The Influence of Defect Engineering on the Electronic Structure of Active Centers on the Catalyst Surface
Abstract
1. Introduction
- Multidimensional regulatory strategies: We systematically dissect how defect engineering modulate the electronic structures of active sites, including electronic density-of-states reconstruction, coordination microenvironment tuning, charge transfer dynamics, and localization effects. Based on this, we summarized the main defect types in nanocatalysts and their regulatory effects on the electronic structure, as shown in Table 1.
- Multiscale characterization techniques: We critically examined advanced techniques used for probing defect-electronic structure–property relationships. These includes defect-specific spectroscopies, electronic structure analyses and computational modeling approaches.
- Performance benchmarks: We highlight representative catalytic systems where defect-mediated electronic tailoring leads to enhanced performance, with a focused on hydrogen evolution reaction (HER), oxygen evolution reaction (OER) and other relevant reaction systems.
2. Mechanisms Underlying Defect Engineering-Mediated Modulation of Electronic Structures at Active Sites
2.1. Electronic Density of States Reconstruction
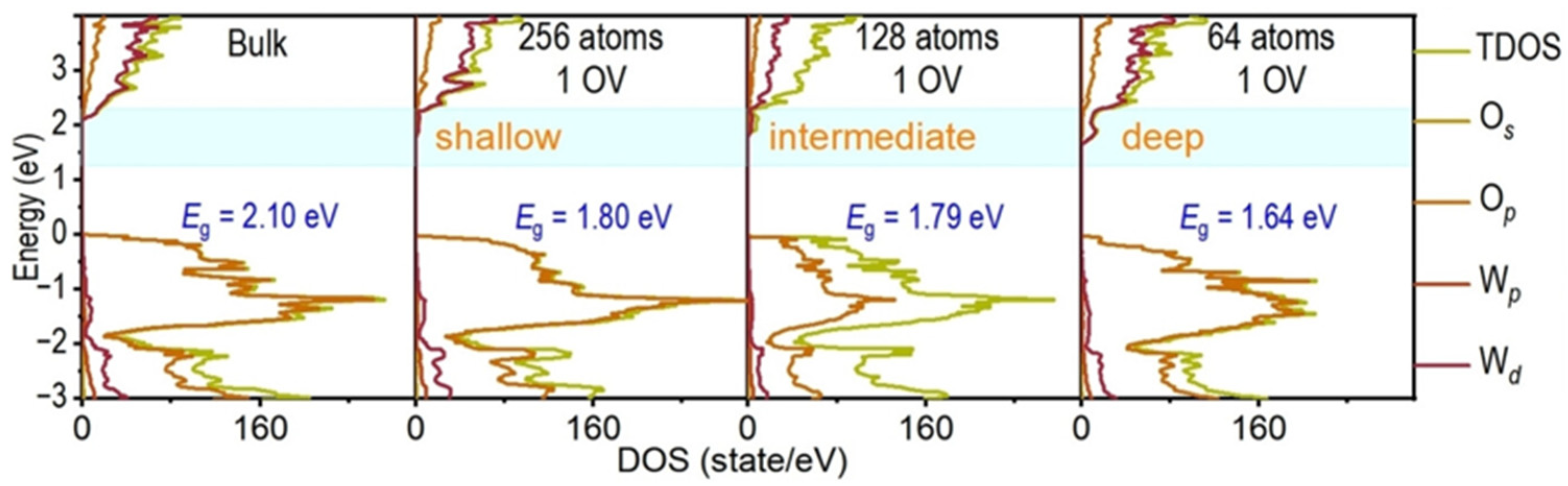
2.1.1. Defect Energy Level Engineering
2.1.2. Fermi Level Shift
2.2. Coordination Environment Engineering
