.; Rasheed, Z. Increase in Food Scarcity, Agricultural Challenges, and Their Management: Pakistan Perspectives. In Managing Plant Production Under Changing Environment; Hasanuzzaman, M., Ahammed, G.J., Nahar, K., Eds.; Springer Nature Singapore: Singapore, 2022; pp. 437–458. ISBN 978-981-16-5058-1. [Google Scholar] [CrossRef]
- Rajan, C.S.R. Nanotechnology in Groundwater Remediation. Int. J. Environ. Sci. Dev. 2011, 2, 182–187. [Google Scholar] [CrossRef]
- Chelghoum, H.; Nasrallah, N.; Tahraoui, H.; Seleiman, M.F.; Bouhenna, M.M.; Belmeskine, H.; Zamouche, M.; Djema, S.; Zhang, J.; Mendil, A. Eco-Friendly Synthesis of ZnO Nanoparticles for Quinoline Dye Photodegradation and Antibacterial Applications Using Advanced Machine Learning Models. Catalysts 2024, 14, 831. [Google Scholar] [CrossRef]
- Kebir, M.; Bouallouche, R.; Nasrallah, N.; Tahraoui, H.; Elboughdiri, N.; Ait Merzeg, F.; Dergal, F.; Amirouche, S.; Assadi, A.A.; Amrane, A. Sustainable Photodegradation of Amoxicillin in Wastewater with a Nickel Aluminate and ZnO Heterosystem Oxides: Experimental and Gaussian Process Regression Modeling Studies. Catalysts 2024, 14, 875. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, X.; Hu, X.; Hu, J.; Zhang, X. Two-dimensional Nanomaterials for Photocatalytic Water Disinfection: Recent Progress and Future Challenges. J. Chem. Technol. Biotechnol. 2019, 94, 22–37. [Google Scholar] [CrossRef]
- Teow, Y.H.; Mohammad, A.W. New Generation Nanomaterials for Water Desalination: A Review. Desalination 2019, 451, 2–17. [Google Scholar] [CrossRef]
- Liu, X.; Ma, R.; Wang, X.; Ma, Y.; Yang, Y.; Zhuang, L.; Zhang, S.; Jehan, R.; Chen, J.; Wang, X. Graphene Oxide-Based Materials for Efficient Removal of Heavy Metal Ions from Aqueous Solution: A Review. Environ. Pollut. 2019, 252, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Yang, Y.; Shi, H.; Zhu, J.; Tan, X.; Luan, Y.; Jiang, Z.; Wang, P.; Qin, J. Hollow Dodecahedra Graphene Oxide-Cuprous Oxide Nanocomposites with Effective Photocatalytic and Bactericidal Activity. Front. Chem. 2021, 9, 755836. [Google Scholar] [CrossRef] [PubMed]
- Anbarasu, K.; Devarajan, Y. Nanomaterials-Based Wastewater Treatment: Addressing Challenges and Advancing Sustainable Solutions. BioNanoScience 2025, 15, 149. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and Disadvantages of Techniques Used for Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Zhou, C.; Lai, C.; Xu, P.; Zeng, G.; Huang, D.; Zhang, C.; Cheng, M.; Hu, L.; Wan, J.; Liu, Y.; et al. In Situ Grown AgI/Bi12O17Cl2 Heterojunction Photocatalysts for Visible Light Degradation of Sulfamethazine: Efficiency, Pathway and Mechanism. ACS Sustain. Chem. Eng. 2018, 6, 4174–4184. [Google Scholar] [CrossRef]
- Lu, K.; Qin, J.; Hu, M.; Hu, L.; Mao, M.; Li, X.; Lin, Z.; Liu, W. High-Efficiency Nickel Recovery from Spent Electroless Nickel Plating Solution: Effective Degradation of High-Concentration Nickel Complexes to Form a Nickel Ferrite Nanomaterial via Fe3O4 Catalytic Oxidation. Environ. Sci. Nano 2024, 11, 900–910. [Google Scholar] [CrossRef]
