Sustainable Photocatalytic Treatment of Real Pharmaceutical Wastewater Using a Novel ZnO/MIP-202(Zr) Bio-MOF Hybrid Synthesized via a Green Approach
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Characteristics of the Synthesized Materials
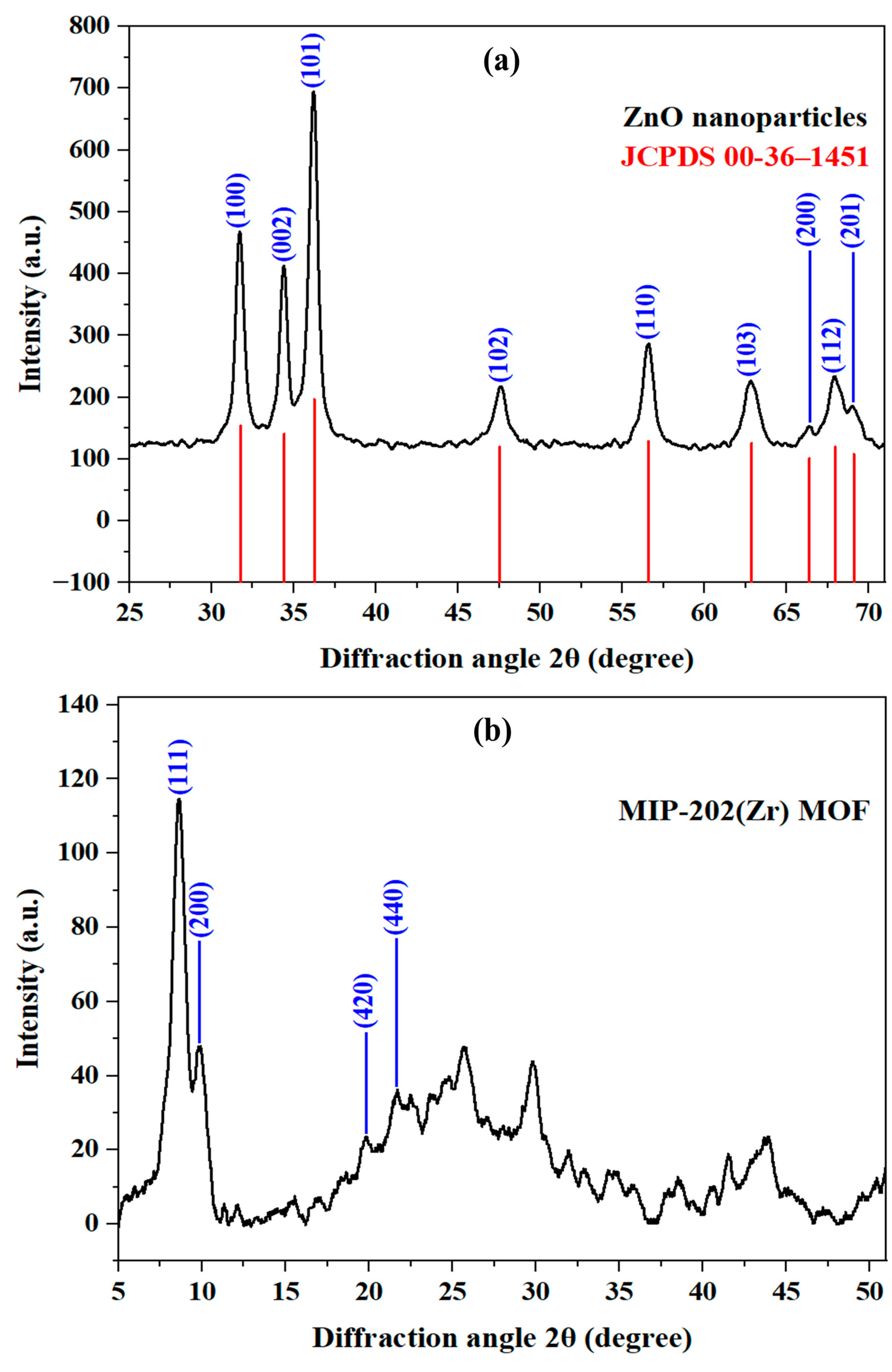
2.1.1. X-Ray Diffraction (XRD)
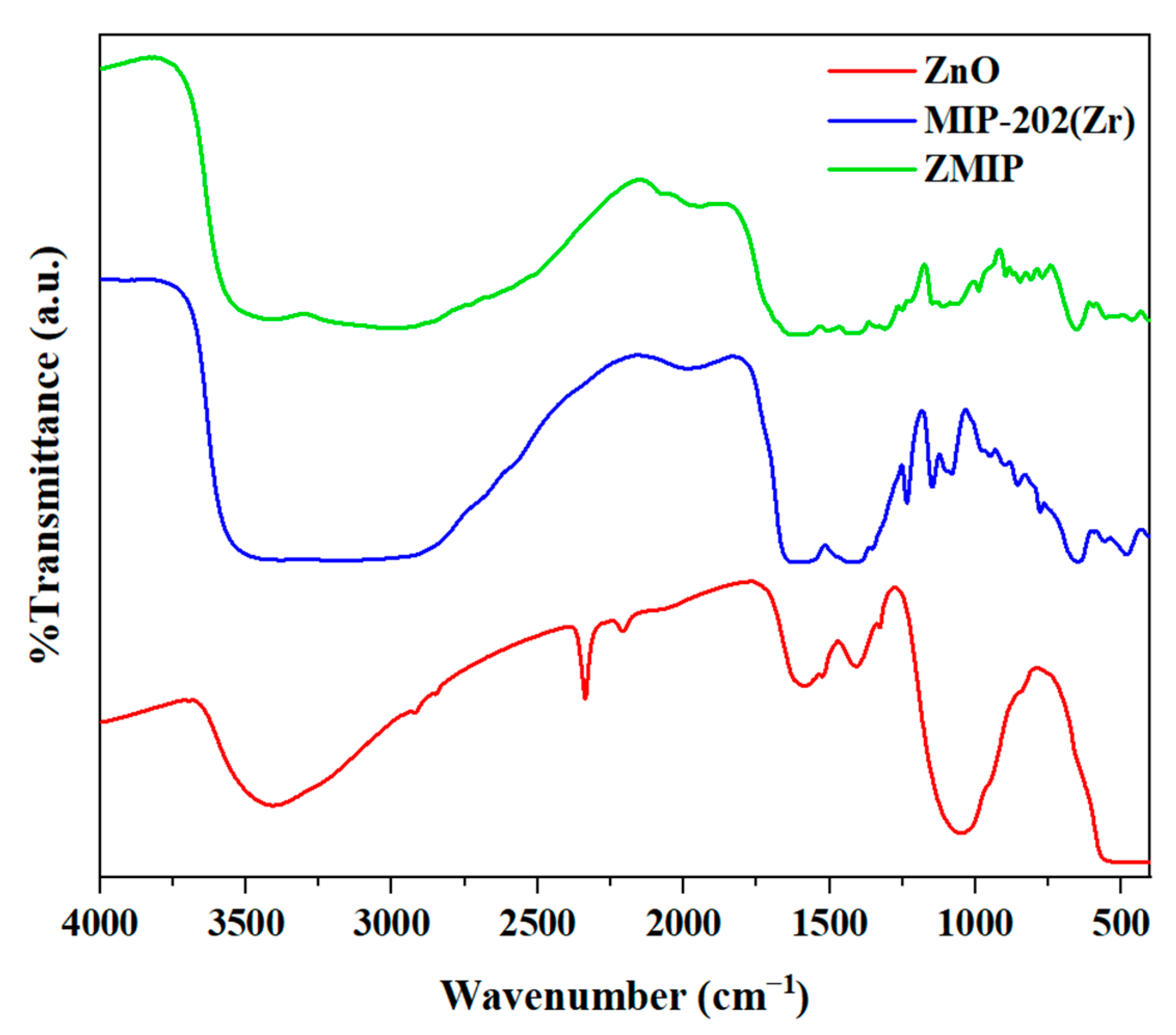
2.1.2. Fourier-Transform Infrared (FTIR) Spectroscopy
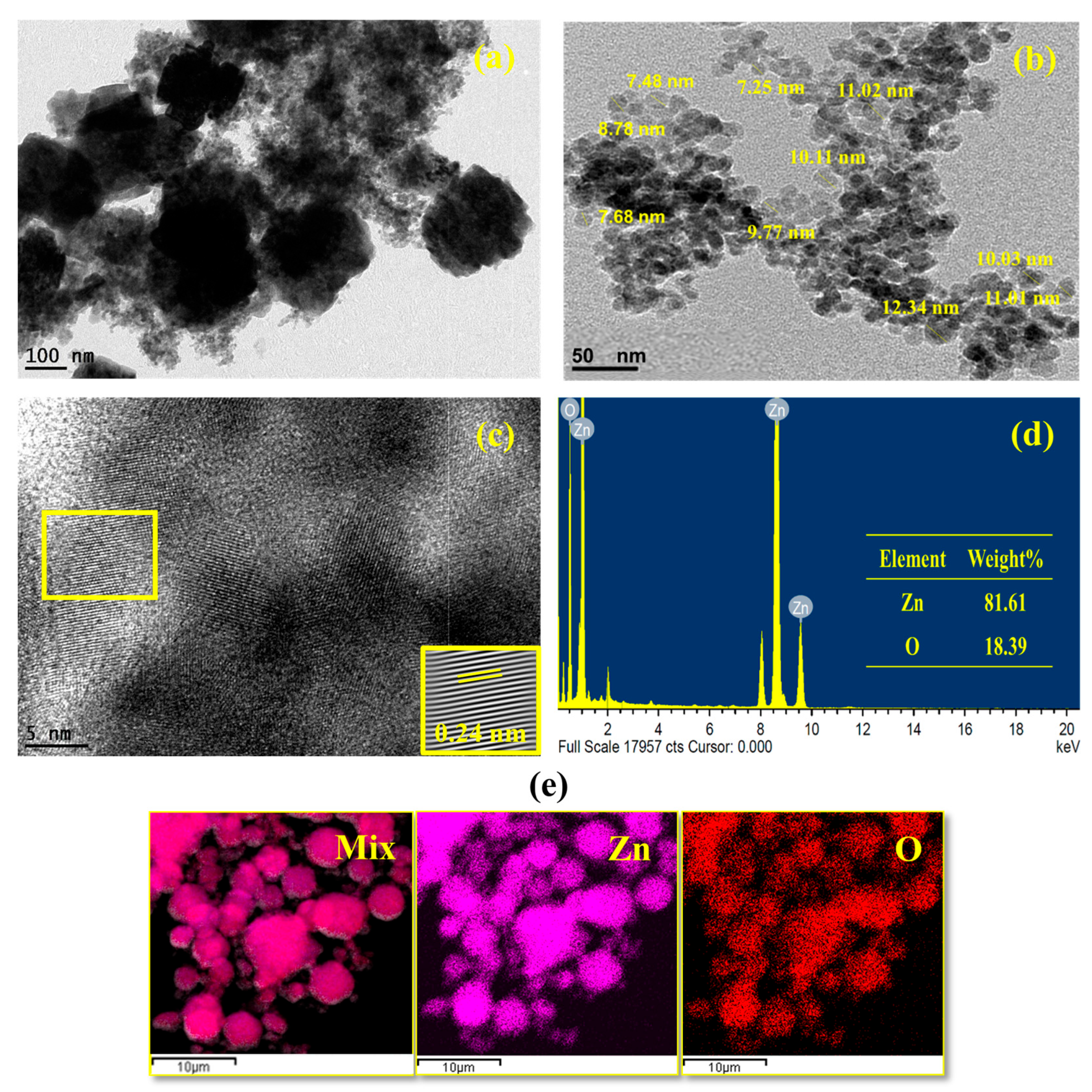
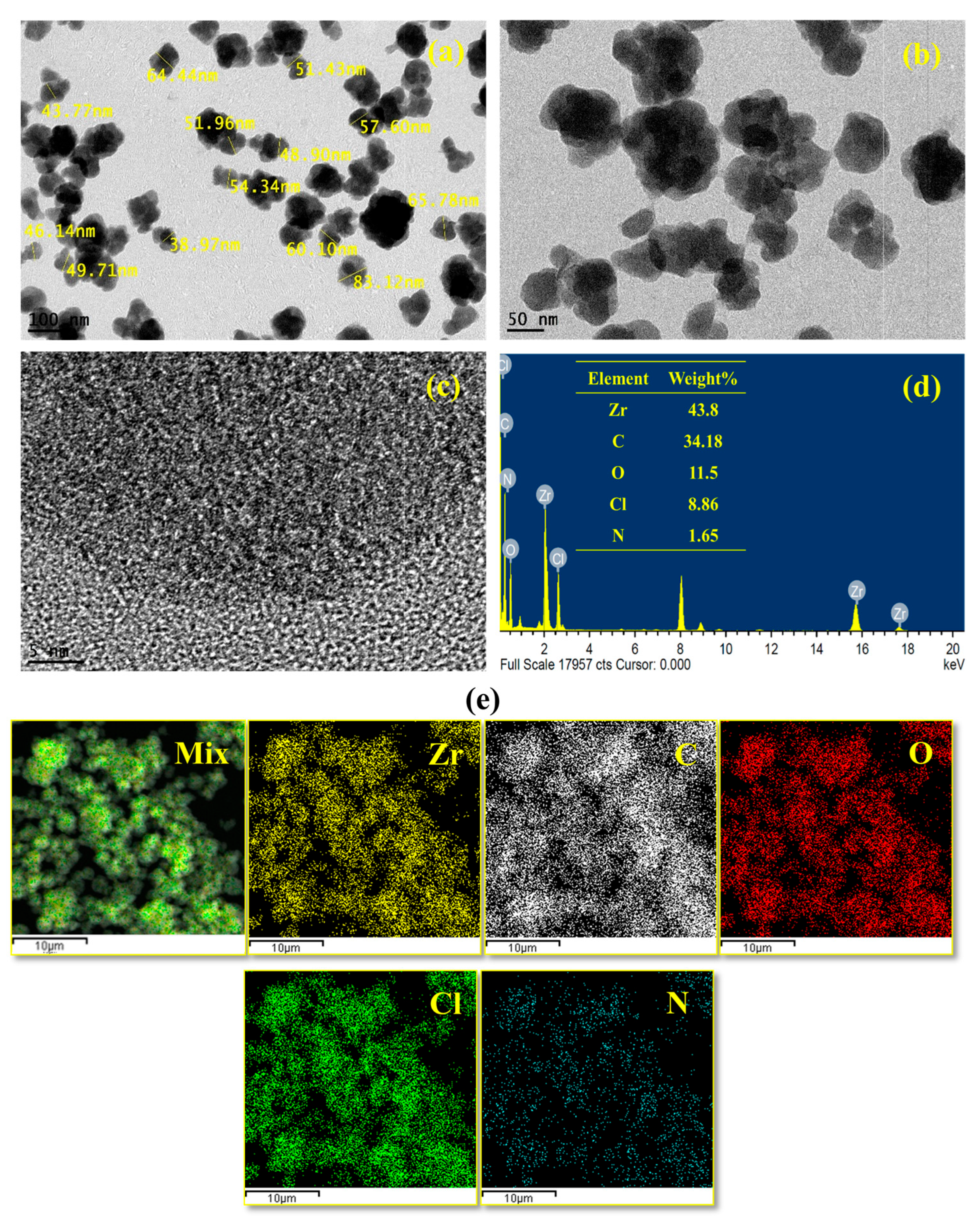
2.1.3. Structural Morphology and Elemental Profiling
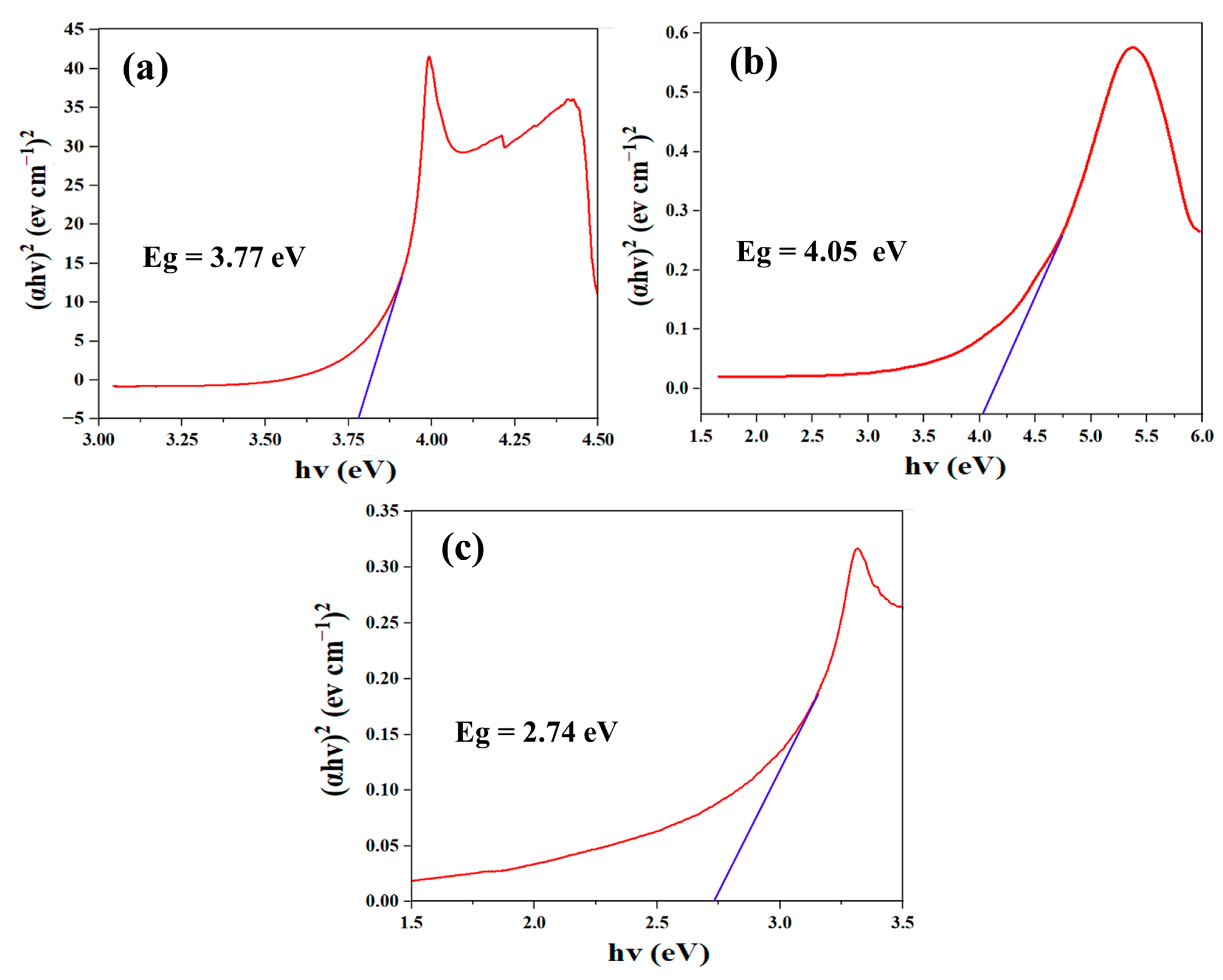
2.2. Optical Performance of the Synthesized Materials
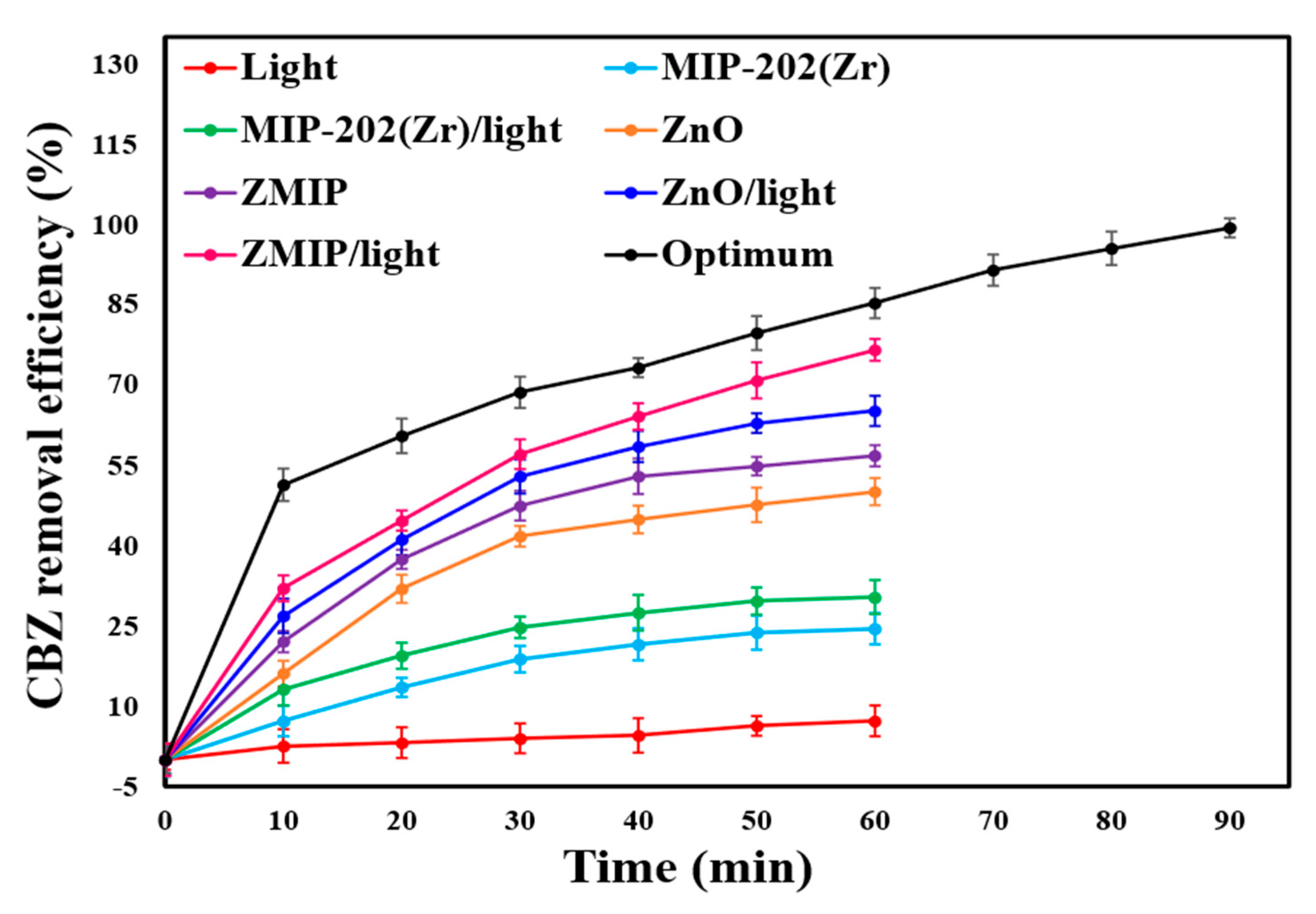
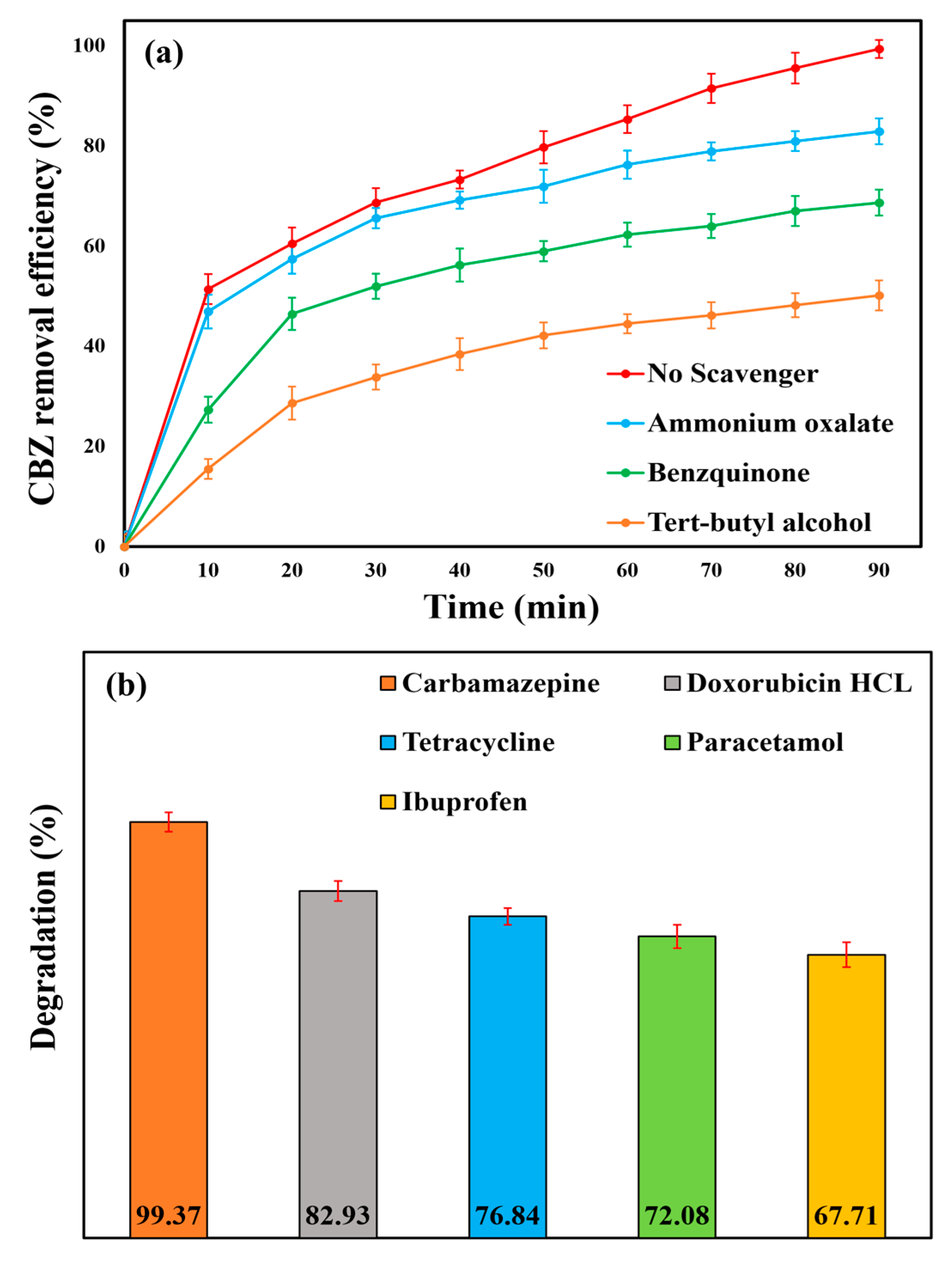
2.3. Contribution of Multiple Processes
2.4. Model Generation, Optimization, and Validation
2.5. Analysis of Variance
2.6. Influence of Operational Factors
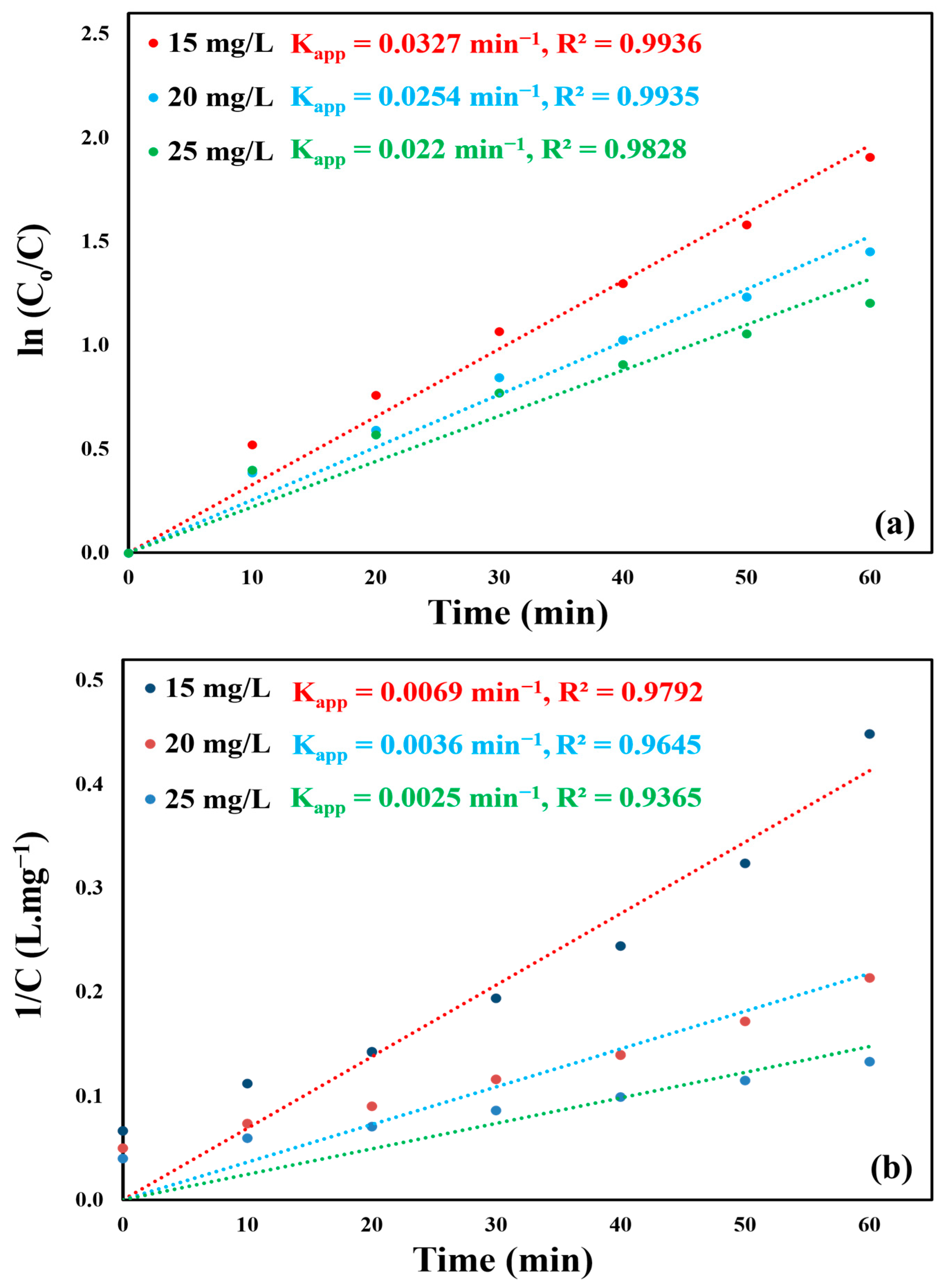
2.7. Photocatalytic Degradation Kinetics of CBZ
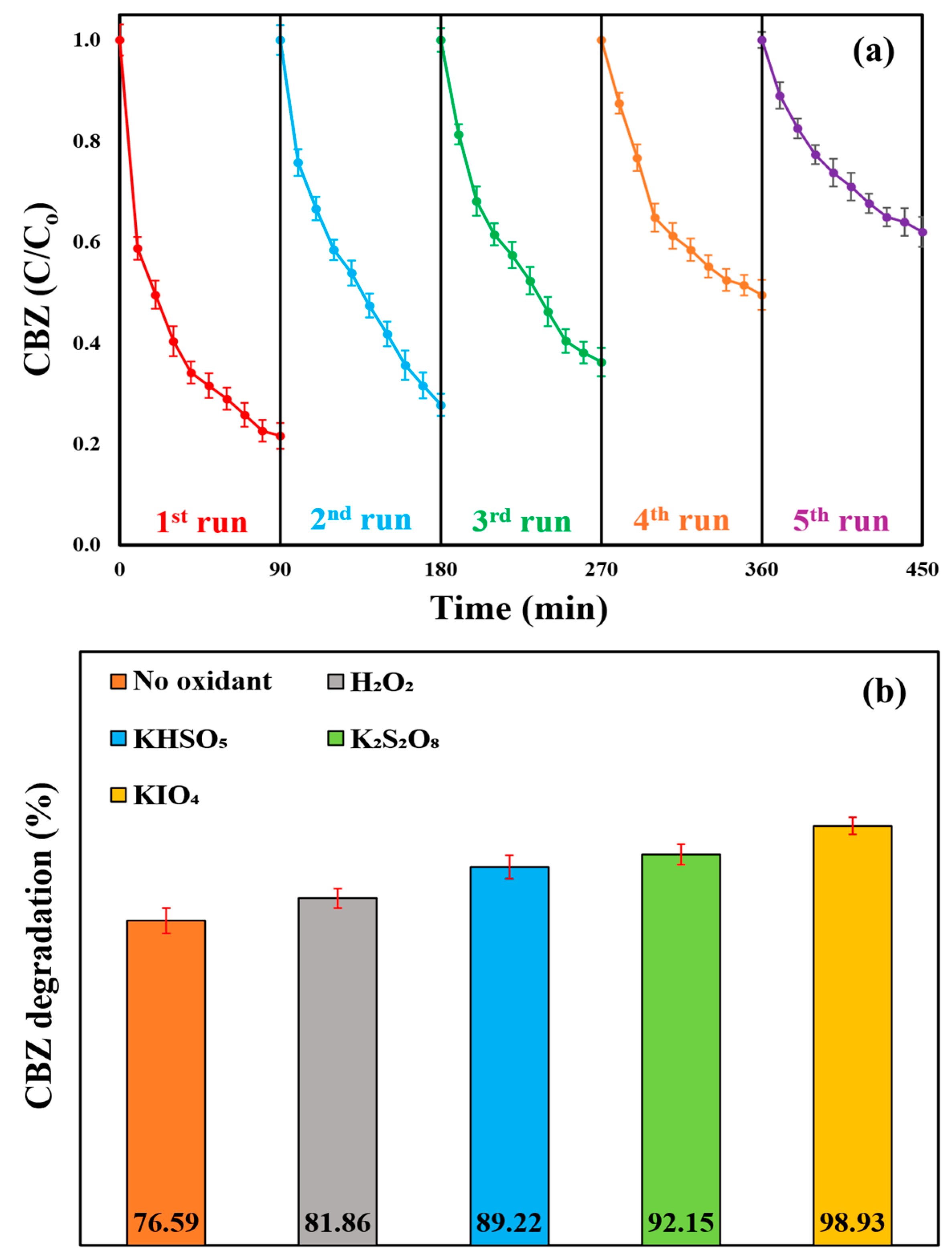
2.8. The Reusability of the ZMIP Photocatalyst
2.9. Total Organic Carbon (TOC) Mineralization in the ZMIP/Light System
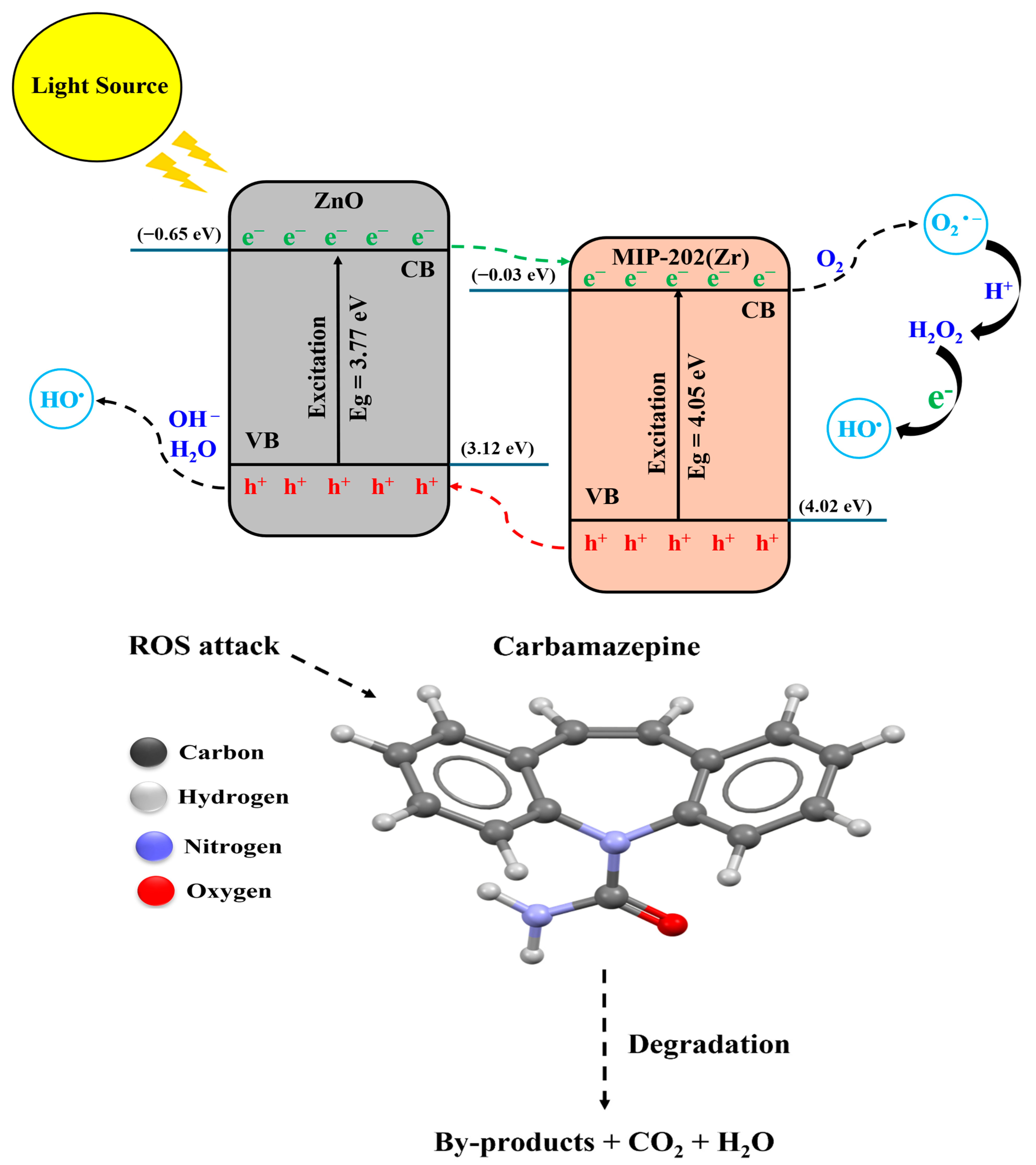
2.10. Photocatalytic Degradation Mechanism of CBZ
2.11. Degradation of Other Organic Pollutants
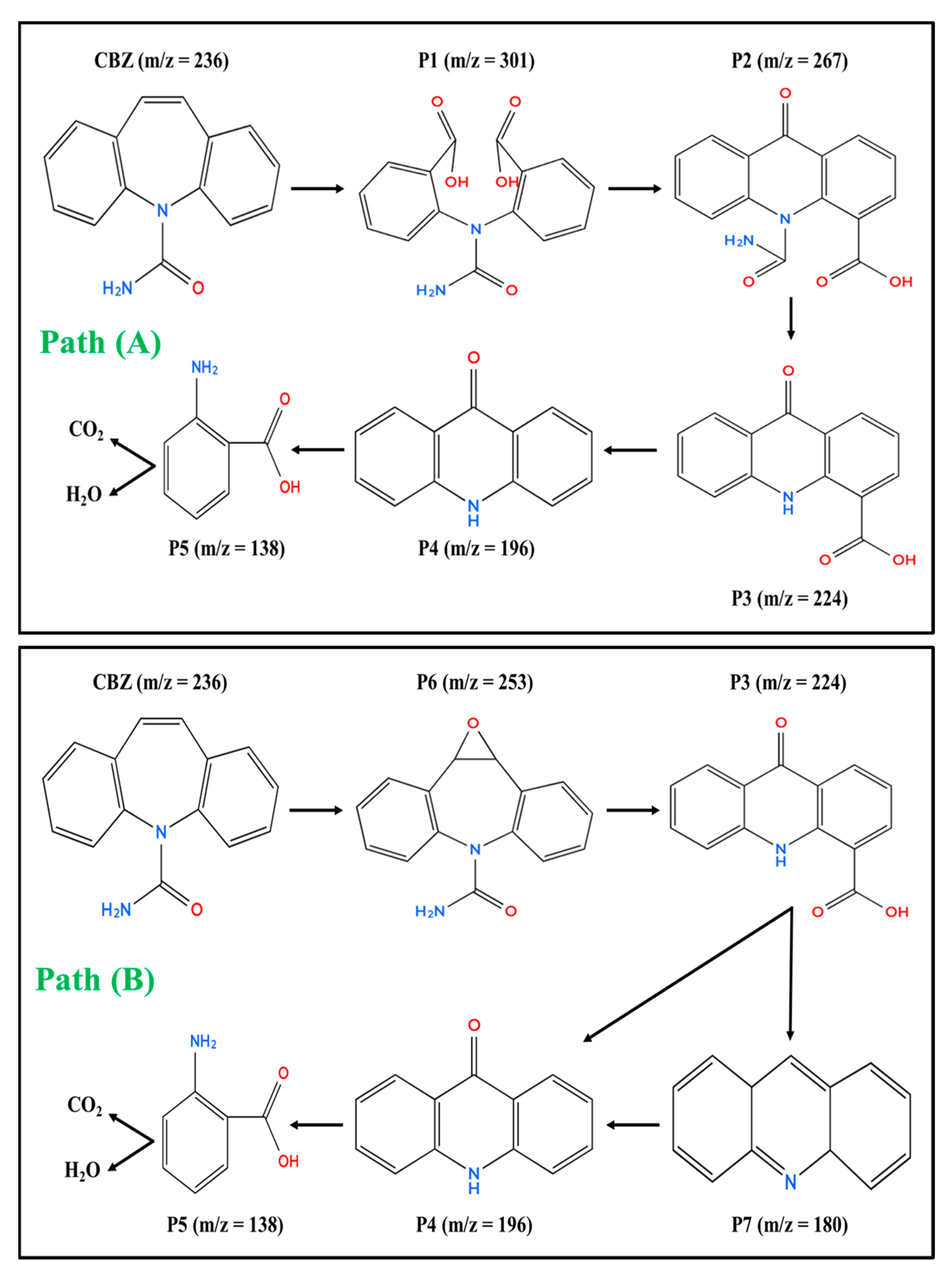
2.12. CBZ Proposed Degradation Paths
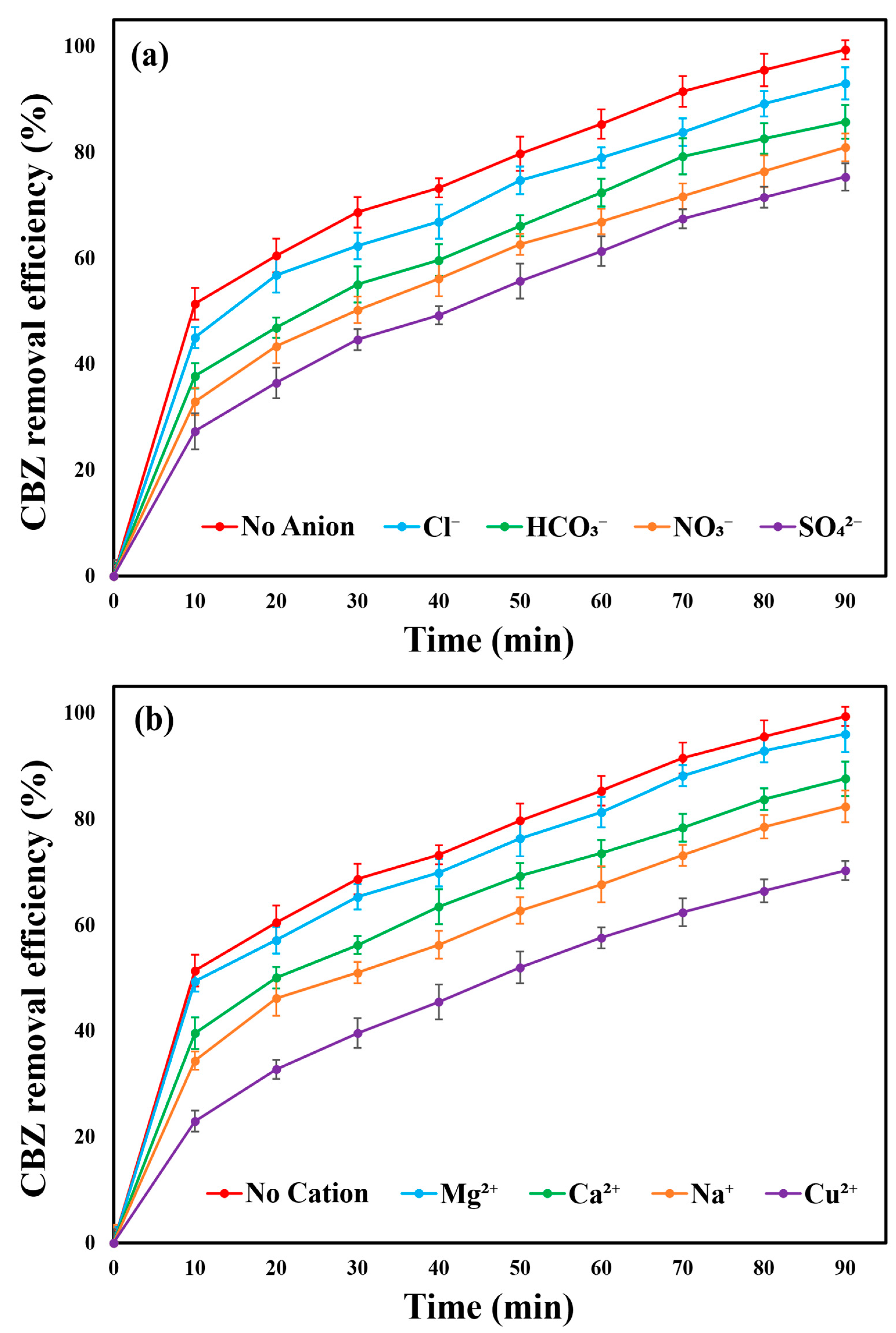
2.13. Effect of Coexisting Substances
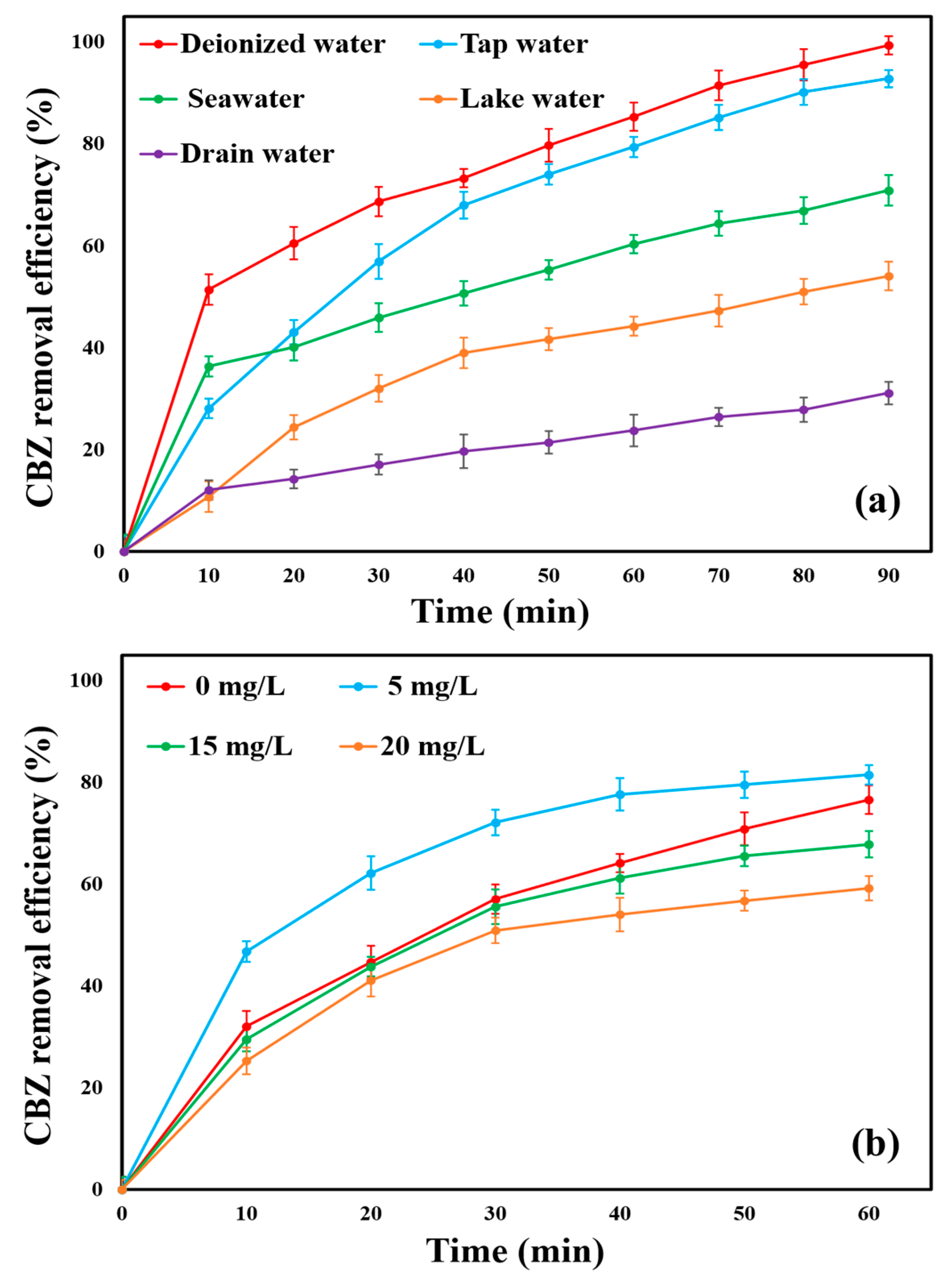
2.14. Remediation of Real Industrial Discharge
2.15. Effect of Introducing Highly Reactive Inorganic Oxidizing Agents
3. Materials and Methodology
3.1. Real Wastewater Samples
3.2. Chemicals and Materials
3.3. Green Synthesis of the ZnO Nanoparticles
3.4. Construction of the MIP-202(Zr) Bio-MOF
3.5. Fabrication of the ZMIP Nanocomposite
3.6. Physicochemical Characterization of the Synthesized Materials
3.7. Point of Zero Charge
3.8. Optical Properties
3.9. Experimental Set-Up and Degradation Study
3.10. Experimental Design and Statistical Analysis
3.11. Analytical Methods
3.12. Kinetic Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bakhadur, A.; Alem, H.; Carré, V.; Gries, T.; Balan, L.; Cantin, J.L.; Medjahdi, G.; Orazov, Z.; Uralbekov, B.; Schneider, R. Porous Nitrogen-Doped TiO2/Graphene Oxide Derived from H2N-MIL-125(Ti)/Graphene Oxide Composites as Highly Efficient Visible Light Active Photocatalysts for the Degradation of Dyes and Carbamazepine. Appl. Surf. Sci. 2025, 714, 164466. [Google Scholar] [CrossRef]
- Mohammed-Amine, E.; Kaltoum, B.; El Mountassir, E.M.; Abdelaziz, A.T.; Stephanie, R.; Stephanie, L.; Anne, P.; Pascal, W.W.C.; Alrashed, M.M.; Salah, R. Novel Sol-Gel Synthesis of TiO2/BiPO4 Composite for Enhanced Photocatalytic Degradation of Carbamazepine under UV and Visible Light: Kinetic, Identification of Photoproducts and Mechanistic Insights. J. Water Process Eng. 2025, 70, 107098. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B. Photocatalytic Treatment of High Concentration Carbamazepine in Synthetic Hospital Wastewater. J. Hazard Mater. 2012, 199, 135–142. [Google Scholar] [CrossRef]
- Hai, F.I.; Yang, S.; Asif, M.B.; Sencadas, V.; Shawkat, S.; Sanderson-Smith, M.; Gorman, J.; Xu, Z.Q.; Yamamoto, K. Carbamazepine as a Possible Anthropogenic Marker in Water: Occurrences, Toxicological Effects, Regulations and Removal by Wastewater Treatment Technologies. Water 2018, 10, 107. [Google Scholar] [CrossRef]
- Novikov, M.V.; Snytnikova, O.A.; Fedunov, R.G.; Yanshole, V.V.; Grivin, V.P.; Plyusnin, V.F.; Xu, J.; Pozdnyakov, I.P. A New View on the Mechanism of UV Photodegradation of the Tricyclic Antidepressant Carbamazepine in Aqueous Solutions. Chemosphere 2023, 329, 138652. [Google Scholar] [CrossRef] [PubMed]
- Samy, M.; Ibrahim, M.G.; Fujii, M.; Diab, K.E.; ElKady, M.; Gar Alalm, M. CNTs/MOF-808 Painted Plates for Extended Treatment of Pharmaceutical and Agrochemical Wastewaters in a Novel Photocatalytic Reactor. Chem. Eng. J. 2021, 406, 127152. [Google Scholar] [CrossRef]
- He, Y.; Ding, L.; Zhou, J.; Liu, D.; Zhang, Y. Synergistic Activation of Photocatalysis and Peroxymonosulfate via Fe2O3/Bi2O3 p-n Heterojunction for Enhanced Degradation of Tetracycline. Chem. Phys. Lett. 2025, 876, 142301. [Google Scholar] [CrossRef]
- Li, H.; Wang, K.; Xu, J.; Li, T.; Lu, Y.; Zou, R.; Zheng, G. Pre-Reduction of Goethite by Hydroxylamine Hydrochloride Enhances Sulfamethoxazole Degradation via Accelerated Fe(III)/Fe(II) Cycling in Fenton-like Systems. Chem. Eng. J. 2025, 522, 167877. [Google Scholar] [CrossRef]
- Montenegro-Apraez, D.; Graça, C.A.L.; Machuca-Martinez, F.; Soares, O.S.G.P. Catalytic Ozonation of Lamotrigine with Modified Activated Carbon: Degradation, Mineralization and by-Products Analysis. J. Water Process Eng. 2025, 77, 108434. [Google Scholar] [CrossRef]
- Butola, D.; Purohit, L.P. Synergistic G-C3N4@ZnO/SnO2 Heterojunction Nanocomposites for Multifunctional Applications in Photocatalysis and Gas Sensing. Surf. Interfaces 2025, 72, 106963. [Google Scholar] [CrossRef]
- Quan, Y.; Lu, S.; Wang, Q.; Wang, H.; Hu, E.; Xin, X.; Su, Y.; Zhang, Y.; Bao, J. Elucidating Structure-Activity Relationships in CdS/ZnO Heterojunctions for Synergistic Adsorption-Photocatalysis of Uranium (VI) Removal. Sep. Purif. Technol. 2025, 375, 133806. [Google Scholar] [CrossRef]
- Sonia; Kumari, H.; Chahal, S.; Suman; Kumar, S.; Mahak; Kumar, P.; Kumar, A. Hydrothermally Synthesized ZnFe2O4/ZnO Heterojunction Nanocomposites for Enhanced RB Dye Degradation via Z-Scheme Photocatalysis. Mater. Chem. Phys. 2024, 322, 129560. [Google Scholar] [CrossRef]
- Jayathilake, K.M.P.I.; Manage, P.M.; Idroos, F.S. Development of Water Lettuce (Pistia Spp.) Based Biochar Filter for the Treatment of Industrial Wastewater: A Green Approach. Curr. Sci. 2024, 127, 1208–1218. [Google Scholar] [CrossRef]