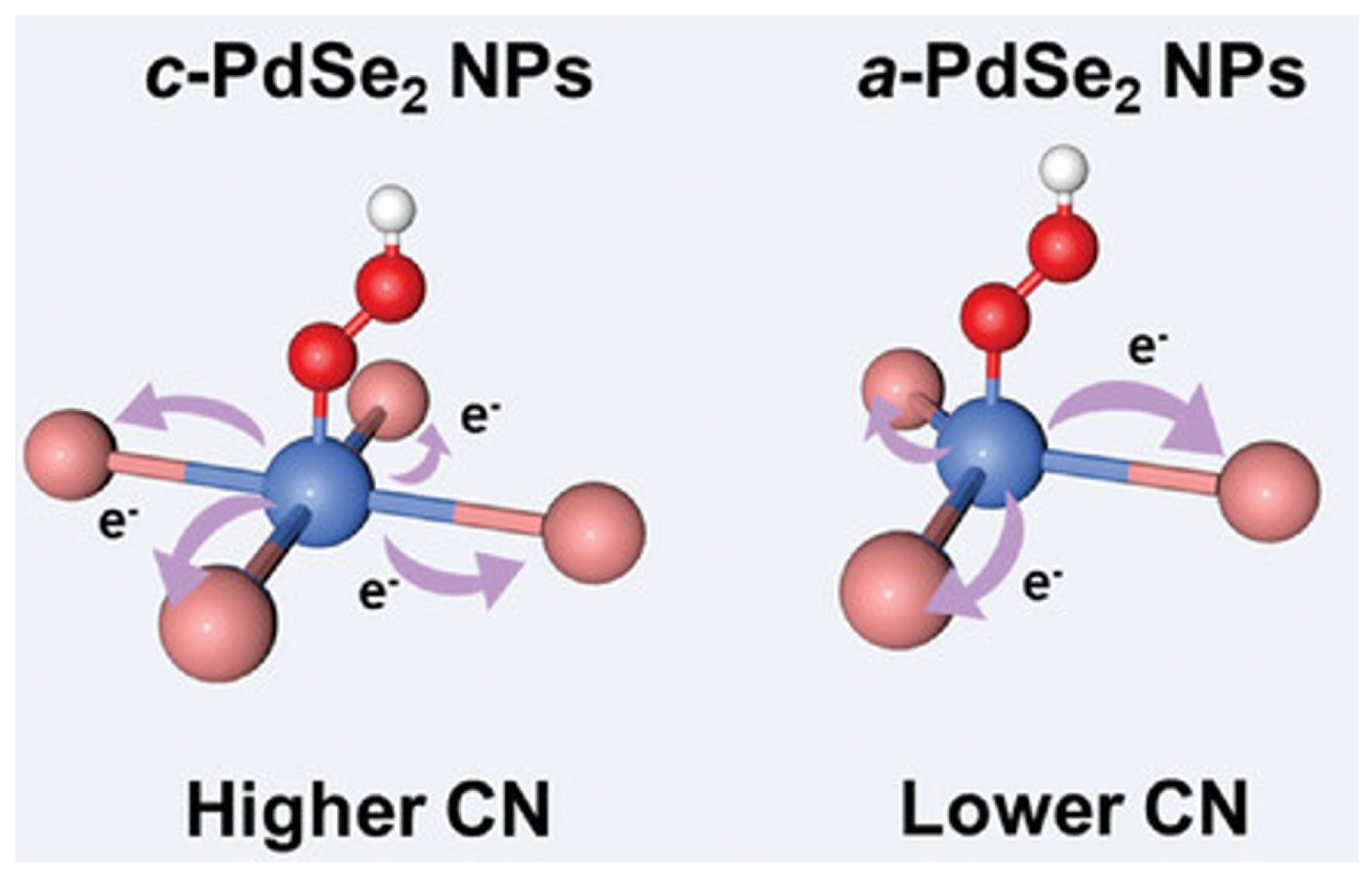
2.2.1. Low-Coordination Environments Induce the Exposure of Catalytically Active Sites
2.2.2. Lattice Distortion Engineering for Electronic Structure Modulation
2.3. Charge Transfer and Localization Effects
2.3.1. Defects as Charge Traps
2.3.2. The Metal-Support Interaction
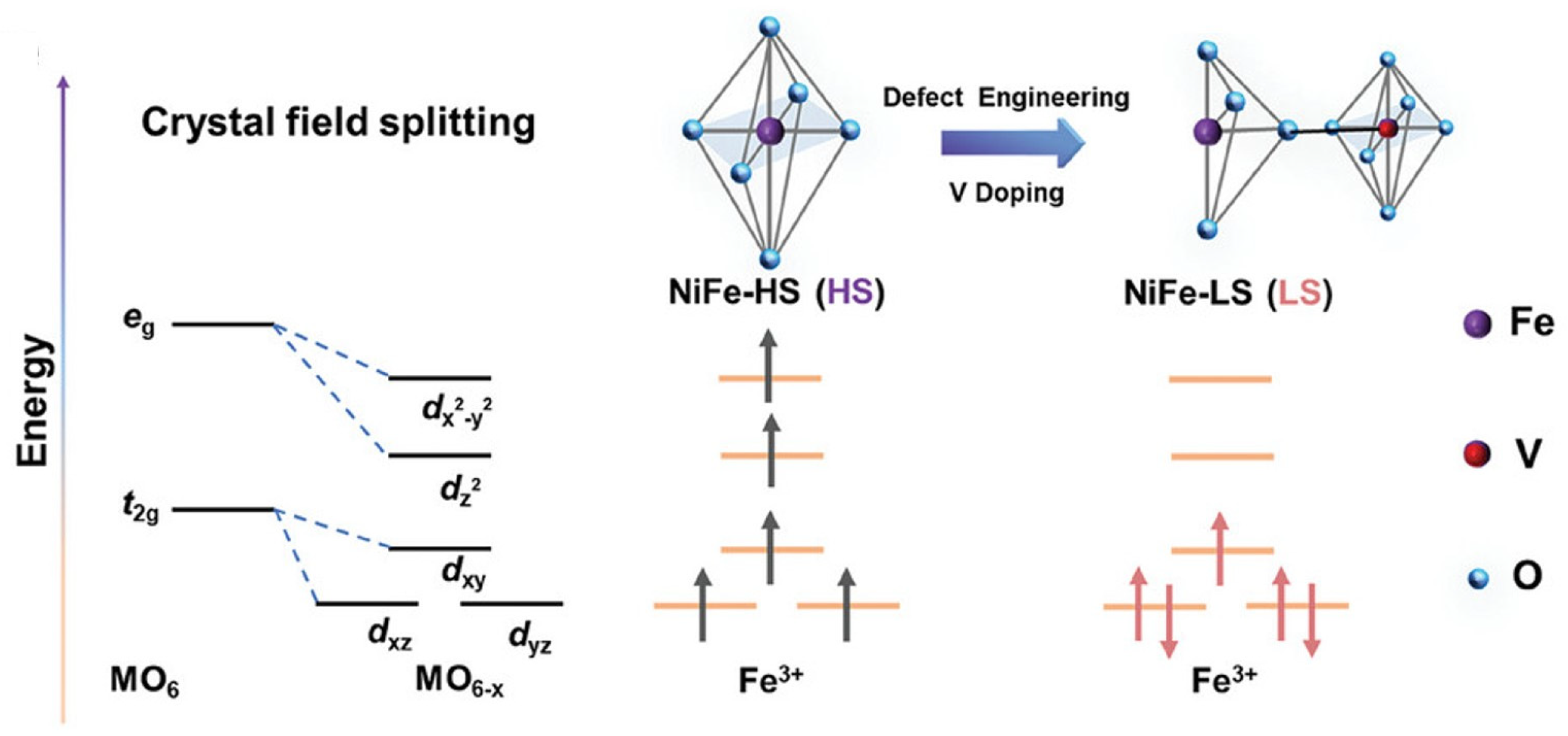
2.4. Modulation of Spin States and Magnetic Coupling
2.4.1. Impact of Spin-State Transitions on Catalytic Reactions
2.4.2. Defect-Engineered Magnetic Synergy: Tailoring Cooperative Spin Interactions via Structural Imperfections
2.5. Dynamic Defect and Interface Engineering
2.5.1. Defect Reconfiguration Under Reaction Conditions
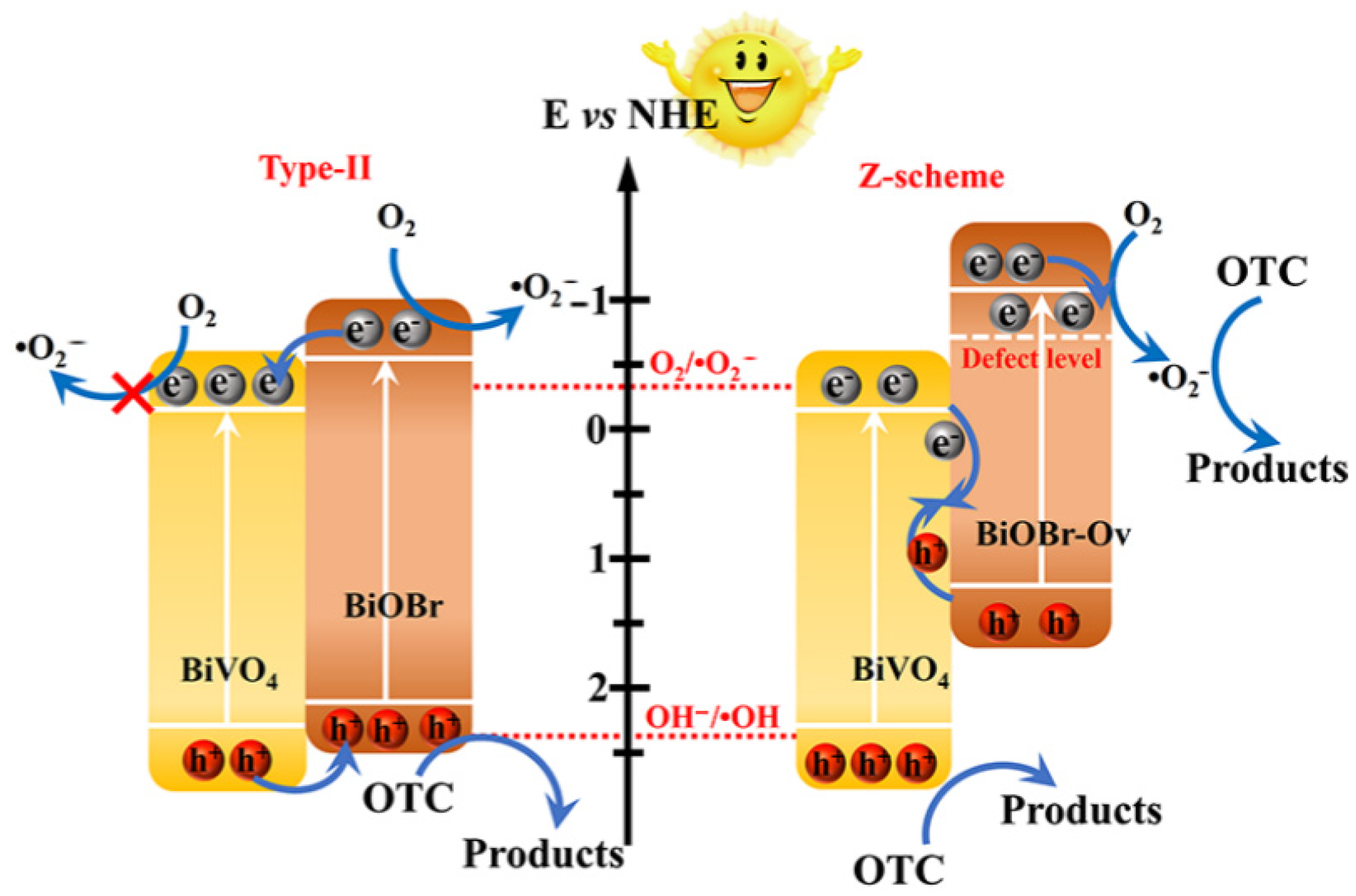
2.5.2. Heterojunction Interface Engineering
3. Multiscale Characterization of Defect–Electronic Structure–Performance Correlations
3.1. Defects Characterization
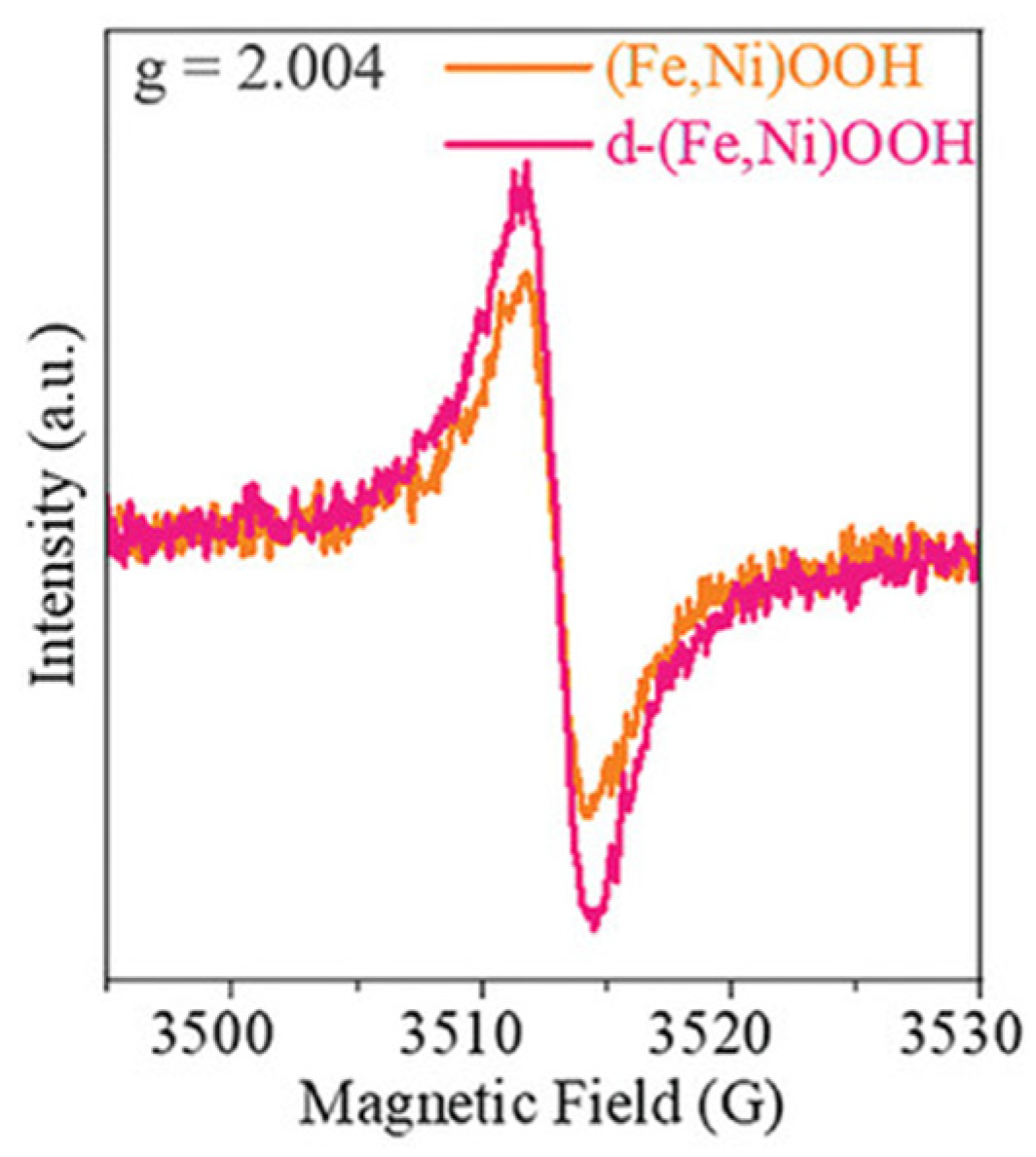
3.1.1. Electron Paramagnetic Resonance (EPR)
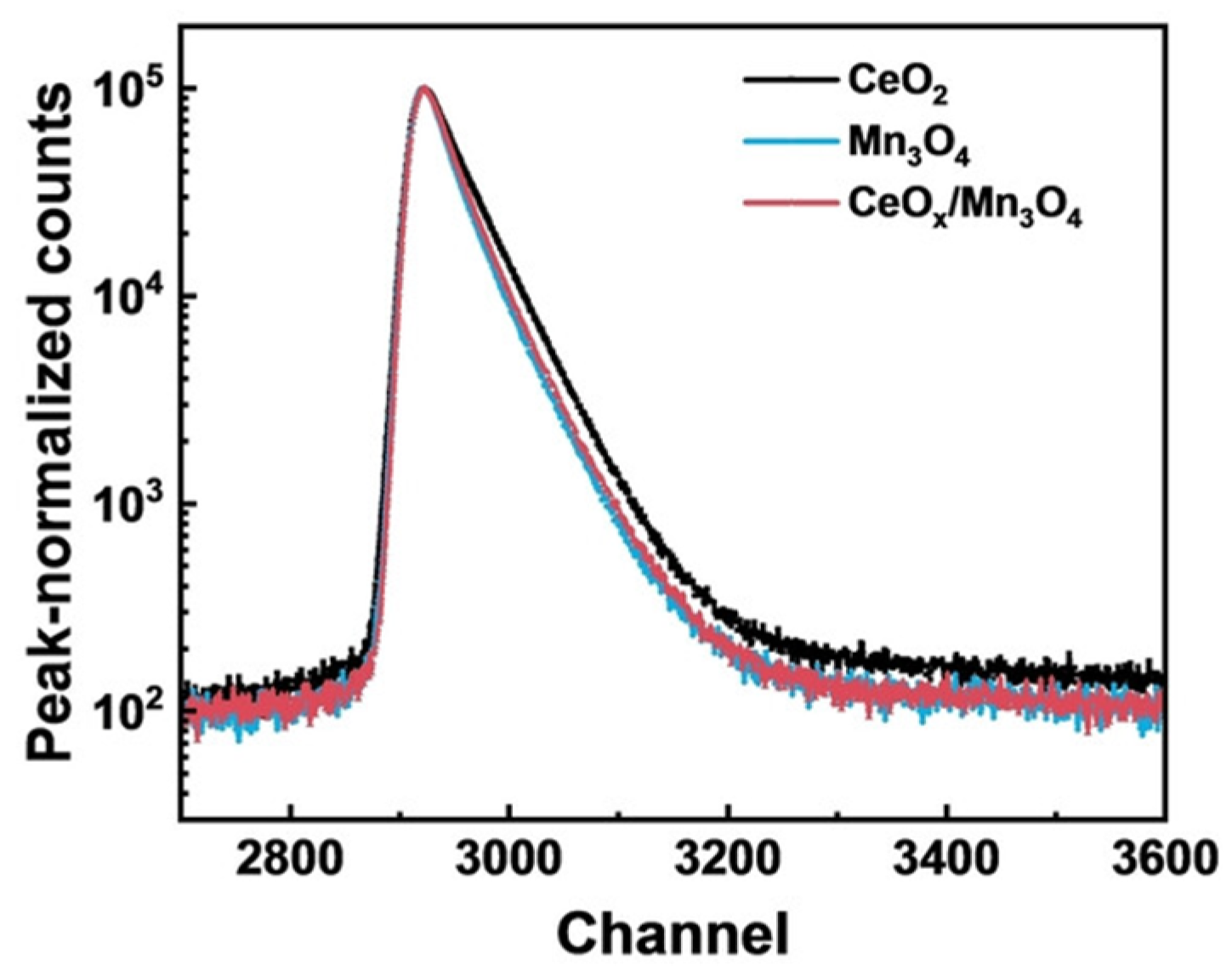
3.1.2. Positron Annihilation Lifetime Spectroscopy (PALS)
3.2. Electronic Structure Characterization
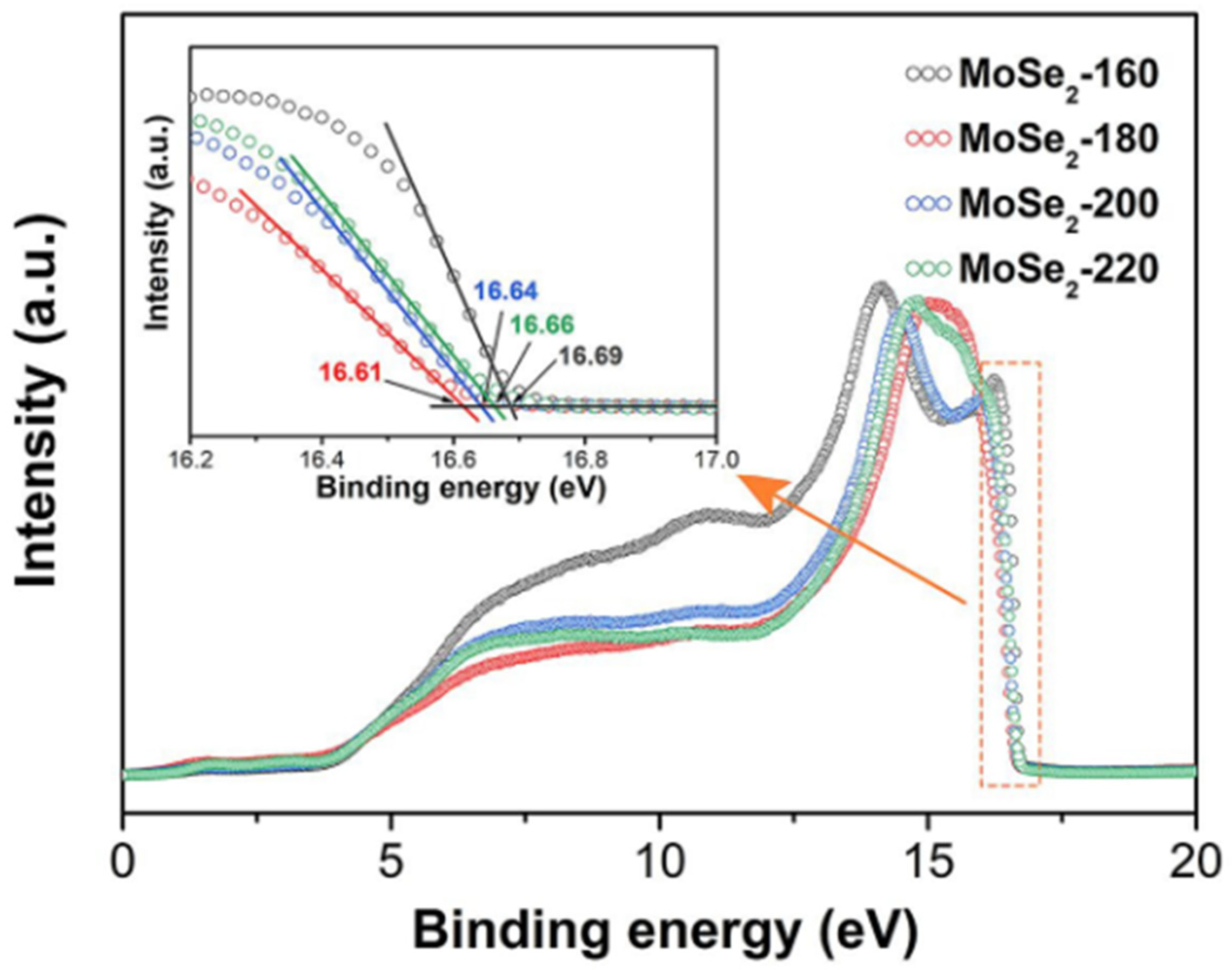
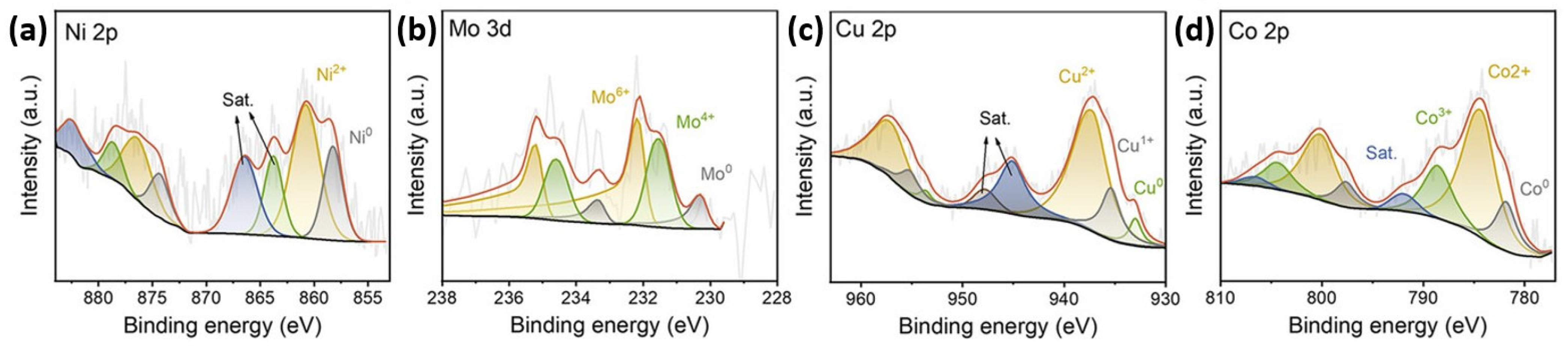
3.2.1. X-Ray Photoelectron Spectroscopy (XPS) and Ultraviolet Photoelectron Spectroscopy (UPS)
3.2.2. X-Ray Fine Structure Analysis (XAFS)
3.2.3. Electron Energy Loss Spectroscopy (EELS)
3.3. Theoretical Calculations
3.3.1. Density Functional Theory (DFT) Calculations
