Diastereoselective Synthesis of 2-Amino-spiro[4.5]decane-6-ones Through Synergistic Photocatalysis and Organocatalysis for [3 + 2] Cycloaddition of Cyclopropylamines with Olefins
Abstract
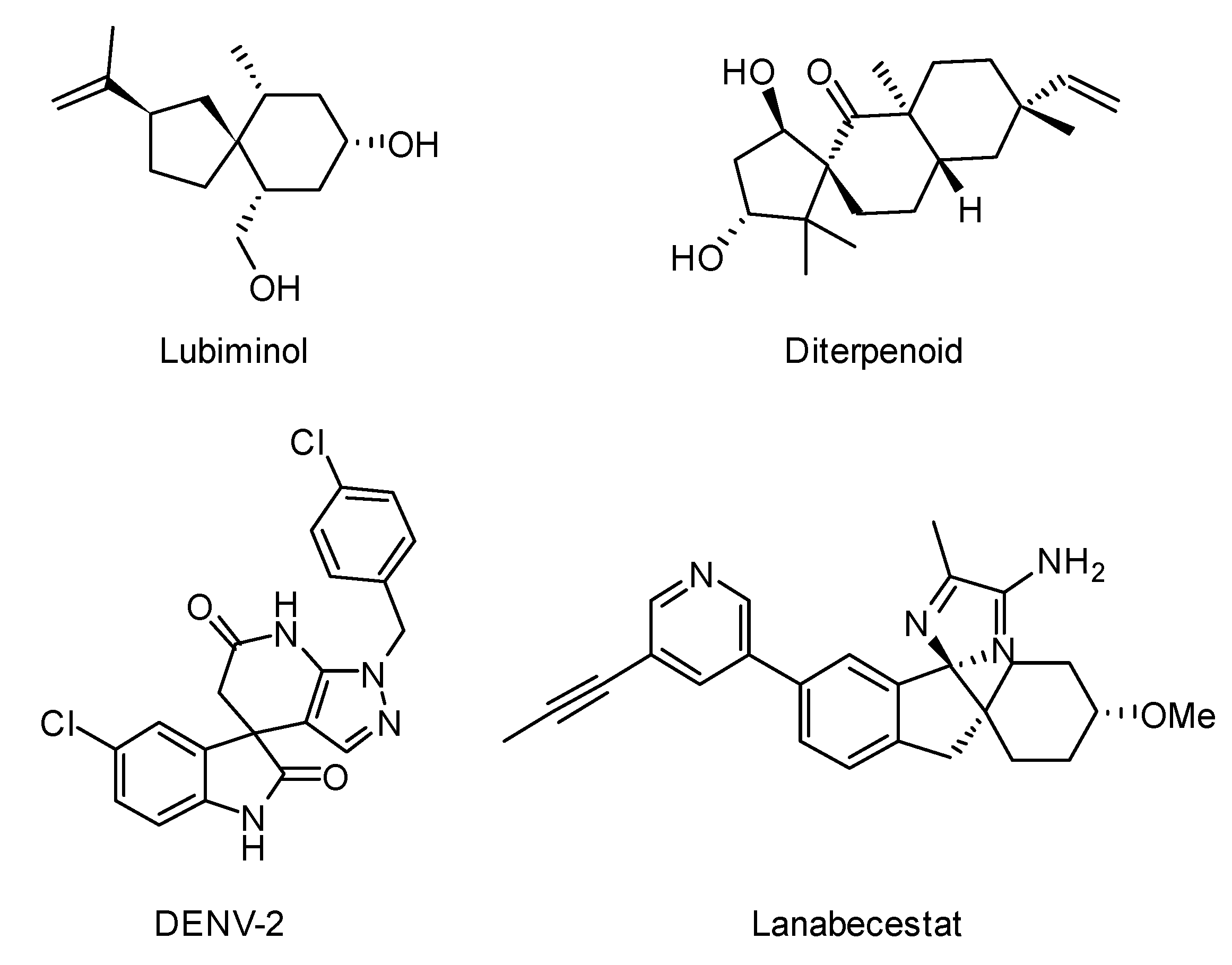
1. Introduction
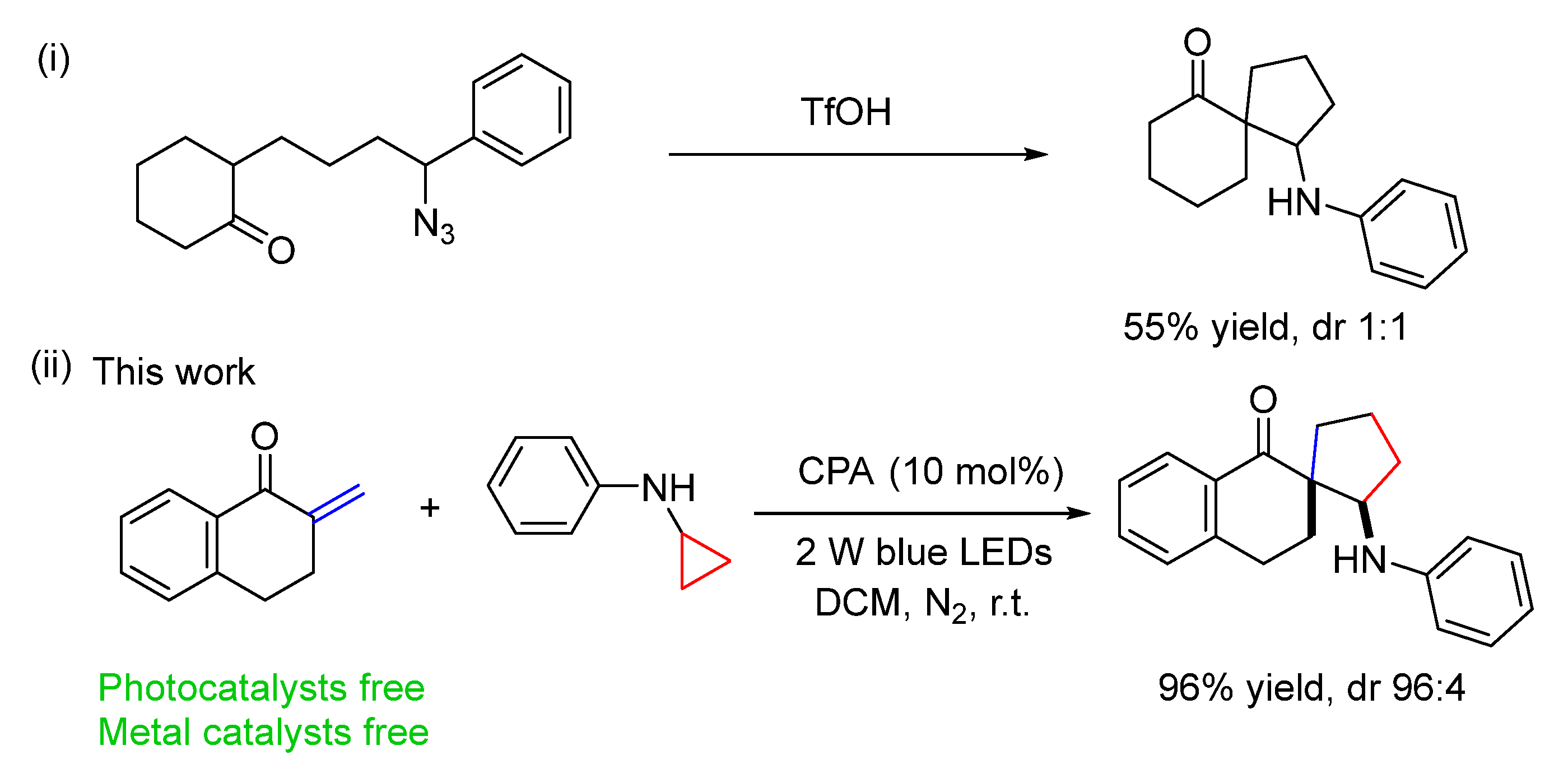
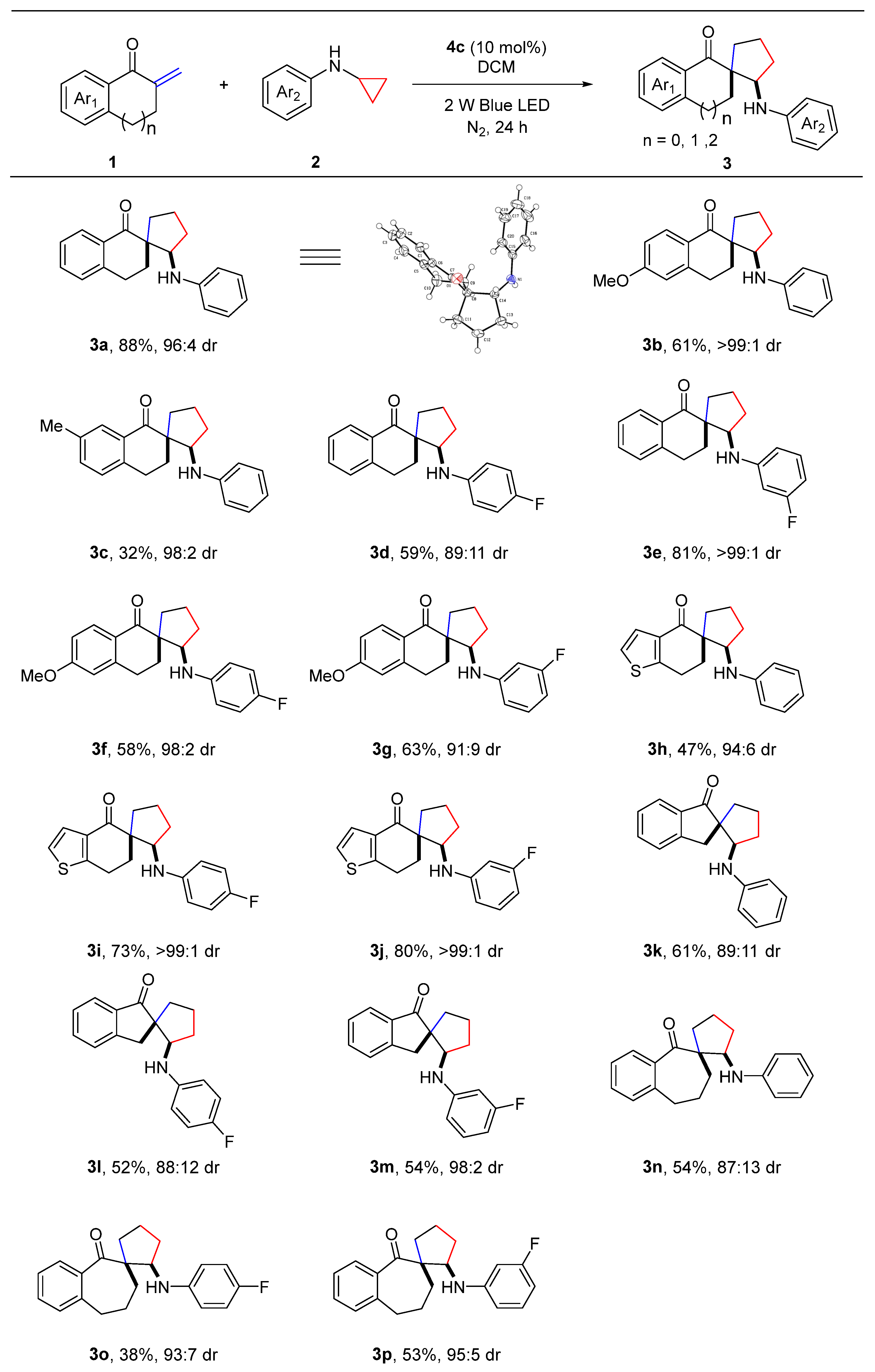
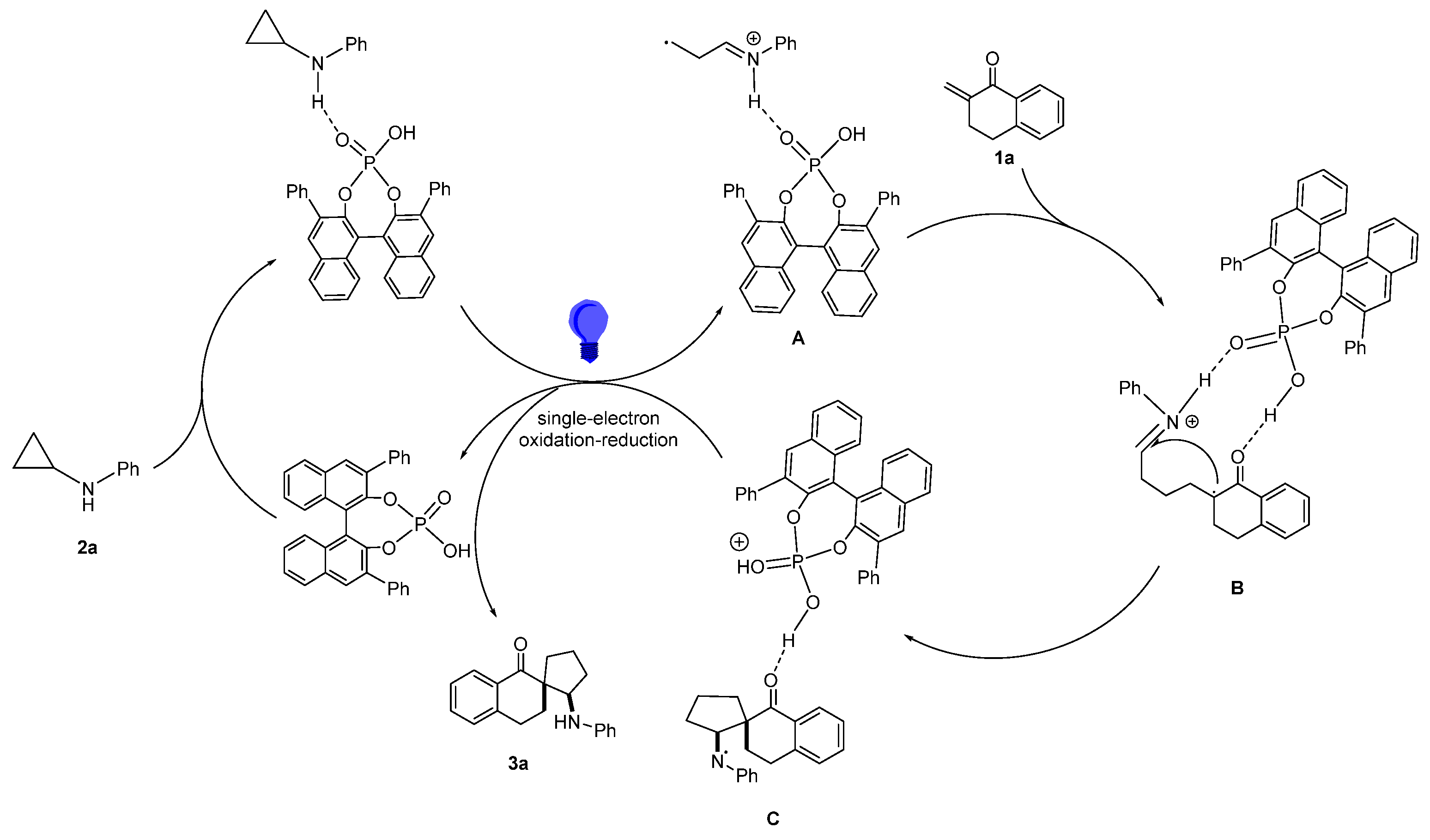
2. Results and Discussion
3. Materials and Methods
3.1. General Informatin
3.2. General Procedure for Synthesis of 3
3.3. Characterization Data for Products
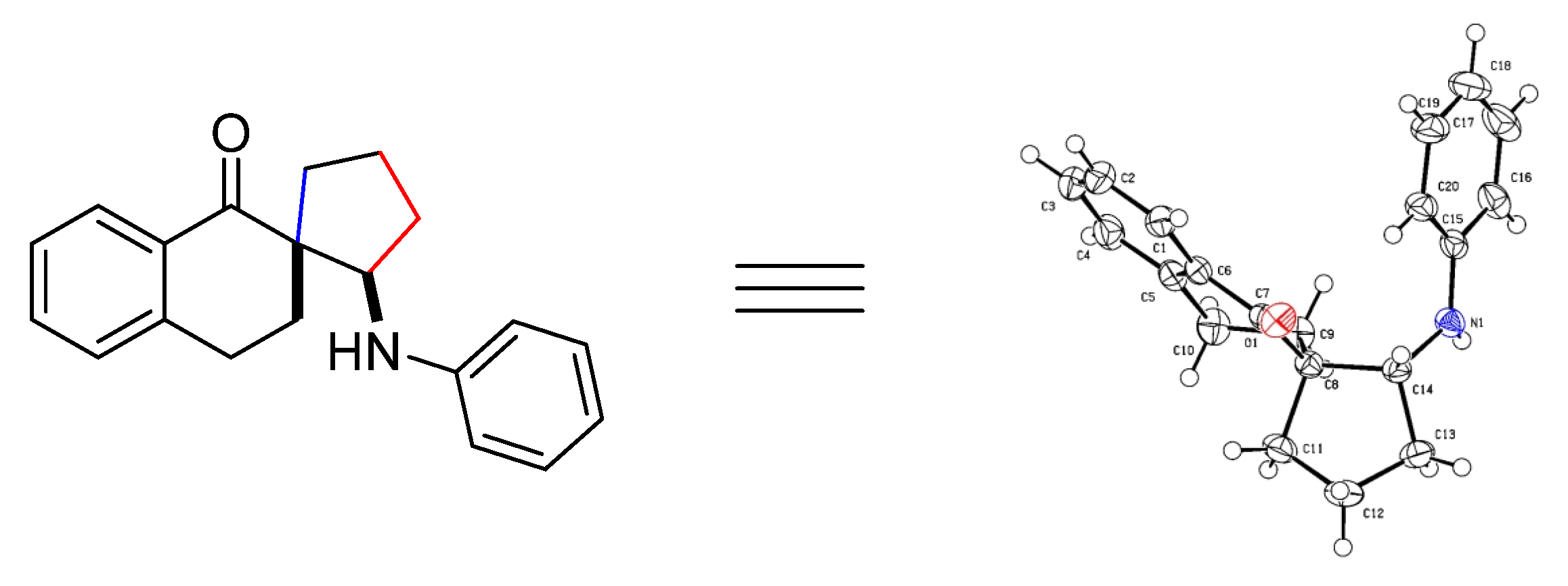
3.4. X-Ray Structure and Crystal Data of 3a
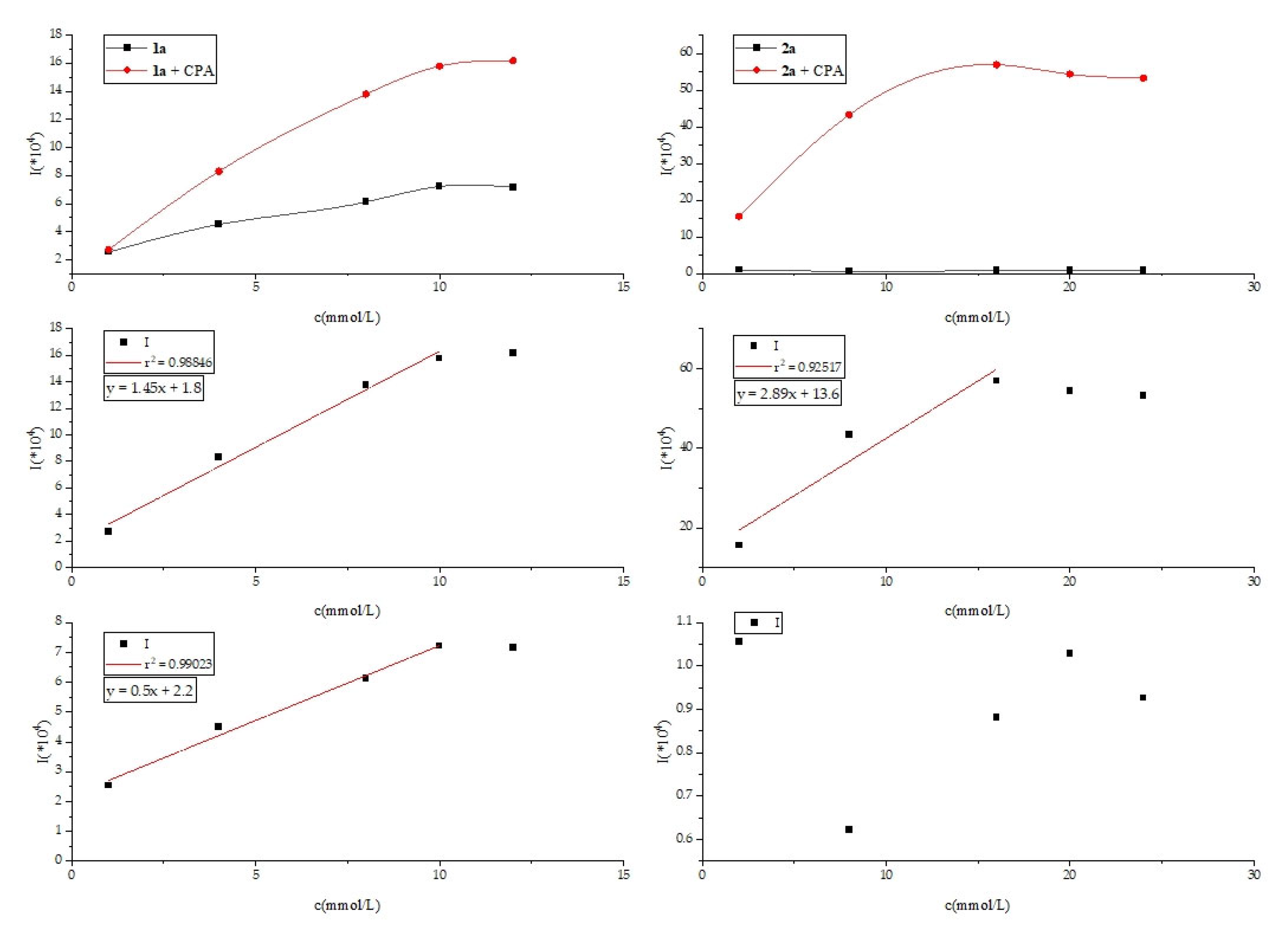
3.5. Ultraviolet Fluorescence Method Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vessally, E.; Babazadeh, M.; Didehban, K.; Hosseinian, A.; Edjlali, L. Intramolecular ipso-Cyclization of N-Arylpropiolamides: A Novel and Straightforward Synthetic Approach for Azaspiro [4.5]decatrien-2-ones. Curr. Org. Chem. 2018, 22, 286–297. [Google Scholar] [CrossRef]