- Thongam, D.D.; Hang, D.R.; Gupta, J. Highly Active Sunlight-Driven Photocatalysis: Harnessing Z-Scheme Charge Transfer in g-C3N4/ZnO Heterojunction for Effective Advanced Oxidation Processes. Surf. Interfaces 2025, 72, 107048. [Google Scholar] [CrossRef]
- Qin, B.; Chen, J.; Xu, F.; Zou, J.; Wang, W.; Lv, Y.; Tian, L.; Zhou, L.; Feng, J. ZIF-8 Derived ZnS/ZnO Type-II Heterojunction for Efficient Photocatalytic Hydrogen Peroxide Production and Norfloxacin Degradation. J. Alloys Compd. 2025, 1038, 182866. [Google Scholar] [CrossRef]
- Oliveira, R.A.; Castro, M.A.M.; Porto, D.L.; Aragão, C.F.S.; Souza, R.P.; Silva, U.C.; Bomio, M.R.D.; Motta, F.V. Immobilization of Bi2MoO6/ZnO Heterojunctions on Glass Substrate: Design of Drug and Dye Mixture Degradation by Solar-Driven Photocatalysis. J. Photochem. Photobiol. A Chem. 2024, 452, 115619. [Google Scholar] [CrossRef]
- Luo, H.; Feng, M.; Zheng, S.; Wang, W.; Li, X. Enhancing Sunlight-Driven Photocatalysis of Ag/AgCl@MOF-808 Heterojunction via Synergistic Effects of Plasmonic Ag QDs and Heterostructure. J. Environ. Chem. Eng. 2025, 13, 117456. [Google Scholar] [CrossRef]
- Berehe, B.A.; Desalew, A.A.; Derbe, G.W.; Misganaw, D.M.; Mohammed, K.S.; Chang, J.Y.; Girma, W.M. Enhanced Photocatalytic Degradation of Methylene Blue Dye via Valorization of a Polyethylene Terephthalate Plastic Waste-Derived Metal–Organic Framework-Based ZnO@Co-BDC Composite Catalyst. Nanoscale Adv. 2025, 7, 3834–3845. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Liao, J.; Zhou, J.; Zhao, L.; Liu, Y.; Zhang, Y.; Chen, W.; Tang, S. A New Green Approach to Synthesizing MIP-202@porous Silica Microspheres for Positional Isomer/Enantiomer/Hydrophilic Separation. Chin. Chem. Lett. 2025, 36, 109985. [Google Scholar] [CrossRef]
- Sandhu, S.S.; Kotagiri, Y.G.; Ashvin Iresh Fernando, I.P.U.; Kalaj, M.; Tostado, N.; Teymourian, H.; Alberts, E.M.; Thornell, T.L.; Jenness, G.R.; Harvey, S.P.; et al. Green MIP-202(Zr) Catalyst: Degradation and Thermally Robust Biomimetic Sensing of Nerve Agents. J. Am. Chem. Soc. 2021, 143, 18261–18271. [Google Scholar] [CrossRef]
- Roushree, R.R.; Haimbodi, R. Recent Advances in ZnO/MOFs: Synthesis, Characterization, and Applications in Sustainable Antibiotic Wastewater Treatment. Chin. J. Anal. Chem. 2025, 100619. [Google Scholar] [CrossRef]
- Zhang, Z.; Bai, Z.; Yu, S.; Meng, X.; Xiao, S. Photo-Fenton Efficient Degradation of Organic Pollutants over S-Scheme ZnO@NH2-MIL-88B Heterojunction Established for Electron Transfer Channel. Chem. Eng. Sci. 2024, 288, 119789. [Google Scholar] [CrossRef]
- Fu, S.; Xi, W.; Ren, J.; Wei, H.; Sun, W. Study on the Photocatalytic Properties of Metal–Organic Framework-Derived C-, N-Co-Doped ZnO. Materials 2024, 17, 855. [Google Scholar] [CrossRef]
- Jin, C.; Ge, C.; Jian, Z.; Wei, Y. Facile Synthesis and High Photocatalytic Degradation Performance of ZnO-SnO2 Hollow Spheres. Nanoscale Res. Lett. 2016, 11, 526. [Google Scholar] [CrossRef]
- Piri, A.; Kaykhaii, M.; Khajeh, M.; Oveisi, A.R. Application of a Magnetically Separable Zr-MOF for Fast Extraction of Palladium before Its Spectrophotometric Detection. BMC Chem. 2024, 18, 63. [Google Scholar] [CrossRef]
- Abdelghani, G.M.; Ahmed, A.B.; Al-Zubaidi, A.B. Synthesis, Characterization, and the Influence of Energy of Irradiation on Optical Properties of ZnO Nanostructures. Sci. Rep. 2022, 12, 20016. [Google Scholar] [CrossRef] [PubMed]
- Murthy, K.R.S.; Raghu, G.K.; Binnal, P. Zinc Oxide Nanostructured Material for Sensor Application. J. Biotechnol. Bioeng. 2021, 5, 25–29. [Google Scholar] [CrossRef]
- Flores-Loyola, D.M.; Márquez-Guerrero, E.; Galindo-Guzman, S.Y.; Marszalek, M.; Green, J.E.; Monserrat Sánchez-Pérez, D.; Flores-Loyola, E.; Yuridia Márquez-Guerrero, S.; Galindo-Guzman, M.; Marszalek, J.E. Green Synthesis and Characterization of Zinc Oxide Nanoparticles Using Larrea tridentata Extract and Their Impact on the In-Vitro Germination and Seedling Growth of Capsicum annuum. Sustainability 2023, 15, 3080. [Google Scholar] [CrossRef]
- Ramesh, P.; Saravanan, K.; Manogar, P.; Johnson, J.; Vinoth, E.; Mayakannan, M. Green Synthesis and Characterization of Biocompatible Zinc Oxide Nanoparticles and Evaluation of Its Antibacterial Potential. Sens. Biosens. Res. 2021, 31, 100399. [Google Scholar] [CrossRef]
- Hashem, T.; Ibrahim, A.H.; Wöll, C.; Alkordi, M.H. Grafting Zirconium-Based Metal-Organic Framework UiO-66-NH2 Nanoparticles on Cellulose Fibers for the Removal of Cr(VI) Ions and Methyl Orange from Water. ACS Appl. Nano Mater. 2019, 2, 5804–5808. [Google Scholar] [CrossRef]
- Bagherzadeh, M.; Chegeni, M.; Bayrami, A.; Amini, M. Superior and Efficient Performance of Cost-Effective MIP-202 Catalyst over UiO-66-(CO2H)2 in Epoxide Ring Opening Reactions. Sci. Rep. 2024, 14, 17730. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Qian, Q.; Mizrahi Rodriguez, K.; Smith, Z.P. Hydrothermal Synthesis of Sub-20 Nm Amine-Functionalized MIL-101(Cr) Nanoparticles with High Surface Area and Enhanced CO2Uptake. Ind. Eng. Chem. Res. 2020, 59, 7888–7900. [Google Scholar] [CrossRef]
- Xu, X.; Xia, L.; Zheng, C.; Liu, Y.; Yu, D.; Li, J.; Zhong, S.; Li, C.; Song, H.; Liu, Y.; et al. Unravelling Nonclassical Beam Damage Mechanisms in Metal-Organic Frameworks by Low-Dose Electron Microscopy. Nat. Commun. 2025, 16, 261. [Google Scholar] [CrossRef] [PubMed]
- Gaber, M.M.; Shokry, H.; Samy, M.; El-Bestawy, E.A. Green Approach for Fabricating Hybrids of Food Waste-Derived Biochar/Zinc Oxide for Effective Degradation of Bromothymol Blue Dye in a Photocatalysis/Persulfate Activation System. Chemosphere 2024, 364, 143245. [Google Scholar] [CrossRef] [PubMed]
