Photocatalytic and Enzymatic Degradation of Microplastics: Current Status, Comparison, and Combination
Abstract
1. Introduction
| Title | Main Topic | References |
|---|---|---|
| From macro to micro: The key parameters influencing the degradation mechanism and the toxicity of microplastics in the environment | The mechanisms (photodegradation, thermal, mechanochemical, photocatalytic, and biodegradation) influencing MP degradation and its ecotoxicological impacts on terrestrial and aquatic ecosystems | [48] |
| Light-driven degradation of microplastics: Mechanisms, technologies, and future directions | Photocatalytic degradation mechanisms, catalyst advancements (e.g., TiO2 and ZnO), and value-added upcycling of microplastics into hydrogen/chemicals | [49] |
| Microplastics: a global threat to life and living | Mechanisms of action, degradation pathways (biological/chemical/photodegradation), and analytical techniques for MPs | [50] |
| Advances in chemical removal and degradation technologies for microplastics in the aquatic environment: A review | Chemical removal and degradation technologies for MPs in aquatic environments (such as coagulation, advanced oxidation, and photocatalysis), analyzing their efficiency, mechanisms, and influencing factors | [51] |
| Review of Soil Microplastic Degradation Pathways and Remediation Techniques | Degradation pathways (e.g., pyrolysis, hydrolysis, and biodegradation) and photocatalytic remediation techniques for MPs in soil environments, with emphasis on agricultural mulch film | [52] |
| From bulk to bits: understanding the degradation dynamics from plastics to microplastics, geographical influences and analytical approaches | Degradation dynamics from plastics to microplastics with emphasis on geographical influences and analytical characterization techniques | [53] |
| Occurrence, Degradation Pathways, and Potential Synergistic Degradation Mechanism of Microplastics in Surface Water: A Review | Occurrence, accumulation, and synergistic degradation mechanisms (physical–chemical–biological coupling and microbial community interactions) of MPs, specifically in surface waters (rivers, lakes, and reservoirs) | [54] |
| On the degradation of (micro)plastics: Degradation methods, influencing factors, environmental impacts | Degradation methods, influencing factors (intrinsic properties and external environment), and environmental impacts of degradation products for (micro)plastics. | [55] |
| Microbial strategies for effective microplastics biodegradation: Insights and innovations in environmental remediation | Microbial consortia and enzymatic pathways for enhancing MP biodegradation, with emphasis on pretreatment strategies and environmental remediation applications | [56] |
| Engineered technologies for the separation and degradation of microplastics in water: A review | State-of-the-art engineered technologies for both the separation and degradation of MPs in freshwater, identifying knowledge gaps and future research directions for real-scale application | [57] |
2. Photocatalytic Degradation
2.1. Ultraviolet-Induced Photocatalysis
2.2. Visible Light-Induced Photocatalysis
| Type | Photocatalyst | MPs | Light Source Type | Degradation Efficiency (%) | Time | References |
|---|---|---|---|---|---|---|
| TiO2 | TiO2 | PP | Visible light | Mass loss, 50.5 ± 0.5% | 50 h | [82] |
| Pac-Man TiO2 | PS | UV light | 28% | 70 h | [83] | |
| Doped TiO2 | N-TiO2 | PE | Visible light | Mass loss, 6.4% | 20 h | [69] |
| N-TiO2 | HDPE, LDPE | Visible light | Mass loss, 4.65 ± 0.35% | 50 h | [70] | |
| MJMPs | PMMA, PE, PS | Visible light | 34.6%, 48.9%, and 11.3% | 36 h | [84] | |
| Nb2O5 | PE, PP, PET | Visible light | 100% | 55–64 h | [85] | |
| Heterojunctions | In2O3-rGO | PS | Visible light | 56% | 12 h | [71] |
| Cs3Bi3Br9/BiOCl S | PS | Visible light | Mass loss, 42.3 ± 3.89% | 50 h | [72] | |
| CuO/TiO2 | Nylon | UV-Vis light | - | 5 h | [86] | |
| Fe1−xS/FeMoO4/MoS2 | PS | Visible light | 58.46% | 30 h | [87] | |
| CdS-16% CuInSe2 | PET | Visible light | - | - | [88] | |
| C,N-TiO2/SiO2 | PET | Visible light | 9.35–16.22% | - | [89] | |
| g-C3N4/TiO2/WCT-AC | PE | Visible light | 67.58% | 60 h | [90] | |
| 0D Co3O4@2D Co (OH)2 | PE | Visible light | 40% | 9 h | [91] | |
| FeB/TiO2 | PS | UV light | 92.3% | 12 h | [76] | |
| TiOX/ZnO | PET, PES | UV light | 100% | 480 h | [92] | |
| NH2-UiO-66 | PS | Visible light | 98% | 30 min | [93] | |
| CuO/Bi2O3/g-C3N4 | PET | Visible light | Mass loss, 41.6% | 240 h | [94] | |
| g-C3N4/CQD/FeNi-BTC | PS, PET | Visible light | 82.16% | 72 h | [95] | |
| MOFs | BiOI/MIL101 | PE | Visible light | CI increased by 0.127 | 6 h | [73] |
| Ag2O/Fe-MOF | PEG | Visible light | Mass loss, 5.5% | 3 h | [96] | |
| Plasmonic Photocatalyst | Ionophore-assisted Hopcalite | PS | Visible light | 98.4% | 1 h | [75] |
| Plasmonic Platinum/ZnO | LDPE | Visible light | 78% | - | [74] |
| Characteristics | UV-Induced Photocatalysis | Visible Light-Induced Photocatalysis |
|---|---|---|
| Light source | UV light | Visible light |
| Photocatalysts | Wide Bandgap Semiconductors TiO2 ZnO | Narrow-bandgap or modified semiconductors g-C3N4 Modified TiO2 CdS Composite materials |
| Advantages | Catalysts (such as TiO2) are stable, non-toxic, and low-cost and have strong redox ability and high reactivity. | It can efficiently use solar energy and has greater application potential, which is the current research hotspot. |
| Disadvantages | The utilization rate of solar light is extremely low (UV light only accounts for 3–5%), and additional UV light sources are usually required, resulting in high energy consumption and cost. | Many catalysts (such as CdS) have poor stability and are prone to photocorrosion. The design and preparation of catalysts are more complicated, and the cost may be higher. The oxidation ability is sometimes weaker than that of the ultraviolet catalytic system. |
