Production of Syngas and Hydrogen-Rich Gas from Lignocellulosic Biomass via Ru/Al2O3 Catalyst-Assisted Slow Pyrolysis
Abstract
1. Introduction
2. Results and Discussion
2.1. Mass Balance of the Process
2.2. Composition and H2/CO Ratio of Pyrolysis Gas
2.3. Composition of Bio-Oil
2.4. Composition and Properties of Biochar
2.5. Possibilities of Re-Use or Regeneration of Catalyst Used
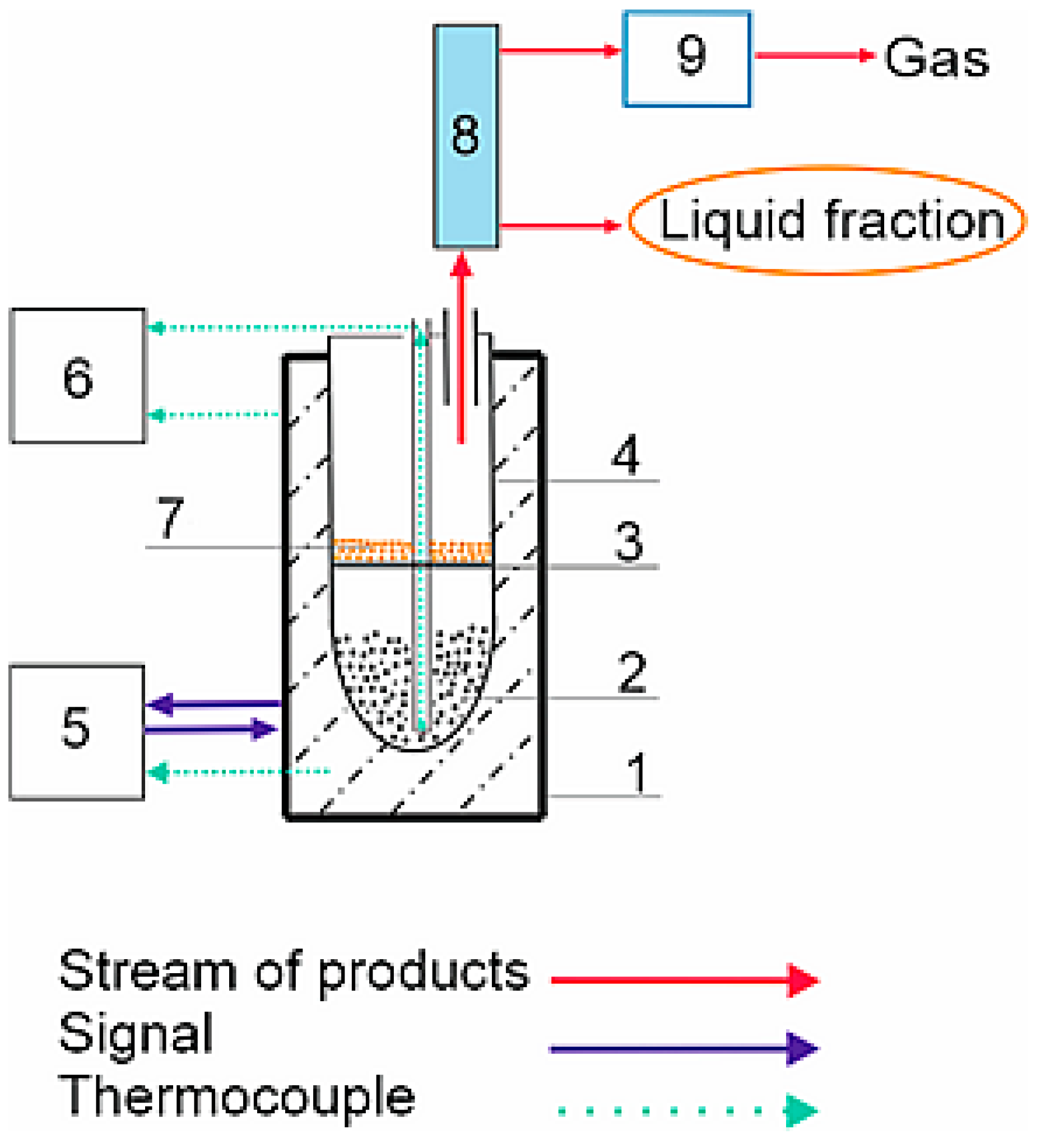
3. Materials and Methods
3.1. Materials
3.2. Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ashfaq, M.M.; Tüzemen, G.B.; Noor, A. Exploiting agricultural biomass via thermochemical processes for sustainable hydrogen and bioenergy: A critical review. Int. J. Hydrogen Energy 2024, 84, 1068–1084. [Google Scholar] [CrossRef]
- Raza, M.; Inayat, A.; Ahmed, A.; Jamil, F.; Ghenai, C.; Naqvi, S.R.; Shanableh, A.; Ayoub, M.; Waris, A.; Park, Y.-K. Progress of the Pyrolyzer Reactors and Advanced Technologies for Biomass Pyrolysis Processing. Sustainability 2021, 13, 11061. [Google Scholar] [CrossRef]
- Sun, W.; Yan, Y.; Wei, Y.; Ma, J.; Niu, Z.; Hu, G. Catalytic Pyrolysis of Biomass: A Review of Zeolite, Carbonaceous, and Metal Oxide Catalysts. Nanomaterials 2025, 15, 493. [Google Scholar] [CrossRef]
- Yang, H.; Cui, Y.; Jin, Y.; Lu, X.; Han, T.; Sandström, L.; Jönsson, P.G.; Yang, W. Evaluation of Engineered Biochar-Based Catalysts for Syngas Production in a Biomass Pyrolysis and Catalytic Reforming Process. Energy Fuels 2023, 37, 5942–5952. [Google Scholar] [CrossRef]
- Lan, K.; Qin, Z.; Li, Z.; Hu, R.; Xu, X.; He, W.; Li, J. Syngas production by catalytic pyrolysis of rice straw over modified Ni-based catalyst. BioResources 2020, 15, 2293–2309. [Google Scholar] [CrossRef]
- Akubo, K.; Nahil, M.A.; Williams, P.T. Pyrolysis-catalytic steam reforming of agricultural biomass wastes and biomass components for production of hydrogen/syngas. J. Energy Inst. 2019, 92, 1987–1996. [Google Scholar] [CrossRef]
- Shang, S.; Qin, Z.; Lan, K.; Wang, Y.; Zhang, J.; Xiong, T.; He, W.; Li, J. Hydrogen-rich syngas production via catalytic gasification of biomass using Ni/Zr-MOF catalyst. BioResources 2020, 15, 1716–1731. [Google Scholar] [CrossRef]
- He, W.; Liu, K.; Zhang, L.; Liu, M.; Ni, Z.; Li, Y.; Xu, D.; Cui, M.; Zhao, Y. Catalytic pyrolysis and in situ carbonization of walnut shells: Poly-generation and enhanced electrochemical performance of carbons. RSC Adv. 2024, 14, 12255–12264. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Xu, J.; Wu, Y. Hydrogen-Rich Gas Production from Two-Stage Catalytic Pyrolysis of Pine Sawdust with Calcined Dolomite. Catalysts 2022, 12, 131. [Google Scholar] [CrossRef]
- Kuhn, J.N.; Zhao, Z.; Felix, L.G.; Slimane, R.B.; Choi, C.W.; Ozkan, U.S. Olivine catalysts for methane- and tar-steam reforming. Appl. Catal. B Environ. 2008, 81, 14–26. [Google Scholar] [CrossRef]