- Chada, R.R.; Karna, N.; Puthiya, P.V.; Uprety, A.; Rene, G. Electrochemical Domino Sulfonylation/Dearomative ipso-Annulation of 2-Alkynyl Biaryls to Access Spiro(indenyl)cycylohexadienones. Eur. J. Org. Chem. 2024, 48, e202400990. [Google Scholar]
- Nagaoka, T.; Goto, K.; Watanabe, A.; Sakata, Y.; Yoshihara, T. Sesquiterpenoids in root exudates of Solanum aethiopicum. Z. Naturforschung C 2001, 56, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; An, L.; Xu, J.; Guo, Y. Euphnerins A and B, Diterpenoids with a 5/6/6 Rearranged Spirocycylic Carbon Skeleton from the Stems of Euphorbia neriifolia. J. Nat. Prod. 2020, 83, 2592–2596. [Google Scholar] [CrossRef]
- Zou, B.; Chan, W.L.; Ding, M.; Leong, S.Y.; Nilar, S.; Seah, P.G.; Liu, W.; Karuna, R.; Blasco, F.; Yip, A.; et al. Lead Optimization of Spiropyrazolopyridones: A New and Potent Class of Dengue Virus Inhibitors. ACS Med. Chem. Lett. 2015, 6, 344–348. [Google Scholar] [CrossRef]
- Wessels, A.M.; Tariot, P.N.; Zimmer, J.A.; Selzer, K.J.; Bragg, S.M.; Andersen, S.W.; Landry, J.; Krull, J.H.; Downing, A.C.M.; Willis, B.A.; et al. Efficacy and Safety of Lanabecestat for Treatment of Early and Mild Alzheimer Disease: The AMARANTH and DAYBREAK-ALZ Randomized Clinical Trials. JAMA Neurol. 2020, 77, 199–209. [Google Scholar] [CrossRef]
