Influence of Basic/Acidic Treatment on *BEA Zeolite and WO3 Impregnation in Alcohol Dehydration Reactions
Abstract
1. Introduction
2. Results and Discussion
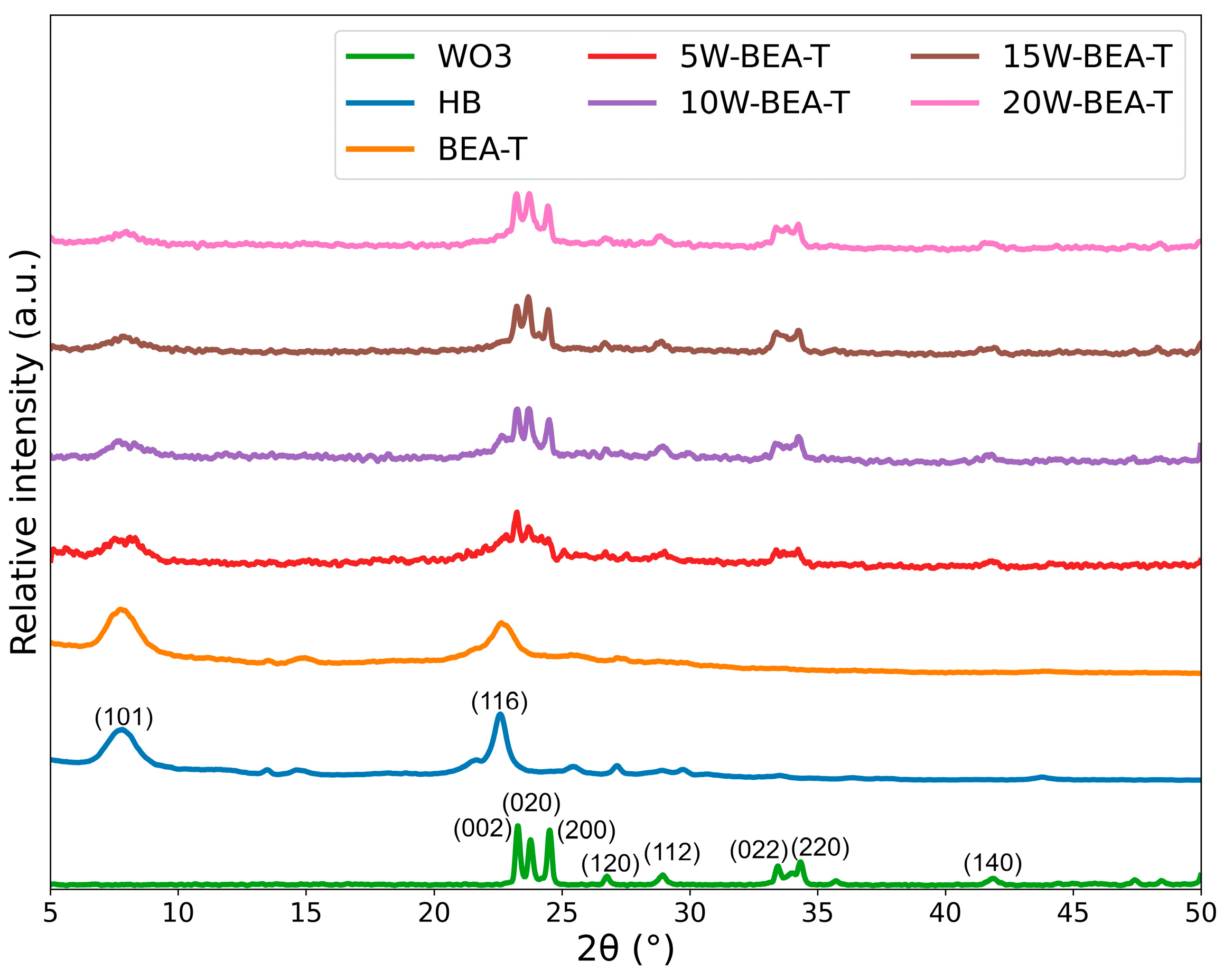
2.1. Elemental and XRD Analyses
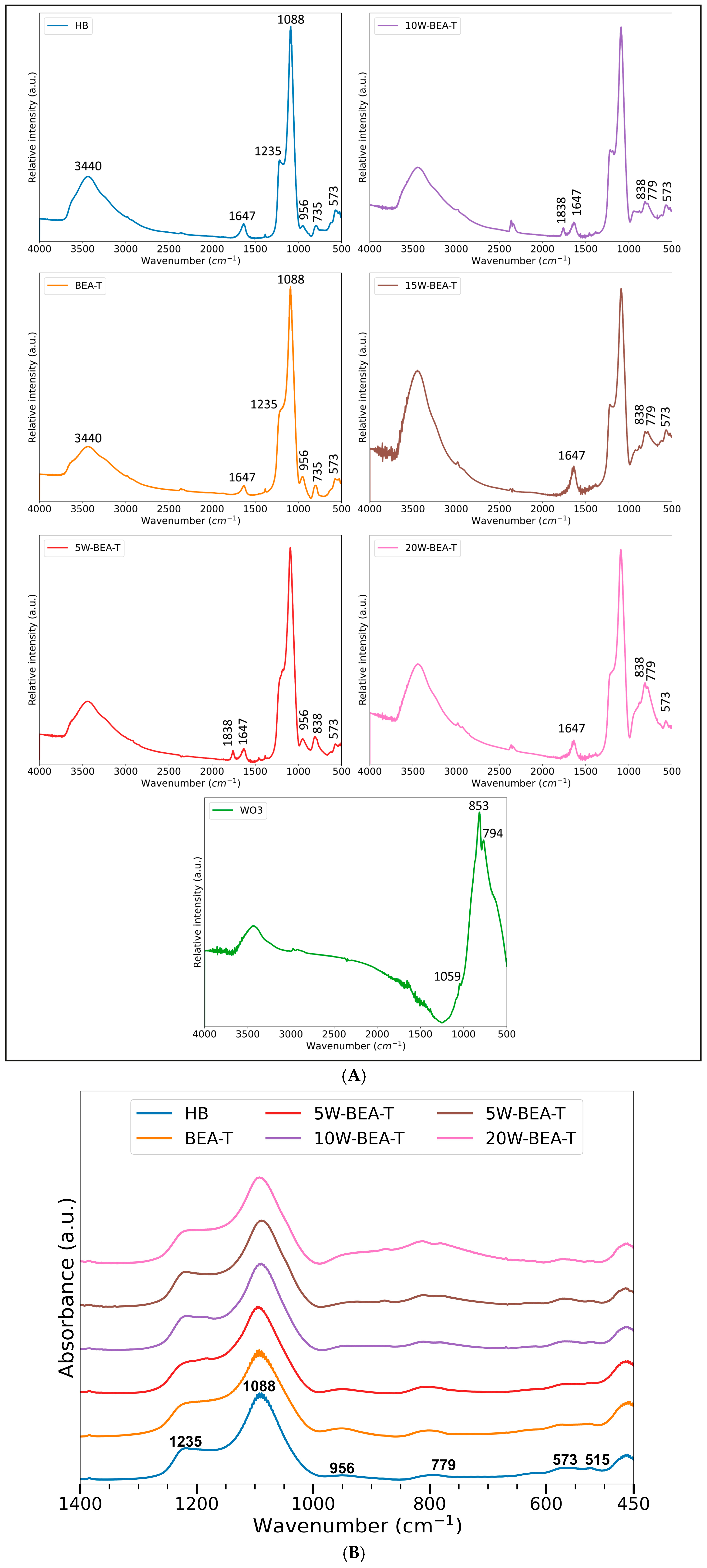
2.2. FT-IR and Raman Spectroscopies
2.3. Main Textural Properties Determined by N2 Adsorption/Desorption Isotherms
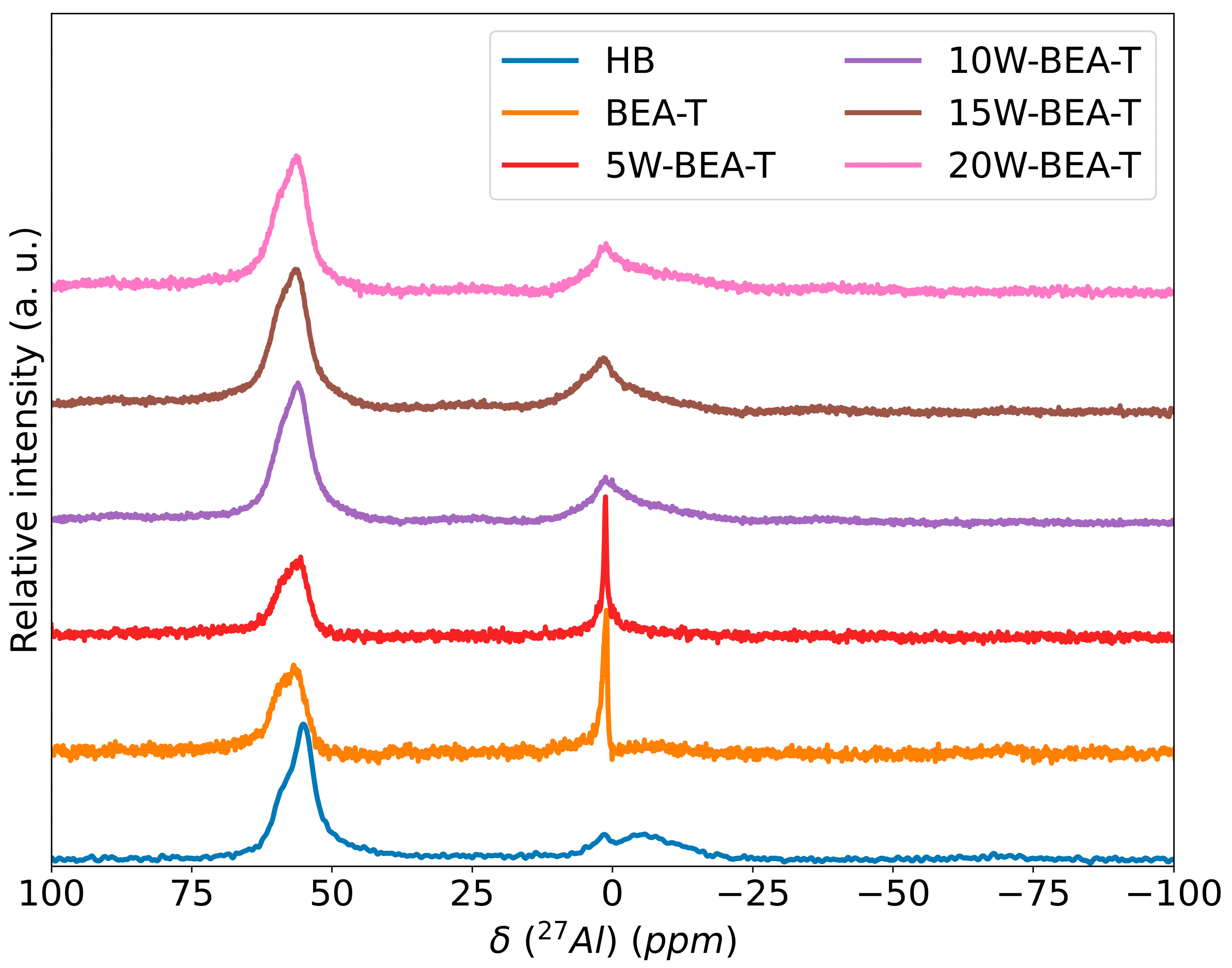
2.4. Solid State (MAS) 27Al and 29Si NMR Spectroscopy
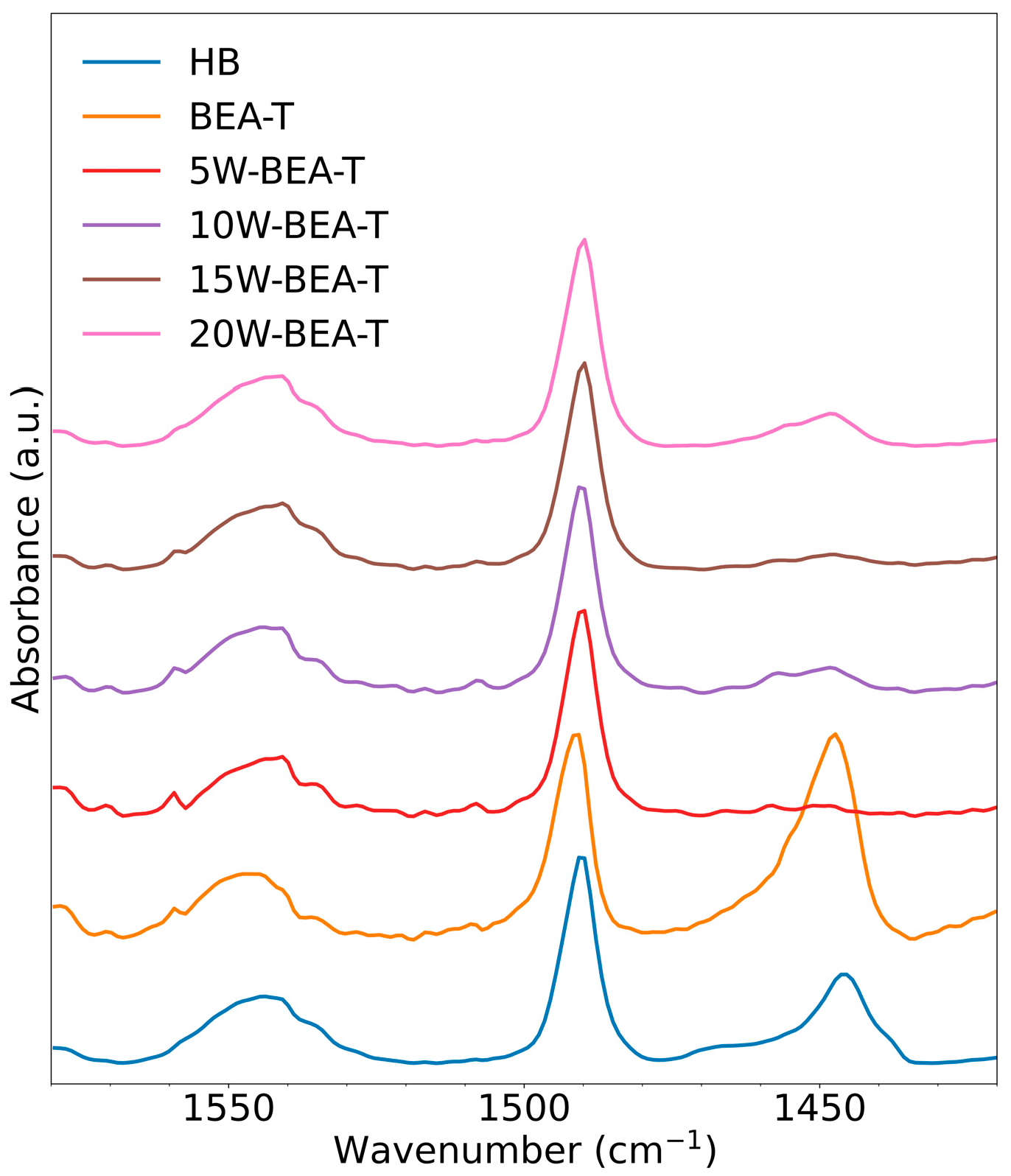
2.5. Acidity of the Catalysts Determined by Pyridine Adsorption
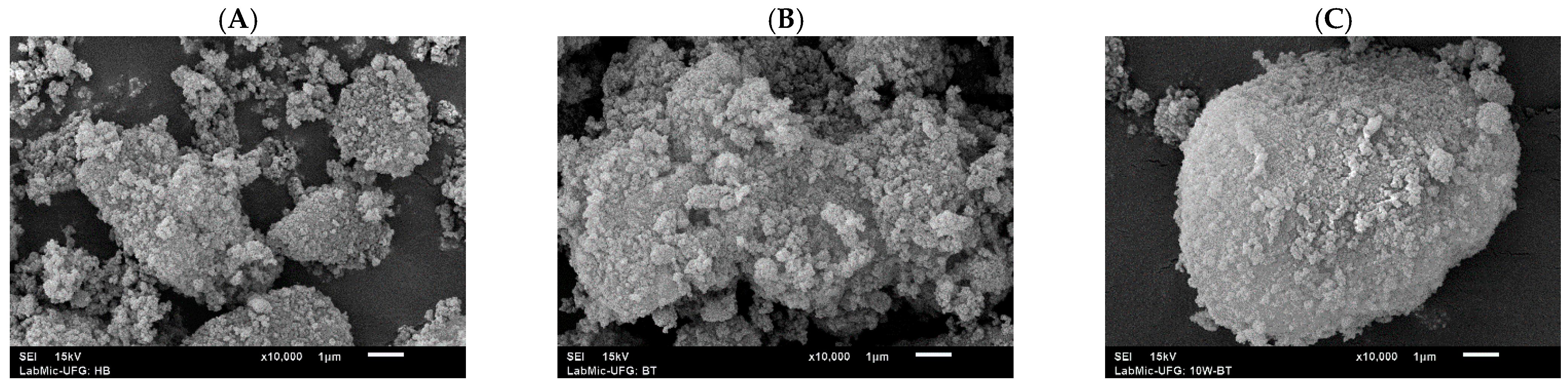
2.6. Analyses by SEM Micrographs
2.7. Ethanol and 1-Propanol Catalytic Dehydrations
3. Materials and Methods
3.1. Hierarchization of *BEA Zeolite and Impregnation of WO3
3.2. Experimental Methods of Characterization
3.2.1. Powder X-Ray Diffraction (XRD)
3.2.2. Elemental Analysis
3.2.3. Fourier-Transform Infrared Spectroscopy (FT-IR)
3.2.4. Raman Spectroscopy
3.2.5. Textural Properties
3.2.6. Microscopy Analysis
3.2.7. Solid-State 27Al and 29Si Magic Angle Spinning Nuclear Magnetic Resonance
3.2.8. Acidity of the Catalysts
3.2.9. Catalytic Dehydration Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Energy Institute. Statistical Review of World Energy, 73rd ed.; Energy Institute: London, UK, 2024. [Google Scholar]
- Alabdullah, M.A.; Shoinkhorova, T.; Dikhtiarenko, A.; Ould-Chikh, S.; Rodriguez-Gomez, A.; Chung, S.; Alahmadi, A.O.; Hita, I.; Pairis, S.; Hazemann, J.; et al. Understanding Catalyst Deactivation during the Direct Cracking of Crude Oil. Catal. Sci. Technol. 2022, 12, 5657–5670. [Google Scholar] [CrossRef]
