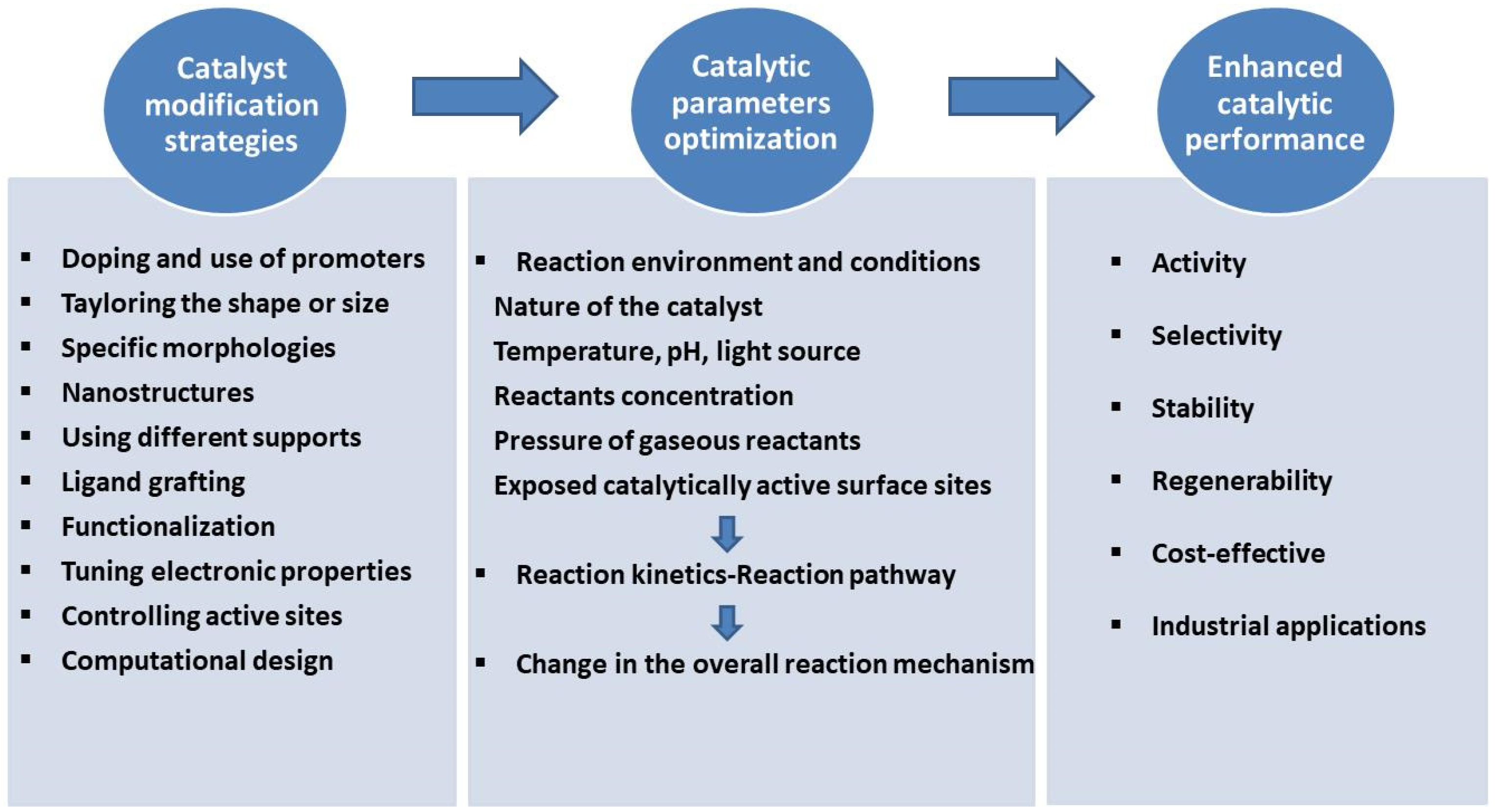
One of the major challenges in catalysis is developing catalysts that demonstrate superior catalytic activity, selectivity, and durability. The complex structural and compositional aspects of the catalytic system demand a comprehensive, systematic approach to uncover the properties of the catalyst’s active sites [
1].