- Cheah, S.; Gaston, K.R.; Parent, Y.O.; Jarvis, M.W.; Vinzant, T.B.; Smith, K.M.; Thornburg, N.E.; Nimlos, M.R.; Magrini-Bair, K.A. Nickel cerium olivine catalyst for catalytic gasification of biomass. Appl. Catal. B Environ. 2013, 134, 34–45. [Google Scholar] [CrossRef]
- Dang, Q.; Zhang, X.; Zhou, Y.; Jia, X. Prediction and optimization of syngas production from a kinetic-based biomass gasification process model. Fuel Process. Technol. 2021, 212, 106604. [Google Scholar] [CrossRef]
- Ammendola, P.; Lisi, L.; Piriou, B.; Ruoppolo, G. Rh-perovskite catalysts for conversion of tar from biomass pyrolysis. Chem. Eng. J. 2009, 154, 361–368. [Google Scholar] [CrossRef]
- Tomishige, K.; Asadullah, M.; Kunimori, K. Syngas production by biomass gasification using Rh/CeO2/SiO2 catalysts and flu-idized bed reactor. Catal. Today 2004, 89, 389–403. [Google Scholar] [CrossRef]
- Loy, A.C.M.; Yusup, S.; Lam, M.K.; Chin, B.L.F.; Shahbaz, M.; Yamamoto, A.; Acda, M.N. The effect of industrial waste coal bottom ash as catalyst in catalytic pyrolysis of rice husk for syngas production. Energy Convers. Manag. 2018, 165, 541–554. [Google Scholar] [CrossRef]
- Zsinka, V.; Miskolczi, N.; Juzsakova, T.; Jakab, M. Pyrolysis-gasification of biomass using nickel modified catalysts: The effect of the catalyst regeneration on the product properties. J. Energy Inst. 2022, 105, 16–24. [Google Scholar] [CrossRef]
- Xu, T.; Zheng, X.; Xu, J.; Wu, Y. Hydrogen-Rich Gas Production from Two-Stage Catalytic Pyrolysis of Pine Sawdust with Nano-NiO/Al2O3 Catalyst. Catalysts 2022, 12, 256. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, X.; Chen, L.; Yuan, Q.; Sun, L.; Xie, X.; Zhao, B.; Si, H. Characteristics and Catalytic Properties of Bi-Functional Fe/CaO Catalyst for Syngas Production from Pyrolysis of Biomass. J. Biobased Mater. Bioenergy 2016, 10, 415–422. [Google Scholar] [CrossRef]
- Manić, N.G.; Janković, B.B.; Dodevski, V.M.; Stojiljković, D.D.; Jovanović, V.V. Multicomponent Modelling Kinetics and Simultaneous Thermal Analysis of Apricot Kernel Shell Pyrolysis. J. Sustain. Dev. Energy, Water Environ. Syst. 2020, 8, 766–787. [Google Scholar] [CrossRef]
- Demiral, I.; Kul, Ş.Ç. Pyrolysis of apricot kernel shell in a fixed-bed reactor: Characterization of bio-oil and char. J. Anal. Appl. Pyrolysis 2014, 107, 17–24. [Google Scholar] [CrossRef]
- Al Arni, S. Comparison of slow and fast pyrolysis for converting biomass into fuel. Renew. Energy 2018, 124, 197–201. [Google Scholar] [CrossRef]
- Dokuzparmak, T.U.; Bozkurt, P.A. Catalytic pyrolysis of apricot kernel shell over metal supported MCM-41 catalysts obtained by synthesizing sonochemical method. J. Energy Inst. 2024, 117, 101764. [Google Scholar] [CrossRef]
- Shah, M.A.; Khan, N.; Kumar, V.; Qurashi, A. Pyrolysis of walnut shell residues in a fixed bed reactor: Effects of process parameters, chemical and functional properties of bio-oil. J. Environ. Chem. Eng. 2021, 9, 105564. [Google Scholar] [CrossRef]
- Demirbas, A. Effect of temperature on pyrolysis products from four nut shells. J. Anal. Appl. Pyrol. 2006, 76, 285–289. [Google Scholar] [CrossRef]