3.3.2. Machine Learning(ML)-Driven Prediction
- Automated Data Extraction: A line-recognition algorithm and thermogravimetric analysis curve evaluation method were developed to automate data extraction and batch calculations, minimizing manual errors and improving efficiency.
- Pure-Phase Subspace Delineation: Given the sensitivity of UiO-66(Ce) synthesis to parameters, ML screened >200,000 experimental protocols to define a tailored synthesis subspace, preventing impurity phases like CSUST-1 or Ce(HCO2)3.
- Closed-Loop Optimization: A hybrid utility function combining probability of achievement and weighted-sum techniques balanced defect density and thermal stability.
4. Defect Engineering-Driven High-Efficiency Catalytic Applications
4.1. Hydrogen Evolution Reaction (HER)
4.2. Oxygen Evolution Reaction (OER)
4.3. Carbon Dioxide Reduction(CO2RR)
4.4. Ammonia Synthesis Catalysis
4.5. Volatile Organic Compounds (VOCs) Degradation
4.6. Other Catalytic Reactions
5. Conclusions and Perspectives
- Precise Structure–Property Relationships: Quantitative models linking defect types (point/line/surface), spatial distribution (monodispersed/clustered), and concentrations to electronic responses are lacking. Breakthroughs require ultrafast spectroscopy (e.g., femtosecond X-ray absorption) combined with machine learning to establish a “defect configuration–electronic response” database.
