A Radio Frequency Plasma-Modified
Abstract
1. Introduction
2. Results and Discussion
2.1. Hammett Titration
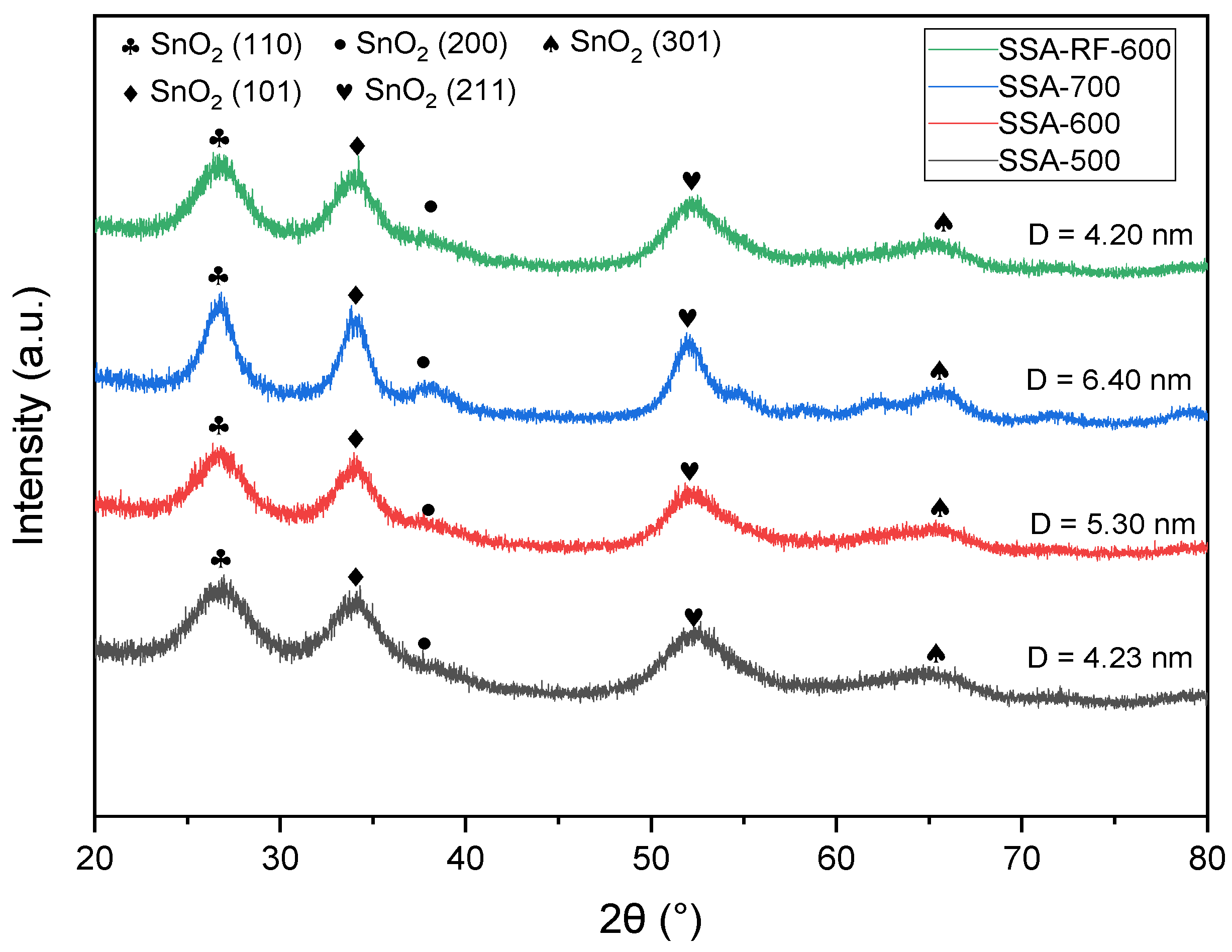
2.2. XRD
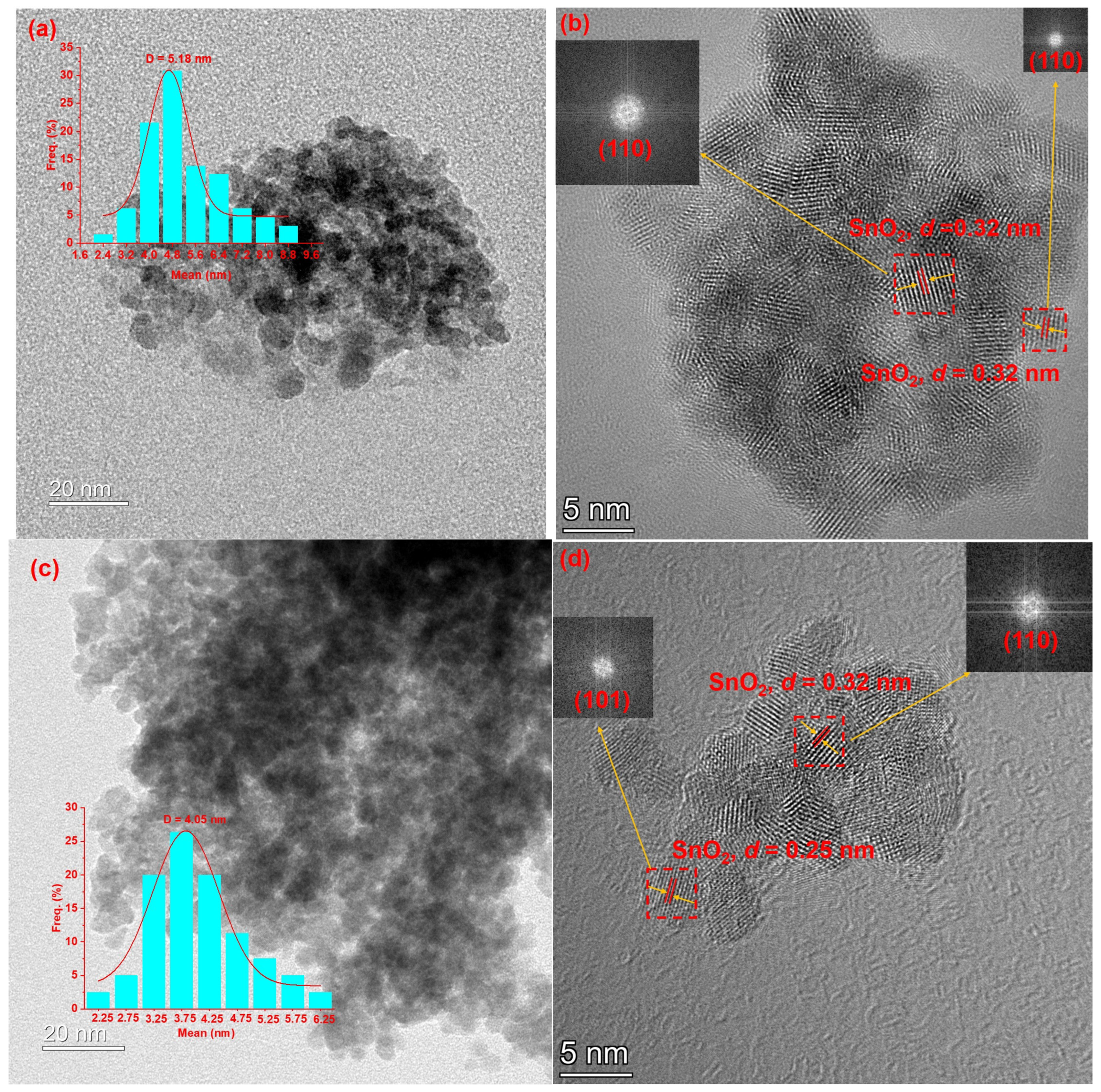
2.3. HRTEM
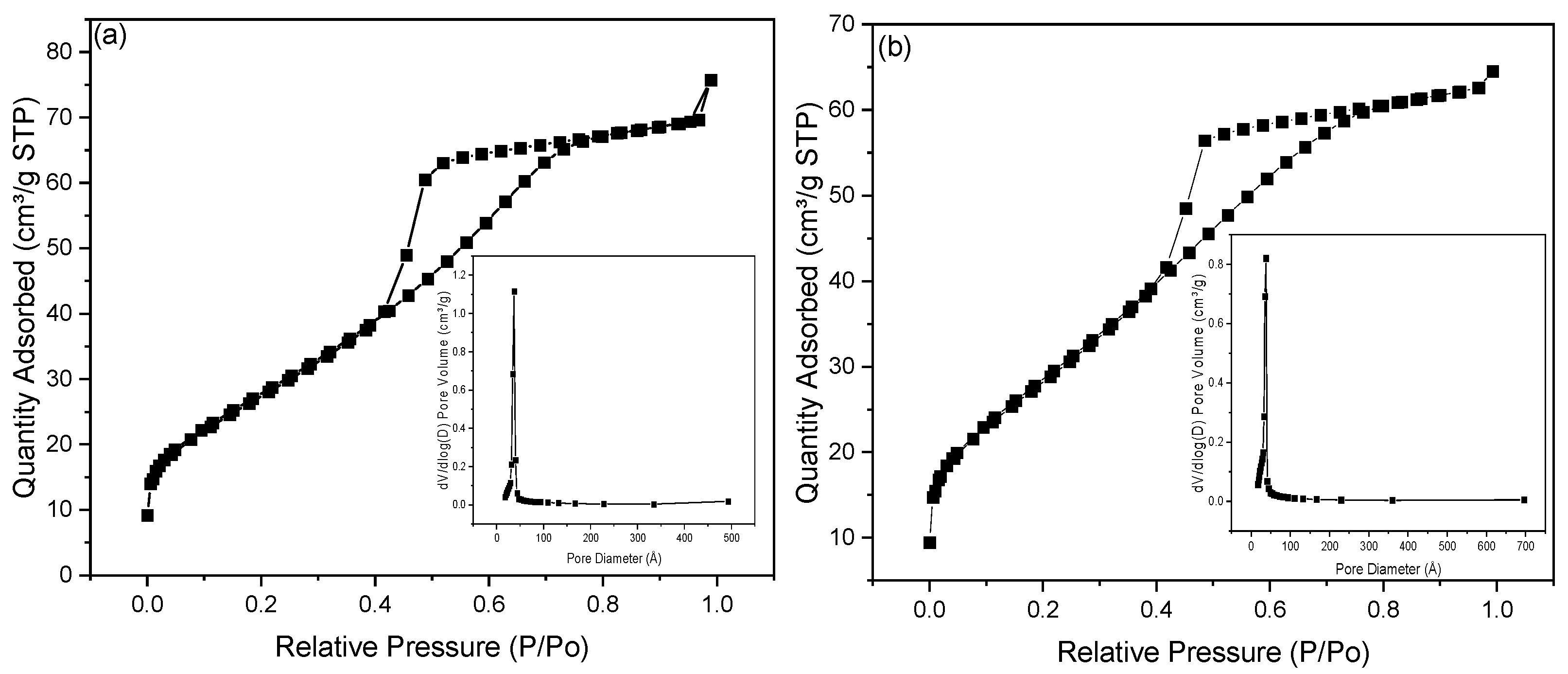
2.4. Nitrogen Adsorption—Desorption
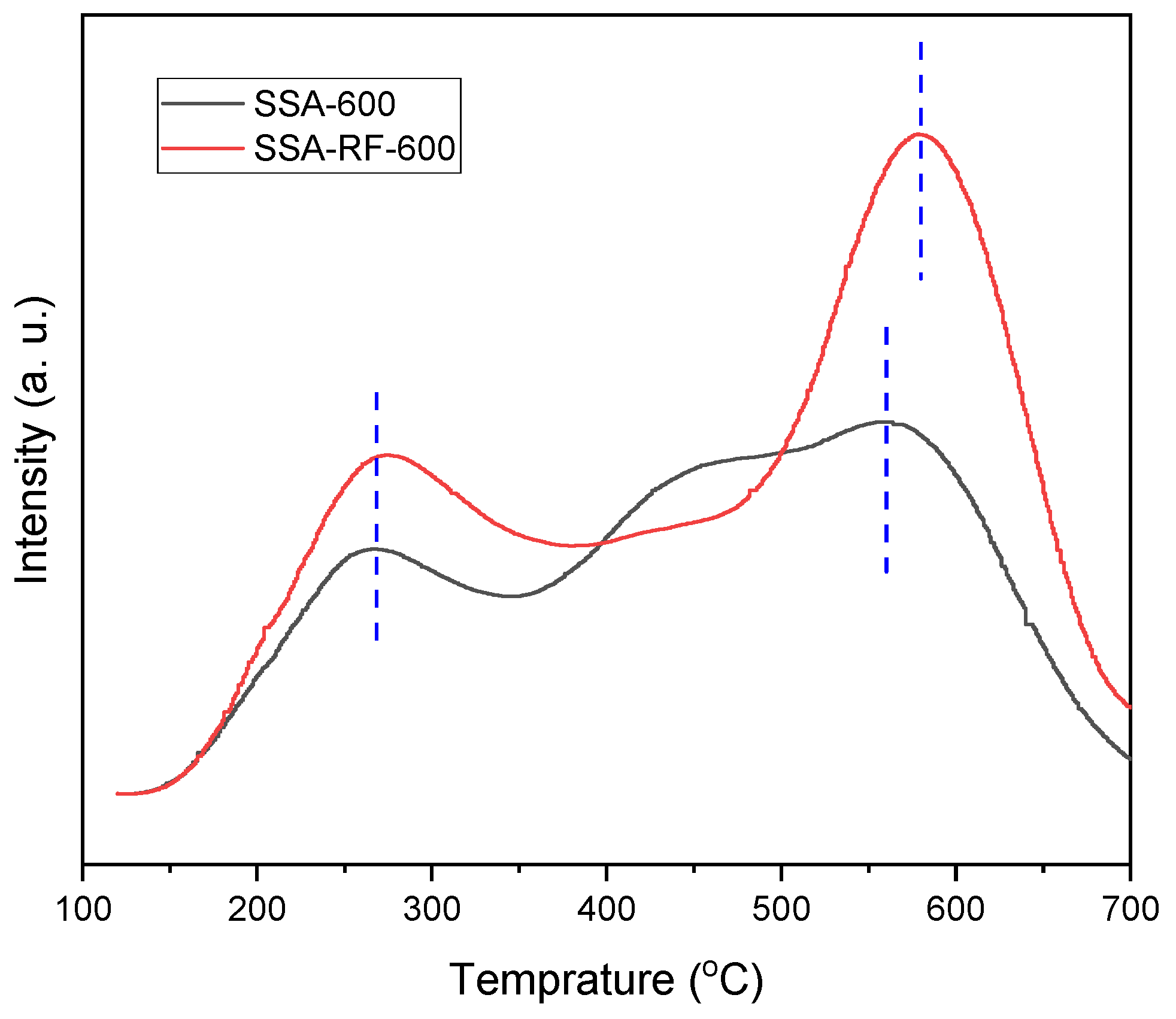
2.5. NH3-TPD
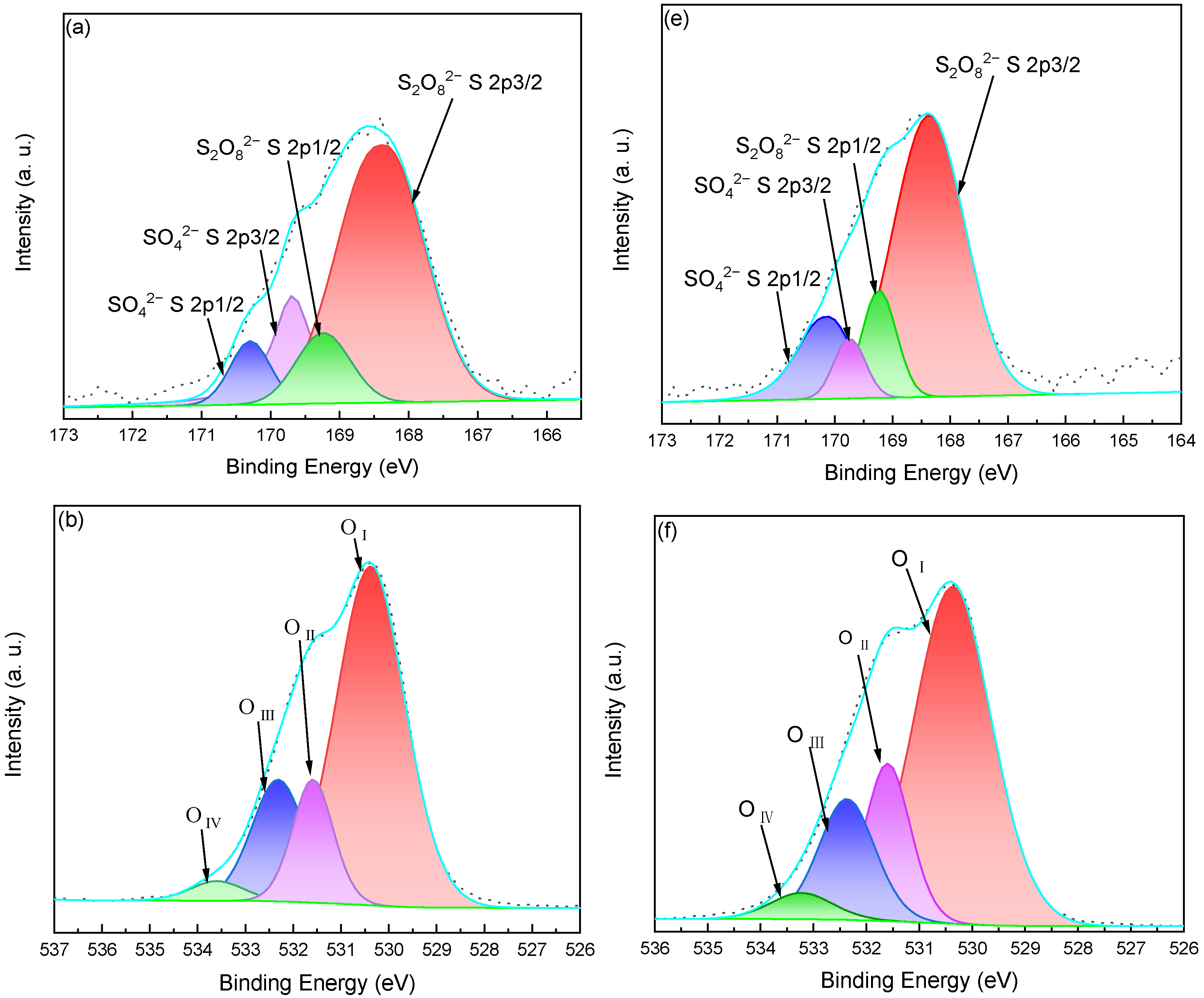
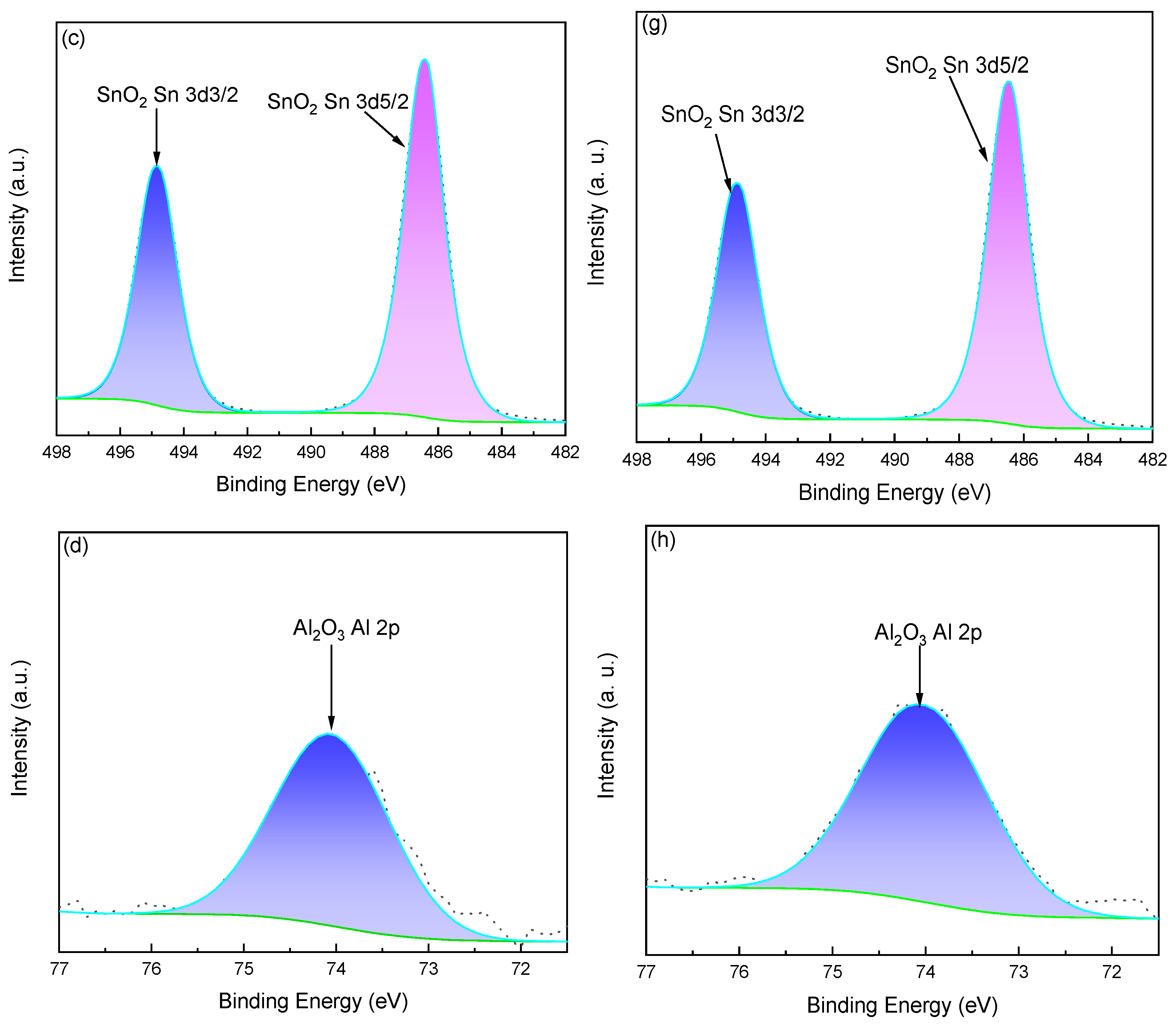
2.6. XPS
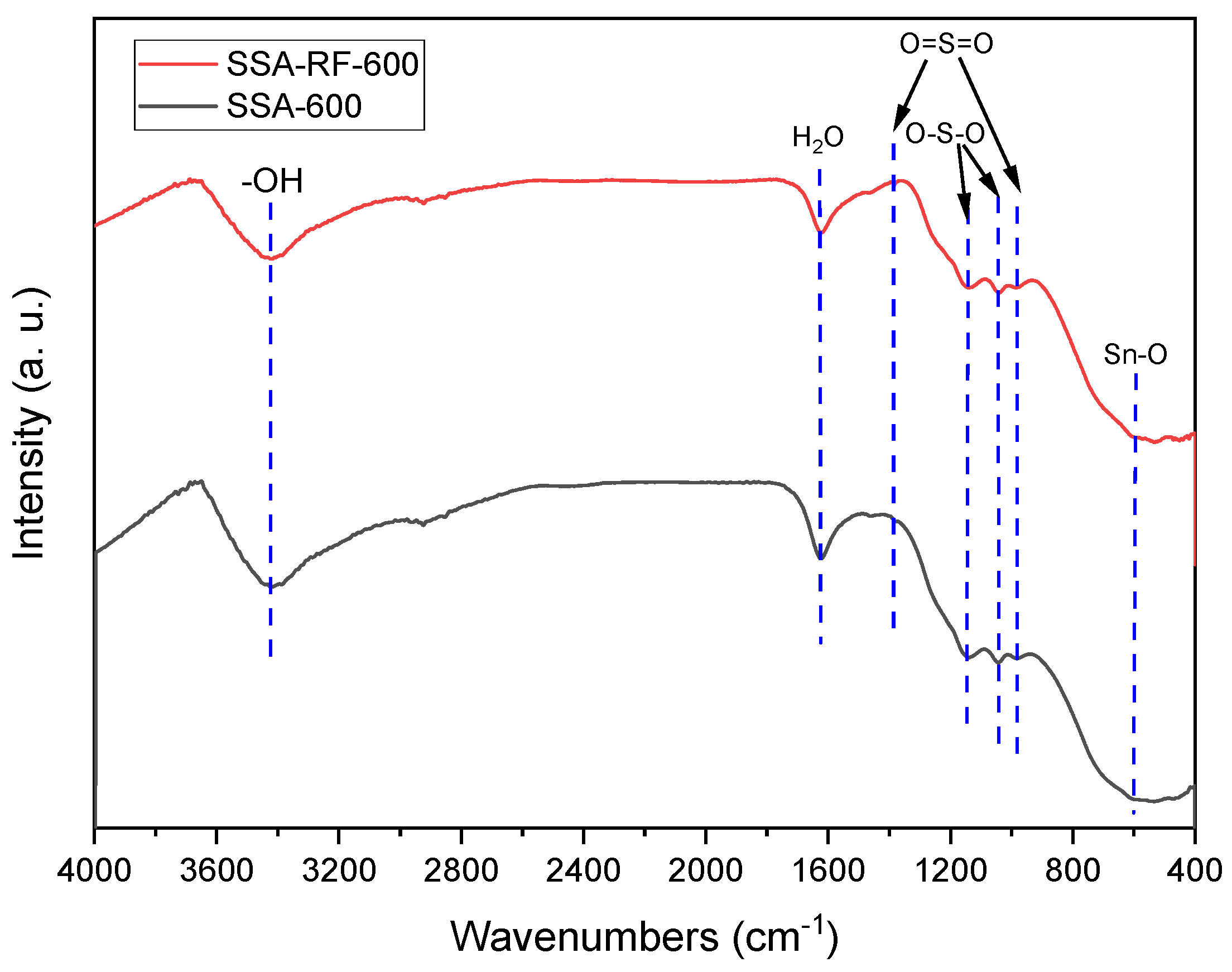
2.7. FT-IR
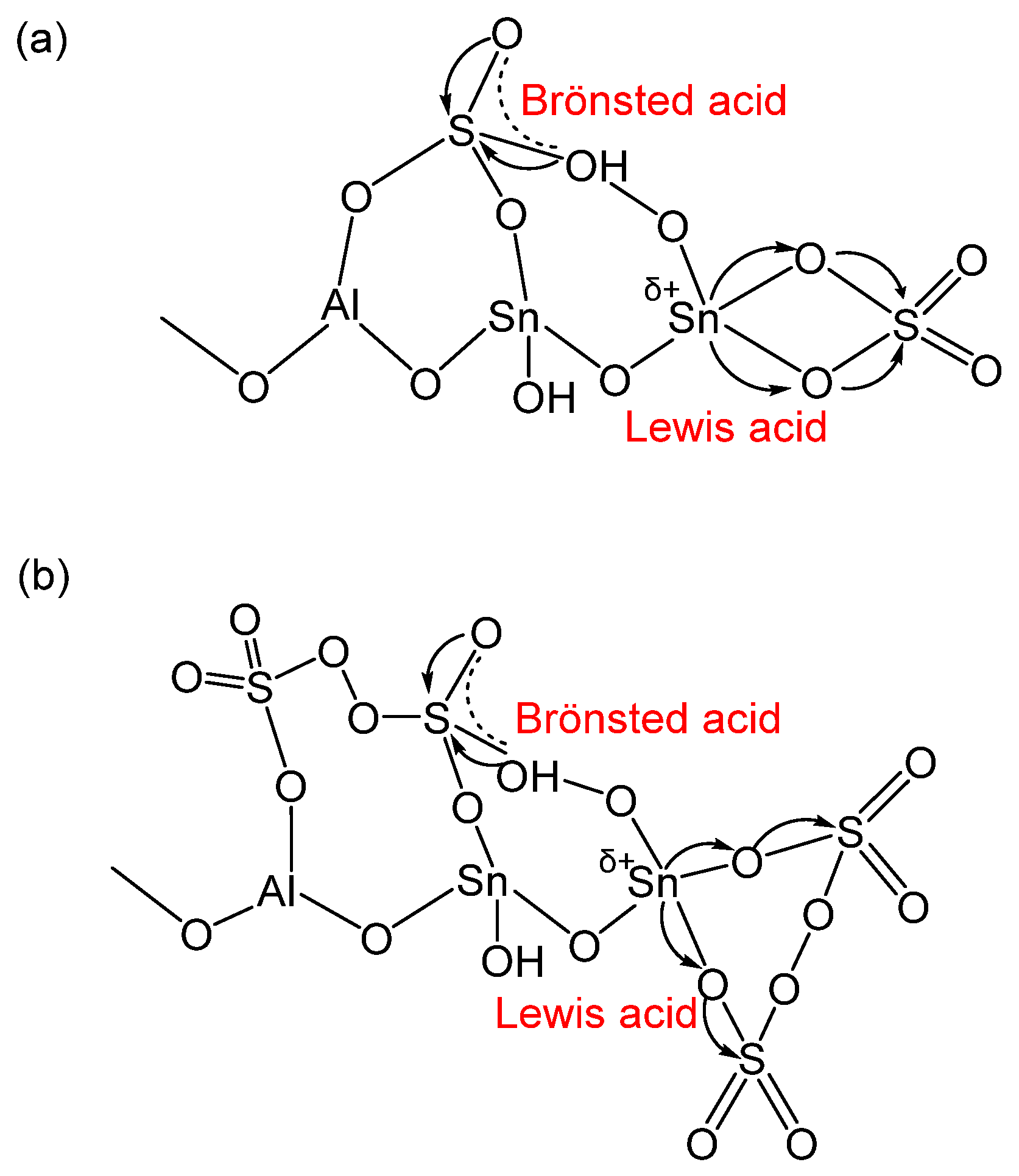
2.8. Mechanism of Acid Site Formation and Catalysis
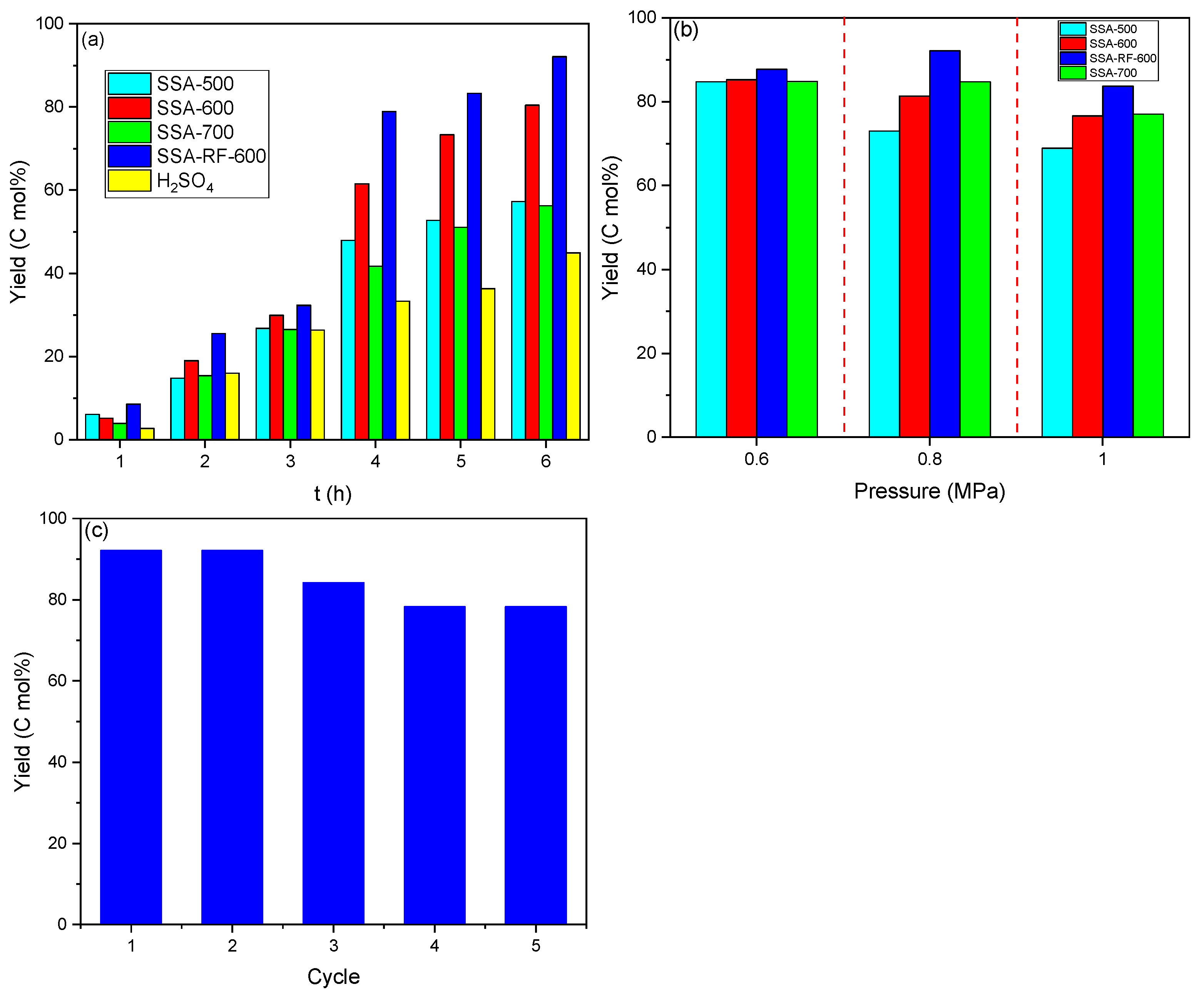
2.9. Catalytic Performance
3. Materials and Methods
3.1. Reagents
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalytic Esterification
3.5. Recyclability Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vinod, V.; Sashidhar, R.; Sarma, V.; Raju, S.S. Comparative amino acid and fatty acid compositions of edible gums kondagogu (Cochlospermum gossypium) and karaya (Sterculia urens). Food Chem. 2010, 123, 57–62. [Google Scholar] [CrossRef]
- Anand, R.C.; Vimal. A mild and convenient procedure for the esterification of amino acids. Synth. Commun. 1998, 28, 1963–1965. [Google Scholar] [CrossRef]
- He, J.; Wen, X.; Wu, L.; Chen, H.; Hu, J.; Hou, X. Dielectric barrier discharge plasma for nanomaterials: Fabrication, modification and analytical applications. TrAC Trends Anal. Chem. 2022, 156, 116715. [Google Scholar] [CrossRef]
- Liu, C.-j.; Vissokov, G.P.; Jang, B.W.-L. Catalyst preparation using plasma technologies. Catal. Today 2002, 72, 173–184. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, Q.Q.; Xiao, Z.H.; Li, X.Y.; Dai, L.M. Plasma-engraved Co3O4 nanosheets with oxygen vacancies and high surface area for the oxygen evolution reaction. Angew. Chem. 2016, 128, 5363–5367. [Google Scholar] [CrossRef]
- Di, L.; Zhang, J.; Zhang, X.; Wang, H.; Li, H.; Li, Y.; Bu, D. Cold plasma treatment of catalytic materials: A review. J. Phys. D Appl. Phys. 2021, 54, 333001. [Google Scholar] [CrossRef]