- Dubbelman, M.A.; Hendriksen, H.M.A.; Harrison, J.E.; Vijverberg, E.G.B.; Prins, N.D.; Kroeze, L.A.; Ottenhoff, L.; Van Leeuwenstijn, M.M.S.S.A.; Verberk, I.M.W.; Teunissen, C.E.; et al. Cognitive and Functional Change Over Time in Cognitively Healthy Individuals According to Alzheimer Disease Biomarker-Defined Subgroups. Neurology 2024, 102, e207978. [Google Scholar] [CrossRef]
- Acosta-Quiroga, K.; Rojas-Peña, C.; Nerio, L.S.; Gutiérrez, M.; Polo-Cuadrado, E. Spirocyclic derivatives as antioxidante: A review. RSC Adv. 2021, 11, 21926–21954. [Google Scholar] [CrossRef]
- Feng, Y.; Ren, Y.Y.; Tang, K.-H.; Hu, Y.; Wang, J.; Huang, D.; Lv, X.; Hu, Y. Synthesis of difluoromethylated spiropyrazolones via [3 + 2] cycloaddition of difluoroacetohydrazonoyl bromides with alkylidene pyrazolones. Org. Biomol. Chem. 2024, 22, 2797–2812. [Google Scholar] [CrossRef]
- Elumalai, G.; Namboothiri Irishi, N.N. Synthesis of Fused Bromofurans via Mg-Mediated Dibromocyclopropanation of Cycloalkanone-Derived Chalcones and Cloke-Wilson Rearrangement. J. Org. Chem. 2013, 78, 910–919. [Google Scholar]
- Li, M.; Scheeff, S.; Chen, J.; Johnston, R.C.; Rizzo, A.; Krenske, E.H.; Chiu, P. Diastereo- and Enantioselective Construction of Stereochemical Arrays Exploiting Non-Classical Hydrogen Bonding in Enolborates. Chem. Eur. J. 2024, 30, e202401485. [Google Scholar] [CrossRef] [PubMed]
- Markó, I.E.; Ates, A.; Gautier, A.; Leroy, B.; Plancher, J.-M.; Quesnel, Y.; Vanherck, J.-C. Cerium(IV)-Catalyzed Deportection of Acetals and Ketals under Mildly Basic Conditions. Angew. Chem. Int. Ed. 1999, 38, 3207–3209. [Google Scholar] [CrossRef]
- Guo, L.-D.; Xu, Z.; Tong, R. Asymmetric Total Synthesis of Indole Diterpenes Paspalicine, Paspalinine, and Paspalinine-13-ene. Angew. Chem. Int. Ed. 2021, 61, e202115384. [Google Scholar] [CrossRef] [PubMed]
- Wrobleski, A.; Aubé, J. Intramolecular Reactions of Benzylic Azides with Ketones: Competition between Schmidt and Mannich Pathways. J. Org. Chem. 2001, 66, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, H.; Kawase, A.; Doi, T. Asymmetric palladium-catalyzed umpolung cyclization of allylic acetate-aldehyde using formate as a reductant. Chem. Commun. 2015, 51, 8027–8030. [Google Scholar] [CrossRef]
- Jia, Z.-L.; An, X.-T.; Deng, Y.-H.; Wang, H.-B.; Gan, K.-J.; Zhang, J.; Zhao, X.-H.; Fan, C.-A. Palladium-Catalyzed Asymmetric (2+3) Annulation of p-Quinone Methides with Trimethylenemethanes: Enantioselective Synthesis of Functionalized Chiral Spirocyclopentyl p-Dienones. Org. Lett. 2020, 22, 4171–4175. [Google Scholar] [CrossRef]
- Lv, S.; Xu, W.-F.; Yang, T.-Y.; Lan, M.-X.; Xiao, R.-X.; Mou, X.-Q.; Chen, Y.-Z.; Cui, B.-D. Iron(II)-Catalyzed Radical [3 + 2] Cyclization of N- Aryl Cyclopropylamines for the Synthesis of Polyfunctionalized Cyclopentylamines. Org. Lett. 2024, 26, 3151–3157. [Google Scholar] [CrossRef]
- Ha, J.D.; Lee, J.; Blackstock, S.C.; Cha, J.K. Intramolecular [3 + 2] Annulation of Olefin-Tethered Cyclopropylamines. J. Org. Chem. 1998, 63, 8510–8514. [Google Scholar] [CrossRef]