- Ikram, M.; Rashid, M.; Haider, A.; Naz, S.; Haider, J.; Raza, A.; Ansar, M.T.; Uddin, M.K.; Ali, N.M.; Ahmed, S.S.; et al. A Review of Photocatalytic Characterization, and Environmental Cleaning, of Metal Oxide Nanostructured Materials. Sustain. Mater. Technol. 2021, 30, e00343. [Google Scholar] [CrossRef]
- Zhuang, Q.; Li, X.; Lian, X.; Hu, H.; Wang, N.; Wu, J.; Miao, K.; Feng, G.; Luo, X. Catalysis Enhancement of Co3O4 through the Epitaxial Growth of Inert ZnO in Peroxymonosulfate Activation: The Catalytic Mechanism of Surface Hydroxyls in Singlet Oxygen Generation. Cryst. Growth Des. 2025, 25, 319–329. [Google Scholar] [CrossRef]
- Ali, T.; Tripathi, P.; Azam, A.; Raza, W.; Ahmed, A.S.; Ahmed, A.; Muneer, M. Photocatalytic Performance of Fe-Doped TiO2 Nanoparticles under Visible-Light Irradiation. Mater. Res. Express 2017, 4, 015022. [Google Scholar] [CrossRef]
- Hasija, V.; Raizada, P.; Sudhaik, A.; Sharma, K.; Kumar, A.; Singh, P.; Jonnalagadda, S.B.; Thakur, V.K. Recent Advances in Noble Metal Free Doped Graphitic Carbon Nitride Based Nanohybrids for Photocatalysis of Organic Contaminants in Water: A Review. Appl. Mater. Today 2019, 15, 494–524. [Google Scholar] [CrossRef]
- Anpo, M.; Ichihashi, Y.; Takeuchi, M.; Yamashita, H. Design of Unique Titanium Oxide Photocatalysts by an Advanced Metal Ion-Implantation Method and Photocatalytic Reactions under Visible Light Irradiation. Res. Chem. Intermed. 1998, 24, 143–149. [Google Scholar] [CrossRef]
- Guediri, M.K.; Chebli, D.; Bouguettoucha, A.; Bourzami, R.; Amrane, A. Novel Fe2TiO5/Reduced Graphene Oxide Heterojunction Photocatalyst with Improved Adsorption Capacity and Visible Light Photoactivity: Experimental and DFT Approach. Environ. Sci. Pollut. Res. 2021, 28, 8507–8519. [Google Scholar] [CrossRef]
- Muthuvel, A.; Jothibas, M.; Mohana, V.; Manoharan, C. Green Synthesis of Cerium Oxide Nanoparticles Using Calotropis Procera Flower Extract and Their Photocatalytic Degradation and Antibacterial Activity. Inorg. Chem. Commun. 2020, 119, 108086. [Google Scholar] [CrossRef]
- Xing, Z.; Zhang, J.; Cui, J.; Yin, J.; Zhao, T.; Kuang, J.; Xiu, Z.; Wan, N.; Zhou, W. Recent Advances in Floating TiO2-Based Photocatalysts for Environmental Application. Appl. Catal. B Environ. 2018, 225, 452–467. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A Review of ZnO Nanoparticles as Solar Photocatalysts: Synthesis, Mechanisms and Applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Li, X.; Peng, T.; Zhang, Y.; Wen, Y.; Nan, Z. A New Efficient Visible-Light Photocatalyst Made of SnO2 and Cyclized Polyacrylonitrile. Mater. Res. Bull. 2018, 97, 517–522. [Google Scholar] [CrossRef]
- Rasheed, H.M.; Aroosh, K.; Meng, D.; Ruan, X.; Akhter, M.; Cui, X. A Review on Modified ZnO to Address Environmental Challenges through Photocatalysis: Photodegradation of Organic Pollutants. Mater. Today Energy 2025, 48, 101774. [Google Scholar] [CrossRef]
- Chikhi, B.; Gouasmi, M.; Mounia, A.; Gasem, L.; Saadi, A.; Mekaoui, N.; Bachari, K.; Boudjemaa, A. Propyl Paraben Removal Using Cu2O/ZnO-NPs Photocatalyst Elaborated via Green Method. Environ. Sci. Pollut. Res. 2025, 32, 2107–2122. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Adil, S.F.; Al-Mayouf, A. Metal Oxides as Photocatalysts. J. Saudi Chem. Soc. 2015, 19, 462–464. [Google Scholar] [CrossRef]
