1. Introduction
The fabrication of supported metal oxides with high loading and fine dispersion is of significant importance, mainly because of the obtainment of sufficient exposed sites and hence excellent performance in areas like adsorption and catalysis [
1,
2,
3,
4,
5]. Nickel-based catalysts are among the most widely explored because of their rich reserves and superior activities in many industrially important (de)hydrogenation reactions [
6,
7,
8]. Conventionally, to maximize the utilization of active sites, the nickel precursors are deposited onto a rigid support like SiO
2 or activated carbon. Compared to solid supports, supports with porous or hollow structures are highly expected, mainly due to the possession of a large surface area for immobilization of guest species. More importantly, it is anticipated that these peculiar structures could offer a confined environment for outstanding performance [
9].
As a famous mesoporous silica, SBA-15 is widely explored in the context of guest species dispersal [
10]. Thus, extensive attentions have been paid to encapsulate nanoarchitectures (nanoparticle, nanowire and nanorod) into the channels of SBA-15 [
11,
12,
13,
14,
15]. For application in catalysis, the exclusive formation of discrete nanoparticles with the maximum number of exposed atoms is of great interest. Until now, several strategies have been developed to construct pore-confined NiO nanoparticles in SBA-15. Richards et al. reported a so-called grafting method to prepare well-dispersed NiO nanoparticles [
16]. It was established that the surface reaction between the precursor (Bis(1,5-cyclooctadiene) nickel) and surface silanol allowed for the preparation of uniform NiO nanoparticles of sizes smaller than 5 nm. However, it was noticeable that due to the limited silanol available on the surface of SBA-15, the NiO loadings were restricted, and the final value was lower than 3
wt.%. A similar phenomenon was also reported by Lu et al. during the preparation of Ni/silica catalyst with nickel acetylacetonate as a metal precursor through the use of a grafting method [
17]. Alternatively, Yi and colleagues devised an in situ encapsulation method for anchoring NiO on SBA-15 by introducing PEO into the synthetic process of SBA-15 [
18]. They found that the NiO loadings were intimately related to the PEO/P123 ratios and the highest loading value reached 8.5
wt.%. Nevertheless, in consideration of the large pore volume (~1 cm
3 g
−1) of SBA-15, the theoretic loading capacity can be roughly calculated by employing a solution of nickel precursor with a density of 1.578 g cm
−3, resulting in a value of 21.26
wt.%, which considerably exceeds the above values [
19]. Thus, the utilization efficiency of mesopores is still poor, and concerted efforts are needed to extend the loadings.
As the most used technique for the fabrication of supported catalysts, the impregnation of a porous support with a soluble metal precursor (e.g., nitrate or acetate) solution exhibits the merits of simple operation and the ability to handle a wide range of loadings. However, the commonly employed wet impregnation route always induces random distributions of guest species, generating particles both inside and outside of the pores. This disadvantage was recently resolved by two independent groups via modified impregnation methods. Based on a method of controlled thermal decomposition that was achieved by varying the category of calcination gases, de Jong and colleagues demonstrated that the strategy was effective in synthesizing moderately small Ni particles (~4 nm) at high loading (24
wt.% in terms of NiO) in a reductive calcination atmosphere [
20]. However, the additive requirement needed for the NO/He atmosphere would inevitably limit its application on a larger scale. Another strategy was reported by Cheng and colleagues [
19]. In their work, the formation of discrete and highly loaded (18.55
wt.%) NiO nanoparticles confined in SBA-15 was achieved by a so-called hydrophobic encapsulation process. By introducing a Pechini process during the complexing of Ni
2+ with citric acid and the removal of outer precursor solutions with a hydrophobic solvent, the final NiO nanoparticles with small sizes (~3 nm) were found to be exclusively located in the mesopores and displayed excellent activity during methanation reaction. Although it approached the goal of high loading and good dispersion, drawbacks such as its tedious operational process and the leaching of the precursor were encountered, and the latter may simultaneously add environmental burden. Therefore, a facile and efficient synthesis of highly dispersed NiO nanoparticles confined in SBA-15 with high loadings continues to be of great importance but its achievement remains a challenge.
To insert guest species into a porous support, it is necessary for the precursor to first be translated into a mobile phase. As a unique property of many transitional metal nitrates, they will present as molten salts at temperatures higher than their melting points. In a previous study [
21], Yue et al. successfully fabricated a family of mesoporous single crystals (Co
3O
4, NiO, CeO
2, and Cr
2O
3) by using mesoporous silica as a hard template and metal nitrates as precursors through a so-called solid–liquid route. The result revealed that molten salts can be introduced into mesopores at large amounts. However, it is still unclear whether this method holds true for the fabrication of highly dispersed nanoparticles, without the coexistence of rod-like particles in the pore channels and external particles, and no related studies have been reported. On the other hand, the simple, cost-effective and environmentally benign features of this novel method encourage further exploration of its possible applications. Hence, in the present study, the efficiency of a solvent-free method for preparing highly loaded NiO nanoparticles confined in SBA-15 was investigated. The exclusively homogeneous dispersion of guest species in the pore channels derived in the absence of a solvent was subsequently emphasized. Moreover, the confinement of mesopores on the decomposition and coarsening of guest species was explored. Lastly, the advantage of encapsulated NiO in heterogeneous catalysis is exemplified by the reaction of NH
3 decomposition in the production of CO
x-free H
2, which is usually accomplished at relatively high temperatures and is thus suitable for exploring both the reactivity and sintering resistance of the prepared catalyst.
