Abstract
In an enzyme-based fuel cell system, glucose oxidase and laccase were immobilized on carbon paper as the anode and cathode electrodes. A conductive polymer (polypyrrole) was added to improve conductivity. The mediator and enzymes were mixed in a phosphate-buffer solution for entrapment. A Nafion 212 membrane separated the two half-cells. Power density measurements were taken at a glucose concentration of 10 mM across different operating voltages. Potassium hexacyanoferrate III was used as a redox mediator in the anode and 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) in the cathode to boost power output. The biofuel cells, constructed from acrylic (40 × 50 × 50 mm) with a working volume of 20 × 30 × 40 mm, were assembled using a rubber gasket to secure the Nafion membrane. The use of micropore tape covering the electrodes extended the system’s operational lifespan. Without the micropore tape, the maximum power density was 57.6 μW/cm2 at 0.24 V. With the micropore tape, the cell achieved a maximum power density of 324.9 μW/cm2 at 0.57 V, sustaining performance for 20 days. Thus, micropore tape effectively enhances enzyme retention and biofuel cell performance.
1. Introduction
Since the nineteenth-century industrial revolution, continuous industrial advancements have made energy a vital element of development and progress [1]. Energy issues remain critical today, underscoring their essential role in daily life and technological advancement as drivers of human civilization. The invention of the steam engine in the nineteenth century ignited the Industrial Revolution, while the rise of information technology fueled the second Industrial Revolution in the twentieth century. In the twenty-first century, biotechnology is anticipated to profoundly shape human civilization (Biotechnology Industry White Paper, 2012) [2].
Biotechnology and nanotechnology are considered the leading industries of the new century. Humans have been using biotechnology for thousands of years, with techniques such as pickling, brewing, and baking being early examples [3]. Biotechnology uses biological procedures, cells, biofermentation, and biological metabolites to manufacture products, improve traditional production processes, and enhance human life science and technology [4,5]. Biotechnology is an interdisciplinary science with applications in life sciences, medicine, agriculture, marine studies, energy, environmental protection, and chemistry. It is expected to drive a new wave of technological revolution, following petrochemical, aviation, nuclear, and information technology breakthroughs. Advanced countries prioritize biotechnology development, positioning it as one of the most promising industries of the 21st century [6]. Biotechnology research is in ascension, and the development of the biotechnology industry has just started. The future of medicine, healthcare, agriculture, environmental protection, specialization, food, and other fields will be significantly impacted. Many countries will see the biotechnology industry as the future of emerging industries, with strong potential for promotion [7].
The increasing global demand for sustainable energy sources has driven significant interest in biofuel cells as a promising alternative for clean energy generation. Enzyme-based biofuel cells (EBFCs), in particular, offer a unique approach due to their use of biological catalysts for energy conversion, making them both eco-friendly and efficient [8]. However, optimizing their design for long-term stability, higher power output, and reduced enzyme degradation remains a critical challenge. This study aimed to explore innovative electrode designs and material combinations to address these limitations and enhance the performance and operational lifespan of biofuel cells for potential real-world energy applications [9]. In 2001, a self-powered glucose sensor was developed to monitor diabetes mellitus and blood glucose levels in the body using glucose as fuel [10]. Using enzymes in the system is crucial for streamlining the overall chemical reaction. This approach removes the necessity for intact microorganisms, which often complicate biochemical reactions and introduce unpredictable interference [11]. However, enzymes are difficult to obtain and expensive. Adding enzymes to the system, such as glucose oxidase (GOx) at the anode and laccase (Lac) at the cathode, introduces competition and complexity. Enzyme activity varies with environmental factors like temperature and pH, further complicating system design. Additionally, electron and proton transfer must be carefully considered. Electron transfer involves moving the biological catalyst to the electron transfer solution through the metabolic pathway. The number of electrons released by the biological catalyst and captured by the electron transport medium directly influences the current magnitude [12,13]. Advancements in enzyme immobilization are crucial for the operational stability and efficiency of EBFCs. The layer-by-layer assembly technique has been highlighted for its ability to form controllable enzyme layers, allowing high-density enzyme loading and improved electron transfer. This method enhances the stability and performance of EBFCs [14]. The enzyme immobilization technique has been applied in biofuel production to improve enzyme performance by providing physical support, thereby enhancing reaction rates and stability [15]. Recent approaches have focused on achieving high-power performance and stable operation in glucose-based EBFCs. Strategies include optimizing enzyme–electrode interfaces and employing innovative materials with linkers and 3D host electrodes to enhance electron transfer and operational stability [16].
This study investigated enzyme-based biofuel cells, optimizing electrode design, enzyme immobilization, and conductive polymers to enhance power output, stability, and operational lifespan. This study immobilized GOx, a conductive polymer, and a dielectric on the electrode surface at the anode end [17], while Lac and a conductive polymer were immobilized on the cathode end. It is necessary to design an enzyme fuel cell with the best output voltage (V), a high power density, and a prolonged output time [18]. To optimize the battery’s overall efficiency, the electrode must have high conductivity, surface area, porosity, and biocompatibility [19]. This study used a microtape to entrap the enzymes, immobilizing them in the electrode, while maintaining enzyme activity similar to that of free enzymes. This approach increases power density and allows for prolonged operation without reducing the activity.
2. Results and Discussion
2.1. Cyclic Voltammetry Analysis of the Electrodes
Cyclic voltammetry (CV) of half-cell analysis is a powerful electrochemical technique that studies an electrode’s redox reaction mechanisms with IUPAC convention voltammograms. An anodic (oxidation) current is positive, and a cathodic (reduction) current is negative. This technique involves sweeping the electrode potential from a lower to a higher voltage and back, allowing the system to reach equilibrium. During this process, the electrode’s potential and the resulting current are measured, providing valuable insights into the tested system’s electrochemical behavior, reaction kinetics, and diffusion properties [20,21].
A copper-type (CP) electrode, as prepared in Section 3.3, was tested to evaluate the performance of conductive polymers, specifically polypyrrole (PPy) [22] or polyaniline (PANI), mixed with phosphate-buffer solutions (PBSs) of varying pH levels using CV with a scanning rate of 0.01 V/s. The electrode was immersed in glucose (10 mM) and PBS (pH 7) for 0 and 1 h. The CV test results revealed that for electrodes fabricated using the same conductive polymer, the oxidation and reduction potentials remained nearly identical despite using PBS with different pH values. However, the polarization peak values varied. For the six tested electrodes showing quasi-reversible reactions in Table 1, the oxidation potential of the PPy conductive polymer ranged from −0.58 to −0.55 V (vs. Ag/AgCl). The difference in polarization peaks was observed at 7.8%, 6%, and 15.2%, respectively.

