1. Introduction
Industrial wastewater pollution is one of the most significant threats to the ecological state of ecosystems and to the sustainable operation of industrial enterprises. According to the current legislation, industries must necessarily use treatment facilities to neutralize the negative impact of wastewater. The presence of dyes in the receiving waterways significantly limits the penetration of light through the water surface and, as a result, reduces photosynthetic activity [
1]. These organic compounds are resistant to conventional industrial wastewater treatment processes and are very resistant to natural biodegradation. Wastewater containing dyes is of particular concern due to the impact imparted by their color. The development of effective treatment methods for this type of wastewater has been the subject of numerous studies [
2].
Dyes are classified as anionic or cationic based on chemical functionality and are largely toxic and harmful to humans and to the environment. Among dyes, methylene blue (MB) is a well-known aromatic heterocyclic basic cationic and primary thiazine dye [
3], with the molecular formula C
16H
18N
3ClS [
4], with a model and structure as shown in
Figure 1. It is highly soluble in water and thus forms a stable solution with water at room temperature [
5].
MB molecules are able to receive and release electrons and protons, transforming them into various intermediate forms differing in its spectral properties [
6]. Under the action of O
2 of air dissolved in aqueous media, MB is oxidized, mainly in the form of the cation radical MB
+, which has a maximum absorption band at 660 nm. A method and device to control the rate of discoloration of MB by microorganisms of activated sludge (AS) were also proposed, and studies were conducted showing the prospects of using an optical version of a reduction test to analyze the state of AS and assess the quality of wastewater treatment [
7].
Several other available options have been used to combat water pollution caused by dyes, including physicochemical, biological, ozonation, membrane filtration, and integrated purification processes [
8]. Still, all of them have revealed full or partial limitations, including insufficient mineralization of dyes, use of dense solutions, high energy consumption, high operating costs, and excessive sediment formation [
9]. Therefore, there is an urgent demand for highly efficient and progressive new technologies for completely removing pollutants from the aquatic environment [
10]. Among new alternative wastewater treatment technologies, advanced oxidation processes (AOPs) appear as effective methods that can be used to convert toxic and persistent chemicals into environmentally friendly minerals. Highly efficient decomposition of compounds present in wastewater is achievable using the direct oxidation method [
11]. However, most technologies demand harsh operating conditions (for example, high temperature and pressure) for the decomposition of selected compounds, increasing the total cost of the process [
12]. In this sense, catalytic wet peroxide oxidation (CWPO) appears as a suitable alternative and one of the most effective oxidation methods [
13].
CWPO is based on the formation of strong oxidizing radicals, mainly hydroxyl radicals (HO
•), in the presence of a catalyst under relatively mild reaction conditions [
14]. These radicals have the dominant ability to eliminate most of the pollutants present in wastewater and can transform synthetic dyes into benign products [
15].
Specifically, in the case of CWPO, hydrogen peroxide (H
2O
2) is the oxidizing agent used for HO
• formation (E
0HO•
/H2O2 = 2.73 V). Catalytic wet peroxide oxidation (CWPO), regarded as the heterogeneous counterpart of the conventional Fenton process, was developed to overcome the main drawbacks of homogeneous treatments, such as the generation of secondary pollution caused by iron sludge and the excessive consumption of reagents [
16]. On the other hand, CWPO represents a more efficient oxidation process due to the ease of catalyst recovery. This advantage helps to reduce the consumption of reactants and, consequently, the overall operational cost. However, the immobilization of iron or other transition metals on solid supports [
17] often results in a decrease in catalytic activity during oxidation reactions [
18]. Therefore, the careful selection of supports with a high specific surface area is essential, as it ensures better dispersion of active metal species, uniform particle size distribution, and enhanced catalytic performance.
Despite a significant amount of research on Fe–clay and Fe3O4-based catalysts for Fenton-like oxidation processes, most of these systems work effectively only in an acidic environment (pH 2–4), where the Fe3+/Fe2+ redox cycle is provided. Under neutral conditions, the activity decreases sharply due to surface passivation and iron leaching, which limits their practical application. In addition, surface immobilization of Fe3O4 leads to the aggregation of nanoparticles and instability of active centers. In contrast to these approaches, this work implements the interlayer intercalation of Fe3+ and Mn2+ ions into a natural aluminosilicate matrix followed by the formation of a highly dispersed MnFe2O4 ferritic spinel. This configuration increases stability, reduces metal leaching, and ensures effective activation of H2O2 at moderate pH 6 due to the synergy of Fe–Mn redox cycles. The use of natural Kazakhstani clays as a carrier further improves textural and sorption properties, forming a cheap, stable, and magnetically extractable catalyst for CWPO.
The application of magnetic supports [
19,
20], or the incorporation of immobilized magnetic nanoparticles, provides a dual benefit: they not only act as active catalytic sites for catalytic wet peroxide oxidation (CWPO) but also allow for the easy magnetic separation and recovery of the catalyst [
21]. Moreover, coupling CWPO with an electromagnetic field or microwave irradiation has been reported to significantly improve process efficiency. Consequently, extensive research has been devoted to the design and optimization of highly efficient catalysts that can facilitate the decomposition of H
2O
2 into hydroxyl radicals (HO
•), in parallel with the oxidation of organic contaminants in aqueous media, while simultaneously minimizing the overall treatment cost [
22].
Of the countless materials used for this purpose, clays are highly suitable for wastewater treatment [
23]. Clay minerals have excellent physical and chemical characteristics, making them an ideal choice due to their high availability, low cost, and classification as an environmentally friendly material. For instance, clays have been extensively employed as supports for titanium dioxide (TiO
2) nanoparticles in water treatment applications [
24]. Moreover, the intercalation of metal oxides into clay structures through pillaring techniques has been shown to enhance their physicochemical properties, leading to increased surface area, the formation of multi-charged sites, expanded interlayer spacing, and improved thermal stability [
25]. In addition, clays are inherently porous materials composed of layered SiO
4 and AlO
6 units containing exchangeable ions between the layers, which facilitate ion exchange processes [
26]. Magnetic separation has recently been used in many fields for preliminary concentration and quantitative determination in analytical chemistry, for the capture and separation of pollutants from the aqueous medium [
27], as well as for the adsorption and decomposition of dyes. One of the most promising alternatives for removing various dyes from wastewater is the combination of excellent adsorption advantages of clays with improved catalytic reactivity [
28]. Clay adsorbents can be used as such or in a modified form, depending on the nature of the target pollutants. They are well known as adsorbents of heavy metals, medicines, pesticides, and organic substances [
29].
This work aims to develop a new method for the synthesis of magnetic clay-based composites (Mn–Fe PILCs), exploring different proportions of clay and Mn
2+ and Fe
3+ ions, and further evaluation of their catalytic properties in CWPO [
30], using the well-known cationic dye methylene blue (MB) as model compound.
2. Results and Discussion
2.1. General Physical–Chemical Properties of the Synthesized MnFe2O4/Shymkent and MnFe2O4/Ural Composites
2.1.1. Elemental Composition
The results of elemental composition of the natural and modified clays are found in
Table 1. As it is noted, Shymkent is rich in iron (6.23%), silicon (28.66%), and aluminum (9.83%). In the modified MnFe
2O
4/Shymkent, the Fe and Mn content increases compared to those values in the natural clay, indicating the exchange and fixation of intercalating metals in the interlayer space (15.04% of Fe and 10.83% of Mn were found). In the MnFe
2O
4/Ural, similar observations are obtained when compared to Ural, the metallic values being 20.04% for Fe and 11.65% for Mn.