A thorough understanding of the composition, structure, and nature of active sites offers the opportunity to tailor catalytic properties to enhance catalytic performance, which encompasses both activity and selectivity. The properties that can be targeted through synthesis include controlled morphology [
2], sintering resistance [
3], a large specific surface area, optimal acid-base properties [
4], lattice vacancies, interactions between metal and support [
5], catalyst design, and conductivity, among others [
6,
7,
8]. Consequently, alterations in catalyst characteristics result in adjusted kinetic parameters (such as reaction rate and activation energy) [
9,
10] and influence the reaction mechanism, as schematically represented below (
Scheme 1).
Enhancing catalytic efficiency through catalyst modification is vital in today’s global landscape, as it mitigates environmental harm and promotes the sustainable utilization of natural resources. This issue aims to showcase cutting-edge strategies for catalyst modification, with the intention of making a significant contribution to the advancement of research in this field from both practical and theoretical perspectives.
In continuation of the previously released edition [
11], the objective of this Special Issue, named “Effect of the Modification of Catalysts on the Catalytic Performance, 2nd Edition”, was to assemble a collection of papers that showcase novel methods for modifying catalysts, which support the formation of relationships between composition and performance, in addition to structure and performance. In the following sections, we summarize some conclusions derived from the articles published in this volume.
The process of eliminating pollutants from wastewater utilizing semiconductor photocatalysts in the presence of either artificial or natural light is an effective method. TiO
2 stands out among the photocatalytic semiconductors due to its extensive study, attributed to its remarkable activity, durability, and non-harmful characteristics. To increase the efficiency and widen the application range of TiO
2 in photocatalytic reactions, a variety of synthesis strategies have been adopted. Earlier research has shown that the incorporation of activated carbon (AC) and metals into TiO
2 enhances its photocatalytic efficiency [
12]. In Contribution 1, for the synthesis of mesoporous TiO
2, Negoescu et al. utilized a range of surfactants, which included a nonionic surfactant (polyethylene glycol hexadecyl ether—Brij 58), an anionic surfactant (sodium dodecyl sulfate—SDS), and a cationic surfactant (cetyltrimethylammonium bromide—CTAB). Additionally, Brij 58 was combined with activated carbon (AC). The photocatalysts were produced by modifying these supports with Ag, Fe, and AgFe, with each metal comprising approximately 1% by mass. To assess the potential use of the prepared catalysts, photodegradation experiments involving certain textile dyes were carried out. The model dyes selected were Congo Red (CR) and Crystal Violet (CV). The correlation between the structure, morphology, texture, composition, optical properties, and photo-catalytic performance in the degradation of CV and CR dyes from water was linked to the effects of precursors, surfactants, activated carbon, and metals. Also, the proposed reaction mechanisms were discussed. The influence of Ag or Fe association via immobilization on mesoporous TiO
2 or TiO
2–AC composites has been explored. This comparative study regarding the effects of anionic, cationic, and nonionic surfactants on the morphological, structural, and textural characteristics provides novel information for the synthesis of mesoporous titanium dioxide.
Primary amines are considered crucial compounds in the fields of agriculture, pharmaceuticals, textiles, and personal care. 1,3-cyclohexanediamine (1,3-CHDA) is commonly employed as an important intermediate in the chemical industry. The development of a viable industrial process for the synthesis of 1,3-CHDA is discussed in Contribution 2. Motivated by their earlier research [
13], Sun et al. outline three synthesis strategies for the production of 1,3-CHDA by varying the substrate and amine reagents. These strategies include the one-pot reductive amination of resorcinol (RES) with ammonia and molecular hydrogen, the reductive amination of 1,3-cyclohexandione (1,3-CHD), and the oximation–hydrogenation of 1,3-CHD. They established and optimized the reaction conditions for each pathway to achieve the highest yield of 1,3-CHDA, and finally developed a competitive route for the synthesis of 1,3-CHDA. RES was chosen as the starting material, and through hydrogenation, 1,3-CHD was produced. Following this, oximation–hydrogenation was performed to yield the final product, 1,3-CHDA, achieving a total yield of 75.2%. By optimizing the raw materials and reaction conditions, they successfully created an effective methodology for the synthesis of 1,3-CHDA.