- Ye, Z.; Zhao, L.; Nikiforov, A.; Giraudon, J.-M.; Chen, Y.; Wang, J.; Tu, X. A review of the advances in catalyst modification using nonthermal plasma: Process, Mechanism and Applications. Adv. Colloid Interface Sci. 2022, 308, 102755. [Google Scholar] [CrossRef]
- Sripada, S.; Kastner, J.R. Catalytic esterification using solid acid carbon catalysts synthesized by sustainable hydrothermal and plasma sulfonation techniques. Ind. Eng. Chem. Res. 2022, 61, 3928–3940. [Google Scholar] [CrossRef]
- Qin, L.; Takeuchi, N.; Takahashi, K.; Kang, J.; Kim, K.H.; Li, O.L. N2/Ar plasma-induced surface sulfonation on graphene nanoplatelets for catalytic hydrolysis of cellulose to glucose. Appl. Surf. Sci. 2021, 545, 149051. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Liu, C.-j. Mechanism of template removal for the synthesis of molecular sieves using dielectric barrier discharge. Catal. Today 2015, 256, 137–141. [Google Scholar] [CrossRef]
- Nicolaï, B.; Mahé, N.; Céolin, R.; Rietveld, I.B.; Barrio, M.; Tamarit, J.-L. Tyrosine alkyl esters as prodrug: The structure and intermolecular interactions of L-tyrosine methyl ester compared to L-tyrosine and its ethyl and n-butyl esters. Struct. Chem. 2011, 22, 649–659. [Google Scholar] [CrossRef]
- Rietveld, I.B.; Barrio, M.; Tamarit, J.L.; Nicolaï, B.; Van de Streek, J.; Mahé, N.; Ceolin, R.; Do, B. Dimorphism of the prodrug L-tyrosine ethyl ester: Pressure–temperature state diagram and crystal structure of phase II. J. Pharm. Sci. 2011, 100, 4774–4782. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Dong, J.; Zhu, H.; Teng, X.; Ai, S.; Mang, M. A simple and sensitive fluorescent sensor for methyl parathion based on l-tyrosine methyl ester functionalized carbon dots. Biosens. Bioelectron. 2015, 68, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Abrash, H.I.; Niemann, C. Steric hindrance in α-chymotrypsin-catalyzed reactions*. Biochemistry 1963, 2, 947–953. [Google Scholar] [CrossRef]
