A Comparative Evaluation of Bimetallic Alumina-Supported Catalysts: Synthesis, Characterization and Catalytic Performance in Pyrolysis of Expanded Polystyrene Waste
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.1.1. N2-Physisorption
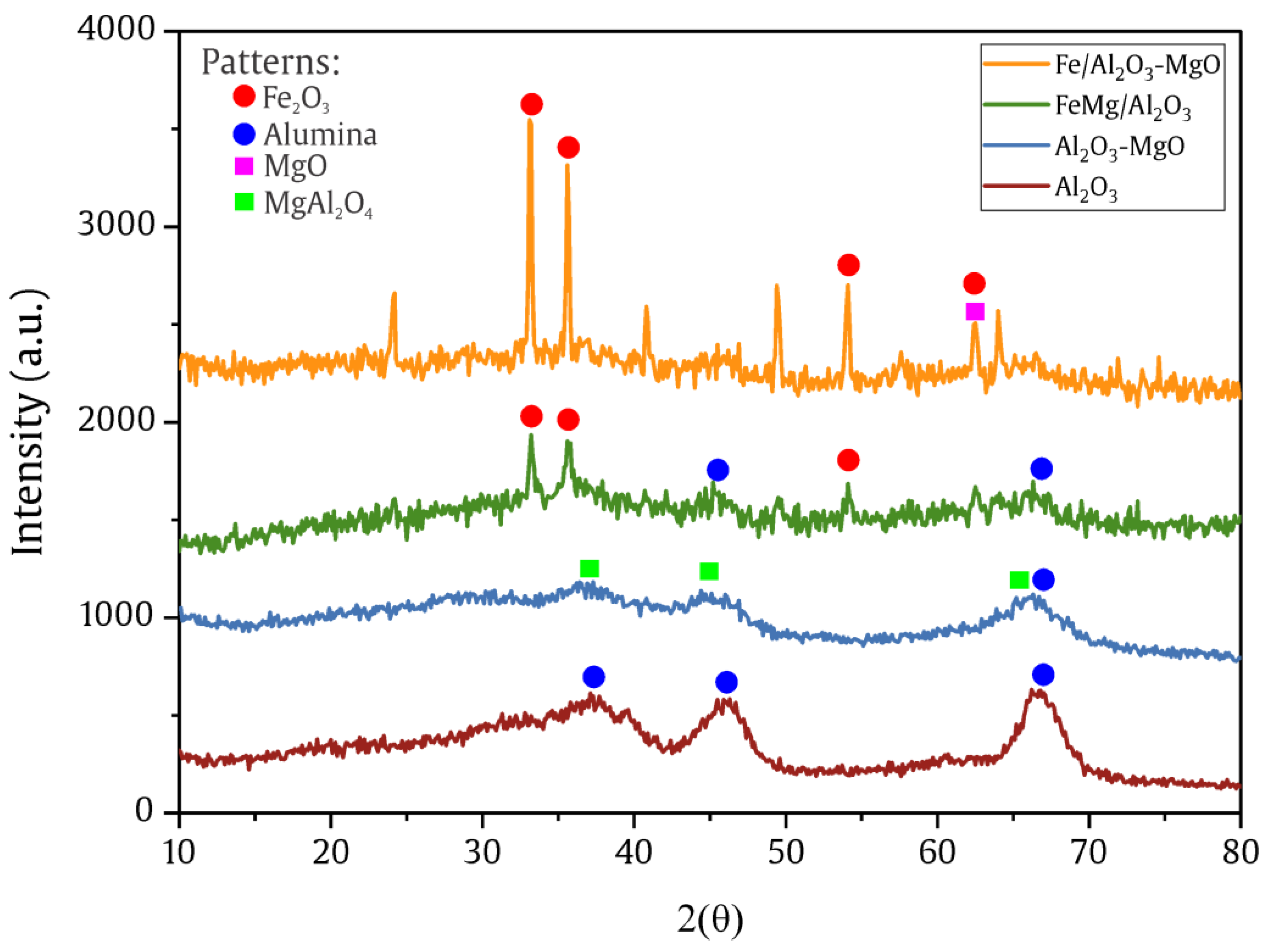
2.1.2. X-Ray Diffraction (XRD)
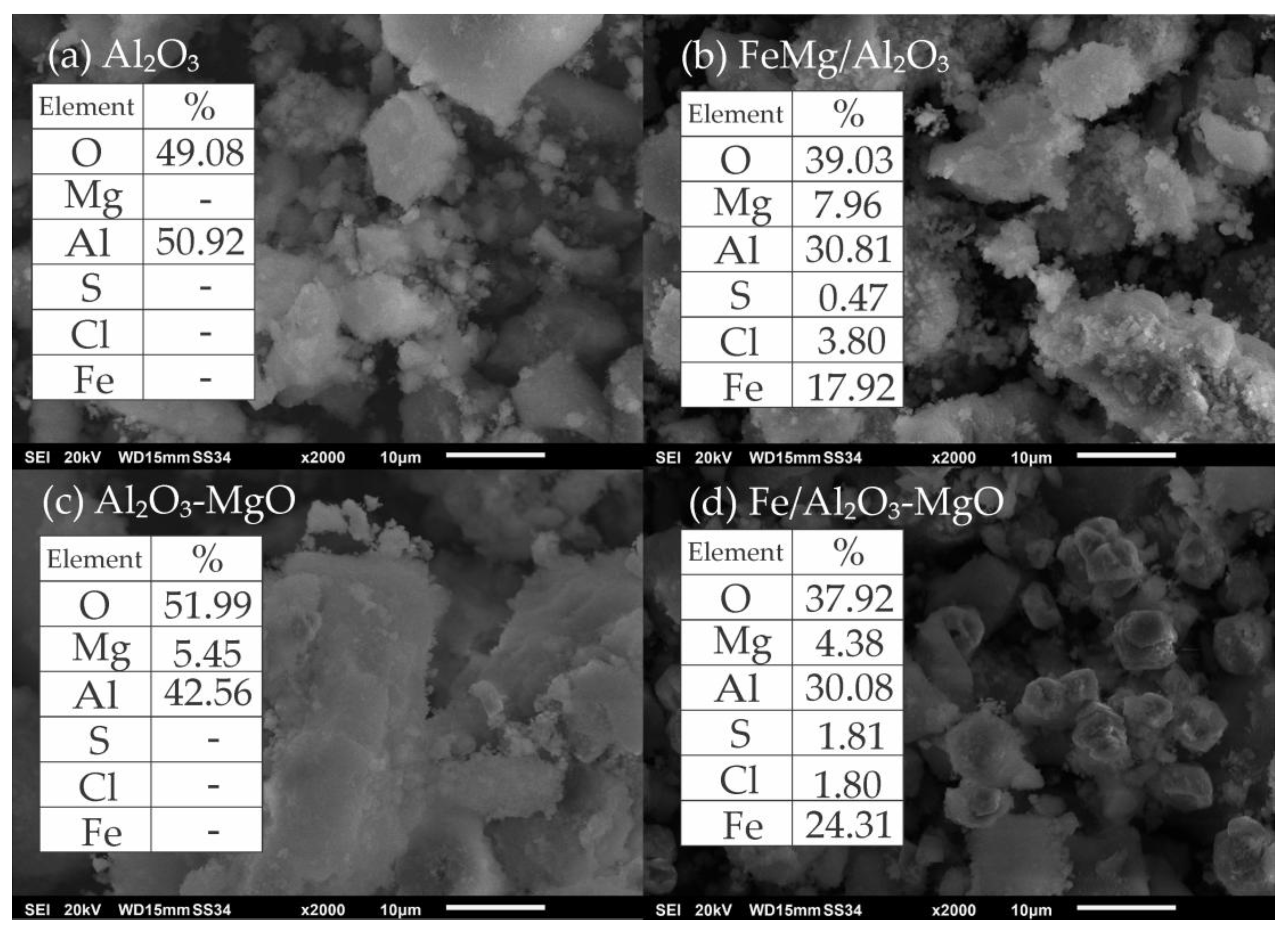
