Photocatalytic Activity of Green-Synthesized Semiconductor CuO/ZnO Nanocomposites Against Organic Dye: An Assessment of Antimicrobial and Cytotoxicity Investigations
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of CuO/ZnO Nanocomposite
2.1.1. Fourier Transform Infrared (FT-IR)
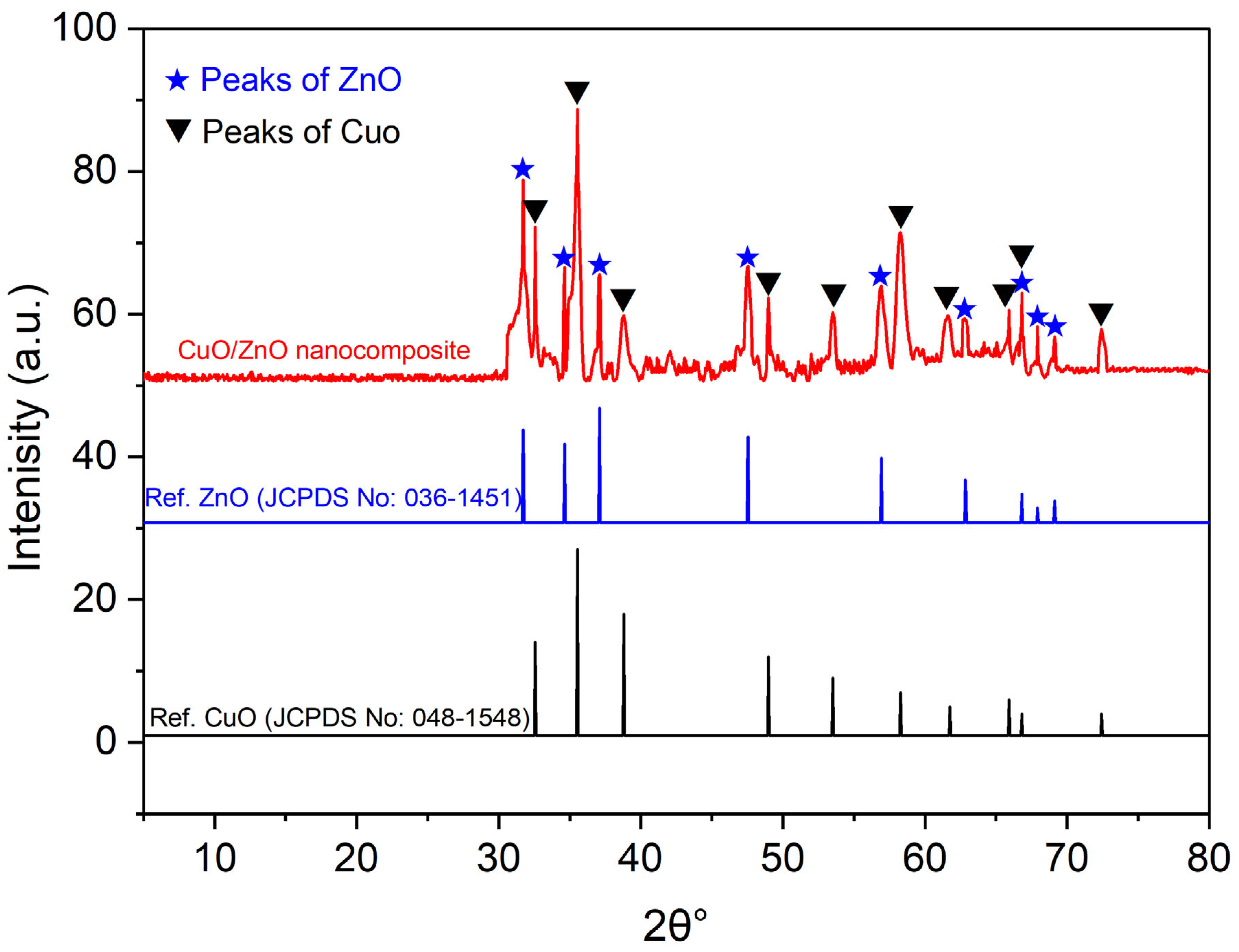
2.1.2. X-Ray Diffraction (XRD)
2.1.3. Morphological and Composition Analyses
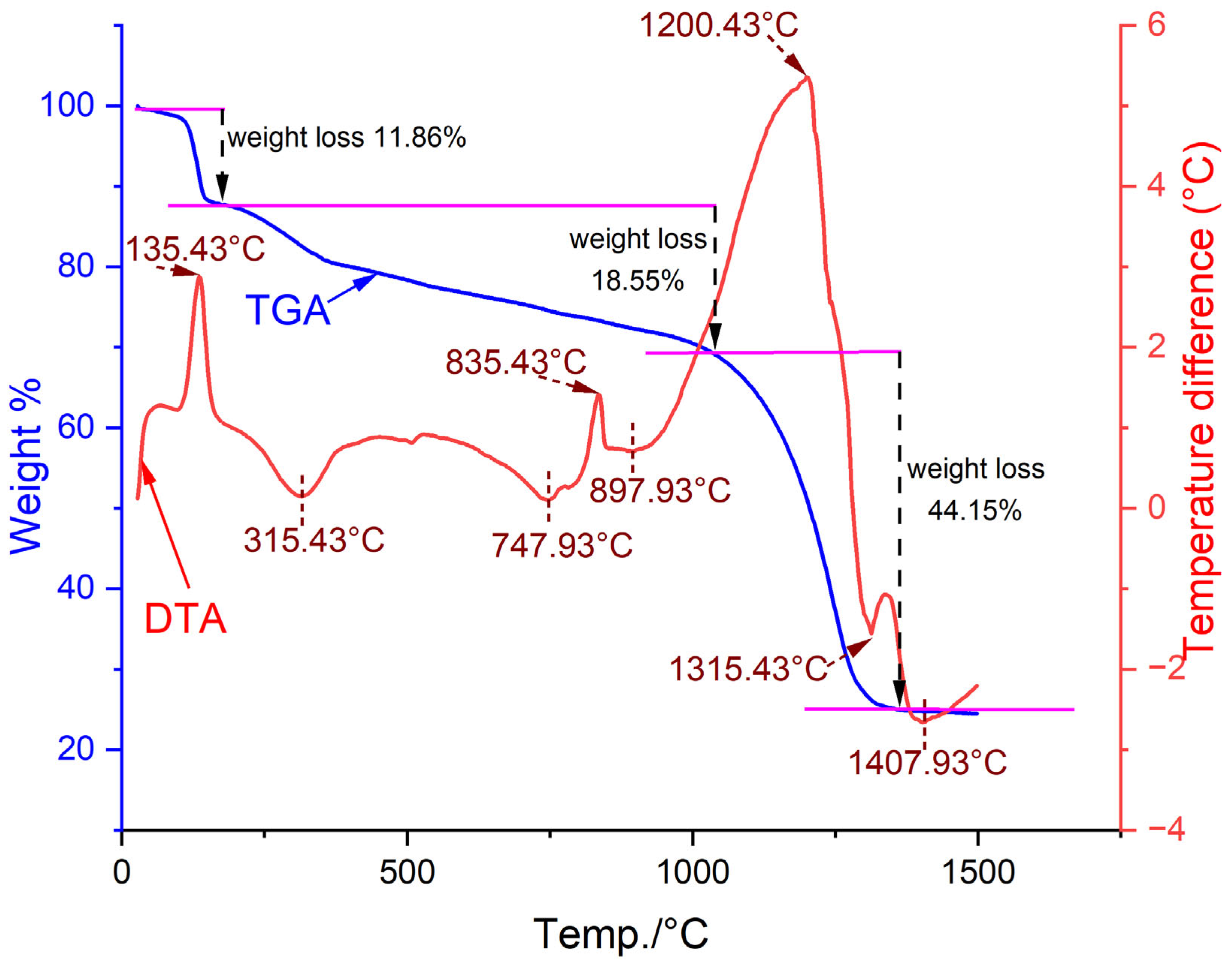
2.1.4. Thermal Stability
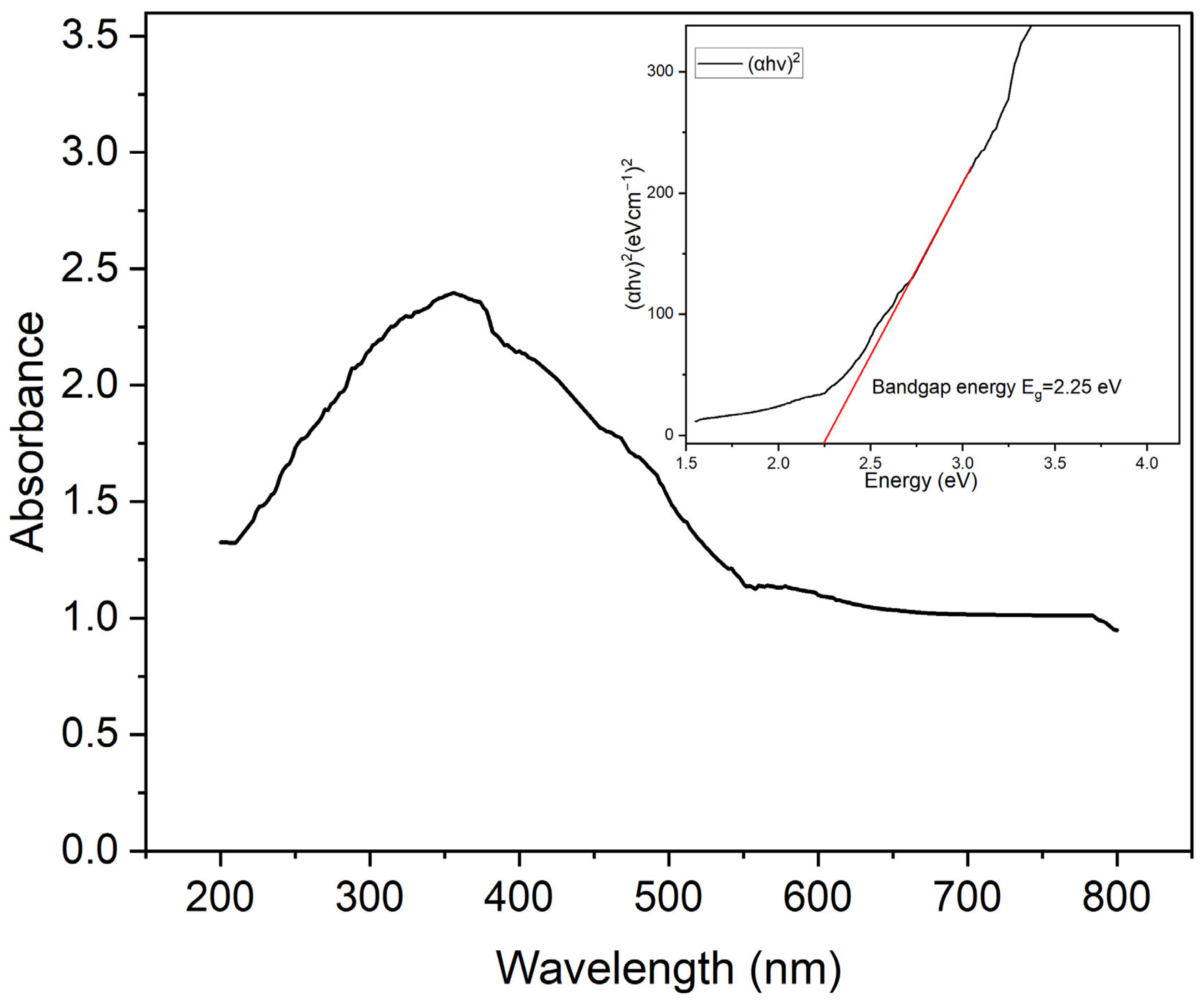
2.1.5. Optical Features and Bandgap Detection
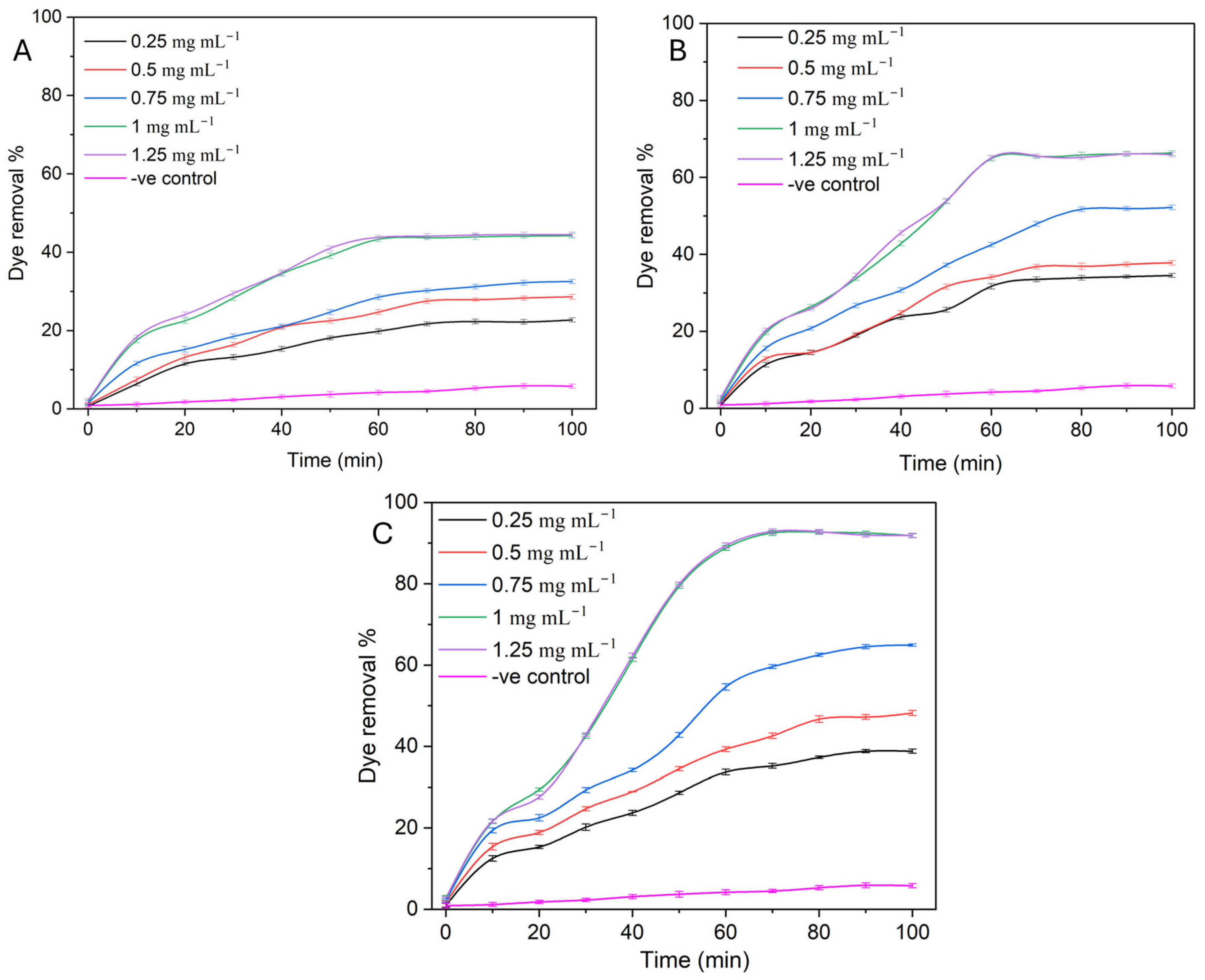
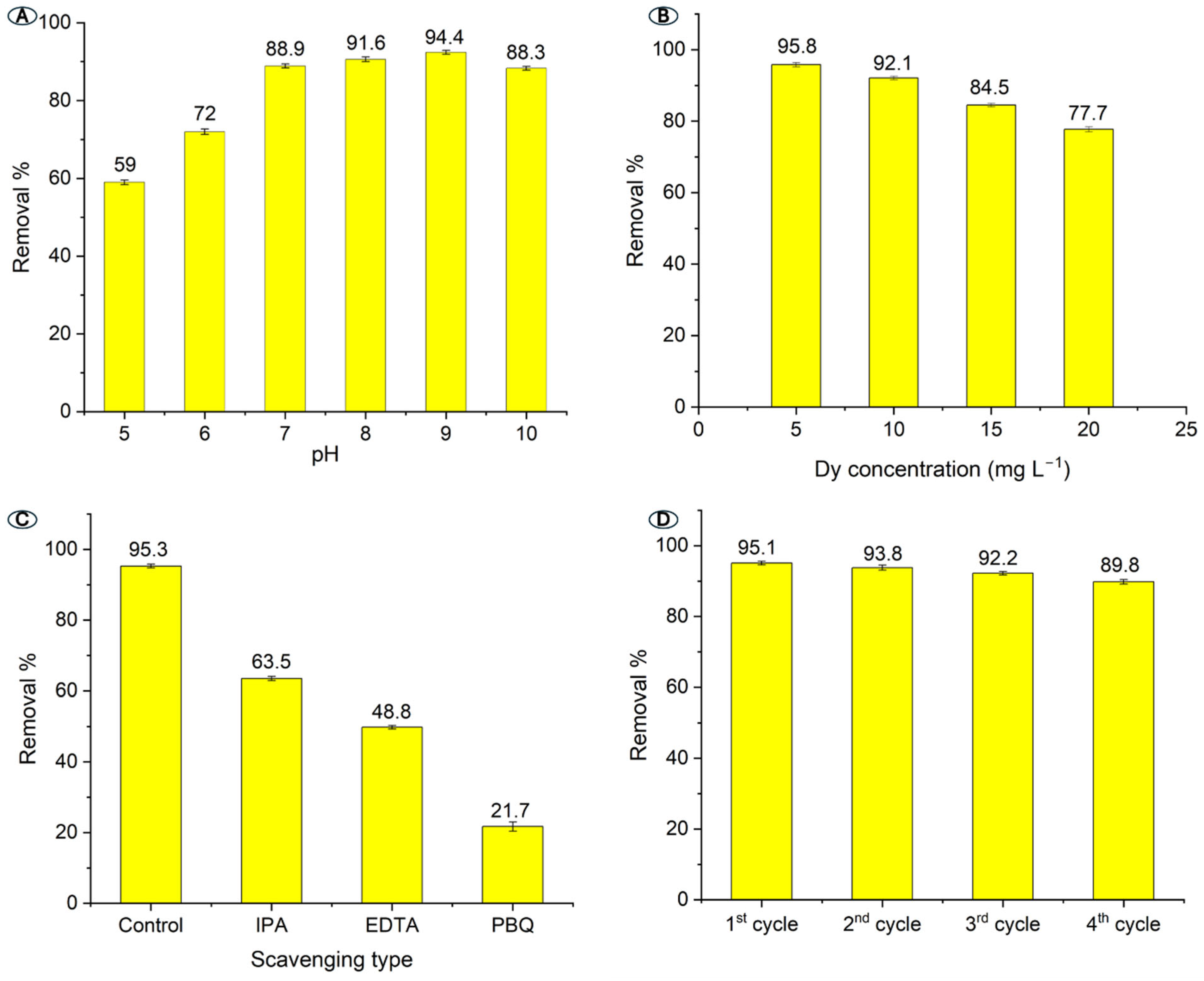
2.2. Photocatalytic Reaction
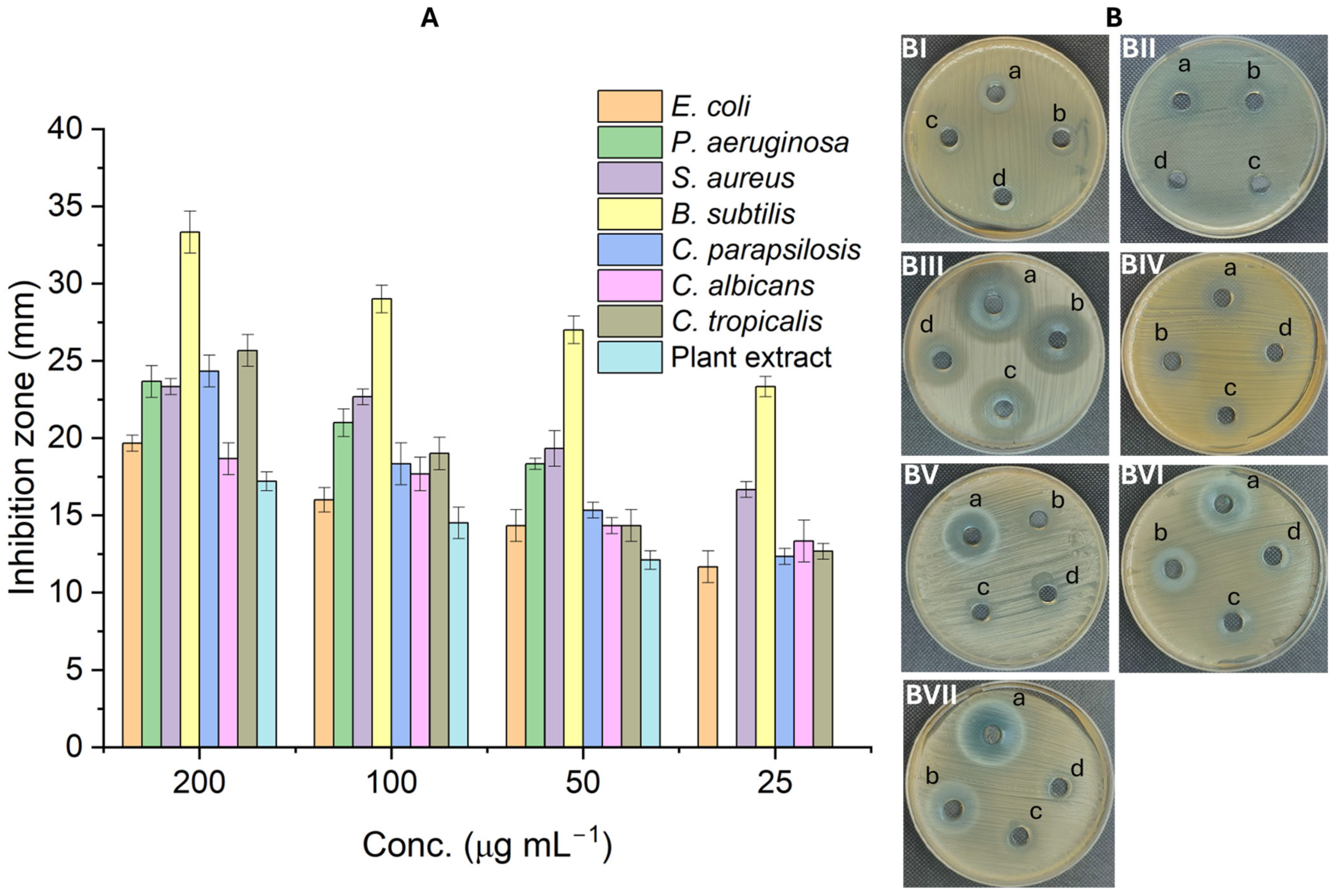
2.3. Antimicrobial Activity
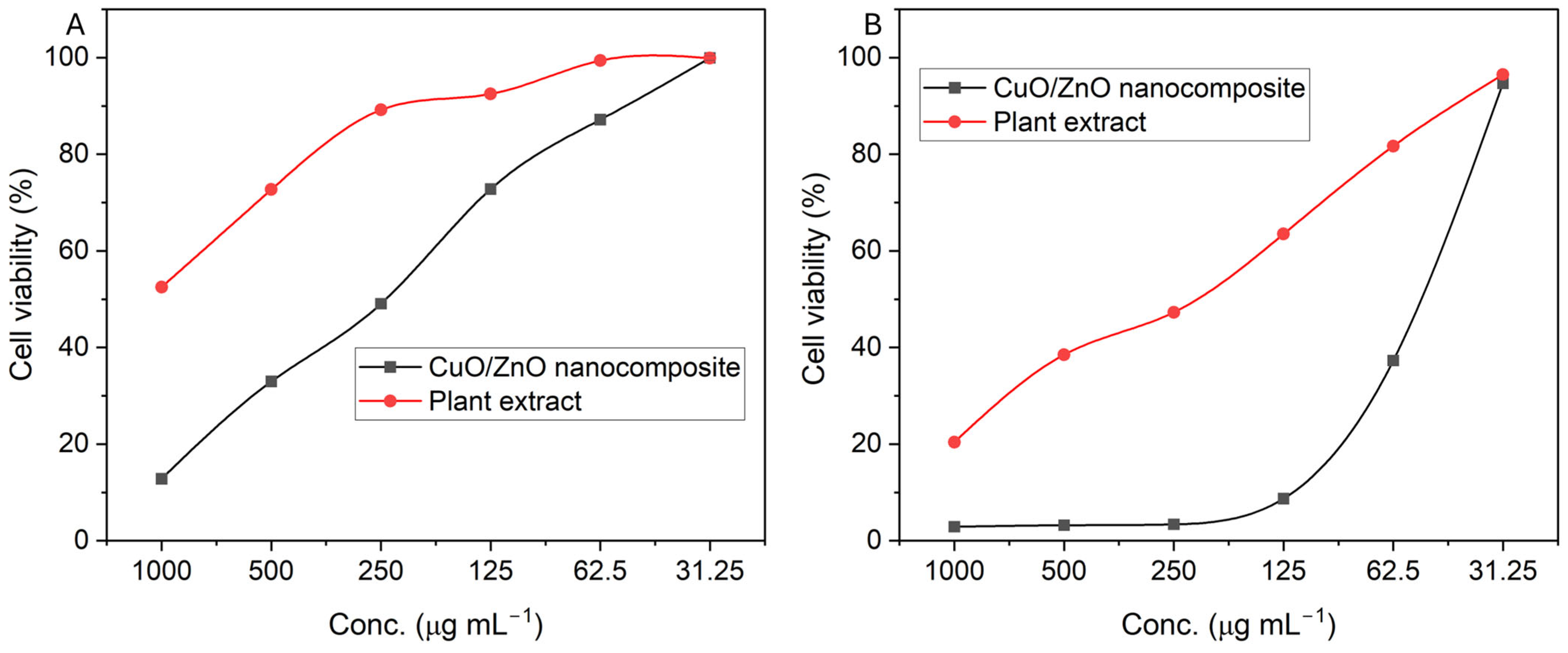
2.4. In Vitro Cytotoxicity
3. Materials and Methods
3.1. Urtica Urens-Mediated Biosynthesis of CuO/ZnO Nanocomposites
3.2. Nanocomposite Characterization
3.3. Photocatalytic Experiment
3.4. Antimicrobial Activity
3.5. Cytotoxicity Assay
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fouda, A.; Hassan, S.E.-D.; Saied, E.; Hamza, M.F. Photocatalytic degradation of real textile and tannery effluent using biosynthesized magnesium oxide nanoparticles (MgO-NPs), heavy metal adsorption, phytotoxicity, and antimicrobial activity. J. Environ. Chem. Eng. 2021, 9, 105346. [Google Scholar] [CrossRef]
- Mrabet, C.; Jaballah, R.; Mahdhi, N.; Boukhachem, A.; Amlouk, M. CuO-ZnO nanocomposites-based thin films: Characterization, physical properties and sunlight photocatalytic degradation of organic pollutants. J. Alloys Compd. 2023, 968, 172252. [Google Scholar] [CrossRef]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Cuprys, A.K.; Maletskyi, Z.; Ratnaweera, H. Treatment Trends and Combined Methods in Removing Pharmaceuticals and Personal Care Products from Wastewater—A Review. Membranes 2023, 13, 158. [Google Scholar] [CrossRef] [PubMed]
- Magaña-Chavez, C.A.; Villegas-Fuentes, J.A.; Nava, O.J.; Vilchis-Nestor, A.R.; Luque, P.A. Green synthesis of semiconductor nanoparticles (ZnO, CuO, and SnO2) using Physalis philadelphica peel extract: Characterization and photocatalytic studies on five organic dyes. J. Mater. Sci. Mater. Electron. 2025, 36, 1728. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: An Overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef] [PubMed]
- Khoshsang, H.; Abbasi, K.; Ghaffarinejad, A. Biosynthesis of ZnO and CuO nanoparticles using sunflower petal extract. Inorg. Chem. Commun. 2023, 155, 111083. [Google Scholar] [CrossRef]
- Zhigang, Z.; Wensi, C.; Yong, Z. Metal Oxide Semiconductors: State-of-the-Art and New Challenges. In Metal Oxide Semiconductors: Synthesis, Properties, and Devices; Wiley: Hoboken, NJ, USA, 2024; pp. 1–13. [Google Scholar] [CrossRef]
- Sonkar, R.; Mondal, N.J.; Boro, B.; Ghosh, M.P.; Chowdhury, D. Cu doped ZnO nanoparticles: Correlations between tuneable optoelectronic, antioxidant and photocatalytic activities. J. Phys. Chem. Solids 2024, 185, 111715. [Google Scholar] [CrossRef]