- Ruan, C.; Rodgers, M.T. Cation-π interactions: structures and energetics of complexation of Na+ and K+ with the aromatic amino acids, phenylalanine, tyrosine, and tryptophan. J. Am. Chem. Soc. 2004, 126, 14600–14610. [Google Scholar] [CrossRef]
- Theobaldo, F.C.; Lira, E.; Cheng, E.; Irokawa, A.; Tominaga, M. Esterification of n-protected-tyrosine by alpha-chymotrypsin in a high concentration of ethanol. Biotechnol. Tech. 1991, 5, 73–76. [Google Scholar] [CrossRef]
- Salvitti, C.; de Petris, G.; Troiani, A.; Managò, M.; Di Noi, A.; Ricci, A.; Pepi, F. Sulfuric acid catalyzed esterification of amino acids in thin film. J. Am. Soc. Mass Spectrom. 2023, 34, 2748–2754. [Google Scholar] [CrossRef]
- Boyle, T.P. Design and Synthesis of Novel Cationic Peptide Antibiotics. Ph.D. Thesis, University of Wollongong, Wollongong, Australia, 2004. [Google Scholar]
- Zhu, C.; Wang, K.; Luo, J.; Tian, B.; Sun, J.; Liu, X.; Zhu, W.; Zou, Z. Solid superacid SO42−-S2O82−/SnO2-Nd2O3-catalyzed esterification of α-aromatic amino acids. Mol. Catal. 2023, 535, 112833. [Google Scholar] [CrossRef]
- Paul, M.A.; Long, F.A. H0 and related indicator acidity function. Chem. Rev. 1957, 57, 1–45. [Google Scholar] [CrossRef]
- Yang, P.; Fan, S.; Chen, Z.; Bao, G.; Zuo, S.; Qi, C. Synthesis of Nb2O5 based solid superacid materials for catalytic combustion of chlorinated VOCs. Appl. Catal. B Environ. 2018, 239, 114–124. [Google Scholar] [CrossRef]
- Chen, X.; Pfender, E. Behavior of small particles in a thermal plasma flow. Plasma Chem. Plasma Process. 1983, 3, 351–366. [Google Scholar] [CrossRef]
- Liu, D.; Li, H.; Song, L.; Zhu, X.; Qin, Y.; Zu, H.; He, J.; Yang, Z.; Wang, F. Modulating electrical and photoelectrical properties of one-step electrospun one-dimensional SnO2 arrays. Nanotechnology 2020, 31, 335202. [Google Scholar] [CrossRef] [PubMed]