- Madi, K.; Chebli, D.; Ait Youcef, H.; Tahraoui, H.; Bouguettoucha, A.; Kebir, M.; Zhang, J.; Amrane, A. Green Fabrication of ZnO Nanoparticles and ZnO/rGO Nanocomposites from Algerian Date Syrup Extract: Synthesis, Characterization, and Augmented Photocatalytic Efficiency in Methylene Blue Degradation. Catalysts 2024, 14, 62. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, P.; Zang, Z. High Performance CsPbBr3 Quantum Dots Photodetectors by Using Zinc Oxide Nanorods Arrays as an Electron-Transport Layer. Appl. Phys. Lett. 2020, 116, 162103. [Google Scholar] [CrossRef]
- Li, C.; Han, C.; Zhang, Y.; Zang, Z.; Wang, M.; Tang, X.; Du, J. Enhanced Photoresponse of Self-Powered Perovskite Photodetector Based on ZnO Nanoparticles Decorated CsPbBr3 Films. Sol. Energy Mater. Sol. Cells 2017, 172, 341–346. [Google Scholar] [CrossRef]
- Dejen, K.D.; Sabir, F.K.; Ananda Murthy, H.C.; Ayanie, G.T.; Shume, M.S.; Bekele, E.T. Green Synthesis of Nanomaterials for Environmental Remediation. In Green Nanoremediation; Policarpo Tonelli, F.M., Roy, A., Ananda Murthy, H.C., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 27–65. ISBN 978-3-031-30557-3. [Google Scholar]
- Duan, H.; Wang, D.; Li, Y. Green Chemistry for Nanoparticle Synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef]
- Iravani, S. Green Synthesis of Metal Nanoparticles Using Plants. Green Chem. 2011, 13, 2638. [Google Scholar] [CrossRef]
- Bandeira, M.; Giovanela, M.; Roesch-Ely, M.; Devine, D.M.; Da Silva Crespo, J. Green Synthesis of Zinc Oxide Nanoparticles: A Review of the Synthesis Methodology and Mechanism of Formation. Sustain. Chem. Pharm. 2020, 15, 100223. [Google Scholar] [CrossRef]
- Jena, S.K.; Sadasivam, R.; Packirisamy, G.; Saravanan, P. Nanoremediation: Sunlight Mediated Dye Degradation Using Electrospun PAN/CuO–ZnO Nanofibrous Composites. Environ. Pollut. 2021, 280, 116964. [Google Scholar] [CrossRef]
- Alavi-Tabari, S.A.R.; Khalilzadeh, M.A.; Karimi-Maleh, H. Simultaneous Determination of Doxorubicin and Dasatinib as Two Breast Anticancer Drugs Uses an Amplified Sensor with Ionic Liquid and ZnO Nanoparticle. J. Electroanal. Chem. 2018, 811, 84–88. [Google Scholar] [CrossRef]
- Chandran, S.P.; Chaudhary, M.; Pasricha, R.; Ahmad, A.; Sastry, M. Synthesis of Gold Nanotriangles and Silver Nanoparticles Using Aloe Vera Plant Extract. Biotechnol. Prog. 2006, 22, 577–583. [Google Scholar] [CrossRef]
- Song, J.Y.; Kim, B.S. Rapid Biological Synthesis of Silver Nanoparticles Using Plant Leaf Extracts. Bioprocess Biosyst. Eng. 2009, 32, 79–84. [Google Scholar] [CrossRef]
- Rathnasamy, R.; Thangasamy, P.; Thangamuthu, R.; Sampath, S.; Alagan, V. Green Synthesis of ZnO Nanoparticles Using Carica Papaya Leaf Extracts for Photocatalytic and Photovoltaic Applications. J. Mater. Sci. Mater. Electron. 2017, 28, 10374–10381. [Google Scholar] [CrossRef]
- Sangeetha, G.; Rajeshwari, S.; Venckatesh, R. Green Synthesis of Zinc Oxide Nanoparticles by Aloe Barbadensis Miller Leaf Extract: Structure and Optical Properties. Mater. Res. Bull. 2011, 46, 2560–2566. [Google Scholar] [CrossRef]
- Ramesh, M.; Anbuvannan, M.; Viruthagiri, G. Green Synthesis of ZnO Nanoparticles Using Solanum Nigrum Leaf Extract and Their Antibacterial Activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 864–870. [Google Scholar] [CrossRef]
