Ultrasound-Induced Construction of CuxCo3−xO4/Attapulgite for Catalytic Degradation of Toluene
Abstract
1. Introduction
2. Results and Discussion
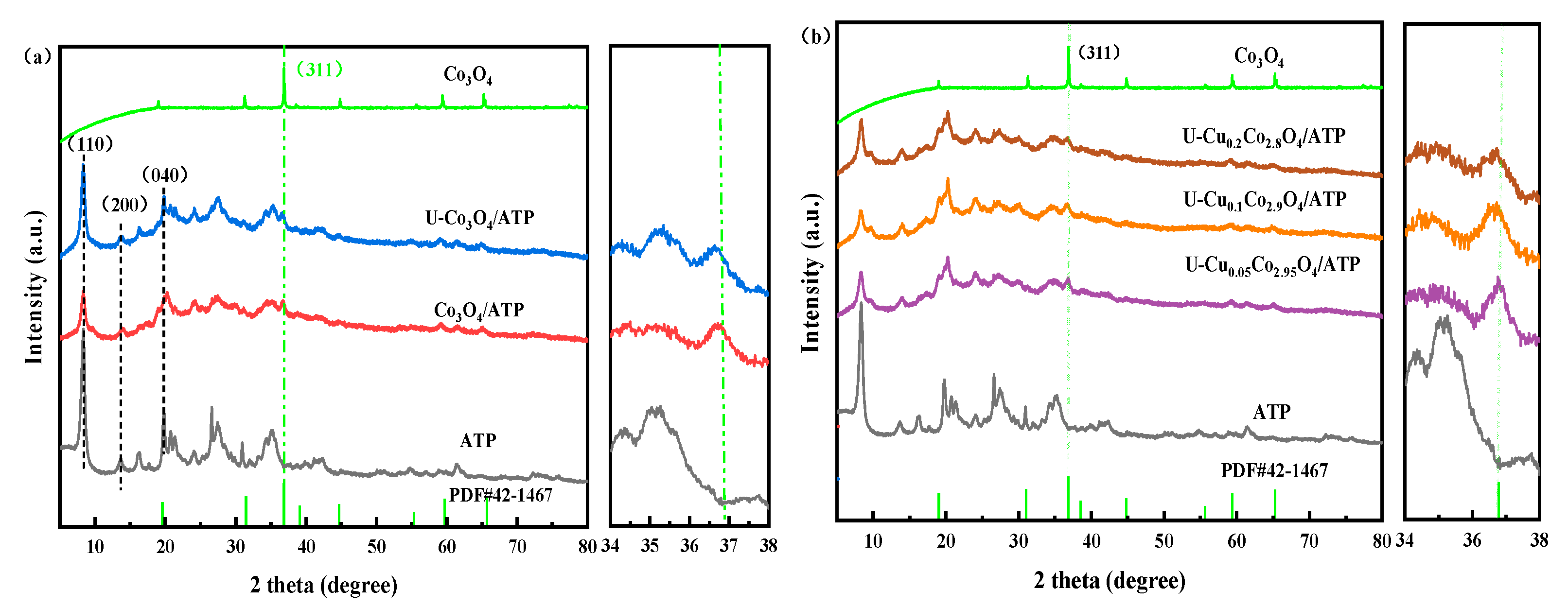
2.1. XRD Analysis
2.2. TEM Analysis
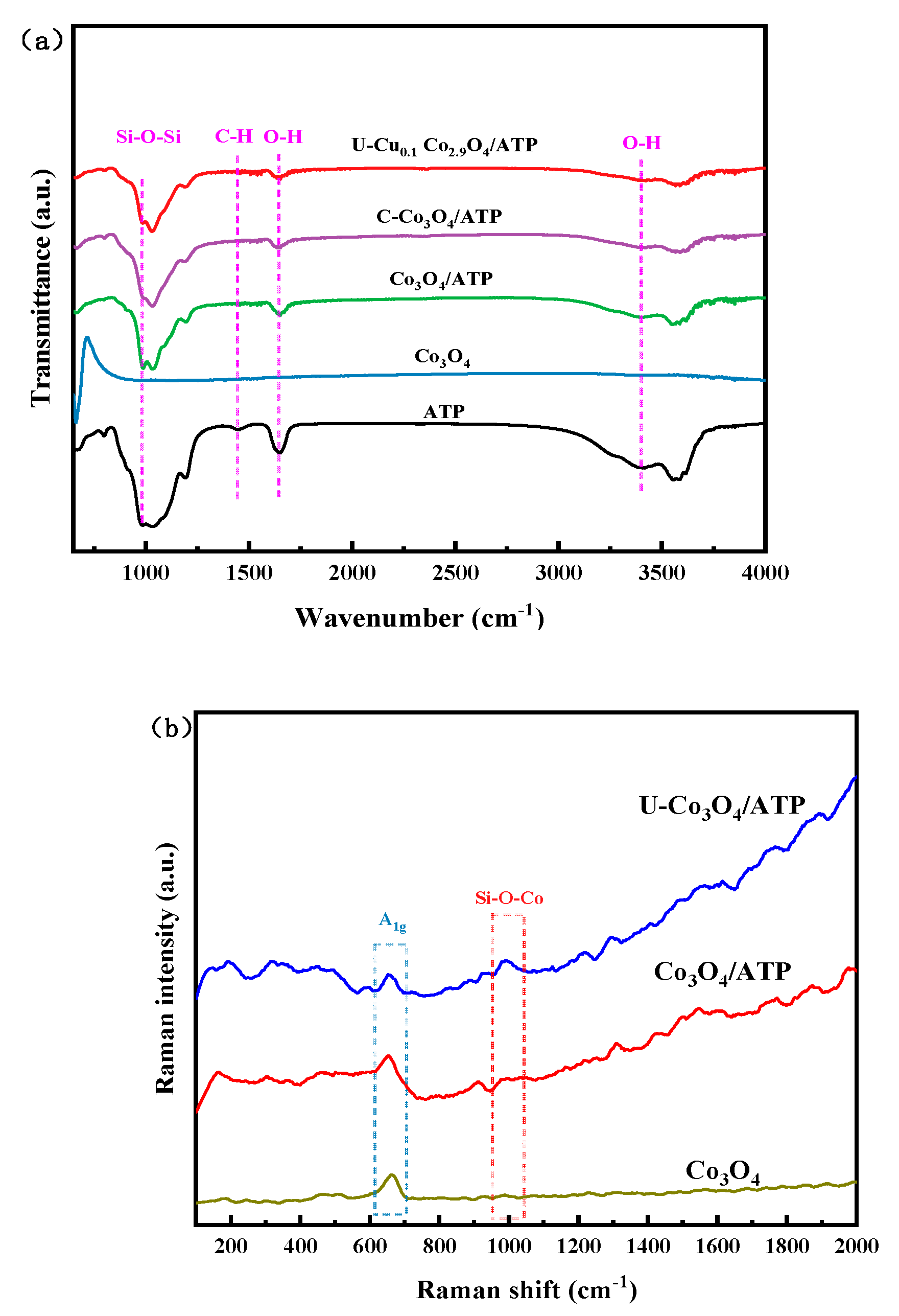
2.3. FT-IR and Raman Analysis
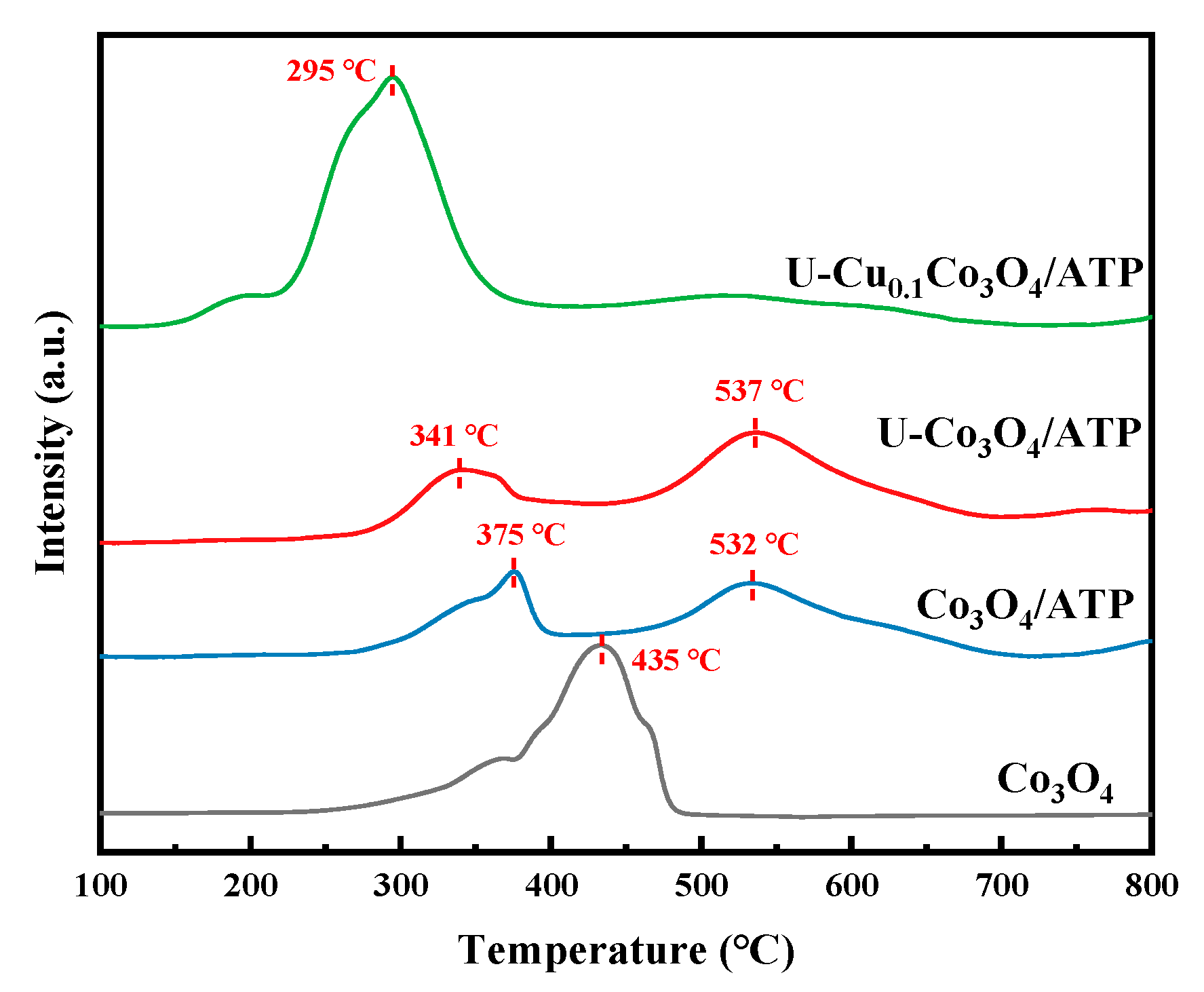
2.4. H2-TPR Analysis
2.5. XPS Analysis
2.6. Catalytic Oxidation Performance
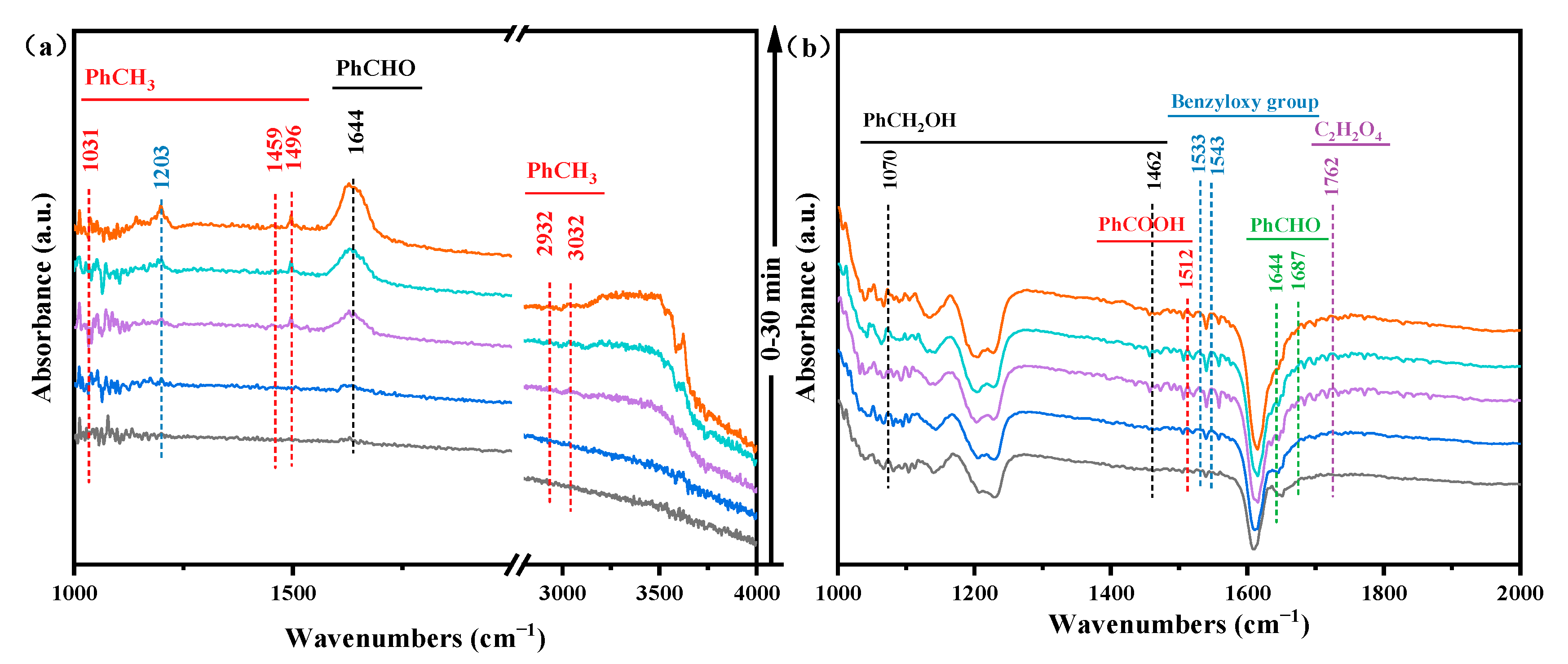
2.7. In-Situ DRIFTS Analysis
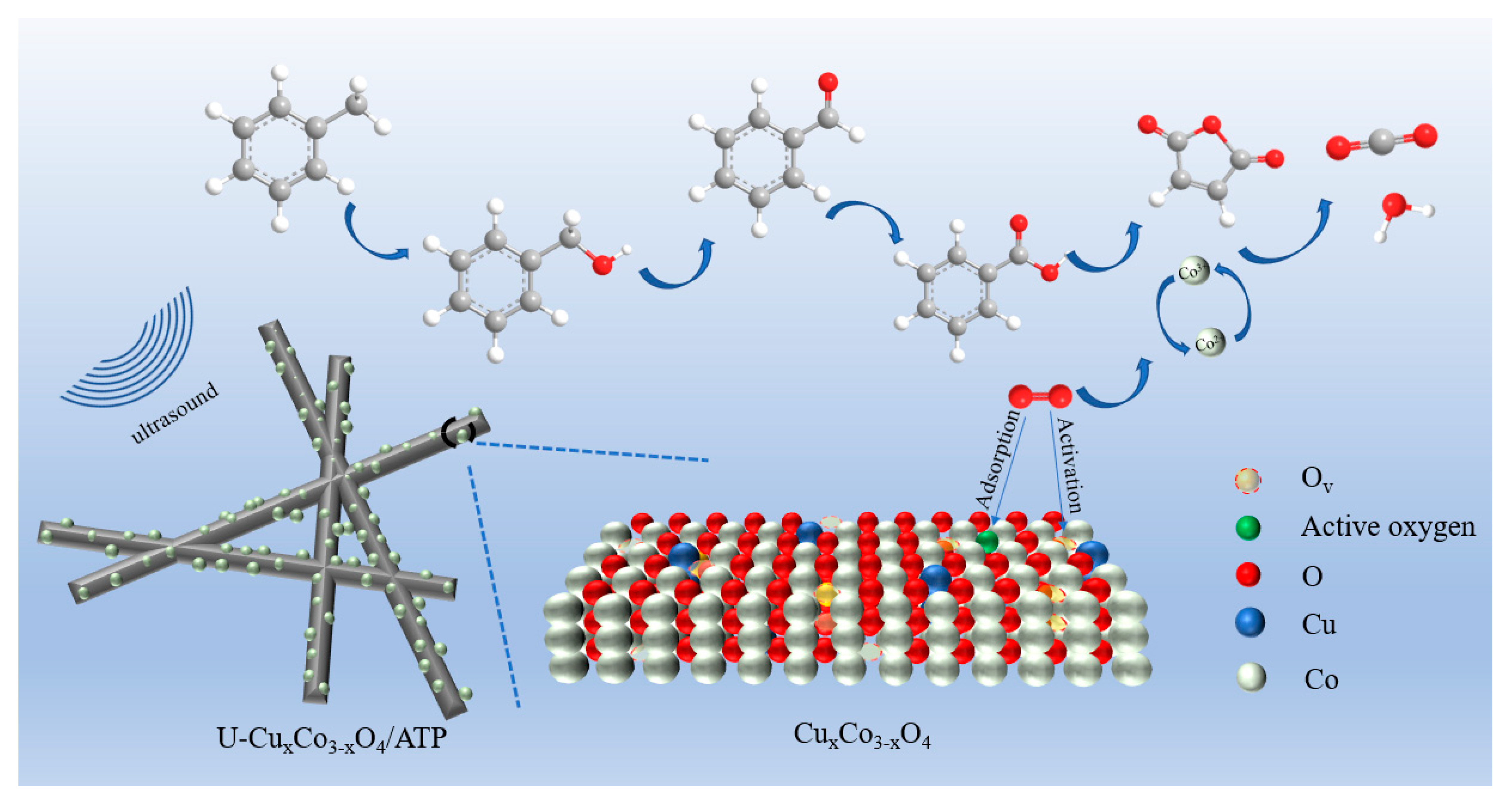
2.8. Catalytic Oxidation Degradation Mechanism
3. Experimental
3.1. Materials
3.2. Preparation of U-CuxCo3−xO4/ATP
3.3. Material Characterization
3.4. Catalytic Evaluation and Kinetic Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hui, L.; Liu, X.; Tan, Q.; Feng, M.; An, J.; Qu, Y.; Zhang, Y.; Cheng, N. VOC characteristics, sources and contributions to SOA formation during haze events in Wuhan, Central China. Sci. Total Environ. 2019, 650, 2624. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhou, J.; Zhuge, X.; Xie, Z.; Du, K. Enhanced oxygen mobility of Mn-Ce solid solution catalysts for polysaccharide-assisted catalytic oxidation of toluene. Surf. Interfaces 2024, 46, 103993. [Google Scholar] [CrossRef]