As the most fundamental hydrocarbon, methane (CH
4) demonstrates significant versatility, leading to its use in a diverse range of essential applications [
14]. The oxidative coupling of methane (OCM) represents a promising method for the direct conversion of methane into C
2 species under oxidative conditions. Among the various materials proposed as effective OCM catalysts, catalyst-based Pt-OCM at short contact time shows the potential to directly produce acetylene (C
2H
2) during the operation of an auto-thermal reactor; the exothermic reactions facilitated by platinum allow for the reaction to sustain itself following a brief ignition phase, without requiring additional energy input. As a result, Pt-OCM is fundamentally appealing from an economic perspective; however, achieving high catalyst durability and product selectivity poses significant challenges. Given that precious metals like platinum are costly and increasingly limited, in Contribution 3, Schardt et al. explore the impact of dopants on Pt-OCM, aiming to sustain high methane conversion and to enhance C
2 product formation—specifically targeting elevated C
2H
2 selectivity—while minimizing the use of noble metals. To achieve this, Pt-X (X = Ni, Sn, and V
2O
5) catalysts are synthesized in the weight ratios 3:1 and 1:3, with a total loading of 1 wt.% active material. Following the washcoating of the acquired powder catalysts onto monolithic cordierite substrates, the catalyst samples underwent evaluation for their OCM performance. The monometallic catalysts, specifically 1 wt.% and 0.5 wt.% Pt/Al
2O
3, are utilized as reference points. The objective is to clarify how the composition of the catalyst influences the selectivity of different products and to comprehend the role of dopants in these bimetallic systems, ensuring that the total metal loading of the catalyst remains unchanged. To conclude, their investigation demonstrates that the introduction of dopants can enhance the overall C
2 selectivity of platinum-based catalytic systems during OCM while preserving high levels of methane conversion. The use of bimetallic systems permits a reduction in platinum content by up to seventy-five percent, and their research supports the movement towards replacing expensive and scarce noble metals with non-noble metals in heterogeneous catalyst formulations, thereby reducing dependence on precious metals.
In Contribution 4, Munsif et al. investigated the catalytic combustion of benzene, toluene, ethylbenzene, and xylene (BTEX), utilizing a PdPt/Al
2O
3 catalyst, with hydrogen-ignited methanol serving as the initiator for the complete oxidation of these aromatic volatile organic compounds (VOCs). In addition to previous research [
15], their work investigates innovative methods for the abatement of volatile organic compounds, assessing the effects of different VOCs, a concentration of 0.01% methanol, and 3% hydrogen (H
2) on catalytic combustion. Additionally, it compares the energy consumption associated with conventional electric heating against that of hydrogen-ignited methanol combustion. The experimental results indicate that the addition of hydrogen raises the bed temperature and promotes methanol combustion, resulting in the emergence of a CO
2 peak alongside a temperature increase of approximately 450 °C over time. This elevated temperature is suitable for methanol to enhance the combustion of VOCs and confirms the occurrence of complete combustion at this high temperature. The study revealed that the bimetallic catalyst exhibited superior catalytic activity in methanol catalytic combustion, highlighting its increased efficiency and reactivity in comparison to conventional combustion methods.