Table 1.
Comparison of electrode polarization peaks under different conductive polymers and pH levels.
Additionally, the polarization peak of the PPy electrode was higher than that of the PANI electrode (Figure 1), suggesting that PPy exhibits better electrochemical oxidation activity than PANI. The prepared CP electrode was immersed in glucose (10 mM) and PBS solution (pH 7) for 1 h and then tested using CV under the conditions previously described. Comparing the CV results before and after the 1 h immersion revealed an increase in the electrode’s polarization peak and a shift in the polarization curve in the absence of glucose (Figure 1). This suggests that after immersion, the electrode exhibited an upward trend in glucose oxidative activity and improved hydrogen evolution performance.
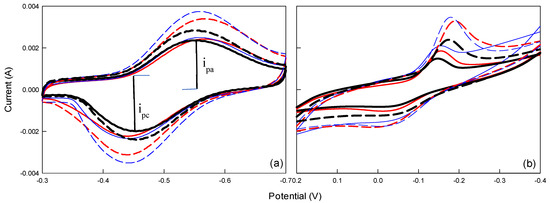
Figure 1.
CV diagrams using different polymers: (a) PPy and (b) PANI for different pH levels. ( : pH5
: pH5 : pH 6
: pH 6 : pH 7), immersed for 0 h in a glucose and PBS solution; (
: pH 7), immersed for 0 h in a glucose and PBS solution; ( : pH 5
: pH 5 : pH 6
: pH 6 : pH 7), immersed for 1 h in a glucose and PBS solution.
: pH 7), immersed for 1 h in a glucose and PBS solution.
 : pH5
: pH5 : pH 6
: pH 6 : pH 7), immersed for 0 h in a glucose and PBS solution; (
: pH 7), immersed for 0 h in a glucose and PBS solution; ( : pH 5
: pH 5 : pH 6
: pH 6 : pH 7), immersed for 1 h in a glucose and PBS solution.
: pH 7), immersed for 1 h in a glucose and PBS solution.
The CV test results in Figure 2 indicate that differences in the conductive polymers affected the electrochemical performance of the prepared buffer solution with the same pH value. Higher electroconductivity and reduction potential were observed with increased conductive polymer content, leading to a higher electrode position current. Overall, the polarization area of the PPy electrode was more extensive than that of the PANI electrode, as shown in Figure 2. This suggests that the PPy electrode exhibited more significant glucose oxidation activity and enhanced hydrogen evolution performance than the PANI electrode.
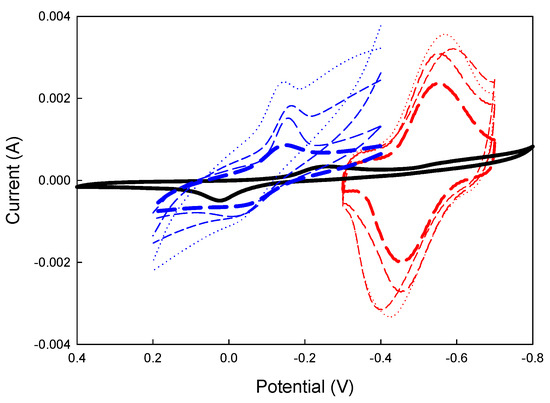
Figure 2.
Comparison of CV between the PPy and PANI electrodes. PPy (mM) = ( : 0.1,
: 0.1,  : 0.2,
: 0.2,  : 0.3,
: 0.3,  : 0.4); PANI (mM) = (
: 0.4); PANI (mM) = ( : 0.1,
: 0.1,  : 0.2,
: 0.2,  : 0.3,
: 0.3,  : 0.4); blank (mM) =
: 0.4); blank (mM) =  : 0, GOx (5 U/10 μL), Fe(CN)63−(10 mM), pH = 7.
: 0, GOx (5 U/10 μL), Fe(CN)63−(10 mM), pH = 7.
 : 0.1,
: 0.1,  : 0.2,
: 0.2,  : 0.3,
: 0.3,  : 0.4); PANI (mM) = (
: 0.4); PANI (mM) = ( : 0.1,
: 0.1,  : 0.2,
: 0.2,  : 0.3,
: 0.3,  : 0.4); blank (mM) =
: 0.4); blank (mM) =  : 0, GOx (5 U/10 μL), Fe(CN)63−(10 mM), pH = 7.
: 0, GOx (5 U/10 μL), Fe(CN)63−(10 mM), pH = 7.
The CV test results in Figure 3 show that the integrated areas of the anodic oxidation and reduction polarization curves were nearly equal for electrodes A1 to A9, indicating quasi-reversible reactions. The anode compositions of A1 to A9 are listed in Section 3.3. The primary differences among the electrodes were their electro-oxidation activity toward glucose and the extent of hydrogen evolution. Based on the results in Figure 3, the electro-oxidation activity was ranked as A3 > A6 > A2 > A9 > A5 > A8 > A4 > A1 > A7. In Figure 1a, Figure 2, and Figure 3, anodic and cathodic currents of similar magnitudes were observed. This finding indicates that enzymatic reactions occurred in both the anodic and cathodic directions at a similar rate.
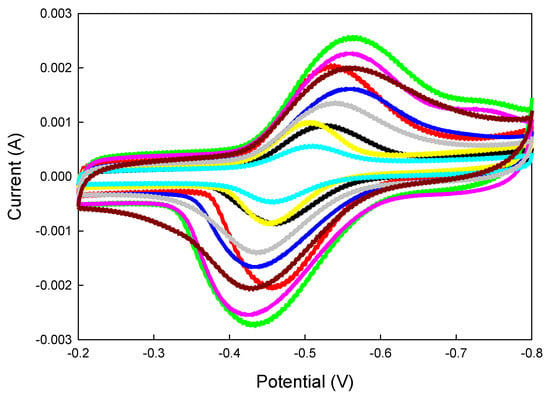
Figure 3.
Comparison of the CV of anode A1 to A9 electrodes.  A1,
A1,  A2,
A2,  A3,
A3,  A4,
A4,  A5,
A5,  A6,
A6,  A7,
A7,  A8,
A8,  A9.
A9.
 A1,
A1,  A2,
A2,  A3,
A3,  A4,
A4,  A5,
A5,  A6,
A6,  A7,
A7,  A8,
A8,  A9.