- Demirbaş, A. Yields of hydrogen-rich gaseous products via pyrolysis from selected biomass samples. Fuel 2001, 80, 1885–1891. [Google Scholar] [CrossRef]
- Oni, B.A.; Sanni, S.E.; Oyedepo, S.O.; Ibegbu, A.J. Catalytic reforming of tar and volatiles from walnut shell pyrolysis over a novel Ni/olivine/La2O3 supported on ZrO2. J. Energy Inst. 2022, 103, 33–46. [Google Scholar] [CrossRef]
- Li, N.; Gao, Z.; Yi, W.; Li, Z.; Wang, L.; Fu, P.; Li, Y.; Bai, X. Fast pyrolysis of birch wood in a bubbling fluidized bed reactor with recycled non-condensable gases. BioResources 2019, 14, 8114–8134. [Google Scholar] [CrossRef]
- Stepacheva, A.A.; Lugovoy, Y.V.; Manaenkov, O.V.; Kosivtsov, Y.Y.; Sulman, M.G. Thermocatalytic Conversion of Pine and Birch Sawdust over H-ZSM-5 Zeolite Catalyst. Chem. Eng. Trans. 2023, 103, 787–792. [Google Scholar]
- Wang, S.; Wang, H.; Yin, Q.; Zhu, L.; Yin, S. Methanation of bio-syngas over a biochar supported catalyst. New J. Chem. 2014, 38, 4471–4477. [Google Scholar] [CrossRef]
- Ostadi, M.; Rytter, E.; Hillestad, M. Boosting carbon efficiency of the biomass to liquid process with hydrogen from power: The effect of H2/CO ratio to the Fischer-Tropsch reactors on the production and power consumption. Biomass Bioenergy 2019, 127, 105282. [Google Scholar] [CrossRef]
- Yu, Z.; Xie, H.; Wang, L.; Shao, Z.; Huang, C.; Yang, S. Combination with biomass pyrolysis and dry/steam reforming for adjustable H2/CO syngas production. J. Therm. Anal. Calorim. 2024, 149, 3497–3512. [Google Scholar] [CrossRef]
- Saxena, R.C.; Seal, D.; Kumar, S.; Goyal, H.B. Thermo-chemical routes for hydrogen rich gas from biomass: A review. Renew. Sustain. Energy Rev. 2008, 12, 1909–1927. [Google Scholar] [CrossRef]
- Chen, R.; Sheng, Q.; Dai, X.; Dong, B. Upgrading of sewage sludge by low temperature pyrolysis: Biochar fuel properties and combustion behavior. Fuel 2021, 300, 121007. [Google Scholar] [CrossRef]
- DePablo, R.S.; Harrington, D.E.; Bramstedt, W.R. Ruthenium Recovery Process. U.S. Patent No. US4132569A, 2 January 1979. [Google Scholar]
- Applicant BASF AG. Recycling of Ruthenium from a Used Ruthenium Catalyst Comprises Treating the Catalyst Containing Ruthenium Oxide in a Hydrogen Stream and Treating the Carrier Material Containing Ruthenium Metal with Hydrochloric Acid. Patent No. DE102005061954A1, 5 July 2007. [Google Scholar]
- Nippon Mining Holdings Inc. Method for Recycling Ruthenium from Ruthenium-Containing Waste. Patent No. CN101519732A, 10 August 2011. [Google Scholar]
- EN ISO 16948; Solid Biofuels—Determination of Total Content of Carbon, Hydrogen and Nitrogen. ISO: Geneva, Switzerland, 2015.
- EN ISO 16994; Solid Biofuels—Determination of Total Content of Sulfur and Chlorine. ISO: Geneva, Switzerland, 2016.
- EN ISO 18122; Solid Biofuels—Determination of Ash Content. ISO: Geneva, Switzerland, 2022.
- EN ISO 18134; Solid Biofuels—Determination of Moisture Content. ISO: Geneva, Switzerland, 2022.