2.1.2. X-Ray Diffractometric Analysis
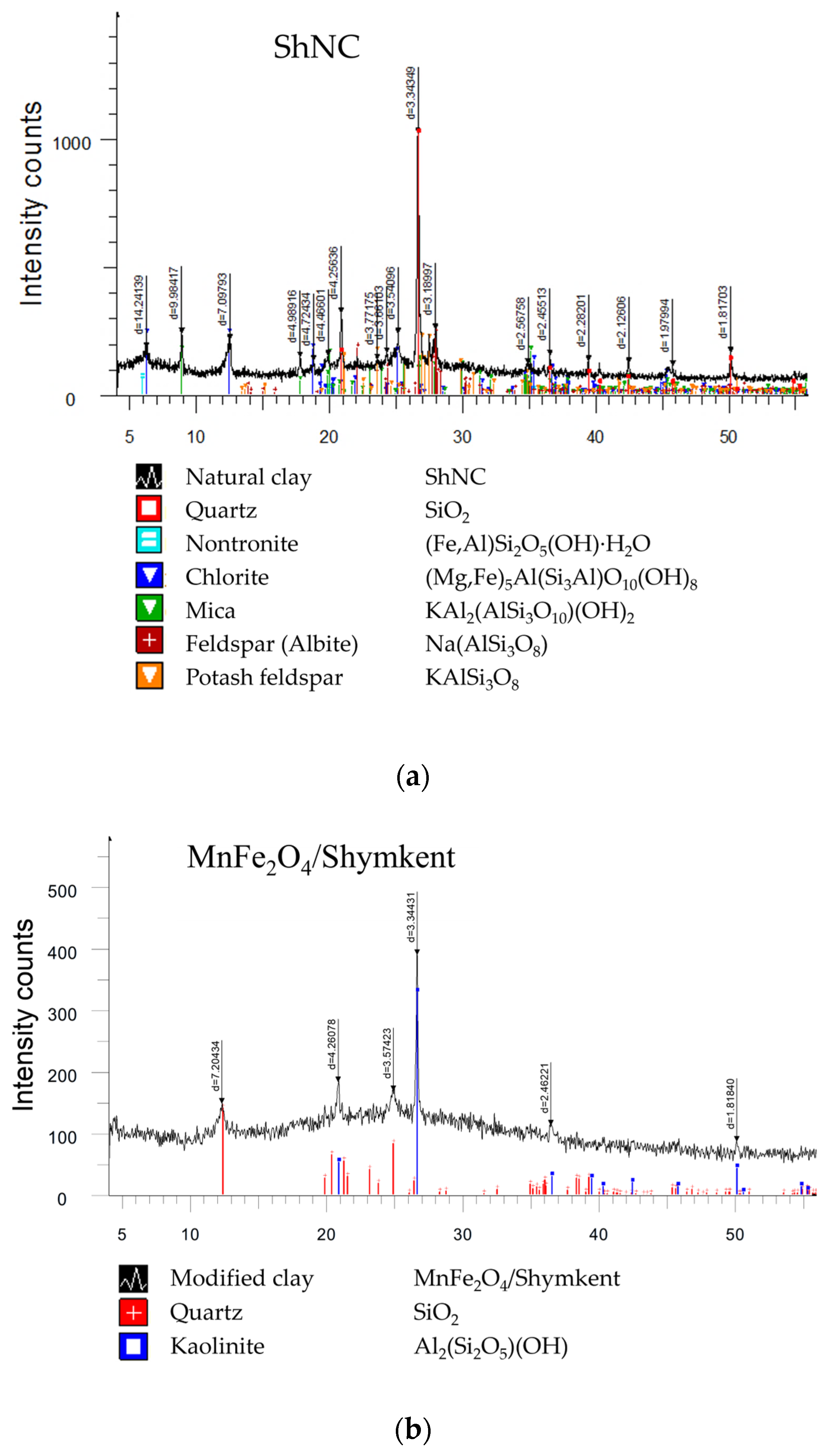
X-ray diffractograms of ShNC and UNC and of the corresponding MnFe
2O
4/Shymkent and MnFe
2O
4/Ural are shown in
Figure 2 and
Figure 3, respectively. X-ray phase analysis was performed semi-quantitatively using diffractograms of powder samples. The quantitative ratios of crystalline phases were determined based on the analysis of X-ray diffraction patterns. The interpretation of the diffractograms was performed using the ICDD database (PDF-2, powder diffraction file) and reference diffractograms of impurity-free mineral standards. The results of the semi-quantitative XRD phase analysis are summarized in
Table 2.
The bulk of natural and modified Shymkent clay samples is represented by X-ray amorphous material. The crystalline phase of the initial sample, represented by the mineral potash feldspar (KAlSi
3O
8), is 5.2% (
Figure 2a,
Table 2). The X-ray image of the modified sample shows no reflexes responsible for the crystalline phases (
Figure 2b,
Table 2).
In both the natural UNC clay and the modified MnFe2O4/Ural sample, the major reflections correspond to quartz (SiO2), kaolinite (Al2(Si2O5)(OH)4), and minor mica-type phases. After modification, additional weak and broadened features appear at approximately 2θ ≈ 30.1°, 35.4°, 43.0°, 57.3°, and 62.9°, which can be assigned to the (220), (311), (400), (511), and (440) planes of the MnFe2O4 spinel (JCPDS 10-0319). The low intensity of these peaks is consistent with the nanocrystalline nature and partial dispersion of the ferrite phase within the clay framework. Such overlap and broadening effects are commonly observed for ferrite–clay composites synthesized by co-precipitation methods.
Although the diffraction patterns of the modified clays (MnFe2O4/Shymkent and MnFe2O4/Ural) remain partially dominated by the reflections of the aluminosilicate matrix, weak additional peaks can be distinguished at approximately 2θ ≈ 30.1°, 35.4°, 43.0°, 57.3°, and 62.9°, which correspond to the (220), (311), (400), (511), and (440) planes of MnFe2O4 (JCPDS 10-0319). Their low intensity and broad profile are attributed to the small crystallite size of the ferrite phase (18–25 nm, estimated from the (311) reflection using the Scherrer equation as described in the methodological section) and to its partial incorporation within the layered clay framework, which limits long-range ordering. Similar peak broadening and overlap between MnFe2O4 and silicate phases have been reported for ferrite–clay composites synthesized by co-precipitation. These results, together with the Fe–O/Mn–O vibrations observed in the FTIR spectra at 520–547 cm−1, confirm the successful formation of the nanostructured MnFe2O4 spinel phase within the clay matrix.
2.1.3. Scanning Electron Microscopy
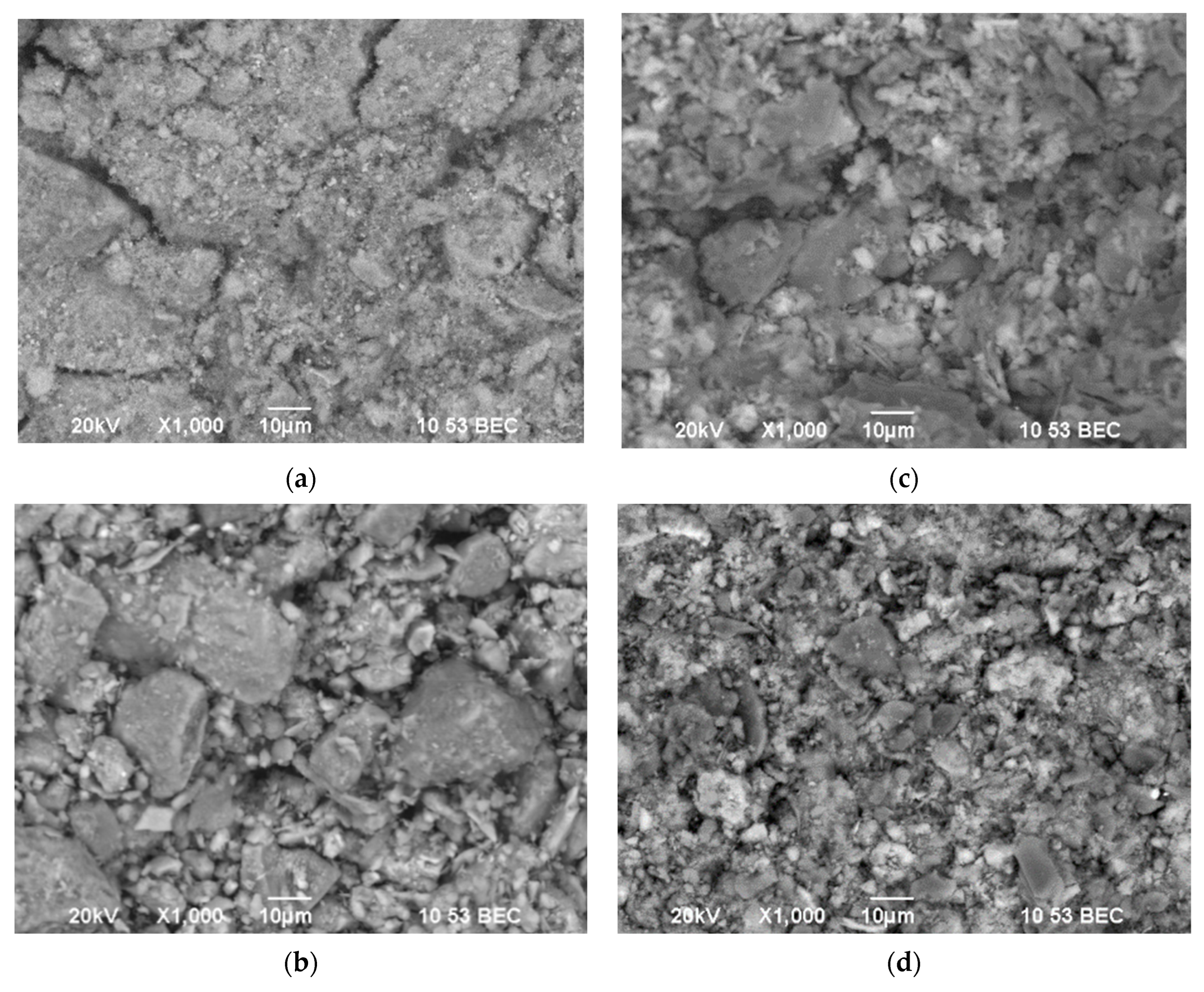
The morphological structure of the materials was obtained by scanning electron microscopy (SEM), the results obtained are shown in
Figure 4. SEM images were taken at an acceleration voltage of 20 kV and a magnification of 1000×, which was chosen to demonstrate the general morphology and surface structure of natural and modified clays. The purpose of this analysis was to show a change in texture after modification—the transition from dense lamellar aggregates to a more friable, porous, and “fluffy” structure typical of MnFe
2O
4/PILCs. Agglomerated particles are observed on MnFe
2O
4/PILCs micrographs (
Figure 4c,d) compared with scattered uneven clay particles on natural clay micrographs (
Figure 4a,b). This can be explained by the loss of some cations during alkaline treatment and the loss of some volatile organic substances during calcination, which all occurred during the change in the surface area of the Fe element at the surface of the natural clays.