2.1.3. Scanning Electron Microscopy (SEM) and Energy Dispersive X-Ray Spectroscopy (EDS)
2.2. Catalyst Evaluation
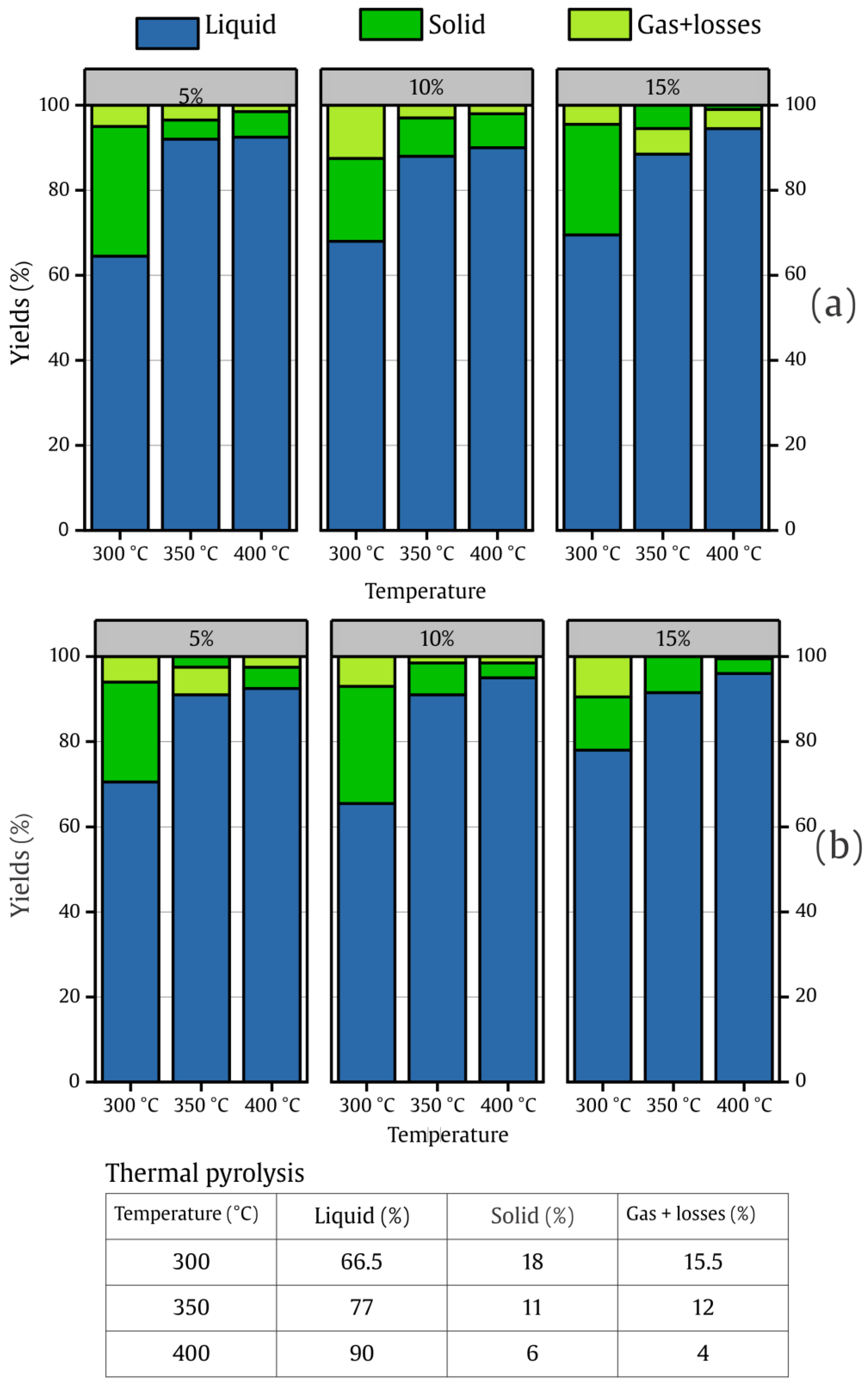
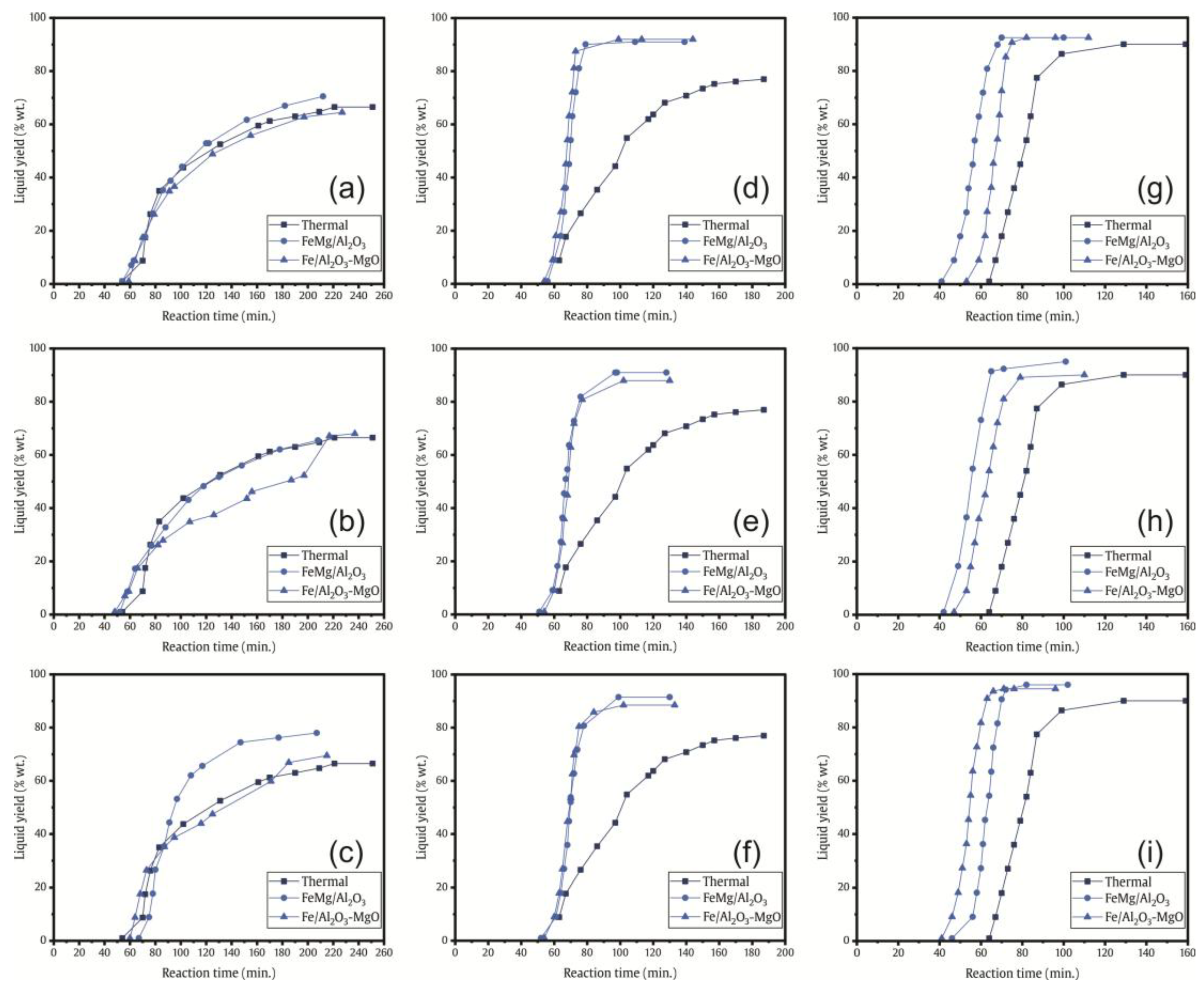
2.2.1. Comparative of Product Yields
2.2.2. Conversion Rate of Catalytic Pyrolysis vs. Thermal Pyrolysis
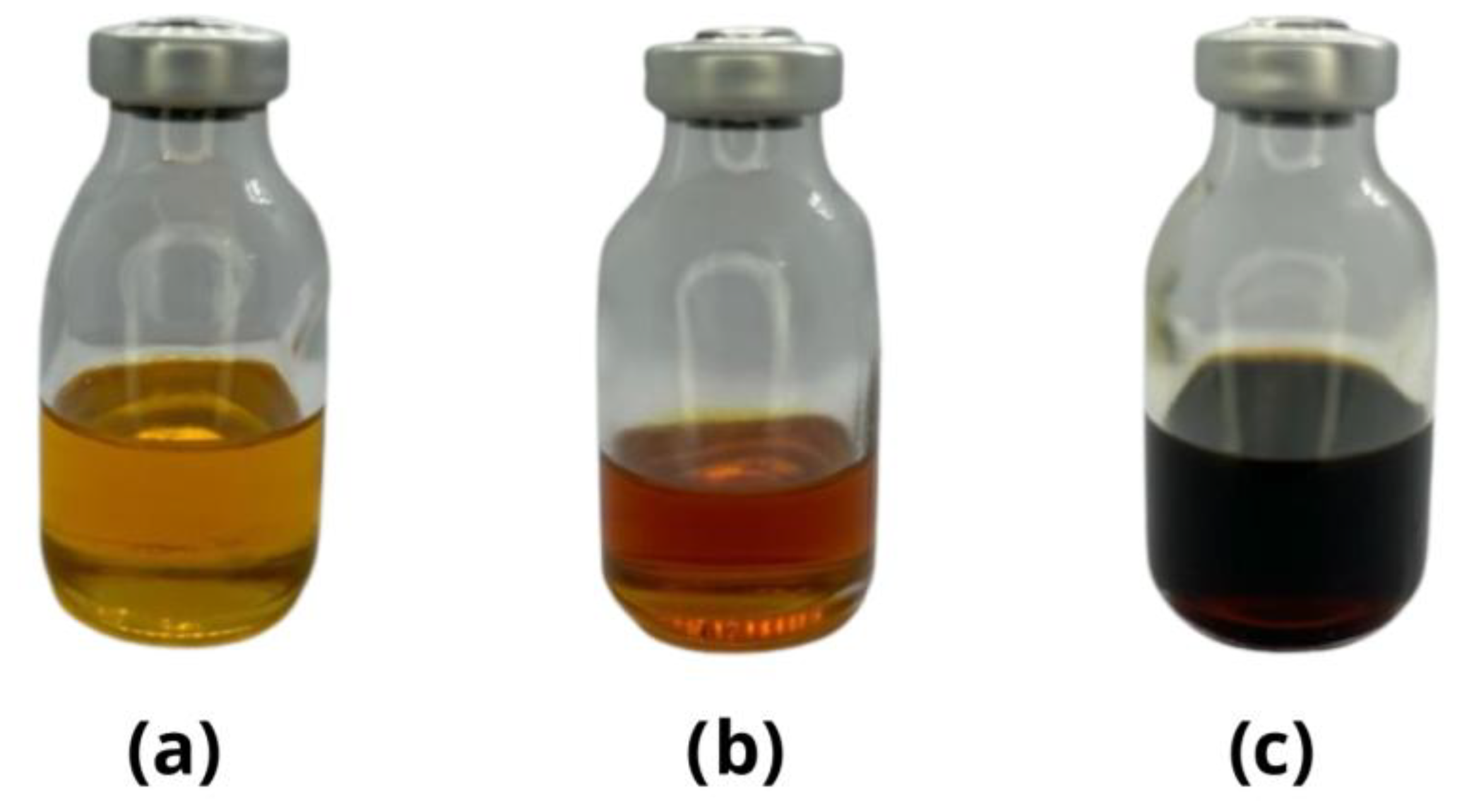
2.2.3. Aromatic Composition of Liquid Products
2.3. Performance Comparison with Literature
