Carbon-Coated Cobalt-Catalyzed Hydrodeoxygenation of Lipids to Alcohols
Abstract
1. Introduction
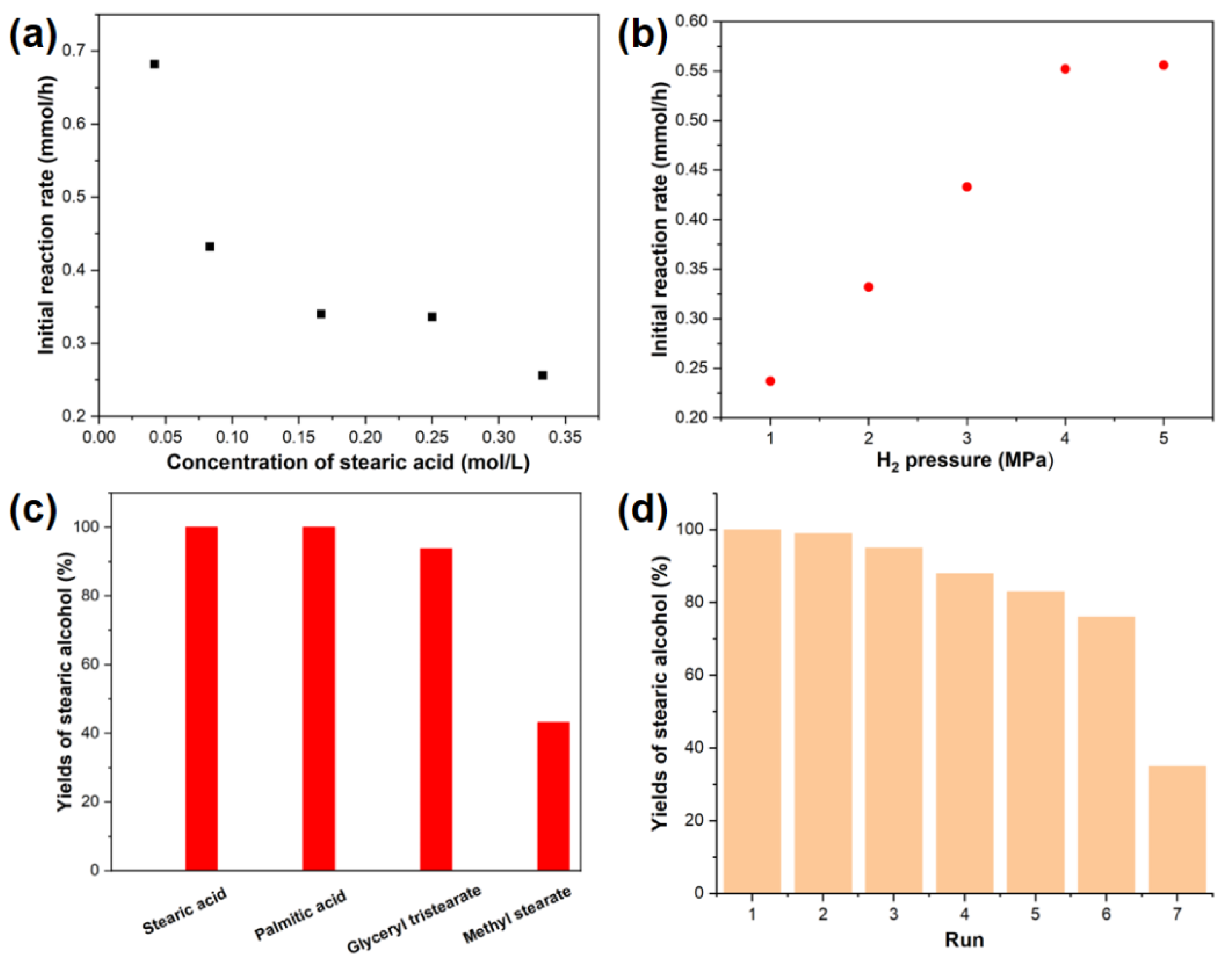
2. Results and Discussion
3. Materials and Methods
3.1. Chemical
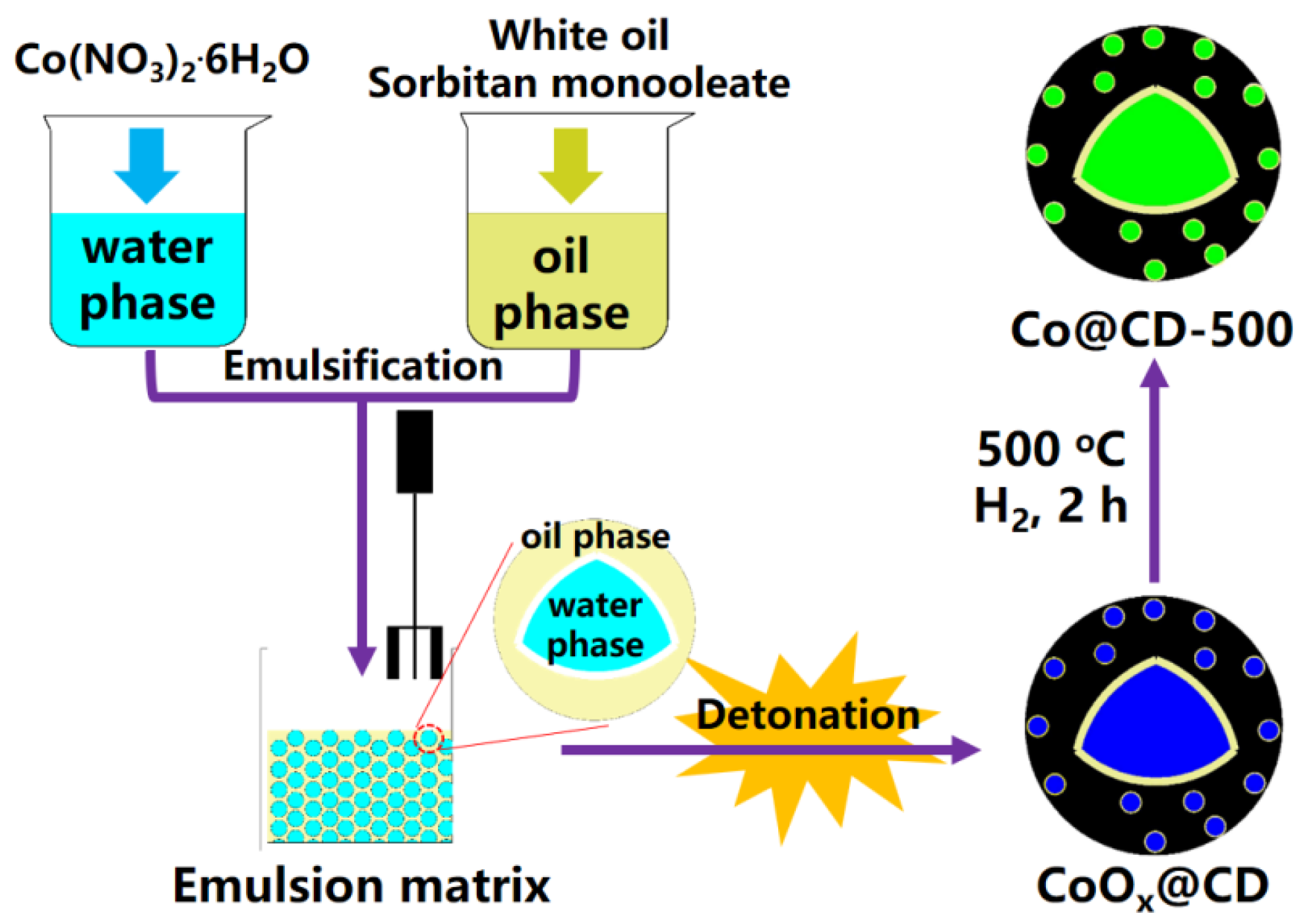
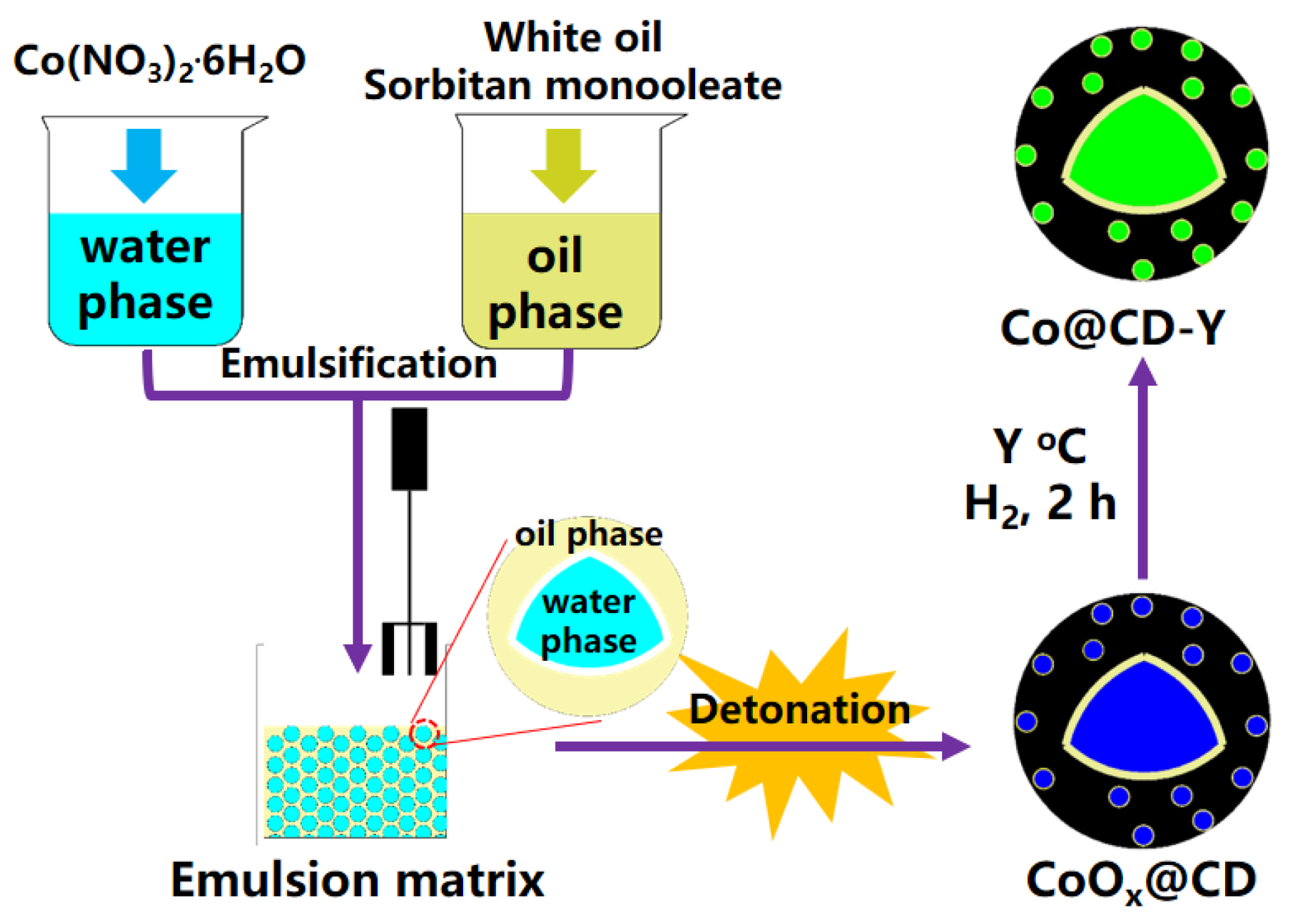
3.2. The Synthesis of Co@CD-Y
3.3. The Synthesis of Co/C
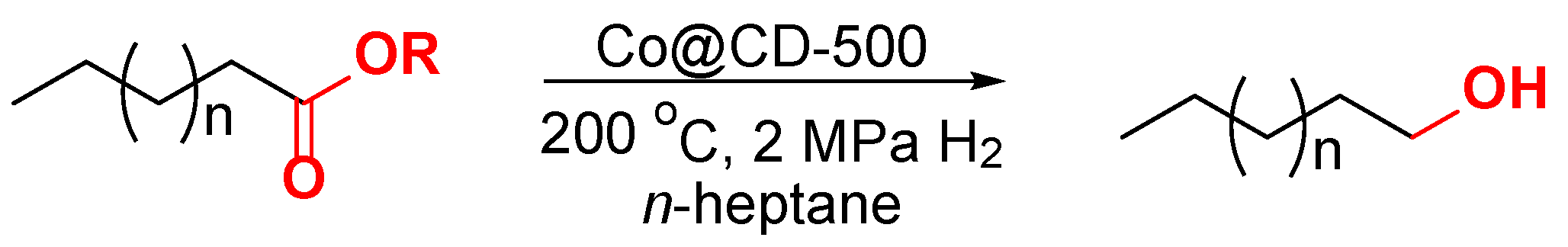
3.4. The Procedures for the HDO of Lipids to Alcohols
3.5. The H2-TPD Experiments
3.6. Material Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Behera, P.; Sethi, L.; Sethi, N. Balancing India’s energy trilemma: Assessing the role of renewable energy and green technology innovation for sustainable development. Energy 2024, 308, 132842. [Google Scholar] [CrossRef]
- Alhasnawi, B.N.; Almutoki, S.M.M.; Hussain, F.F.K.; Harrison, A.; Bazooyar, B.; Zanker, M.; Bureš, V. A new methodology for reducing carbon emissions using multi-renewable energy systems and artificial intelligence. Sustain. Cities Soc. 2024, 114, 105721. [Google Scholar] [CrossRef]
- Kanani, B.; Zahedi, A. Step toward sustainable development through the integration of renewable energy systems with fuel cells: A review. Sustain. Energy Technol. Assess. 2024, 70, 103935. [Google Scholar] [CrossRef]
- Lin, Y.; Yu, J.; Zhang, X.; Fang, J.; Lu, G.P.; Huang, H. Carbohydrate-derived porous carbon materials: An ideal platform for green organic synthesis. Chin. Chem. Lett. 2022, 33, 186–196. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Xu, X.; Lu, G.P. Alkali-promoted transition-metal-free oxidative condensation of biomass-derived furfural and alcohols. Tetrahedron 2024, 155, 133932. [Google Scholar] [CrossRef]