The SEM images of the natural clays (
Figure 4a,b) show that clays have a structure similar to large flakes/plates that are able to stick to each other, forming large lumps. After modification of the clays, they become fluffy (
Figure 4c,d), which provides more opportunities for adsorption of dyes into the structure, which enables further reaction with oxygen reactive species formed in the CWPO process.
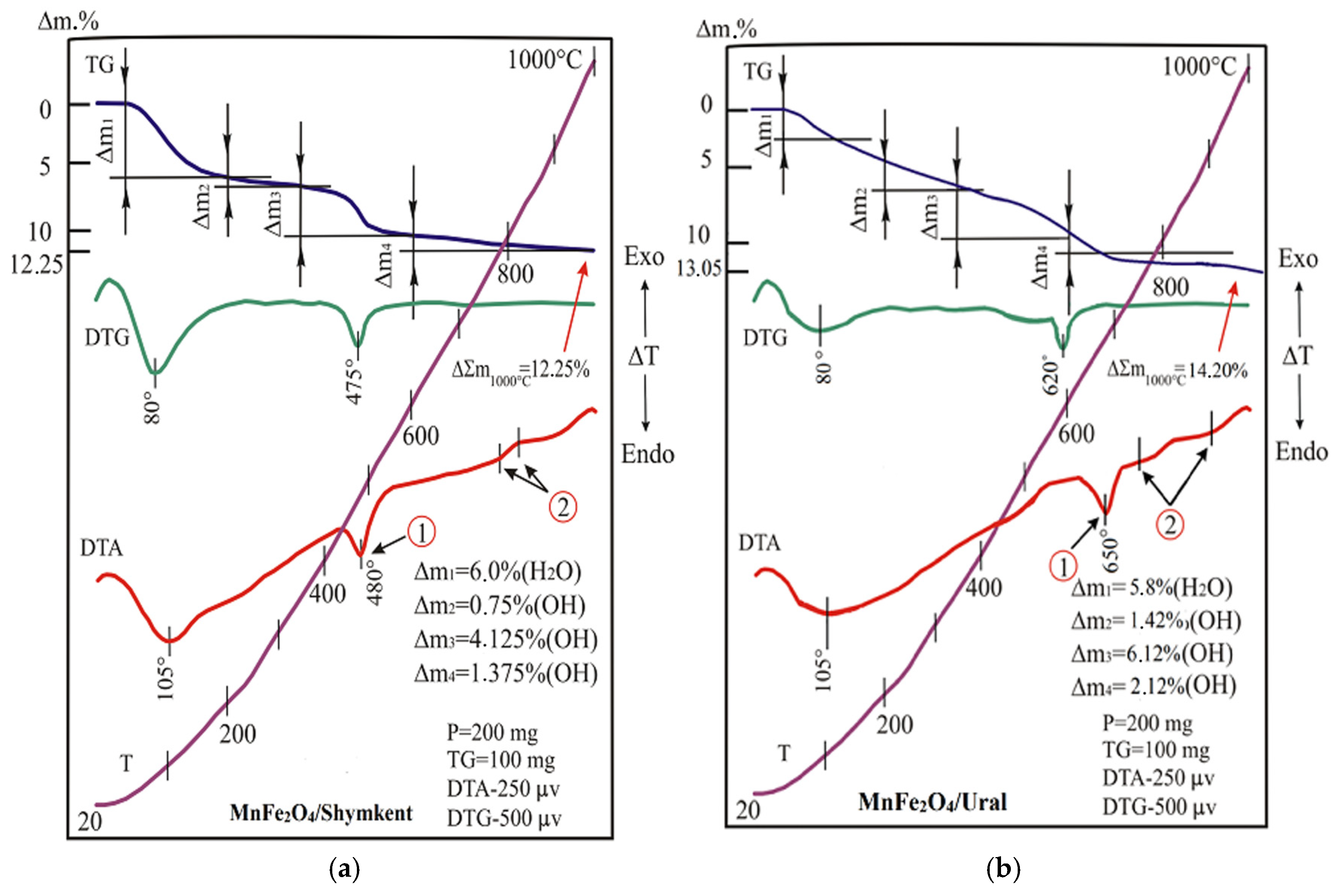
2.1.4. Thermal Analysis (DTA and TGA)
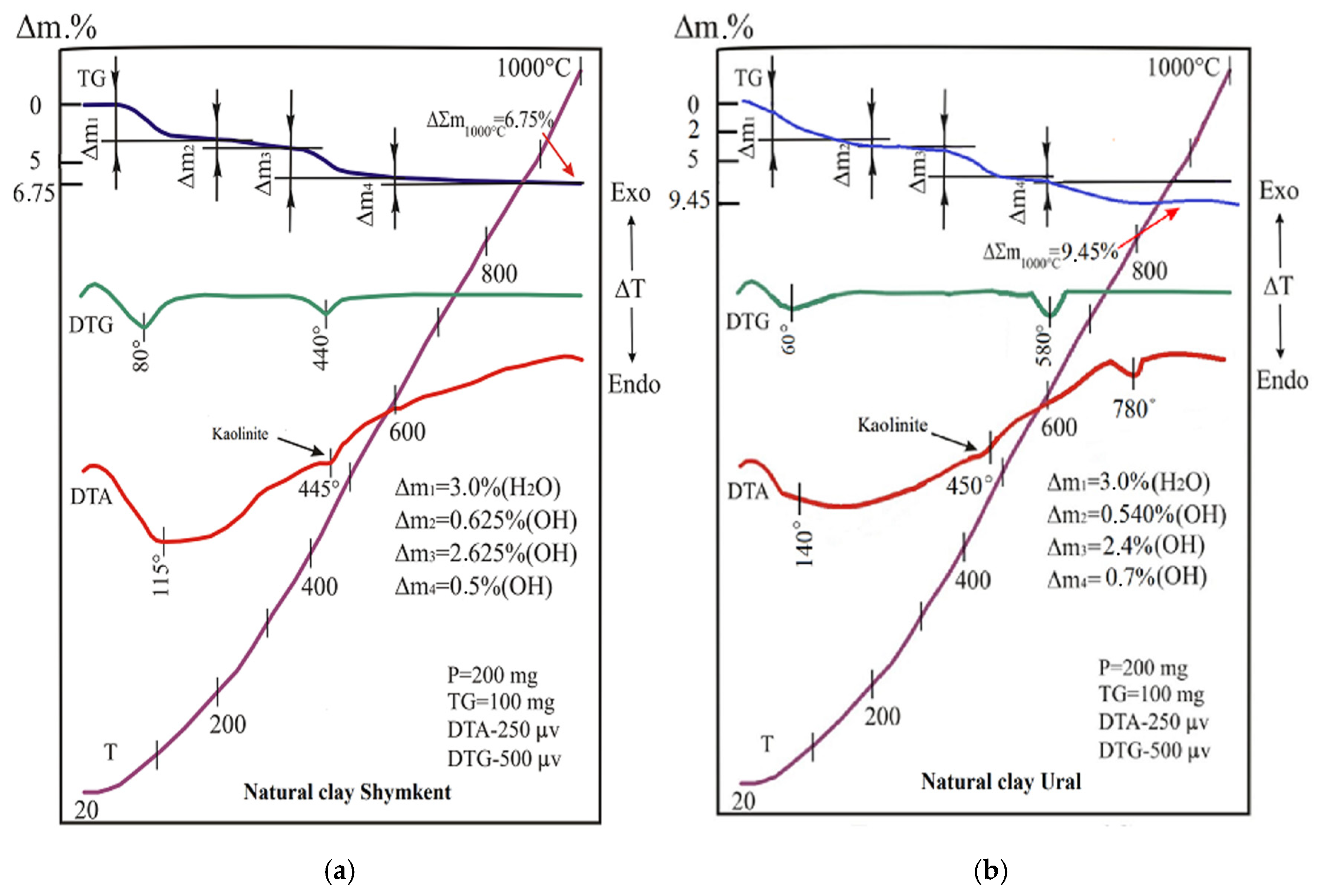
The results of thermal analysis (DTA and TGA) of the natural clay ShNC are shown in
Figure 5 and in
Table 3.
At 20–200 °C, a mass removal is observed, caused by the dehydration of montmorillonite and hydromica. In addition to layered silicate minerals, the samples also contained silica, represented by quartz—a thermally inert mineral. Quartz undergoes a reversible polymorphic transformation upon heating at approximately 500 °C. In this temperature range, the endothermic peak observed on the DTA curve, corresponding to the dehydroxylation and structural breakdown of kaolinite, completely overlaps with the thermal effect associated with the quartz phase transition.
The results of thermal analysis (DTA and TGA) of the modified MnFe
2O
4/Shymkent and MnFe
2O
4/Ural clays are shown in
Figure 6 and in
Table 4. It is observed that the modified clays, when heated, registers on their curves all the same manifestations that were observed when the original natural clays were heated. A significant difference in the development of these reactions in modified clays is the increased intensity of their course,
Figure 6. Similar changes in favor of increased weight loss at all stages are demonstrated by the thermogravimetric (TG) curve of the tested samples, as shown in
Table 5. Since the content of clay minerals in the sample is controlled by the mass of H
2O and OH released into the atmosphere, the amounts of montmorillonite, hydroslude, kaolinite, and chlorite calculated in the sample of the modified MnFe
2O
4/Shymkent clay turned out to be higher than those presented in the composition of the original natural clay, as evidenced in
Table 5. The content of thermally inert inclusions in the composition of the studied sample corresponds to <15%.