3. Enzymatic Degradation
3.1. Main Enzymes Involved in the Degradation of MPs
3.1.1. Hydrolase
3.1.2. Oxidoreductase
3.2. Strategies to Improve the Efficiency of the Enzymatic Degradation of MPs
| Enzyme Type | Enzymes | MPs | Possible Sources | References |
|---|---|---|---|---|
| Hydrolases | Esterases | PU, PE, PET, PVC | Pseudomonas spp. | [117] |
| Lipases | PET, PCL | Thermomyces Lanuginosus | [118] | |
| PETase | PET | Ideonella sakaiensis | [105] | |
| MHETase | PET | Ideonella sakaiensis | [105] | |
| Cutinases | PET, PCL, PU | Resulting from phytopathogenic fungus infection | [106] | |
| Oxidoreductases | Laccases | PET, PE, PS, PVC | Ascomycetes, Basidiomycetes, and Deuteromycetes fungi | [119] |
| LiP | PVC, PET, PE, PP, PS | White-rot fungus | [108] | |
| MnP | PVC, PE, PP | Phanerochaete chrysosporium | [109] |
4. Comparison and Combination Between Photocatalytic and Enzymatic Degradation
5. Conclusions and Prospects
5.1. Conclusion
5.2. Challenges and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MPs | microplastics |
| NPs | nanoplastics |
| PE | polyethylene |
| PP | polypropylene |
| PET | polyethylene terephthalate |
| PA | polyamide |
| PS | polystyrene |
| PVC | polyvinyl chloride |
| PU | polyurethane |
| PCL | polycaprolactone |
| UV | ultraviolet |
| CI | carbonyl |
| HI | hydroxyl |
| LDHs | layered double hydroxides |
| MOFs | metal–organic frameworks |
| AOPs | advanced oxidation processes |
| ROS | reactive oxygen species |
| HDPE | high-density polyethylene |
| LDPE | low-density polyethylene |
| C/O | carbon/oxygen |
| MNMs | micro/nanomotors |
References
- Jacques, O.; Prosser, R.S. A probabilistic risk assessment of microplastics in soil ecosystems. Sci. Total Environ. 2021, 757, 143987. [Google Scholar] [CrossRef]
- Teresa, R.-S.; Fang, W.; Guilherme, M.; Damià, B. Micro(nano)plastics in the environment. J. Hazard. Mater. Adv. 2022, 8, 100181. [Google Scholar] [CrossRef]
- Solange, M.; Luís, A.; Bruno, M.; Anabela, R.; Graça, R.M.d. Microplastics in Ecosystems: From Current Trends to Bio-Based Removal Strategies. Molecules 2020, 25, 3954. [Google Scholar] [CrossRef]
- Nene, A.; Sadeghzade, S.; Viaroli, S.; Yang, W.; Uchenna, U.P.; Kandwal, A.; Liu, X.; Somani, P.; Galluzzi, M. Recent advances and future technologies in nano-microplastics detection. Environ. Sci. Eur. 2025, 37, 7. [Google Scholar] [CrossRef]
- Liu, S.; Wang, J.; Zhu, J.; Wang, J.; Wang, H.; Zhan, X. The joint toxicity of polyethylene microplastic and phenanthrene to wheat seedlings. Chemosphere 2021, 282, 130967. [Google Scholar] [CrossRef]
- Meng, S.; Zhang, Z. Effects of microplastics on soil environment and land plant growth: A review. Environ. Monit. Assess. 2025, 197, 861. [Google Scholar] [CrossRef] [PubMed]
- Fendall, L.S.; Sewell, M.A. Contributing to marine pollution by washing your face: Microplastics in facial cleansers. Mar. Pollut. Bull. 2009, 58, 1225–1228. [Google Scholar] [CrossRef]
- Browne, M.A.; Galloway, T.; Thompson, R. Microplastic—An emerging contaminant of potential concern? Integr. Environ. Assess. Manage. 2007, 3, 559–561. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Ren, Z.; Gui, X.; Xu, X.; Zhao, L.; Qiu, H.; Cao, X. Microplastics in the soil-groundwater environment: Aging, migration, and co-transport of contaminants—A critical review. J. Hazard. Mater. 2021, 419, 126455. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.Y.; Tang, Y.; Cheng, P.; Song, Y.; Li, X.; Lou, J.; Iqbal, B.; Zhao, X.; Hameed, R.; Li, G.; et al. Effects of degradable and non-degradable microplastics and oxytetracycline co-exposure on soil N2O and CO2 emissions. Appl. Soil Ecol. 2024, 197, 105331. [Google Scholar] [CrossRef]
- Ansari, I.; Arora, C.; Verma, A.; Mahmoud, A.E.D.; El-Kady, M.M.; Rajarathinam, R.; Verma, D.K.; Mahish, P.K. A Critical Review on Biological Impacts, Ecotoxicity, and Health Risks Associated with Microplastics. Water Air Soil Pollut. 2025, 236, 88. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; Kim, S.; Sarkar, B.; Oleszczuk, P.; Sang, M.K.; Haque, M.N.; Ahn, J.H.; Bank, M.S.; Ok, Y.S. Effects of microplastics on the terrestrial environment: A critical review. Environ. Res. 2022, 209, 112734. [Google Scholar] [CrossRef] [PubMed]
- Payanthoth, N.S.; Mut, N.N.N.; Samanta, P.; Li, G.; Jung, J. A review of biodegradation and formation of biodegradable microplastics in soil and freshwater environments. Appl. Biol. Chem. 2024, 67, 110. [Google Scholar] [CrossRef]
- Guo, X.; Wang, J. The chemical behaviors of microplastics in marine environment: A review. Mar. Pollut. Bull. 2019, 142, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Suman, T.Y.; Jia, P.-P.; Li, W.-G.; Junaid, M.; Xin, G.-Y.; Wang, Y.; Pei, D.-S. Acute and chronic effects of polystyrene microplastics on brine shrimp: First evidence highlighting the molecular mechanism through transcriptome analysis. J. Hazard. Mater. 2020, 400, 123220. [Google Scholar] [CrossRef] [PubMed]
- Ugwu, K.; Herrera, A.; Gomez, M. Microplastics in marine biota: A review. Mar. Pollut. Bull. 2021, 169, 112540. [Google Scholar] [CrossRef]
- Shen, M.; Song, B.; Zhou, C.; Almatrafi, E.; Hu, T.; Zeng, G.; Zhang, Y. Recent advances in impacts of microplastics on nitrogen cycling in the environment: A review. Sci. Total Environ. 2022, 815, 152740. [Google Scholar] [CrossRef]
- Iqbal, B.; Zhao, T.; Yin, W.; Zhao, X.; Xie, Q.; Khan, K.Y.; Zhao, X.; Nazar, M.; Li, G.; Du, D. Impacts of soil microplastics on crops: A review. Appl. Soil Ecol. 2023, 181, 104680. [Google Scholar] [CrossRef]