A9.
2.2. EBFC Performance Test Analysis
A bipolar system was used to analyze the enzyme-based biofuel cell system. A 4-channel thermocouple differential analog input module measured the voltage, current, and temperature, with operating conditions controlled and data analyzed using LabVIEW 2012 software. The temperature was kept below 40 °C during enzyme immobilization on the electrode surface to prevent enzyme inactivation.
The CP electrodes were tested with nine different concentrations of anions and cations (Section 3.3). The fuel cells consisted of anode enzyme electrodes (A1 to A9) and cathode enzyme electrodes (C1 to C9). Each fuel cell configuration was assembled by placing the electrodes in the anode and cathode compartments of the fuel cell setup. Figure 4 shows the potential-time plots for different anode and cathode electrodes in biofuel cells.
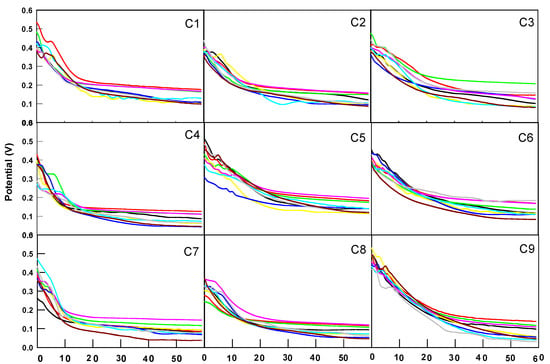
Figure 4.
Plot of potential against time in biofuel cells for different anode ( A1,
A1,  A2,
A2,  A3,
A3,  A4,
A4,  A5,
A5,  A6,
A6,  A7,
A7,  A8,
A8,  A9) and cathode electrodes (subfigure number).
A9) and cathode electrodes (subfigure number).
 A1,
A1,  A2,
A2,  A3,
A3,  A4,
A4,  A5,
A5,  A6,
A6,  A7,
A7,  A8,
A8,  A9) and cathode electrodes (subfigure number).
A9) and cathode electrodes (subfigure number).
The computer sampled data at three points per second, storing a set of data every 1/3 s, totaling 10,800 data points over 60 min. To compare the power, current, and potential differences among the 81 enzyme battery groups and minimize errors from single-point measurements, the average potential and power over the first minute were recorded for each group (Table 2). The total output energy over 60 min was also integrated and is summarized in Table 3. The performance results are as follows:
A1 electrode: The best initial performance was observed with A1C5 and A1C9, achieving potentials above 0.5 V and power outputs exceeding 250 μW/cm2 in Table 2. The total energy output after 60 min exceeded 800 J for both batteries, as shown in Table 3.
A2 electrode: The best initial performance was observed with A2C1 and A2C9, with potentials above 0.5 V and power outputs exceeding 250 μW/cm2. The total energy output after 60 min was highest for A2C1, A2C3, A2C5, A2C6, and A2C9, with A2C9 reaching over 1000 J.
A3 electrode: The best initial performance was observed with A3C9, achieving a potential of 0.513 V and a power output of 263 μW/cm2. The total energy output after 60 min exceeded 800 J for A3C3, A3C5, and A3C9, with A3C9 approaching 1000 J.
A4 electrode: The best initial performance was observed with A4C9, which reached a potential of 0.542 V and a power output of 293 μW/cm2. After 60 min, only A4C9 performed well, reaching 921 J of energy output.
A5 electrode: The performance was generally lower, with all nine groups showing potential below 0.5 V. Only A5C9 performed better after 60 min, reaching 868 J.
A6 electrode: Similar results were observed, with all nine groups having potentials below 0.5 V. However, A6C5 and A6C9 showed superior performance, with energy outputs exceeding 900 J.
A7 electrode: The most effective combinations were A7C7 and A7C9, which achieved potentials of 0.472 and 0.459 V, with power outputs of 222 and 211 μW/cm2, respectively. After 60 min, only the A7C9 configuration exceeded 844 J in total energy generation.
A8 electrode: Optimal performance was observed with A8C5 and A8C9, recording potentials of 0.482 and 0.446 V and power outputs of 233 and 199 μW/cm2, respectively. Notably, the total energy generated by A8C3 and A8C6 exceeded 800 J over the same duration.
A9 electrode: The highest performance was observed with A9C9, which recorded a potential of 0.512 V and a power output of 262 μW/cm2. After 60 min, A9C5 and A9C9 achieved total energy outputs exceeding 800 J.



Table 2.
Enzyme battery potential and power density.
Table 2.
Enzyme battery potential and power density.