- Ma, D.; Liu, W.; Huang, Y.; Xia, D.; Lian, Q.; He, C. Enhanced Catalytic Ozonation for Eliminating CH3SH via Stable and Circular Electronic Metal-Support Interactions of Si-O-Mn Bonds with Low Mn Loading. Environ. Sci. Technol. 2022, 56, 3678. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117. [Google Scholar] [CrossRef]
- Yang, C.; Miao, G.; Pi, Y.; Xia, Q.; Wu, J.; Li, Z.; Xiao, J. Abatement of various types of VOCs by adsorption/catalytic oxidation: A review. Chem. Eng. J. 2019, 370, 1128. [Google Scholar] [CrossRef]
- Zhang, J.; Shu, M.; Niu, Y.; Yi, L.; Yi, H.; Zhou, Y.; Zhao, S.; Tang, X.; Gao, F. Advances in CO catalytic oxidation on typical noble metal catalysts: Mechanism, performance and optimization. Chem. Eng. J. 2024, 495, 153523. [Google Scholar] [CrossRef]
- Liu, P.; Wei, G.; Liang, X.; Chen, D.; He, H.; Chen, T.; Xi, Y.; Chen, H.; Han, D.; Zhu, J. Synergetic effect of Cu and Mn oxides supported on palygorskite for the catalytic oxidation of formaldehyde: Dispersion, microstructure, and catalytic performance. Appl. Clay Sci. 2018, 161, 265. [Google Scholar] [CrossRef]
- Ren, Q.; Mo, S.; Peng, R.; Feng, Z.; Zhang, M.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Controllable synthesis of 3D hierarchical Co3O4 nanocatalysts with various morphologies for the catalytic oxidation of toluene. J. Mater. Chem. A 2018, 6, 498. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, S.; Zeng, Y.; Zhong, Q. Spinel-structured catalyst formed by Fe, Cu, and Co composites for efficient toluene oxidation. J. Environ. 2024, 12, 113598. [Google Scholar] [CrossRef]