- Dai, Y.; Liang, S.; Zeng, G.; Huang, H.; Zhao, X.; Cao, S.; Jiang, Z. Asymmetric [3 + 2] photocycloadditions of cyclopropylamines with electron-rich and electron-neutral olefins. Chem. Sci. 2022, 13, 3787–3795. [Google Scholar] [CrossRef]
- Luo, Z.; Cao, B.; Song, T.; Xing, Z.; Ren, J.; Wang, Z. Visible-Light Organophotoredox-Mediated [3 + 2] Cycloaddition of Arylcyclopropylamine with Structurally Diverse Olefins for the Construction of Cyclopentylamines and Spiro[4.n] Skeletons. J. Org. Chem. 2022, 87, 15511–15529. [Google Scholar] [CrossRef]
- Gras, J.-L.; Gombatz, K.J.; Büchi, G. Methylene Ketones and Aldehydes by simple, direct Methylene transfer: 2-Methylene-1-oxo-1,2,3,4-tetrahydronaphthalene. Org. Synth. 1981, 60, 88. [Google Scholar]
- Maity, S.; Zhu, M.; Shinabery, R.S.; Zheng, N. Intermolecular [3 + 2] Cycloaddition of Cyclopropylamines with Olefins by Visible-Light Photocatalysis. Angew. Chem. Int. Ed. 2012, 51, 222–226. [Google Scholar] [CrossRef]

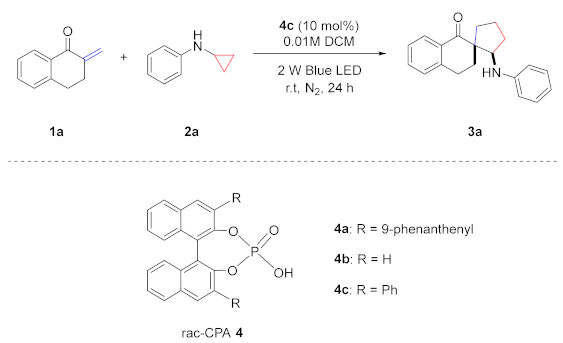 | |||
|---|---|---|---|
| Entry | Conditions | Yield (%) [b] | d.r. [c] |
| 1 | None | 88 | 96:4 |