- Lu, T.; Yan, W.; Xu, R. Chiral Zeolite Beta: Structure, Synthesis, and Application. Inorg. Chem. Front. 2019, 6, 1938–1951. [Google Scholar] [CrossRef]
- Treacy, M.M.J.; Newsam, J.M. Two New Three-Dimensional Twelve-Ring Zeolite Frameworks of Which Zeolite Beta is a Disordered Intergrowth. Nature 1988, 332, 249–251. [Google Scholar] [CrossRef]
- Smith, K.; El-Hiti, G.A. Use of Zeolites for Greener and More Para-Selective Electrophilic Aromatic Substitution Reactions. Green Chem. 2011, 13, 1579–1608. [Google Scholar] [CrossRef]
- Groen, J.C.; Abelló, S.; Villaescusa, L.A.; Pérez-Ramírez, J. Mesoporous Beta Zeolite Obtained by Desilication. Microporous Mesoporous Mater. 2008, 114, 93–102. [Google Scholar] [CrossRef]
- Hartmann, M.; Machoke, A.G.; Schwieger, W. Catalytic Test Reactions for the Evaluation of Hierarchical Zeolites. Chem. Soc. Rev. 2016, 45, 3313–3330. [Google Scholar] [CrossRef] [PubMed]
- Verboekend, D.; Mitchell, S.; Pérez-Ramírez, J. Hierarchical Zeolites Overcome All Obstacles: Next Stop Industrial Implementation. Chimia 2013, 67, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-H.; Sun, M.-H.; Wang, Z.; Yang, W.; Xie, Z.; Su, B.-L. Hierarchically Structured Porous Materials: From Nanoscience to Catalysis, Separation, Optics, Energy, and Life Science. Chem. Rev. 2020, 120, 11194–11294. [Google Scholar] [CrossRef] [PubMed]
- Valadares, D.S.; Carvalho, W.H.R.; Fonseca, A.L.F.; Machado, G.F.; Silva, M.R.; Campos, P.T.A.; Dias, J.A.; Dias, S.C.L. Different Routes for the Hierarchization of *BEA Zeolite, Followed by Impregnation with Niobium and Application in Ethanol and 1-Propanol Dehydration. Catalysts 2025, 15, 340. [Google Scholar] [CrossRef]