- Emisha, L.; Wilfred, N.; Kavitha, S.; Halder, G.; Haldar, D.; Patel, A.K.; Singhania, R.R.; Pandey, A. Biodegradation of microplastics: Advancement in the strategic approaches towards prevention of its accumulation and harmful effects. Chemosphere 2024, 346, 140661. [Google Scholar] [CrossRef]
- Zhang, T.; Luo, X.-S.; Xu, J.; Yao, X.; Fan, J.; Mao, Y.; Song, Y.; Yang, J.; Pan, J.; Khattak, W.A. Dry-wet cycle changes the influence of microplastics (MPs) on the antioxidant activity of lettuce and the rhizospheric bacterial community. J. Soils Sed. 2023, 23, 2189–2201. [Google Scholar] [CrossRef]
- Khan, I.; Tariq, M.; Alabbosh, K.F.; Rehman, A.; Jalal, A.; Khan, A.A.; Farooq, M.; Li, G.; Iqbal, B.; Ahmad, N.; et al. Soil microplastics: Impacts on greenhouse gasses emissions, carbon cycling, microbial diversity, and soil characteristics. Appl. Soil Ecol. 2024, 197, 105343. [Google Scholar] [CrossRef]
- Qian, J.; Tang, S.; Wang, P.; Lu, B.; Li, K.; Jin, W.; He, X. From source to sink: Review and prospects of microplastics in wetland ecosystems. Sci. Total Environ. 2021, 758, 143633. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Huang, Z.Y.; Li, Y.F.; Lu, X.L.; Li, G.R.; Qi, S.S.; Khan, I.U.; Li, G.L.; Dai, Z.C.; Du, D.L. The Degradability of Microplastics May Not Necessarily Equate to Environmental Friendliness: A Case Study of Cucumber Seedlings with Disturbed Photosynthesis. Agriculture 2023, 14, 53. [Google Scholar] [CrossRef]
- Lakhiar, I.A.; Yan, H.; Zhang, J.; Wang, G.; Deng, S.; Bao, R.; Zhang, C.; Syed, T.N.; Wang, B.; Zhou, R.; et al. Plastic Pollution in Agriculture as a Threat to Food Security, the Ecosystem, and the Environment: An Overview. Agronomy 2024, 14, 548. [Google Scholar] [CrossRef]
- Thuy-Hanh, P.; Huu-Tuan, D.; Lan-Anh Phan, T.; Singh, P.; Raizada, P.; Wu, J.C.-S.; Van-Huy, N. Global challenges in microplastics: From fundamental understanding to advanced degradations toward sustainable strategies. Chemosphere 2021, 267, 129275. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Redondo-Hasselerharm, P.E.; Nor, N.H.M.; de Ruijter, V.N.; Mintenig, S.M.; Kooi, M. Risk assessment of microplastic particles. Nat. Rev. Mater. 2022, 7, 138–152. [Google Scholar] [CrossRef]
- Ziani, K.; Ionita-Mindrican, C.-B.; Mititelu, M.; Neacsu, S.M.; Negrei, C.; Morosan, E.; Draganescu, D.; Preda, O.-T. Microplastics: A Real Global Threat for Environment and Food Safety: A State of the Art Review. Nutrients 2023, 15, 617. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, L. Microplastic migration and transformation pathways and exposure health risks. Environ. Pollut. 2025, 368, 125700. [Google Scholar] [CrossRef] [PubMed]
- Jenner, L.C.; Rotchell, J.M.; Bennett, R.T.; Cowen, M.; Tentzeris, V.; Sadofsky, L.R. Detection of microplastics in human lung tissue using μFTIR spectroscopy. Sci. Total Environ. 2022, 831, 154907. [Google Scholar] [CrossRef]
- Virk, M.S.; Virk, M.A.; He, Y.; Tufail, T.; Gul, M.; Qayum, A.; Rehman, A.; Rashid, A.; Ekumah, J.-N.; Han, X.; et al. The Anti-Inflammatory and Curative Exponent of Probiotics: A Comprehensive and Authentic Ingredient for the Sustained Functioning of Major Human Organs. Nutrients 2024, 16, 546. [Google Scholar] [CrossRef]
- Shen, M.; Song, B.; Zhu, Y.; Zeng, G.; Zhang, Y.; Yang, Y.; Wen, X.; Chen, M.; Yi, H. Removal of microplastics via drinking water treatment: Current knowledge and future directions. Chemosphere 2020, 251, 126612. [Google Scholar] [CrossRef]
- Pan, Y.; Gao, S.-H.; Ge, C.; Gao, Q.; Huang, S.; Kang, Y.; Luo, G.; Zhang, Z.; Fan, L.; Zhu, Y.; et al. Removing microplastics from aquatic environments: A critical review. Environ. Sci. Ecotechnol. 2023, 13, 100222. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.B.T.; Ha, D.T.; Tong, H.D.; Trinh, T.T. Molecular investigation of pyrolysis and thermal gasification pathways in polyethylene microplastics degradation. Chem. Eng. Process.-Process Intensif. 2025, 213, 110285. [Google Scholar] [CrossRef]
- Ramirez-Escarcega, K.J.; Amaya-Galvan, K.J.; Garcia-Prieto, J.C.; Silerio-Vazquez, F.d.J.; Proal-Najera, J.B. Advancing photocatalytic strategies for microplastic degradation in aquatic systems: Insights into key challenges and future pathways. J. Environ. Chem. Eng. 2025, 13, 115594. [Google Scholar] [CrossRef]
- Ullah, Z.; Peng, L.; Lodhi, A.F.; Kakar, M.U.; Mehboob, M.Z.; Iqbal, I. The threat of microplastics and microbial degradation potential; a current perspective. Sci. Total Environ. 2024, 955, 177045. [Google Scholar] [CrossRef]
- Zeng, L.; Li, L.; Xiao, J.; Zhou, P.; Han, X.; Shen, B.; Dai, L. Microplastics in the Environment: A Review Linking Pathways to Sustainable Separation Techniques. Separations 2025, 12, 82. [Google Scholar] [CrossRef]
- Yuan, J.; Ma, J.; Sun, Y.; Zhou, T.; Zhao, Y.; Yu, F. Microbial degradation and other environmental aspects of microplastics/plastics. Sci. Total Environ. 2020, 715, 136968. [Google Scholar] [CrossRef] [PubMed]
- Sacco, N.A.; Zoppas, F.M.; Devard, A.; Munoz, M.d.P.G.; Garcia, G.; Marchesini, F.A. Recent Advances in Microplastics Removal from Water with Special Attention Given to Photocatalytic Degradation: Review of Scientific Research. Microplastics 2023, 2, 278–303. [Google Scholar] [CrossRef]
- Hermabessiere, L.; Dehaut, A.; Paul-Pont, I.; Lacroix, C.; Jezequel, R.; Soudant, P.; Duflos, G. Occurrence and effects of plastic additives on marine environments and organisms: A review. Chemosphere 2017, 182, 781–793. [Google Scholar] [CrossRef]
- Gottschalk, F.; Nowack, B. The release of engineered nanomaterials to the environment. J. Environ. Monit. 2011, 13, 1145–1155. [Google Scholar] [CrossRef]