The surface platinization of TiO
2, introduced by Kraeutler and Bard [
16], has emerged as a prevalent technique for modifying photocatalysts, attributed to the enhanced activity of platinized TiO
2 (Pt/TiO
2) across various photocatalytic reactions. The characteristics of platinized TiO
2 can vary significantly based on the synthesis conditions. These properties are influenced by several factors, including the characteristics of the substrate (TiO
2), the quantity of Pt deposited, the oxidation state of Pt, the size of Pt particles, their dispersion, and morphology, among others [
17]. In Contribution 5, Vasile et al. analyzed the surface topology of the Pt/TiO
2 photocatalyst. They synthesized three nanostructured Pt/TiO
2 materials characterized by different surface configurations, using novel thermosensitive polymers as structure-directing agents in an aqueous solution at room temperature. The study examined the photocatalytic activity of platinum nanoparticles that are supported on titanium dioxide for hydrogen production under simulated solar irradiation, revealing a correlation between fractal dimensions and catalytic characteristics. The varying distributions and dimensions of the Pt nanoparticles on the TiO
2 surface enhance the H
2 production rate and influence the photoactivity of Pt/TiO
2-3TB, prepared with a molar percentage of N-t-butyl amide (TB) of 3 mol%. These effects are partially associated with the oxidation state and the local environment of Pt on the TiO
2 surface. Fractal analysis characterization indicates that for the Pt/TiO
2-3TB catalyst, the particles create densely packed agglomerations and exhibit a high correlation over short distances. Conversely, the agglomerations of Pt nanoparticles (packets) are spread out over longer distances. This arrangement may lead to a more robust interaction with the TiO
2 surface, facilitating the transfer of photo-generated electrons from the conduction band (CB) of titania to the CB of platinum, thereby enhancing the separation of the photo-generated charge carriers (electrons, e
−, and holes, h
+).
Manganese oxides (MnO
x) are extensively utilized for the elimination of formaldehyde (HCHO), owing to their significant activity, minimal toxicity, and ready availability. The redox method is frequently employed to synthesize manganese oxides, which produce specific MnO
x through the redox reaction involving the manganese source precursor (KMnO
4) and the designated reducing agents (MnSO
4 and Mn(NO
3)
2). In Contribution 6, Zhang et al. demonstrate that modifying the preparation parameters of the redox method significantly influences the crystal morphology, microstructure, redox properties, and the degradation efficiency of the target pollutants associated with the MnO
x catalyst. Following the earlier studies on a MnO
2 catalyst created via a one-step oxidation and reduction process [
18], the authors expanded their research to investigate the impact of pH on the microstructural and surface attributes of MnO
x. They also sought to identify the most favorable pH conditions for the MnO
x catalysts synthesis that demonstrate remarkable catalytic efficiency in HCHO oxidation at room temperature, explaining the mechanisms at play. In addition, this research highlights the significance of pH in determining the properties of MnO
x catalysts, focusing on optimizing conditions to achieve enhanced catalytic performance in HCHO oxidation. The results from XRD, SEM, TEM, and BET analyses indicated that the crystal plane spacing of layered-MnO
2 increased as the pH of the precursor solution rose from 7 to 13. This structural change facilitates the formation of abundant Mn vacancies and active sites, which enhances the adsorption of active oxygen species and improves catalytic activity for the degradation of formaldehyde. Overall, the best catalyst, 13-MnO
2, demonstrates remarkable catalytic performance at room temperature, low concentration, and high flow rates, contributing positively to the design and advancement of compact indoor air purifiers.
For the production of industrial vinyl chloride monomer (VCM), acetylene (C
2H
2) hydrochlorination has been widely employed in countries where acetylene can be economically sourced from coal, using mercuric chloride (HgCl
2) supported on activated carbon as the industrial heterogeneous catalyst. With the issues related to significant mercury loss from industrial units, it is crucial to develop a new, non-mercury heterogeneous catalyst for the C
2H
2 hydrochlorination process. Thus, the development of non-mercury catalysts for C
2H
2 hydrochlorination is concentrated on the engineering of supporting ligands through additive-based modifications and the creation of novel metal complexes [
19]. In Contribution 7, Wang et al. theoretically investigated the ruthenium-based catalyst for its potential in high-performance catalysis for C
2H
2 hydrochlorination. Their research considered the PNP pincer ligands
PNPL1 and
PNPL2 for the generation of RuII-chloride complexes. In contrast to the conventional nucleophilic chloro-metalation of C
2H
2, the findings indicate that the hydrochlorination of C
2H
2 occurs through electrophilic proton-metalation or synergistic addition, particularly when the catalyst’s metal center is electron-rich. This investigation presents a novel viewpoint for the design of ruthenium complex catalysts that exhibit enhanced potential activity, focusing not on altering concentration to influence chemical equilibrium, but rather on developing alternative reaction mechanisms that provide greater energy efficiency.