- Groen, J.C.; Moulijn, J.A.; Pérez-Ramírez, J. Decoupling mesoporosity formation and acidity modification in ZS-5 zeolites by sequential desilication-dealumination. Microporous Mesoporous Mater. 2005, 87, 153–161. [Google Scholar] [CrossRef]
- Roberg, D.M.; Hausmann, H.; Hölderich, W.F. Dealumination of zeolite bea by acid leaching: A new insight with two-dimensional multi-quantum and cross polarization 27Al MAS NMR. Phys. Chem. Chem. Phys. 2002, 4, 3128–3135. [Google Scholar] [CrossRef]
- Can, F.; Courtois, X.; Duprez, D. Tungsten-Based Catalysts for Environmental Applications. Catalysts 2021, 11, 703. [Google Scholar] [CrossRef]
- Knowles, W.V.; Nutt, M.O.; Wong, M.S. Supported Metal Oxides and the Surface Density Metric. In Catalyst Preparation, Science & Engineering; Regalbuto, J., Ed.; CRC Press: Boca Raton, FL, USA, 2007; Chapter 11; pp. 251–282. [Google Scholar]
- Tohdee, K.; Semmas, S.; Nonthawong, J.; Praserthdam, P.; Pungpo, P.; Jongsomjit, B. Characteristics and Catalytic Properties of WO3 Supported on Zeolite A-Derived from Fly Ash of Sugarcane Bagasse via Esterification of Ethanol and Lactic Acid. S. Afr. J. Chem. Eng. 2024, 49, 273–284. [Google Scholar] [CrossRef]
- Said, S.; Riad, M. Catalysts for the Non-Oxidative Dehydrogenation of Ethanol Supported by WO3-Based Micro-Mesoporous Composites. J. Phys. Chem. Solids 2025, 205, 112789. [Google Scholar] [CrossRef]
- Valadares, D.S.; Clemente, M.C.H.; Freitas, E.; Martins, G.A.V.; Dias, J.A.; Dias, S.C.L. Niobium on BEA Dealuminated Zeolite for High Selectivity Dehydration Reactions of Ethanol and Xylose into Diethyl Ether and Furfural. Nanomaterials 2020, 10, 1269. [Google Scholar] [CrossRef]