- Mali, J.M.; Arbuj, S.S.; Ambekar, J.D.; Rane, S.B.; Mulik, U.P.; Amalnerkar, D.P. Synthesis of SnO2 Nano Rods and Their Photocatalytic Properties. J. Nanoeng. Nanomanuf. 2013, 3, 121–125. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, S.; Deng, D.; Deng, S.; Chen, J.; Xu, N. Growth direction manipulation of few-layer graphene in the vertical plane with parallel arrangement. Carbon 2013, 56, 103–108. [Google Scholar] [CrossRef]
- Liu, G.; Cai, W. Morphological and structural control of organic monolayer colloidal crystal based on plasma etching and its application in fabrication of ordered gold nanostructured arrays. Crystals 2016, 6, 126. [Google Scholar] [CrossRef]
- Hong, S.-K.; Hanada, T.; Ko, H.-J.; Chen, Y.; Yao, T.; Imai, D.; Araki, K.; Shinohara, M.; Saitoh, K.; Terauchi, M. Control of crystal polarity in a wurtzite crystal: ZnO films grown by plasma-assisted molecular-beam epitaxy on GaN. Phys. Rev. B 2002, 65, 115331. [Google Scholar] [CrossRef]
- Adelkhani, H.; Ghaemi, M.; Ruzbehani, M. Evaluation of the porosity and the nano-structure morphology of MnO2 prepared by pulse current electrodeposition. Int. J. Electrochem. Sci. 2011, 6, 123–135. [Google Scholar] [CrossRef]
- Kleitz, F.; Schmidt, W.; Schüth, F. Evolution of mesoporous materials during the calcination process: Structural and chemical behavior. Microporous Mesoporous Mater. 2001, 44–45, 95–109. [Google Scholar] [CrossRef]
- Li, W.; Fan, G.; Yang, L.; Li, F. Surface Lewis acid-promoted copper-based nanocatalysts for highly efficient and chemoselective hydrogenation of citral to unsaturated allylic alcohols. Catal. Sci. Technol. 2016, 6, 2337–2348. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, H.; Li, H.; Cai, B.; Lv, W.; Cai, C.; Wang, C.; Yan, L.; Liu, Q.; Ma, L. Selective hydrodeoxygenation of 5-hydroxymethylfurfural to 2, 5-dimethylfuran over alloyed Cu− Ni encapsulated in biochar catalysts. ACS Sustain. Chem. Eng. 2019, 7, 19556–19569. [Google Scholar] [CrossRef]