- Thema, F.T.; Manikandan, E.; Dhlamini, M.S.; Maaza, M. Green Synthesis of ZnO Nanoparticles via Agathosma Betulina Natural Extract. Mater. Lett. 2015, 161, 124–127. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Mathimani, T.; Alharbi, S.A.; Chinnathambi, A.; Karuppusamy, I.; Brindhadevi, K.; Kheawfu, K.; Pikulkaew, S. In Vitro Efficacy of Green Synthesized ZnO Nanoparticles against Biofilm and Virulence of Serratia Marcescens. Prog. Org. Coat. 2022, 166, 106781. [Google Scholar] [CrossRef]
- Khoso, S.; Mehar, S.; Anam; Naheed, N.; Saeed, F.; Khan, N.; Abbasi, B.H. Green synthesis of ZnO nanoparticles from foeniculum vulgare, its characterization and biological potential against bacteria. J. Anim. Plant Sci. 2021, 32, 229–237. [Google Scholar] [CrossRef]
- Etape, E.P.; Foba-Tendo, J.; Ngolui, L.J.; Namondo, B.V.; Yollande, F.C.; Nguimezong, M.B.N. Structural Characterization and Magnetic Properties of Undoped and Ti-Doped ZnO Nanoparticles Prepared by Modified Oxalate Route. J. Nanomater. 2018, 2018, 9072325. [Google Scholar] [CrossRef]
- Bourzami, R.; Guediri, M.K.; Chebli, D.; Bouguettoucha, A.; Amrane, A. Bottom-up Construction of Reduced-Graphene-Oxide-Anchored Spinel Magnet Fe2.02Ni1.01O3.22, Anatase TiO2 and Metallic Ag Nanoparticles and Their Synergy in Photocatalytic Water Reduction. J. Environ. Chem. Eng. 2021, 9, 105307. [Google Scholar] [CrossRef]
- Demissie, M.G.; Sabir, F.K.; Edossa, G.D.; Gonfa, B.A. Synthesis of Zinc Oxide Nanoparticles Using Leaf Extract of Lippia adoensis (Koseret) and Evaluation of Its Antibacterial Activity. J. Chem. 2020, 2020, 7459042. [Google Scholar] [CrossRef]
- Mallika, A.N.; Reddy, A.R.; Reddy, K.V. Annealing Effects on the Structural and Optical Properties of ZnO Nanoparticles with PVA and CA as Chelating Agents. J. Adv. Ceram. 2015, 4, 123–129. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Liao, C.-H.; Chueh, Y.-L.; Lai, C.-C.; Chen, L.-Y.; Chu, A.-K.; Kuo, C.-T.; Wang, H.-C. High Performance Cu2O/ZnO Core-Shell Nanorod Arrays Synthesized Using a Nanoimprint GaN Template by the Hydrothermal Growth Technique. Opt. Mater. Express 2014, 4, 1473. [Google Scholar] [CrossRef]
- Caliman, F.A.; Robu, B.M.; Smaranda, C.; Pavel, V.L.; Gavrilescu, M. Soil and Groundwater Cleanup: Benefits and Limits of Emerging Technologies. Clean Technol. Environ. Policy 2011, 13, 241–268. [Google Scholar] [CrossRef]
- Oniya, E.O.; Olubi, O.E.; Ibitoye, A.; Agbi, J.I.; Agbeni, S.K.; Faweya, E.B. Effect of Milling Equipment on the Level of Heavy Metal Content of Foodstuff. Phys. Sci. Int. J. 2018, 20, 1–8. [Google Scholar] [CrossRef]
- Naiel, B.; Fawzy, M.; Halmy, M.W.A.; Mahmoud, A.E.D. Green synthesis of zinc oxide nanoparticles using Sea Lavender (Limonium pruinosum L. Chaz.) extract: Characterization, evaluation of anti-skin cancer, antimicrobial and antioxidant potentials. Sci. Rep. 2022, 12, 20370. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Sharma, J.K.; Kansay, V.; Sharma, V.D.; Sharma, A.; Kumar, S.; Sharma, A.K.; Bera, M.K. The Effect of Calcination Temperatures on the Structural and Optical Properties of Zinc Oxide Nanoparticles and Their Influence on the Photocatalytic Degradation of Leather Dye. Chem. Phys. Impact 2023, 6, 100196. [Google Scholar] [CrossRef]