2. Results and Discussion
Figure 1 displays the small-angle XRD pattern of a representative sample (20Ni-NO
3-550) from the solvent-free method, and the pattern of pristine SBA-15 is registered for comparison. For SBA-15, four peaks (noted as 100, 110, 200, 210) indexed to the hexagonally arranged structures are observable, indicating the obtainment of highly ordered mesopores. After NiO introduction, the (210) reflection vanishes but the other peaks are still present, despite their disturbed intensity when compared to the pristine SBA-15. This tells us that the ordered framework does not suffer severe damage during grinding and the subsequent process of air calcination. Notably, when considering the introduction of guest species, the weakening of reflections can be reasonably ascribed to poorer scattering contrasts between the silica walls and the encapsulated mesopores, which is a well-reported phenomenon in occluded mesoporous materials [
22].
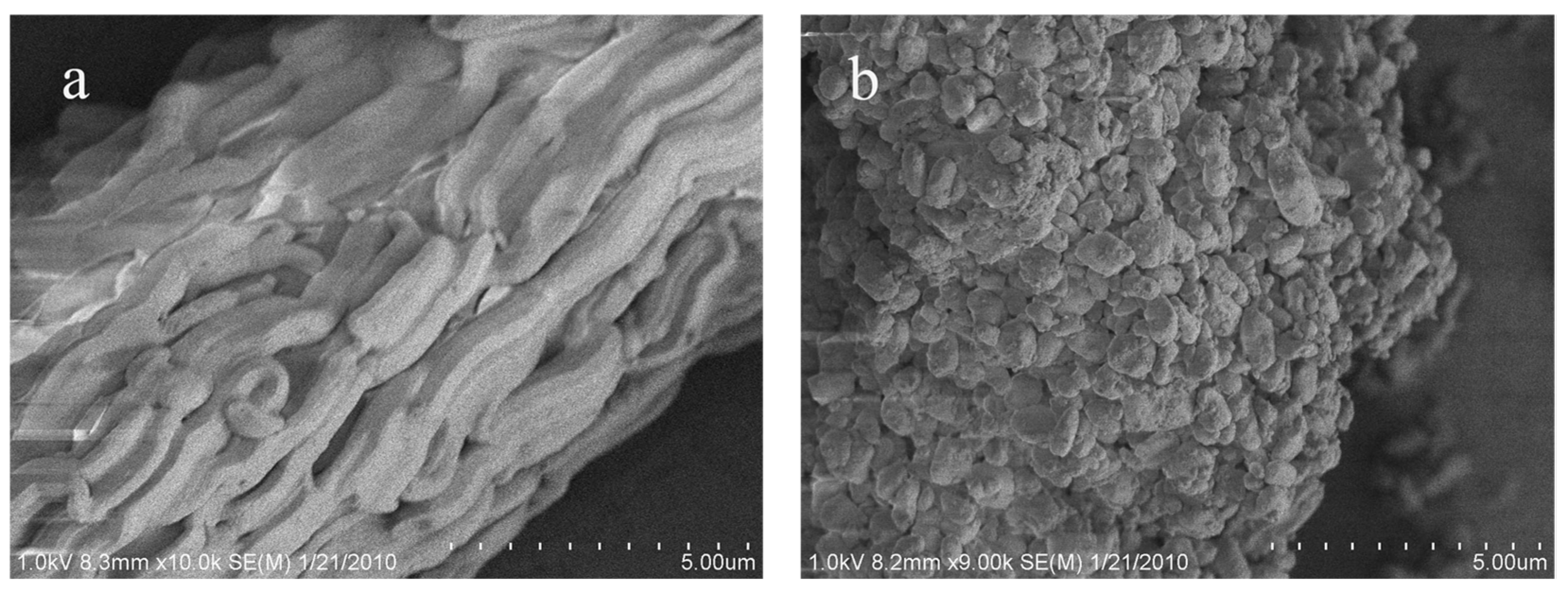
The effect of manual grinding on the size and morphology of the final product is examined by scanning electron microscope (SEM). For pristine SBA-15 (
Figure 2a), its macroscopic morphology is represented by rope-like domains with relatively uniform sizes of 2–3 μm in length and 0.5 μm in width, which are aggregated into wheat-like microstructures. Because of the homogeneous condensation of siliceous species onto the soft template, the surface of SBA-15 is smooth, and no bumps appear. This result is consistent with Zhao’s investigation [
10]. Interestingly, after manual grinding, distinct changes are observed on the size and morphology of the primary particles. The rope-like domains are transformed into irregular particles, and their sizes decrease to the sub-micrometer level. On the other hand, the surface of the particles is no longer smooth, and tiny bumps are observed. Notably, most of these bumps are smaller than one hundred nanometers. In concerning of the amorphous nature and relatively poor mechanical stability of the silica matrix [
23], the decrease in the size of the mesoporous matrix is probably due to the cutting effect from manual grinding, which is also supported by the partial reduction in textual parameters (
Table S1). The concomitant small bumps can thus be interpreted as the silica fragments. This unexpected finding is of certain significance. As is well acknowledged, the nanochannels of SBA-15 run along the long axis and their length is in the scale of micrometers. Molecular diffusion through the lengthy pore channels is one of the main concerns when applying these materials for sorption and catalysis. Hence, the present solvent-free method in terms of manual grinding can “cut” the lengthy pores into short ones, which will in turn provide greater pore accessibility and facilitate molecular diffusion.