- Ghareeb, A.; Fouda, A.; Kishk, R.M.; El Kazzaz, W.M. Unlocking the potential of titanium dioxide nanoparticles: An insight into green synthesis, optimizations, characterizations, and multifunctional applications. Microb. Cell Fact. 2024, 23, 341. [Google Scholar] [CrossRef]
- Alsaiari, N.S.; Alzahrani, F.M.; Amari, A.; Osman, H.; Harharah, H.N.; Elboughdiri, N.; Tahoon, M.A. Plant and Microbial Approaches as Green Methods for the Synthesis of Nanomaterials: Synthesis, Applications, and Future Perspectives. Molecules 2023, 28, 463. [Google Scholar] [CrossRef]
- Pandit, C.; Roy, A.; Ghotekar, S.; Khusro, A.; Islam, M.N.; Emran, T.B.; Lam, S.E.; Khandaker, M.U.; Bradley, D.A. Biological agents for synthesis of nanoparticles and their applications. J. King Saud. Univ. Sci. 2022, 34, 101869. [Google Scholar] [CrossRef]
- El-Sawaf, A.K.; El-Moslamy, S.H.; Kamoun, E.A.; Hossain, K. Green synthesis of trimetallic CuO/Ag/ZnO nanocomposite using Ziziphus spina-christi plant extract: Characterization, statistically experimental designs, and antimicrobial assessment. Sci. Rep. 2024, 14, 19718. [Google Scholar] [CrossRef]
- Ayu Larasati, D.; Lismawenning Puspitarum, D.; Imani Istiqomah, N.; Partini, J.; Ali, H.; Ali, D.; Suharyadi, E. Novel green synthesis approach of eco-friendly magnetic/semiconductor nanocomposites as magnetically separable and reusable photocatalyst for organic dye degradation. Results Chem. 2024, 10, 101693. [Google Scholar] [CrossRef]
- Farooq, U.; Zahra, S.T.; Najeeb, J.; Naseem, K.; Khan, M.E.; Ali, W.; Alomar, M.S.; Ali, S.K.; Hassan, M.; Al Zoubi, W.; et al. Harnessing the potential of green synthesized Co-Fe-ZnS nanocomposites for successive solar-driven degradation of amoxicillin, hydrogen production, and CO2 reduction: Optimization of reaction parameters by response surface methodology. Sustain. Mater. Technol. 2025, 44, e01377. [Google Scholar] [CrossRef]
- Puspitarum, D.L.; Istiqomah, N.I.; Larasati, D.A.; Kusumaatmaja, A.; Aliah, H.; Suharyadi, E. Photocatalytic mechanism and properties of recyclable hybrid magnetic/semiconductor nanocomposites synthesized via green route for organic dye degradation. Results Mater. 2023, 19, 100439. [Google Scholar] [CrossRef]
- Grauso, L.; de Falco, B.; Lanzotti, V.; Motti, R. Stinging nettle, Urtica dioica L.: Botanical, phytochemical and pharmacological overview. Phytochem. Rev. 2020, 19, 1341–1377. [Google Scholar] [CrossRef]
- Carvalho, A.R.; Costa, G.; Figueirinha, A.; Liberal, J.; Prior, J.A.V.; Lopes, M.C.; Cruz, M.T.; Batista, M.T. Urtica spp.: Phenolic composition, safety, antioxidant and anti-inflammatory activities. Food Res. Int. 2017, 99, 485–494. [Google Scholar] [CrossRef]
- Prabu, P.; Losetty, V. Green synthesis of copper oxide nanoparticles using Macroptilium Lathyroides (L) leaf extract and their spectroscopic characterization, biological activity and photocatalytic dye degradation study. J. Mol. Struct. 2024, 1301, 137404. [Google Scholar] [CrossRef]