The estimation of the phase composition was based on the sequence and magnitude of mass losses during heating of the samples in the range of 20–1000 °C. Each stage of mass loss (Δm1–Δm4) corresponds to certain processes occurring with different phases of clay.
The first stage (20–200 °C, Δm
1) is associated with the removal of physically adsorbed moisture (H
2O), characteristic of montmorillonite and hydrosludes. The second stage (200–345 °C, Δm
2) reflects the dehydroxylation of layered hydrosilicates, mainly montmorillonite and hydrous mica. The third stage (345–600 °C, Δm
3) corresponds to the destruction of hydroxyl groups of kaolinite and partially chlorite. The fourth stage (600–1000 °C, Δm
4) is associated with the further decomposition of stable aluminosilicate structures and partial dehydroxylization of chlorite. The total mass loss at 1000 °C was 12.25% for MnFe
2O
4/Shymkent and 14.20% for MnFe
2O
4/Ural, indicating an increased content of clay minerals with thermolabile hydroxyl groups in the modified samples. Based on a quantitative assessment of the mass losses associated with the release of H
2O and OH groups and the known thermochemical characteristics of minerals, the relative contribution of individual phases was calculated, shown in
Table 5. Thus, in the MnFe
2O
4/Shymkent sample, the content of montmorillonite, kaolinite, hydroslude, and chlorite increased to 19.6%, 15.7%, 16.9%, and 5.8%, respectively, compared to 10–8% in the original natural ShNC clay. At the same time, the proportion of quartz decreased from ~40% to ~25%, and the number of thermally inert components decreased to ~17%, which confirms the formation of a more homogeneous and active clay matrix.
Thus, the phase composition of the modified clays was calculated based on integral mass losses according to TGA data, taking into account the temperature ranges of dehydration and dehydroxylation characteristic of montmorillonite, kaolinite, hydrous mica, and chlorite. The data obtained indicate an increased content of clay minerals and a decrease in the proportion of inert impurities, which is consistent with the observed increase in the catalytic activity of MnFe2O4-modified samples.
2.1.5. Fourier Transform Infrared Spectroscopy (FT-IR)
The natural clays of the Shymkent and Ural deposits were analyzed by IR-Fourier Spectroscopy. At the same time, changes in the absorption bands on the spectra of these clays were analyzed after modification with Mn (II) and Fe (III) (
Figure 7).
IR spectroscopy can indeed reveal the Fe-O–Si interaction in modified clays through characteristic shifts, widenings, and changes in band intensities associated with Si–O and Fe–O valence and deformation vibrations. This is exactly the pattern observed in our spectra. After modification, the bands associated with Si–O–Si valence vibrations (1022 and 1009 cm−1) shift and partially flatten, while the bands in the 450–560 cm−1 region (characteristic of Fe–O/Mn–O) become wider and less symmetrical; at the same time, there is an increase/appearance of a band of about 520 cm−1. The totality of these changes is consistent with the formation of chemical contacts between the ferritic phase and the silicate matrix (Fe–O–Si), and not only with physical adsorption.
Valence vibrations of Si–O–Si bridge bonds in layered silicates are usually observed in the range of about 1000–1100 cm−1; in the initial samples, are observed at 1022 and 1009 cm−1. When a silicon atom (or bridging oxygen) is coordinated with a metal ion (Fe, Mn), the local force constants, and/or the effective reduced mass of the oscillatory system change. As a rule, during the formation of the M–O–Si bond, bands may shift to the region of lower wavenumbers (due to an increase in the reduced mass and/or weakening of Si–O) and a decrease in the intensity of the initial “pure” Si–O group. Spectra show a partial blurring/decrease in the clarity of the 1022/1009 cm−1 bands after modification, which is consistent with the partial coordination of oxygen to Fe/Mn and the formation of Fe–O–Si bonds. Fe–O fluctuations in spinels and oxides are usually in the range of ≈400–600 cm−1. Bands/features observed at 547 and 457 cm−1 have become more blurred after modification, as well as the appearance of a band around 520 cm−1. This broadening and slight shift in the Fe–O modes is a typical indicator of changes in the local iron environment: the formation of the Fe-O–Si interface will lead to an inhomogeneous environment of Fe (some ions are associated with acidic centers of the silicate matrix), which manifests itself in a weakening of symmetry, band broadening, and small frequency shifts.
An increase in the intensity/width of the Oh–H band indicates an increase in the number of adsorbed water molecules and/or hydroxyl groups on the surface. These groups often participate in bridging between the metal oxide and the silicate matrix (M–OH…O–Si), which additionally supports the assumption of a chemical interaction. A set of observations—displacement and attenuation of Si–O bands (≈1000 cm−1), broadening and displacement of Fe–O bands (≈450–560 cm−1), appearance/strengthening of the band ≈520 cm−1, and change in O–H bands—collectively correlate with the formation of the Fe–O–Si interface. This indicates the presence of chemical bonds between the ferritic phase and the clay matrix, rather than solely physical adsorption.
The FTIR spectra analysis revealed that the major absorption bands correspond to the stretching vibrations of Si–O and O–H bonds. In the high-frequency region of the olivine band, low-intensity peaks were observed at 1080 and 1150 cm
−1. The bands at 1022 and 1009 cm
−1 are assigned to Si–O–Si(Al) stretching vibrations in the crystal lattice [
31]. Partial substitution of silicon within the lattice causes most of the valence bands of layered minerals (1000–900 cm
−1) to shift toward lower frequencies. The weak bands at 794 and 547 cm
−1 are attributed to Si–O–Si(Al) vibrations in distorted tetrahedral and octahedral layers, where the 547 cm
−1 band corresponds to Si–O–Si bending vibrations involving bridged oxygen atoms and the 794 cm
−1 band reflects symmetric Si–O–Si stretching typical of SiO
4 tetrahedra. Two intense absorption peaks at around 1442 cm
−1 in the modified samples indicate the formation of ferrite phases. The broad region between 400 and 700 cm
−1 is associated with Fe–O vibrations, confirming the presence of a spinel ferrite structure. In such ferrites, metal cations occupy both tetrahedral and octahedral sites within the crystal lattice. High-frequency bands near 875 cm
−1 correspond to vibrations in the tetrahedral sites, while low-frequency bands near 629 cm
−1 correspond to octahedral sites. The sharpness of these peaks suggests a high degree of crystallinity in the MnFe
2O
4 structures. The broad absorption band observed at 3449–3421 cm
−1 is attributed to O–H stretching vibrations of adsorbed water molecules, indicating a higher concentration of surface hydroxyl groups. In the modified clay samples, the absorption bands at 547 and 457 cm
−1 became more diffuse with slight shifts in position. The appearance of the band at 457 cm
−1 is associated with Fe–O stretching vibrations, while the disappearance of the 621 cm
−1 band (typical for the γ-Fe
2O
3 phase) and the emergence of a new band at 520 cm
−1 suggest a possible phase transformation from γ-Fe
2O
3 to α-Fe
2O
3 [
32]. No pronounced peaks corresponding to the phases of Fe
2O
3 are observed in X-ray diffractograms. This is probably because the content of iron oxides in the composite is low, as well as their high dispersion and possible amorphous or nanocrystalline state, in which X-ray diffraction does not detect clear reflexes. Nevertheless, weak changes in the range of 520–550 cm
−1 observed in the IR spectra are interpreted as manifestations of fluctuations in Fe–O bonds, which is consistent with the literature data for nanostructured MnFe
2O
4/clay systems. These bands do not indicate the formation of the Fe
2O
3 crystalline phase but rather reflect partial oxidation of iron and the presence of surface oxide groups.