The efficiency of the solvent-free method when fabricating highly loaded NiO particles on SBA-15 is comprehensively investigated by selecting two loads (20
wt.% and 30
wt.%) and choosing two kinds of commonly used nickel precursors, i.e., nickel nitrate hydrate (Ni(NO
3)·6H
2O) and nickel acetate hydrate (Ni(CH
3COO)
2·3H
2O). The results of wide-angle XRD are displayed in
Figure 3. For comparison purposes, the pattern of the sample prepared via the conventional impregnation method is also presented. In general, all samples display the peaks (2θ = 37.2°, 43.2° and 62.8°) which can be indexed to the diffractions of (111), (200) and (220) planes of a face-centered cubic crystalline NiO (JCPDS 75-0269), and no reflections from other nickel species demonstrate the complete conversion of precursors into NiO. In addition, the broad peak centered at around 2θ = 23 originated from the amorphous silica matrix. Noticeably, the intensity of diffraction peaks for NiO varies greatly with different methods and precursors, indicating that the state (size, location) of the synthesized NiO may be different. For the samples prepared using the solvent-free method with nickel nitrate as a precursor, the line broadening of profiles is obvious, as compared to the reference sample of 20NiO/SBA-15. It reveals the formation of NiO particles of nanometric dimensions and verifies that the solvent-free method can disperse guest species well on the mesoporous support, even at the highest reported metal oxide loading value of 30
wt.%. This result is of great interest and significant importance, especially concerning the facile operation and the lack of demand for special facilities and expensive reagents. Further screening of the XRD peak shapes shows that there are bimodal crystal-size distributions in the pattern of 30Ni-NO
3-550, which are similar to the results for the sample calcined in helium atmosphere [
20], indicating that the slight aggregation of NiO particles may take place at higher loadings. On the other hand, for the sample prepared with nickel acetate as a precursor, it is interesting to note that the intensity of NiO diffraction peaks is much sharper than that of 20Ni-NO
3-550 and 20NiO/SBA-15 when at the same loading value, suggesting that the properties of precursors exert a predominant influence on the final dispersion and size of NiO.
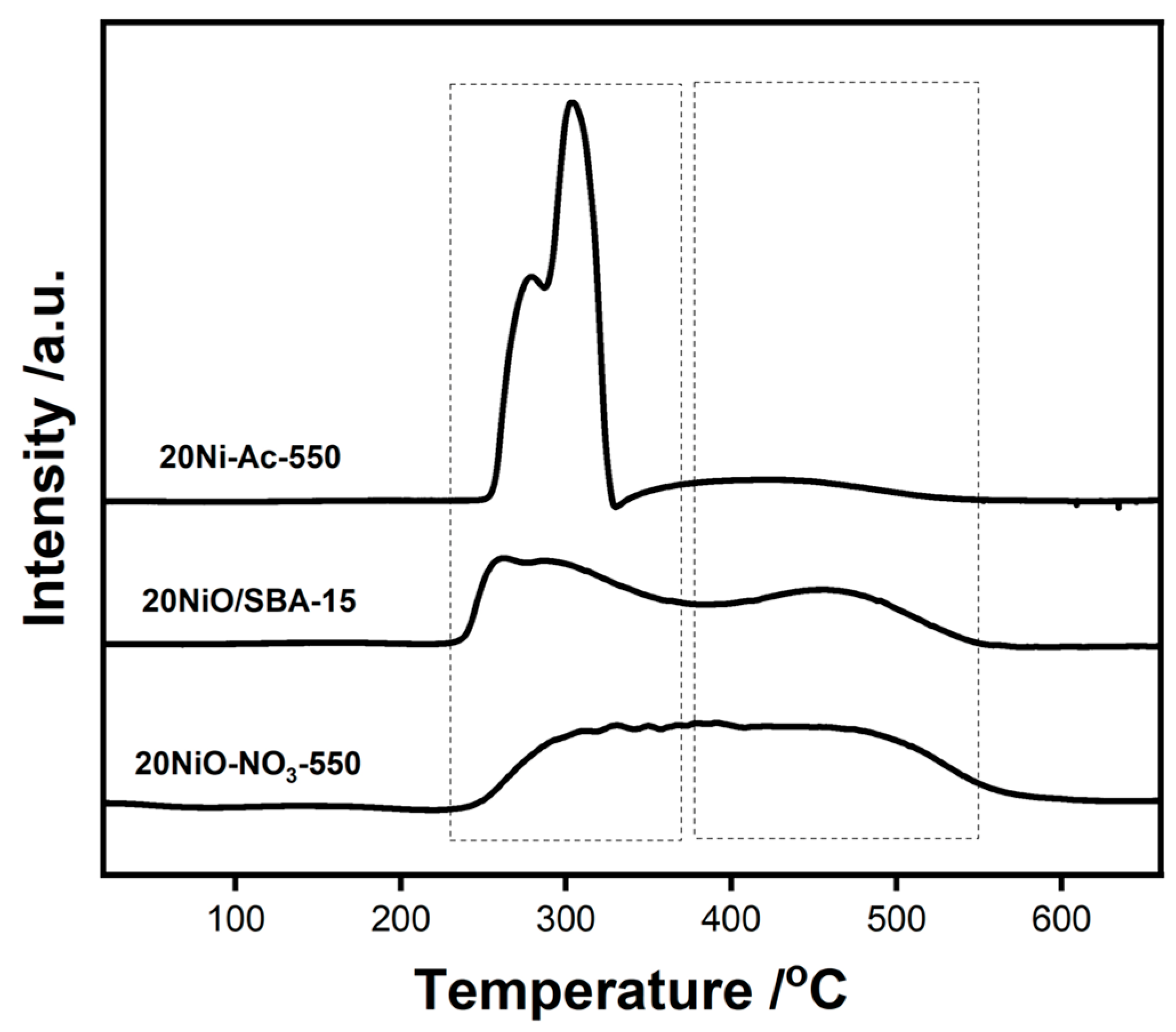
The formation of NiO of different particle sizes can be reflected by its interaction with the silica support. As such, H
2-TPR measurement is performed. It can be seen from
Figure 4 that the samples display distinctly different reduction profiles. For 20Ni-Ac-550, most of the reduction is accomplished at the temperature range of 200–400 °C, revealing that the facile reduction in bulk NiO is due to the limited interaction with silica support. For the other two samples, in addition to the low temperature peak, another reduction between 400 and 550 °C is obvious, suggesting the existence of NiO in strong interactions with SBA-15.