| 2 | With 4a in Et2O at −20 °C | 31 | 97:3 |
| 3 | With 4a in MeCN at −20 °C | 8 | 93:7 |
| 4 | With 4a in DCE at −20 °C | 32 | 96:4 |
| 5 | With 4a in DCM at −20 °C | 36 | 80:20 |
| 6 | With 4b in DCM at −20 °C | 13 | 92:8 |
| 7 | With 4c in DCM at −20 °C | 30 | 96:4 |
| 8 | With Diphenylphosphate in DCM at −20 °C | 28 | 92:8 |
| 9 | With 4c in DCM at 40 °C | 87 | 94:6 |
| 10 | 1a:2a = 1:1 | 70 | 98:2 |
| 11 | 1a:2a = 2:1 | 97 | 89:11 |
| 12 | C(1a) = 0.005 M | 62 | 94:6 |
| 13 | C(1a) = 0.02 M | 83 | 88:12 |
| 14 | With Eosin Y (5 mol%) | 64 | 97:3 |
| 15 | With 4CzIPN (5 mol%) | 41 | 99:1 |
| 16 | With Rhodamine B (5 mol%) | 35 | 92:8 |
| 17 | Reaction in 18 h | 50 | 98:2 |
| 18 | Reaction in 30 h | 91 | 92:8 |
| 19 | Purple light | N.R. | - |
| 20 | Green light | 80 | 96:4 |
| 21 | White light | 62 | 92:8 |
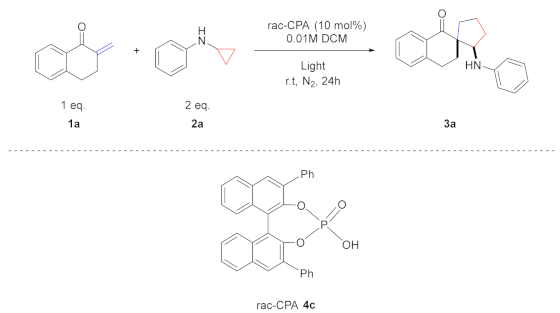 | ||||
|---|---|---|---|---|
| Entry | rac-CPA | Light | Yield (%) [b] | d.r. [c] |
| 1 | 4c | - | N.R | - |
| 2 [d] | 4c | 2 W Blue | N.R | - |