- Lima, J.P.V.; Campos, P.T.A.; Paiva, M.F.; Linares, J.J.; Dias, S.C.L.; Dias, J.A. Dehydration of Fructose to 5-Hydroxymethylfurfural: Effects of Acidity and Porosity of Different Catalysts in the Conversion, Selectivity, and Yield. Chemistry 2021, 3, 1189–1202. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, S.; Gao, H.; Wen, D. Metal-Modified Zeolites for Catalytic Dehydration of Bioethanol to Ethylene: Mechanisms, Preparation, and Performance. Catalysts 2025, 15, 791. [Google Scholar] [CrossRef]
- Han, X.; Xiao, Z.; Guo, X.; Zhang, C.; Wang, J.; Feng, M.; Zou, J.; Li, G.; Wang, D. Advancements in Catalyst Design and Reactor Engineering for Efficient Olefin Synthesis from Alcohol (C2+) Dehydration. Chem. Eng. Sci. 2026, 321, 122719. [Google Scholar] [CrossRef]
- Resende, M.A.; Clemente, M.C.H.; Martins, G.A.V.; Da Silva, L.C.C.; Fantini, M.C.A.; Dias, S.C.L.; Dias, J.A. Silver Salts of 12-Tungstophosphoric Acid Supported on SBA-15: Effect of Enhanced Specific Surface Area on Ethanol Dehydration. Res. Chem. Intermed. 2025, 51, 111–124. [Google Scholar] [CrossRef]
- Kerstens, D.; Smeyers, B.; Waeyenberg, J.V.; Zhang, Q.; Yu, J.; Sels, B.F. State of the Art and Perspectives of Hierarchical Zeolites: Practical Overview of Synthesis Methods and Use in Catalysis. Adv. Mater. 2020, 32, 2004690. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Xiao, P.; Bekhti, S.; Kunkes, E.; Iemhoff, A.; Bottke, N.; Vos, D.E.; Meng, X.; Xiao, F.; et al. Needle-like Hierarchical Beta Zeolite Synthesis via Postsynthetic Morphology Control in the Presence of Cetyltrimethylammonium Bromide for Catalytic Dehydration of Sorbitol. ACS Appl. Nano Mater. 2025, 8, 1042–1053. [Google Scholar] [CrossRef]