| Cell | A1C1 | A1C2 | A1C3 | A1C4 | A1C5 | A1C6 | A1C7 | A1C8 | A1C9 |
| Initial voltage (V) | 0.422 | 0.377 | 0.390 | 0.409 | 0.513 | 0.458 | 0.262 | 0.329 | 0.500 |
| Power density (μW/cm2) | 178.1 | 141.8 | 152.3 | 167.3 | 263.3 | 209.4 | 68.7 | 107.9 | 249.7 |
| Cell | A2C1 | A2C2 | A2C3 | A2C4 | A2C5 | A2C6 | A2C7 | A2C8 | A2C9 |
| Initial voltage (V) | 0.534 | 0.355 | 0.414 | 0.428 | 0.491 | 0.406 | 0.380 | 0.277 | 0.515 |
| Power density (μW/cm2) | 285.5 | 125.9 | 171.3 | 183.3 | 241.5 | 164.8 | 144.1 | 76.6 | 264.8 |
| Cell | A3C1 | A3C2 | A3C3 | A3C4 | A3C5 | A3C6 | A3C7 | A3C8 | A3C9 |
| Initial voltage (V) | 0.482 | 0.374 | 0.476 | 0.387 | 0.429 | 0.377 | 0.415 | 0.244 | 0.513 |
| Power density (μW/cm2) | 232.4 | 139.6 | 226.3 | 149.7 | 184.4 | 142.3 | 172.5 | 59.6 | 263.2 |
| Cell | A4C1 | A4C2 | A4C3 | A4C4 | A4C5 | A4C6 | A4C7 | A4C8 | A4C9 |
| Initial voltage (V) | 0.394 | 0.390 | 0.381 | 0.397 | 0.407 | 0.399 | 0.359 | 0.303 | 0.542 |
| Power density (μW/cm2) | 155.2 | 151.9 | 145.2 | 157.4 | 166.0 | 159.1 | 129.0 | 91.8 | 293.5 |
| Cell | A5C1 | A5C2 | A5C3 | A5C4 | A5C5 | A5C6 | A5C7 | A5C8 | A5C9 |
| Initial voltage (V) | 0.433 | 0.370 | 0.361 | 0.376 | 0.308 | 0.442 | 0.366 | 0.324 | 0.469 |
| Power density (μW/cm2) | 187.1 | 137.1 | 130.3 | 141.6 | 94.7 | 195.3 | 133.7 | 105.2 | 220.2 |
| Cell | A6C1 | A6C2 | A6C3 | A6C4 | A6C5 | A6C6 | A6C7 | A6C8 | A6C9 |
| Initial voltage (V) | 0.388 | 0.400 | 0.400 | 0.270 | 0.406 | 0.417 | 0.396 | 0.362 | 0.479 |
| Power density (μW/cm2) | 150.3 | 160.3 | 159.6 | 73.1 | 165.0 | 173.7 | 156.7 | 131.2 | 229.2 |
| Cell | A7C1 | A7C2 | A7C3 | A7C4 | A7C5 | A7C6 | A7C7 | A7C8 | A7C9 |
| Initial voltage (V) | 0.421 | 0.379 | 0.398 | 0.271 | 0.373 | 0.391 | 0.472 | 0.329 | 0.459 |
| Power density (μW/cm2) | 177.5 | 144.0 | 158.4 | 73.7 | 139.4 | 153.1 | 222.4 | 108.5 | 211.0 |
| Cell | A8C1 | A8C2 | A8C3 | A8C4 | A8C5 | A8C6 | A8C7 | A8C8 | A8C9 |
| Initial voltage (V) | 0.397 | 0.437 | 0.441 | 0.287 | 0.482 | 0.344 | 0.432 | 0.328 | 0.446 |
| Power density (μW/cm2) | 157.9 | 191.3 | 194.5 | 82.4 | 232.8 | 118.4 | 186.8 | 107.6 | 199.3 |
| Cell | A9C1 | A9C2 | A9C3 | A9C4 | A9C5 | A9C6 | A9C7 | A9C8 | A9C9 |
| Initial voltage (V) | 0.380 | 0.353 | 0.377 | 0.378 | 0.455 | 0.382 | 0.354 | 0.369 | 0.512 |
| Power density (μW/cm2) | 144.2 | 124.3 | 142.3 | 142.6 | 206.6 | 145.6 | 125.2 | 136.0 | 261.7 |

Table 3.
Total output energy (TOE) for different anode and cathode electrodes.
Table 3.
Total output energy (TOE) for different anode and cathode electrodes.
| Cell | A1C1 | A1C2 | A1C3 | A1C4 | A1C5 | A1C6 | A1C7 | A1C8 | A1C9 |
| TOE (J) | 655 | 700 | 672 | 498 | 801 | 756 | 438 | 486 | 919 |
| Cell | A2C1 | A2C2 | A2C3 | A2C4 | A2C5 | A2C6 | A2C7 | A2C8 | A2C9 |
| TOE (J) | 877 | 738 | 819 | 574 | 902 | 808 | 484 | 529 | 1020 |
| Cell | A3C1 | A3C2 | A3C3 | A3C4 | A3C5 | A3C6 | A3C7 | A3C8 | A3C9 |
| TOE (J) | 774 | 673 | 941 | 577 | 876 | 719 | 576 | 501 | 981 |
| Cell | A4C1 | A4C2 | A4C3 | A4C4 | A4C5 | A4C6 | A4C7 | A4C8 | A4C9 |
| TOE (J) | 611 | 647 | 574 | 423 | 650 | 673 | 496 | 432 | 922 |
| Cell | A5C1 | A5C2 | A5C3 | A5C4 | A5C5 | A5C6 | A5C7 | A5C8 | A5C9 |
| TOE (J) | 655 | 546 | 684 | 388 | 646 | 684 | 480 | 430 | 868 |
| Cell | A6C1 | A6C2 | A6C3 | A6C4 | A6C5 | A6C6 | A6C7 | A6C8 | A6C9 |
| TOE (J) | 764 | 748 | 754 | 530 | 907 | 800 | 654 | 635 | 948 |
| Cell | A7C1 | A7C2 | A7C3 | A7C4 | A7C5 | A7C6 | A7C7 | A7C8 | A7C9 |
| TOE (J) | 671 | 582 | 602 | 442 | 782 | 697 | 515 | 456 | 844 |
| Cell | A8C1 | A8C2 | A8C3 | A8C4 | A8C5 | A8C6 | A8C7 | A8C8 | A8C9 |
| TOE (J) | 631 | 611 | 803 | 424 | 784 | 809 | 493 | 490 | 777 |
| Cell | A9C1 | A9C2 | A9C3 | A9C4 | A9C5 | A9C6 | A9C7 | A9C8 | A9C9 |
| TOE (J) | 640 | 561 | 568 | 391 | 813 | 548 | 336 | 415 | 920 |
Total output energy.
A consistent trend emerged with the cathode electrodes C5 and C9, which frequently outperformed the other combinations across various anode types, indicating superior catalytic activity and efficient electron transfer. The pH of the buffer solution also played a significant role, with C5 and C9 prepared with CBS (pH 5) consistently demonstrating enhanced performance. The acidic conditions optimized Lac activity in the cathode chamber, leading to better biofuel cell performance.
Regarding the anode’s pH influence, the combinations A2 to A6 prepared with PBS (pH 6 and 7) exhibited better glucose oxidation activity, suggesting that GOx operates more effectively under neutral or slightly acidic conditions. Electrode composition also significantly impacted performance, with the anode electrodes A2, A3, A8, and A9 and the cathode electrodes C2, C3, C6, C8, and C9 showing a higher PPy content. This higher PPy concentration correlated with improved conductivity and electron transfer, consistently resulting in better performance.
Battery lifetime analysis revealed that the longest-lasting batteries included the anode electrodes A2, A3, A7, and A9 and the cathode electrodes C2, C3, C6, C8, and C9. A correlation between higher PPy content and improved energy retention was evident, as batteries with this composition demonstrated prolonged operational life and stability.
The choice of cathode electrode proved more critical to overall enzyme fuel cell performance than the anode electrode. The cathode electrodes C5 and C9 prepared with CBS at pH 5 and a higher PPy content delivered the most consistent and optimal results. Enzyme activity was also pH-dependent, with acidic conditions favoring Lac and neutral conditions favoring GOx. Ultimately, the combination of a higher PPy content and optimized buffer conditions significantly enhanced the performance and longevity of the enzyme biofuel cells.