- Albo Hay Allah, M.A.; Ibrahim, H.K.; Alshamsi, H.A.; Radhi Saud, H. Eco-friendly synthesis of biochar supported with zinc oxide as a heterogeneous catalyst for photocatalytic decontamination of Rhodamine B under sunlight illumination. J. Photochem. Photobiol. A Chem. 2024, 449, 115413. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Ajiboye, T.O.; Omotola, E.O.; Oyewola, O.J. Methylene blue dye: Toxicity and potential elimination technology from wastewater. Results Eng. 2022, 16, 100678. [Google Scholar] [CrossRef]
- Binsalah, M.; Devanesan, S.; AlSalhi, M.S.; Nooh, A.; Alghamdi, O.; Nooh, N. Biomimetic Synthesis of Silver Nanoparticles Using Ethyl Acetate Extract of Urtica diocia Leaves; Characterizations and Emerging Antimicrobial Activity. Microorganisms 2022, 10, 789. [Google Scholar] [CrossRef]
- Mohamed, A.E.; Elgammal, W.E.; Eid, A.M.; Dawaba, A.M.; Ibrahim, A.G.; Fouda, A.; Hassan, S.M. Synthesis and characterization of new functionalized chitosan and its antimicrobial and in-vitro release behavior from topical gel. Int. J. Biol. Macromol. 2022, 207, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; JohnWiley & Sons Ltd.: West Sussex, England, 2006; pp. 10815–10837. [Google Scholar]
- Hamza, M.F.; Mubark, A.E.; Wei, Y.; Vincent, T.; Guibal, E. Quaternization of Composite Algal/PEI Beads for Enhanced Uranium Sorption—Application to Ore Acidic Leachate. Gels 2020, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Nassar, A.A.; Eid, A.M.; Atta, H.M.; El Naghy, W.S.; Fouda, A. Exploring the antimicrobial, antioxidant, anticancer, biocompatibility, and larvicidal activities of selenium nanoparticles fabricated by endophytic fungal strain Penicillium verhagenii. Sci. Rep. 2023, 13, 9054. [Google Scholar] [CrossRef] [PubMed]
- Pasieczna-Patkowska, S.; Cichy, M.; Flieger, J. Application of Fourier Transform Infrared (FTIR) Spectroscopy in Characterization of Green Synthesized Nanoparticles. Molecules 2025, 30, 684. [Google Scholar] [CrossRef]
- Khalil, M.M.H.; Ismail, E.H.; El-Magdoub, F. Biosynthesis of Au nanoparticles using olive leaf extract: 1st Nano Updates. Arab. J. Chem. 2012, 5, 431–437. [Google Scholar] [CrossRef]
- Das, R.K.; Borthakur, B.B.; Bora, U. Green synthesis of gold nanoparticles using ethanolic leaf extract of Centella asiatica. Mater. Lett. 2010, 64, 1445–1447. [Google Scholar] [CrossRef]
- Karthik, K.V.; Raghu, A.V.; Reddy, K.R.; Ravishankar, R.; Sangeeta, M.; Shetti, N.P.; Reddy, C.V. Green synthesis of Cu-doped ZnO nanoparticles and its application for the photocatalytic degradation of hazardous organic pollutants. Chemosphere 2022, 287, 132081. [Google Scholar] [CrossRef]
- Bekru, A.G.; Tufa, L.T.; Zelekew, O.A.; Goddati, M.; Lee, J.; Sabir, F.K. Green Synthesis of a CuO–ZnO Nanocomposite for Efficient Photodegradation of Methylene Blue and Reduction of 4-Nitrophenol. ACS Omega 2022, 7, 30908–30919. [Google Scholar] [CrossRef]