2.2. Adsorption Experiments
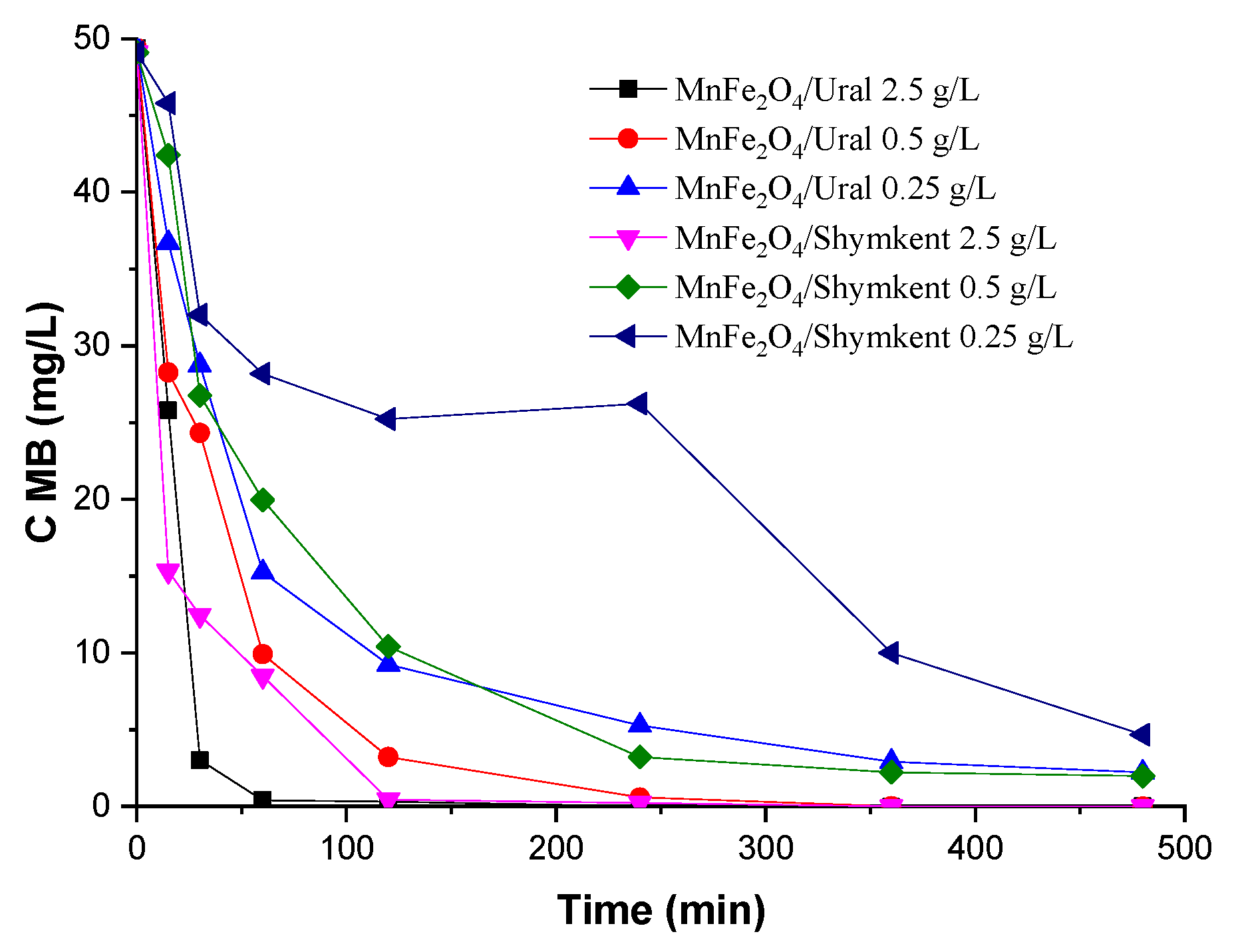
2.2.1. Effect of Adsorbent Dose
To evaluate the capacity of the developed materials to adsorb MB, and to understand its influence in the mechanism of CWPO (results presented in
Section 2.3), adsorption experiments were carried out at different adsorbent doses and different pHs. This effect was studied using a dose of modified clays of 0.25, 0.5, and 2.5 g/L. The results obtained are shown in
Figure 8 and reveal that the percentage of adsorption increases with an increase in the dose of MnFe
2O
4/Shymkent and MnFe
2O
4/Ural. It is obvious that, with an increase in the dose of modified clays, the number of adsorption centers available for adsorbent-MB interaction increases, leading to an increase in the percentage of MB removal from the solution. A decrease in the capacity of the adsorbent, i.e., the amount of MB adsorbed per unit mass of the adsorbent, with an increase in modified clays from 0.25 to 0.5 g/L, and to 2.5 g/L, may be due to two reasons. An increase in the adsorbent dose at a constant concentration and volume of MB will lead to unsaturation of adsorption centers during adsorption and, secondly, may be associated with particle interaction [
20], such as aggregation because of a high dose of adsorbent (
Figure 8). Such aggregation would lead to a decrease in the total surface area of the adsorbent and to an increase in the length of the diffusion path.
The effect of the adsorbent dosage in the adsorption of MB at an initial concentration of 50 mg/L of the dye, in
Figure 8, shows that adsorbents with the lowest MnFe
2O
4/PILCs dose of 0.25 g/L demonstrate the lowest removal values, which averaged 70%, when at a material dose of 2.5 g/L, the removal increases to 99% (with MnFe
2O
4/Ural) and to 97.81% (with MnFe
2O
4/Shymkent).
2.2.2. Effect of pH
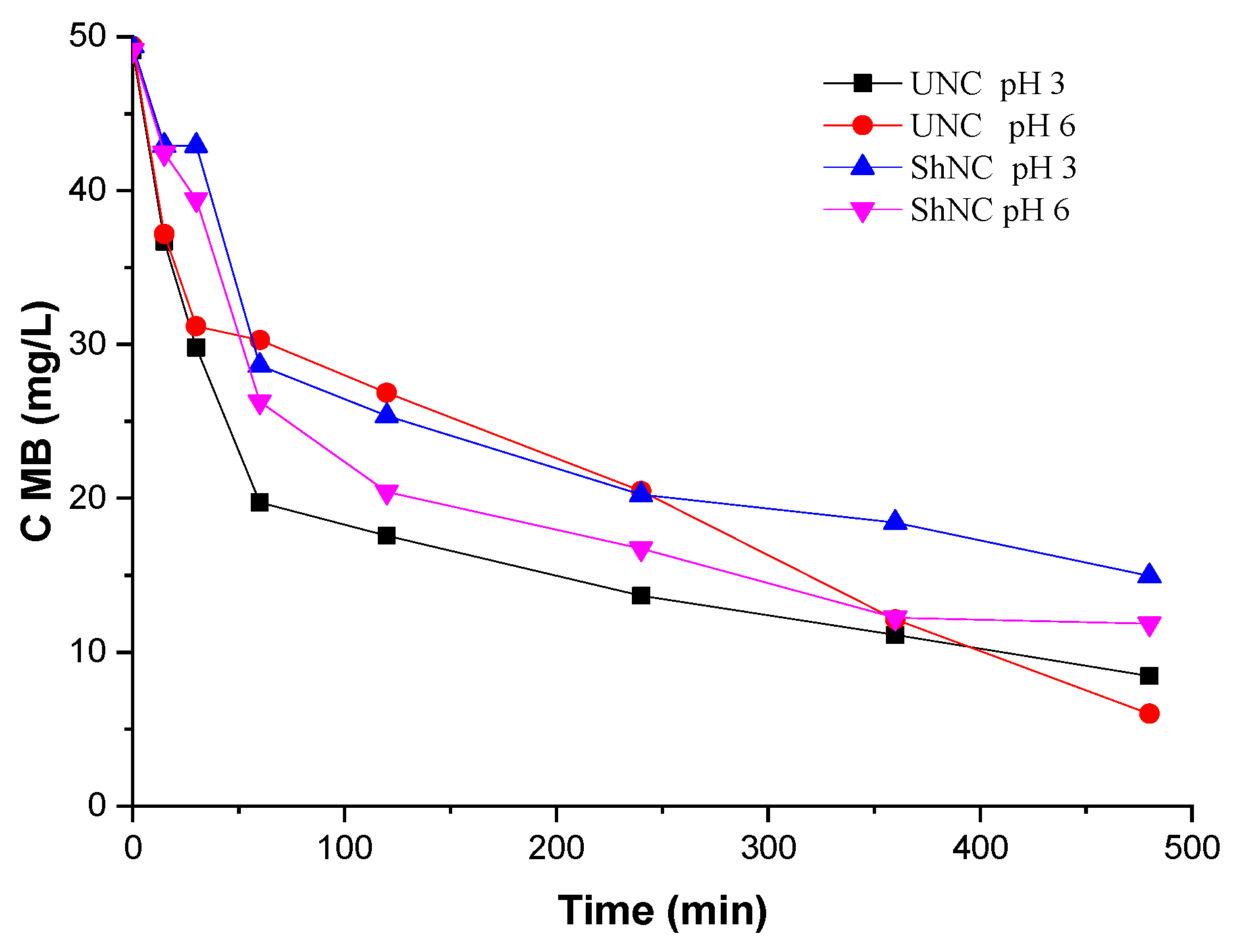
The pH of the dye solution plays an essential role in the entire adsorption process, especially with respect to the adsorption capacity.
Figure 9 and
Figure 10 shows the effect of pH on the adsorption of MB on the natural and modified clays, respectively, at an initial MB concentration of 50 mg/L and with a dose of adsorbent of 0.5 g/L, at a stirring speed of 200 rpm and 50 °C. When the pH of the dye solution is changed from 3 to 6, the adsorption removal of the modified clays increases (
Figure 9), irrespectively of the clay used. Since MB is a cationic dye, the positive charge causes electrostatic repulsion of the dye molecules, leading to their lower adsorption, while at higher pH, a negatively charged surface facilitates the adsorption of cationic contaminants.