- da Trindade, L.G.; Borba, K.M.N.; Trench, A.B.; Zanchet, L.; Teodoro, V.; Pontes, F.M.L.; Longo, E.; Mazzo, T.M. Effective Strategy to Coupling Zr-MOF/ZnO: Synthesis, Morphology and Photoelectrochemical Properties Evaluation. J. Solid. State Chem. 2021, 293, 121794. [Google Scholar] [CrossRef]
- Taddei, M.; Schukraft, G.M.; Warwick, M.E.A.; Tiana, D.; McPherson, M.J.; Jones, D.R.; Petit, C. Band Gap Modulation in Zirconium-Based Metal–Organic Frameworks by Defect Engineering. J. Mater. Chem. A Mater. 2019, 7, 23781–23786. [Google Scholar] [CrossRef]
- Parsa Amouzesh, S.; Zandjou, M.; Ali Khodadadi, A.; Mortazavi, Y.; Hooriabad Saboor, F.; Saris, S.; Javanmard, A.; Alirezayi, S.; Asgari, M. Innovative Photocatalyst Design: Advancing ZnO/MIL-100(Fe) through Atomic Layer Deposition in Hydrogen Evolution. ChemCatChem 2024, 16, e202401016. [Google Scholar] [CrossRef]
- Harisankar, A.; Preethi, P.C.; Sreeja, T.G.; Rejani, P.; Murali, M.; Raghunandan, R. Zinc Oxide Functionalized MOF-5 for the Adsorptive Removal of Pb(II) Metal Ions and Photocatalytic Degradation of Methylene Blue Dye in Aqueous Medium. Ionics 2024, 30, 2313–2331. [Google Scholar] [CrossRef]
- Wang, J.; Bi, L.; Fu, Q.; Jen, A.K.Y. Methods for Passivating Defects of Perovskite for Inverted Perovskite Solar Cells and Modules. Adv. Energy Mater. 2024, 14, 2401414. [Google Scholar] [CrossRef]
- Ul Muazzam, U.; Muralidharan, R.; Raghavan, S.; Nath, D.N. Investigation of Optical Functions, Sub-Bandgap Transitions, and Urbach Tail in the Absorption Spectra of Ga2O3 Thin Films Deposited Using Mist-CVD. Opt. Mater. 2023, 145, 114373. [Google Scholar] [CrossRef]
- Vetokhina, V.; Nepomniashchaia, N.; de Prado, E.; Pacherova, O.; Kocourek, T.; Anandakrishnan, S.S.; Bai, Y.; Dejneka, A.; Tyunina, M. Tuning Optical Absorption in Perovskite (K,Na)NbO3 Ferroelectrics. Mater. Adv. 2024, 5, 8901–8908. [Google Scholar] [CrossRef]
- Ganesha Krishna, V.S.; Mahesha, M.G. (Mg,Mn)-Dual Doping Synergism towards Luminescence and Electrical Properties of ZnO/p-Si Heterojunction Diodes. RSC Adv. 2023, 13, 32282–32295. [Google Scholar] [CrossRef]
- Klein, J.; Kampermann, L.; Mockenhaupt, B.; Behrens, M.; Strunk, J.; Bacher, G.; Klein, J.; Kampermann, L.; Bacher, G.; Mockenhaupt, B.; et al. Limitations of the Tauc Plot Method. Adv. Funct. Mater. 2023, 33, 2304523. [Google Scholar] [CrossRef]
- Sohrabnezhad, S.; Pourahmad, A.; Karimi, M.F. Magnetite-Metal Organic Framework Core@shell for Degradation of Ampicillin Antibiotic in Aqueous Solution. J. Solid. State Chem. 2020, 288, 121420. [Google Scholar] [CrossRef]
- Zhao, W.; Yan, M.; Chen, Y.; Shen, J.; Hong, X.; Mu, F.; Li, S.; Zhang, S.; Wang, Q.; Dai, B.; et al. Rational Design of Novel Metal-Organic Framework/Bi4O7 S-Scheme Heterojunction Photocatalyst for Boosting Carbamazepine Degradation. Appl. Surf. Sci. 2023, 622, 156876. [Google Scholar] [CrossRef]
- Goh, J.W.; Xiong, Y.; Wu, W.; Huang, Z.; Ong, S.L.; Hu, J.Y. Degradation of Carbamazepine by HF-Free-Synthesized MIL-101(Cr)@Anatase TiO2 Composite under UV-A Irradiation: Degradation Mechanism, Wastewater Matrix Effect, and Degradation Pathway. Water 2022, 14, 3964. [Google Scholar] [CrossRef]
- Liu, N.; Shang, Q.; Gao, K.; Cheng, Q.; Pan, Z. Construction of ZnO/ZIF-9 Heterojunction Photocatalyst: Enhanced Photocatalytic Performance and Mechanistic Insight. New J. Chem. 2020, 44, 6384–6393. [Google Scholar] [CrossRef]
- Du, Q.; Wu, P.; Sun, Y.; Zhang, J.; He, H. Selective Photodegradation of Tetracycline by Molecularly Imprinted ZnO@NH2-UiO-66 Composites. Chem. Eng. J. 2020, 390, 124614. [Google Scholar] [CrossRef]
- Samy, M.; Ibrahim, M.G.; Gar Alalm, M.; Fujii, M. MIL-53(Al)/ZnO Coated Plates with High Photocatalytic Activity for Extended Degradation of Trimethoprim via Novel Photocatalytic Reactor. Sep. Purif. Technol. 2020, 249, 117173. [Google Scholar] [CrossRef]
- Mohsen Mousavi, S.; Chamack, M.; Fakhri, H. Study of New Hybrid Material of ZnO/CuO and Metal-Organic Framework as Photocatalyst for Removal of Tetracycline from Water. J. Nanostruct 2022, 12, 1097–1107. [Google Scholar] [CrossRef]
- Fakhri, H.; Bagheri, H. Highly Efficient Zr-MOF@WO3/Graphene Oxide Photocatalyst: Synthesis, Characterization and Photodegradation of Tetracycline and Malathion. Mater. Sci. Semicond. Process 2020, 107, 104815. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Shi, X.; Bi, F.; Yang, Y.; Wang, Y. Synergistic Effects of Octahedral TiO2-MIL-101(Cr) with Two Heterojunctions for Enhancing Visible-Light Photocatalytic Degradation of Liquid Tetracycline and Gaseous Toluene. J. Colloid. Interface Sci. 2020, 579, 37–49. [Google Scholar] [CrossRef]
- Wu, J.; Fang, X.; Zhu, Y.; Ma, N.; Dai, W. Well-Designed TiO2@UiO-66-NH2Nanocomposite with Superior Photocatalytic Activity for Tetracycline under Restricted Space. Energy Fuels 2020, 34, 12911–12917. [Google Scholar] [CrossRef]
- Hu, P.; Yao, C.; Yang, L.; Xin, Y.; Miao, Y. Boosted Photodegradation of Tetracycline Hydrochloride over Z-Scheme MIL-88B(Fe)/Bi2WO6 Composites under Visible Light. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127248. [Google Scholar] [CrossRef]
- Yin, S.; Chen, Y.; Li, M.; Hu, Q.; Ding, Y.; Shao, Y.; Di, J.; Xia, J.; Li, H. Construction of NH2-MIL-125(Ti)/Bi2WO6 Composites with Accelerated Charge Separation for Degradation of Organic Contaminants under Visible Light Irradiation. Green. Energy Environ. 2020, 5, 203–213. [Google Scholar] [CrossRef]
- Mirzaei, A.; Chen, Z.; Haghighat, F.; Yerushalmi, L. Removal of Pharmaceuticals and Endocrine Disrupting Compounds from Water by Zinc Oxide-Based Photocatalytic Degradation: A Review. Sustain. Cities Soc. 2016, 27, 407–418. [Google Scholar] [CrossRef]