- Gan, Q.; Zhou, W.; Zhang, X.; Lin, Y.; Huang, S.; Lu, G.P. Selective Hydrodeoxygenation of Lignin-Derived Phenolic Monomers to Cyclohexanol over Tungstated Zirconia Supported Ruthenium Catalysts. ChemSusChem 2024, 17, e202400644. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhou, W.; Cao, X.; Xu, X.; Lin, Y.; Wang, K.; Lu, G.P. Single atom Cu anchored graphitic-C3N5 for photocatalytic selective oxidation of biomass-derived furfurals to maleic anhydride. J. Mater. Chem. A 2024, 12, 23897–23909. [Google Scholar] [CrossRef]
- Dreher, J.; Weißmüller, M.; Herrmann, K.; Terjung, N.; Gibis, M.; Weiss, J. Influence of protein and solid fat content on mechanical properties and comminution behavior of structured plant-based lipids. Food Res. Int. 2021, 145, 110416. [Google Scholar] [CrossRef]
- Sharma, H.; Giriprasad, R.; Goswami, M. Animal fat-processing and its quality control. J. Food Process. Technol. 2013, 4, 1000252. [Google Scholar]
- Ratjen, I.; Morze, J.; Enderle, J.; Both, M.; Borggrefe, J.; Müller, H.P.; Lieb, W. Adherence to a plant-based diet in relation to adipose tissue volumes and liver fat content. Am. J. Clin. Nutr. 2020, 112, 354–363. [Google Scholar] [CrossRef]
- Biermann, U.; Bornscheuer, U.; Meier, M.A.; Metzger, J.O.; Schäfer, H.J. Oils and fats as renewable raw materials in chemistry. Angew. Chem. Int. Ed. 2011, 50, 3854–3871. [Google Scholar] [CrossRef] [PubMed]
- Shylesh, S.; Gokhale, A.A.; Ho, C.R.; Bell, A.T. Novel strategies for the production of fuels, lubricants, and chemicals from biomass. Acc. Chem. Res. 2017, 50, 2589–2597. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, T.; Yang, S.; Sun, K.; Yan, H.; Feng, X.; Yan, N. Intensifying Hydrogen Heterocracking via Regulating the ZnO Overlayer for Enhanced Fatty Acid Ester Hydrogenation. ACS Catal. 2023, 13, 16126–16135. [Google Scholar] [CrossRef]