3. Materials and Methods
3.1. EPS Feedstock
3.2. Reagents
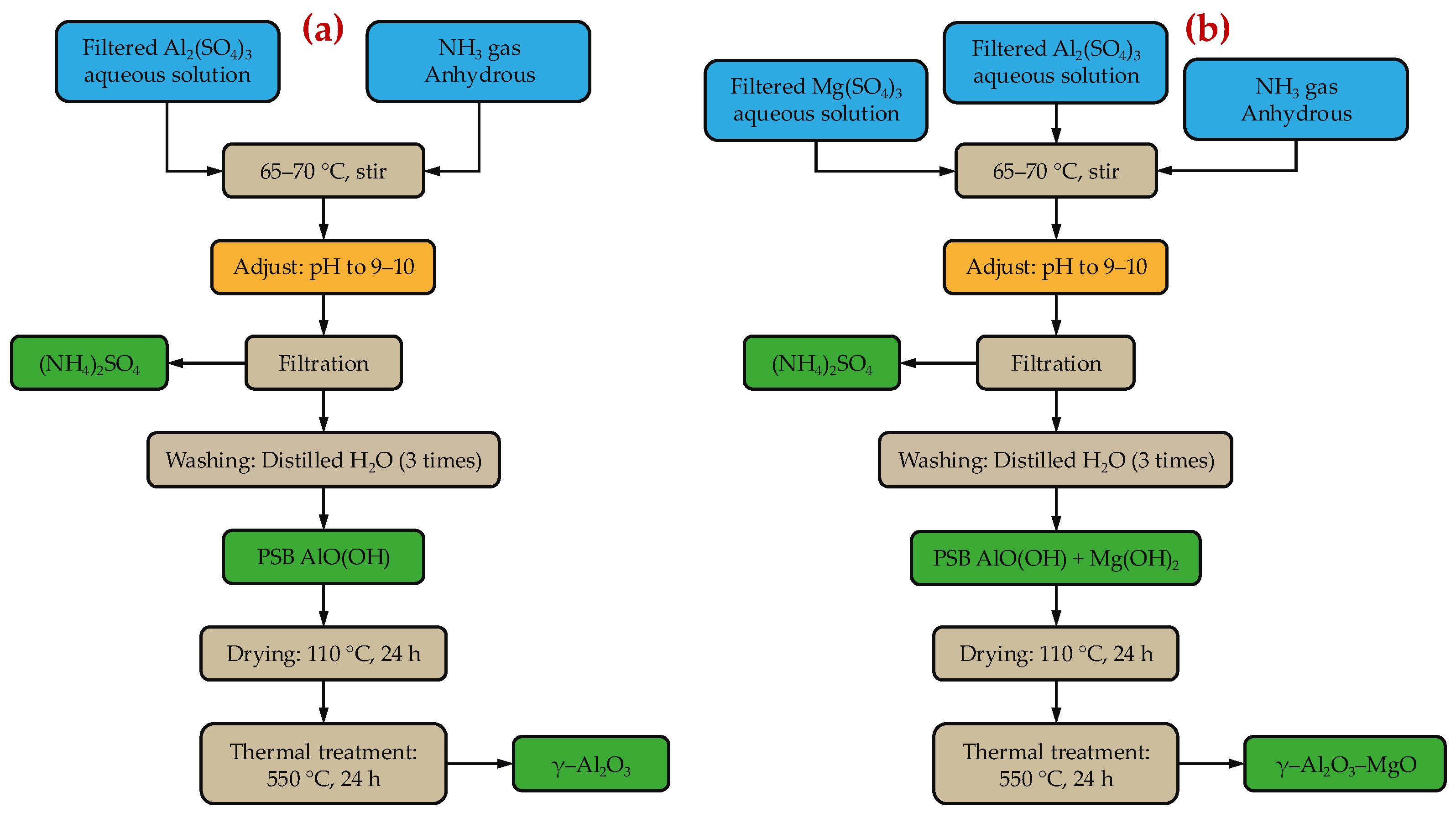
3.3. Synthesis of Catalyst Supports
3.4. Catalysts Formulation
3.5. Catalyst Characterization
3.5.1. N2-Physisorption
3.5.2. X-Ray Diffraction (XRD)
3.5.3. Scanning Electron Microscopy (SEM)
3.5.4. Energy Dispersive X-Ray Spectroscopy (EDS)
3.6. Liquid Fraction Characterization
3.7. Catalyst Performance Studies
4. Conclusions
- Global evaluation of catalytic pyrolysis showed that the incorporation of Fe significantly modifies the pyrolysis performance, improving the yield and selectivity toward the liquid phase. However, the final behavior depends significantly on the nature of the support and the method in which the metallic components are integrated into the structure.