With the rapid evolution of nanotechnology and characterization techniques, materials science is moving into a new era characterized by atomic-scale design [
20]. Defect engineering, the practice of deliberately introducing defects such as vacancies, dopants, and grain boundaries, is reshaping the essential paradigms in catalysis, optoelectronic devices, and energy storage. As a primary strategy for modulating electronic structures, its fundamental nature is rooted in exploiting defect-induced local atomic rearrangements and electronic state reconstructions to address the limitations posed by ideal crystalline lattices, thereby offering multidimensional degrees of freedom for the development of new material systems. In Contribution 8, Zhang et al. conduct a systematic exploration of the mechanisms involved in defect-mediated regulation of electronic structures, synthesizing recent insights into the defect electronic structure and properties, while establishing a conceptual framework for the rational design of defects within specific electronic architectures. Their article review emphasizes the mechanisms of regulation, characterization, and the impact of defect-engineering strategies on the electronic structure of nanocatalyst surfaces. It offers a thorough analysis of how defect engineering influences electronic structures and provides a comprehensive summary of research strategies, including defect characterization, electronic structure characterization, theoretical calculations, and machine learning. The authors provide a systematic explanation of the coupling mechanisms that exist between defects and electronic properties. They summarize sophisticated characterization techniques and computational tools, while also emphasizing recent advancements in defect-engineered catalysis. The key mechanisms discussed encompass defect-induced energy levels, shifts in the Fermi level, tuning of the coordination environment, lattice distortion, charge trapping, interactions between metal and support, transitions in spin states, ferromagnetic coupling, dynamics of defects, and the engineering of interfaces.
Ozone is extensively utilized in a range of industrial and daily applications, including food sterilization, treatment of organic pollutants, and disinfection of tap water, due to its potent oxidative characteristics. Nevertheless, these uses frequently result in the release of residual ozone, which poses risks to human health and the environment. Among the various techniques for ozone decomposition, the catalytic decomposition method is recognized as the most effective. In Contribution 9, Ma et al. concentrate on the introduction of copper-based materials for ozone decomposition and their optimization strategies [
21], while also addressing the challenges and opportunities currently encountered in the domain of ozone catalytic decomposition. Recent studies on catalysts for ozone decomposition predominantly emphasize powder materials; however, these powder catalysts present certain drawbacks, such as their impracticality in usage, as they frequently necessitate supplementary binders for application, complicating the preparation process. Furthermore, powder catalysts are prone to surface deactivation, which can reduce their catalytic efficiency over time and impact their overall effectiveness. In comparison, monolithic catalyst materials demonstrate advantages in applications due to their improved stability and durability, removing the requirement for binders and facilitating a more convenient and efficient catalyst application process. This review has improved the design of copper-based materials from the viewpoints of morphology, semiconductor properties, and heterostructure, and has developed integrated catalytic devices that exploit the inherent features of copper materials. The investigation covers powder copper-based catalysts (morphology, heterostructure) and monolithic copper-based catalysts, while also presenting the mechanisms of ozone decomposition and the significant factors in these processes. Additionally, the role of these catalysts in ozone wastewater treatment technology was addressed.
In conclusion, the Special Issue entitled “Effect of the Modification of Catalysts on the Catalytic Performance, 2nd Edition” is expected to be of considerable interest to all researchers active in this scientific area, particularly regarding: the synthesis and characterization of different catalysts or catalytic materials; catalytic performance (activity and selectivity); the synergetic effect; the modification of catalysts or appropriate promoters that are utilized to modify the catalyst structure, thereby enhancing stability or improving catalytic reactions for better activity or selectivity; as well as the reaction mechanism and kinetic parameters (such as reaction rate and activation energy).
We extend our sincere appreciation to the MDPI Editorial Team and the Catalysts Journal for granting us the opportunity to serve as Guest Editors, improving the contemporary benchmarks of achievement in this crucial area of catalysis and catalytic materials. Thanks to the excellent collaboration with Section Managing Editors Dr. Patty Ge and Dr. Maeve Yue, as well as the exceptional efforts of the entire editorial team, we successfully published nine articles, which include two reviews. Moreover, we want to thank all the authors of these publications for their outstanding research and contributions to this Special Issue.