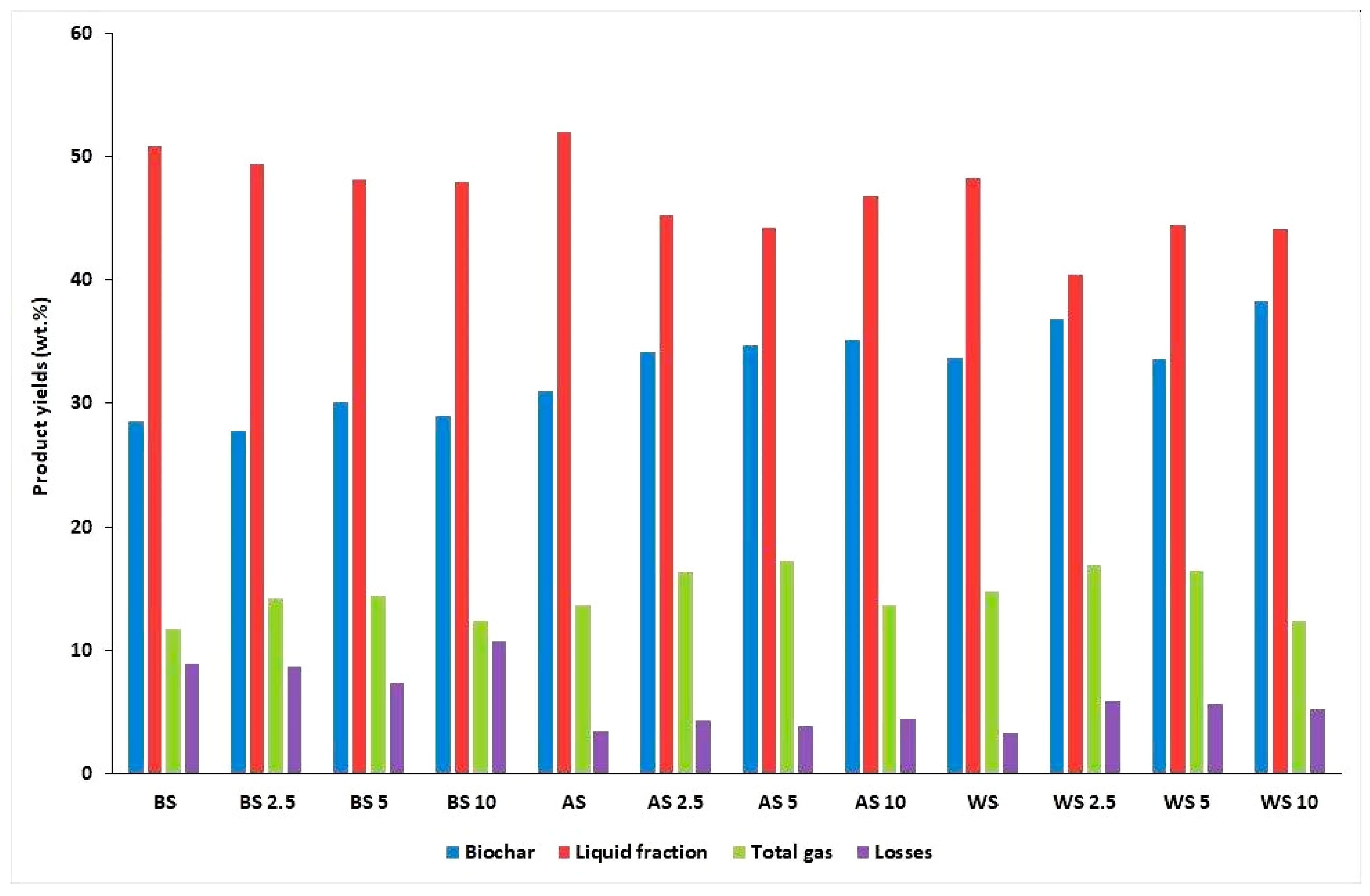
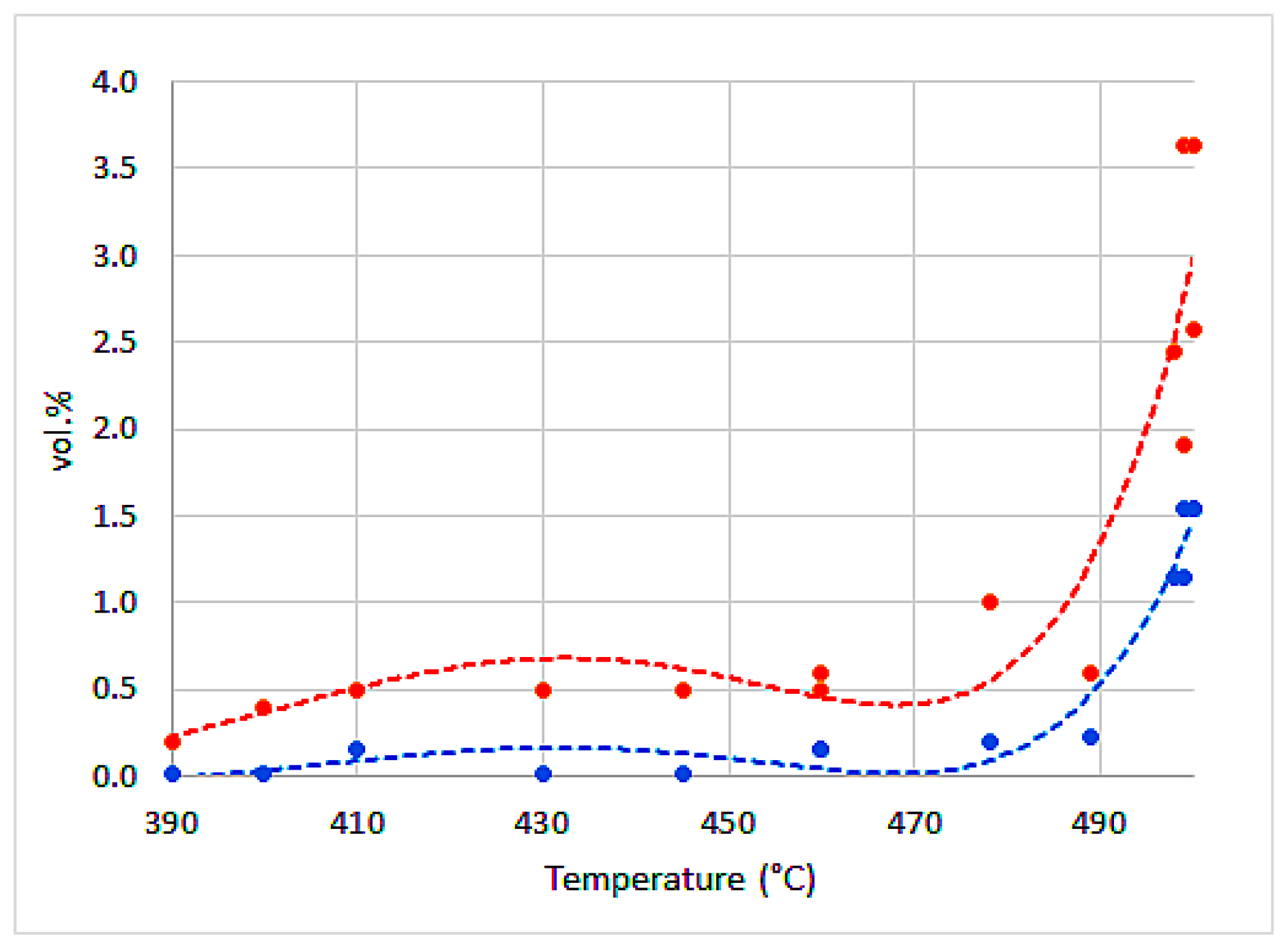
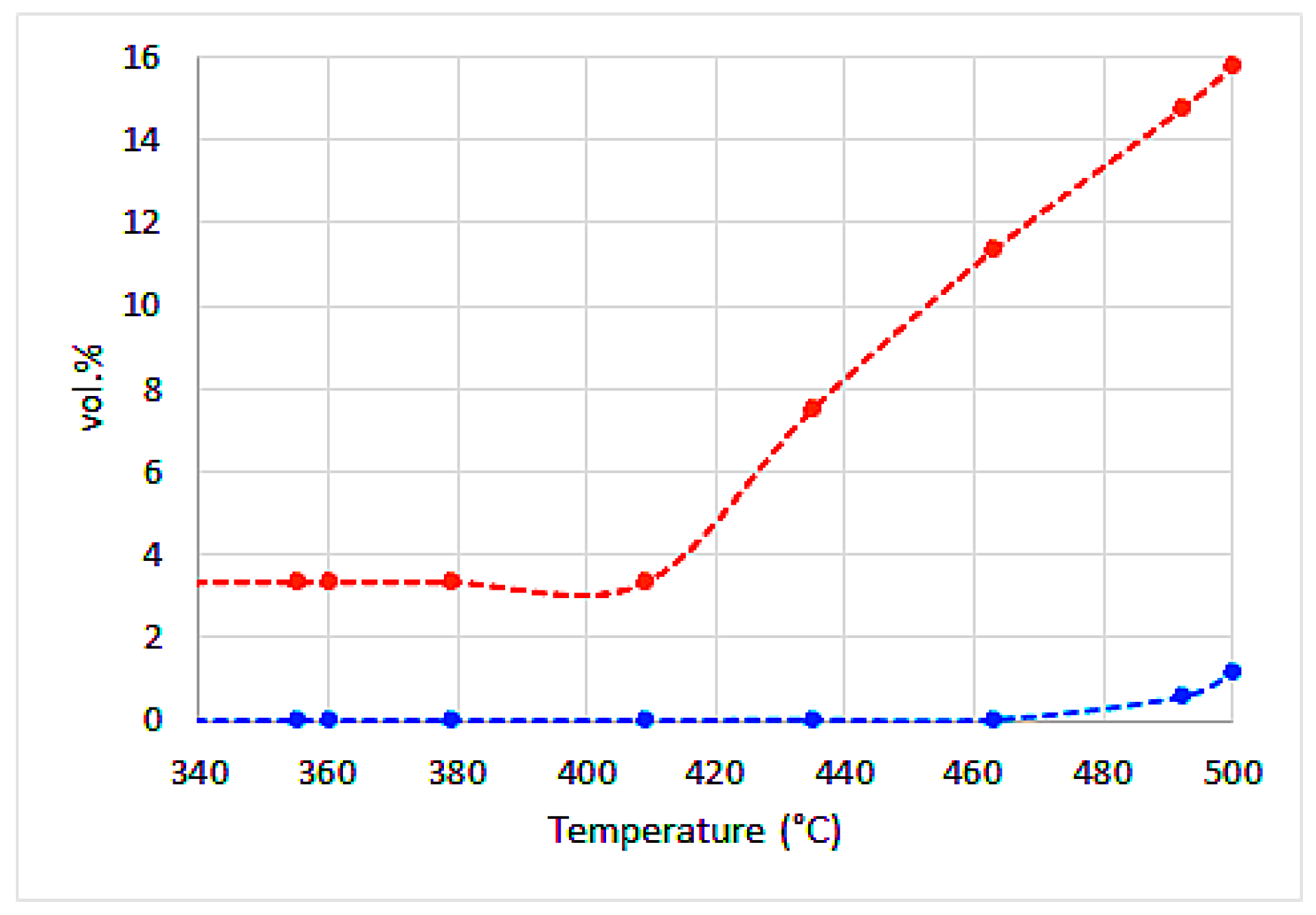
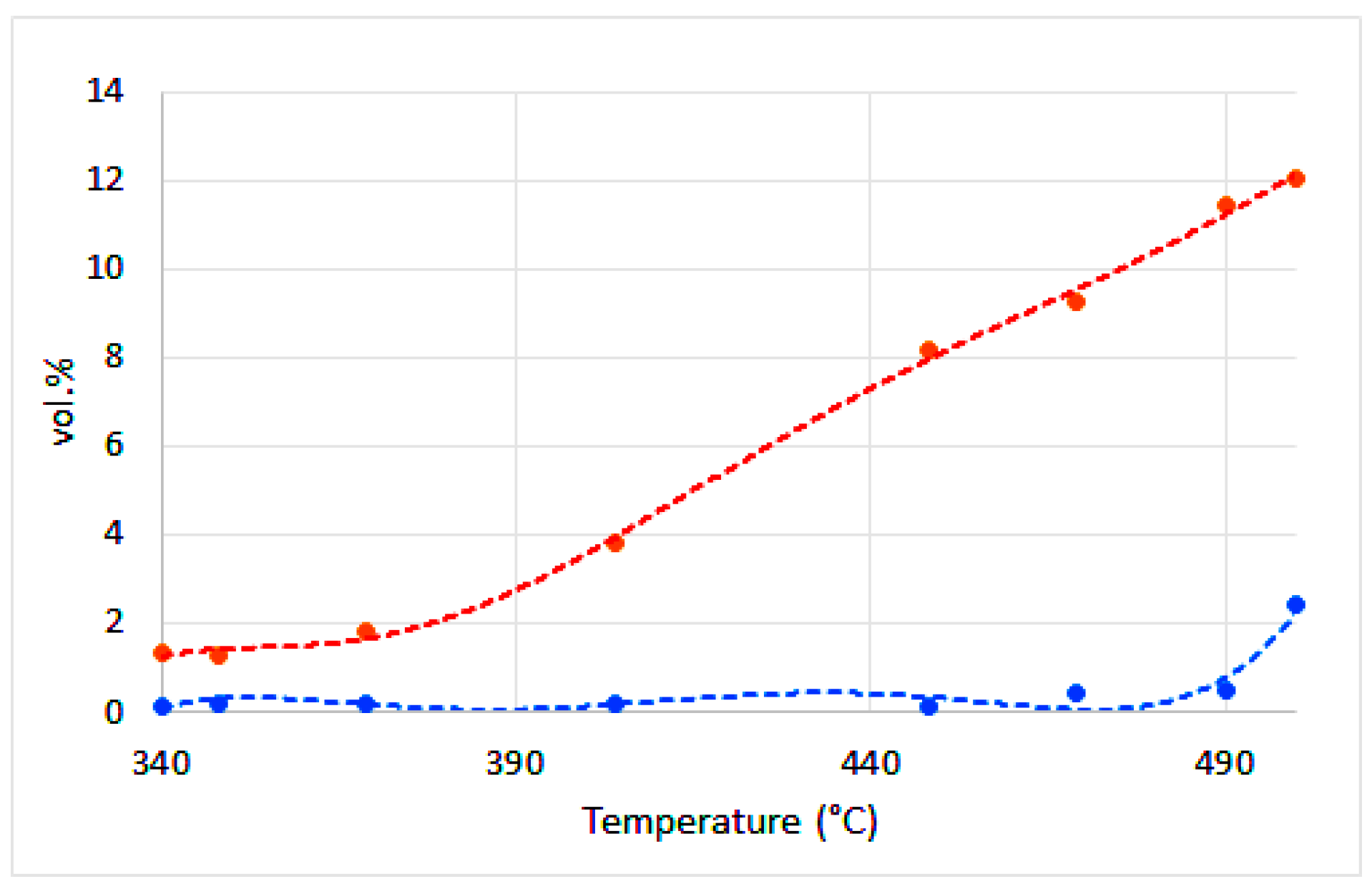

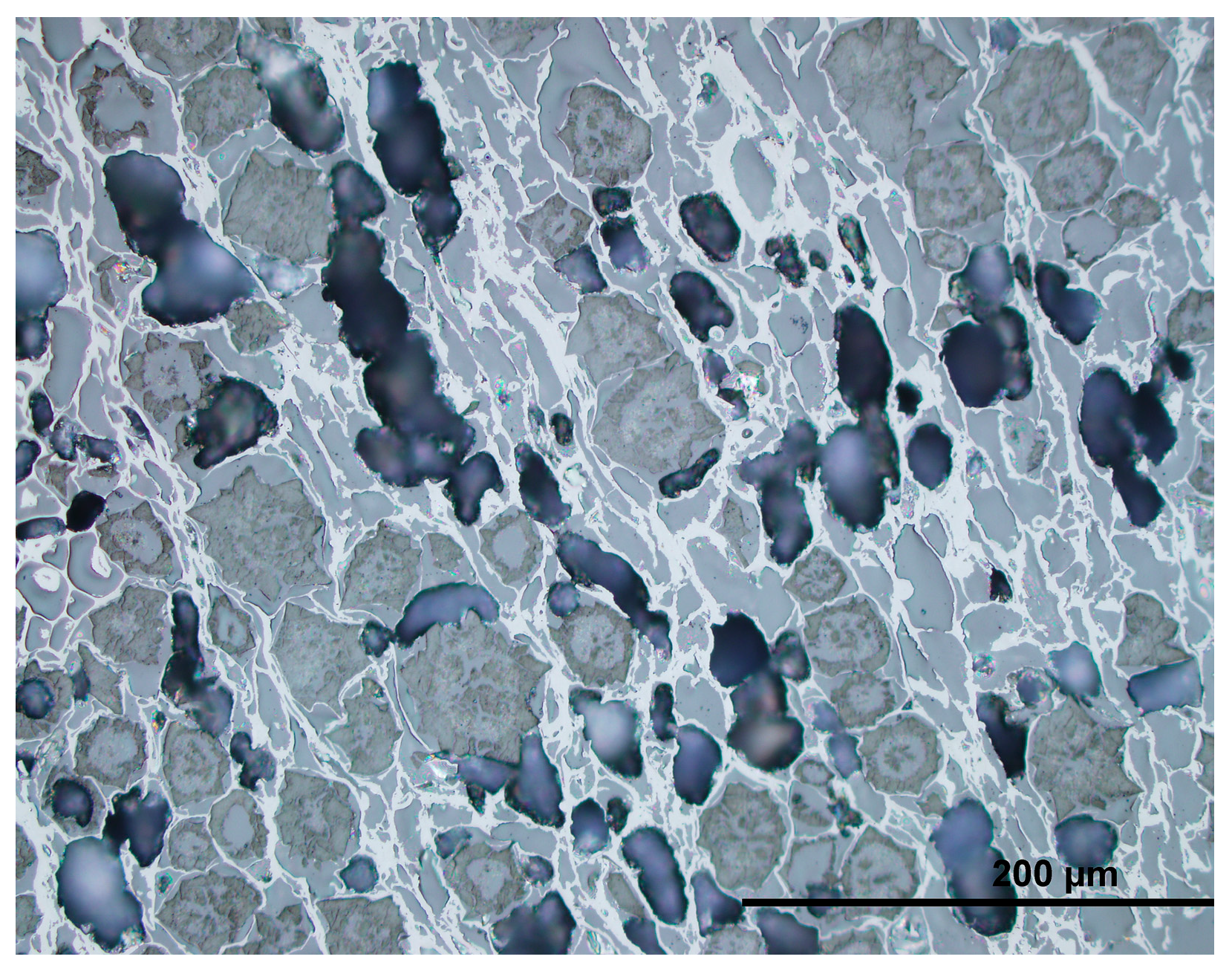



| Water | Ash | VM | FC | H | C | Sorg | N | O | |
|---|---|---|---|---|---|---|---|---|---|
| BS | 6.51 | 4.32 | 72.82 | 16.35 | 5.65 | 46.64 | 0.04 | 0.55 | 36.29 |
| AS | 4.87 | 2.30 | 81.53 | 11.30 | 7.78 | 56.08 | 0.17 | 6.63 | 22.17 |
| WS | 7.86 | 2.60 | 71.53 | 18.02 | 5.88 | 48.50 | 0.05 | 1.30 | 33.81 |
| Lignin | Cellulose | Hemicellulose | Extractives | |
|---|---|---|---|---|
| BS | 23.01 | 44.58 | 27.27 | 2.54 |
| AS | 31.33 | 25.37 | 29.45 | 11.44 |
| WS | 48.07 | 25.71 | 22.07 | 2.33 |
| Ru/Al2O3 (g) | CH4 | C2H4 | C2H6 | C3H6 | N2 | CO | CO2 | H2 | H2/CO | |
|---|---|---|---|---|---|---|---|---|---|---|
| BS | 0 | 15.02 | 0.06 | 0.13 | 0.00 | 0.13 | 43.22 | 13.90 | 27.54 | 0.64 |
| 2.5 | 8.74 | 0.01 | 0.02 | 0.01 | 0.88 | 41.50 | 6.46 | 42.39 | 0.97 | |
| 5 | 8.21 | 0.04 | 0.06 | 0.00 | 0.93 | 25.22 | 14.34 | 51.20 | 2.03 | |