- Fiore, A.M.; Romanazzi, G.; Leonelli, C.; Mastrorilli, P.; Dell’Anna, M.M. Partial hydrogenation of soybean and waste cooking oil biodiesel over recyclable-polymer-supported Pd and Ni nanoparticles. Catalysts 2022, 12, 506. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.; Zhang, X.; Hou, J.; Liu, X.; Yuan, Y.; Liao, X. Highly effective CoOx for catalytic transfer hydrogenation of plant oil to fatty alcohols. Catal. Commun. 2022, 165, 106448. [Google Scholar] [CrossRef]
- Spiekermann, M.L.; Seidensticker, T. Catalytic processes for the selective hydrogenation of fats and oils: Reevaluating a mature technology for feedstock diversification. Catal. Sci. Technol. 2024, 14, 4390. [Google Scholar] [CrossRef]
- Smirnov, A.; Wang, W.; Kikhtyanin, O.; Xiao, L.; Wu, W.; Kubička, D. Hydroconversion of sunflower oil to fatty alcohols and hydrocarbons using CuZn and CuZn-HBEA-based catalysts. Catal. Today 2023, 424, 113841. [Google Scholar] [CrossRef]
- Han, D.; Yin, W.; Wang, S.; Xia, S. Fabrication of a NiFe alloy oxide catalyst via surface reconstruction for selective hydrodeoxygenation of fatty acid to fatty alcohol. ACS Sustain. Chem. Eng. 2021, 9, 15027–15041. [Google Scholar] [CrossRef]
- Biermann, U.; Bornscheuer, U.T.; Feussner, I.; Meier, M.A.; Metzger, J.O. Fatty acids and their derivatives as renewable platform molecules for the chemical industry. Angew. Chem. Int. Ed. 2021, 60, 20144–20165. [Google Scholar] [CrossRef]
- Zhou, Y.; Remón, J.; Jiang, Z.; Matharu, A.S.; Hu, C. Tuning the selectivity of natural oils and fatty acids/esters deoxygenation to biofuels and fatty alcohols: A review. Green Energy Environ. 2023, 8, 722–743. [Google Scholar] [CrossRef]