As reported elsewhere, the NiO particles outside of the mesopores usually exhibit large sizes under elevated thermal treatment [
24,
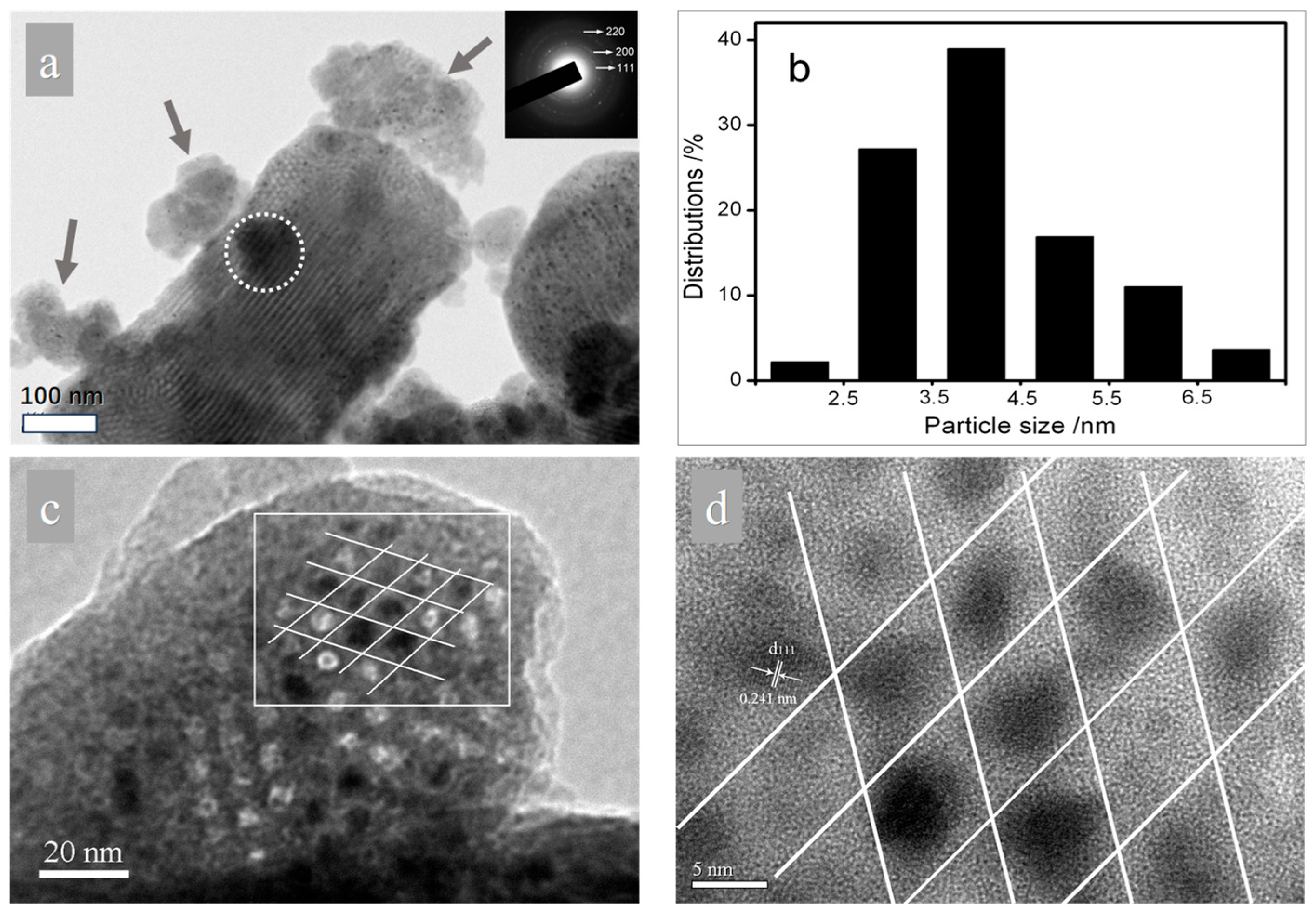
25]. Thus, it is presumed that the varied grain sizes of NiO that have been derived from different methods and precursors are related to their altered locations in SBA-15. To prove this assumption, further characterization via transmission electron microscope is operated. The representative TEM images of 20Ni-NO
3-550 are displayed in
Figure 5. To fully elucidate the location and dispersion of the NiO particles on the porous support, both images viewed from the directions perpendicular to (
Figure 5a) and parallel to (
Figure 5c,d) the pore channels are displayed. As for support, the alternating clear and dark stripes are visible (
Figure 5a), indicating the preservation of ordered mesostructures and supporting the small-angle XRD result. Noticeably, apart from the presence of the bulk silica matrix with sizes in the sub-micrometer range, there are also some smaller silica fragments present (indicated by arrows in
Figure 5a), which is in accordance with the SEM result. Thus, the darker area circled can be well-explained by the deposition of silica fragments on the bulk silica matrix. When it comes to guest species, the selected area electron diffraction (SAED) pattern (inset in
Figure 5a) confirms that they are NiO crystallites. It can be clearly observed that the NiO particles are homogeneously dispersed on the support and no aggregation to large particles is found, and this corresponds with the wide-angle XRD result. The corresponding particle size distribution (PSD) that were statistically analyzed by the counting of about 200 particles is shown in
Figure 5b and demonstrates that the present solvent-free method is fairly effective in preparing monodisperse NiO nanoparticles with narrow PSD. The histogram shows that most of the NiO nanoparticles (>80%) are centered at 3–5 nm. Based on
Figure 5a, the dispersion of NiO is clearly established. However, the evidence is not very persuasive regarding the determination of these fine particles in the mesopores. Complementarily, the image viewed from the direction parallel to pore channels is displayed. To analyze the inner part of the pores, it is preferential for the electron beam to be oriented in the direction parallel to the mesopores, and it is then possible to illuminate the content of the mesopores [
26,
27]. In
Figure 5c, still no aggregated particles are observed to have been deposited on the surface of the silica. Meanwhile, we can observe that some of the white spots representing pore channels are replaced by dark spots, and the enlarged image (
Figure 5d) reveals the dark spots to be crystalline NiO that is packed in a hexagonal arrangement, which convincingly confirms the NiO nanoparticles are essentially studded in the pore channels of SBA-15. The encapsulation of NiO in mesopores can also be evidenced by the analysis of the N
2 sorption result (
Table S1).
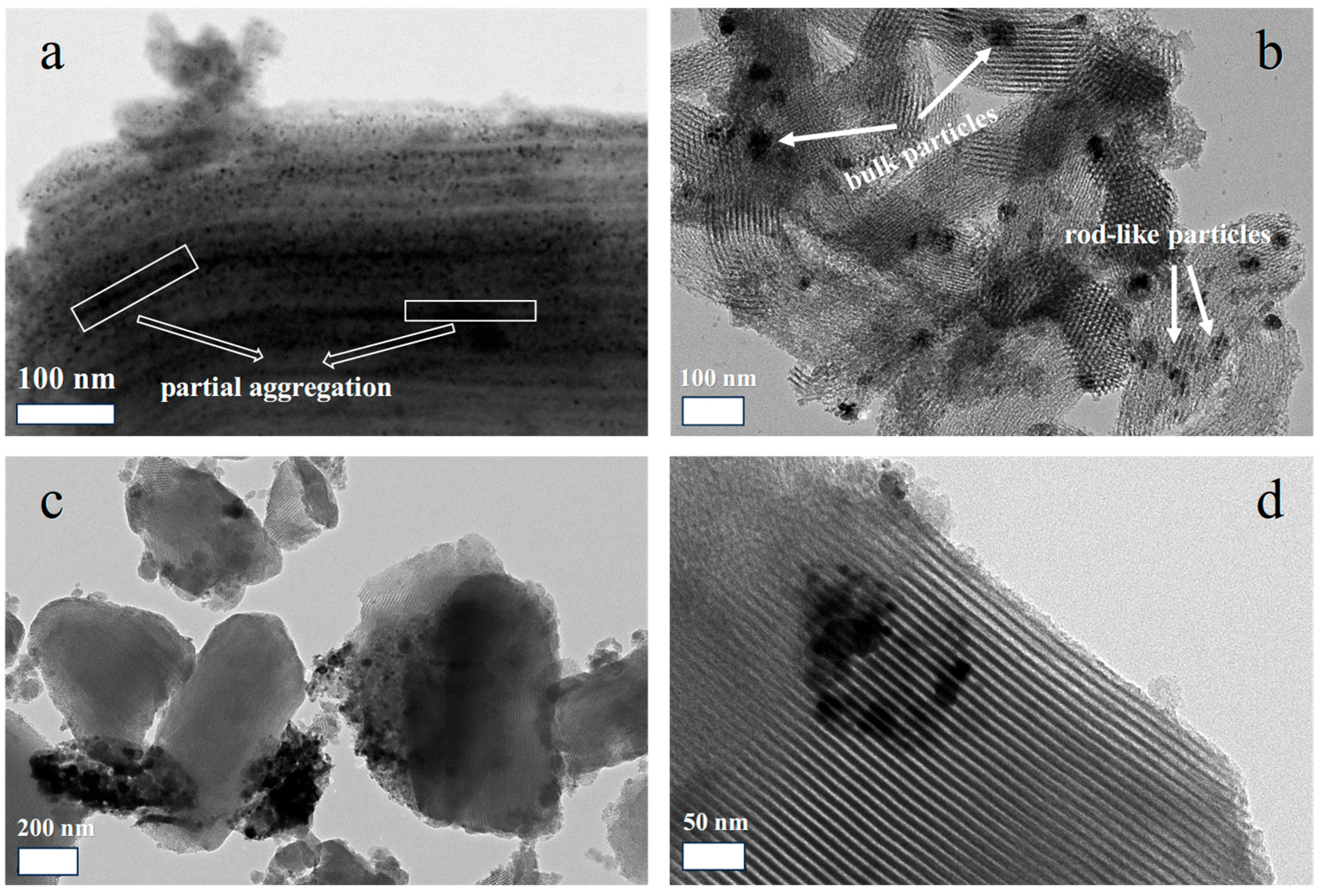
The dispersions of NiO in other samples are also characterized by TEM. As can be seen from
Figure 6a, the silica fragments are still present in 30Ni-NO