| 10 | 6.02 | 0.06 | 0.13 | 0.00 | 0.34 | 25.79 | 15.65 | 52.01 | 2.02 | |
| AS | 0 | 19.71 | 0.00 | 0.02 | 0.02 | 0.87 | 48.12 | 1.48 | 29.78 | 0.51 |
| 2.5 | 10.01 | 0.02 | 0.02 | 0.00 | 0.83 | 50.07 | 12.87 | 26.18 | 0.52 | |
| 5 | 9.89 | 0.03 | 0.07 | 0.00 | 0.90 | 19.88 | 13.87 | 55.36 | 2.78 | |
| 10 | 6.61 | 0.01 | 0.04 | 0.00 | 0.93 | 18.21 | 12.89 | 61.31 | 3.37 | |
| WS | 0 | 17.18 | 0.03 | 0.04 | 0.00 | 0.66 | 48.30 | 17.21 | 16.58 | 0.34 |
| 2.5 | 0.97 | 0.00 | 0.00 | 0.00 | 0.62 | 15.82 | 1.04 | 82.17 | 5.19 | |
| 5 | 3.16 | 0.04 | 0.07 | 0.00 | 0.71 | 16.88 | 12.42 | 66.73 | 3.95 | |
| 10 | 1.04 | 0.00 | 0.00 | 0.00 | 0.76 | 23.91 | 4.35 | 69.94 | 2.93 |
| Biomass | Ru/Al2O3 (g/wt.%) | H2 (vol.%) | HHV (MJ m−3) | LHV (MJ m−3) | d (kg m−3) |
|---|---|---|---|---|---|
| BS | 5 g/10% | 51.20 | 13.06 | 11.73 | 0.717 |
| 10 g/20% | 52.01 | 12.43 | 11.16 | 0.729 | |
| AS | 5 g/10% | 55.36 | 13.59 | 12.11 | 0.656 |
| 10 g/20% | 61.31 | 12.80 | 11.33 | 0.597 | |
| WS | 2.5 g/5% | 82.17 | 12.88 | 11.22 | 0.307 |
| 5 g/10% | 66.73 | 11.99 | 10.54 | 0.549 | |
| 10 g/20% | 69.94 | 12.37 | 10.95 | 0.465 |