- Wu, N.; Yu, H.; Liu, Z.; Di, S.; Zhao, H.; Wang, Z.; Wang, Z.; Wang, X.; Qi, P. The underestimated environmental risk of tris (2-chloroethyl) phosphate photodegradation in aqueous environment induced by polystyrene microplastics. Water Res. 2025, 273, 123048. [Google Scholar] [CrossRef] [PubMed]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D. Wastewater treatment plants as a pathway for microplastics: Development of a new approach to sample wastewater-based microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Li, R.; Li, J.; Zou, J. Polystyrene nanoplastics promote colitis-associated cancer by disrupting lipid metabolism and inducing DNA damage. Environ. Int. 2025, 195, 109258. [Google Scholar] [CrossRef] [PubMed]
- Sahith, V.N.; Kumar, J.A.; Sruthi, V.S.; Sathish, S.; Venkatesan, D.; Prabu, D.; Samrot, A.V. Microbial and enzymatic biodegradation of microplastics and nanoplastics: Advances, challenges, and sustainable solutions for Environmental Remediation. Desalination Water Treat. 2025, 324, 101450. [Google Scholar] [CrossRef]
- Choi, J.; Kim, H.; Ahn, Y.-R.; Kim, M.; Yu, S.; Kim, N.; Lim, S.Y.; Park, J.-A.; Ha, S.-J.; Lim, K.S. Recent advances in microbial and enzymatic engineering for the biodegradation of micro-and nanoplastics. RSC Adv. 2024, 14, 9943–9966. [Google Scholar] [CrossRef]
- Ma, F.; Wang, W.; Dong, J.; Zhou, X.; Lin, Z.; Zheng, P.; Nian, X. Ecotoxicological impacts of polystyrene microplastics on rainbow trout: A multidisciplinary analysis of gut microbiota dysbiosis, oxidative stress, and cellular senescence for environmental risk assessment. Process Saf. Environ. Prot. 2025, 199, 107323. [Google Scholar] [CrossRef]
- Kumari, S.; Yadav, D.; Yadav, S.; Selvaraj, M.; Sharma, G.; Karnwal, A.; Yadav, S. From macro to micro: The key parameters influencing the degradation mechanism and the toxicity of microplastics in the environment. Polym. Degrad. Stab. 2025, 233, 111174. [Google Scholar] [CrossRef]
- Wu, Y.; Yi, R.; Wang, Y.; Zhang, C.; Zheng, J.; Ning, P.; Shan, D.; Wang, B. Light-driven degradation of microplastics: Mechanisms, technologies, and future directions. J. Hazard. Mater. Adv. 2025, 17, 100628. [Google Scholar] [CrossRef]
- Alva, P.P.; Thomas, T.A. Microplastics: A global threat to life and living. Environ. Monit. Assess. 2025, 197, 725. [Google Scholar] [CrossRef]
- Zhou, T.; Song, S.; Min, R.; Liu, X.; Zhang, G. Advances in chemical removal and degradation technologies for microplastics in the aquatic environment: A review. Mar. Pollut. Bull. 2024, 201, 116202. [Google Scholar] [CrossRef]
- Tingting, X.; Xiyuan, W.; Qingdong, S.; Huapeng, L.; Yutong, C.; Jia, L. Review of Soil Microplastic Degradation Pathways and Remediation Techniques. Int. J. Environ. Res. 2024, 18, 77. [Google Scholar] [CrossRef]
- Payel, S.; Pahlevani, F.; Ghose, A.; Sahajwalla, V. From bulk to bits: Understanding the degradation dynamics from plastics to microplastics, geographical influences and analytical approaches. Environ. Toxicol. Chem. 2025, 44, 895–915. [Google Scholar] [CrossRef]
- Niu, L.; Wang, Y.; Li, Y.; Lin, L.; Chen, Y.; Shen, J. Occurrence, Degradation Pathways, and Potential Synergistic Degradation Mechanism of Microplastics in Surface Water: A Review. Curr. Pollut. Rep. 2023, 9, 312–326. [Google Scholar] [CrossRef]
- Liu, L.; Xu, M.; Ye, Y.; Zhang, B. On the degradation of (micro)plastics: Degradation methods, influencing factors, environmental impacts. Sci. Total Environ. 2022, 806, 151312. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Zhang, Y.; Ju, C.; Zhao, T.; Meng, Q.; Cong, J. Microbial strategies for effective microplastics biodegradation: Insights and innovations in environmental remediation. Environ. Res. 2024, 263, 120046. [Google Scholar] [CrossRef]
- Rodriguez-Narvaez, O.M.; Goonetilleke, A.; Perez, L.; Bandala, E.R. Engineered technologies for the separation and degradation of microplastics in water: A review. Chem. Eng. J. 2021, 414, 128692. [Google Scholar] [CrossRef]
- He, J.; Han, L.; Wang, F.; Ma, C.; Cai, Y.; Ma, W.; Xu, E.G.; Xing, B.; Yang, Z. Photocatalytic strategy to mitigate microplastic pollution in aquatic environments: Promising catalysts, efficiencies, mechanisms, and ecological risks. Crit. Rev. Environ. Sci. Technol. 2023, 53, 504–526. [Google Scholar] [CrossRef]
- Bi, X.; Li, L.; Luo, L.; Liu, X.; Li, J.; You, T. A ratiometric fluorescence aptasensor based on photoinduced electron transfer from CdTe QDs to WS2 NTs for the sensitive detection of zearalenone in cereal crops. Food Chem. 2022, 385, 132657. [Google Scholar] [CrossRef]
- Sharma, A.S.; Ali, S.; Sabarinathan, D.; Murugavelu, M.; Li, H.; Chen, Q. Recent progress on graphene quantum dots-based fluorescence sensors for food safety and quality assessment applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5765–5801. [Google Scholar] [CrossRef]
- Lu, W.; Dai, X.; Yang, R.; Liu, Z.; Chen, H.; Zhang, Y.; Zhang, X. Fenton-like catalytic MOFs driving electrochemical aptasensing toward tracking lead pollution in pomegranate fruit. Food Control 2025, 169, 111006. [Google Scholar] [CrossRef]
- Yu, Q.; Shi, T.; Xiong, Z.; Yuan, L.; Hong, H.; Gao, R.; Bao, Y. Oxidation affects dye binding of myofibrillar proteins via alteration in net charges mediated by a reduction in isoelectric point. Food Res. Int. 2023, 163, 112204. [Google Scholar] [CrossRef]
- Umair, M.; Sultana, T.; Xun, S.; Jabbar, S.; Rajoka, M.S.R.; Albahi, A.; Abid, M.; Ranjha, M.M.A.N.; El-Seedi, H.R.R.; Xie, F.; et al. Advances in the application of functional nanomaterial and cold plasma for the fresh-keeping active packaging of meat. Food Sci. Nutr. 2023, 11, 5753–5772. [Google Scholar] [CrossRef]
- Ali, S.S.; Moawad, M.S.; Hussein, M.A.; Azab, M.; Abdelkarim, E.A.; Badr, A.; Sun, J.; Khalil, M. Efficacy of metal oxide nanoparticles as novel antimicrobial agents against multi-drug and multi-virulent Staphylococcus aureus isolates from retail raw chicken meat and giblets. Int. J. Food Microbiol. 2021, 344, 109116. [Google Scholar] [CrossRef]