- Martínez-Prieto, L.M.; Puche, M.; Cerezo-Navarrete, C.; Chaudret, B. Uniform Ru nanoparticles on N-doped graphene for selective hydrogenation of fatty acids to alcohols. J. Catal. 2019, 377, 429–437. [Google Scholar] [CrossRef]
- Lin, M.; Jiang, D.; Yan, Y.; Li, X.; Song, X.; Li, R.; Wu, Y. Temperature–Controlled hydrothermal hydrogenation of palmitic acid to alkanol or alkanes over Co@CN-x catalysts derived from ZIF-67. Chem. Eng. J. 2024, 481, 148565. [Google Scholar] [CrossRef]
- Rahmawati, Z.; Anderson, J.A.; McCue, A.J. Selective hydrogenation of stearic acid to stearyl alcohol over cobalt alumina catalysts. Appl. Catal. A Gen. 2023, 666, 119437. [Google Scholar] [CrossRef]
- Wu, S.; Long, F.; Cao, X.; Liu, P.; Lu, Y.; Xu, J. Highly selective and efficient hydrogenation of fatty acids to alcohols using NiMo@C catalyst. Fuel 2024, 357, 129830. [Google Scholar] [CrossRef]
- Cui, Y.; Yu, S.; Sun, Z.; Niu, H.; Liang, C.; Li, C. Identifying the Active Site and Structure-Activity Relationship in Hydrodeoxygenation of Ester to Alcohols over an Al2O3@CuZn Core–Shell Catalyst. ACS Sustain. Chem. Eng. 2024, 12, 10340–10350. [Google Scholar] [CrossRef]
- Zheng, X.; Yu, P.; Liu, Y.; Ma, Y.; Cao, Y.; Cai, Z.; Jiang, L. Efficient hydrogenation of methyl palmitate to hexadecanol over Cu/m-ZrO2 catalysts: Synergistic effect of Cu species and oxygen vacancies. ACS Catal. 2023, 13, 2047–2060. [Google Scholar] [CrossRef]
- Lu, G.P.; Wang, B.; Li, Y.; Lin, Y.; Hu, J.; Chen, Z.; Chen, F. Insights into the route of 5-hydroxymethylfurfural hydrodeoxygenation to 2,5-dimethylfuran over N-doped carbon anchored CoMo bimetallic catalyst. Appl. Catal. A Gen. 2023, 661, 119240. [Google Scholar] [CrossRef]
- Chen, L.; Wu, J.; Lu, G.P.; Zhang, Q.; Su, T.; Cai, C. Al (PO3)3 supported NiMo bimetallic catalyst for selective synthesis of fatty alcohols from lipids. Mol. Catal. 2022, 530, 112596. [Google Scholar] [CrossRef]