In
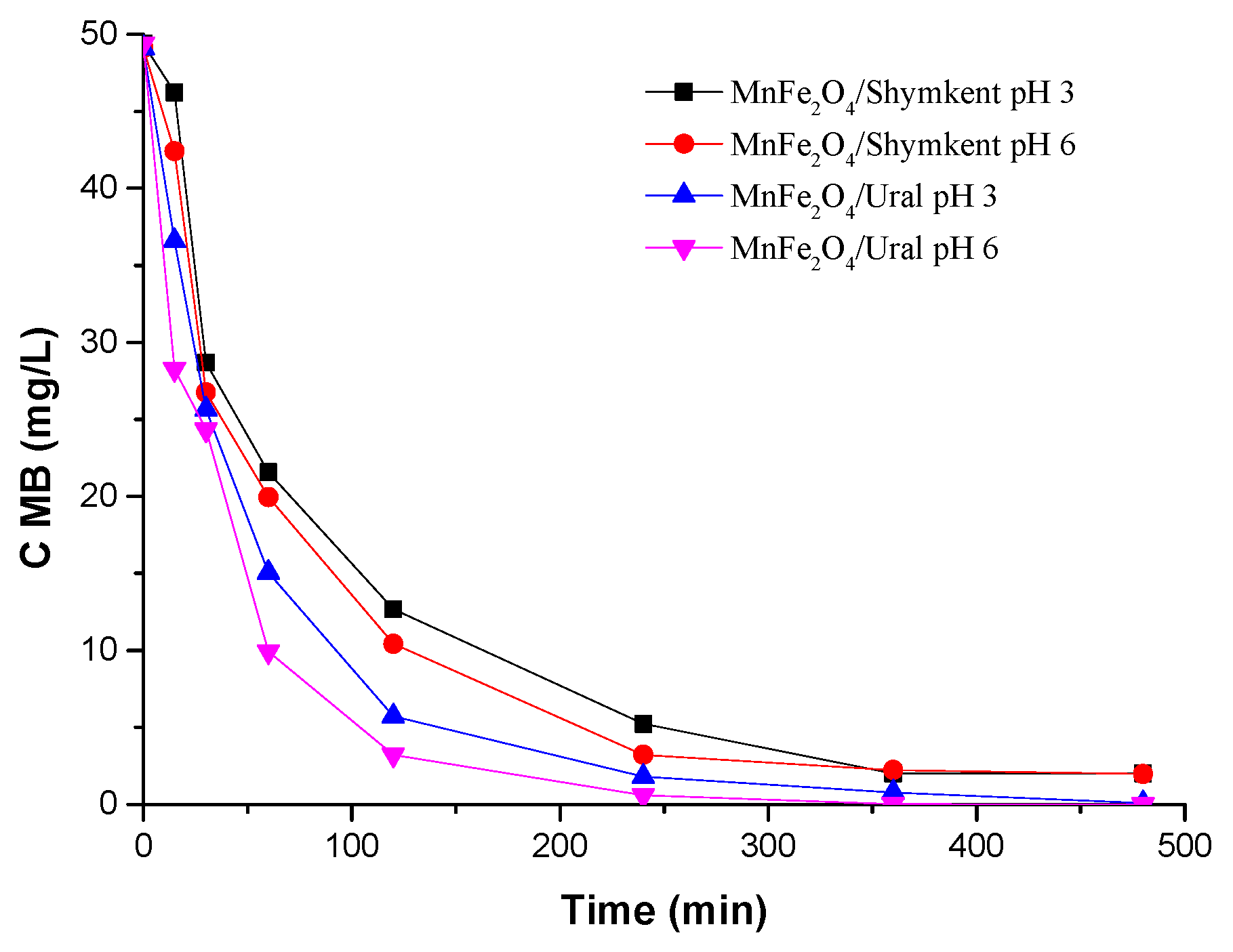
Figure 9 it is also possible to observe that for about the first 15 min, the adsorption of methylene blue at pH = 3 was similar for UNC and ShNC and that at pH = 6, both samples adsorb MB significantly better. For 15 to 30 min, the concentration of methylene blue decreased more visibly for UNC and ShNC, decreasing after gradually until 480 min, when maximum removal values were obtained. When UNC and ShNC come into contact with MB for 480 min, 86% of the pollutant can be removed. The amount of adsorbed MB was not complete, because the binding of functional groups on the surface of the adsorbent and MB become weaker as the concentration decreased, so residual MB remained in solution. The longer the contact time for adsorption, the greater the collision between the adsorbate particle and the adsorbent, and the steady increase in removal from the solution.
Figure 10 shows that during the first 15 min, the adsorption of MB at pH = 3 and pH = 6 with MnFe
2O
4/Shymkent and MnFe
2O
4/Ural samples significantly reduced the concentration of MB when compared to natural clays. From 120 to 240 min, the concentration of adsorbed MB was complete (100% removal) with the MnFe
2O
4/Ural sample at pH = 6. From 360 to 480 min, all the samples showed excellent results (100% removal).
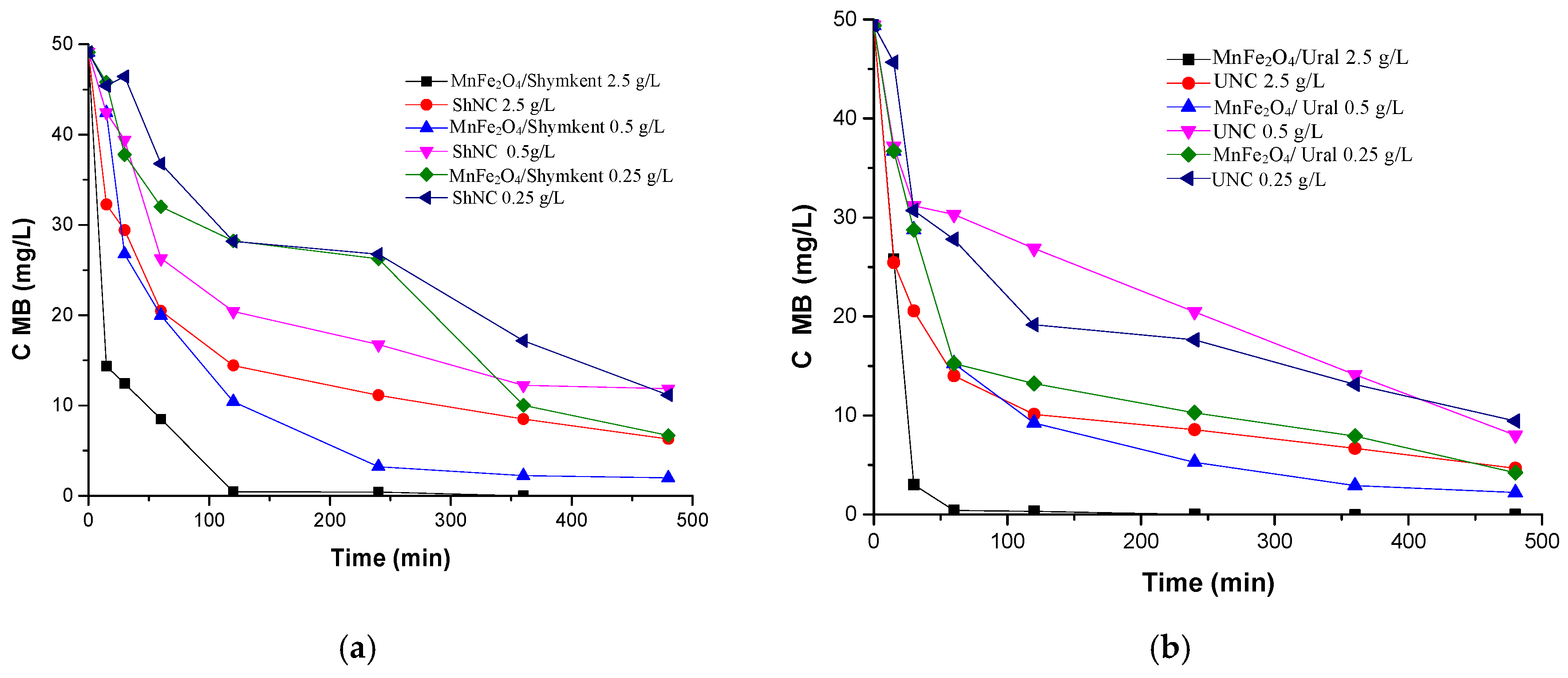
2.3. Activity of the Modified Clays in CWPO
The aim of the work was to develop effective magnetically modified clays (PILC) based on natural clays from the Shymkent and Ural deposits activated with Fe
3+ and Mn
2+ ions and to evaluate their performance in catalytic wastewater treatment through the process of an improved analogue of “chemical oxidative peroxidation” (CWPO) of methylene blue (MB). The results shown in
Figure 11 demonstrate that the MnFe
2O
4/Shymkent and MnFe
2O
4 samples/Ural have a significantly higher capacity to remove MB when compared to natural clays. Full MB removal is achieved by sampling most of the materials, while the initial clays show minimal efficiency. An analysis of the removals depending on the dose of the material (0.25; 0.5; 2.50 g/L) revealed the following pattern: despite the improved textural characteristics (increased specific surface area, from 21 to 74 m
2/g; and increased pore volume, from 0.029 to 1.77 cm
3/g) of the modified samples, at the lowest dose (0.25 g/L) they exhibit a relatively low degree of MB removal. This indicates that insufficient concentration of active sites limits the rate and completeness of the process. At doses of 0.5 g/L and above, a rapid decrease in MB concentration is observed, and for iron-containing samples, complete removal is achieved within ~60 min, which confirms the key role of the Fe phase as the active phase in the process. An in-depth analysis shows that the removal capacity depends on several interrelated factors: (i) the presence of the active transition of Fe
3+/Fe
2+ and Mn
2+, cyclic providing radicals HO
•, and other reactive oxygen species for removal by reaction [
33]; (ii) textural availability of these centers, that is, the access of reactant molecules MB and H
2O
2 to the catalytic sites; (iii) adsorption properties of a matrix of clay, providing an accumulation of MB on the surface of the material, contributing for the removal by adsorption. Of particular note is the role of iron; as shown earlier, spinel ferrites of the type MnFe
2O
4 can effectively activate H
2O
2 or other oxidizing agents due to the transition of Fe
2+ → Fe
3+ and reverse reduction, generating HO
• radicals. At the same time, manganese performs a stabilizing function, improving the distribution of ferritic particles and reducing aggregation, which preserves the active area. Our interpretation is that in MnFe
2O
4/PILC samples the active centers are distributed on the clay surface, which increases contact with reagents and accelerates kinetics. We additionally note that higher doses of the catalyst increase the density of active sites, thereby reducing the time to complete MB conversion. It is also important that the adsorption capacity of clay leads to the concentration of MB near the active centers, improving the efficiency of catalysis. Thus, the combination of adsorption (clay) with catalysis (MnFe
2O
4) forms a synergistic effect [
34], occurring simultaneously in the CWPO process.