3-550, verifying the proposed cutting effect made by manual grinding. In addition, most of the NiO nanoparticles are well scattered on the silica support with sizes of 4–5 nm. Typically, no bulk NiO is observed on the external surface of the support, powerfully demonstrating the advantage of the solvent-free method in preparing exclusive pore confined nanoarchitectures. Nevertheless, in certain areas, as framed in the image, rod-like particles appear. It suggests that partial aggregation of small nanoparticles into nanorods occurs when overwhelming amounts of metal precursor are introduced, which is in agreement with the wide-angle XRD result. In most cases, the appearance of nanorods is indicative of the blocking of mesopores, which is not favorable for molecular diffusion in the pores. For the reference sample of 20NiO/SBA-15 (
Figure 6b), the dispersion of NiO particles is not good. That is, bulk particles with sizes of 20–100 nm are detected, and they are expected to disperse on the exterior of SBA-15. Meanwhile, rod-like particles are also observable, suggesting the difficulty in controlling the proper distribution of guest species on SBA-15 through wet impregnation. To further examine the effect of different precursors on the dispersion of NiO, the TEM images of 20Ni-Ac-550 are shown in
Figure 6c,d. From the low magnification image (
Figure 6c), the most severe aggregation of NiO is observed among the three samples, as evidenced by the presence of aggregated NiO particles at sizes of hundreds of nanometers. The majority of NiO particles are either completely separated from the silica matrix or just in loose contact with the fringe of SBA-15. In the high magnification image (
Figure 6d), the agglomeration of bulk NiO particles is clearly observed and no particles are actually confined in the pore channels, even in the form of aggregated nanorods. The result reveals that the solvent-free method is not an innate technique for the fabrication of pore confined NiO particles and its successful encapsulation is much dependent on the properties of the selected precursors.
On the basis of the combined analyses from wide-angle XRD, N
2 physisorption and TEM, the dispersion and location of NiO from different methods and precursors are confirmed. The results show that both the preparation method and precursors have great influence on the final state of NiO. For the conventional wet impregnation method, it is generally achieved by the impregnation of the porous support with a solution of metal precursor, followed by the evaporation of the solvent (drying step) and calcination. As seemingly facile as the practical execution is, the fundamental phenomena underlying impregnation and drying are extremely complex. Therefore, many intensive studies are carried out to investigate the influences of each individual step in preparing supported catalysts. Generally, the soluble precursor can be homogeneously dispersed onto the support in the impregnation step, and in certain cases, with the assistance of ultrasonic treatment. For SBA-15, it is reported the precursor is introduced into the pores during this step, resulting in its homogenous distribution. Subsequently, in the drying step, owing to the limited interactions between precursor and inert support, the diffusion of surface species with solvent evaporation results in the redistribution of the active phase [