- Liao, F.; Chu, Y.; Chen, X.; Xu, D.; Yang, L. Regulation of hydrogenation products of fatty acids by tuning the acidity of cobalt catalysts. New J. Chem. 2024, 48, 12864–12868. [Google Scholar] [CrossRef]
- Jia, W.; Xu, G.; Liu, X.; Zhou, F.; Ma, H.; Zhang, Y.; Fu, Y. Direct selective hydrogenation of fatty acids and jatropha oil to fatty alcohols over cobalt-based catalysts in water. Energy Fuels 2018, 32, 8438–8446. [Google Scholar] [CrossRef]
- Lin, M.; Zhang, X.; Zhan, L.; Li, X.; Song, X.; Wu, Y. Product distribution-tuned and excessive hydrocracking inhibiting in fatty acid deoxygenation over amorphous Co@SiO2 porous nanorattles. Fuel 2022, 318, 123605. [Google Scholar] [CrossRef]
- Wang, J.; Nie, R.; Xu, L.; Lyu, X.; Lu, X. Catalytic transfer hydrogenation of oleic acid to octadecanol over magnetic recoverable cobalt catalysts. Green Chem. 2019, 21, 314–320. [Google Scholar] [CrossRef]
- Chen, L.; Wu, J.; Lu, G.P.; Su, T.; Cai, C. Carbon-Coated NiMoOx Bimetallic Catalyst for Selective Hydrodeoxygenation of Lipids to Fatty Alcohols. ACS Sustain. Chem. Eng. 2024, 12, 3800–3807. [Google Scholar] [CrossRef]
- Xiang, S.; Dong, L.; Wang, Z.Q.; Han, X.; Daemen, L.L.; Li, J.; Wang, Y. A unique Co@CoO catalyst for hydrogenolysis of biomass-derived 5-hydroxymethylfurfural to 2, 5-dimethylfuran. Nature Communications 2022, 13, 3657. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Y.; Lin, W.; Cheng, X.; Wang, J.; Liu, X.; Nie, R. Catalytically efficient Co-CoOx-Al2O3 interface for mild temperature fatty alcohol production via fatty acid transfer hydrogenation. Fuel 2023, 345, 128136. [Google Scholar] [CrossRef]