- Borade, R.B.; Clearfield, A. Effect of fluoride ions on the acidic and catalytic properties of beta zeolite. J. Chem. Soc. Faraday Trans. 1995, 91, 539–547. [Google Scholar] [CrossRef]
- Shao, J.; Cheng, S.; Li, Z.; Huang, B. Enhanced catalytic performance of hierarchical MnOx/ZSM-5 catalyst for the low-temperature NH3-SCR. Catalysts 2020, 10, 311. [Google Scholar] [CrossRef]
- Liu, L.; Xu, K.; Su, S.; He, L.; Qing, M.; Chi, H.; Liu, T.; Hu, S.; Wang, Y.; Xiang, J. Efficient Sm modified Mn/TiO2 catalysts for selective catalytic reduction of NO with NH3 at low temperature. Appl. Catal. A Gen. 2020, 592, 117413. [Google Scholar] [CrossRef]
- Du, X.; Wang, L.; Fu, Y.; Wang, H.; Yuan, M.; Xia, Q.; Hu, Q.; Zhou, A. Enhancing the electrochemical performance of d-Mo2CTx MXene in lithium-ion batteries and supercapacitors by sulfur decoration. Ceram. Int. 2023, 49, 19737–19745. [Google Scholar] [CrossRef]
- Peng, L.; Shang, Y.; Gao, B.; Xu, X. Co3O4 anchored in N, S heteroatom co-doped porous carbons for degradation of organic contaminant: Role of pyridinic N-Co binding and high tolerance of chloride. Appl. Catal. B Environ. 2021, 282, 119484. [Google Scholar] [CrossRef]
- Seid-Mohammadi, A.; Shabanloo, A.; Fazlzadeh, M.; Poureshgh, Y. Degradation of acid blue 113 by US/H2O2/Fe2+ and US/S2O82−/Fe2+ processes from aqueous solutions. Desalination Water Treat. 2017, 78, 273–280. [Google Scholar] [CrossRef]
- Supothina, S.; De Guire, M.R. Characterization of SnO2 thin films grown from aqueous solutions. Thin Solid Films 2000, 371, 1–9. [Google Scholar] [CrossRef]
- Tabata, K.; Hirano, Y.; Suzuki, E. XPS studies on the oxygen species of LaMn1−xCuxO3+λ. Appl. Catal. A Gen. 1998, 170, 245–254. [Google Scholar] [CrossRef]
- Liu, B.; Li, C.; Zhang, G.; Yao, X.; Chuang, S.S.; Li, Z. Oxygen vacancy promoting dimethyl carbonate synthesis from CO2 and methanol over Zr-doped CeO2 nanorods. ACS Catal. 2018, 8, 10446–10456. [Google Scholar] [CrossRef]