- Nabi, I.; Bacha, A.-U.-R.; Li, K.; Cheng, H.; Wang, T.; Liu, Y.; Ajmal, S.; Yang, Y.; Feng, Y.; Zhang, L. Complete Photocatalytic Mineralization of Microplastic on TiO2 Nanoparticle Film. Iscience 2020, 23, 101326. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R. Metal Oxides-Based Nano/Microstructures for Photodegradation of Microplastics. Adv. Sustain. Syst. 2023, 7, 2300033. [Google Scholar] [CrossRef]
- Noor, S.F.M.; Paiman, S.H.; Nordin, A.H.; Ngadi, N.; Malek, N.A.N.N.; Hamed, N.K.A. Solid- and aqueous-phase approaches on zinc oxide-based photocatalytic system for degradation of plastics and microplastics: A review. Chem. Eng. Res. Des. 2024, 201, 194–208. [Google Scholar] [CrossRef]
- Xie, A.; Jin, M.; Zhu, J.; Zhou, Q.; Fu, L.; Wu, W. Photocatalytic Technologies for Transformation and Degradation of Microplastics in the Environment: Current Achievements and Future Prospects. Catalysts 2023, 13, 846. [Google Scholar] [CrossRef]
- Camila Ariza-Tarazona, M.; Francisco Villarreal-Chiu, J.; Barbieri, V.; Siligardi, C.; Iveth Cedillo-Gonzalez, E. New strategy for microplastic degradation: Green photocatalysis using a protein-based porous N-TiO2 semiconductor. Ceram. Int. 2019, 45, 9618–9624. [Google Scholar] [CrossRef]
- Llorente-Garcia, B.E.; Hernandez-Lopez, J.M.; Zaldivar-Cadena, A.A.; Siligardi, C.; Cedillo-Gonzalez, E.I. First Insights into Photocatalytic Degradation of HDPE and LDPE Microplastics by a Mesoporous N-TiO2 Coating: Effect of Size and Shape of Microplastics. Coatings 2020, 10, 658. [Google Scholar] [CrossRef]
- Devi, P.; Singh, J.P. High-Efficiency photocatalytic degradation of polystyrene microplastics using In2O3-rGO nanocomposite catalysts under visible Light. J. Polym. Res. 2025, 32, 154. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Luo, C.; Li, Y.; Zhang, L.; Li, C.; Zhang, X.; Liao, J.; Zhou, W. Cs3Bi2Br9/BiOCl S-scheme heterojunction photocatalysts with solid built-in electric field for efficient polystyrene microplastics degradation. Appl. Catal. B-Environ. Energy 2025, 371, 125288. [Google Scholar] [CrossRef]
- Gu, X.; Li, L.; Wu, Y.; Dong, W. Enhancement of microplastics degradation with MIL-101 modified BiOI photocatalyst under light and dark alternated system. J. Environ. Chem. Eng. 2024, 12, 112958. [Google Scholar] [CrossRef]
- Tofa, T.S.; Ye, F.; Kunjali, K.L.; Dutta, J. Enhanced Visible Light Photodegradation of Microplastic Fragments with Plasmonic Platinum/Zinc Oxide Nanorod Photocatalysts. Catalysts 2019, 9, 819. [Google Scholar] [CrossRef]
- Sima, J.; Song, J.; Du, X.; Lou, F.; Zhu, Y.; Lei, J.; Huang, Q. Complete degradation of polystyrene microplastics through non-thermal plasma-assisted catalytic oxidation. J. Hazard. Mater. 2024, 480, 136313. [Google Scholar] [CrossRef]
- He, J.; Han, L.; Ma, W.; Chen, L.; Ma, C.; Xu, C.; Yang, Z. Efficient photodegradation of polystyrene microplastics integrated with hydrogen evolution: Uncovering degradation pathways. Iscience 2023, 26, 106833. [Google Scholar] [CrossRef]
- He, Y.; Rehman, A.U.; Xu, M.; Not, C.A.; Ng, A.M.C.; Djurisic, A.B. Photocatalytic degradation of different types of microplastics by TiOx/ZnO tetrapod photocatalysts. Heliyon 2023, 9, e22562. [Google Scholar] [CrossRef] [PubMed]
- Gayathri, P.V.; Joseph, S.; Mohan, M.; Pillai, D. Advanced oxidation processes for the degradation of microplastics from the environment: A review. Water Environ. J. 2023, 37, 686–701. [Google Scholar] [CrossRef]
- Hao, D.; Yuqun, X.; Jun, W. Microplastic degradation methods and corresponding degradation mechanism: Research status and future perspectives. J. Hazard. Mater. 2021, 418, 126377. [Google Scholar] [CrossRef]
- Song, Z.; Gao, H.; Li, J.; Zhao, Z.; Zhang, W.; Wang, D. Slag-based Z-scheme heterojunction visible light-driven photocatalyst for efficient degradation of tetracycline antibiotics in water. J. Water Process Eng. 2025, 71, 107195. [Google Scholar] [CrossRef]
- Atstaja, D. Renewable Energy for Sustainable Development: Opportunities and Current Landscape. Energies 2025, 18, 196. [Google Scholar] [CrossRef]
- Jeyaraj, J.; Baskaralingam, V.; Stalin, T.; Muthuvel, I. Mechanistic vision on polypropylene microplastics degradation by solar radiation using TiO2 nanoparticle as photocatalyst. Environ. Res. 2023, 233, 116366. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, P.; Ariza-Tarazona, M.C.; Cedillo-Gonzalez, E.I.; Siligardi, C.; Simmchen, J. Combining photocatalytic collection and degradation of microplastics using self-asymmetric Pac-Man TiO2. Nanoscale 2023, 15, 14774–14781. [Google Scholar] [CrossRef]
- Yu, H.; Hou, Z.; Wang, B.; Zhu, H.; Zhang, Y. Scalable self-growth of magnetic Janus microparticles for microplastics degradation. Surf. Interfaces 2025, 63, 106331. [Google Scholar] [CrossRef]
- Rehman, A.U.; Han, K.D.; Ali, M.U.; He, Y.; Sergeev, A.A.; Yuan, Z.; Dong, C.; Gao, X.; Not, C.A.; Ng, A.M.C.; et al. Niobium Oxide for Microplastics Degradation-the Effect of Crystal Structure and Morphology. Small Struct. 2025, 6, 2500124. [Google Scholar] [CrossRef]
- Rodriguez-Olivares, A.E.; Guzman-Mar, J.L.; Quero-Jimenez, P.C.; Montemayor, S.M.; Maya-Trevino, L.; Hinojosa-Reyes, L. Analytical approaches to track nylon 6 microplastic fiber degradation using HKUST-1(Cu/Fe)-derived CuO/TiO2 photocatalyst. J. Water Process Eng. 2025, 71, 107192. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, Y.; Liu, W.; Jin, Q.; Lin, H. Piezo-photocatalytic enhanced microplastic degradation on hetero-interpenetrated Fe1-xS/FeMoO4/ MoS2 by producing H2O2 and self-Fenton action. Chem. Eng. J. 2025, 508, 160935. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, Q.; Ning, S.; Gan, Y.; Fujita, T.; Wei, Y.; Wang, X.; Zeng, D. CuInSe2 nanoplatelets decorated CdS nanosheets as 2D-2D S-scheme photocatalyst for photocatalytic H2 generation coupled with benzyl alcohol oxidation and microplastic degradation. J. Solid State Chem. 2024, 333, 124645. [Google Scholar] [CrossRef]