- Yao, S.; Zhang, T.; Tang, X.; Li, D.; Zhang, W.; Lin, D.; Yang, C. Octadecanol production from methyl stearate by catalytic transfer hydrogenation over synergistic Co/HAP catalysts. Energy Fuels 2021, 35, 9970–9982. [Google Scholar] [CrossRef]
- Song, S.; Wang, D.; Di, L.; Wang, C.; Dai, W.; Wu, G.; Li, L. Robust cobalt oxide catalysts for controllable hydrogenation of carboxylic acids to alcohols. Chin. J. Catal. 2018, 39, 250–257. [Google Scholar] [CrossRef]
- Lin, M.; Jiang, D.; Yan, Y.; Zhou, S.; Li, R.; Song, X.; Wu, Y. Directional hydrothermal hydrogenation of palmitic acid to alcohol over carbon-encapsulated highly dispersed Co catalyst. Chem. Eng. Sci. 2024, 296, 120236. [Google Scholar] [CrossRef]
- Gao, X.; Zhu, S.; Dong, M.; Fan, W. MOF-derived hcp-Co nanoparticles encapsulated in ultrathin graphene for carboxylic acids hydrogenation to alcohols. J. Catal. 2021, 399, 201–211. [Google Scholar] [CrossRef]
- Hengfei, Q.; Shifei, K.; Yangang, W.; Huan, L.; Zhijiang, N.; Yongkui, H.; Xi, L. Lignin-based fabrication of Co@C core–shell nanoparticles as efficient catalyst for selective fischer–tropsch synthesis of C5+ compounds. ACS Sustain. Chem. Eng. 2016, 4, 1240–1247. [Google Scholar]
- Ghogia, A.C.; Machado, B.F.; Cayez, S.; Nzihou, A.; Serp, P.; Soulantica, K.; Minh, D.P. Beyond confinement effects in Fischer-Tropsch Co/CNT catalysts. J. Catal. 2021, 397, 156–171. [Google Scholar] [CrossRef]
- Zhang, X.; Gan, Q.; Zhou, P.; Chen, Z.; Zhang, Z.; Lu, G.P. Boosting strong metal-support interactions between Ru and sodium titanate nanowire for hydrogenolysis of polyolefins under mild conditions. Appl. Catal. B Environ. 2024, 344, 123626. [Google Scholar] [CrossRef]
- Cao, X.; Zhao, J.; Long, F.; Zhang, X.; Xu, J.; Jiang, J. Al-modified Pd@ mSiO2core-shell catalysts for the selective hydrodeoxygenation of fatty acid esters: Influence of catalyst structure and Al atoms incorporation. Appl. Catal. B Environ. 2022, 305, 121068. [Google Scholar] [CrossRef]