- Multi-Defect Synergy: Single defects often suffer from limited functionality or instability. Cooperative defects (e.g., oxygen vacancies with metal dopants) can induce interfacial charge redistribution and strain–field coupling. Future work should focus on in situ nanofabrication for spatially ordered assembly of multi-defect systems.
- Dynamic Defect Quantum Control: Current studies emphasize static defects, yet dynamic evolution under operando conditions (e.g., electrochemical-bias-induced vacancy migration) may trigger electronic state reorganization or quantum phase transitions. Atomic-resolution spatiotemporal characterization (e.g., in situ TEM or time-resolved X-ray probes) is essential.
- Sustainable Application: Advancing green synthesis and scalable manufacturing will align defect engineering with global sustainability and carbon neutrality goals.
- AI-Driven Design: Graph neural networks could predict defect-induced electronic perturbations, while automated platforms like droplet microreactors enable tailored synthesis for energy catalysis or optoelectronics.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, J.; Meng, D.; Guo, J.; Liu, X.; Zhou, L.; Wang, Z. Defect Engineering for Enhanced Electrocatalytic Oxygen Reaction on Transition Metal Oxides: The Role of Metal Defects. Adv. Mater. 2024, 36, 2405129. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Sun, Y.; Liu, Y.; Cai, Q.; Zhao, J. Boosting the Hydrogen Evolution Reaction on NiTe Monolayers via Defect Engineering: A Computational Study. Inorg. Chem. Front. 2024, 11, 1279–1288. [Google Scholar] [CrossRef]
- Yang, J.; An, L.; Wang, S.; Zhang, C.; Luo, G.; Chen, Y.; Yang, H.; Wang, D. Defects Engineering of Layered Double Hydroxide-Based Electrocatalyst for Water Splitting. Chin. J. Catal. 2023, 55, 116–136. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, Y.; Yuan, Z.; Kang, N.; Mehdi, S.; Xing, C.; Liu, X.; Fan, Y.; Li, B.; Liu, B. Interfacial Active Sites on Co-Co2C@carbon Heterostructure for Enhanced Catalytic Hydrogen Generation. Rare Met. 2023, 42, 1935–1945. [Google Scholar] [CrossRef]
- Lu, W.; Yan, L.; Ye, W.; Ning, J.; Zhong, Y.; Hu, Y. Defect Engineering of Electrode Materials towards Superior Reaction Kinetics for High-Performance Supercapacitors. J. Mater. Chem. A 2022, 10, 15267–15296. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, T.; Wu, Z.; Shi, T.; Xie, W. Band Structure Engineering in 2D BA2PbI4/InSe Perovskite Heterostructures and Superlattices. Appl. Phys. Lett. 2025, 126, 032104. [Google Scholar] [CrossRef]
- Han, G.; Sun, M.; Zhao, R.; Junaid, Q.M.; Hou, G.; Mohmand, N.Z.K.; Jia, C.; Liu, Y.; Van Der Voort, P.; Shi, L.; et al. Defect Engineered Ti-MOFs and Their Applications. Chem. Soc. Rev. 2025, 54, 5081–5107. [Google Scholar] [CrossRef]
- Deng, Z.; Liu, Y.; Lin, J.; Chen, W. Rational Design and Energy Catalytic Application of High-Loading Single-Atom Catalysts. Rare Met. 2024, 43, 4844–4866. [Google Scholar] [CrossRef]
- Muhammad, P.; Zada, A.; Rashid, J.; Hanif, S.; Gao, Y.; Li, C.; Li, Y.; Fan, K.; Wang, Y. Defect Engineering in Nanocatalysts: From Design and Synthesis to Applications. Adv. Funct. Mater. 2024, 34, 2314686. [Google Scholar] [CrossRef]
- Dou, Y.; He, C.; Zhang, L.; Yin, H.; Al-Mamun, M.; Ma, J.; Zhao, H. Approaching the Activity Limit of CoSe2 for Oxygen Evolution via Fe Doping and Co Vacancy. Nat. Commun. 2020, 11, 1664. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Yuan, Z.; Sun, Y.; Tang, Z.; Gao, X.; Zhang, H.; Li, J.; Wang, S.; Yang, D.; et al. Optimizing Electrocatalytic Nitrogen Reduction via Interfacial Electric Field Modulation: Elevating d-Band Center in WS2–WO3 for Enhanced Intermediate Adsorption. Angew. Chem. 2023, 135, e202303794. [Google Scholar] [CrossRef]
- Ren, B.; Huang, J.; Yuan, Q.; Liu, X.; Li, P.; Zhu, G.; Xu, W.; Dong, B. Tungsten Defect-Induced Surface Reconstruction of Ce2W2O9 Arrays for Enhanced Oxygen Evolution Reaction Performance. Adv. Funct. Mater. 2025, 2501328. [Google Scholar] [CrossRef]
- Xu, F.; He, Y.; Zhang, J.; Liang, G.; Liu, C.; Yu, J. Prolonging Charge Carrier Lifetime via Intraband Defect Levels in S-Scheme Heterojunctions for Artificial Photosynthesis. Angew. Chem. Int. Ed. 2025, 64, e202414672. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Sun, K.; Wan, J.; Ma, Y.-A.; Wang, Y.; Liu, L.; Zhu, B.; Fu, F. Dual-Site Oxygen Activation for Enhanced Photocatalytic Aerobic Oxidation by S-Scheme Ni2P/Bi3O4Br-OVs Heterojunction. Chem. Eng. J. 2023, 452, 139425. [Google Scholar] [CrossRef]
- Ma, X.; Ding, B.; Yang, Z.; Liu, S.; Liu, Z.; Meng, Q.; Chen, H.; Li, J.; Li, Z.; Ma, P.; et al. Sulfur-Vacancy-Engineered Two-Dimensional Cu@SnS2–x Nanosheets Constructed via Heterovalent Substitution for High-Efficiency Piezocatalytic Tumor Therapy. J. Am. Chem. Soc. 2024, 146, 21496–21508. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Huang, X.; Yong, X.; Li, X.; Li, J.; Wang, J.; Wang, N.; Hao, H. N-Doped Synergistic Porous Thin-Walled g-C3N4 Nanotubes for Efficient Tetracycline Photodegradation. Chem. Eng. J. 2023, 455, 140570. [Google Scholar] [CrossRef]