- Jeevarathinam, M.; Asharani, I.V. Synthesis of CuO, ZnO nanoparticles, and CuO-ZnO nanocomposite for enhanced photocatalytic degradation of Rhodamine B: A comparative study. Sci. Rep. 2024, 14, 9718. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Nguyen, Y.N.N.; Tran, X.T.; Nguyen, T.T.T.; Tran, T.V. Green synthesis of CuO, ZnO and CuO/ZnO nanoparticles using Annona glabra leaf extract for antioxidant, antibacterial and photocatalytic activities. J. Environ. Chem. Eng. 2023, 11, 111003. [Google Scholar] [CrossRef]
- Holder, C.F.; Schaak, R.E. Tutorial on Powder X-ray Diffraction for Characterizing Nanoscale Materials. ACS Nano 2019, 13, 7359–7365. [Google Scholar] [CrossRef]
- Filippov, S.K.; Khusnutdinov, R.; Murmiliuk, A.; Inam, W.; Zakharova, L.Y.; Zhang, H.; Khutoryanskiy, V.V. Dynamic light scattering and transmission electron microscopy in drug delivery: A roadmap for correct characterization of nanoparticles and interpretation of results. Mater. Horiz. 2023, 10, 5354–5370. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Dhahad, H.A.; El-Shorbagy, M.A.; Alijani, H.Q.; Zakeri, M.; Heydari, A.; Bahonar, E.; Slouf, M.; Khatami, M.; Naderifar, M.; et al. Green synthesis of bimetallic ZnO–CuO nanoparticles and their cytotoxicity properties. Sci. Rep. 2021, 11, 23479. [Google Scholar] [CrossRef] [PubMed]
- Turabik, M.; Özdemir, S.; Akinbingol, G.; Gonca, S.; Gecgel, C. Comparison of antioxidant, antimicrobial, DNA cleavage, cell viability, and biofilm inhibition activities of mono- and bimetallic copper and zinc nanoparticles. Inorg. Chem. Commun. 2023, 155, 111072. [Google Scholar] [CrossRef]
- Hitkari, G.; Chowdhary, P.; Kumar, V.; Singh, S.; Motghare, A. Potential of Copper-Zinc Oxide nanocomposite for photocatalytic degradation of congo red dye. Clean. Chem. Eng. 2022, 1, 100003. [Google Scholar] [CrossRef]
- Riaz, T.; Assey, N.; Javed, M.; Shahzadi, T.; Zaib, M.; Shahid, S.; Iqbal, S.; Elkaeed, E.B.; Alzhrani, R.M.; Alsaab, H.O.; et al. Biogenic plant mediated synthesis of monometallic zinc and bimetallic Copper/Zinc nanoparticles and their dye adsorption and antioxidant studies. Inorg. Chem. Commun. 2022, 140, 109449. [Google Scholar] [CrossRef]
- Madeshwaran, K.; Venkatachalam, R. Green synthesis of bimetallic ZnO–CuO nanoparticles using Annona muricata l. extract: Investigation of antimicrobial, antioxidant, and anticancer properties. J. Ind. Eng. Chem. 2024, 140, 454–467. [Google Scholar] [CrossRef]
- Fouda, A.; Saied, E.; Eid, A.M.; Kouadri, F.; Alemam, A.M.; Hamza, M.F.; Alharbi, M.; Elkelish, A.; Hassan, S.E. Green Synthesis of Zinc Oxide Nanoparticles Using an Aqueous Extract of Punica granatum for Antimicrobial and Catalytic Activity. J. Funct. Biomater. 2023, 14, 205. [Google Scholar] [CrossRef]
- Hamza, M.F.; Ibrahim, A.G.; Yin, X.; Salih, K.A.M.; Eid, A.M.; Wei, Y.; Abdel-Rahman, M.A.; Hassan, S.E.-D.; Fouda, A. Efficacy of green synthesized TiO2/ZnO nanocomposite for photocatalytic sorption and removal of uranium: A sustainable approach for environmental remediation. J. Water Process Eng. 2025, 74, 107870. [Google Scholar] [CrossRef]
- Rami, J.M.; Patel, C.D.; Patel, C.M.; Patel, M.V. Thermogravimetric analysis (TGA) of some synthesized metal oxide nanoparticles. Mater. Today Proc. 2021, 43, 655–659. [Google Scholar] [CrossRef]