- Cao, X.; Long, F.; Zhang, G.; Xu, J.; Jiang, J. Selective hydrogenation of methyl palmitate to cetyl alcohol via ternary synergistic catalysis of Ni, oxygen vacancies, and Lewis acid sites under mild reaction conditions. ACS Sustain. Chem. Eng. 2021, 9, 9789–9801. [Google Scholar] [CrossRef]
- Cao, X.; Zhao, J.; Long, F.; Liu, P.; Jiang, X.; Zhang, X.; Jiang, J. Efficient low-temperature hydrogenation of fatty acids to fatty alcohols and alkanes on a Ni-Re bimetallic catalyst: The crucial role of NiRe alloys. Appl. Catal. B Environ. 2022, 312, 121437. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, X.; Jiang, S.; Dong, Z.; Lu, R.; Xu, Y.; Lu, G.P. Iron single-atom anchored N-doped carbon: An efficient catalyst for the one-pot, scale-up pentazolate synthesis. J. Mater. Chem. A 2024, 12, 18096–18103. [Google Scholar] [CrossRef]
- Xu, J.; Chen, F.; Xu, X.; Lu, G.P. The selective hydrogenation of nitroarenes and alkenes catalyzed by Pd@MOFs: The role of electronic interactions between Pd nanoparticles and MOFs on the reaction. Mol. Catal. 2020, 495, 111157. [Google Scholar] [CrossRef]
- Lu, G.P.; Li, X.; Zhong, L.; Li, S.; Chen, F. Ru@UiO-66 (Ce) catalyzed acceptorless dehydrogenation of primary amines to nitriles: The roles of Lewis acid–base pairs in the reaction. Green Chem. 2019, 21, 5386–5393. [Google Scholar] [CrossRef]


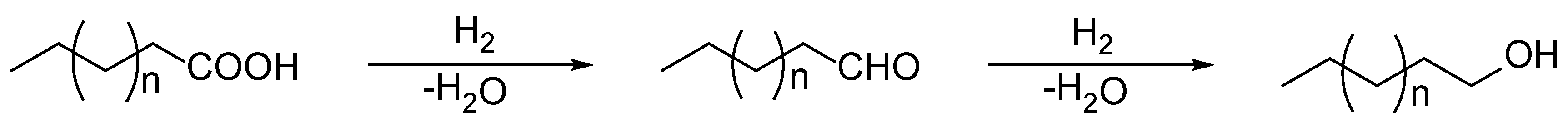
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Catalyst | Conversion (%) | Selectivity b | |||
| n-C17H36 | n-C18H38 | n-C18H38OH | TON g | |||
| 1 | Co@CD-400 | 5 | 0 | 0 | >99 | 0.12 |
| 2 | Co@CD-500 | >99 | 0 | 0 | >99 | 2.39 |
| 3 | Co@CD-700 | 24 | 0 | 0 | >99 | 0.58 |