- Wu, P.; Jin, X.; Qiu, Y.; Ye, D. Recent Progress of Thermocatalytic and Photo/Thermocatalytic Oxidation for VOCs Purification over Manganese-based Oxide Catalysts. Environ. Sci. Technol. 2021, 55, 4268. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Qian, K.; Huang, W. Metal–Support Interactions in Metal/Oxide Catalysts and Oxide–Metal Interactions in Oxide/Metal Inverse Catalysts. ACS Catal. 2022, 12, 1268. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, T.; Naren, T.; Jiang, Y.; Wang, Y.; Liu, F.; Zeng, S. Halloysite-assisted strong binary oxides-support interactions on multisite catalysts for enhanced stability in preferential CO oxidation. J. Environ. 2023, 11, 110545. [Google Scholar] [CrossRef]
- Ouyang, T.; Jin, B.; Mao, Y.; Wei, D.; Liang, Z. Control of strong electronic oxide-support interaction in iron-based redox catalysts for highly efficient chemical looping CO2 conversion. Appl. Catal. B 2024, 343, 123531. [Google Scholar] [CrossRef]
- Ji, J.; Liu, J.; Shi, L.; Guo, S.; Cheng, N.; Liu, P.; Gu, Y.; Yin, H.; Zhang, H.; Zhao, H. Ruthenium Oxide Clusters Immobilized in Cationic Vacancies of 2D Titanium Oxide for Chlorine Evolution Reaction. Small Struct. 2024, 5, 2300240. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Gong, M.; Wang, W.; Hu, Y.M.Z.