- The highest liquid yield achieved was 96% using FeMg/Al2O3 with 15 wt.% of catalyst loading at 400 °C, and the lowest yield obtained was 69.5% using Fe/Al2O3-MgO at 300 °C at the same catalyst loading.
- In terms of process intensification, the use of 15 wt.% of FeMg/Al2O3 reduced the time required to achieve 91.5% of liquid yield by around 45% compared to thermal pyrolysis at 350 °C.
- Fe/Al2O3-MgO exhibited less dispersion and lower accessibility to active sites, requiring longer operating times to achieve competitive yields. However, the possible formation of MgAl2O4 spinel may lead to improved operational durability by enhancing thermal and structural stability.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BET | Brunauer-Emmett-Teller |
| BJH | Barret-Joyner-Halenda |
| CNTs | Carbon nanotubes |
| EDS | Energy dispersive X-ray spectroscopy |
| EPS | Expanded polystyrene |
| GC | Gas Chromatography |
| IUPAC | International Union of Pure and Applied Chemistry |
| PS | Polystyrene |
| PSB | Pseudo-boehmite |
| PSD | Particle Size Distribution |
| SEM | Scanning Electron Microscopy |
| XRD | X-ray diffraction |
References
- Wu, J.; Xiao, H.; Cai, N.; Yang, H.; Chen, H.; Yang, Y. Sustainable Conversion of Polypropylene into High-Value Carbon Nanotubes Using Chelating Agent-Modified Fe/Al2O3 Catalysts. J. Energy Inst. 2026, 124, 102320. [Google Scholar] [CrossRef]
- PlasticsEurope. Plastics the Fast Facts. 2025. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2025/ (accessed on 3 November 2025).
- Arora, Y.; Sharma, S.; Sharma, V. Microalgae in Bioplastic Production: A Comprehensive Review. Arab. J. Sci. Eng. 2023, 48, 7225–7241. [Google Scholar] [CrossRef]
- Li, X.; Mahadas, N.A.; Zhang, M.; DePodesta, J.; Stefik, M.; Tang, C. Sustainable High-Density Polyethylene via Chemical Recycling: From Modification to Polymerization Methods. Polymer 2024, 295, 126698. [Google Scholar] [CrossRef]
- Sun, K.; Themelis, N.J.; Bourtsalas, A.C.; Huang, Q. Selective Production of Aromatics from Waste Plastic Pyrolysis by Using Sewage Sludge Derived Char Catalyst. J. Cleaner Prod. 2020, 268, 122038. [Google Scholar] [CrossRef]
- Bashirgonbadi, A.; Saputra Lase, I.; Delva, L.; Van Geem, K.M.; De Meester, S.; Ragaert, K. Quality Evaluation and Economic Assessment of an Improved Mechanical Recycling Process for Post-Consumer Flexible Plastics. Waste Manag. 2022, 153, 41–51. [Google Scholar] [CrossRef]
- Raby, H.S.; Rahman, M.M.; Mohammed, M.G.; Siddiquee, M.N. Oxidative Depolymerization of Polyethylene (PE), Polypropylene (PP) and Polystyrene (PS) Wastes to Value-Added Chemicals. Polym. Degrad. Stab. 2025, 242, 111709. [Google Scholar] [CrossRef]
- Karacaoglan, G.; Thibault, M.; Roger, J.; Pirio, N.; Fajerwerg, K.; Kahn, M.L.; Demirci, U.B.; Hierso, J.-C. Chemical Hydrogen Storage Materials-Boranes and Silanes Catalytic Solvolysis and Dehydrogenation: A Mechanistic and Regeneration Perspective. Coord. Chem. Rev. 2026, 548, 217094. [Google Scholar] [CrossRef]
- Kemp, A.; Rahman, T.; Jahromi, H.; Adhikari, S. Production of Aviation Fuel-Range Hydrocarbons Through Catalytic Co-Pyrolysis of Polystyrene and Southern Pine. Catalysts 2024, 14, 806. [Google Scholar] [CrossRef]
- Jang, T.; Shin, I.; Choi, J.; Lee, S.; Hwang, H.; Kim, M.; Kim, B.H. Advancements in Chemical Recycling Catalysts for Plastic Waste in South Korea. Catalysts 2025, 15, 414. [Google Scholar] [CrossRef]
- Kek, H.Y.; Wong, S.J.; Tan, H.; Othman, M.H.D.; Tan, K.Y.; Fong, W.C.W.; Woon, K.S.; Wang, X.-C.; Chiong, M.C.; Wong, K.Y. Plastic-to-Hydrogen through Pyrolysis and Gasification: Life-Cycle Implications, Techno-Economic, and Digital Optimisation. J. Anal. Appl. Pyrolysis 2026, 193, 107440. [Google Scholar] [CrossRef]
- Vuppaladadiyam, S.S.V.; Vuppaladadiyam, A.K.; Sahoo, A.; Urgunde, A.; Murugavelh, S.; Šrámek, V.; Pohořelý, M.; Trakal, L.; Bhattacharya, S.; Sarmah, A.K.; et al. Waste to Energy: Trending Key Challenges and Current Technologies in Waste Plastic Management. Sci. Total Environ. 2024, 913, 169436. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, Y.; Wang, D.; Yan, M.; Zhang, J.; Zhang, P.; Ding, T.; Chen, L.; Chen, C. Current Technologies for Plastic Waste Treatment: A Review. J. Cleaner Prod. 2021, 282, 124523. [Google Scholar] [CrossRef]
- Habib, M.A.; Abdulrahman, G.A.Q.; Alquaity, A.B.S.; Qasem, N.A.A. Hydrogen Combustion, Production, and Applications: A Review. Alex. Eng. J. 2024, 100, 182–207. [Google Scholar] [CrossRef]
- Faizan, M.; Jallow, A.; Siddiqui, M.N.; Jameel, A.G.A. Pyrolytic Upcycling of Plastic Waste into Graphene and Carbon Nanostructures. J. Anal. Appl. Pyrolysis 2026, 193, 107407. [Google Scholar] [CrossRef]
- Gines, R.; Montalvo, C.; Luna, G.; Montalvo, D.; Cerón, R.M.; Cerón, J.G.; Ginés, S.; García, A.; Aguilar, C.A. Photodegradation of Pyridine in a Fluidized Bed Photocatalytic Reactor Using Pt-ZnO Supported on Al2O3 as a Catalyst. Catalysts 2025, 15, 772. [Google Scholar] [CrossRef]