- Lin, H.; Xin, X.; Xu, L.; Li, P.; Chen, D.; Turkevych, V.; Li, Y.; Wang, H.; Xu, J.; Wang, L. Defect-Mediated Fermi Level Modulation Boosting Photo-Activity of Spatially-Ordered S-Scheme Heterojunction. J. Colloid Interface Sci. 2024, 676, 310–322. [Google Scholar] [CrossRef]
- Liu, C.; Mao, S.; Shi, M.; Hong, X.; Wang, D.; Wang, F.; Xia, M.; Chen, Q. Enhanced Photocatalytic Degradation Performance of BiVO4/BiOBr through Combining Fermi Level Alteration and Oxygen Defect Engineering. Chem. Eng. J. 2022, 449, 137757. [Google Scholar] [CrossRef]
- Yu, Z.; Lv, S.; Yao, Q.; Fang, N.; Xu, Y.; Shao, Q.; Pao, C.; Lee, J.; Li, G.; Yang, L.; et al. Low-Coordinated Pd Site within Amorphous Palladium Selenide for Active, Selective, and Stable H2O2 Electrosynthesis. Adv. Mater. 2023, 35, 2208101. [Google Scholar] [CrossRef]
- Zhao, W.; Cui, C.; Xu, Y.; Liu, Q.; Zhang, Y.; Zhang, Z.; Lu, S.; Rong, Z.; Li, X.; Fang, Y.; et al. Triggering Pt Active Sites in Basal Plane of Van Der Waals PtTe2 Materials by Amorphization Engineering for Hydrogen Evolution. Adv. Mater. 2023, 35, 2301593. [Google Scholar] [CrossRef]
- Wang, X.; Gao, R.; Fan, G.; Guo, Y.; Han, C.; Gao, Y.; Shen, A.; Wu, L.; Gu, X. Dual Defects-Induced Iron Single Atoms Immobilized in Metal-Organic Framework-Derived Hollow BiOBr Microtubes for Low-Barrier Photocatalytic Nitrogen Reduction. Angew. Chem. Int. Ed. 2025, 64, e202501297. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Song, L.; Lv, X.; Wang, H.; Zhang, F.; Hao, S.; Wei, R.; Zhang, L.; Han, Q.; Zheng, G. Low-Coordination Triangular Cu3 Motif Steers CO2 Photoreduction to Ethanol. Angew. Chem. Int. Ed. 2025, 64, e202500928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qian, J.; Ran, J.; Xi, P.; Yang, L.; Gao, D. Engineering Lower Coordination Atoms onto NiO/Co3O4 Heterointerfaces for Boosting Oxygen Evolution Reactions. ACS Catal. 2020, 10, 12376–12384. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Li, H.; Kim, M.G.; Duan, Z.; Talat, K.; Lee, J.Y.; Wu, M.; Lee, H. Effectiveness of Strain and Dopants on Breaking the Activity-Stability Trade-off of RuO2 Acidic Oxygen Evolution Electrocatalysts. Nat. Commun. 2025, 16, 1717. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Liu, H.; Feng, Y.; Du, X.; Xie, Z.; Zhou, J.; Liu, Y.; Song, Y.; Wang, F.; et al. Electroreduction-Driven Distorted Nanotwins Activate Pure Cu for Efficient Hydrogen Evolution. Nat. Mater. 2025, 24, 424–432. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, M.; He, X.; Zhao, E.; Liang, H.; Shang, J.; Liu, K.; Chen, J.; Zuo, S.; Zhou, M. Intrinsic Strain of Defect Sites Steering Chlorination Reaction for Water Purification. Nat. Commun. 2025, 16, 2652. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, Y.; Wang, Y.; Jia, Y.; He, J.; Tang, Q.; Liu, L.; Dong, J. Manganese Atom-Induced Lattice Distortion in Fe3O4 to Boost Selective Transfer Hydrogenation of Unsaturated Oxygenates. ACS Catal. 2025, 15, 7505–7515. [Google Scholar] [CrossRef]
- Ma, Z.; Ma, X.; Zhang, L.; Cheng, H.; Shi, F. 1T-WS2/NCN Photocatalyst with Unique Trion Behavior and Cyano-Defects: Photocatalytic Hydrogen Evolution and Mechanistic Insights. Sep. Purif. Technol. 2023, 325, 124743. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, X.; Xu, G.; Biswal, B.K.; Balasubramanian, R. Accumulation of Long-Lived Photogenerated Holes at Indium Single-Atom Catalysts via Two Coordinate Nitrogen Vacancy Defect Engineering for Enhanced Photocatalytic Oxidation. Adv. Mater. 2024, 36, 2309205. [Google Scholar] [CrossRef]
- Su, H.; Yin, H.; Orbell, W.; Li, Y.; Wang, G.; Wang, Y.; Mori, K.; Chen, Z.; Li, H.; Yamashita, H.; et al. Asymmetric Triple-Atom Sites Combined with Oxygen Vacancy for Selective Photocatalytic Conversion of CO2 to Propionic Acid. Angew. Chem. Int. Ed. 2025, 64, e202425446. [Google Scholar] [CrossRef]
- Bai, H.; Yang, Y.; Huang, Y.; Yuan, H.; Dong, M.; Ni, C.; Liu, X. Quantum Confinement and Defect Engineering Mediated “Two Birds with One Stone” Strategy for Co/Bi Self-Regeneration and Effective Photogenerated Carrier Separation. Appl. Catal. B Environ. Energy 2025, 365, 125001. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Z.; Si, Y.; Li, B.; Deng, F.; Yang, L.; Liu, X.; Dai, W.; Luo, S. Gradient Hydrogen Migration Modulated with Self-Adapting S Vacancy in Copper-Doped ZnIn2S4 Nanosheet for Photocatalytic Hydrogen Evolution. ACS Nano 2021, 15, 15238–15248. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Dai, F.; Guo, H.; Du, P.; Xu, X.; Liu, J.; Zhang, Z.; Lu, X. Improving Electrocatalytic Nitrogen Reduction Selectivity and Yield by Suppressing Hydrogen Evolution Reaction via Electronic Metal-Support Interaction. Adv. Energy Mater. 2023, 13, 2203032. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, Y.; Jiang, Z.; Deng, B.; Xin, Y.; Jiang, Z. Defect Engineering Promoted Ultrafine Ir Nanoparticle Growth and Sr Single-Atom Adsorption on TiO2 Nanowires to Achieve High-Performance Overall Water Splitting in Acidic Media. Adv. Energy Mater. 2024, 14, 2303987. [Google Scholar] [CrossRef]