- Ma, Y.; Rigolet, S.; Michelin, L.; Paillaud, J.; Mintova, S.; Khoerunnisa, F.T.; Daou, J.; Ng, E. Facile and Fast Determination of Si/Al Ratio of Zeolites Using FTIR Spectroscopy Technique. Microporous Mesoporous Mater. 2021, 311, 110683. [Google Scholar] [CrossRef]
- Mihailova, B.; Valtchev, V.; Mintova, S.; Faust, A.-C.; Petkova, N.; Bein, T. Interlayer stacking disorder in zeolite beta family: A Raman spectroscopic study. Phys. Chem. Chem. Phys. 2005, 7, 2756–2763. [Google Scholar] [CrossRef]
- Su, C.-Y.; Lin, H.-C.; Lin, C.-K. Fabrication and optical properties of Ti-doped W18O49 nanorods using a modified plasma-arc gas-condensation technique. J. Vac. Sci. Technol. B 2009, 27, 2170–2174. [Google Scholar] [CrossRef]
- Oulhakem, O.; Rezki, B. In Situ Growth of Tungstite (WO3·H2O) on Microporous and Mesoporous Silicas for Enhanced Oxygen-Evolving Photocatalysis. J. Phys. Chem. C 2025, 129, 10518–10530. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Shestakova, P.; Martineau, C.; Mavrodinova, V.; Popova, M. Solid State NMR Characterization of Zeolite Beta Based Drug Formulations Containing Ag and Sulfadiazine. RSC Adv. 2015, 5, 81957–81964. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, S.; Hu, M.Y.; Bao, X.; Peden, C.H.F.; Hu, J. Investigation of Aluminum Site Changes of Dehydrated Zeolite H-Beta during a Rehydration Process by High-Field Solid-State NMR. J. Phys. Chem. C 2015, 119, 1410–1417. [Google Scholar] [CrossRef]