- Wang, J.; Yang, X.; Ma, Q.; Gao, X.; Zhang, J.; Fan, S.; Zhao, T.-S. Promotion of Al2O3 to Ni/In2O3 in the Low-Temperature CO2 + H2 Reaction to Methanol. Fuel 2026, 405, 136814. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Song, Y.; Xu, P.; Wang, L.; Sun, X.; Xiang, S. Cu/γ-Al2O3-Y Molecular Sieve Composite Supported Catalyst for Ethylene Oxychlorination Reaction and Mechanism Study. Mol. Catal. 2026, 588, 115557. [Google Scholar] [CrossRef]
- Wiyantoko, B.; Saviola, A.J.; Hutama, A.S.; Fatimah, I.; Hauli, L.; Oh, W.-C.; Sagadevan, S.; Wijaya, K. Fixing Silicotungstic Acid onto Activated Bentonite Assisted by Ultrasonic Waves and Its Application as a Solid Acid Catalyst for Levulinic Acid Esterification into Butyl Levulinate. Inorg. Chem. Commun. 2026, 183, 115721. [Google Scholar] [CrossRef]
- Ayoob, H.W.; Taieh, N.K.; Abdullah, A.S.; Homod, R.Z.; Medina, F. Salt Effect and Comparative Analysis of Micro and Nano-Bentonite in Blue Dye Removal: Surface Morphology and Adsorption Efficiency. Powder Technol. 2026, 468, 121666. [Google Scholar] [CrossRef]
- Ke, Z.-W.; Liu, Y.-X.; Wei, S.-J.; Shen, P.; Yang, K.; Chen, Y.-M.; Li, Y.-C. Hexadecyltrimethylammonium-Modified Bentonite for Enhanced Adsorption of per- and Polyfluoroalkyl Substances: Experiment and Thermodynamic Mechanism. Appl. Clay Sci. 2026, 279, 108022. [Google Scholar] [CrossRef]
- Dhaniswara, T.K.; Sardi, B.; Juliastuti, S.R.; Mahfud, M. Non-Catalytic and Catalytic Pyrolysis of Polystyrene, Polypropylene, and Polyethylene for Liquid Fuel Production Using a Reactor Integrated with Fractionation Column. Cleaner Waste Syst. 2025, 11, 100305. [Google Scholar] [CrossRef]
- Gil-Díaz, M.; Mancho, C.; Pérez, R.A.; Alonso, J.; Padilla, I.; López-Delgado, A.; Lobo, M.C. Viability of Zeolites Obtained from Hazardous Aluminum Industry Waste for Soil Remediation. Environ. Technol. Innov. 2025, 40, 104582. [Google Scholar] [CrossRef]
- Barzallo, D.; Lazo, R.; Medina, C.; Guashpa, C.; Tacuri, C.; Palmay, P. Synthesis and Application of ZSM-5 Catalyst Supported with Zinc and/or Nickel in the Conversion of Pyrolytic Gases from Recycled Polypropylene and Polystyrene Mixtures under Hydrogen Atmosphere. Polymers 2023, 15, 3329. [Google Scholar] [CrossRef] [PubMed]
- Kate, A.; Sahu, L.K.; Pandey, J.; Mishra, M.; Sharma, P.K. Green Catalysis for Chemical Transformation: The Need for the Sustainable Development. Curr. Res. Green Sustain. Chem. 2022, 5, 100248. [Google Scholar] [CrossRef]
- Altalbawy, F.M.A.; Arbab, A.M.; Makasana, J.; Renuka Jyothi, S.; Kumari, B.; Bhanot, D.; Khelif, A. Iodine Source Heterogenized on Fe3O4@SiO2 Modified with Dopamine as a Green and Reusable Nanocatalyst for the Synthesis of 2,4,5-Triaryl Imidazoles. Polyhedron 2025, 268, 117355. [Google Scholar] [CrossRef]
- Ross, J.R.H. An Introduction to Heterogeneous Catalysis and Its Development Through the Centuries-Chemistry in Two Dimensions. In Contemporary Catalysis-Fundamentals and Current Applications; Elsevier: Limerick, Ireland, 2019; pp. 3–38. ISBN 978-0-444-63474-0. [Google Scholar]
- Abbas-Abadi, M.S. The Effect of Process and Structural Parameters on the Stability, Thermo-mechanical and Thermal Degradation of Polymers with Hydrocarbon Skeleton Containing PE, PP, PS, PVC, NR, PBR and SBR. J. Therm Anal. Calorim. 2021, 143, 2867–2882. [Google Scholar] [CrossRef]
- Shah, J.; Jan, M.R. Adnan Tertiary Recycling of Waste Polystyrene Using Magnesium Impregnated Catalyst into Valuable Products. J. Anal. Appl. Pyrolysis 2015, 114, 163–171. [Google Scholar] [CrossRef]
- Amjad, U.-S.; Ishaq, M.; Rehman, H.U.; Ahmad, N.; Sherin, L.; Hussain, M.; Mustafa, M. Diesel and Gasoline like Fuel Production with Minimum Styrene Content from Catalytic Pyrolysis of Polystyrene. Environ. Prog. Sustain. Energy 2021, 40, e13493. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Y.; Li, S.; Yue, C. Catalytic Pyrolysis of Polystyrene in Different Reactors: Effects of Operating Conditions on Distribution and Composition of Products. J. Anal. Appl. Pyrolysis 2024, 177, 106366. [Google Scholar] [CrossRef]
- Adnan; Shah, J.; Rasul Jan, M. Polystyrene Degradation Studies Using Cu Supported Catalysts. J.l Anal. Appl. Pyrolysis 2014, 109, 196–204. [Google Scholar] [CrossRef]
- Sukkasem, T.; Yoojaroen, N.; Truadnog, Y.; Prayutte, P.; Junpirom, S.; Sukjit, E. Converting Polystyrene Waste into Valuable Oils: High Performance of SUZ-4 Zeolite for Selective Oil Component Conversion in Catalytic Pyrolysis. Fuel 2026, 405, 136456. [Google Scholar] [CrossRef]
- Gonzalez-Aguilar, A.M.; Pérez-García, V.; Riesco-Ávila, J.M. A Thermo-Catalytic Pyrolysis of Polystyrene Waste Review: A Systematic, Statistical, and Bibliometric Approach. Polymers 2023, 15, 1582. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Sharma, S.; Pramanik, H. Pyrolysis of Waste Expanded Polystyrene and Reduction of Styrene via In-Situ Multiphase Pyrolysis of Product Oil for the Production of Fuel Range Hydrocarbons. Waste Manag. 2021, 120, 330–339. [Google Scholar] [CrossRef]