- Mishra, S.R.; Gadore, V.; Ahmaruzzaman, M. Insights into Persulfate-Activated Photodegradation of Tinidazole and Photoreduction of Hexavalent Chromium through β-In2S3 Anchored on Ag-Doped Fish Scale-Derived HAp Composite Quantum Dots. J. Clean. Prod. 2023, 427, 139221. [Google Scholar] [CrossRef]
- Dehghan, A.; Dehghani, M.H.; Nabizadeh, R.; Ramezanian, N.; Alimohammadi, M.; Najafpoor, A.A. Adsorption and Visible-Light Photocatalytic Degradation of Tetracycline Hydrochloride from Aqueous Solutions Using 3D Hierarchical Mesoporous BiOI: Synthesis and Characterization, Process Optimization, Adsorption and Degradation Modeling. Chem. Eng. Res. Des. 2018, 129, 217–230. [Google Scholar] [CrossRef]
- Gar Alalm, M.; Samy, M.; Ookawara, S.; Ohno, T. Immobilization of S-TiO2 on Reusable Aluminum Plates by Polysiloxane for Photocatalytic Degradation of 2,4-Dichlorophenol in Water. J. Water Process Eng. 2018, 26, 329–335. [Google Scholar] [CrossRef]
- Liu, X.; Lv, P.; Yao, G.; Ma, C.; Huo, P.; Yan, Y. Microwave-Assisted Synthesis of Selective Degradation Photocatalyst by Surface Molecular Imprinting Method for the Degradation of Tetracycline onto ClTiO2. Chem. Eng. J. 2013, 217, 398–406. [Google Scholar] [CrossRef]
- Fathinia, M.; Khataee, A.R. Residence Time Distribution Analysis and Optimization of Photocatalysis of Phenazopyridine Using Immobilized TiO2 Nanoparticles in a Rectangular Photoreactor. J. Ind. Eng. Chem. 2013, 19, 1525–1534. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Li, H.; Ao, X.; Sun, W.; Li, Z. Reactive Oxygen Species Generated in Situ During Carbamazepine Photodegradation at 222 Nm Far-UVC: Unexpected Role of H2O Molecules. Environ. Sci. Technol. 2024, 58, 19070–19079. [Google Scholar] [CrossRef]
- Zayyat, R.M.; Yahfoufi, R.; Al-Hindi, M.; Kordahi, M.A.; Ayoub, G.M.; Ahmad, M.N. Elucidating the Dynamics of Carbamazepine Uptake Using Date Pit-Derived Activated Carbon: A Comprehensive Kinetic and Thermodynamic Analysis. Heliyon 2024, 10, e39068. [Google Scholar] [CrossRef]
- Abumelha, H.M.; Alzahrani, S.O.; Alrefaee, S.H.; Al-bonayan, A.M.; Alkhatib, F.; Saad, F.A.; El-Metwaly, N.M. Evaluation of Tetracycline Removal by Magnetic Metal Organic Framework from Aqueous Solutions: Adsorption Isotherm, Kinetics, Thermodynamics, and Box-Behnken Design Optimization. J. Saudi Chem. Soc. 2023, 27, 101706. [Google Scholar] [CrossRef]
- Ahmad, F.A. The Use of Agro-Waste-Based Adsorbents as Sustainable, Renewable, and Low-Cost Alternatives for the Removal of Ibuprofen and Carbamazepine from Water. Heliyon 2023, 9, e16449. [Google Scholar] [CrossRef]
- Kumar, S.; Dhiman, V.; Kumar, R.; Kaur, S.; Sharma, P.; Singh, K. Unveiling the Photocatalytic Properties of Cadmium Oxide for Sustainable Approach towards Water Remediation: A Review. Coord. Chem. Rev. 2025, 539, 216713. [Google Scholar] [CrossRef]
- Li, Z.; Tao, W.; Wang, Y.; Ye, X.; Chen, Y.; Han, B.; Lee, L.Y.S. Corrosion-Resistant MoO3/Fe2O3/MoS2 Heterojunctions Stabilize OH- Adsorption for Efficient Light-Assisted Seawater Electrooxidation. J. Am. Chem. Soc. 2025, 147, 24461. [Google Scholar] [CrossRef]
- Décima, M.A.; Marzeddu, S.; Barchiesi, M.; Di Marcantonio, C.; Chiavola, A.; Boni, M.R. A Review on the Removal of Carbamazepine from Aqueous Solution by Using Activated Carbon and Biochar. Sustainability 2021, 13, 11760. [Google Scholar] [CrossRef]
- Sultana, M.; Mohapatra, S.R.; Ahmaruzzaman, M. Spherical Magnetic MgO-CeO2-Fe3O4@JB Heterojunction for Enhanced Photodegradation of Pesticide and Complex Anionic Dyes: Understanding the Degradation Process and Diverse Water Systems. Chem. Eng. J. 2024, 502, 157549. [Google Scholar] [CrossRef]
- Mohtaram, M.S.; Sabbaghi, S.; Rasouli, J.; Rasouli, K. Photocatalytic Degradation of Tetracycline Using a Novel WO3–ZnO/AC under Visible Light Irradiation: Optimization of Effective Factors by RSM-CCD. Environ. Pollut. 2024, 347, 123746. [Google Scholar] [CrossRef] [PubMed]
- Quy, B.M.; Thu, N.T.N.; Xuan, V.T.; Hoa, N.T.H.; Linh, N.T.N.; Tung, V.Q.; Le, V.T.T.; Thao, T.T.; Ngan, N.T.K.; Tho, P.T.; et al. Photocatalytic Degradation Performance of a Chitosan/ZnO–Fe3O4 Nanocomposite over Cationic and Anionic Dyes under Visible-Light Irradiation. RSC Adv. 2025, 15, 1590–1603. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.D.; Nguyen, D.Q.; Do, P.T.; Tran, U.N.P. Kinetics of Photocatalytic Degradation of Organic Compounds: A Mini-Review and New Approach. RSC Adv. 2023, 13, 16915–16925. [Google Scholar] [CrossRef]
- Bloh, J.Z. A Holistic Approach to Model the Kinetics of Photocatalytic Reactions. Front. Chem. 2019, 7, 440665. [Google Scholar] [CrossRef] [PubMed]
- Gaber, M.M.; Toghan, A.; Shokry, H.; Samy, M. Efficient Oxidative Degradation of Organic Pollutants in Real Industrial Effluents Using a Green-Synthesized Magnetite Supported on Biochar Catalyst. RSC Adv. 2025, 15, 31522–31538. [Google Scholar] [CrossRef]
- Gaber, M.M.; Rashid, N.; Alzahrani, A.; Alanazi, F. Utilization of Incense Stick Residues as a Sustainable Catalyst for Efficient Peroxydisulfate Activation in the Oxidative Degradation of Atrazine from Real Industrial Effluents. J. Environ. Chem. Eng. 2025, 13, 116031. [Google Scholar] [CrossRef]
- Abdel-Salam, M.O.; Farghal, H.H.; El Sawy, E.; Yoon, T.; El-Sayed, M.M.H. Activation of Peroxymonosulfate for Rhodamine-B Removal from Water: Enhanced Efficiency with Cobalt-Enriched, Magnetically Recoverable CNTs. RSC Adv. 2025, 15, 6371–6383. [Google Scholar] [CrossRef]
- El-Bestawy, E.A.; Gaber, M.; Shokry, H.; Samy, M. Effective Degradation of Atrazine by Spinach-Derived Biochar via Persulfate Activation System: Process Optimization, Mechanism, Degradation Pathway and Application in Real Wastewater. Environ. Res. 2023, 229, 115987. [Google Scholar] [CrossRef]