-H. α-MnO2/Palygorskite composite as an effective catalyst for heterogeneous activation of peroxymonosulfate (PMS) for the degradation of Rhodamine B. Sep. Purif. 2020, 230, 115877. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, H.; Jiang, Z.; Wang, J.; Kong, C.; Yang, Z.; Wang, T.; Zhu, H. Fabrication of acid-activated dispersive palygorskite nanorods supported copper manganese composite oxide for catalytic oxidation of toluene. Mater. Today Commun. 2024, 38, 108269. [Google Scholar] [CrossRef]
- Tan, Z.; Zhang, S.; Zhao, F.; Zhang, R.; Tang, F.; You, K.; Luo, H.A.; Zhang, X. SnO2/ATP catalyst enabling energy-efficient and green amine-based CO2 capture. Chem. Eng. J. 2023, 453, 139801. [Google Scholar] [CrossRef]
- Li, X.; Lu, X.; Meng, Y.; Yao, C.; Chen, Z. Facile synthesis and catalytic oxidation property of palygorskite/mesocrystalline Ce1−xMnxO2 nanocomposites. J. Alloys Compd. 2013, 562, 56. [Google Scholar] [CrossRef]
- Xing, X.; Li, N.; Cheng, J.; Sun, Y.; Zhang, Z.; Zhang, X.; Hao, Z. Synergistic effects of Cu species and acidity of Cu-ZSM-5 on catalytic performance for selective catalytic oxidation of n-butylamine. J. Environ. Sci. 2020, 96, 55. [Google Scholar] [CrossRef]
- Wang, Y.; Han, J.; Du, J.; Liu, R.; Wan, F. The toughening of pyrochlore La2Zr2O7 by a ferroelastic NdAlO3 second phase for potential thermal barrier coating applications. J. Am. Ceram. Soc. 2021, 104, 3508. [Google Scholar] [CrossRef]