- Zemlyanov, D.Y.; Savinova, E.; Scheybal, A.; Doblhofer, K.; Schlögl, R. XPS observation of OH groups incorporated in an Ag (111) electrode. Surf. Sci. 1998, 418, 441–456. [Google Scholar] [CrossRef]
- Lin, X.; He, L.; Zhang, J.; Wang, X.; Wang, T. Study on surface morphology and microstructure of Al2O3 nano-films prepared by ion beam sputtering deposition. Trans. Mater. Heat Treat. 2009, 30, 166–169. [Google Scholar]
- Li, S.; Shi, Z.; Dong, J.; Ma, R. Multifunctional urea in modification of SnO2 interface for high efficiency planar perovskite solar cells. Surf. Interfaces 2024, 46, 104044. [Google Scholar] [CrossRef]
- Iatsunskyi, I.; Kempiński, M.; Jancelewicz, M.; Załęski, K.; Jurga, S.; Smyntyna, V. Structural and XPS characterization of ALD Al2O3 coated porous silicon. Vacuum 2015, 113, 52–58. [Google Scholar] [CrossRef]
- Bezrodna, T.; Puchkovska, G.; Shymanovska, V.; Baran, J.; Ratajczak, H. IR-analysis of H-bonded H2O on the pure TiO2 surface. J. Mol. Struct. 2004, 700, 175–181. [Google Scholar] [CrossRef]
- Zhang, B.; Tian, Y.; Zhang, J.X.; Cai, W. Structural, optical, electrical properties and FTIR studies of fluorine doped SnO2 films deposited by spray pyrolysis. J. Mater. Sci. 2011, 46, 1884–1889. [Google Scholar] [CrossRef]
- Song, H.; Zhao, L.; Meng, Y.; Song, H.; Li, F. Comparison of Ni-S2O82–/ZrO2–Al2O3 catalysts prepared by microemulsion and impregnation methods and their performance for isomerisation. Prog. React. Kinet. Mech. 2016, 41, 356–364. [Google Scholar] [CrossRef]
- Zhao, Q.; Meng, S.; Wang, J.; Li, Z.; Liu, J.; Guo, Y. Preparation of solid superacid S2O82−/TiO2–exfoliated graphite (EG) and its catalytic performance. Ceram. Int. 2014, 40, 16183–16187. [Google Scholar] [CrossRef]
- Liu, X.; Wang, K.; Liu, B.; Guo, Z.; Zhang, C.; Lv, Z. Novel WO3/SO42−-ZrO2–TiO2 double bridge coordination catalyst hfor oxidation of cyclohexene. J. Solid State Chem. 2021, 300, 122239. [Google Scholar] [CrossRef]
- Rinaldi, R.; Schüth, F. Design of solid catalysts for the conversion of biomass. Energ. Environ. Sci. 2009, 2, 610–626. [Google Scholar] [CrossRef]