- Choi, H.; Lee, E.; Jin, M.; Park, Y.K.; Kim, J.M.; Jeon, J.K. Catalytic Properties of Highly Ordered Crystalline Nanoporous Tungsten Oxide in Butanol Dehydration. J. Nanosci. Nanotechnol. 2014, 14, 8828–8833. [Google Scholar] [CrossRef][Green Version]
- Yamamoto, S.; Sugiyama, S.; Matsuoka, O.; Kohmura, K.; Honda, T.; Banno, Y.; Nozoye, H. Dissolution of Zeolite in Acidic and Alkaline Aqueous Solutions as Revealed by AFM Imaging. J. Phys. Chem. 1996, 100, 18474–18482. [Google Scholar] [CrossRef]
- dos Santos, G.M.; Paiva, M.F.; de França, J.O.C.; Dias, S.C.L.; Dias, J.A. Synthesis and Properties of *BEA Zeolite Modified with Iron(III) Oxide. Inorganics 2025, 13, 383. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Catalysis and Sustainable (Green) Chemistry. Catal. Today 2003, 77, 287–297. [Google Scholar] [CrossRef]
- Phung, T.K.; Pham, T.L.M.; Vu, K.B.; Busca, G. (Bio)Propylene production processes: A critical review. J. Environ. Chem. Eng. 2021, 9, 105673. [Google Scholar] [CrossRef]
- Motte, J.; Nachtergaele, P.; Mahmoud, M.; Vleeming, H.; Thybaut, J.W.; Poissonnier, J.; Dewulf, J. Developing Circularity, Renewability and Efficiency Indicators for Sustainable Resource Management: Propanol Production as a Showcase. J. Clean. Prod. 2022, 379, 134843. [Google Scholar] [CrossRef]
- Kim, S.; Lee, M.S.; Camaioni, D.M.; Gutiérrez, O.Y.; Glezakou, V.A.; Govind, N.; Huthwelker, T.; Zhao, R.; Rousseau, R.; Fulton, J.L.; et al. Self-Organization of 1-Propanol at H-ZSM-5 Brønsted Acid Sites. JACS Au 2023, 3, 2487–2497. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Y.; Shi, H.; Mu, L.; Liu, Y.; Mei, D.; Camaioni, D.M.; Lercher, J.A. Dehydration Pathways of 1-Propanol on HZSM-5 in the Presence and Absence of Water. J. Am. Chem. Soc. 2015, 137, 15781–15794. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.E.; Amsler, J.; Spiske, L.; Plessow, P.N.; Asare, T.; Carravetta, M.; Raja, R.; Cox, P.A.; Studt, F.; Armstrong, L.M. Combining Theoretical and Experimental Methods to Probe Confinement within Microporous Solid Acid Catalysts for Alcohol Dehydration. ACS Catal. 2023, 13, 5955–5968. [Google Scholar] [CrossRef]







| Catalyst | Si/Al Ratio | WO3 (%) | C (%) |
|---|---|---|---|
| HB | 13 | 0 | 100 |
| BEA-T | 39 | 0 | 85 |
| 5W-BEA-T | 41 | 4.9 | 83 |
| 10W-BEA-T | 42 | 10.2 | 80 |
| 15W-BEA-T | 42 | 14.6 | 74 |
| 20W-BEA-T | 43 | 19.4 | 70 |
| Catalyst | SBET a (m2/g) | SMicro b (m2/g) | SMeso c (m2/g) | Vp d (cm3/g) | VMicro e (cm3/g) | VMeso f (cm3/g) |
|---|---|---|---|---|---|---|
| HB | 661 | 410 | 251 | 0.90 | 0.17 | 0.75 |
| BEA-T | 616 | 347 | 269 | 1.01 | 0.14 | 0.89 |
| WO3 | 12.4 | 0 | 12.4 | 0.03 | 0.002 | 0.028 |
| 5W-BEA-T | 373 | 191 | 182 | 0.96 | 0.08 | 0.88 |
| 10W-BEA-T | 346 | 125 | 221 | 0.56 | 0.06 | 0.50 |
| 15W-BEA-T | 277 | 80 | 197 | 0.54 | 0.02 | 0.52 |
| 20W-BEA-T | 240 | 107 | 134 | 0.55 | 0.04 | 0.51 |
| Catalyst | Al-Td | Al-Oh | Si-Q4 | Si-Q3 |
|---|---|---|---|---|
| HB | 62 | 38 | 80 | 20 |
| BEA-T | 76 | 24 | 74 | 26 |
| 5W-BEA-T | 76 | 24 | 76 | 24 |
| 10W-BEA-T | 71 | 29 | 71 | 29 |
| 15W-BEA-T | 70 | 30 | 72 | 28 |
| 20W-BEA-T | 71 | 29 | 73 | 27 |
| Catalyst | NPy (mmol/g) |
|---|---|
| HB | 0.62 |
| BEA-T | 0.55 |
| 5W-BEA-T | 0.52 |
| 10W-BEA-T | 0.49 |