- Ren, G.; Zhao, J.; Zhao, Z.; Li, Z.; Wang, L.; Zhang, Z.; Li, C.; Meng, X. Defects-Induced Single-Atom Anchoring on Metal-Organic Frameworks for High-Efficiency Photocatalytic Nitrogen Reduction. Angew. Chem. Int. Ed. 2024, 63, e202314408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, F.; Yang, X.; Guo, Y.; Zhang, X.; Dong, H.; Wang, W.; Lu, F.; Lu, Z.; Liu, H.; et al. Electronic Metal-Support Interaction Modulates Cu Electronic Structures for CO2 Electroreduction to Desired Products. Nat. Commun. 2025, 16, 1956. [Google Scholar] [CrossRef]
- Singhvi, C.; Sharma, G.; Verma, R.; Paidi, V.K.; Glatzel, P.; Paciok, P.; Patel, V.B.; Mohan, O.; Polshettiwar, V. Tuning the Electronic Structure and SMSI by Integrating Trimetallic Sites with Defective Ceria for the CO2 Reduction Reaction. Proc. Natl. Acad. Sci. USA 2025, 122, e2411406122. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Hou, X.; Cui, T.; Zhuang, Z.; Zhao, Y.; Wang, H.; Wei, W.; Xu, M.; Fu, Q.; et al. Low-Spin Fe3+ Evoked by Multiple Defects with Optimal Intermediate Adsorption Attaining Unparalleled Performance in Water Oxidation. Adv. Mater. 2024, 36, 2412598. [Google Scholar] [CrossRef]
- Pei, W.; Ma, X.; Wu, Y.; Wang, Y.; Zhou, L.; Lei, J.; Yamashita, H.; Zhang, J. A-Site Defect Regulates d-Band Center in Perovskite-Type Catalysts Enhancing Photo-Assisted Peroxymonosulfate Activation for Levofloxacin Removal via High-Valent Iron-Oxo Species. Appl. Catal. B Environ. Energy 2025, 371, 125273. [Google Scholar] [CrossRef]
- Huang, G.; Xia, Y.; Yang, F.; Long, W.; Liu, J.; Liao, J.; Zhang, M.; Liu, J.; Lan, Y. On-Off Switching of a Photocatalytic Overall Reaction through Dynamic Spin-State Transition in a Hofmann Clathrate System. J. Am. Chem. Soc. 2023, 145, 26863–26870. [Google Scholar] [CrossRef]
- Sun, T.; Tang, Z.; Zang, W.; Li, Z.; Li, J.; Li, Z.; Cao, L.; Dominic Rodriguez, J.S.; Mariano, C.O.M.; Xu, H.; et al. Ferromagnetic Single-Atom Spin Catalyst for Boosting Water Splitting. Nat. Nanotechnol. 2023, 18, 763–771. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, H.; Zhang, C.; Li, J.; Xu, R.; Pan, F.; Chen, R.; Cai, C.; Liu, S.; Zhou, Y.; et al. Vacancy Defect Activation Spin Magnetic Effect of Ni(OH)2 Enhanced Oxygen Catalysis. Int. J. Hydrogen Energy 2024, 72, 201–208. [Google Scholar] [CrossRef]
- Li, M.; Li, H.; Fan, H.; Liu, Q.; Yan, Z.; Wang, A.; Yang, B.; Wang, E. Engineering Interfacial Sulfur Migration in Transition-Metal Sulfide Enables Low Overpotential for Durable Hydrogen Evolution in Seawater. Nat. Commun. 2024, 15, 6154. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hung, S.; Zhao, S.; Wang, Y.; Bi, S.; Li, S.; Ma, J.; Zhang, C.; Zhang, Y.; Li, L.; et al. Modulating the Covalency of Ru–O Bonds by Dynamic Reconstruction for Efficient Acidic Oxygen Evolution. Nat. Commun. 2025, 16, 3502. [Google Scholar] [CrossRef]
- Alina, A.K.; Kadyrzhanov, K.K.; Kozlovskiy, A.A.; Konuhova, M.; Popov, A.I.; Shlimas, D.D.; Borgekov, D.B. WO3/ZnWO4 Microcomposites with Potential Application in Photocatalysis. Opt. Mater. 2024, 150, 115280. [Google Scholar] [CrossRef]
- Lisitsyn, V.M.; Musakhanov, D.A.; Korzhneva, T.G.; Strelkova, A.V.; Lisitsyna, L.A.; Golkovsky, M.G.; Zhunusbekov, A.M.; Karipbaev, J.T.; Kozlovsky, A.L. Synthesis and Characterization of Ceramics BaxMg(2-x)F4 Activated by Tungsten. GLASS Phys. Chem. 2023, 49, 288–292. [Google Scholar] [CrossRef]
- Huang, Q.; Xia, G.; Huang, B.; Xie, D.; Wang, J.; Wen, D.; Lin, D.; Xu, C.; Gao, L.; Wu, Z.; et al. Activating Lattice Oxygen by a Defect-Engineered Fe2O3–CeO2 Nano-Heterojunction for Efficient Electrochemical Water Oxidation. Energy Environ. Sci. 2024, 17, 5260–5272. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.; Deng, X.; Wang, Y.; Meng, G.; Liu, R.; Huang, J.; Tu, M.; Xu, C.; Peng, Y.; et al. Structure and Defect Engineering Synergistically Boost High Solar-to-Chemical Conversion Efficiency of Cerium Oxide/Au Hollow Nanomushrooms for Nitrogen Photofixation. Angew. Chem. Int. Ed. 2024, 63, e202316384. [Google Scholar] [CrossRef]
- Zu, D.; Ying, Y.; Wei, Q.; Xiong, P.; Ahmed, M.S.; Lin, Z.; Li, M.; Li, M.; Xu, Z.; Chen, G.; et al. Oxygen Vacancies Trigger Rapid Charge Transport Channels at the Engineered Interface of S-Scheme Heterojunction for Boosting Photocatalytic Performance. Angew. Chem. Int. Ed. 2024, 63, e202405756. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, J.; Qi, K.; Liang, G.; Xu, F.; Yu, J. Ultrafast Electron Transfer at the In2O3/Nb2O5 S-Scheme Interface for CO2 Photoreduction. Nat. Commun. 2024, 15, 4807. [Google Scholar] [CrossRef]
- Wu, L.; Ning, M.; Xing, X.; Wang, Y.; Zhang, F.; Gao, G.; Song, S.; Wang, D.; Yuan, C.; Yu, L.; et al. Boosting Oxygen Evolution Reaction of (Fe,Ni)OOH via Defect Engineering for Anion Exchange Membrane Water Electrolysis Under Industrial Conditions. Adv. Mater. 2023, 35, 2306097. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Guo, D.; Wang, N.; Wang, H.; Yang, X.; Shen, K.; Chen, L.; Li, Y. Defect Engineered Metal-Organic Framework with Accelerated Structural Transformation for Efficient Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2023, 62, e202311909. [Google Scholar] [CrossRef]