- Ma, X.; Guo, D.; Jiang, Q.; Ma, Z.; Ma, Z.; Ye, W.; Li, C. Preparation and characterization of SO42−/TiO2 and S2O82−/TiO2 catalysts. Front. Chem. Sci. Eng. 2007, 1, 45–49. [Google Scholar] [CrossRef]
- Su, F.; Guo, Y. Advancements in solid acid catalysts for biodiesel production. Green. Chem. 2014, 16, 2934–2957. [Google Scholar] [CrossRef]
- Alaba, P.A.; Sani, Y.M.; Daud, W.M.A.W. Efficient biodiesel production via solid superacid catalysis: A critical review on recent breakthrough. RSC Adv. 2016, 6, 78351–78368. [Google Scholar] [CrossRef]
- Wachs, I.E.; Chen, Y.; Jehng, J.-M.; Briand, L.E.; Tanaka, T. Molecular structure and reactivity of the Group V metal oxides. Catal. Today 2003, 78, 13–24. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, X.; Li, X.; Wang, B.; Dong, Y.; Sun, F.; Zhou, D.; Xia, Q. Construction of strong Lewis acidity through pre-calcining octahedral Zr-MOF to exhibit high activity for the selective isomerization of α-epoxypinane. Mol. Catal. 2022, 526, 112380. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Chen, L.; Xu, Y.; Ma, L. Effect of calcination temperature of Ni/SiO2-ZrO2 catalyst on its hydrodeoxygenation of guaiacol. Chin. J. Catal. 2014, 35, 302–309. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, P.; He, M.; Zhang, T.; Yang, C.; Li, Z. Rare earth nanomaterials in electrochemical reduction of carbon dioxide. Coord. Chem. Rev. 2024, 516, 215983. [Google Scholar] [CrossRef]
- Khorsand Zak, A.; Majid, W.H.A.; Ebrahimizadeh Abrishami, M.; Yousefi, R.; Parvizi, R. Synthesis, magnetic properties and X-ray analysis of Zn0.97X0.03O nanoparticles (X = Mn, Ni, and Co) using Scherrer and size–strain plot methods. Solid State Sci. 2012, 14, 488–494. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.; Pérez-Ramírez, J. Pore size determination in modified micro-and mesoporous materials. Pitfalls and limitations in gas adsorption data analysis. Microporous Mesoporous Mater. 2003, 60, 1–17. [Google Scholar] [CrossRef]