| Compounds | BS | AS | WS | Note | |||
|---|---|---|---|---|---|---|---|
| with | Without | with | Without | with | Without | ||
| Liquid hydrocarbons C6–C17 | 0.24 | 0.21 | 1.75 | 1.60 | 0.09 | 1.10 | Including cyclohexanes 0.14 wt.% at BS; no cyclohexanes were detected at AS and WS. |
| Alkylbenzenes | 2.40 | 2.42 | 0.35 | 0.35 | 0.69 | 0.67 | |
| Naphthalenes | 0.15 | 0.08 | 0.00 | 0.22 | 0.10 | 0.09 | |
| Phenols | 32.57 | 20.66 | 17.50 | 26.19 | 32.62 | 33.73 | |
| Hydroquinone | 5.13 | 23.62 | 2.69 | 16.07 | 4.01 | 17.47 | |
| Carboxylic acids | 33.42 | 37.04 | 46.16 | 46.62 | 41.92 | 36.45 | Including esters of lower acids in a significant minority. |
| Benzoates | 2.74 | 0.00 | 1.37 | 0.00 | 0.85 | 0.00 | |
| Stearates | 4.88 | 0.00 | 26.38 | 0.00 | 3.25 | 0.00 | |
| Ketones | 14.25 | 12.25 | 2.88 | 3.46 | 9.52 | 4.54 | Cyclopentanones, cyclohexanones, 2-hexanone. |
| Acetophenone derivatives | 1.54 | 0.00 | 0.92 | 0.00 | 2.28 | 0.00 | |
| Subst. benzaldehydes | 2.68 | 3.72 | 0.00 | 5.49 | 4.67 | 5.95 | |
| Sum | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | |
| Initial Biomass | Catalyst Amount (g) | Water | Ash | C | H | N | Sorg | O | HHV | LHV |
|---|---|---|---|---|---|---|---|---|---|---|
| BS | 0 | 2.09 | 3.85 | 83.55 | 2.28 | 1.05 | 0.07 | 7.11 | 23.90 | 23.35 |
| 2.5 | 2.04 | 2.21 | 86.59 | 3.01 | 0.33 | 0.05 | 5.77 | 33.78 | 33.07 | |
| 5 | 2.41 | 4.18 | 84.41 | 2.93 | 0.25 | 0.07 | 5.75 | 32.27 | 31.57 | |
| 10 | 3.55 | 5.65 | 83.20 | 2.95 | 0.14 | 0.10 | 4.41 | 31.71 | 30.98 | |
| AS | 0 | 3.48 | 2.90 | 81.34 | 2.97 | 0.78 | 0.13 | 8.40 | 31.46 | 30.73 |
| 2.5 | 1.75 | 2.32 | 85.30 | 3.02 | 0.91 | 0.05 | 6.65 | 33.01 | 32.31 | |
| 5 | 1.37 | 5.24 | 83.41 | 2.97 | 0.69 | 0.05 | 6.27 | 32.25 | 31.57 | |
| 10 | 1.59 | 5.32 | 84.73 | 2.92 | 0.84 | 0.05 | 4.55 | 32.27 | 31.59 | |
| WS | 0 | 3.14 | 2.34 | 87.50 | 2.89 | 0.57 | 0.06 | 3.50 | 33.29 | 32.58 |
| 2.5 | 2.15 | 3.87 | 84.97 | 3.05 | 0.08 | 0.05 | 5.83 | 32.41 | 31.69 | |
| 5 | 1.65 | 7.74 | 82.77 | 2.61 | 0.07 | 0.05 | 5.11 | 27.86 | 27.25 | |
| 10 | 2.67 | 8.46 | 80.10 | 2.80 | 0.17 | 0.05 | 5.75 | 30.61 | 29.93 |
| Initial Biomass | Smicro (m2 g−1) | n |