- Siniard, K.M.; Li, M.; Yang, S.Z.; Zhang, J.; Polo-Garzon, F.; Wu, Z.; Yang, Z.; Dai, S. Ultrasonication-Induced Strong Metal-Support Interaction Construction in Water Towards Enhanced Catalysis. Angew. Chem. Int. Ed. Engl. 2023, 62, e202214322. [Google Scholar] [CrossRef] [PubMed]
- Cano, F.J.; Coste, S.; Reyes-Vallejo, O.; Makowska-Janusik, M.; Velumani, S.; de la Luz Olvera, M.; Kassiba, A. Influence of go oxidation degrees on the organization and physical features of TiO2-go-based nanocomposites for water dye removal. Surf. Interfaces 2024, 46, 104004. [Google Scholar] [CrossRef]
- Sun, L.; Liang, X.; Liu, H.; Cao, H.; Liu, X.; Jin, Y.; Li, X.; Chen, S.; Wu, X. Activation of Co-O bond in (110) facet exposed Co3O4 by Cu doping for the boost of propane catalytic oxidation. J. Hazard. Mater. 2023, 452, 131319. [Google Scholar] [CrossRef]
- Kong, D.; Zhou, W.; Han, J.; Gao, Y.; Gao, Y.; Zhao, L.; Sun, P.; Lu, G. Mesoporous Co3O4 nanosheets with exposed Co2+-rich crystal facets for improved toluene detection. Appl. Surf. Sci. 2023, 619, 156714. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Y.; Ding, H.; Lu, A.; Li, Y.; Ji, X.; Zhang, Y.; Wang, C. Vibrational and structural insight into silicate minerals by mid-infrared absorption and emission spectroscopies. Phys. Chem. Miner. 2022, 49, 6. [Google Scholar] [CrossRef]