- Mathew, R.A.; Kanmani, S. Photocatalytic Degradation of Carbamazepine Using Ozonation and Photocatalytic Ozonation with TiO2 and WO3. Water Pr. Technol. 2020, 15, 645–651. [Google Scholar] [CrossRef]
- Gaber, M.M.; Samy, M.; El-Bestawy, E.A.; Shokry, H. Effective Degradation of Tetracycline and Real Pharmaceutical Wastewater Using Novel Nanocomposites of Biosynthesized ZnO and Carbonized Toner Powder. Chemosphere 2024, 352, 141448. [Google Scholar] [CrossRef] [PubMed]
- Franz, S.; Falletta, E.; Arab, H.; Murgolo, S.; Bestetti, M.; Mascolo, G. Degradation of Carbamazepine by Photo(Electro)Catalysis on Nanostructured TiO2 Meshes: Transformation Products and Reaction Pathways. Catalysts 2020, 10, 169. [Google Scholar] [CrossRef]
- Grinnell, C.; Samokhvalov, A. Exploring the Electronic Structure of Aluminum Metal-Organic Framework Basolite A100: Solid-State Synchronous Fluorescence Spectroscopy Reveals New Charge Excitation/Relaxation Pathways. Phys. Chem. Chem. Phys. 2018, 20, 26947–26956. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Sharma, G.; Al-Muhtaseb, A.H.; Naushad, M.; Ghfar, A.A.; Stadler, F.J. Quaternary Magnetic BiOCl/g-C3N4/Cu2O/Fe3O4 Nano-Junction for Visible Light and Solar Powered Degradation of Sulfamethoxazole from Aqueous Environment. Chem. Eng. J. 2018, 334, 462–478. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and Mechanisms of Photocatalytic Dye Degradation on TiO2 Based Photocatalysts: A Comparative Overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Zhou, C.; Lai, C.; Xu, P.; Zeng, G.; Huang, D.; Li, Z.; Zhang, C.; Cheng, M.; Hu, L.; Wan, J.; et al. Rational Design of Carbon-Doped Carbon Nitride/Bi12O17Cl2 Composites: A Promising Candidate Photocatalyst for Boosting Visible-Light-Driven Photocatalytic Degradation of Tetracycline. ACS Sustain. Chem. Eng. 2018, 6, 6941–6949. [Google Scholar] [CrossRef]
- Samy, M.; Ibrahim, M.G.; Gar Alalm, M.; Fujii, M. Effective Photocatalytic Degradation of Sulfamethazine by CNTs/LaVO4 in Suspension and Dip Coating Modes. Sep. Purif. Technol. 2020, 235, 116138. [Google Scholar] [CrossRef]
- Zeng, H.; Chen, Y.; Xu, J.; Li, S.; Wu, J.; Li, D.; Zhang, J. Iron-Based Materials for Activation of Periodate in Water and Wastewater Treatment Processes: The Important Role of Fe Species. Chem. Eng. J. 2024, 482, 148885. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, J.; Xie, M.; Jing, L.; Xu, H.; She, X.; Li, H.; Xie, J. Construction of Novel CNT/LaVO4 Nanostructures for Efficient Antibiotic Photodegradation. Chem. Eng. J. 2019, 357, 487–497. [Google Scholar] [CrossRef]
- Hammad, M.; Angel, S.; Al-kamal, A.K.; Asghar, A.; Kräenbring, M.A.; Amin, A.; Wiedemann, H.T.A.; Amin, A.S.; Vinayakumar, V.; Schmidt, T.C.; et al. Spray-Flame Synthesis of LaCo0.2Mn0.8O3 for Selective Peroxymonosulfate Activation into Singlet Oxygen towards Efficient Degradation of Carbamazepine. Process Saf. Environ. Prot. 2025, 194, 1347–1359. [Google Scholar] [CrossRef]
- Mafa, P.J.; Malefane, M.E.; Idris, A.O.; Mamba, B.B.; Liu, D.; Gui, J.; Kuvarega, A.T. Cobalt Oxide/Copper Bismuth Oxide/Samarium Vanadate (Co3O4/CuBi2O4/SmVO4) Dual Z-Scheme Heterostructured Photocatalyst with High Charge-Transfer Efficiency: Enhanced Carbamazepine Degradation under Visible Light Irradiation. J. Colloid. Interface Sci. 2021, 603, 666–684. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Ji, H.; Du, P.; Huang, T.; Wang, C.; Liu, W. Insights into Catalytic Activation of Peroxymonosulfate for Carbamazepine Degradation by MnO2 Nanoparticles In-Situ Anchored Titanate Nanotubes: Mechanism, Ecotoxicity and DFT Study. J. Hazard Mater. 2021, 402, 123779. [Google Scholar] [CrossRef]
- Chen, G.; Wang, H.; Dong, W.; Huang, Y.; Zhao, Z.; Zeng, Y. Graphene Dispersed and Surface Plasmon Resonance-Enhanced Ag3PO4 (DSPR-Ag3PO4) for Visible Light Driven High-Rate Photodegradation of Carbamazepine. Chem. Eng. J. 2021, 405, 126850. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, L.; Ni, C.; He, E.; Yu, L.; Li, X. 3D/2D MOF-Derived CoCeOx/g-C3N4 Z-Scheme Heterojunction for Visible Light Photocatalysis: Hydrogen Production and Degradation of Carbamazepine. J. Alloys Compd. 2022, 890, 161786. [Google Scholar] [CrossRef]
- Qudsieh, I.Y.; Ali, M.A.; Maafa, I.M. Effect of Water Matrix on Photocatalytic Degradation of Organic Pollutants in Water: A Literature Review. Rev. Chem. Eng. 2025, 41, 539–573. [Google Scholar] [CrossRef]
- Heredia Deba, S.A.; Wols, B.A.; Yntema, D.R.; Lammertink, R.G.H. Effects of the Water Matrix on the Degradation of Micropollutants by a Photocatalytic Ceramic Membrane. Membranes 2022, 12, 1004. [Google Scholar] [CrossRef]
- Pavel, M.; Anastasescu, C.; State, R.N.; Vasile, A.; Papa, F.; Balint, I. Photocatalytic Degradation of Organic and Inorganic Pollutants to Harmless End Products: Assessment of Practical Application Potential for Water and Air Cleaning. Catalysts 2023, 13, 380. [Google Scholar] [CrossRef]
- Gaber, M.M.; Samy, M.; Azam, A.; Shokry, H. Remediation of Paracetamol-Contaminated Water by Novel Burned Hookah Charcoal Residues via Persulfate Activation under Visible Light: Optimization, Mechanism, and Real Pharmaceutical Wastewater Application. J. Environ. Chem. Eng. 2024, 12, 114399. [Google Scholar] [CrossRef]
- Gaber, M.M.; Samy, M.; Shokry, H. Effective Degradation of Synthetic Micropollutants and Real Textile Wastewater via a Visible Light-Activated Persulfate System Using Novel Spinach Leaf-Derived Biochar. Environ. Sci. Pollut. Res. 2024, 31, 25163–25181. [Google Scholar] [CrossRef] [PubMed]