| 4 | CoMoOx@CD-500 | >99 | 0 | 0 | >99 | 2.39 |
| 5 | Co/C | 0 | 0 | 0 | 0 | 0 |
| 6 | Co@CD-500 c | 51 | 0 | 0 | >99 | 1.22 |
| 7 | Co@CD-500 d | 59 | 2 | 0 | 98 | 1.41 |
| 8 | Co@CD-500 e | 69 | 0 | 0 | >99 | 3.31 |
| 9 | Co@CD-500 f | 94 | 0 | 0 | >99 | 0.12 |
| 10 | CoMoOx@CD-500 f | 86 | 0 | 0 | >99 | 2.39 |
| Entry | Catalyst (g/mol) | Conditions | C/S a | Ref. |
|---|---|---|---|---|
| 1 | Co-350 (41) | 200 °C, iPrOH, 4 h | 100/92 | [32] |
| 2 | CoAl-LDO600 (162) | 200 °C, iPrOH, 3 h | 100/94 | [35] |
| 3 | Co/HAP (200) | 290 °C, MeOH, 5 h | 95/68 | [36] |
| 4 | Co/Al2O3 (57) | 300 °C, 5 MPa H2, 15 h | 91/73 | [23] |
| 5 | Co3O4-573 (67) | 200 °C, 2 MPa H2, 3 h | 100/98 | [37] |
| 6 | Co@C (128) | 200 °C, 4 MPa H2, 1 h | 98/97 | [38] |
| 7 | Co@CN-900 (128) | 220 °C, 4 MPa H2, 0.5 h | 90/97 | [22] |
| 8 | hcp-Co@G400 (50) | 220 °C, 5 MPa H2, 24 h | >99/91 | [39] |
| 9 | Co@C-500 (30) | 200 °C, 2 MPa H2, 5 h | >99/>99 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Wu, J.; Chang, A.; Lu, G.-P.; Cai, C. Carbon-Coated Cobalt-Catalyzed Hydrodeoxygenation of Lipids to Alcohols. Catalysts 2025, 15, 254. https://doi.org/10.3390/catal15030254
Chen L, Wu J, Chang A, Lu G-P, Cai C. Carbon-Coated Cobalt-Catalyzed Hydrodeoxygenation of Lipids to Alcohols. Catalysts. 2025; 15(3):254. https://doi.org/10.3390/catal15030254
Chicago/Turabian StyleChen, Long, Jing Wu, Ai Chang, Guo-Ping Lu, and Chun Cai. 2025. "Carbon-Coated Cobalt-Catalyzed Hydrodeoxygenation of Lipids to Alcohols" Catalysts 15, no. 3: 254. https://doi.org/10.3390/catal15030254
APA StyleChen, L., Wu, J., Chang, A., Lu, G.-P., & Cai, C. (2025). Carbon-Coated Cobalt-Catalyzed Hydrodeoxygenation of Lipids to Alcohols. Catalysts, 15(3), 254. https://doi.org/10.3390/catal15030254