- Adamu, H.; Bello, U.; Tafida, U.I.; Garba, Z.N.; Galadima, A.; Lawan, M.M.; Abba, S.I.; Qamar, M. Harnessing bio and (Photo)catalysts for microplastics degradation and remediation in soil environment. J. Environ. Manage. 2024, 370, 122543. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, L.; Nekliudov, A. Construction of loading g-C3N4/TiO2 on waste cotton-based activated carbon S-scheme heterojunction for enhanced photocatalytic degradation of microplastics: Performance, DFT calculation and mechanism study. Opt. Mater. 2024, 154, 115786. [Google Scholar] [CrossRef]
- Greco, R.; Baxauli-Marin, L.; Temerov, F.; Daboczi, M.; Eslava, S.; Niu, Y.; Zakharov, A.; Zhang, M.; Li, T.; Cao, W. Activation of 2D cobalt hydroxide with 0D cobalt oxide decoration for microplastics degradation and hydrogen evolution. Chem. Eng. J. 2023, 471, 144569. [Google Scholar] [CrossRef]
- Kasuske, Z.A.; Arole, K.; Green, M.J.; Anderson, T.A.; Canas-Carrell, J.E. Photo-induced degradation of single-use polyethylene terephthalate microplastics under laboratory and outdoor environmental conditions. Environ. Toxicol. Chem. 2025, 44, 1525–1537. [Google Scholar] [CrossRef]
- Farahbakhsh, J.; Golgoli, M.; Najafi, M.; Haeri, S.Z.; Khiadani, M.; Razmjou, A.; Zargar, M. An innovative NH2-UiO-66/NH2-MIL-125 MOF-on-MOF structure to improve the performance and antifouling properties of ultrafiltration membranes. Sep. Purif. Technol. 2025, 353 Pt A, 128273. [Google Scholar] [CrossRef]
- Musthafa, J.M.; Mandal, B.K. CuO/Bi2O3/g-C3N4 nanoparticles for sunlight-mediated degradation of polyethylene terephthalate microplastic films. Opt. Mater. 2024, 154, 115701. [Google Scholar] [CrossRef]
- Nguyen, M.B.; Doan, H.V.; Tan, D.L.H.; Lam, T.D. Advanced g-C3N4 and bimetallic FeNi-BTC integration with carbon quantum dots for removal of microplastics and antibiotics in aqueous environments. J. Environ. Chem. Eng. 2024, 12, 112965. [Google Scholar] [CrossRef]
- Qin, J.; Dou, Y.; Wu, F.; Yao, Y.; Andersen, H.R.; Helix-Nielsen, C.; Lim, S.Y.; Zhang, W. In-situ formation of Ag2O in metal-organic framework for light-driven upcycling of microplastics coupled with hydrogen production. Appl. Catal. B-Environ. Energy 2022, 319, 121940. [Google Scholar] [CrossRef]
- Zhai, C.; Yu, Y.; Han, J.; Hu, J.; He, D.; Zhang, H.; Shi, J.; Mohamed, S.R.; Dawood, D.H.; Wang, G.; et al. Isolation, Characterization, and Application of Clostridium sporogenes F39 to Degrade Zearalenone under Anaerobic Conditions. Foods 2022, 11, 1194. [Google Scholar] [CrossRef]
- Xing, R.; Zhai, K.; Du, X.; Chen, X.; Chen, Z.; Zhou, S. Hybrid mechanism of microplastics degradation via biological and chemical process during composting. Bioresour. Technol. 2024, 408, 131167. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Q.; Ngea, G.L.N.; Godana, E.A.; Yang, Q.; Zhang, H. Biodegradation of patulin in fresh pear juice by an aldo-keto reductase from Meyerozyma guilliermondii. Food Chem. 2024, 436, 137696. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, Y.; Sun, Q.; Guo, Z.; Zhang, D.; Zou, X. Enzyme-assisted patulin detoxification: Recent applications and perspectives. Trends Food Sci. Technol. 2024, 146, 104383. [Google Scholar] [CrossRef]
- Schenone, L.; Capitani, L.; Lora, U.; Setala, O.; Kaartokallio, H.; Seppala, J.; Lehtiniemi, M. Microbial plankton uptake enhances the degradation of a biodegradable microplastic. Environ. Pollut. 2025, 374, 126252. [Google Scholar] [CrossRef]
- Cao, Y.; Bian, J.; Han, Y.; Liu, J.; Ma, Y.; Feng, W.; Deng, Y.; Yu, Y. Progress and Prospects of Microplastic Biodegradation Processes and Mechanisms: A Bibliometric Analysis. Toxics 2024, 12, 463. [Google Scholar] [CrossRef]
- Kristina, V.; Ren, W.; Lara, P.; Daniel, B.; Hassan, A.-F.; Christian, O.; Irina, E.-L.; Tom, V.; Agnes, S.; Hauke, H.; et al. Enzymatic degradation of polyethylene terephthalate nanoplastics analyzed in real time by isothermal titration calorimetry. Sci. Total Environ. 2021, 773, 145111. [Google Scholar] [CrossRef]
- Menzel, T.; Weigert, S.; Gagsteiger, A.; Eich, Y.; Sittl, S.; Papastavrou, G.; Ruckdaeschel, H.; Altstaedt, V.; Hoecker, B. Impact of Enzymatic Degradation on the Material Properties of Poly(Ethylene Terephthalate). Polymers 2021, 13, 3885. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades and assimilates poly(ethylene terephthalate). Science 2016, 351, 1196–1199. [Google Scholar] [CrossRef]
- Sahu, S.; Kaur, A.; Khatri, M.; Singh, G.; Arya, S.K. A review on cutinases enzyme in degradation of microplastics. J. Environ. Manage. 2023, 347, 119193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Hou, Y.; Hou, S.; Wang, Y.; Wang, Q. Enhancement of microplastics degradation efficiency: Microbial laccase-driven radical chemical coupling catalysis. Chem. Eng. J. 2025, 507, 160579. [Google Scholar] [CrossRef]
- Ramamurthy, K.; Thomas, N.P.; Gopi, S.; Sudhakaran, G.; Haridevamuthu, B.; Namasivayam, K.R.; Arockiaraj, J. Is Laccase derived from Pleurotus ostreatus effective in microplastic degradation? A critical review of current progress, challenges, and future prospects. Int. J. Biol. Macromol. 2024, 276 Pt 2, 133971. [Google Scholar] [CrossRef]
- Sundaramoorthy, M.; Gold, M.H.; Poulos, T.L. Ultrahigh (0.93Å) resolution structure of manganese peroxidase from Phanerochaete chrysosporium: Implications for the catalytic mechanism. J. Inorg. Biochem. 2010, 104, 683–690. [Google Scholar] [CrossRef]
- Jain, R.; Gaur, A.; Suravajhala, R.; Chauhan, U.; Pant, M.; Tripathi, V.; Pant, G. Microplastic pollution: Understanding microbial degradation and strategies for pollutant reduction. Sci. Total Environ. 2023, 905, 167098. [Google Scholar] [CrossRef]