| 3 | - | 2 W Blue | 45 | 79:21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, T.; Lin, X. Diastereoselective Synthesis of 2-Amino-spiro[4.5]decane-6-ones Through Synergistic Photocatalysis and Organocatalysis for [3 + 2] Cycloaddition of Cyclopropylamines with Olefins. Catalysts 2025, 15, 107. https://doi.org/10.3390/catal15020107
Hu T, Lin X. Diastereoselective Synthesis of 2-Amino-spiro[4.5]decane-6-ones Through Synergistic Photocatalysis and Organocatalysis for [3 + 2] Cycloaddition of Cyclopropylamines with Olefins. Catalysts. 2025; 15(2):107. https://doi.org/10.3390/catal15020107
Chicago/Turabian StyleHu, Tianxiao, and Xufeng Lin. 2025. "Diastereoselective Synthesis of 2-Amino-spiro[4.5]decane-6-ones Through Synergistic Photocatalysis and Organocatalysis for [3 + 2] Cycloaddition of Cyclopropylamines with Olefins" Catalysts 15, no. 2: 107. https://doi.org/10.3390/catal15020107
APA StyleHu, T., & Lin, X. (2025). Diastereoselective Synthesis of 2-Amino-spiro[4.5]decane-6-ones Through Synergistic Photocatalysis and Organocatalysis for [3 + 2] Cycloaddition of Cyclopropylamines with Olefins. Catalysts, 15(2), 107. https://doi.org/10.3390/catal15020107