Based on initial battery performance statistics, the best-performing enzyme batteries were the anode electrodes A1, A2, and A3 and the cathode electrodes C1, C5, and C9. The combination of different anode and cathode electrodes showed varying results without a clear overall trend. However, the conductive polymer PPy content influenced oxidative activity, with the following observed activation order: A3 > A6 > A2 > A9 > A5 > A8 > A4 > A1 > A7. The concentration of PPy was observed to influence the electrode’s oxidative performance but not the overall energy release in the EBFC system.
For cathode performance, the cathode electrodes C5 and C9 consistently showed superior initial efficiency and total energy output. Modulating enzyme fuel cells with C5 and C9 showed improved energy generation, indicating that the cathode plays a more critical role than the anode in fuel cell efficiency.
During continuous operation testing for the CP vs. CP3M electrodes in Figure 5, the sample concentration combination (enzyme concentration = 5 U/10 μL, conductive polymer concentration = 1.5 mM, mediator concentration = 10 mM, buffer solution pH = 5) was tested for continuous operation over seven days using the CP and CP3M electrodes. On the third day, the buffer solution (anode side) and ultrapure water (cathode side) were replaced. The CP3M electrode resulted in a voltage drop rate of 0.32 V at 102.4 μW/cm2 by day 2, and voltage stabilized around 0.3 V after buffer solution replacement on day 3. The CP3M electrode maintained better voltage stability due to the enzyme immobilization effect and breathable tape coverage, preventing rapid glucose depletion.
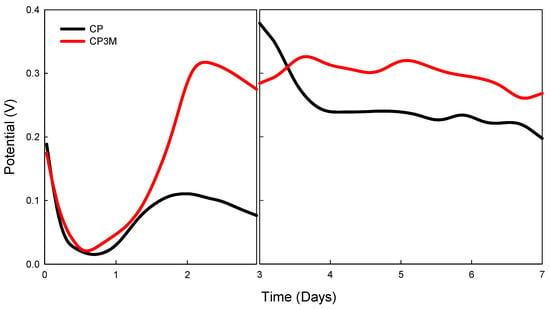
Figure 5.
The potential of enzymatic fuel cells for Cp and CPM3 electrodes vs. time on stream. Enzyme concentration = 5 U/10 μL, conductive polymer concentration conditions = 1.5 mM, mediator concentration = 10 mM, buffer solution pH = 5. Anode: PBS as buffer solution (containing a glucose concentration of 10 mM); cathode: ultrapure water. On the third day, the buffer solution (anode side) and ultrapure water (cathode side) were replaced. The electrodes were not immersed in the solution before assembly.
The CP electrode resulted in the voltage dropping significantly by day 2, likely due to glucose depletion. After buffer solution replacement on day 3, the voltage spiked to 0.38 V but gradually reduced to 0.24 V. The power density was 57.6 μW/cm2, indicating enzyme immobilization was effective but less stable than CP3M.
In potential–time analysis (first 12 h), the CP electrode showed a faster initial performance, possibly due to the absence of a breathable tape barrier allowing more immediate enzyme interaction. The CP3M electrode exhibited delayed but stronger performance after 1.5 h, likely due to the breathable tape creating a diffusion barrier that initially slowed the reaction but stabilized the enzyme immobilization for long-term activity.
For the first 30 h, adding breathable tape did not significantly affect the overall efficiency and initially slowed the reaction. The voltage increased as the system stabilized. The voltage drop after peak output may be linked to the limitations of the Nafion 212 proton exchange membrane. As the current density increased, mass transfer limitations affected ion exchange and glucose availability, reducing performance. The cathode electrode significantly influenced the enzyme fuel cell performance more than the anode electrode. A higher conductive polymer content enhanced the oxidative performance but did not directly impact the energy release. The CP3M electrode, with breathable tape, showed better long-term stability and enzyme retention, while the CP electrode exhibited faster initial performance but lower stability over time. Cathode combinations (C5 and C9) with slightly acidic conditions (pH 5) and a higher PPy content yielded the best results.
A PBS with a glucose concentration of 10 mM was used in the anode tank, while ultrapure water was introduced into the cathode tank. A Nafion 212 proton exchange membrane was placed between the compartments to facilitate proton transfer. However, the membrane allowed proton flow in both directions instead of exclusively from the anode to the cathode. Due to the higher glucose concentration in the anode compared to the cathode, a concentration gradient developed. This caused water dissociation in the cathode, generating H⁺ and OH⁻ ions. The H⁺ ions migrated back into the anode, disrupting proton transfer from the glucose oxidation catalyzed by GOx and reducing electron flow in the external circuit. In Figure 5, the initial phase of operation involved enzyme diffusion and stabilization on the electrode surface. On day 1, GOx at the anode and laccase at the cathode became more effectively engaged in redox reactions. The enzymes might not have been fully active due to initial diffusion limitations and incomplete immobilization. By day 2, a steady-state concentration of reactants and products developed near the electrode surfaces, enhancing the redox reaction rates and causing a rapid potential increase. The enzymes reached an equilibrium state, increasing their catalytic efficiency and improving electron transfer, leading to a sharp rise in potential.
The CP and CP3M electrodes were prepared using the optimal enzyme solution. The anode electrode was immersed in PBS with 10 mM glucose, while the cathode electrode was soaked in ultrapure water for two days before assembly. The fuel cells were tested continuously for 20 days, as shown in Figure 6.
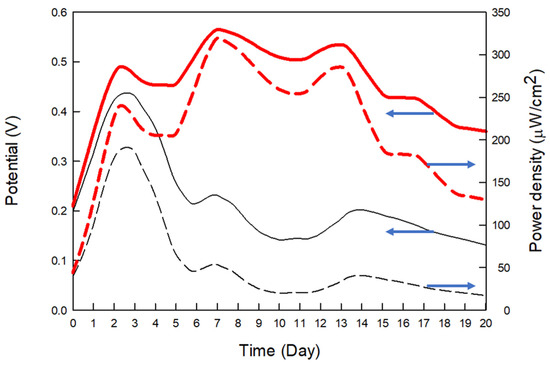
Figure 6.