- Othman, A.R.; Abu Hasan, H.; Muhamad, M.H.; Ismail, N.I.; Abdullah, S.R.S. Microbial degradation of microplastics by enzymatic processes: A review. Environ. Chem. Lett. 2021, 19, 3057–3073. [Google Scholar] [CrossRef]
- Ding, K.; Lin, H.; Liu, L.; Jia, X.; Zhang, H.; Tan, Y.; Liang, X.; He, Y.; Liu, D.; Han, L.; et al. Effect of ball milling on enzymatic sugar production from fractionated corn stover. Ind. Crops Prod. 2023, 196, 116502. [Google Scholar] [CrossRef]
- Guo, R.-T.; Li, X.; Yang, Y.; Huang, J.-W.; Shen, P.; Liew, R.K.; Chen, C.-C. Natural and engineered enzymes for polyester degradation: A review. Environ. Chem. Lett. 2024, 22, 1275–1296. [Google Scholar] [CrossRef]
- Ma, Y.; Tayefi, S.H.; Mogharabi-Manzari, M.; Luo, X. Advances in immobilized enzyme systems for enhanced microplastic biodegradation: A review. Int. J. Biol. Macromol. 2025, 328 Pt 2, 147656. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; Fehn, S.; Steegmuller, T.; Rauwolf, S.; Loewe, H.; Pflueger-Grau, K.; Berensmeier, S. Immobilization of PETase enzymes on magnetic iron oxide nanoparticles for the decomposition of microplastic PET. Nanoscale Adv. 2021, 3, 4395–4399. [Google Scholar] [CrossRef]
- Zurier, H.S.; Goddard, J.M. Directed Immobilization of PETase on Mesoporous Silica Enables Sustained Depolymerase Activity in Synthetic Wastewater Conditions. ACS Appl. Bio Mater. 2022, 5, 4981–4992. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.; Dutta, S.P.; Mukherjee, G.; Basu, A.; Majumder, D.; Sil, A.K. Cloning, expression and characterization of PURase gene from Pseudomonas sp. AKS31. Arch. Microbiol. 2022, 204, 498. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, T.; Long, L.; Zhang, R.; Ding, S. Efficient enzymatic degradation of poly (ɛ-caprolactone) by an engineered bifunctional lipase-cutinase. Polym. Degrad. Stab. 2019, 160, 120–125. [Google Scholar] [CrossRef]
- Rivera-Hoyos, C.M.; Morales-Alvarez, E.D.; Poutou-Pinales, R.A.; Pedroza-Rodriguez, A.M.; Rodriguez-Vazquez, R.; Del-gado-Boada, J.M. Fungal laccases. Fungal Biol. Rev. 2013, 27, 67–82. [Google Scholar] [CrossRef]
- Wafaa, D.M.; Sadik, M.W.; Eissa, H.F.; Tonbol, K. Biodegradation of low-density polyethylene LDPE by marine bacterial strains Gordonia alkanivorans PBM1 and PSW1 isolated from Mediterranean Sea, Alexandria, Egypt. Sci. Rep. 2025, 15, 16769. [Google Scholar] [CrossRef]
- Guo, H.; Yang, K.; Cui, L. Microbial Degradation of Environmental Microplastics. Prog. Chem. 2025, 37, 112–123. [Google Scholar] [CrossRef]
- Yang, G.; Quan, X.; Shou, D.; Guo, X.; Ouyang, D.; Zhuang, L. New insights into microbial degradation of polyethylene microplastic and potential polyethylene-degrading bacteria in sediments of the Pearl River Estuary, South China. J. Hazard. Mater. 2025, 486, 137061. [Google Scholar] [CrossRef]
- Ujuagu, G.I.; Ejeromedoghene, O.; Enwemiwe, V.; Mgbechidinma, C.L.; Omoniyi, A.O.; Oladipo, A.; Gu, J. Exploring the toxicology, socio-ecological impacts and biodegradation of microplastics in Africa: Potentials for resource conservation. Toxicol. Rep. 2025, 14, 101873. [Google Scholar] [CrossRef]
- Bai, F.; Fan, J.; Zhang, X.; Wang, X.; Liu, S. Biodegradation of polyethylene with polyethylene-group-degrading enzyme delivered by the engineered Bacillus velezensis. J. Hazard. Mater. 2025, 488, 137330. [Google Scholar] [CrossRef]
- Gowthami, A.; Syed Marjuk, M.; Santhanam, P.; Thirumurugan, R.; Muralisankar, T.; Perumal, P. Marine microalgae—Mediated biodegradation of polystyrene microplastics: Insights from enzymatic and molecular docking studies. Chemosphere 2025, 370, 144024. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Yi, J.; Wang, J.; Qian, Q.; Chen, Q.; Cao, C.; Zhou, W. Enhancing Microplastic Degradation through Synergistic Photocatalytic and Pretreatment Approaches. Langmuir 2024, 40, 22582–22590. [Google Scholar] [CrossRef]
- Amparan, M.A.A.; Palacios, A.; Flores, G.M.; Olivera, P.M.C. Review and future outlook for the removal of microplastics by physical, biological and chemical methods in water bodies and wastewaters. Environ. Monit. Assess. 2025, 197, 429. [Google Scholar] [CrossRef]
- Sharara, A.; Samy, M.; Mossad, M.; Alalm, M.G. Enhanced depolymerization of microplastic debris in water by a hybrid ZnO-based photocatalysis-persulfate activation system. J. Water Process Eng. 2025, 72, 107633. [Google Scholar] [CrossRef]
- Biao, W.; Hashim, N.A.; Rabuni, M.F.B.; Lide, O.; Ullah, A. An innovative strategy for polyester microplastic fiber elimination from laundry wastewater via coupled separation and degradation using TiO2-based photocatalytic membrane reactor. Sep. Purif. Technol. 2025, 356, 129929. [Google Scholar] [CrossRef]
- Oliva, J.; Valle-Garcia, L.S.; Garces, L.; Oliva, A.I.; Valadez-Renteria, E.; Hernandez-Bustos, D.A.; Campos-Amador, J.J.; Gomez-Solis, C. Using NIR irradiation and magnetic bismuth ferrite microparticles to accelerate the removal of polystyrene microparticles from the drinking water. J. Environ. Manage. 2023, 345, 118784. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, A.L.G.; Dari, D.N.; Silva, M.F.d.M.; Vieira, R.d.S.; Aires, F.I.d.S.; de Sousa Junior, P.G.; dos Santos, K.M.; dos Santos, J.C.S. Advances in enzymatic degradation of microplastics: Mechanisms, optimization strategies, and future directions. Mol. Catal. 2025, 585, 115392. [Google Scholar] [CrossRef]
- Kushbu, R.; Madhu, M. Isolation and screening of microplastics from Nodularia spumigena and Phaeodactylum tricornutum and determining the efficacy of biodegradation of microplastics obtained using the synergistic consortium. Res. J. Biotechnol. 2024, 19, 63–75. [Google Scholar] [CrossRef]