- Yu, X.; Dang, X.; Li, S.; Li, Y.; Wang, H.; Jing, K.; Dong, H.; Liu, X. Enhanced activity of plasma catalysis for trichloroethylene decomposition via metal-support interaction of SiOCo/Mn bonds over CoMnOx/ZSM-5. Sep. Purif. 2023, 305, 122553. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, J.; Li, X.; Luo, K.; Xiong, Z.; Zhou, Z.; Zhu, W.; Luo, Z.; Huang, J.; Li, Y. Steering surface reconstruction of hybrid metal oxides for efficient oxygen evolution reaction in water splitting and zinc-air batteries. J. Energy Chem. 2024, 92, 383. [Google Scholar] [CrossRef]
- Zhong, J.; Zeng, Y.; Chen, D.; Mo, S.; Zhang, M.; Fu, M.; Wu, J.; Su, Z.; Chen, P.; Ye, D. Toluene oxidation over Co3+-rich spinel Co3O4: Evaluation of chemical and by-product species identified by in situ DRIFTS combined with PTR-TOF-MS. J. Hazard. Mater. 2020, 386, 121957. [Google Scholar] [CrossRef]
- Zhang, J.; Qin, X.; Chu, X.; Chen, M.; Chen, X.; Chen, J.; He, H.; Zhang, C. Tuning Metal-Support Interaction of Pt-CeO2 Catalysts for Enhanced Oxidation Reactivity. Environ. Sci. Technol. 2021, 55, 16687. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Z.; Wang, S.; Jin, P.; Li, F.; Dang, D. Metal organic framework template method for the preparation of bimetallic oxide CuCoOx catalysts for the catalytic oxidation of volatile organic compounds. Chem. Eng. J. 2023, 476, 146853. [Google Scholar] [CrossRef]