- Agnieszka, K.-R.; Ewa, M.; Teofil, J. Structural Characterisation of ZnO Particles Obtained by the Emulsion Precipitation Method. J. Nanomater. 2012, 656353. [Google Scholar] [CrossRef]
- Khan, S.H.; R, S.; Pathak, B.; Fulekar, M. Photocatalytic Degradation of Organophosphate Pesticides (Chlorpyrifos) Using Synthesized Zinc Oxide Nanoparticle by Membrane Filtration Reactor under UV Irradiation. Front. Nanosci. Nanotechnol. 2015, 1, 23–27. [Google Scholar] [CrossRef]
- Christobel, G.J. Vibrational Spectroscopy of ZnO-ZnS Nanoparticles. Int. J. Sci. Res. 2013, 5, 2228–2230. [Google Scholar]
- Veiga, A.; Toledo, M.D.G.T.; Rossa, L.S.; Mengarda, M.; Stofella, N.C.F.; Oliveira, L.J.; Gonçalves, A.G.; Murakami, F.S. Colorimetric Microdilution Assay: Validation of a Standard Method for Determination of MIC, IC50%, and IC90% of Antimicrobial Compounds. J. Microbiol. Methods 2019, 162, 50–61. [Google Scholar] [CrossRef]
- Bourzami, R.; AitYoucef, H.C.; Hamdouni, N.; Sebais, M. Synthesis, Crystal Structure, Vibrational Spectra and Thermal Properties of Novel Ionic Organic-Inorganic Hybrid Material. Chem. Phys. Lett. 2018, 711, 220–226. [Google Scholar] [CrossRef]
- Ouksel, L.; Chafaa, S.; Bourzami, R.; Hamdouni, N.; Sebais, M.; Chafai, N. Crystal Structure, Vibrational, Spectral Investigation, Quantum Chemical DFT Calculations and Thermal Behavior of Diethyl [Hydroxy (Phenyl) Methyl] Phosphonate. J. Mol. Struct. 2017, 1144, 389–395. [Google Scholar] [CrossRef]
- Redjili, S.; Ghodbane, H.; Bourzami, R.; Mayouf, F.; Chebli, D.; Boudechicha, A.; Djelloul, C. Green Synthesis of Silver Oxide Nanoparticles: Eco-Friendly Approach for Sustainable Solutions. Ind. Crops Prod. 2025, 223, 120168. [Google Scholar] [CrossRef]
- Boudiaf, M.; Messai, Y.; Bentouhami, E.; Schmutz, M.; Blanck, C.; Ruhlmann, L.; Bezzi, H.; Tairi, L.; Eddine Mekki, D. Green Synthesis of NiO Nanoparticles Using Nigella Sativa Extract and Their Enhanced Electro-Catalytic Activity for the 4-Nitrophenol Degradation. J. Phys. Chem. Solids 2021, 153, 110020. [Google Scholar] [CrossRef]
- Liu, H.R.; Shao, G.X.; Zhao, J.F.; Zhang, Z.X.; Zhang, Y.; Liang, J.; Liu, X.G.; Jia, H.S.; Xu, B.S. Worm-Like Ag/ZnO Core–Shell Heterostructural Composites: Fabrication, Characterization, and Photocatalysis. J. Phys. Chem. C 2012, 116, 16182–16190. [Google Scholar] [CrossRef]
- Garg, R. Characterization and Performance Evaluation of Synthesized ZnO Nanoflowers, Nanorods, and Their Hybrid Nanocomposites with Graphene Oxide for Degradation of Orange G. Environ. Sci. Pollut. Res. 2021, 28, 57009–57029. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, Z.; Liu, D.; Gao, Z. Preparation of ZnO Photocatalyst for the Efficient and Rapid Photocatalytic Degradation of Azo Dyes. Nanoscale Res. Lett. 2017, 12, 143. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, M.; Mahjoub, A.R.; Mehri, B. Enhanced Photocatalytic Degradation of Congo Red by Solvothermally Synthesized CuInSe2–ZnO Nanocomposites. RSC Adv. 2014, 4, 21757. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, G.; Umar, A. ZnO Nano-Mushrooms for Photocatalytic Degradation of Methyl Orange. Mater. Lett. 2013, 97, 100–103. [Google Scholar] [CrossRef]