- Gonzalez-Aguilar, A.M.; Cabrera-Madera, V.P.; Vera-Rozo, J.R.; Riesco-Ávila, J.M. Effects of Heating Rate and Temperature on the Thermal Pyrolysis of Expanded Polystyrene Post-Industrial Waste. Polymers 2022, 14, 4957. [Google Scholar] [CrossRef]
- Hussain, Z.; Imtiaz, M.; Khan, K.M.; Naz, M.Y.; Khaled, U.; Khan, Y. White Cement and Burnt Brick Powder Catalyzed Pyrolysis of Waste Polystyrene for Production of Liquid and Gaseous Fuels. Asia—Pac. J. Chem. Eng. 2020, 15, e2391. [Google Scholar] [CrossRef]
- Van der Westhuizen, S.; Collard, F.-X.; Görgens, J. Pyrolysis of Waste Polystyrene into Transportation Fuel: Effect of Contamination on Oil Yield and Production at Pilot Scale. J. Anal. Appl. Pyrolysis 2022, 161, 105407. [Google Scholar] [CrossRef]
- Xie, M.; Cheng, M.; Yang, Y.; Huang, Z.; Zhou, T.; Zhao, Y.; Xiao, P.; Cen, Q.; Liu, Z.; Li, B. A Review on Catalytic Pyrolysis of Textile Waste to High-Value Products: Catalytic Mechanisms, Products Application and Perspectives. Chem. Eng. J. 2024, 498, 155120. [Google Scholar] [CrossRef]
- Yao, D.; Wu, C.; Yang, H.; Zhang, Y.; Nahil, M.A.; Chen, Y.; Williams, P.T.; Chen, H. Co-Production of Hydrogen and Carbon Nanotubes from Catalytic Pyrolysis of Waste Plastics on Ni-Fe Bimetallic Catalyst. Energy Conv. Manag. 2017, 148, 692–700. [Google Scholar] [CrossRef]
- Li, J.; Xia, H.; Wu, Q.; Hu, Z.; Hao, Z.; Zhu, Z. Hydrocracking of the Crude Oil from Thermal Pyrolysis of Municipal Wastes over Bi-Functional Mo-Ni Catalyst. Catal. Today 2016, 271, 172–178. [Google Scholar] [CrossRef]
- Aljabri, N.M.; Lai, Z.; Huang, K.-W. Selective Conversion of Polystyrene into Renewable Chemical Feedstock under Mild Conditions. Waste Manag. 2018, 78, 871–879. [Google Scholar] [CrossRef]
- Ghorai, S.; Pant, K.K. Investigations on the Column Performance of Fluoride Adsorption by Activated Alumina in a Fixed-Bed. Chem. Eng. J. 2004, 98, 165–173. [Google Scholar] [CrossRef]
- Hao, O.J.; Huang, C.P. Adsorption Characteristics of Fluoride onto Hydrous Alumina. J. Environ. Eng. 1986, 112, 1054–1069. [Google Scholar] [CrossRef]
- Aljabri, N.M.; Lai, Z.; Hadjichristidis, N.; Huang, K.-W. Renewable Aromatics from the Degradation of Polystyrene under Mild Conditions. J. Saudi Chem. Society 2017, 21, 983–989. [Google Scholar] [CrossRef]
- Powder Diffraction File (PDF); International Centre for Diffraction Data: Newton Square, PA, USA, 2023.
- Hodala, J.L.; Moon, D.J.; Reddy, K.R.; Reddy, C.V.; Kumar, T.N.; Ahamed, M.I.; Raghu, A.V. Catalyst Design for Maximizing C5+ Yields during Fischer-Tropsch Synthesis. Int. J. Hydrog. Energy 2021, 46, 3289–3301. [Google Scholar] [CrossRef]
- Guo, J.; Lou, H.; Zhao, H.; Chai, D.; Zheng, X. Dry Reforming of Methane over Nickel Catalysts Supported on Magnesium Aluminate Spinels. Appl. Catal. A Gen. 2004, 273, 75–82. [Google Scholar] [CrossRef]
- Kim, J.-S.; Lee, W.-Y.; Lee, S.-B.; Kim, S.-B.; Choi, M.-J. Degradation of Polystyrene Waste over Base Promoted Fe Catalysts. Catalysis Today 2003, 87, 59–68. [Google Scholar] [CrossRef]
- Thybaut, J.W.; Marin, G.B. Multiscale Aspects in Hydrocracking. In Advances in Catalysis; Elsevier: Amsterdam, The Netherlands, 2016; Volume 59, pp. 109–238. ISBN 978-0-12-811004-1. [Google Scholar]
- Shah, J.; Jan, M.R. Adnan Catalytic Activity of Metal Impregnated Catalysts for Degradation of Waste Polystyrene. J. Ind. Eng. Chem. 2014, 20, 3604–3611. [Google Scholar] [CrossRef]
- Inayat, A.; Fasolini, A.; Basile, F.; Fridrichova, D.; Lestinský, P. Chemical Recycling of Waste Polystyrene by Thermo-Catalytic Pyrolysis: A Description for Different Feedstocks, Catalysts and Operation Modes. Polym. Degrad. Stab. 2022, 201, 109981. [Google Scholar] [CrossRef]
- Martínez-Rosales, J.M. Control de textura de Alúminas Activadas vía Sustitución de Líquido Intermicelar. Master’s Thesis, Universidad de Guanajuato, Guanajuato, Mexico, 1994. [Google Scholar]
- Leyva-Ramos, R.; Medellin-Castillo, N.A.; Jacobo-Azuara, A.; Mendoza-Barron, J.; Landin-Rodriguez, L.E.; Martínez-Rosales, J.M.; Aragon-Piña, A. Fluoride Removal from Water Solution by Adsorption on Activated Alumina Prepared from Pseudo-Boehmite. J. Environ. Manag. 2008, 18, 303–311. [Google Scholar]
- Ross, J.R.H. Catalyst Characterization. In Contemporary Catalysis- Fundamentals and Current Applications; Elsevier: Limerick, Ireland, 2019; pp. 121–132. ISBN 978-0-444-63474-0. [Google Scholar]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface Area and Pore Texture of Catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- ASTM D86; Test Method for Distillation of Petroleum Products and Liquid Fuels at Atmospheric Pressure. Advancing Standards Transforming Markets Standard (ASTM): West Conshohocken, PA, USA, 2024.