The magnetic phase further enables easy post-reaction recovery, confirming the potential of these composites as stable, reusable catalysts for CWPO under mild operating conditions. The obtained results demonstrated stable catalytic behavior of the synthesized materials in repeated CWPO cycles, which confirms their chemical and structural stability. This is consistent with recent studies of similar MnFe
2O
4/clay catalysts, which have shown good repeatability and stability in cyclic tests [
35,
36,
37].
In experiments with CWPO, the materials were tested at different loads (0.25, 0.5, and 2.50 g/L) under identical operating conditions (C
MB,0 = 50 mg/L, C
H2O2,0 = 450 mg/L, C
cat = 0.25, 0.5, or 2.50 g/L, pH0 = 6.0, and
T = 50 °C). As shown in
Figure 11, all modified materials exhibit high efficiency, with complete removal of MB achieved in most cases within 60–120 min after the end of the process. On the contrary, unmodified natural clays (ShNC and UNC) exhibit the lowest capacity for removal of MB, which confirms the effectiveness of Fe–Mn modification. The removal increases with an increase in the dose of the material: 2.5 g/L MnFe
2O
4/Shymkent and MnFe
2O
4/Ural completely decomposes MB within 60 min, whereas at 0.25 g/L the conversion remains partial, not exceeding 20–30% after 480 min. This difference can be explained by the larger surface area and the increased number of active sites available at a higher catalyst load.
The results in
Figure 12 illustrate the effect of pH on the efficiency of modified clays. Since pH determines the degree of ionization and the surface charge of both adsorbent and adsorbate, it plays a crucial role in the removal efficiency. At pH 3.0, the removal of MB is relatively small due to the competition of H
+ ions with the cationic dye for the sites of adsorption and electrostatic repulsion between the positively charged clay surface and MB molecules. Under these acidic conditions, the concentration of MB gradually decreases, reaching about 10–15 mg/L after 120 min, and is almost completely removed only after 480 min for the most active samples.
However, when the pH was increased to 6.0, there was a noticeable increase in the removal of MB (
Figure 12). The surface of the modified clays becomes negatively charged, which enhances the electrostatic attraction between the adsorbent and the cationic dye molecules. As a result, the decomposition rate of MB increased significantly: at a catalyst dosage of 2.5 g/L, almost complete discoloration of MB occurred within 60–90 min, while at 0.5 g/L, the concentration dropped below 5 mg/L after 240 min. This indicates that a higher pH level favors both adsorption and catalytic oxidation processes.
In general, catalytic experiments confirm that the modified MnFe2O4 clays developed in this work have excellent capacity of the removal of methylene blue. Fe3+ ions in the spinel phase play a dominant role in the Fenton-like formation of hydroxyl radicals, accelerating the oxidative degradation of the dye, occurring simultaneously with adsorption. The combined effect of metal modification, catalyst dosage, and pH results in improved decomposition kinetics, demonstrating the high efficiency of the synthesized materials for the rapid and effective removal of organic pollutants from aqueous media.
The influence of pH and catalyst dose on the CWPO performance was evaluated under identical conditions. It was observed that, at strongly acidic pH (≈3), the apparent MB removal during CWPO was sometimes lower than that obtained by adsorption alone. This behavior can be explained by the scavenging of hydroxyl radicals and the unproductive decomposition of H2O2 under excess oxidant or high proton concentration, as widely reported for heterogeneous Fenton systems. At pH 6, however, these side reactions are minimized, and the MnFe2O4/clay materials display high efficiency: both MnFe2O4/Shymkent and MnFe2O4/Ural achieve nearly complete MB removal within 60–90 min, whereas unmodified clays reach only partial adsorption equilibrium. This confirms that the synthesized materials can activate H2O2 through Fe3+/Fe2+ and Mn2+/Mn3+ redox cycles, generating HO• radicals that drive MB oxidation under mild conditions. The coexistence of adsorption and catalysis thus results in a synergistic effect, consistent with previous studies on ferrite–clay catalysts.
Despite the fact that AOPs are usually effective in a wide pH range (usually from 4 to 9), in this study the reaction was carried out at pH = 3, which is more typical for heterogeneous Fenton processes. The use of such acidic conditions is justified by several factors. First, the surface chemistry of the MnFe2O4 catalyst plays a key role. At low pH values, the oxide surface acquires a positive charge, which affects the adsorption of cationic dyes such as methylene blue. However, the presence of two types of ions in the spinel structure—iron and manganese—ensures the existence of several redox pairs (Fe2+/Fe3+ and Mn2+/Mn3+) capable of generating active hydroxyl radicals (HO•), even in an acidic environment. Secondly, according to the literature data, spinel-type catalysts, including MnFe2O4, exhibit the greatest catalytic activity in the pH range of 3–4 during the decomposition of hydrogen peroxide. This is due to the optimal ratio between the rate of generation of active radicals and the stability of the catalyst.
2.4. Potential Advantages and Limitations of MnFe2O4/Clay Catalysts for Scalable and Sustainable Applications
The developed MnFe2O4/clay composites combine catalytic, magnetic, and structural properties, providing high CWPO efficiency under mild conditions (pH 3–6. 50 °C). Embedding ferritic phase MnFe2O4 in natural clay matrix creates a stable active centers of Fe2+/Fe3+ and Mn2+/Mn3+ responsible for the generation of HO• radicals. The use of available Kazakhstani clays (ShNC, UNC) reduces the cost and increases the environmental friendliness of the process. Due to the ferromagnetic properties of MnFe2O4, the catalyst is easily removed by a magnet (<30 s), which simplifies regeneration and reduces waste. Strong Fe–O–Si bonds ensure low metal leaching (<1%), preventing secondary contamination and complying with the principles of “green catalysis”. The synthesis of MnFe2O4/clay by in situ co-deposition proceeds under moderate conditions (T < 80 °C, pH ≈ 9–10) and does not require expensive equipment. The technology is easily adaptable for continuous or batch synthesis and preserves the morphology of ferritic particles. The use of local mineral raw materials makes the process economically and environmentally sustainable.
The main limitations of the developed MnFe2O4/clay system include partial leaching of Fe and Mn at pH < 3, decreased activity at excessive H2O2 concentrations (>600 mg L−1), limited diffusion of large organic molecules due to partial pore blockage, and the need for occasional purification of the catalyst to remove surface precipitates, as well as proper management of spent material through regeneration by acid etching or disposal as solid non-hazardous waste. Despite these limitations, the MnFe2O4/clay composite represents an environmentally sustainable alternative to conventional Fenton catalysts, operating efficiently under mild conditions without forming Fe(OH)3 sludge, allowing easy magnetic separation, and avoiding the generation of toxic by-products. Its performance aligns with the principles of the Zero Liquid Discharge concept and makes it a promising candidate for integration into hybrid water treatment technologies.
3. Materials and Methods
3.1. Chemicals and Materials
Natural clays of Kazakhstan from the deposits of Shymkent (ShNC, located in the south of Kazakhstan) and Urals (UNC, located in the western region of Kazakhstan) were used as raw materials for the preparation of the magnetically modified clays for the removal of MB.
Sodium hydroxide (NaOH, 98 wt.%), iron (III) sulfate nonahydrate (Fe2(SO4)3∙9H2O, 98 wt.%), and manganese chloride tetrahydrate (MnCl2∙4H2O, 99 wt.%) were purchased from Laborfarma LLP. Methylene blue (CAS 61-73-4,C16H18N3SCl99 wt.%) and hydrogen peroxide (H2O2, 60 wt.%), used as an oxidizer in the treatment of synthetic wastewater, were purchased from Fluka. Titanium (IV) oxysulfate (TiOSO4, 15 wt.% in dilute sulfuric acid, 99.99%) and sodium sulfate (CAS 7757-83-7, Na2SO3 wt.%) were obtained from Aldrich. All chemicals were used as received without further purification. Distilled and ultrapure water were used during the work.