- Toloman, D.; Popa, A.; Stan, M.; Stefan, M.; Vlad, G.; Ulinici, S.; Baisan, G.; Silipas, T.D.; Macavei, S.; Leostean, C.; et al. Visible-light-driven photocatalytic degradation of different organic pollutants using Cu doped ZnO-MWCNT nanocomposites. J. Alloys Compd. 2021, 866, 159010. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Bopape, D.A.; Hintsho-Mbita, N.C. Commelina benghalensis-mediated CuO–ZnO nanocomposite: Effect of the p-n heterojunction on the photocatalytic activity against Congo red and carbamazepine. Inorg. Chem. Commun. 2025, 178, 114529. [Google Scholar] [CrossRef]
- Pathania, D.; Sharma, A.; Kumar, S.; Srivastava, A.K.; Kumar, A.; Singh, L. Bio-synthesized Cu–ZnO hetro-nanostructure for catalytic degradation of organophosphate chlorpyrifos under solar illumination. Chemosphere 2021, 277, 130315. [Google Scholar] [CrossRef] [PubMed]
- Basit, R.A.; Abbasi, Z.; Hafeez, M.; Ahmad, P.; Khan, J.; Khandaker, M.U.; Al-Mugren, K.S.; Khalid, A. Successive Photocatalytic Degradation of Methylene Blue by ZnO, CuO and ZnO/CuO Synthesized from Coriandrum sativum Plant Extract via Green Synthesis Technique. Crystals 2023, 13, 281. [Google Scholar] [CrossRef]
- Dien, N.D.; Thu Ha, P.T.; Vu, X.H.; Trang, T.T.; Thanh Giang, T.D.; Dung, N.T. Developing efficient CuO nanoplate/ZnO nanoparticle hybrid photocatalysts for methylene blue degradation under visible light. RSC Adv. 2023, 13, 24505–24518. [Google Scholar] [CrossRef]
- Saravanan, R.; Karthikeyan, S.; Gupta, V.K.; Sekaran, G.; Narayanan, V.; Stephen, A. Enhanced photocatalytic activity of ZnO/CuO nanocomposite for the degradation of textile dye on visible light illumination. Mater. Sci. Eng. C 2013, 33, 91–98. [Google Scholar] [CrossRef]
- Azabi, W.; Gherraf, N.; Romero, A.; Abdullah, J.A.A. Synergetic green synthesis of CuO, ZnO, and CuO-ZnO nanocomposite nanoparticles using Genista hispanica L. extract for enhanced photocatalytic and antioxidant properties. Res. Chem. Intermed. 2025, 51, 4491–4517. [Google Scholar] [CrossRef]
- Yu, Q.; Su, Y.; Tursun, R.; Zhang, J. Synthesis and characterization of low density porous nickel zinc ferrites. RSC Adv. 2019, 9, 13173–13181. [Google Scholar] [CrossRef]
- Yin, X.; Eid, A.M.; Wei, Y.; Hamza, M.F.; Abdel-Rahman, M.A.; Zheng, N.; Fouda, A. Biogenic iron oxide nanoparticles with enhanced photocatalytic activity for effective removal of uranium and microbial decontamination. J. Water Process Eng. 2025, 70, 107093. [Google Scholar] [CrossRef]
- Xu, M.; Wang, Y.; Mu, Z.; Li, S.; Li, H. Dissolution of copper oxide nanoparticles is controlled by soil solution pH, dissolved organic matter, and particle specific surface area. Sci. Total Environ. 2021, 772, 145477. [Google Scholar] [CrossRef]
- Pawar, R.C.; Choi, D.-H.; Lee, J.-S.; Lee, C.S. Formation of polar surfaces in microstructured ZnO by doping with Cu and applications in photocatalysis using visible light. Mater. Chem. Phys. 2015, 151, 167–180. [Google Scholar] [CrossRef]