- Mutalib, A.A.A. ZnO Photocatalysts Applications in Abating the Organic Pollutant Contamination: A Mini Review. Total. Environ. Res. Themes 2022, 3, 100013. [Google Scholar] [CrossRef]
- Naha, U. Rapid Precipitation-Free Synthesis of Zinc Oxide Nanowire Arrays. Mater. Today Proc. 2023, 79, 132–138. [Google Scholar] [CrossRef]
- Yashni, G.; Al-Gheethi, A.; Al-Kahtani, A.A.; Al-Sahari, M.; Hazhar, N.J.N.; Noman, E.; Alkhadher, S. Bio-Inspired ZnO NPs Synthesized from Citrus Sinensis Peels Extract for Congo Red Removal from Textile Wastewater via Photocatalysis: Optimization, Mechanisms, Techno-Economic Analysis. Chemosphere 2021, 281, 130661. [Google Scholar] [CrossRef]
- Gawade, V.V.; Gavade, N.L.; Shinde, H.M.; Babar, S.B.; Kadam, A.N.; Garadkar, K.M. Green Synthesis of ZnO Nanoparticles by Using Calotropis Procera Leaves for the Photodegradation of Methyl Orange. J. Mater. Sci. Mater. Electron. 2017, 28, 14033–14039. [Google Scholar] [CrossRef]
- Madhumitha, G.; Fowsiya, J.; Gupta, N.; Kumar, A.; Singh, M. Green Synthesis, Characterization and Antifungal and Photocatalytic Activity of Pithecellobium Dulce Peel–Mediated ZnO Nanoparticles. J. Phys. Chem. Solids 2019, 127, 43–51. [Google Scholar] [CrossRef]
- Sherly, E.D.; Vijaya, J.J.; Selvam, N.C.S.; Kennedy, L.J. Microwave Assisted Combustion Synthesis of Coupled ZnO–ZrO2 Nanoparticles and Their Role in the Photocatalytic Degradation of 2,4-Dichlorophenol. Ceram. Int. 2014, 40, 5681–5691. [Google Scholar] [CrossRef]
- Shore, A. Expression of Concern: One-Pot Sol–Gel Synthesis of Reduced Graphene Oxide Uniformly Decorated Zinc Oxide Nanoparticles in Starch Environment for Highly Efficient Photodegradation of Methylene Blue. RSC Adv. 2018, 8, 10332. [Google Scholar] [CrossRef]
- Kumar Sur, U.; Ankamwar, B.; Karmakar, S.; Halder, A.; Das, P. Green Synthesis of Silver Nanoparticles Using the Plant Extract of Shikakai and Reetha. Mater. Today Proc. 2018, 5, 2321–2329. [Google Scholar] [CrossRef]
- Suppiah, D.D.; Julkapli, N.M.; Sagadevan, S.; Johan, M.R. Eco-Friendly Green Synthesis Approach and Evaluation of Environmental and Biological Applications of Iron Oxide Nanoparticles. Inorg. Chem. Commun. 2023, 152, 110700. [Google Scholar] [CrossRef]
- Kavitha, A.; Doss, A.; Praveen Pole, R.P.; Pushpa Rani, T.P.K.; Prasad, R.; Satheesh, S. A Mini Review on Plant-Mediated Zinc Oxide Nanoparticles and Their Antibacterial Potency. Biocatal. Agric. Biotechnol. 2023, 48, 102654. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. Do Iron Oxide Nanoparticles Have Significant Antibacterial Properties? Antibiotics 2021, 10, 884. [Google Scholar] [CrossRef]
- Manjamadha, V.P.; Muthukumar, K. Ultrasound Assisted Green Synthesis of Silver Nanoparticles Using Weed Plant. Bioprocess Biosyst. Eng. 2016, 39, 401–411. [Google Scholar] [CrossRef]
- Raffi, M.; Hussain, F.; Bhatti, T.M.; Akhter, J.I.; Hameed, A.; Hasan, M.M. Antibacterial Characterization of Silver Nanoparticles against E. coli ATCC-15224. J. Mater. Sci. Technol. 2008, 24, 192–196. [Google Scholar]