Potential and power density of the enzymatic electrode vs. time on stream. Enzyme concentration = 5 U/10 μL, conductive polymer concentration = 1.5 mM, mediator concentration = 10 mM, buffer solution pH = 5. Anode: PBS as buffer solution (containing a glucose concentration of 10 mM); cathode: ultrapure water. Black line: CP electrode, red line: CP3M electrode. The anode electrode was immersed in a PBS solution with 10 mM glucose, while the cathode electrode was soaked in ultrapure water for two days before assembly.
After assembly, the CP and CP3M batteries showed an upward voltage trend during the first three days. The CP3M battery maintained a stable voltage of around 0.5 V, while the CP battery voltage decreased to approximately 0.2 V, indicating better enzyme immobilization in the CP3M electrode.
After 14 days, the battery voltage declined, likely due to glucose depletion in the anode tank. Overall, the CP3M electrode extended the fuel cell’s operational lifespan to 20 days, achieving a peak voltage of 0.57 V and a power density of 324.9 μW/cm2. Its stability and performance were superior to the CP electrode, as summarized in Table 4.

Table 4.
Comparison of two kinds of electrode production for EBFCs.
The results of this study, compared to those from the literature (Table 5), show variations in battery performance despite using GOx and Lac as the primary oxidation enzymes for the anode and cathode electrodes, respectively. These differences can be attributed to the addition of conductive polymers, mediator materials, and buffer solutions, which influenced the overall power output. Electrode design also affected the performance. Covering the electrode surface with breathable tape led to an upward trend in battery power output, offering a promising direction for future research and fuel cell optimization [23]. The comparisons in Table 5 highlight the strengths of this study, particularly in terms of voltage, power density, and operational stability. The use of carbon paper covered with breathable tape in PBS (pH 7.0) and CBS (pH 5.0) systems resulted in a significantly higher potential (0.57 V) and power density (324.9 μW/cm2) compared to previously reported biofuel cells. Most of the other studies in Table 5 achieved lower voltages and power outputs, with power densities ranging from 10 to 178 μW/cm2 and operational times from 1 to 144 h.

Table 5.
Comparison of enzyme-based biofuel cells using GOx and Lac across different electrode materials, electrolyte conditions, and power outputs.
One of the most notable advantages of this study is the extended operational lifespan of 480 h, which surpasses that previously reported for biofuel cells. Incorporating breathable tape for enzyme immobilization enhanced stability and prolonged enzyme activity, reducing the rapid degradation commonly observed in biofuel cells. Additionally, the strategic use of carbon paper as the electrode material and the optimization of pH conditions (CBS, pH 5.0 for the cathode) provided a highly effective environment for enzyme activity, resulting in superior performance.
3. Materials and Methods
3.1. Chemicals
PBS, PPy (Mw = 11060), PANI (Mw = 15000), 2,2-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), potassium ferrocyanide (III) K3Fe(CN6), GOx, and Lac were purchased from Sigma-Aldrich (St. Louis, MO, USA). Glucose, N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide, and N-hydroxysuccinimide were obtained from Fisher Chemical (Leicestershire, UK). The carbon paper (GDL 10 AA) was purchased from the SGL Group (Meitingen, Germany). The Nafion® 212 proton-exchange membrane was obtained from DuPont (Wilmington, DE, USA). Oxygen (O2) and nitrogen (N2) gases were purchased from Ming-Young Co. (Taoyuan, Taiwan).
3.2. Electrolyte Solution
To prepare PBS at pH 7 and a 0.2 M concentration, 0.477 mol of monosodium phosphate and 0.523 mol of disodium sulfate were dissolved in distilled water. The pH was measured using a pH meter (JENWAY 3510, Barloworld Scientific Ltd., Dunmow, Essex, UK) and adjusted to neutrality using sulfuric acid or sodium hydroxide. The solution volume was then increased to 1 L with distilled water, creating a stock solution of 1 M PBS. This stock was later diluted to prepare buffers with concentrations of 0.4, 0.5, and 0.1 M as needed. Sorensen’s PBS (pKa = 7.2, pH 5.8–8.0, 0.1 M), consisting of NaH2PO4 and Na2SO4, was specifically used in this research. Additionally, CBS (pH 5) was prepared by combining 100 mL of citric acid (0.1 M) with 100 mL of sodium citrate (0.1 M).
3.3. Preparation of the Enzyme Solution and Immobilization Technique
Two buffer solutions, PBS and CBS, were prepared and used for the anode and cathode solutions, respectively, with pH values of 5.0, 6.0, and 7.0. A glucose solution (0.01 M) was prepared by dissolving 0.36 g of glucose powder in 200 mL of PBS (pH 7.0) and reconstituted the day before each experiment.
The anode GOx solution was prepared by mixing PPy (0.5 mM, 0.0885 g), potassium hexacyanoferrate (III) (K3Fe(CN)6) (1 mM, 0.00525 g), and GOx (5 U/10 μL) in 16 mL of PBS (pH 5.0) and mixed with magnetic stirring until all were completely dissolved. PPy concentration refers to the concentration of PPy chains. The cathode Lac solution was prepared with PPy (0.5 mM, 0.0885 g), 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS, 10 mM, 0.08775 g), and Lac (5 U/10 μL) in 16 mL of CBS (pH 5.0).
Table 6 and Table 7 detail the compositions of the GOx solution in the anode and the Lac solution in the cathode, respectively.

Table 6.
Composition of the GOx solution in the anode cells.

Table 7.
Composition of the Lac solution in the cathode cells.
The electrodes were prepared using a copper wire (2 mm diameter) covered with two copper tapes (10 × 10 mm) and carbon paper. Using a pipette, the bioanode and biocathode were created by drop-coating 40 μL of the respective anode and cathode enzyme solutions onto the carbon paper surface. Once the enzyme solution was uniformly coated and completely dried, enzyme immobilization was considered complete. This electrode was referred to as the CP electrode (Figure 7a). A layer of 3M ventilation tape (3M™ Micropore™ Surgical Tape, Minnesota Mining and Manufacturing Company, Taoyuan, Taiwan) was then applied over the CP electrode, creating the CP3M electrode (Figure 7b). The electrode was stored at 4 °C in a refrigerator. A 10 mM PBS glucose solution was introduced into the anode and cathode cells for testing.
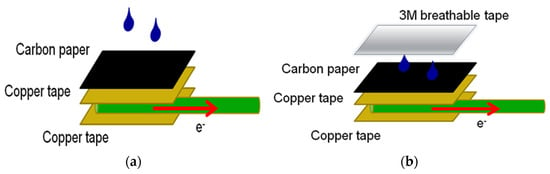
Figure 7.