- Fouda, A.; Salem, S.S.; Wassel, A.R.; Hamza, M.F.; Shaheen, T.I. Optimization of green biosynthesized visible light active CuO/ZnO nano-photocatalysts for the degradation of organic methylene blue dye. Heliyon 2020, 6, e04896. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Chundawat, T.S.; Surolia, P.K.; Vaya, D. Photocatalytic degradation of textile dyes using β-CD-CuO/ZnO nanocomposite. J. Phys. Chem. Solids 2022, 165, 110691. [Google Scholar] [CrossRef]
- Takele, E.; Feyisa Bogale, R.; Shumi, G.; Kenasa, G. Green Synthesis, Characterization, and Antibacterial Activity of CuO/ZnO Nanocomposite Using Zingiber officinale Rhizome Extract. J. Chem. 2023, 2023, 3481389. [Google Scholar] [CrossRef]
- Jayanetti, M.; Thambiliyagodage, C.; Liyanaarachchi, H.; Ekanayake, G.; Mendis, A.; Usgodaarachchi, L. In vitro influence of PEG functionalized ZnO–CuO nanocomposites on bacterial growth. Sci. Rep. 2024, 14, 1293. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.; Amin, M.A.-A.; Ismail, M.; Ismail, M.; Sarker, T.C.; Elnaggar, M.; Abdelkareem, E. Selenium/copper oxide nanoparticles prepared with Urtica urens extract: Their antimicrobial, antioxidant, antihemolytic, anticoagulant, and plant growth effects. BioResources 2025, 20, 2791–2810. [Google Scholar] [CrossRef]
- Daimari, J.; Deka, A.K. Anticancer, antimicrobial and antioxidant activity of CuO–ZnO bimetallic nanoparticles: Green synthesised from Eryngium foetidum leaf extract. Sci. Rep. 2024, 14, 19506. [Google Scholar] [CrossRef]
- Hasanin, M.S.; Hashem, A.H.; Al-Askar, A.A.; Haponiuk, J.; Saied, E. A novel nanocomposite based on mycosynthesized bimetallic zinc-copperoxide nanoparticles, nanocellulose and chitosan: Characterization, antimicrobial and photocatalytic activities. Electron. J. Biotechnol. 2023, 65, 45–55. [Google Scholar] [CrossRef]
- Dhage, S.B.; Sah, P.M.; Lakkakula, J.; Raut, R.W.; Malghe, Y.S.; Roy, A.; Verma, D.; Kaur, K. ZnO-CuO Nanocomposite Synthesized by Co-precipitation: Characterization and Antibacterial Properties. Biointerface Res. Appl. Chem. 2024, 14, 59. [Google Scholar] [CrossRef]
- Shaheen, T.I.; Salem, S.S.; Fouda, A. Current Advances in Fungal Nanobiotechnology: Mycofabrication and Applications. In Microbial Nanobiotechnology: Principles and Applications; Lateef, A., Gueguim-Kana, E.B., Dasgupta, N., Ranjan, S., Eds.; Springer: Singapore, 2021; pp. 113–143. [Google Scholar] [CrossRef]
- Jan, T.; Azmat, S.; Mansoor, Q.; Waqas, H.M.; Adil, M.; Ilyas, S.Z.; Ahmad, I.; Ismail, M. Superior antibacterial activity of ZnO-CuO nanocomposite synthesized by a chemical Co-precipitation approach. Microb. Pathog. 2019, 134, 103579. [Google Scholar] [CrossRef]
- Aziz, S.N.; Abdulwahab, A.M.; Aldeen, T.S.; Alqabili, D.M.A. Synthesis, characterization, and evaluation of antibacterial and antifungal activities of CuO-ZnO-Co3O4 nanocomposites. Heliyon 2024, 10, e37802. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.; Eid, A.M.; Awad, M.A.; Althumayri, K.; Badr, N.F.; Hamza, M.F. Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity. Green. Process. Synth. 2022, 11, 931–950. [Google Scholar] [CrossRef]