|---|---|---|
| BS | 356 ± 15 | 7 |
| AS | 421 ± 11 | 5 |
| WS | 409 ± 22 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Straka, P.; Cihlář, J.; Bičáková, O. Production of Syngas and Hydrogen-Rich Gas from Lignocellulosic Biomass via Ru/Al2O3 Catalyst-Assisted Slow Pyrolysis. Catalysts 2025, 15, 1033. https://doi.org/10.3390/catal15111033
Straka P, Cihlář J, Bičáková O. Production of Syngas and Hydrogen-Rich Gas from Lignocellulosic Biomass via Ru/Al2O3 Catalyst-Assisted Slow Pyrolysis. Catalysts. 2025; 15(11):1033. https://doi.org/10.3390/catal15111033
Chicago/Turabian StyleStraka, Pavel, Jaroslav Cihlář, and Olga Bičáková. 2025. "Production of Syngas and Hydrogen-Rich Gas from Lignocellulosic Biomass via Ru/Al2O3 Catalyst-Assisted Slow Pyrolysis" Catalysts 15, no. 11: 1033. https://doi.org/10.3390/catal15111033
APA StyleStraka, P., Cihlář, J., & Bičáková, O. (2025). Production of Syngas and Hydrogen-Rich Gas from Lignocellulosic Biomass via Ru/Al2O3 Catalyst-Assisted Slow Pyrolysis. Catalysts, 15(11), 1033. https://doi.org/10.3390/catal15111033