3.2. Synthesis of MnFe2O4/Shymkent and MnFe2O4/Ural
Kazakhstan’s natural clays from the Shymkent (ShNC) and Ural (UNC) deposits were used to produce magnetically modified clays via the method of joint deposition followed by heat treatment. An aqueous solution of the precursors was prepared by dissolving MnCl
2∙4H
2O and Fe
2(SO
4)
3∙9H
2O in a molar ratio of Mn:Fe = 1:2 at a total metal concentration of 0.3 mol/L. 5.0 g of pre-dried and sifted natural clays (ShNC and UNC) and then added to the resulting solution, after which the suspension was intensively mixed for 30 min at room temperature. Precipitation of the ferritic phase was initiated by gradually adding a solution of NaOH (5 mol/L) until a pH of 10 ± 0.2 was reached, with constant stirring. After that, the system was kept at 95–100 °C for 2 h to complete precipitation and partial crystallization of MnFe
2O
4. The resulting precipitate was cooled to room temperature, repeatedly washed with distilled water (T ≈ 50 °C) to a neutral pH, and magnetically separated. The materials were dried at 105 °C for 2 h and then calcined in a muffle furnace at 400 °C for 3 h (heating rate 5 °C/min) to remove volatile components and finally form a ferritic structure. As a result, stable magnetic composites MnFe
2O
4/Shymkent and MnFe
2O
4/Ural were obtained, characterized by a uniform distribution of the ferritic phase inside the layered clay matrix [
35,
36].
The formation of the MnFe
2O
4 magnetic phase in the structure of natural clays was carried out by the in situ co-deposition method, in which ferritic nanoparticles were formed directly in the interlayer and pore spaces of the clay. This approach ensured a uniform distribution of the active phase and a strong interaction of ferrite with the silicate matrix. Unlike the traditional impregnation method, in situ synthesis prevents aggregation of particles and increases their dispersion. The formation mechanism included three stages: (1) ion exchange of Fe
3+ and Mn
2+ with interlayer Na
+, (2) precipitation of hydroxides in pores and on the surface, and (3) ferritization during calcination to form a stable spinel structure. This method provides high uniformity, stability, and magnetic recoverability of the catalyst, which corresponds to current data on similar systems [
37,
38].
3.3. Chemical and Textural Characterization
To characterize the materials, various analyses were used. The elemental composition of the natural clays was obtained using Electron Microprobe (EMP) analysis. X-ray diffraction analysis of samples of natural (UNC) and modified clay (MnFe2O4/Ural and MnFe2O4/Shymkent) were performed using an automated DRON-3 diffractometer (Burevestnik, Russia) with CuKα radiation (λ = 1.5406 Å) and a nickel β filter to eliminate secondary radiation. Diffraction patterns were recorded in the θ–2θ geometry under the following operating conditions: tube voltage U = 35 kV, current I = 20 mA, scanning speed 2°/min, step size 0.02°, and angular range 2θ = 5–70°. To ensure accuracy and minimize statistical errors, each measurement was performed at least three times, and the averaged results were used for further analysis. Before the study, clay samples were dried at a temperature of 105 ± 5° C to obtain constant mass and crushed in an agate mortar to a powder with a particle size of less than 40 μm. The diffraction data were processed and analyzed using the ICDD PDF-2 database (powder diffraction file) and reference diffraction patterns of pure minerals. The phases were identified based on characteristic interplane distances (d-values) and relative reflection intensities. Special attention was paid to the MnFe2O4 spinel type phase, which exhibits the most intense reflections at 2θ ≈ 30.1°, 35.4°, 43.0°, 57.3°, and 62.9°. The quantitative determination of the MnFe2O4 content was carried out by a semi-quantitative method using the equal weight method and an artificial mixture. For artificial mixtures of standard materials containing known concentrations of MnFe2O4 (in the range from 0 to 50 wt.%) calibration curves were prepared and constructed based on the intensity dependence of the most characteristic MnFe2O4 (I = 35.4°). The peak depends on the concentration of ferrite. To normalize the diffraction intensity, the reflection of quartz (SiO2, 2θ ≈ 26.6°), which is present in all samples, was used as an internal standard. The relative quantitative ratios of the crystalline phases were calculated based on the average integral intensities of their respective peaks. Broad reflections of low intensity were observed in natural clays (UNC), indicating the presence of amorphous or weakly crystallized phases (for example, kaolinite and aluminum hydroxides). On the contrary, the modified samples showed distinct reflections corresponding to MnFe2O4, which confirms the formation of the crystal structure of spinel after modification. The total relative error in the quantitative determination of MnFe2O4 using the equilibrium and artificial mixture method was estimated at ±5–7%, which is typical for semi-quantitative X-ray phase analysis.
The average crystallite size of the ferrite phase (MnFe
2O
4) was calculated using the Scherrer equation:
where D is the average crystallite size (nm), K = 0.9 is the shape factor, λ = 1.5406 Å is the wavelength of the CuKα radiation, β is the full width at half maximum (FWHM) of the diffraction peak in radians, and θ is the corresponding Bragg diffraction angle. Calculations were performed for the most intense peak (311). The obtained values are 18–22 nm for MnFe
2O
4/Shymkent and 20–25 nm for MnFe
2O
4/Ural, which corresponds to the literature data for nanoferrites synthesized under similar conditions [
39].
Studies of the morphology of the samples were carried out on a scanning electron microscope JSM-6490LV with INCA Energy Microanalysis and HKL-Basic Structural Analysis systems (JEOL, Tokyo, Japan). The multi-purpose scanning electron microscope (useful magnification of 300,000×) combines the possibilities of working in both standard and low-vacuum modes, allowing to examine samples without spraying with a conductive layer. Additionally, it is equipped with the INCA Energy 350 energy dispersive microanalysis system and a prefix for studying the texture and structure of polycrystalline HKL Basic samples. Thermal analysis (DTA and TGA) was conducted in air environment, in the temperature range from 20 to 1000 °C. The heating mode of the furnace is linear (dT/dt = 10 °C/min), and the reference substance is calcined Al2O3.
Fourier-transform infrared (FTIR) spectra of the natural and magnetically modified clays were recorded using an Infraspec spectrometer (Model FSM 2202, St. Petersburg, Russia). Measurements were conducted in the spectral range of 5000–500 cm−1 with a resolution of 1 cm−1. The samples were prepared by thoroughly mixing 1 wt% of clay with potassium bromide (KBr) and pressing the mixture into pellets prior to analysis.
3.4. Adsorption and Reaction Experimental Procedures
3.4.1. Adsorption and CWPO Experiments
The CWPO runs of the dye were carried out in a 200 mL round-bottom glass reactor equipped with a condenser containing the model solution. The initial concentration of MB (C
MB = 50 mg/L) was considered for modeling wastewater containing this contaminant. In a typical run, the reactor loaded with 100 mL of the MB aqueous solution was heated at 50 °C by immersion in an oil bath and its pH was adjusted to the selected initial pH of 3 with H
2SO
4 1 M (experiments were allowed to proceed freely, i.e., without buffering). H
2O
2 was then added to the stoichiometric amount required for the complete mineralization of MB; namely, 0.45 µL of hydrogen peroxide (60% by weight). Finally, the catalyst was added in different doses to work at concentrations of 0.25, 0.5, and 2.5 g/L [
40]. Several samples were taken from the reaction medium at pre-selected reaction times to follow the removal of MB measured using UV-Vis. Pure adsorption experiments were also carried out under the same operating conditions as those used during the reactions (50 °C; pH = 3; 50 mg/L of MB; and 0.25, 0.5, and 2.5 g/L of adsorbent) to compare the removal of MB by adsorption with that obtained by CWPO.
To ensure the reliability of the results obtained, all experiments on the adsorption of methylene blue (MB) and catalytic wet peroxide oxidation (CWPO) were carried out in three parallel repetitions under identical conditions [
41]. The average values of the MB removal degree (R
avg) and standard deviations (±σ) were calculated using the formula:
where
n = 3 is the number of repetitions, and Ri is the degree of MB removal in the ith experiment. The difference between parallel measurements did not exceed ±3% for adsorption and ±5% for CWPO, which confirms the good reproducibility of the experiments. The error in determining the dye concentration did not exceed 2%, and the deviations of the kinetic constants were 5%. To ensure accuracy, constant parameters were maintained: temperature (50 ± 1 °C), initial pH (3, 6 ± 0.1), hydrogen peroxide concentration (450 ± 5 mg/L), and catalyst dose (0.25, 0.5, 2.5 g/L). Between the series of measurements, the pH of the solution was checked by the potentiometric method, and the spread of data was controlled using control series with pure clay (without MnFe
2O
4) and without H
2O
2, revealing no appreciable removal.
3.4.2. Analytical Methods
To determine the concentration of MB in model waters, a calibration curve was plotted at five points in five series of standard solutions of MB (C
MB = 10, 20, 30, 40, 50 mg/L), prepared by diluting a mother solution with ultrapure water [
42]. The empty solution and working standards were analyzed on a UV–visible spectrophotometer at the maximum MB absorption wavelength of 664 nm. During the adsorption and CWPO runs, discoloration was accompanied by a periodic selection of small aliquots (1 mL). To separate the solid phase and avoid possible interference, all samples were centrifugated with a DM0506 centrifuge (DLAB, Beijing, China) at 5000 rpm for 1 min, before analysis. Aliquots taken to determine the concentration of MB were first mixed with Na
2SO
3 to consume non-reacted H
2O
2 and to stop oxidation reactions (previous tests showed that adding Na
2SO
3 does not affect subsequent analysis). The concentration of H
2O
2 was also determined using UV-Vis spectrophotometry at 405 nm by adding 1 mL of aliquot to a 50 mL volumetric flask containing 1 mL of H
2SO
4 solution and 0.1 mL of TiOSO
4 (15% dissolved in 99.99% sulfuric acid), and the volume of the volumetric flask filled with distilled water [
43].