| Sample | SBET [m2·g−1] | SBJH [m2·g−1] | Pore Volume [cm3·g−1] | Pore Size [Å] |
|---|---|---|---|---|
| ϒ-Al2O3 | 286 | 384 | 0.57 | 59.1 |
| Al2O3-MgO | 208 | 334 | 0.47 | 56.5 |
| FeMg/Al2O3 | 171 | 242 | 0.34 | 57.0 |
| Fe/Al2O3-MgO | 162 | 247 | 0.36 | 58.1 |
| Aromatic | FeMg/Al2O3 | Fe/Al2O3-MgO | Thermal | |||
|---|---|---|---|---|---|---|
| Average | ± | Average | ± | Average | ± | |
| Toluene | 7.41 | 1.33 | 8.04 | 1.89 | 6.09 | 1.80 |
| Ethylbenzene | 7.08 | 3.25 | 8.87 | 5.27 | 7.27 | 0.87 |
| Cumene | 0.15 | 0.01 | 0.18 | 0.02 | n.d. | n.d. |
| α-Methyl styrene | 11.68 | 2.20 | 12.52 | 4.36 | 6.00 | 0.49 |
| Styrene | 49.65 | 2.74 | 48.93 | 4.07 | 61.1 | 7.51 |
| Benzene | 0.25 | 0.05 | 0.36 | 0.11 | n.d. | n.d. |
| Xylene | 0.99 | 2.21 | 1.40 | 3.13 | n.d. | n.d. |
| Feedstock | Reactor | Catalyst | Loading (wt.%) | Temperature (°C) | Liquid Yield (%) | Reference |
|---|---|---|---|---|---|---|
| Virgin PS | Batch | FeCu/Al2O3 | 10 | 250 | 66.0 | [45] |
| Virgin PS | Batch | FeCo/Al2O3 | 10 | 250 | 91.0 | [42] |
| EPS waste | Pyrex | Mg/Al2O3 | 30 | 450 | 95.4 | [29] |
| EPS waste | Pyrex | Mg/Al2O3 | 20 | 450 | 87.0 | [51] |
| EPS waste | Pyrex | Fe/Al2O3 | 20 | 450 | 89.2 | [51] |
| Virgin PS | Bench-scale | MgO | 10 | 500 | 93.7 | [52] |
| EPS waste | Semi-batch | FeMg/Al2O3 | 15 | 300 | 78.0 | This work |
| EPS waste | Semi-batch | FeMg/Al2O3 | 15 | 350 | 91.5 | This work |
| EPS waste | Semi-batch | FeMg/Al2O3 | 15 | 400 | 96.0 | This work |
| EPS waste | Semi-batch | Fe/Al2O3-MgO | 15 | 300 | 69.5 | This work |
| EPS waste | Semi-batch | Fe/Al2O3-MgO | 15 | 350 | 91.5 | This work |
| EPS waste | Semi-batch | Fe/Al2O3-MgO | 15 | 400 | 94.5 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Aguilar, A.M.; Riesco-Ávila, J.M.; Martínez-Rosales, M.; Tejeda-del-Cueto, M.E.; Vigueras-Zuniga, M.-O.; Hernández-Hernández, J. A Comparative Evaluation of Bimetallic Alumina-Supported Catalysts: Synthesis, Characterization and Catalytic Performance in Pyrolysis of Expanded Polystyrene Waste. Catalysts 2025, 15, 1094. https://doi.org/10.3390/catal15121094
Gonzalez-Aguilar AM, Riesco-Ávila JM, Martínez-Rosales M, Tejeda-del-Cueto ME, Vigueras-Zuniga M-O, Hernández-Hernández J. A Comparative Evaluation of Bimetallic Alumina-Supported Catalysts: Synthesis, Characterization and Catalytic Performance in Pyrolysis of Expanded Polystyrene Waste. Catalysts. 2025; 15(12):1094. https://doi.org/10.3390/catal15121094
Chicago/Turabian StyleGonzalez-Aguilar, Arantxa M., José M. Riesco-Ávila, Merced Martínez-Rosales, María E. Tejeda-del-Cueto, Marco-Osvaldo Vigueras-Zuniga, and José Hernández-Hernández. 2025. "A Comparative Evaluation of Bimetallic Alumina-Supported Catalysts: Synthesis, Characterization and Catalytic Performance in Pyrolysis of Expanded Polystyrene Waste" Catalysts 15, no. 12: 1094. https://doi.org/10.3390/catal15121094
APA StyleGonzalez-Aguilar, A. M., Riesco-Ávila, J. M., Martínez-Rosales, M., Tejeda-del-Cueto, M. E., Vigueras-Zuniga, M.-O., & Hernández-Hernández, J. (2025). A Comparative Evaluation of Bimetallic Alumina-Supported Catalysts: Synthesis, Characterization and Catalytic Performance in Pyrolysis of Expanded Polystyrene Waste. Catalysts, 15(12), 1094. https://doi.org/10.3390/catal15121094