- Panáček, A.; Kvítek, L.; Prucek, R.; Kolář, M.; Večeřová, R.; Pizúrová, N.; Sharma, V.K.; Nevěčná, T.; Zbořil, R. Silver Colloid Nanoparticles: Synthesis, Characterization, and Their Antibacterial Activity. J. Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Kovochich, M.; Brant, J.; Hotze, M.; Sempf, J.; Oberley, T.; Sioutas, C.; Yeh, J.I.; Wiesner, M.R.; Nel, A.E. Comparison of the Abilities of Ambient and Manufactured Nanoparticles to Induce Cellular Toxicity According to an Oxidative Stress Paradigm. Nano Lett. 2006, 6, 1794–1807. [Google Scholar] [CrossRef] [PubMed]
- Song, H.Y.; Ko, K.K.; Oh, I.H.; Lee, B.T. Fabrication of Silver Nanoparticles and Their Antimicrobial Mechanisms. Eur. Cells Mater. 2006, 11 (Suppl. S1), 58. [Google Scholar]
- Sambhy, V.; MacBride, M.M.; Peterson, B.R.; Sen, A. Silver Bromide Nanoparticle/Polymer Composites: Dual Action Tunable Antimicrobial Materials. J. Am. Chem. Soc. 2006, 128, 9798–9808. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; Agarwal, H.; Kumar, S.R.; Kumar, S.V. Green synthesis of silver nanoparticles using medicinal plant acalypha indica leaf extracts and its application as an antioxidant and antimicrobial agent against foodborne pathogens. Int. J. Appl. Pharm. 2017, 9, 42–50. [Google Scholar] [CrossRef]
- Yadav, J.P.; Kumar, S.; Budhwar, L.; Yadav, A.; Yadav, M. Characterization and Antibacterial Activity of Synthesized Silver and Iron Nanoparticles Using Aloe Vera. J. Nanomed. Nanotechnol. 2016, 7, 3. [Google Scholar] [CrossRef]
- Agarwal, H.; Venkat Kumar, S.; Rajeshkumar, S. A Review on Green Synthesis of Zinc Oxide Nanoparticles—An Eco-Friendly Approach. Resour.-Effic. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Benyagoub, E.; Nabbou, N.; Boukhalkhel, S.; Dehini, I. The In Vitro Evaluation of the Antimicrobial Activity of Quercus robur L. Methanolic and Aqueous Leaves’ Extracts, from the Algerian High Plateaus Against Some Uropathogenic Microbial Strains. Phytothérapie 2020, 18, 262–274. [Google Scholar] [CrossRef]
- Šukele, R.; Skadiņš, I.; Koka, R.; Bandere, D. Antibacterial Effects of Oak Bark (Quercus robur) and Heather Herb (Calluna vulgaris L.) Extracts against the Causative Bacteria of Bovine Mastitis. Vet. World 2022, 15, 2315–2322. [Google Scholar] [CrossRef]

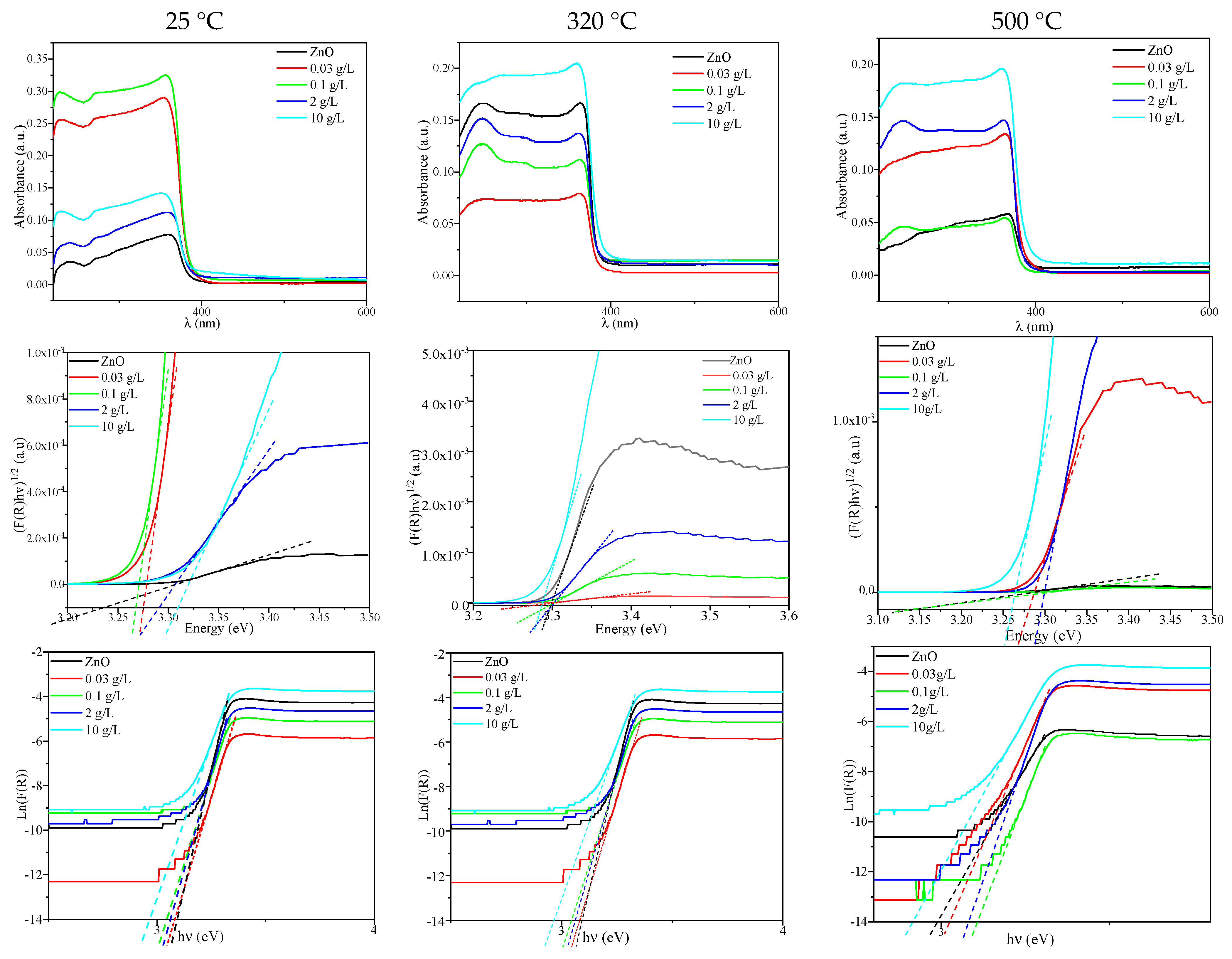


| Concentration (g/L) | ZnO | 0.03 | 0.1 | 2 | 10 |
|---|---|---|---|---|---|
| Direct gap energy (eV) | 3.29 | 3.29 | 3.29 | 3.28 | 3.29 |
| Urbach energy (eV) | 2.94 | 2.90 | 3.01 | 2.97 | 2.90 |
| Concentration (g/L) | ZnO | 0.03 | 0.1 | 2 | 10 |
|---|---|---|---|---|---|
| Direct gap energy (eV) | 3.28 | 3.27 | 3.25 | 3.29 | 3.28 |
| Urbach energy (eV) | 3.05 | 3.03 | 2.99 | 3.01 | 2.90 |
| Concentration (g/L) | ZnO | 0.03 | 0.1 | 2 | 10 |
|---|---|---|---|---|---|
| Direct gap energy (eV) | 2.98 | 3.01 | 3.15 | 3.27 | 3.26 |
| Urbach energy (eV) | 2.89 | 3.01 | 3.10 | 3.07 | 2.90 |
| Concentration (g/L) | 0 | 0.03 | 0.1 | 2 | 10 |
| E% | 50 | 44 | 38 | 35 | 26 |
| k (10−3 min−1) | 4.90 | 3.85 | 3.14 | 2.66 | 1.90 |
| Concentration (g/L) | 0 | 0.03 | 0.1 | 2 | 10 |
| E% | 62 | 74 | 55 | 53 | 47 |
| k (10−3 min−1) | 6.90 | 9.09 | 5.54 | 5.05 | 3.89 |
| Concentration (g/L) | 0 | 0.03 | 0.1 | 2 | 10 |