Electrode construction for (a) the CP electrode and (b) the CP3M electrode.
3.4. Performance Test of the EBFC
The electrode reaction scheme for anode and cathode reactions is shown in Figure 8a. The electrode transfer pathway is shown in Figure 8b [18]. The cell was fabricated from polymethylmethacrylate using a CNC milling machine (Mitsubishi CNC M70, Tokyo, Japan) (Figure 8c). The external dimensions were 40 × 50 × 50 mm, while the inner tank volume measured 20 × 30 × 40 mm. The center of the tank’s bottom was hollowed out to create a groove (8 × 8 × 0.5 mm), as shown in Figure 8d. This groove was designed to accommodate a rotating magnet with a diameter of 20 mm. A circular groove (0.5 mm deep) was machined around the round hole to hold a rubber gasket securely.
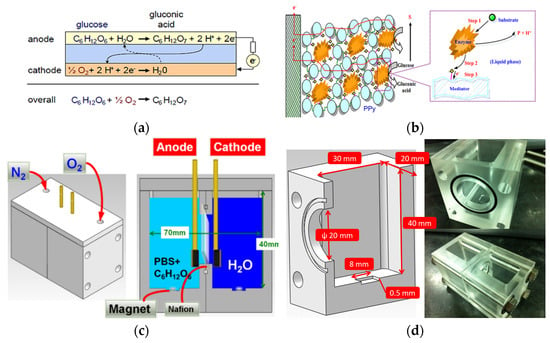
Figure 8.
(a) Electrode reaction in the anode and cathode, (b) electron transfer in the conductive polymer and mediator, (c) structure, and (d) geometry of the enzyme-based biofuel cells.
The tested enzyme-based biofuel cells consisted of GOx, potassium hexacyanoferrate (III) (K3Fe(CN)6), PPy, and a Nafion® 212 proton-exchange membrane. The biocathode chamber (surface area = 1 cm2) was immobilized with Lac and ABTS. Oxygen was bubbled into the system at a 1 mL/min flow rate. The bioanode was in contact with a PBS solution (pH 7) containing 10 mM glucose. The power output performance of the biofuel cell was characterized using a slow scan rate, a two-electrode voltage measurement, and current signals received by a 24-bit differential analogy module. Data were transmitted to a computer for analysis using LabVIEW® 2012 software at a constant temperature of 37 °C.
3.5. Cyclic Voltammetry with Half-Cell Analysis
The CV apparatus was a CH 600 electrochemical analyzer (Bio-Analytical Systems, San Antonio, TX, USA) connected to an Acer computer (Taiwan). A three-electrode cell configuration was employed, consisting of a modified carbon paper electrode (1 × 1 cm) as the working electrode, a platinum wire as the auxiliary electrode, and an Ag/AgCl electrode (3M KCl) as the reference electrode. The working electrode was modified with various combinations of bioanode enzymes, biocathode enzymes, and potassium ferrocyanide (mediator) for each experimental setup. The tests were conducted at 37 °C, with the electrode immersed in a 10 mM glucose solution (pH 7). The scanning rate was set at 0.01 V/s. For PPy as the conductive polymer, the scanning voltage ranged from −0.7 to −0.3 V, while PANI ranged from −0.4 to 0.2 V. Ag/AgCl was used as the reference electrode. The electrodes were allowed to stabilize in the glucose solution for 5 min before testing began.
Each electrode underwent six testing segments, with experimental data collected from segments 3 and 4. For accurate results, each CV scan was considered one segment from the initial to the final potential, with two segments forming a complete cyclic curve.
4. Conclusions
This study successfully demonstrated the effectiveness of the CP3M electrode design, which incorporated breathable tape for enhanced enzyme retention and long-term stability. The use of PPy as a conductive polymer further improved the oxidative activity and hydrogen evolution, particularly under slightly acidic conditions. The CP3M electrode consistently outperformed the CP electrode in both short- and long-term electrochemical performance, achieving higher voltage output, power density, and extended operational lifespan. The optimized buffer conditions (PBS for GOx and CBS for Lac) and the use of PPy significantly contributed to the observed enhanced catalytic efficiency.
The findings also highlight the importance of enzyme retention strategies and membrane design in EBFCs. While the CP3M electrode design demonstrated significant improvements, challenges such as mass transfer limitations and reverse proton migration still need to be addressed. This study provides a strong foundation for future research in EBFCs. The CP3M design enhances enzyme retention and stable power generation and offers a promising pathway for developing sustainable energy solutions. By refining membrane properties, enzyme immobilization techniques, and buffer selection, future studies can further enhance the efficiency, longevity, and applicability of biofuel cells for real-world energy applications.