- De Rivas, B.; López Fonseca, R.; Jiménez González, C.; Gutierrez-Ortiz, J.I. Synthesis, characterisation and catalytic performance of nanocrystalline Co3O4 for gas-phase chlorinated VOC abatement. J. Catal. 2011, 281, 88–97. [Google Scholar] [CrossRef]
- Yue, Z.; Zhi Ying, L.; Xi, L.; Hong Ya, Q.; Peng, Y.; Yan Hua, X. Effect of Ce on the activity of Cu-Co-O catalyst in catalytic combustion of VOCs. China Environ. Sci. 2017, 37, 2087–2091. [Google Scholar]
- Zhao, F.; Zhang, G.; Zeng, P.; Yang, X.; Ji, S. Preparation of CuxCo1−x/Al2O3/Cordierite Monolithic Catalysts and the Catalytic Combustion of Toluene. Chin. J. Catal. 2011, 32, 821–826. [Google Scholar] [CrossRef]
- Cheng, Q.; Yang, Z.; Li, Y.; Wang, J.; Wang, J.; Zhang, G. Amorphous/crystalline Cu1.5Mn1.5O4 with rich oxygen vacancies for efficiently photothermocatalytic mineralization of toluene. Chem. Eng. J. 2023, 471, 144295. [Google Scholar] [CrossRef]
- Fan, S.; Luo, S.; Wang, Y.; Yue, X.; Zheng, D.; Zhang, Z.; Fu, X.; Dai, W. TiO2-based Pd/Fe bimetallic modification for the efficient photothermal catalytic degradation of toluene: The synergistic effect of O2− and ∙OH species. Sep. Purif. 2024, 336, 126256. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, J.; Wang, M.; Qi, F.; Jin, X.; Zhang, L. Enhanced toluene oxidation by photothermal synergetic catalysis on manganese oxide embedded with Pt single-atoms. J. Colloid Interface Sci. 2023, 636, 577. [Google Scholar] [CrossRef]