- Fouad, M.; Gar Alalm, M.; El-Etriby, H.K.; Boffito, D.C.; Ookawara, S.; Ohno, T.; Fujii, M. Visible-Light-Driven Photocatalytic Disinfection of Raw Surface Waters (300–5000 CFU/ML) Using Reusable Coated Ru/WO3/ZrO2. J. Hazard. Mater. 2021, 402, 123514. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Zhou, L.; Tang, Q.; Xiao, X.; Sun, S. Photocatalytic Degradation of Organic Pollutants by Carbon Quantum Dots Functionalized G-C3N4: A Review. Ecotoxicol. Environ. Saf. 2023, 262, 115133. [Google Scholar] [CrossRef]
- Alalm, M.G.; Djellabi, R.; Meroni, D.; Pirola, C.; Bianchi, C.L.; Boffito, D.C. Toward Scaling-Up Photocatalytic Process for Multiphase Environmental Applications. Catalysts 2021, 11, 562. [Google Scholar] [CrossRef]
- Cheng, R.; Xia, J.C.; Shen, L.J.; Shen, Z.P.; Shi, L.; Zheng, X.; Zheng, J.Z. Effect of Humic Acid on Visible Light Photocatalytic Inactivation of Bacteriophage F2 with Electrospinning Cu-TiO2 Nanofibers: Insight into the Mechanisms. Environ. Sci. Pollut. Res. 2024, 31, 30212–30227. [Google Scholar] [CrossRef]
- Hu, M.; Chen, W.; Wang, J. Photocatalytic Degradation of Tetracycline by La-Fe Co-Doped SrTiO3/TiO2 Composites: Performance and Mechanism Study. Water 2024, 16, 210. [Google Scholar] [CrossRef]
- Feng, R.; Chen, L.; Li, W.; Cai, T.; Jiang, C. Activation of Persulfate with Natural Organic Acids (Ascorbic Acid/Catechin Hydrate) for Naproxen Degradation in Water and Soil: Mechanism, Pathway, and Toxicity Assessment. J. Hazard. Mater. 2023, 459, 132152. [Google Scholar] [CrossRef]
- Yang, H.; Joo, J.; Hong, E.; Park, S.J.; Lee, J.; Lee, C.G. Chicken Litter-Derived Catalyst for Persulfate Activation to Remove Acetaminophen: An Organic-Waste-to-Wealth Strategy. Chem. Eng. J. 2023, 471, 144368. [Google Scholar] [CrossRef]
- Tian, C.; Zhao, H.; Mei, J.; Yang, S. Cost-Efficient Graphitic Carbon Nitride as an Effective Photocatalyst for Antibiotic Degradation: An Insight into the Effects of Different Precursors and Coexisting Ions, and Photocatalytic Mechanism. Chem. Asian J. 2019, 14, 162–169. [Google Scholar] [CrossRef]
- Pete, K.Y.; Kabuba, J.; Otieno, B.; Ochieng, A. Modeling Adsorption and Photocatalytic Treatment of Recalcitrant Contaminant on Multi-Walled Carbon/TiO2 Nanocomposite. Environ. Sci. Pollut. Res. 2023, 30, 94154–94165. [Google Scholar] [CrossRef]
- Stavrinou, A.; Theodoropoulou, M.A.; Tsakiroglou, C.D. Synthesis of Titania/Activated Carbon Composites for the Synergistic Adsorption and Photocatalysis of Lindane in Aqueous Solutions. Environ. Sci. Pollut. Res. 2025, 32, 6468–6491. [Google Scholar] [CrossRef]
- Xu, W.; Wang, Q.; He, J.; Liu, F.; Yan, X.; Xu, Y. Visible Light-Assisted Periodate Activation Using Carbon Nitride for the Efficient Elimination of Acid Orange 7. Separations 2024, 11, 274. [Google Scholar] [CrossRef]
- Zhang, X.; Kamali, M.; Yu, X.; Costa, M.E.V.; Appels, L.; Cabooter, D.; Dewil, R. Kinetics and Mechanisms of the Carbamazepine Degradation in Aqueous Media Using Novel Iodate-Assisted Photochemical and Photocatalytic Systems. Sci. Total Environ. 2022, 825, 153871. [Google Scholar] [CrossRef]
- Elmitwalli, T.; Fouad, M.; Mossad, M.; Samy, M. Periodate Activation by Mulukhiyah Stalks and Potato Peels-Derived Biochars for the Efficient Degradation of Sulfamethazine. J. Environ. Chem. Eng. 2024, 12, 112101. [Google Scholar] [CrossRef]
- Mohamed, A.; Mahanna, H.; Samy, M. Synergistic Effects of Photocatalysis-Periodate Activation System for the Degradation of Emerging Pollutants Using GO/MgO Nanohybrid. J. Environ. Chem. Eng. 2024, 12, 112248. [Google Scholar] [CrossRef]
- Moradian, F.; Ramavandi, B.; Jaafarzadeh, N.; Kouhgardi, E. Activation of Periodate Using Ultrasonic Waves and UV Radiation for Landfill Leachate Treatment. Environ. Sci. Pollut. Res. 2022, 29, 90338–90350. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Wu, S.; Han, J.; Dong, T.; Zhu, C.; Li, X.; Xu, L.; Zhang, Y.; Zhou, M.; Pan, Y. Sulfide-Modified Zero-Valent Iron Activated Periodate for Sulfadiazine Removal: Performance and Dominant Routine of Reactive Species Production. Water Res. 2022, 220, 118676. [Google Scholar] [CrossRef]
- Guo, D.; Yao, Y.; You, S.; Jin, L.; Lu, P.; Liu, Y. Ultrafast Degradation of Micropollutants in Water via Electro-Periodate Activation Catalyzed by Nanoconfined Fe2O3. Appl. Catal. B 2022, 309, 121289. [Google Scholar] [CrossRef]
- Monteagudo, J.M.; Durán, A.; San Martín, I.; Carrillo, P. Effect of Sodium Persulfate as Electron Acceptor on Antipyrine Degradation by Solar TiO2 or TiO2/RGO Photocatalysis. Chem. Eng. J. 2019, 364, 257–268. [Google Scholar] [CrossRef]
- Su, S.; Liu, Y.; He, W.; Tang, X.; Jin, W.; Zhao, Y. A Novel Graphene Oxide-Carbon Nanotubes Anchored α-FeOOH Hybrid Activated Persulfate System for Enhanced Degradation of Orange II. J. Environ. Sci. 2019, 83, 73–84. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Zhou, Z.; Wu, Y.; Xing, S. Degradation of Ciprofloxacin by Persulfate Activation with CuO Supported on Mg Al Layered Double Hydroxide. J. Environ. Chem. Eng. 2021, 9, 106178. [Google Scholar] [CrossRef]
- Niu, L.; Zhang, G.; Xian, G.; Ren, Z.; Wei, T.; Li, Q.; Zhang, Y.; Zou, Z. Tetracycline Degradation by Persulfate Activated with Magnetic γ-Fe2O3/CeO2 Catalyst: Performance, Activation Mechanism and Degradation Pathway. Sep. Purif. Technol. 2021, 259, 118156. [Google Scholar] [CrossRef]
- Zhang, H.; Mei, Y.; Zhu, F.; Yu, F.; Komarneni, S.; Ma, J. Efficient Activation of Persulfate by C@Fe3O4 in Visible-Light for Tetracycline Degradation. Chemosphere 2022, 306, 135635. [Google Scholar] [CrossRef]
- Samy, M.; Mensah, K.; El-Fakharany, E.M.; Elkady, M.; Shokry, H. Green Valorization of End-of-Life Toner Powder to Iron Oxide-Nanographene Nanohybrid as a Recyclable Persulfate Activator for Degrading Emerging Micropollutants. Environ. Res. 2023, 223, 115460. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Liu, F.; Ye, X.; Wei, S.; Sun, Y.; He, J. Synergistic Photocatalytic Oxidation and Reductive Activation of Peroxymonosulfate by Bi-Based Heterojunction for Highly Efficient Organic Pollutant Degradation. Nanomaterials 2025, 15, 471. [Google Scholar] [CrossRef]