| E% | 56 | 74 | 62 | 60 | 50 |
| k (10−3 min−1) | 4.52 | 6.41 | 5.49 | 4.93 | 3.70 |
| Concentration (g/L) | 0.25 | 0.5 | 1 | 2 |
| E% | 66 | 44 | 74 | 22 |
| k (10−3 min−1) | 4.54 | 2.61 | 9.57 | 1.88 |
| pH | 4 | neutral | 8 | 10 |
| E% | 42 | 74 | 58 | 51 |
| k (10−3 min−1) | 2.52 | 6.41 | 5.24 | 3.56 |
| Concentration (mg/L) | 10 | 20 | 30 |
| E% | 74 | 48 | 29 |
| k (10−3 min−1) | 9.57 | 3.05 | 1.68 |
| Catalyst | Preparation Method/Plant | Dye | Irradiation | Catalyst Dose/g L−1 | Dye Conc/mg L−1 | Degradation Efficiency/% | Ref. |
|---|---|---|---|---|---|---|---|
| ZnO | Green synthesis | MB | UV-lamp | 1.5 | 10 | 63% | [85] |
| ZnO | Microwave-assisted urea-nitrate combustion | MB | UV-lamp | 0.30 | 75 | >75% | [86] |
| ZnO | Sol gel | MB | UV (Hg lamp 365 nm) | 0.33 | 10 | 37% | [87] |
| ZnO | Green synthesis | MB | UV-lamp | 1 | 10 | 74% | Present work |
| Staphylococcus aureus | Bacillus subtilis | Escherichia coli | Salmonella enterica | ||
|---|---|---|---|---|---|
| ZnO NPs Concentrations (g/L) | Annealing Temperature °C | MIC (μg/mL) | MIC (μg/mL) | MIC (μg/mL) | MIC (μg/mL) |
| c = 0.0 | 25 | 100 | 25 | 100 | 50 |
| 320 | 25 | 25 | 25 | 12.5 | |
| 500 | 25 | 6.25 | 25 | 50 | |
| 0.03 | 25 | 50 | 25 | 100 | 50 |
| 320 | 6.25 | 50 | 100 | 12.5 | |
| 500 | 25 | 12.5 | 100 | 12.5 | |
| 0.1 | 25 | 100 | 50 | 100 | 100 |
| 320 | 25 | 6.25 | 100 | 25 | |
| 500 | 50 | 6.25 | No | 25 | |
| 2 | 25 | No | 25 | 100 | 100 |
| 320 | No | 6.25 | 25 | 25 | |
| 500 | No | 50 | No | No | |
| 10 | 25 | 25 | 25 | 25 | 25 |
| 320 | No | 6.25 | 100 | 100 | |
| 500 | No | 50 | No | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Redjili, S.; Ghodbane, H.; Tahraoui, H.; Abdelouahed, L.; Chebli, D.; Ola, M.S.; Assadi, A.A.; Kebir, M.; Zhang, J.; Amrane, A.; et al. Green Innovation: Multifunctional Zinc Oxide Nanoparticles Synthesized Using Quercus robur for Photocatalytic Performance, Environmental, and Antimicrobial Applications. Catalysts 2025, 15, 256. https://doi.org/10.3390/catal15030256
Redjili S, Ghodbane H, Tahraoui H, Abdelouahed L, Chebli D, Ola MS, Assadi AA, Kebir M, Zhang J, Amrane A, et al. Green Innovation: Multifunctional Zinc Oxide Nanoparticles Synthesized Using Quercus robur for Photocatalytic Performance, Environmental, and Antimicrobial Applications. Catalysts. 2025; 15(3):256. https://doi.org/10.3390/catal15030256
Chicago/Turabian StyleRedjili, Selma, Houria Ghodbane, Hichem Tahraoui, Lokmane Abdelouahed, Derradji Chebli, Mohammad Shamsul Ola, Amine Aymen Assadi, Mohammed Kebir, Jie Zhang, Abdeltif Amrane, and et al. 2025. "Green Innovation: Multifunctional Zinc Oxide Nanoparticles Synthesized Using Quercus robur for Photocatalytic Performance, Environmental, and Antimicrobial Applications" Catalysts 15, no. 3: 256. https://doi.org/10.3390/catal15030256
APA StyleRedjili, S., Ghodbane, H., Tahraoui, H., Abdelouahed, L., Chebli, D., Ola, M. S., Assadi, A. A., Kebir, M., Zhang, J., Amrane, A., & Lekmine, S. (2025). Green Innovation: Multifunctional Zinc Oxide Nanoparticles Synthesized Using Quercus robur for Photocatalytic Performance, Environmental, and Antimicrobial Applications. Catalysts, 15(3), 256. https://doi.org/10.3390/catal15030256