- Bi, F.; Meng, Q.; Zhang, Y.; Chen, H.; Jiang, B.; Lu, H.; Liu, Q.; Zhang, H.; Wu, Z.; Weng, X. Engineering Triple O-Ti-O Vacancy Associates for Efficient Water-Activation Catalysis. Nat. Commun. 2025, 16, 851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Z.; Lin, X.; Gao, X.; Wang, Q.; Huang, R.; Ruan, Y.; Xu, H.; Tian, L.; Ling, C.; et al. Mn-Ce Symbiosis: Nanozymes with Multiple Active Sites Facilitate Scavenging of Reactive Oxygen Species (ROS) Based on Electron Transfer and Confinement Anchoring. Angew. Chem. Int. Ed. 2025, 64, e202416686. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, Z.; Wang, H.; Wang, J.; Xi, G.; Zhao, X.; Zhang, C.; Liao, W.; Ho, J.C. Defect-Engineered Multi-Intermetallic Heterostructures as Multisite Electrocatalysts for Efficient Water Splitting. Adv. Sci. 2025, 2502244. [Google Scholar] [CrossRef]
- Li, Z.; Mao, C.; Pei, Q.; Duchesne, P.N.; He, T.; Xia, M.; Wang, J.; Wang, L.; Song, R.; Ali, F.M.; et al. Engineered Disorder in CO2 Photocatalysis. Nat. Commun. 2022, 13, 7205. [Google Scholar] [CrossRef]
- Yang, P.; Li, J.; Vlachos, D.G.; Caratzoulas, S. Tuning Active Site Flexibility by Defect Engineering of Graphene Ribbon Edge-hosted Fe−N3 Sites. Angew. Chem. Int. Ed. 2024, 63, e202311174. [Google Scholar] [CrossRef]
- Liu, M.; Li, Y.; Yang, L.; Zhao, P.; Li, J.; Tian, L.; Cao, D.; Chen, Z. Defect-Triggered Orbital Hybridization in FeMn Dual-Atom Catalysts Toward Sabatier-Optimized Oxygen Reduction. Angew. Chem. Int. Ed. 2025, e202505268. [Google Scholar] [CrossRef]
- Zhou, J.; Qiu, S.; Hou, X.; Ni, T.; Zhang, C.; Dai, S.; Wang, X.; Wang, G.; Jiang, H.; Huang, M. Defect-Driven Stepwise Activation of Metal-Organic Frameworks Toward Industrial-Level Anion Exchange Membrane Water Electrolysis. Angew. Chem. Int. Ed. 2025, e202503787. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Chen, Y.; Wang, L.; Jiang, S.; Xie, Z.; Zhang, T.; Munroe, P.; Peng, S. Rapid Defect Engineering in FeCoNi/FeAl2O4 Hybrid for Enhanced Oxygen Evolution Catalysis: A Pathway to High-Performance Electrocatalysts. Angew. Chem. Int. Ed. 2024, 63, e202405372. [Google Scholar] [CrossRef]
- Lin, J.; Ban, T.; Li, T.; Sun, Y.; Zhou, S.; Li, R.; Su, Y.; Kasemchainan, J.; Gao, H.; Shi, L.; et al. Machine-learning-assisted Intelligent Synthesis of UiO-66(Ce): Balancing the Trade-off between Structural Defects and Thermal Stability for Efficient Hydrogenation of Dicyclopentadiene. Mater. Genome Eng. Adv. 2024, 2, e61. [Google Scholar] [CrossRef]
- Pei, X.; Bian, J.; Zhang, W.; Hu, Z.; Ng, Y.; Dong, Y.; Zhai, X.; Wei, Z.; Liu, Y.; Deng, J.; et al. Overcoming Defect Limitations in Photocatalysis: Boron-Incorporation Engineered Crystalline Red Phosphorus for Enhanced Hydrogen Production. Adv. Funct. Mater. 2024, 34, 2400542. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, S.; Chai, Y.; Dong, B. Ligand Modulation of Active Sites to Promote Cobalt-Doped 1T-MoS2 Electrocatalytic Hydrogen Evolution in Alkaline Media. Angew. Chem. Int. Ed. 2023, 62, e202313845. [Google Scholar] [CrossRef]
- Zhang, Y.; Yun, S.; Dang, J.; Dang, C.; Yang, G.; Wang, Y.; Liu, Z.; Deng, Y. Defect Engineering via Ternary Nonmetal Doping Boosts the Catalytic Activity of ZIF-Derived Carbon-Based Metal-Free Catalysts for Photovoltaics and Water Splitting. Mater. Today Phys. 2022, 27, 100785. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, M.; Zhang, L.; Lu, N.; Wang, X.; Li, Z.; Zhang, X.; Li, N.; Bu, X. Strategic Defect Engineering Enabled Efficient Oxygen Evolution Reaction in Reconstructed Metal-Organic Frameworks. Adv. Funct. Mater. 2025, 35, 2412406. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, S.; Cao, Y.; Li, Y.; Portniagin, A.S.; Tang, B.; Liu, Q.; Kasák, P.; Zhao, T.; Zheng, X.; et al. High-Density Atomic Level Defect Engineering of 2D Fe-Based Metal-Organic Frameworks Boosts Oxygen and Hydrogen Evolution Reactions. Adv. Sci. 2024, 11, 2405936. [Google Scholar] [CrossRef]
- Gu, M.; Jiang, L.; Wang, H.; Chen, Q.; Hao, Y.; Li, L.; Hu, F.; Zhang, X.; Wu, Y.; Wang, G.; et al. Iodine-Induced Redirection of Active Sources in Cu-Based Catalysts during Efficient and Stable Water Oxidation. J. Am. Chem. Soc. 2025, 147, 19675–19686. [Google Scholar] [CrossRef]
- Zhang, R.; Pan, L.; Guo, B.; Huang, Z.; Chen, Z.; Wang, L.; Zhang, X.; Guo, Z.; Xu, W.; Loh, K.P.; et al. Tracking the Role of Defect Types in Co3O4 Structural Evolution and Active Motifs during Oxygen Evolution Reaction. J. Am. Chem. Soc. 2023, 145, 2271–2281. [Google Scholar] [CrossRef]
- Lai, K.; Sun, Y.; Li, N.; Gao, Y.; Li, H.; Ge, L.; Ma, T. Photocatalytic CO2-to-CH4 Conversion with Ultrahigh Selectivity of 95.93% on S-Vacancy Modulated Spatial In2S3/In2O3 Heterojunction. Adv. Funct. Mater. 2024, 34, 2409031. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, J.; Ge, T.; Chen, F.; Lu, Y.; Chen, R.; Zhang, H.; Ye, B.; Wang, S.; Zhang, Y.; et al. Gradient Cationic Vacancies Enabling Inner-To-Outer Tandem Homojunctions: Strong Local Internal Electric Field and Reformed Basic Sites Boosting CO2 Photoreduction. Adv. Mater. 2023, 35, 2302538. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, R.; Zhang, Y.; Huang, X.; Bi, Y. Dynamic Crystal-Structure and Active-Site of Defective ZnAl-Catalysts During CO2 Photoreduction. Angew. Chem. 2025, e202502834. [Google Scholar] [CrossRef]