- Ali, N.; Khan, A.A.; Wakeel, M.; Khan, I.A.; Din, S.U.; Qaisrani, S.A.; Khan, A.M.; Hameed, M.U. Activation of Peroxymonosulfate by UV-254 Nm Radiation for the Degradation of Crystal Violet. Water 2022, 14, 3440. [Google Scholar] [CrossRef]
- Wang, G.; Hambly, A.C.; Zhao, D.; Wang, G.; Tang, K.; Andersen, H.R. Peroxymonosulfate Activation by Suspended Biogenic Manganese Oxides for Polishing Micropollutants in Wastewater Effluent. Sep. Purif. Technol. 2023, 306, 122501. [Google Scholar] [CrossRef]
- Bouzayani, B.; Lomba-Fernández, B.; Fdez-Sanromán, A.; Elaoud, S.C.; Sanromán, M.Á. Advancements in Copper-Based Catalysts for Efficient Generation of Reactive Oxygen Species from Peroxymonosulfate. Appl. Sci. 2024, 14, 8075. [Google Scholar] [CrossRef]
- Liu, T.; Li, C.X.; Chen, X.; Chen, Y.; Cui, K.; Wei, Q. Peroxymonosulfate Activation by Rice-Husk-Derived Biochar (RBC) for the Degradation of Sulfamethoxazole: The Key Role of Hydroxyl Groups. Int. J. Mol. Sci. 2024, 25, 11582. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Peng, X.; Zhang, S.; Liu, S.; Xiong, Y.; Tian, S.; Fang, J. Activation of Peroxymonosulfate by Nitrogen-Functionalized Sludge Carbon for Efficient Degradation of Organic Pollutants in Water. Bioresour. Technol. 2017, 241, 244–251. [Google Scholar] [CrossRef]
- Cardoso, I.M.F.; Cardoso, R.M.F.; Esteves da Silva, J.C.G. Advanced Oxidation Processes Coupled with Nanomaterials for Water Treatment. Nanomaterials 2021, 11, 2045. [Google Scholar] [CrossRef]
- Mohammed, N.; Palaniandy, P.; Shaik, F.; Mewada, H.; Balakrishnan, D. Comparative Studies of RSM Box-Behnken and ANN-Anfis Fuzzy Statistical Analysis for Seawater Biodegradability Using TiO2 Photocatalyst. Chemosphere 2023, 314, 137665. [Google Scholar] [CrossRef]
- Yousefi, N.; Nabizadeh, R.; Nasseri, S.; Khoobi, M.; Nazmara, S.; Mahvi, A.H. Optimization of the Synthesis and Operational Parameters for NOM Removal with Response Surface Methodology during Nano-Composite Membrane Filtration. Water Sci. Technol. 2018, 77, 1558–1569. [Google Scholar] [CrossRef]
- Gaber, M.M.; Shokry, H.; Hassanin, A.H.; Awad, S.; Samy, M.; Elkady, M. Novel Palm Peat Lignocellulosic Adsorbent Derived from Agricultural Residues for Efficient Methylene Blue Dye Removal from Textile Wastewater. Appl. Water Sci. 2025, 15, 32. [Google Scholar] [CrossRef]
- Karimifard, S.; Alavi Moghaddam, M.R. Application of Response Surface Methodology in Physicochemical Removal of Dyes from Wastewater: A Critical Review. Sci. Total Environ. 2018, 640, 772–797. [Google Scholar] [CrossRef]
- Mirzaei, A.; Yerushalmi, L.; Chen, Z.; Haghighat, F.; Guo, J. Enhanced Photocatalytic Degradation of Sulfamethoxazole by Zinc Oxide Photocatalyst in the Presence of Fluoride Ions: Optimization of Parameters and Toxicological Evaluation. Water Res. 2018, 132, 241–251. [Google Scholar] [CrossRef]
- Diab, K.E.; Salama, E.; Hassan, H.S.; Abd El-moneim, A.; Elkady, M.F. Biocompatible MIP-202 Zr-MOF Tunable Sorbent for Cost-Effective Decontamination of Anionic and Cationic Pollutants from Waste Solutions. Sci. Rep. 2021, 11, 6619. [Google Scholar] [CrossRef] [PubMed]
- Naciri, Y.; Ahdour, A.; Benhsina, E.; Hamza, M.A.; Bouziani, A.; Hsini, A.; Bakiz, B.; Navío, J.A.; Ghazzal, M.N. Ba3(PO4)2 Photocatalyst for Efficient Photocatalytic Application. Glob. Chall. 2024, 8, 2300257. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, B.; Jafari, N.; Abdolahnejad, A.; Farrokhzadeh, H.; Ebrahimi, A. Application of Efficient Photocatalytic Process Using a Novel BiVO/TiO2-NaY Zeolite Composite for Removal of Acid Orange 10 Dye in Aqueous Solutions: Modeling by Response Surface Methodology (RSM). J Environ Chem Eng 2019, 7, 103253. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, Y.; Hammouda, S.B.; Doshi, B.; Guijarro, N.; Min, X.; Tang, C.J.; Sillanpää, M.; Sivula, K.; Wang, S. MIL-101(Fe)/g-C3N4 for Enhanced Visible-Light-Driven Photocatalysis toward Simultaneous Reduction of Cr(VI) and Oxidation of Bisphenol A in Aqueous Media. Appl. Catal. B. 2020, 272, 119033. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, H.; Yin, K.; Li, Z.; Fan, J.; Wu, X.; Wu, Z. Modulating Charge Transfer for Photo-Driven N2O Reduction via Electronegativity Differences between Cu and Support. Appl. Catal. B Environ. Energy 2025, 377, 125528. [Google Scholar] [CrossRef]
- Goudjil, M.B.; Dali, H.; Zighmi, S.; Mahcene, Z.; Bencheikh, S.E. Photocatalytic Degradation of Methylene Blue Dye with Biosynthesized Hematite α-Fe2O3 Nanoparticles under UV-Irradiation. Desalination Water Treat 2024, 317, 100079. [Google Scholar] [CrossRef]
- Roškaric, M.; Žerjav, G.; Zavašnik, J.; Pintar, A. The Influence of Synthesis Conditions on the Visible-Light Triggered Photocatalytic Activity of g-C3N4/TiO2 Composites Used in AOPs. J. Environ. Chem. Eng. 2022, 10, 107656. [Google Scholar] [CrossRef]
- Ni, W.; Khan, A. Modified Metal-Organic Frameworks as Photocatalysts. Metal-Organic Frameworks for Chemical Reactions; Elsevier: Amsterdam, The Netherlands, 2021; pp. 231–270. [Google Scholar] [CrossRef]
- Ran, Z.; Fang, Y.; Sun, J.; Ma, C.; Li, S. Photocatalytic Oxidative Degradation of Carbamazepine by TiO2 Irradiated by UV Light Emitting Diode. Catalysts 2020, 10, 540. [Google Scholar] [CrossRef]
- APHA. AWWA and WEF Standard Methods for the Examination of Water and Wastewater; American Public Works Association: Kansas City, MO, USA, 2017; Available online: https://yabesh.ir/wp-content/uploads/2018/02/Standard-Methods-23rd-Perv.pdf (accessed on 17 September 2025).