20]. For mesoporous silica as a support, the diffusion of the precursor out of the pores always takes place and conforms to the result showing heterogeneous distributions of NiO particles in 20NiO/SBA-15.
To obtain a supported catalyst with a uniform distribution of surface species, it is essential to control the unfavorable redistribution of precursors during the drying step. In brief, there are mainly two strategies to approach the goal. The first is based on the creation of strong interactions between the support and the precursor. In line with this idea, methods like grafting and strong electrostatic adsorption are developed. By preliminarily grafting functional groups like -NH
2 onto the support or directly utilizing the surface hydroxyl, the anchoring sites can bind well to the precursor. As a result, the redistribution of precursors during drying is inhibited, which in turn ensures the high dispersion of active species. As an alternative, the synthesis of well dispersed guest species on silica via electrostatic adsorption is demonstrated [
28,
29]. Due to the presence of SEA, the diffusion of the precursor is prevented and as a result, highly dispersed metals are obtained. To reduce the redistribution of precursors, another strategy is to increase the diffusion resistance of the impregnated solution. This way is mainly based on the employment of chelating salts or the addition of viscosity-increasing agents to aqueous metal nitrate solutions [
30,
31]. With the evaporation of the solvent, the steep increase in viscosity is apparent, and will significantly inhibit the redistribution of precursors during drying. From this point of view, the preparation of highly loaded NiO with good dispersion, as reported by Cheng et al. [
19], can be well explained, as the introduction of citric acid favors the formation of viscous nickel citrate solution.
From the discussion above, it is apparent that the redistribution of precursors during drying is one of the most decisive factors in the poor dispersion of guest species on an inert support. As a matter of fact, redistribution is induced by the diffusion of precursors with the evaporation of the solvent under certain temperatures. Thus, if there is no solvent present and the precursor can be automatically introduced into the mesopores, the redistribution of precursors can be greatly overcome. Moreover, it is assumed that the solvent can exert a competitive adsorption on the support and disturb the host–guest interactions, which in turn hinders the dispersion and anchoring of active components on supports [
32]. This proposition is supported by the experimental result indicating that the dispersion of noble metals (Pd, Au) grafted on SBA-15 is influenced by the use of different solvents [
33]. On the other hand, concerning the filling degree of active species within a single step, the deposition process without the use of a solvent has merit, as it can make full use of the pores without the void space needing to be occupied by a solvent.