- Guerrero Correa, M.; Martínez, F.B.; Vidal, C.P.; Streitt, C.; Escrig, J.; de Dicastillo, C.L. Antimicrobial metal-based nanoparticles: A review on their synthesis, types and antimicrobial action. Beilstein J. Nanotechnol. 2020, 11, 1450–1469. [Google Scholar] [CrossRef] [PubMed]
- Nassar, A.-R.A.; Atta, H.M.; Abdel-Rahman, M.A.; El Naghy, W.S.; Fouda, A. Myco-synthesized copper oxide nanoparticles using harnessing metabolites of endophytic fungal strain Aspergillus terreus: An insight into antibacterial, anti-Candida, biocompatibility, anticancer, and antioxidant activities. BMC Complement. Med. Ther. 2023, 23, 261. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.Z.; Yan, L.; Jiang, Y.Y. The synthesis, regulation, and functions of sterols in Candida albicans: Well-known but still lots to learn. Virulence 2016, 7, 649–659. [Google Scholar] [CrossRef]
- Abdel-Maksoud, G.; Gaballah, S.; Youssef, A.M.; Eid, A.M.; Sultan, M.H.; Fouda, A. Eco-friendly approach for control of fungal deterioration of archaeological skeleton dated back to the Greco-Roman period. J. Cult. Herit. 2023, 59, 38–48. [Google Scholar] [CrossRef]
- Jalal, M.; Azam, A.M.; Ghazanfar, A.S.; Khan, H.M.; Rehman, S. Anticandidal activity of bioinspired ZnO NPs: Effect on growth, cell morphology and key virulence attributes of Candida species. Artif. Cells Nanomed. Biotechnol. 2018, 46, 912–925. [Google Scholar] [CrossRef]
- Amin, M.A.; Algamdi, N.A.; Waznah, M.S.; Bukhari, D.A.; Alsharif, S.M.; Alkhayri, F.; Abdel-Nasser, M.; Fouda, A. An Insight into Antimicrobial, Antioxidant, Anticancer, and Antidiabetic Activities of Trimetallic Se/ZnO/CuO Nanoalloys Fabricated by Aqueous Extract of Nitraria retusa. J. Clust. Sci. 2024, 36, 19. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef]
- Allam, R.M.; El-Nasr, N.M.E.A.; Elbaset, M.A.; Saleh, D.O.; El-Seidy, A.M.A. Unveiling the potency of ZnO and CuO nanocomposites in combating hepatocellular carcinoma by inducing cell death and suppressing migration. Sci. Rep. 2025, 15, 15477. [Google Scholar] [CrossRef]
- Rishikesan, S.; Basha, M.A.M. Synthesis, Characterization and Evaluation of Antimicrobial, Antioxidant & Anticancer Activities of Copper Doped Zinc Oxide Nanoparticles. Acta Chim. Slov. 2020, 67, 235–245. [Google Scholar] [CrossRef]
- Ngwenya, S.; Sithole, N.J.; Ramachela, K.; Mthiyane, D.M.N.; Mwanza, M.; Singh, M.; Onwudiwe, D.C. Eco-friendly synthesis of ZnO, CuO, and ZnO/CuO nanoparticles using extract of spent Pleurotus ostreatus substrate, and their antioxidant and anticancer activities. Discov. Nano 2025, 20, 35. [Google Scholar] [CrossRef] [PubMed]
- Shiny, P.J.; Mukherjee, A.; Chandrasekaran, N. DNA damage and mitochondria-mediated apoptosis of A549 lung carcinoma cells induced by biosynthesised silver and platinum nanoparticles. RSC Adv. 2016, 6, 27775–27787. [Google Scholar] [CrossRef]