Author Contributions
Conceptualization, H.S.W.; methodology, C.Y.C.; investigation, C.Y.C.; writing—original draft preparation, A.A.B.; writing—review and editing, D.D.; supervision, H.S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Notations
| ABTS | 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) |
| CP | copper-type |
| CP3M | copper-type with 3M micropore type |
| EBFC | enzyme-based biofuel cell |
| CV | cyclic voltammetry |
| GOx | glucose oxidase |
| Lac | laccase |
| PANI | polyaniline |
| PBS | phosphate-buffer solution |
| PPy | polypyrrole |
References
- Zebda, A.; Innocent, C.; Renaud, L.; Certin, M.; Pichot, F.; Ferrigno, R.; Tingry, S. Enzyme-Based Microfluidic Biofuel Cell to Generate Micropower in Biofuel’s Engineering Process Technology. In Biofuel’s Engineering Process Technology; Marco Aurelio, D.S.B., Ed.; InTech: London, UK, 2011. [Google Scholar] [CrossRef]
- Kim, J.; Jia, H.; Wang, P. Challenges in biocatalysis for enzyme-based biofuel cells. Biotechnol. Adv. 2006, 24, 296–308. [Google Scholar] [CrossRef]
- Żygowska, M. Design, Fabrication and Characterisation of Components for Microfluidic Enzymatic Biofuel Cells. Ph.D. Thesis, National University of Ireland, Cork, Ireland, April 2014. [Google Scholar]
- Wong, Y.; Yu, J. Laccase-catalyzed Decolorization of Synthetic Dye. Water Res. 1999, 33, 3512–3520. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, C.; Wu, H. Low-cost Immobilized Enzyme Glucose Sensor based on Laminar Flow. J. Phys. Conf. Ser. 2020, 1681, 012008. [Google Scholar] [CrossRef]
- Wong, C.M.; Wong, K.H.; Chen, X.D. Glucose oxidase: Natural occurrence, function, properties and industrial applications. AMBB 2008, 78, 927–938. [Google Scholar] [CrossRef]
- Jaeger, K.E.; Eggert, T. Enantioselective biocatalysis optimized by directed evolution. COBIOT 2004, 15, 305–313. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Bhaskar, T. Recent advances in the role of biocatalyst in biofuel cells and its application: An overview. BGER 2024, 40, 2051–2089. [Google Scholar] [CrossRef]
- Cao, L.; Chen, J.; Pang, J.; Qu, H.; Liu, J.; Gao, J. Research Progress in Enzyme Biofuel Cells Modified Using Nanomaterials and Their Implementation as Self-Powered Sensors. Molecules 2024, 29, 257. [Google Scholar] [CrossRef]
- Blanford, C.F.; Heath, R.S.; Armstrong, F.A. A stable electrode for high-potential, electrocatalytic O2 reduction based on rational attachment of a blue copper oxidase to a graphite surface. Chem. Commun. 2007, 1710–1712. [Google Scholar] [CrossRef]
- Moore, C.M.; Akers, N.L.; Hill, A.D.; Johnson, Z.C.; Minteer, S.D. Improving the Environment for Immobilized Dehydrogenase Enzymes by Modifying Nafion with Tetraalkylammonium Bromides. Biomacromolecules 2004, 5, 1241–1247. [Google Scholar] [CrossRef]
- Heineman, W.R.; Kissinge, P.T. Analytical Electrochemistry: Methodology and Applications of Dynamic Techniques. Anal. Chem. 1980, 52, 138R–151R. [Google Scholar] [CrossRef]
- Cai, J.; Shen, F.; Zhao, J.; Xiao, X. Enzymatic biofuel cell: A potential power source for self-sustained smart textiles. iScience 2024, 27, 108998. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Zhang, L.; Si, Y.; Guo, S.; Su, H.; Liu, J. Layer-by-layer assembly for immobilizing enzymes in enzymatic biofuel cells. Sustain. Energy & Fuels 2020, 4, 68–79. [Google Scholar] [CrossRef]
- Shamsudin, M.; Tan, L.; Tsuji, T. Enzyme immobilization technology in biofuel production: A review. Proc. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1051, 012056. [Google Scholar] [CrossRef]
- Pak, J.; Chang, W.; Kwon, C.H.; Cho, J. Recent Advances in Enzyme-based Biofuel Cells Using Glucose Fuel: Achieving High Power Output and Enhanced Operational Stability. Adv. Funct. Mater. 2024; 2415933early view. [Google Scholar] [CrossRef]
- Ismagilov, R.F.; Stroock, A.D.; Kenis, P.J.A.; Whitesides, G.; Stone, H.A. Experimental and theoretical scaling laws for transverse diffusive broadening in two-phase laminar flows in microchannels. Appl. Phys. Lett. 2000, 76, 2376. [Google Scholar] [CrossRef]
- Dai, D.J.; Chan, D.S.; Wu, H.S. Modified Carbon Nanoball on Electrode Surface Using Plasma in Enzyme-Based Biofuel Cells. Energy Procedia 2012, 14, 1804–1810. [Google Scholar] [CrossRef]
- Roth, G.J.; Calverley, D.C. Aspirin, Platelets, and Thrombosis: Theory and Practice. Blood 1994, 83, 885–898. [Google Scholar] [CrossRef]
- Munauwarah, R.; Bojang, A.A.; Wu, H.S. Characterization of enzyme immobilized carbon electrode using covalent-entrapment with polypyrrole. JCIE 2018, 41, 710–719. [Google Scholar] [CrossRef]
- Bojang, A.A.; Wu, H.S. Characterization of electrode performance in enzymatic biofuel cells using cyclic voltammetry and electrochemical impedance Spectroscopy. Catalysts 2020, 10, 782. [Google Scholar] [CrossRef]
- Khan, M.; Inamuddin. Fabrication and characterization of electrically conducting electrochemically synthesized polypyrrole-based enzymatic biofuel cell anode with biocompatible redox mediator vitamin K3. Sci. Rep. 2024, 14, 3324. [Google Scholar] [CrossRef]
- Hayes, J.R.; Engstrom, A.M.; Friesen, C. Orthogonal flow membraneless fuel cell. J. Power Sources 2008, 183, 257–259. [Google Scholar] [CrossRef]
- Kashyap, D.; Venkateswaran, P.S.; Dwivedi, P.K.; Kim, Y.H.; Kim, G.M.; Sharma, A.; Goel, S. Recent developments in enzymatic biofuel cell: Towards implantable integrated micro-devices. Int. J. Nanoparticles 2015, 8, 61–81. [Google Scholar] [CrossRef]
- Ding, S.N.; Holzinger, M.; Mousty, C.; Cosnier, S. Laccase electrodes based on the combination of single-walled carbon nanotubes and redox layered double hydroxides: Towards the development of biocathode for biofuel cells. J. Power Sources 2010, 195, 4714–4717. [Google Scholar] [CrossRef]
- Klis, M.; Maicka, E.; Michota, A.; Bukowska, J.; Sek, S.; Rogalski, J.; Bilewicz, R. Electroreduction of laccase covalently bound to organothiol monolayers on gold electrodes. Electrochim. Acta 2007, 52, 5591–5598. [Google Scholar] [CrossRef]
- Shim, J.; Kim, G.-Y.; Moon, S.-H. Covalent co-immobilization of glucose oxidase and ferrocenedicarboxylic acid for an enzymatic biofuel cell. JEAC 2011, 653, 14–20. [Google Scholar] [CrossRef]
- Calabrese Barton, S.; Gallaway, J.; Atanassov, P. Enzymatic Biofuel Cells for Implantable and Microscale Devices. Chem. Rev. 2004, 104, 4867–4886. [Google Scholar] [CrossRef]
- Zebda, A.; Renaud, L.; Cretin, M.; Pichot, F.; Innocent, C.; Ferrigno, R.; Tingry, S. A microfluidic glucose biofuel cell to generate micropower from enzymes at ambient temperature. Electrochem. Commun. 2009, 11, 592–595. [Google Scholar] [CrossRef]
- Willner, I.; Yan, Y.M.; Willner, B.; Tel-Vered, R. Integrated Enzyme-Based Biofuel Cells-A Review. Fuel Cells 2009, 9, 7–24. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).