As proved elsewhere, the dry salts can be introduced into the pore channels of SBA-15 [
34]. These dry salts are metal nitrates and will turn into molten salt phase in certain temperature ranges. Apart from its unique property of mobility, the molten salts are also viscous, and both of these properties are supposed to be crucial for determining the dispersion of NiO particles. When the temperature is elevated, nickel nitrate starts to become meltable (evidence is provided by thermogravimetry-differential thermal analysis (TG-DTA) and the direct digital picture,
Figures S1 and S2) and the filling of mesopores begins. Thus, under the driver of capillary imbibition, the molten salt can be easily sucked into the pore channels. As a sharp contrast to the wet impregnation preparation, the simultaneous lack of solvent evaporation and possession of high viscosity of molten salt phase significantly prevents the redistribution of guest species under thermal treatment. As a result, uniform NiO nanoparticles confined in the pore channels are produced when the temperature reaches and then surpasses the decomposition temperature. In terms of nickel acetate as a precursor, due to the thermal property of direct decomposition without involving an intermediate molten salt phase, it cannot be diffused into the pores. Consequently, it displayed bulk NiO particles exclusively outside of the mesopores.
Since the discovery of the ordered mesoporous silica, the expected confinement effects of the pores have attracted tremendous interest. However, there are only few reports concerning the systematic investigation of pore confinement on the properties of guest species. Normally, to fully explore the confinement effect, comparison of the behaviors of guest species exclusive inside and outside of the mesopores is favored. In the present case, the samples prepared from nickel nitrate and nickel acetate via the solvent-free method only just meets this requirement. As most of the catalytic reactions are high-temperature processes, the thermal stability of active species is an important parameter needed to evaluate the catalysts. Hence, the confinement effect of mesopores on the thermal stability of nickel species is investigated by XRD under different calcination temperatures. Owing to the different thermal stabilities, the decomposition processes of the two nickel precursors into NiO are not the same. After being air calcined at 250 °C for 4 h, almost all of the nickel acetate is converted into NiO, as evidenced by the absence of conspicuous diffraction peaks related to nickel species other than NiO (
Figure 7a). Likely because of the relatively low thermal treatment temperature, the line broadening of diffraction peaks is obvious and the coarsening of NiO is not severe. However, for nickel nitrate (
Figure 7b), only partial decomposition of precursor has occurred, as indicated by the presence of a series of diffraction peaks centered at 2θ = 12.8°, 25.8° and 33.6°, attributable to Ni
3(NO
3)
2(OH)
4 (JCPDS 22-0752). The compound had been previously reported as an intermediate between nickel nitrate and the final decomposed product NiO [
22]. By comparing the pattern of 20Ni-NO
3-250 with that of direct decomposed nickel nitrate at 250 °C (for simplicity, denoted as Ni(NO
3)
2-D), it can be assumed that the decomposition of Ni
3(NO
3)
2(OH)
4 into NiO is much retarded, possibly as a result of pore confinement. By increasing the calcination temperatures, the line broadening of diffraction peaks for nickel acetate gradually disappears, indicating the significant sintering of NiO particles under elevated temperatures. For the samples prepared from nickel nitrate, the complete conversion of Ni
3(NO
3)
2(OH)
4 to NiO is observed at 350 °C (
Figure 7c). Strikingly, even after increasing calcination temperatures to 650 °C, the line broadening of diffraction peaks is still obvious, and no distinct changes are detected as compared to the sample air calcined at the lower temperature (350 °C). To quantify the confinement effect, the average grain size calculated by the Sherrer equation as a function of calcination temperature for different precursors is plotted in
Figure 7d. Owing to the incomplete conversion to NiO, the 20Ni-NO
3-250 is missing. All the guest species represented by hollow and solid squares are NiO and the comparison is thus reasonable. The smallest size of NiO is only ca. 2.7 nm, which is obtained from the 20Ni-Ac-250 sample with exclusive external particles. The result indicates that the fine particles are not an inherent property of pore confined NiO, and at low temperatures, it is the temperature rather than the location of guest species that determines the particle size. The size of NiO gradually increases to ca. 5 nm at temperatures below 350 °C. Severe sintering of NiO particles takes place when the temperature is further increased, as evidenced by the sharp increase in sizes to 15 nm and 25 nm at 450 °C and 550 °C, respectively. In contrast, for confined NiO samples (20Ni-NO
3-T), the average grain size of NiO is around 5 nm and almost unchanged within the operated temperature range. The result clearly demonstrates that with the aid of pore confinement, the nanosized NiO particles are highly thermally stable. As a result, the highly dispersed NiO nanoparticles are obtained even at a high calcination temperature (650 °C).
In order to explore the catalytic performance of the prepared samples, the reaction of ammonia decomposition is adopted, in view of the high reaction temperature. Additionally, this reaction has recently been subject to an increasing level of attention due to the possibility of ammonia being used as a hydrogen storage medium in possible hydrogen economy. Compared to conventional processes involving hydrogen production, like stream reforming and partial oxidation of methane, the ammonia decomposition route has the merit of the production of CO
x-free hydrogen, which is highly desirable in current proton-exchange membrane fuel cells (PEMFCs). The supported Ni/silica catalyst is reported to be active for ammonia decomposition, making the evaluation meaningful. Since metallic Ni is well acknowledged to be the active site, the catalyst is subjected to H
2 treatment at 550 °C before activity testing, and most of the NiO can be transformed into metallic Ni at this temperature, in reference to the temperature-programmed reduction by H
2 (H
2-TPR)result and high resolution transmission electron microscopy (HRTEM) characterization (
Figure S3).