- Zhu, J.; Cheng, Y.; Wang, Z.; Zhang, J.; Yue, Y.; Qian, G. Low-energy production of a monolithic catalyst with MnCu-synergetic enhancement for catalytic oxidation of volatile organic compounds. J. Environ. Manag. 2023, 336, 117688. [Google Scholar] [CrossRef]
- Chen, K.; Li, W.; Zhou, Z.; Huang, Q.; Liu, Y.; Duan, Q. Hydroxyl groups attached to Co2+ on the surface of Co3O4: A promising structure for propane catalytic oxidation. Catal. Sci. Technol. 2020, 10, 2573. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Shi, J.; Han, C.; Song, Z.; Xiao, Y.; Li, X. Ultrasound-Induced Construction of CuxCo3−xO4/Attapulgite for Catalytic Degradation of Toluene. Catalysts 2025, 15, 252. https://doi.org/10.3390/catal15030252
Zhang H, Shi J, Han C, Song Z, Xiao Y, Li X. Ultrasound-Induced Construction of CuxCo3−xO4/Attapulgite for Catalytic Degradation of Toluene. Catalysts. 2025; 15(3):252. https://doi.org/10.3390/catal15030252
Chicago/Turabian StyleZhang, Haitao, Jian Shi, Chaoya Han, Zhizhao Song, Yao Xiao, and Xiazhang Li. 2025. "Ultrasound-Induced Construction of CuxCo3−xO4/Attapulgite for Catalytic Degradation of Toluene" Catalysts 15, no. 3: 252. https://doi.org/10.3390/catal15030252
APA StyleZhang, H., Shi, J., Han, C., Song, Z., Xiao, Y., & Li, X. (2025). Ultrasound-Induced Construction of CuxCo3−xO4/Attapulgite for Catalytic Degradation of Toluene. Catalysts, 15(3), 252. https://doi.org/10.3390/catal15030252