- Alidoosti, F.; Giyahchi, M.; Moghimi, H. Synergistic bioremediation: Fungal-bacterial partnership degrades LDPE microplastics twice as fast. Curr. Res. Microb. Sci. 2025, 9, 100450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, J.; Han, J.; Wang, L.; Zhang, W.; Dong, H.; Li, C.; Wang, Y. Photocatalyst/enzyme heterojunction fabricated for high-efficiency photoenzyme synergic catalytic degrading Bisphenol A in water. Chem. Eng. J. 2020, 385, 123764. [Google Scholar] [CrossRef]
- Shi, C.; Wang, K.; Cui, H.; Ma, E.; Wang, H. Bio-templated micromotors for simultaneous adsorption and degradation of microplastics. Surf. Interfaces 2024, 54, 105144. [Google Scholar] [CrossRef]
- Hermanova, S.; Pumera, M. Micromachines for Microplastics Treatment. ACS Nanosci. Au 2022, 2, 225–232. [Google Scholar] [CrossRef]
- Liu, J.; Wan, Y.; Wang, H.; Zhang, Y.; Xu, M.; Song, X.; Zhou, W.; Zhang, J.; Ma, W.; Huo, P. Enhanced activation of peroxymonosulfate by ZIF-67/g-C3N4 S-scheme photocatalyst under visible light assistance for degradation of polyethylene terephthalate. Environ. Pollut. 2024, 360, 124682. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, W.; Xing, R.; Xie, S.; Yang, X.; Cui, P.; Lu, J.; Liao, H.; Yu, Z.; Wang, S.; et al. Enhanced in situ biodegradation of microplastics in sewage sludge using hyperthermophilic composting technology. J. Hazard. Mater. 2020, 384, 121271. [Google Scholar] [CrossRef]
- Dai, L.; Lei, Z.; Cao, Y.; Zhang, M.; Song, X.; Wang, G.; Ma, G.; Zhao, T.; Ren, J. Attapulgite-supported sulfidated nano Zero-Valent Iron activated persulfate advanced oxidation technology for degradation of polyethylene microplastics: Optimal design, change of particle size and degradation mechanisms. J. Environ. Chem. Eng. 2024, 12, 112261. [Google Scholar] [CrossRef]
- Shabib, A.; Maraqa, M.A.; Mohammad, A.F.; Awwad, F. Design, fabrication, and application of electrochemical sensors for microplastic detection: A state-of-the-art review and future perspectives. Environ. Sci. Eur. 2025, 37, 94. [Google Scholar] [CrossRef]
- Ren, J.; Meng, Y.; Wang, Z.; Xie, G. Degradation of Microplastics by Microbial in Combination with a Micromotor. ACS Sustain. Chem. Eng. 2025, 13, 4018–4027. [Google Scholar] [CrossRef]
- Godasiaei, S.H. Predictive modeling of microplastic adsorption in aquatic environments using advanced machine learning models. Sci. Total Environ. 2025, 958, 178015. [Google Scholar] [CrossRef] [PubMed]
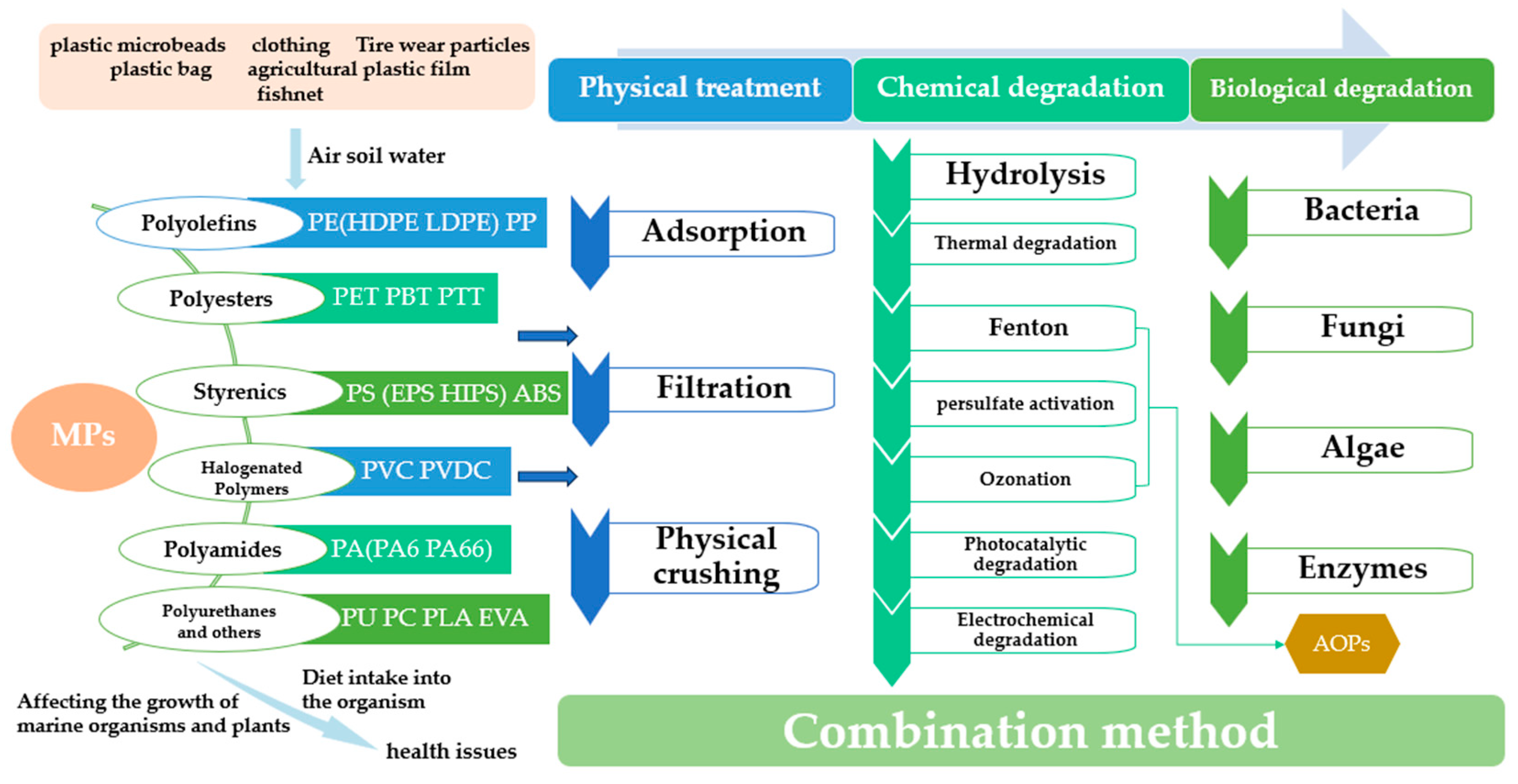
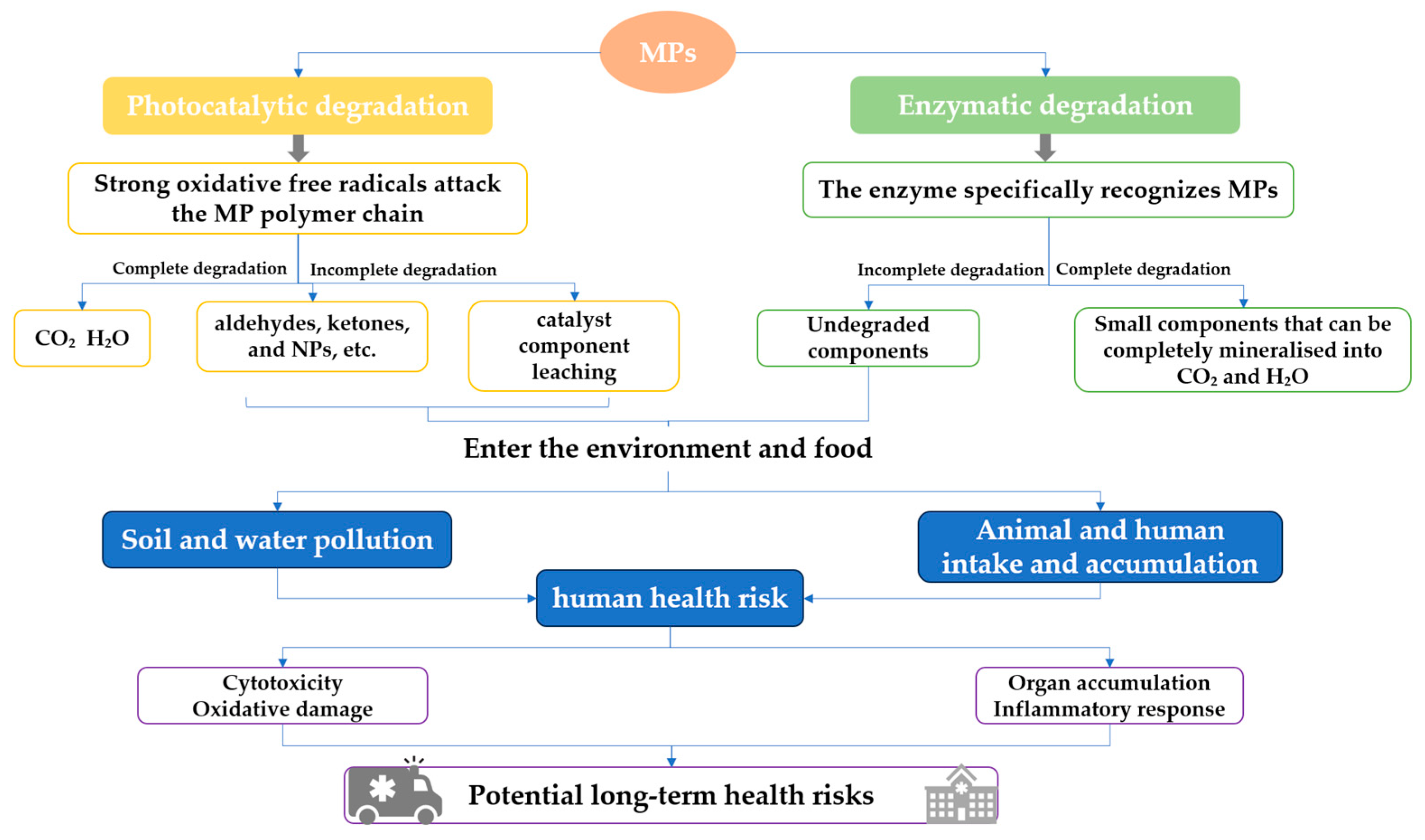
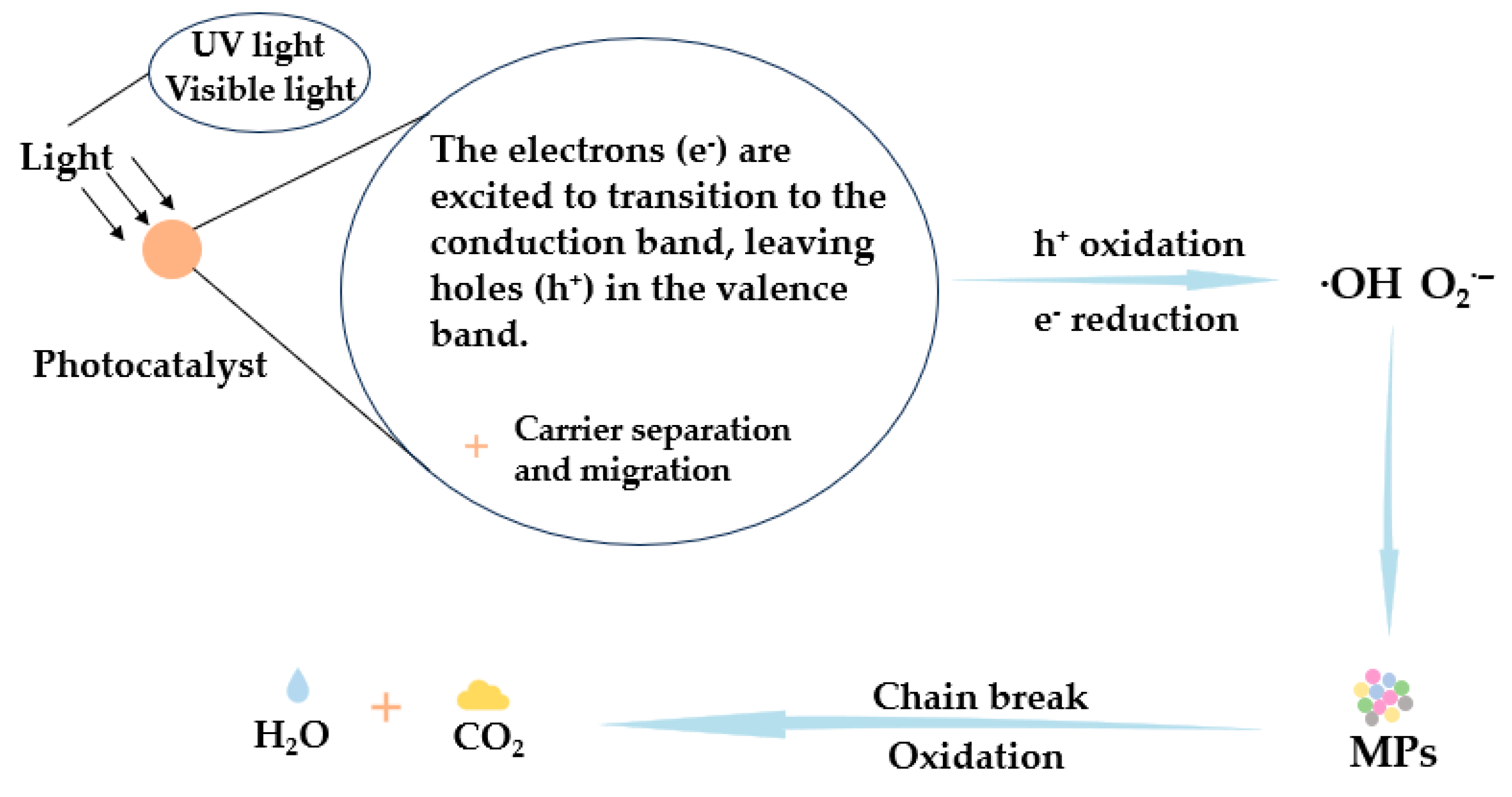

| Method | Photocatalytic Degradation | Enzymatic Degradation |
|---|---|---|
| Reaction mechanism | Radical oxidation reaction. | Enzymatic reaction. |
| Reaction conditions | Requires light (ultraviolet or visible light) and catalysts; affected by temperature, pH, light intensity, etc. | Suitable temperature and pH are required. |
| Degradation efficiency | It is faster in the early stage; however, it may slow down with the accumulation of products. It is effective for a variety of MPs; however, the mineralization rate (completely converted to CO2 and H2O) may not be high. | The efficiency is slow, and the specificity is strong. |
| Advantages | Strong oxidation capability and deep degradation potential, relatively straightforward operation, and readily available catalysts [39]. | Minimal by-products, mild processing conditions, and low energy consumption. Environmentally friendly, with no secondary pollution. |
| Disadvantages | It may not be completely degraded, resulting in secondary pollution, high energy consumption of the light source, and difficult large-scale application. | Enzymes exhibit poor stability, are readily inactivated, degrade slowly, demonstrate limited efficacy on composite MPs, and carry relatively high costs, presenting challenges for large-scale application. |
| Combination | MPs | Degradation Efficiency (%) | Time | References | |
|---|---|---|---|---|---|
| Photocatalytic degradation | Combined with electrostatic adsorption and magnetic separation technology | PS | 100% | 90 min | [130] |
| Combined with the Fenton reaction | PE | CI increased 0.127 | 6 h | [73] | |
| Fixing Ag-TiO2 onto an Al2O3 Ceramic Membrane | PMPF | 23.3% | 28 h | [129] | |
| ZIF-67/g-C3N4 synergistically with PMS under visible light | PET | 60.63% | 6 h | [137] | |
| Enzymatic degradation | Combined with high-temperature composting | - | 43.7% | 45 d | [138] |
| Combined with UV pretreatment | PE | - | 6 d | [38] | |
| Photocatalysis-enzyme | Horseradish peroxidase (HRP) immobilized on mesoporous carbon nitride (MCN) | BPA | 85.7% | - | [134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, G.; Ren, W.; Huo, S.; Zou, B.; Qian, J.; Wang, F.; Ma, A.; Zhuang, G.; Xu, L. Photocatalytic and Enzymatic Degradation of Microplastics: Current Status, Comparison, and Combination. Catalysts 2025, 15, 1015. https://doi.org/10.3390/catal15111015
Guan G, Ren W, Huo S, Zou B, Qian J, Wang F, Ma A, Zhuang G, Xu L. Photocatalytic and Enzymatic Degradation of Microplastics: Current Status, Comparison, and Combination. Catalysts. 2025; 15(11):1015. https://doi.org/10.3390/catal15111015
Chicago/Turabian StyleGuan, Guoqiang, Wenjing Ren, Shuhao Huo, Bin Zou, Jingya Qian, Feng Wang, Anzhou Ma, Guoqiang Zhuang, and Ling Xu. 2025. "Photocatalytic and Enzymatic Degradation of Microplastics: Current Status, Comparison, and Combination" Catalysts 15, no. 11: 1015. https://doi.org/10.3390/catal15111015
APA StyleGuan, G., Ren, W., Huo, S., Zou, B., Qian, J., Wang, F., Ma, A., Zhuang, G., & Xu, L. (2025). Photocatalytic and Enzymatic Degradation of Microplastics: Current Status, Comparison, and Combination. Catalysts, 15(11), 1015. https://doi.org/10.3390/catal15111015