- Xuan, L.; Ju, Z.; Skonieczna, M.; Zhou, P.-K.; Huang, R. Nanoparticles-induced potential toxicity on human health: Applications, toxicity mechanisms, and evaluation models. MedComm 2023, 4, e327. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.; Eid, A.M.; Guibal, E.; Hamza, M.F.; Hassan, S.E.; Alkhalifah, D.H.M.; El-Hossary, D. Green Synthesis of Gold Nanoparticles by Aqueous Extract of Zingiber officinale: Characterization and Insight into Antimicrobial, Antioxidant, and In Vitro Cytotoxic Activities. Appl. Sci. 2022, 12, 2879. [Google Scholar] [CrossRef]
- Alsharif, S.M.; Eid, A.M.; Hassan, S.E.-D.; Abdel-Rahman, M.A.; Abdel-Maksoud, G.; Bukhari, D.A.; Sefrji, F.O.; Alqurashi, A.A.; Waznah, M.S.; Fouda, A. Unveiling the biodeterioration activity of microbial communities to the historical manuscript: Biocontrol using biosynthesized gold and zinc oxide nanoparticles. J. Cult. Herit. 2025, 71, 440–452. [Google Scholar] [CrossRef]
- McFarland, J. Nephelometer: An Instrument for Estimating the Number of Bacteria in Suspensions Used for Calculating the Opsonic Index and for Vaccines. J. Am. Med. Assoc. 1907, 14, 1176–1178. [Google Scholar] [CrossRef]
- Eid, A.M.; Hassan, S.E.; Hamza, M.F.; Selim, S.; Almuhayawi, M.S.; Alruhaili, M.H.; Tarabulsi, M.K.; Nagshabandi, M.K.; Fouda, A. Photocatalytic, Antimicrobial, and Cytotoxic Efficacy of Biogenic Silver Nanoparticles Fabricated by Bacillus amyloliquefaciens. Catalysts 2024, 14, 419. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fouda, A.; Alsharif, S.M.; Eid, A.M.; Albalawi, A.S.; Amin, M.A.; Alraddadi, F.A.; Almutrafy, A.M.; Bukhari, D.A.; Algamdi, N.A.; Abdel-Rahman, M.A. Photocatalytic Activity of Green-Synthesized Semiconductor CuO/ZnO Nanocomposites Against Organic Dye: An Assessment of Antimicrobial and Cytotoxicity Investigations. Catalysts 2025, 15, 1096. https://doi.org/10.3390/catal15121096
Fouda A, Alsharif SM, Eid AM, Albalawi AS, Amin MA, Alraddadi FA, Almutrafy AM, Bukhari DA, Algamdi NA, Abdel-Rahman MA. Photocatalytic Activity of Green-Synthesized Semiconductor CuO/ZnO Nanocomposites Against Organic Dye: An Assessment of Antimicrobial and Cytotoxicity Investigations. Catalysts. 2025; 15(12):1096. https://doi.org/10.3390/catal15121096
Chicago/Turabian StyleFouda, Amr, Sultan M. Alsharif, Ahmed M. Eid, Abeer S. Albalawi, Mohamed A. Amin, Faisal A. Alraddadi, Abeer M. Almutrafy, Duaa A. Bukhari, Noura A. Algamdi, and Mohamed Ali Abdel-Rahman. 2025. "Photocatalytic Activity of Green-Synthesized Semiconductor CuO/ZnO Nanocomposites Against Organic Dye: An Assessment of Antimicrobial and Cytotoxicity Investigations" Catalysts 15, no. 12: 1096. https://doi.org/10.3390/catal15121096
APA StyleFouda, A., Alsharif, S. M., Eid, A. M., Albalawi, A. S., Amin, M. A., Alraddadi, F. A., Almutrafy, A. M., Bukhari, D. A., Algamdi, N. A., & Abdel-Rahman, M. A. (2025). Photocatalytic Activity of Green-Synthesized Semiconductor CuO/ZnO Nanocomposites Against Organic Dye: An Assessment of Antimicrobial and Cytotoxicity Investigations. Catalysts, 15(12), 1096. https://doi.org/10.3390/catal15121096