- Li, P.; Gu, Y.; Yu, Z.; Gao, P.; An, Y.; Li, J. TiO2-SnO2/SO42− mesoporous solid superacid decorated nickel-based material as efficient electrocatalysts for methanol oxidation reaction. Electrochim. Acta 2019, 297, 864–871. [Google Scholar] [CrossRef]
- Baer, D.R.; Artyushkova, K.; Richard Brundle, C.; Castle, J.E.; Engelhard, M.H.; Gaskell, K.J.; Grant, J.T.; Haasch, R.T.; Linford, M.R.; Powell, C.J. Practical guides for X-ray photoelectron spectroscopy: First steps in planning, conducting, and reporting XPS measurements. J. Vac. Sci. Technol. A 2019, 37, 031401. [Google Scholar] [CrossRef]


| Entry | Catalyst | Reaction Conditions | Esterification Rate | Reference |
|---|---|---|---|---|
| 1 | alpha-chymotrypsin | 30 °C, 170 rpm, 120 h | 83% | [16] |
| 2 | H2SO4 | ESI source, 70 °C | 43.9% | [17] |
| 3 | SOCl2 | 0 °C-RT, 16 h | 100% | [18] |
| 4 | -/SnO2-Nd2O3 | 180 °C, 500 rpm, 6 h, 1 MPa | 93.1% | [19] |
| Indicator | 3-NT | 2,4-DNFB |
|---|---|---|
| H0 | −12.0 | −14.5 |
| Result for SSA-600 | + | + |
| Result for SSA-RF-600 | + | + |
| Entry | Cat. | Specific Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Pore Size (nm) |
|---|---|---|---|---|
| 1 | SSA-600 | 102.10 | 0.12 | 3.82 |
| 2 | SSA-RF-600 | 104.44 | 0.11 | 3.43 |
| Entry | Cat. | BA (μmol/g) | LA (μmol/g) | Total Acid (μmol/g) | BA/LA |
|---|---|---|---|---|---|
| 1 | SSA-600 | 36.61 | 92.89 | 129.50 | 0.39 |
| 2 | SSA-RF-600 | 43.18 | 99.68 | 142.86 | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Zhang, X.; Xu, W.; Zheng, Y.; Tian, B.; Chen, X.; Zhu, W.
A Radio Frequency Plasma-Modified
Zhu C, Zhang X, Xu W, Zheng Y, Tian B, Chen X, Zhu W.
A Radio Frequency Plasma-Modified
Zhu, Changhui, Xudong Zhang, Wenling Xu, Yanni Zheng, Baohe Tian, Xi Chen, and Wenchao Zhu.
2025. "A Radio Frequency Plasma-Modified
Zhu, C., Zhang, X., Xu, W., Zheng, Y., Tian, B., Chen, X., & Zhu, W.
(2025). A Radio Frequency Plasma-Modified