- Song, L.; Zhu, B.; Gray, S.; Duke, M.; Muthukumaran, S. Performance of Hybrid Photocatalytic-Ceramic Membrane System for the Treatment of Secondary Effluent. Membranes 2017, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Theodorakopoulos, G.V.; Papageorgiou, S.K.; Katsaros, F.K.; Romanos, G.E.; Beazi-Katsioti, M. Investigation of MO Adsorption Kinetics and Photocatalytic Degradation Utilizing Hollow Fibers of Cu-CuO/TiO2 Nanocomposite. Materials 2024, 17, 4663. [Google Scholar] [CrossRef] [PubMed]
- Tsaviv, J.N.; Eneji, I.S.; Shato’Ato, R.; Ahemen, I.; Jubu, P.R.; Yusof, Y. Photodegradation, Kinetics and Non-Linear Error Functions of Methylene Blue Dye Using SrZrO3 Perovskite Photocatalyst. Heliyon 2024, 10, e34517. [Google Scholar] [CrossRef]
- Pesik, S.; Jobiliong, E.; Steven, E. Comparative Analysis of Photodegradation of Ibuprofen and Clotrimazole Water Pollutant Using UVC Rays in Presence and Absence of ZnO Photocatalyst. Environ. Sci. Proc. 2023, 25, 49. [Google Scholar] [CrossRef]




| Run | Coded Values of Parameters | Actual Values of Parameters | CBZ Removal (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| D | F | G | H | D | F | G | H | Actual | Predicted | |
| 1 | −1 | 0 | −1 | 0 | 30 | 7 | 15 | 1 | 86.87 | 82.43 |
| 2 | −1 | −1 | 0 | 0 | 30 | 3 | 20 | 1 | 63.01 | 66.84 |
| 3 | 0 | 0 | 0 | 0 | 60 | 7 | 20 | 1 | 76.58 | 76.49 |
| 4 | −1 | 1 | 0 | 0 | 30 | 11 | 20 | 1 | 49.23 | 51.49 |
| 5 | 0 | 1 | −1 | 0 | 60 | 11 | 15 | 1 | 65.74 | 67.12 |
| 6 | −1 | 0 | 0 | 1 | 30 | 7 | 20 | 1.25 | 83.17 | 84.08 |
| 7 | 1 | 1 | 0 | 0 | 90 | 11 | 20 | 1 | 72.65 | 71.18 |
| 8 | 0 | 1 | 0 | 1 | 60 | 11 | 20 | 1.25 | 70.00 | 68.07 |
| 9 | 0 | 0 | 0 | 0 | 60 | 7 | 20 | 1 | 76.54 | 76.49 |
| 10 | 0 | 1 | 1 | 0 | 60 | 11 | 25 | 1 | 49.34 | 48.18 |
| 11 | 0 | 1 | 0 | −1 | 60 | 11 | 20 | 0.75 | 48.90 | 49.74 |
| 12 | 1 | 0 | −1 | 0 | 90 | 7 | 15 | 1 | 93.12 | 93.44 |
| 13 | 0 | −1 | −1 | 0 | 60 | 3 | 15 | 1 | 76.73 | 78.64 |
| 14 | 1 | −1 | 0 | 0 | 90 | 3 | 20 | 1 | 79.32 | 79.43 |
| 15 | 0 | −1 | 0 | 1 | 60 | 3 | 20 | 1.25 | 85.77 | 81.83 |
| 16 | 1 | 0 | 0 | −1 | 90 | 7 | 20 | 0.75 | 80.08 | 79.93 |
| 17 | 0 | 0 | 0 | 0 | 60 | 7 | 20 | 1 | 76.59 | 76.49 |
| 18 | 0 | 0 | 1 | 1 | 60 | 7 | 25 | 1.25 | 77.07 | 80.53 |
| 19 | 0 | 0 | 0 | 0 | 60 | 7 | 20 | 1 | 76.32 | 76.49 |
| 20 | 1 | 0 | 0 | 1 | 90 | 7 | 20 | 1.25 | 96.25 | 95.93 |
| 21 | 0 | 0 | −1 | 1 | 60 | 7 | 15 | 1.25 | 90.18 | 92.10 |
| 22 | 0 | −1 | 0 | −1 | 60 | 3 | 20 | 0.75 | 60.75 | 59.58 |
| 23 | −1 | 0 | 1 | 0 | 30 | 7 | 25 | 1 | 62.05 | 58.63 |
| 24 | 0 | −1 | 1 | 0 | 60 | 3 | 25 | 1 | 60.88 | 60.25 |
| 25 | −1 | 0 | 0 | −1 | 30 | 7 | 20 | 0.75 | 58.43 | 59.50 |
| 26 | 0 | 0 | 1 | −1 | 60 | 7 | 25 | 0.75 | 52.68 | 53.14 |
| 27 | 1 | 0 | 1 | 0 | 90 | 7 | 25 | 1 | 78.56 | 79.90 |
| 28 | 0 | 0 | −1 | −1 | 60 | 7 | 15 | 0.75 | 80.00 | 78.91 |
| 29 | 0 | 0 | 0 | 0 | 60 | 7 | 20 | 1 | 76.41 | 76.49 |
| Parameter | Optimum Value |
|---|---|
| Reaction time (min) | 90 |
| pH | 6 |
| Initial CBZ concentration (mg/L) | 15 |
| Photocatalyst dose (g/L) | 1.25 |
| CBZ removal efficiency (Laboratory) | 99.37% |
| CBZ removal efficiency (Model) | 98.84% |
| Photocatalyst | Light Source | Pollutant | Operating Conditions | Removal Ratio (%) | Reference |
|---|---|---|---|---|---|
| Bi4O7/MIL-68(In)-NH2 | Visible light: Xenon lamp (300 W) | CBZ | [Pollutant]o = 50 mg/L, [Catalyst]o = 1 g/L, and reaction time = 120 min. | 92.7 | [45] |
| TiO2/MIL-101(Cr) | UV-A LED (20 W) | CBZ | [Pollutant]o = 12 mg/L, [Catalyst]o = 2 g/L, and reaction time = 60 min. | 99 | [46] |
| ZnO/ZIF-9 | Ultraviolet light | Tetracycline | [Pollutant]o = 20 mg/L, [Catalyst]o = 0.25 g/L, and reaction time = 60 min. | 87.7 | [47] |
| ZnO/UiO-66/NH2 | Visible light: Xenon lamp (50 W, 0.01–105 HZ) | Tetracycline | [Pollutant]o = 20 mg/L, [Catalyst]o = 0.1 g/L, and reaction time = 90 min. | 61 | [48] |
| ZnO/MIL-53(Al) | Visible light: Metal-halide lamp (Venture, 400 W) | Trimethoprim | [Pollutant]o = 10 mg/L, pH = 7, flowrate = 5 mL/min, and reaction time = 240 min. | 93.5 | [49] |
| ZnO-CuO/TMU-5 | Visible light: Metal halide lamp (400 W, λ = 510 nm) | Tetracycline | [Pollutant]o = 30 mg/L, [Catalyst]o = 1 g/L, and reaction time = 180 min. | 62 | [50] |
| GO-WO3/UiO-66 | Visible light: Mercury lamp (400 W) | Tetracycline | [Pollutant]o = 20 mg/L, [Catalyst]o = 1.67 g/L, pH = 7, and reaction time = 70 min. | 84 | [51] |
| TiO2/MIL-101(Cr) | Visible light: Xenon lamp (300 W, λ > 400 nm) | Tetracycline | [Pollutant]o = 10 mg/L, [Catalyst]o = 0.2 g/L, and reaction time = 45 min. | 94.85 | [52] |
| TiO2/UiO-66-NH2 | Visible light: Xenon lamp (350 W) | Tetracycline | [Pollutant]o = 20 mg/L, [Catalyst]o = 0.12 mg TCN/mg catalyst, and reaction time = 60 min. | 75 | [53] |
| Bi2WO6/MIL-88B(Fe) | Visible light: Xenon lamp (500 W) | Tetracycline | [Pollutant]o = 10 mg/L, [Catalyst]o = 50 mg/L, and reaction time = 90 min. | 96.4 | [54] |
| Bi2WO6/MIL-125 (Ti)-NH2 | Visible light: Xenon lamp (300 W) | Tetracycline | [Pollutant]o = 20 mg/L, [Catalyst]o = 0.4 g/L, and reaction time = 120 min. | 69.19 | [55] |
| ZnO/MIP-202(Zr) | Visible light: Metal halide lamp (400 W, λ = 510 nm) | CBZ | [Pollutant]o = 15 mg/L, [Catalyst]o = 1.25 g/L, pH = 6, and reaction time = 90 min. | 99.37 | This study |
| Source | DF | Sum of Squares | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 14 | 4760.41 | 340.03 | 46.18 | 0 |
| Linear | 4 | 3481.67 | 870.42 | 118.2 | 0 |
| D-Reaction time (min) | 1 | 787.64 | 787.64 | 106.96 | 0 |
| F-pH | 1 | 415.36 | 415.36 | 56.41 | 0 |
| G-CBZ concentration (mg/L) | 1 | 1046.45 | 1046.45 | 142.11 | 0 |
| H-Catalyst dose (g/L) | 1 | 1232.21 | 1232.21 | 167.33 | 0 |
| Square | 4 | 1167.02 | 291.76 | 39.62 | 0 |
| D (min) × D (min) | 1 | 54.53 | 54.53 | 7.4 | 0.017 |
| F × F | 1 | 958.05 | 958.05 | 130.1 | 0 |
| G (mg/L) × G (mg/L) | 1 | 4.06 | 4.06 | 0.55 | 0.47 |
| H (g/L) × H (g/L) | 1 | 1.41 | 1.41 | 0.19 | 0.668 |
| 2-Way Interaction | 6 | 111.71 | 18.62 | 2.53 | 0.072 |
| D (min) × F | 1 | 12.64 | 12.64 | 1.72 | 0.211 |
| D (min) × G (mg/L) | 1 | 26.32 | 26.32 | 3.57 | 0.08 |
| D (min) × H (g/L) | 1 | 18.36 | 18.36 | 2.49 | 0.137 |
| F × G (mg/L) | 1 | 0.08 | 0.08 | 0.01 | 0.921 |
| F × H (g/L) | 1 | 3.84 | 3.84 | 0.52 | 0.482 |
| G (mg/L) × H (g/L) | 1 | 50.48 | 50.48 | 6.86 | 0.02 |
| Error | 14 | 103.09 | 7.36 | – | – |
| Lack-of-Fit | 10 | 103.04 | 10.3 | 737.56 | 0 |
| Pure Error | 4 | 0.06 | 0.01 | – | – |
| Total | 28 | 4863.5 | – | – | – |
| Independent Parameter | Code | Unit | Levels | ||
|---|---|---|---|---|---|
| −1 | 0 | 1 | |||
| Reaction time | D | min | 30 | 60 | 90 |
| pH | F | – | 3 | 7 | 11 |
| Initial CBZ concentration | G | mg/L | 15 | 20 | 25 |
| Photocatalyst dose | H | g/L | 0.75 | 1 | 1.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaber, M.M.; Toghan, A.; Eldesoky, A.M.; Al-Hussain, S.A.; Masoud, E.M.; Shokry, H.; Samy, M.; Elkady, M. Sustainable Photocatalytic Treatment of Real Pharmaceutical Wastewater Using a Novel ZnO/MIP-202(Zr) Bio-MOF Hybrid Synthesized via a Green Approach. Catalysts 2025, 15, 1017. https://doi.org/10.3390/catal15111017
Gaber MM, Toghan A, Eldesoky AM, Al-Hussain SA, Masoud EM, Shokry H, Samy M, Elkady M. Sustainable Photocatalytic Treatment of Real Pharmaceutical Wastewater Using a Novel ZnO/MIP-202(Zr) Bio-MOF Hybrid Synthesized via a Green Approach. Catalysts. 2025; 15(11):1017. https://doi.org/10.3390/catal15111017
Chicago/Turabian StyleGaber, Mohamed Mohamed, Arafat Toghan, Ahmed M. Eldesoky, Sami A. Al-Hussain, Emad M. Masoud, Hassan Shokry, Mahmoud Samy, and Marwa Elkady. 2025. "Sustainable Photocatalytic Treatment of Real Pharmaceutical Wastewater Using a Novel ZnO/MIP-202(Zr) Bio-MOF Hybrid Synthesized via a Green Approach" Catalysts 15, no. 11: 1017. https://doi.org/10.3390/catal15111017
APA StyleGaber, M. M., Toghan, A., Eldesoky, A. M., Al-Hussain, S. A., Masoud, E. M., Shokry, H., Samy, M., & Elkady, M. (2025). Sustainable Photocatalytic Treatment of Real Pharmaceutical Wastewater Using a Novel ZnO/MIP-202(Zr) Bio-MOF Hybrid Synthesized via a Green Approach. Catalysts, 15(11), 1017. https://doi.org/10.3390/catal15111017