To make a reliable comparison, the effects from internal and external mass diffusion are first explored. The reaction rates were compared over the 20NiO-NO
3-550 with varied granule sizes (20–40, 40–60, 60–80, 80–100 mesh) and at different dilution ratios with SiC (3:1, 1:1, 3:7, 1:9) while maintaining the same contact time at 400 °C. As can be seen from
Figure S4a, the NH
3 conversion showed similar rates when particle size was smaller than 20–40 mesh. In addition, the reaction rates were kept constant over the explored SiC dilution experiment (
Figure S4b). These results suggested that the mass transfer limitations of catalysts during reactions could be eliminated under the employed reaction conditions. As such, it can be safely concluded that the differences in catalytic behavior accurately reflect the varied reactivity of the active sites.
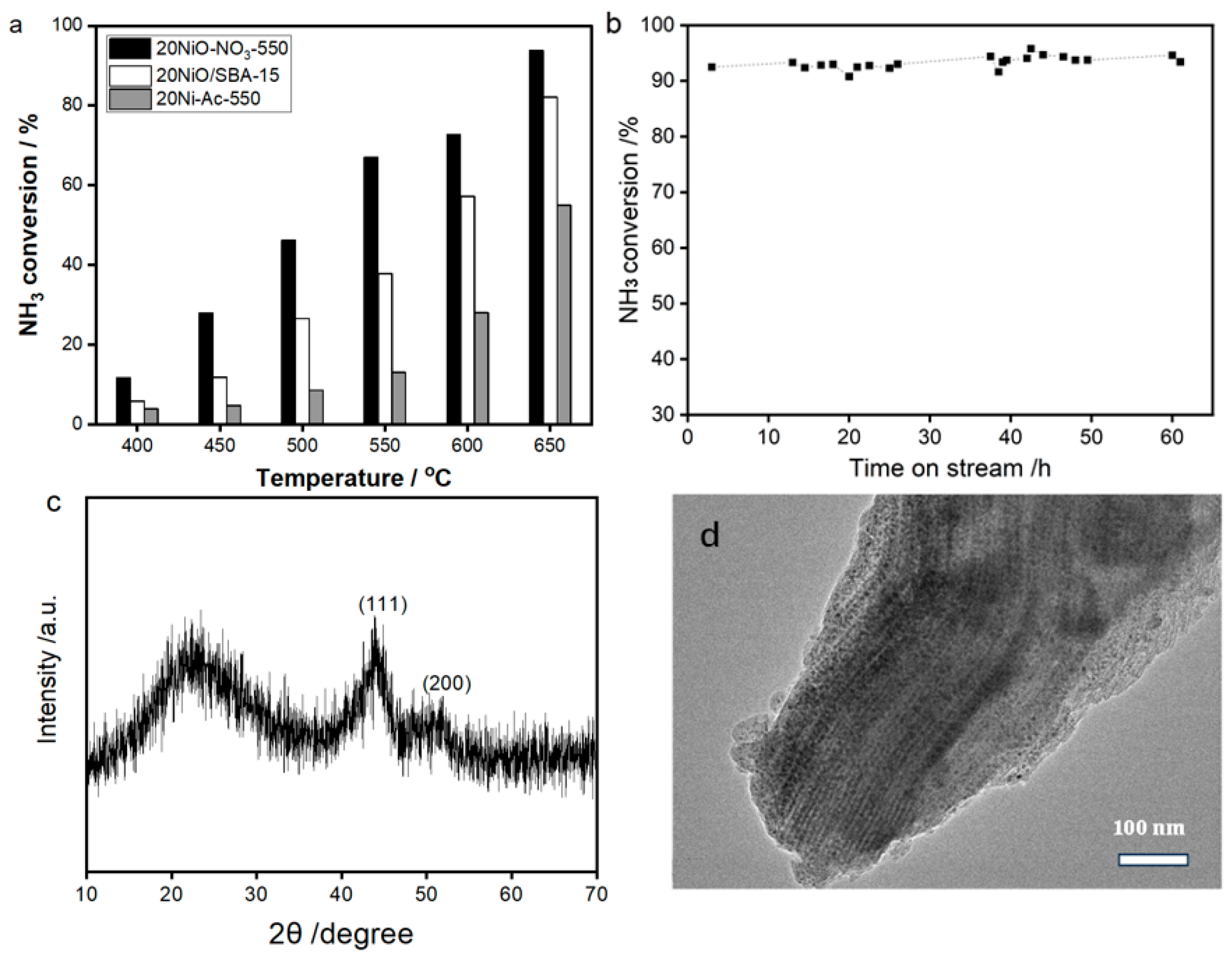
From
Figure 8, it is evident that significant variation in NH
3 conversion are exhibited. That is, the catalytic activity of 20Ni-Ac-550 exhibits the worst performance, with the highest value of 55.0% at 650 °C. For the sample derived from wet impregnation, due to the improvement in NiO dispersion, the conversion efficiency receives distinct enhancement, with the maximum value of 82.1% obtained at 650 °C. Notably, the reaction efficiency is further improved when the catalyst is prepared by using the novel solvent-free method. The value of NH
3 conversion (93.7% at 650 °C) exhibits a much higher value than the counterpart catalysts of 20Ni-Ac-550 and 20NiO/SBA-15. Remarkably, when compared with the literature reports, this value is among the best performance for Ni-base catalysts [
35,
36], and is likely related to both high loading and good dispersion of NiO. On the other hand, the catalyst also reveals superior stability, since the activity is almost the same even after successive running of 60 h at 650 °C (
Figure 8b). The result demonstrates the present solvent-free method is powerful in preparing thermally stable and robust supported catalysts, which is supported by the results of XRD and TEM for the spent catalyst of 20Ni-NO
3-550 (
Figure 8c,d).