- A, B.; Jin, X.; Wang, M.; Wang, Y.; Chen, W.; Wei, Z.; Du, Z.; Liu, X.; Wang, Y.; Zhang, L. Steering CO2 Electroreduction toward C2+ Products by Defect-Engineered Coordination Microenvironments of Cu-MOFs. Chem. Eng. J. 2024, 500, 157076. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, S.; Liu, Y.; Liu, Y.; Zheng, H.; Qing, L.; Song, Y.; Ma, Z.; Zhang, L.; Liu, T. Defect-Enriched Cobalt-Based Coordination Polymers for Selective and Efficient Nitrate Electroreduction to Ammonia. Adv. Funct. Mater. 2025, 2422339. [Google Scholar] [CrossRef]
- Yuan, J.; Feng, W.; Zhang, Y.; Xiao, J.; Zhang, X.; Wu, Y.; Ni, W.; Huang, H.; Dai, W. Unraveling Synergistic Effect of Defects and Piezoelectric Field in Breakthrough Piezo-Photocatalytic N2 Reduction. Adv. Mater. 2024, 36, 2303845. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, G.; Wang, Z.; Li, X.; Li, Y.; Sui, N.L.D.; Fan, W.; Wang, A.; Yuan, B.; Wang, J.; et al. Size Effect of Surface Defects Dictates Reactivity for Nitrogen Electrofixation. Angew. Chem. Int. Ed. 2025, 64, e202425112. [Google Scholar] [CrossRef]
- Guo, X.; Yu, J.; Ren, S.; Gao, R.; Wu, L.; Wang, L. Controlled Defective Engineering on CuIr Catalyst Promotes Nitrate Selective Reduction to Ammonia. ACS Nano 2024, 18, 24252–24261. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Sun, Y.; Qiu, Y.; Li, A.; Chiang, C.; Xiao, H.; Qian, J.; Li, Y. Self-Carbon-Thermal-Reduction Strategy for Boosting the Fenton-like Activity of Single Fe-N4 Sites by Carbon-Defect Engineering. Nat. Commun. 2023, 14, 7549. [Google Scholar] [CrossRef]
- Sun, B.; Li, Q.; Su, G.; Song, M.; Ma, C.; Pang, J.; Zhao, X.; Meng, J.; Shi, B. Sustainable and Efficient Catalytic Oxidation of Chlorinated Volatile Organic Compounds over Ru-Loaded Facet-Engineered {201}-TiO2 Catalyst with Tuned Defects. Appl. Catal. B Environ. Energy 2025, 361, 124582. [Google Scholar] [CrossRef]
- Zhu, Y.; Liang, W.; Zhang, C.; Bin, F.; Tao, Q. Defect-Rich Regulatory Activity Strategy: Disordered Structure for Enhanced Catalytic Interfacial Reaction of Chlorobenzene. Environ. Sci. Technol. 2024, 58, 19385–19396. [Google Scholar] [CrossRef]
- Zhong, H.; Wang, J.; Zhang, B.; Liu, Y.; Gong, Z.; Xiao, X.; Yu, J.; Hou, Y.; Tao, Y.; Yang, H.; et al. Selective Introduction of Pentagon Defects into Co–N4 Sites for Boosting Fenton-Like Activity. Adv. Funct. Mater. 2025, 2501208. [Google Scholar] [CrossRef]
- Shi, Y.; Li, P.; Chen, H.; Wang, Z.; Song, Y.; Tang, Y.; Lin, S.; Yu, Z.; Wu, L.; Yu, J.C.; et al. Photocatalytic Toluene Oxidation with Nickel-Mediated Cascaded Active Units over Ni/Bi2WO6 Monolayers. Nat. Commun. 2024, 15, 4641. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wu, Q.; Han, Y.; Zhang, D.; Zhang, R.; Song, N.; Fang, Y.; Liu, H.; Wang, M.; Chen, J.; et al. Constructing Asymmetric Defects Pairs in Electrocatalysts for Efficient Glycerol Oxidation. J. Am. Chem. Soc. 2025, 147, 18033–18043. [Google Scholar] [CrossRef] [PubMed]

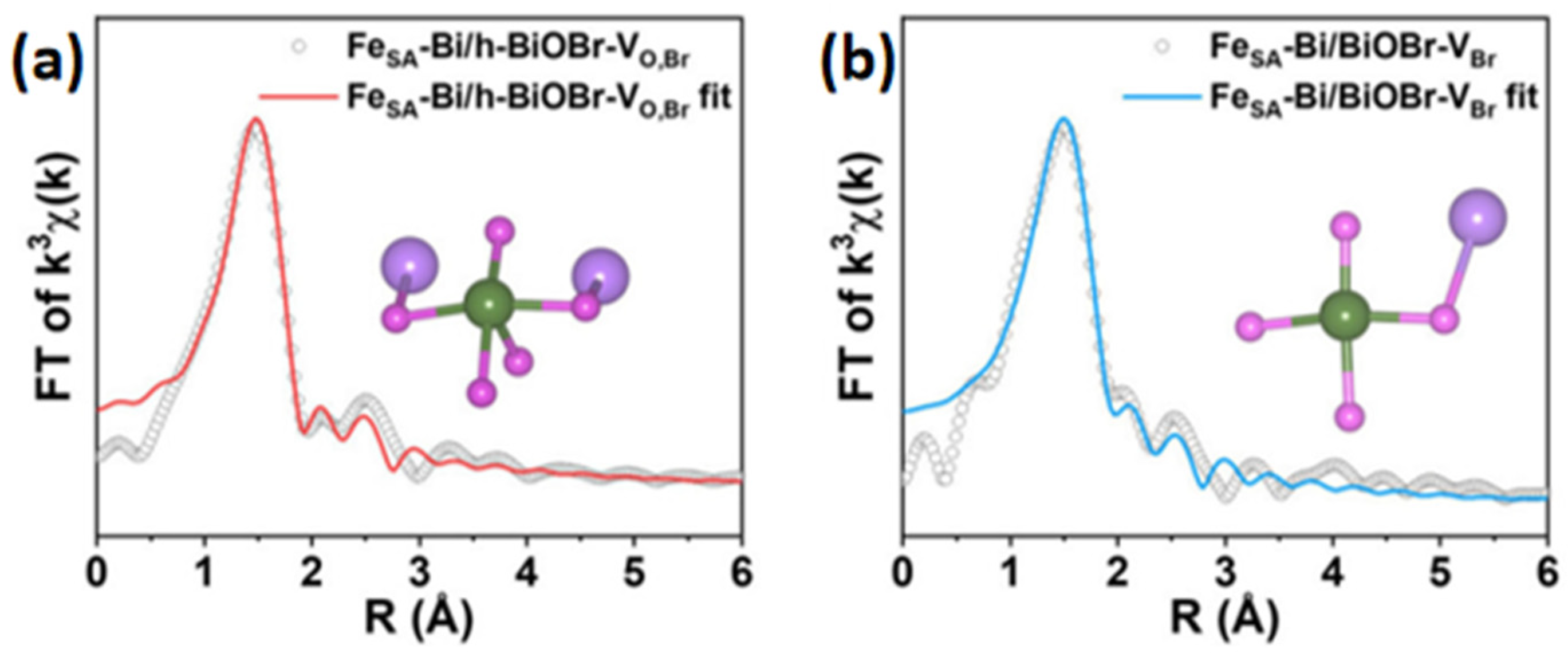
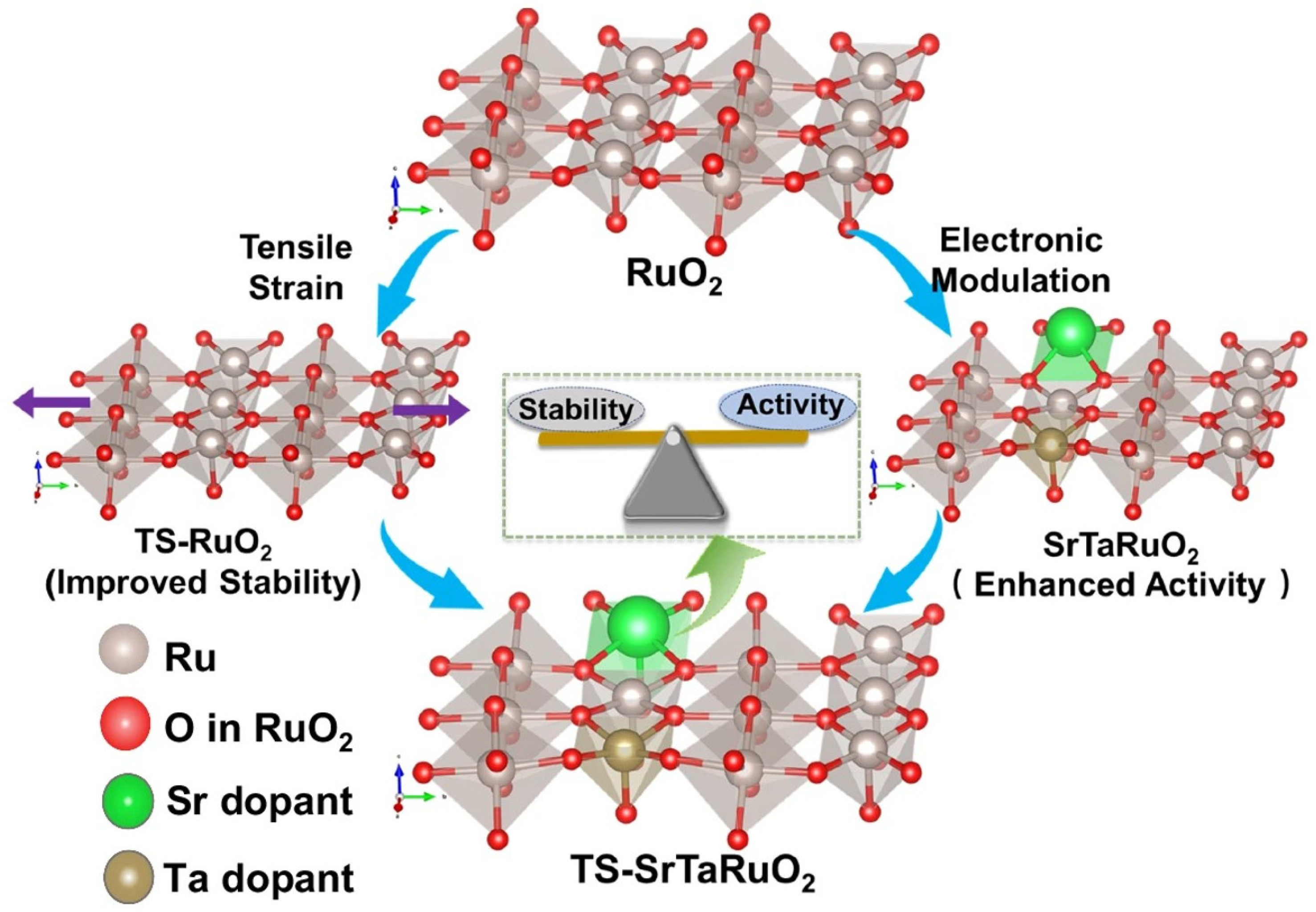
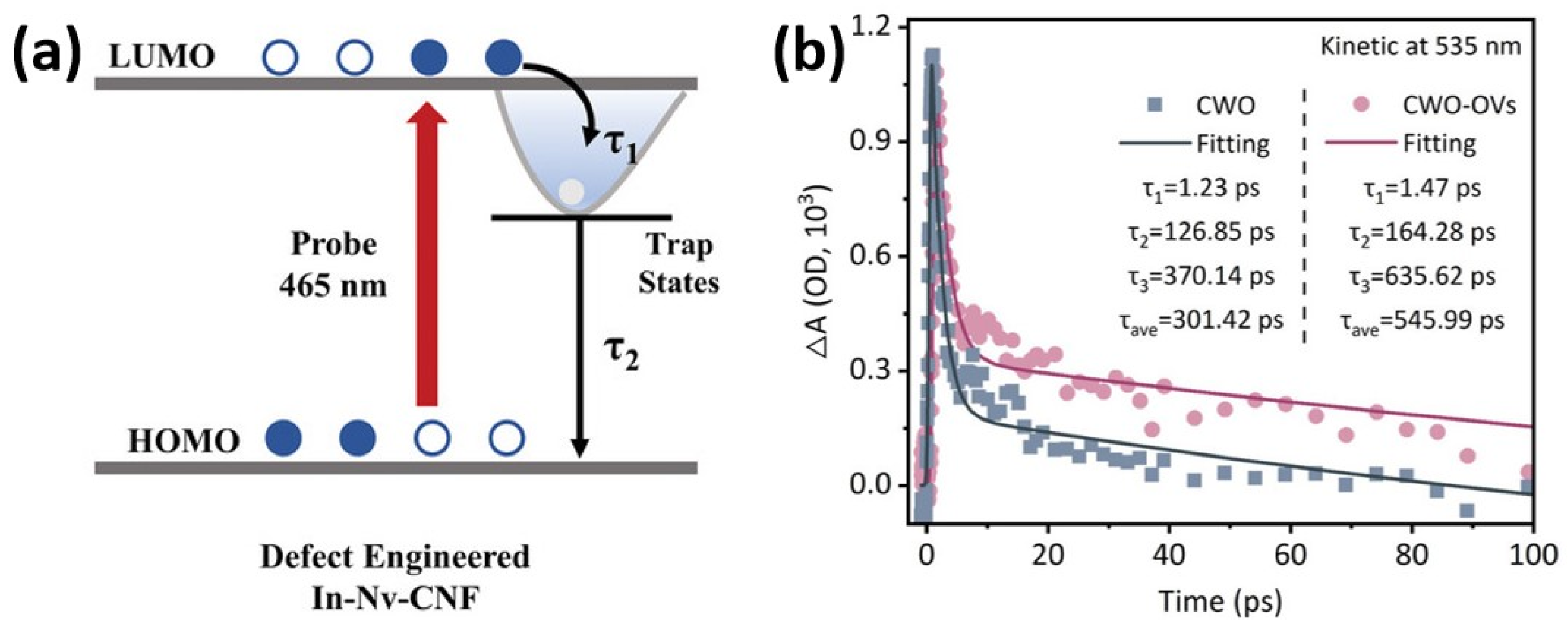
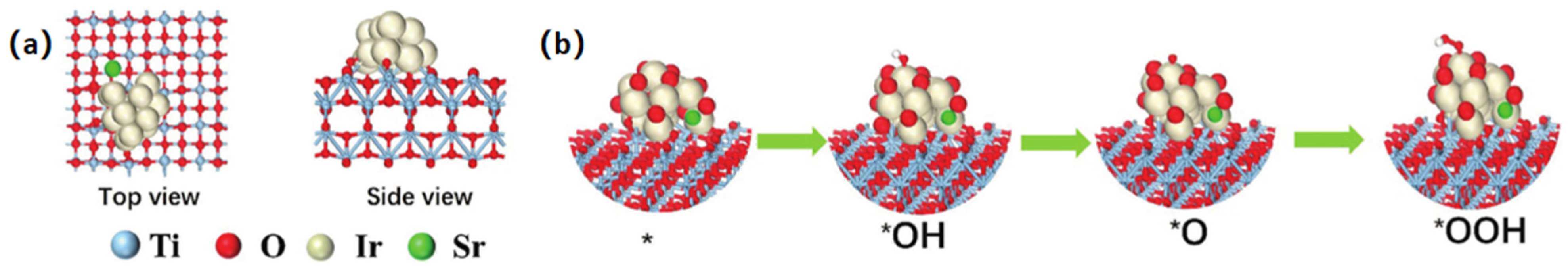

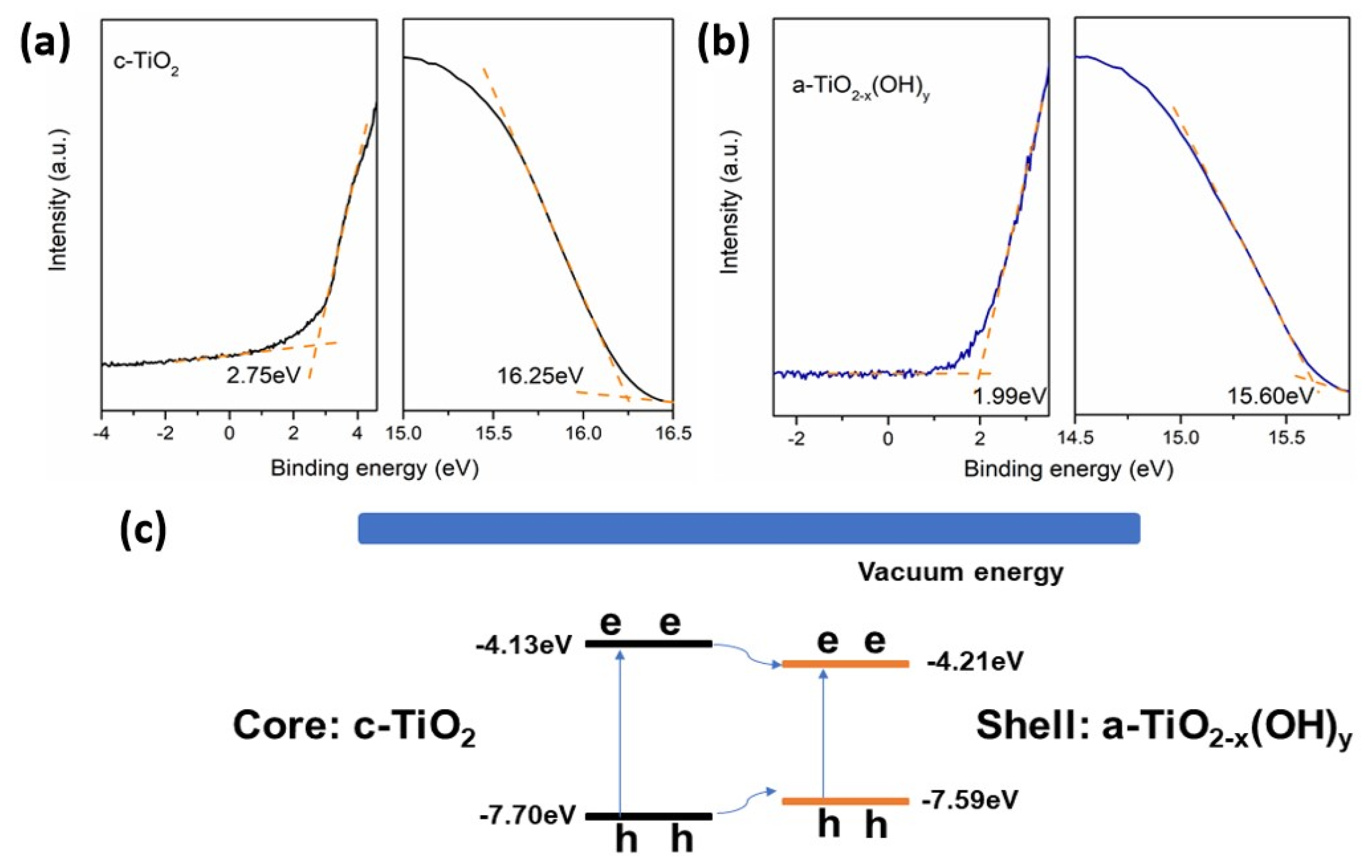

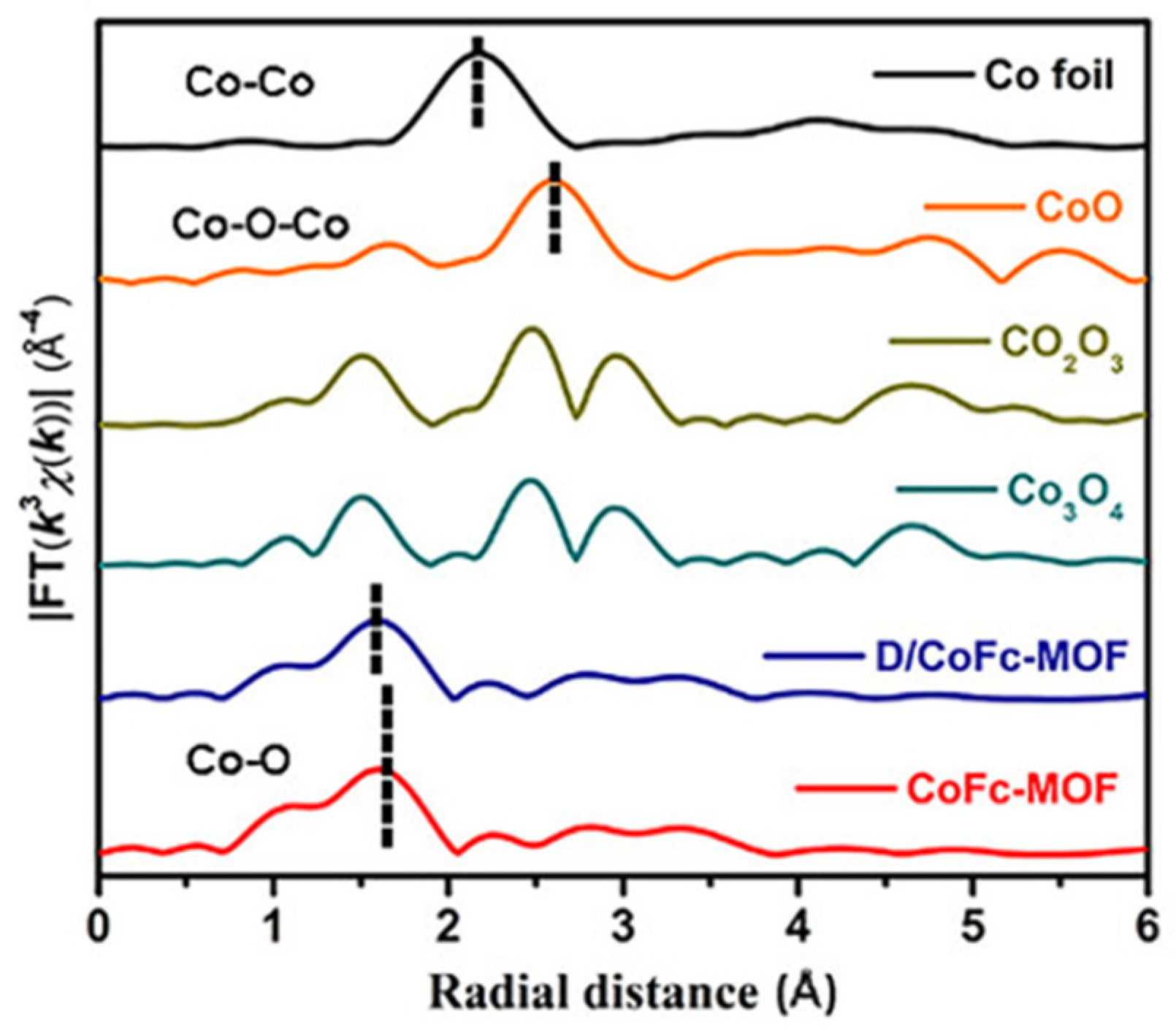

| The Main Types | The Effects on Electronic Structures |
|---|---|
| Vacancy defect | Introduces local electronic states, forms defect energy levels in the band gap, creates charge traps, changes the energy gap, and alters the electron spin |
| Doping defect | Induces charge transfer and orbital hybridization, regulates the position of Fermi energy levels, introduces impurity energy levels, and changes the band gap |
| Coordination defect | Induces the rearrangement of orbital electrons and regulates the position of the Fermi energy level |
| Lattice distortion defect | Changes the bond length, adjusts the electronic state density, and increases the center of the D-band |
| Interface defect | Forms the space charge region at the grain boundaries, generates an interfacial electric field that affects carrier migration, and alters the energy band structure |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Wang, Y.; Guo, T.; Hu, P. The Influence of Defect Engineering on the Electronic Structure of Active Centers on the Catalyst Surface. Catalysts 2025, 15, 651. https://doi.org/10.3390/catal15070651
Zhang Z, Wang Y, Guo T, Hu P. The Influence of Defect Engineering on the Electronic Structure of Active Centers on the Catalyst Surface. Catalysts. 2025; 15(7):651. https://doi.org/10.3390/catal15070651
Chicago/Turabian StyleZhang, Zhekun, Yankun Wang, Tianqi Guo, and Pengfei Hu. 2025. "The Influence of Defect Engineering on the Electronic Structure of Active Centers on the Catalyst Surface" Catalysts 15, no. 7: 651. https://doi.org/10.3390/catal15070651
APA StyleZhang, Z., Wang, Y., Guo, T., & Hu, P. (2025). The Influence of Defect Engineering on the Electronic Structure of Active Centers on the Catalyst Surface. Catalysts, 15(7), 651. https://doi.org/10.3390/catal15070651