| 15W-BEA-T | 0.50 |
| 20W-BEA-T | 0.48 |
| Code | Description |
|---|---|
| HB | Protonic *BEA zeolite |
| BEA-T | HB treated with NaOH and HCl |
| 5W-BEA-T | HB treated with NaOH and HCl and impregnated with 5 wt.% of WO3 |
| 10W-BEA-T | HB treated with NaOH and HCl and impregnated with 10 wt.% of WO3 |
| 15W-BEA-T | HB treated with NaOH and HCl and impregnated with 15 wt.% of WO3 |
| 20W-BEA-T | HB treated with NaOH and HCl and impregnated with 20 wt.% of WO3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valadares, D.d.S.; Fernandes, R.C.; de Carvalho, W.H.R.; Dias, J.A.; Dias, S.C.L. Influence of Basic/Acidic Treatment on *BEA Zeolite and WO3 Impregnation in Alcohol Dehydration Reactions. Catalysts 2025, 15, 1170. https://doi.org/10.3390/catal15121170
Valadares DdS, Fernandes RC, de Carvalho WHR, Dias JA, Dias SCL. Influence of Basic/Acidic Treatment on *BEA Zeolite and WO3 Impregnation in Alcohol Dehydration Reactions. Catalysts. 2025; 15(12):1170. https://doi.org/10.3390/catal15121170
Chicago/Turabian StyleValadares, Deborah da Silva, Roberto Chaves Fernandes, Willian Henrique Ribeiro de Carvalho, José Alves Dias, and Sílvia Cláudia Loureiro Dias. 2025. "Influence of Basic/Acidic Treatment on *BEA Zeolite and WO3 Impregnation in Alcohol Dehydration Reactions" Catalysts 15, no. 12: 1170. https://doi.org/10.3390/catal15121170
APA StyleValadares, D. d. S., Fernandes, R. C., de Carvalho, W. H. R., Dias, J. A., & Dias, S. C. L. (2025). Influence of Basic/Acidic Treatment on *BEA Zeolite and WO3 Impregnation in Alcohol Dehydration Reactions. Catalysts, 15(12), 1170. https://doi.org/10.3390/catal15121170






