Sustainable Biofuel Production from Sludge by Oleaginous Fungi: Effect of Process Variables on Lipid Accumulation
Abstract
1. Introduction
2. Results and Discussions
2.1. Physico-Chemical Characterisation of Wastewater Sludge
2.2. Isolation of Oleagenous Fungi
2.3. Screening of Oleagenous Fungi
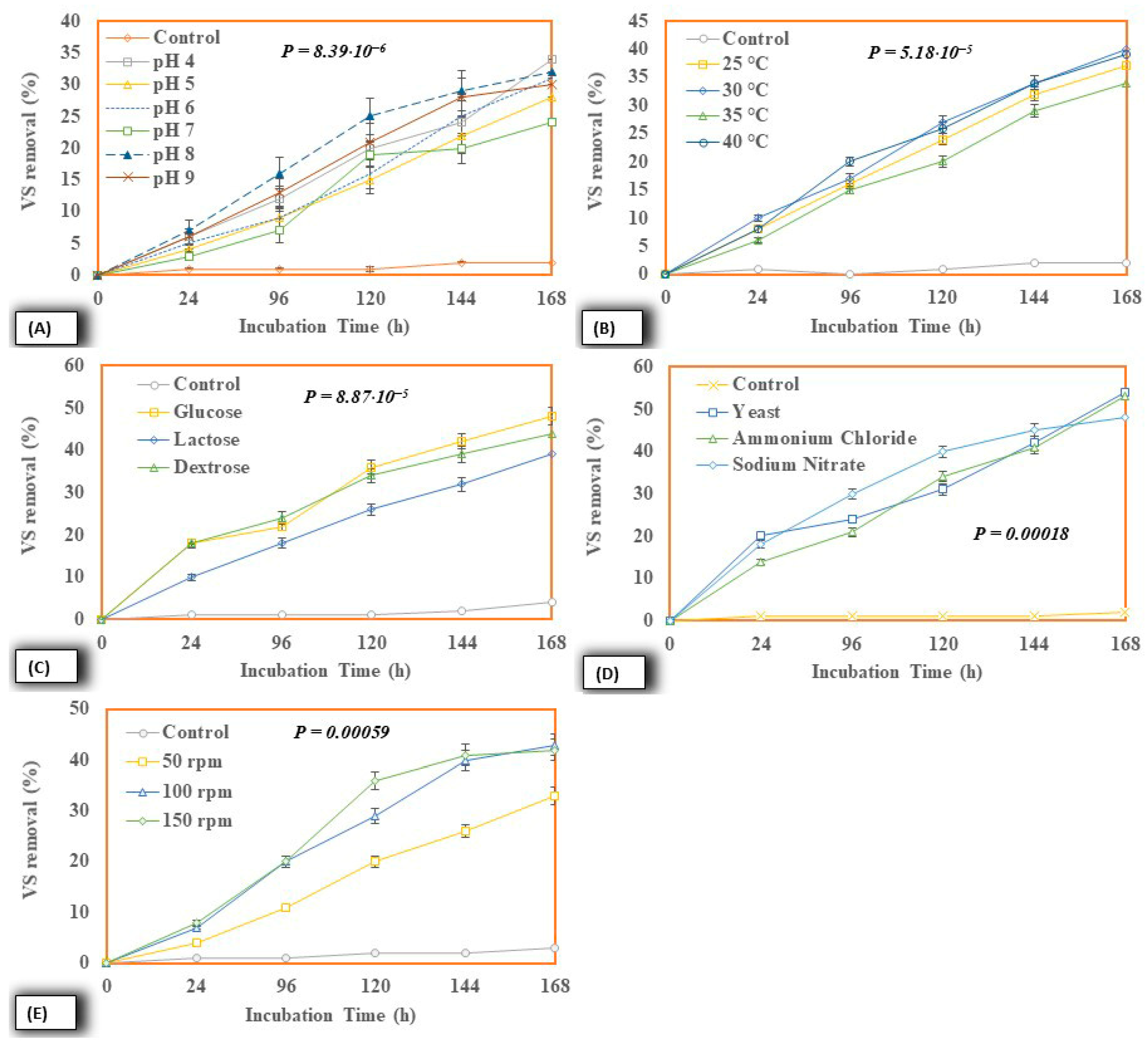
2.4. Effect of Different Process Variables
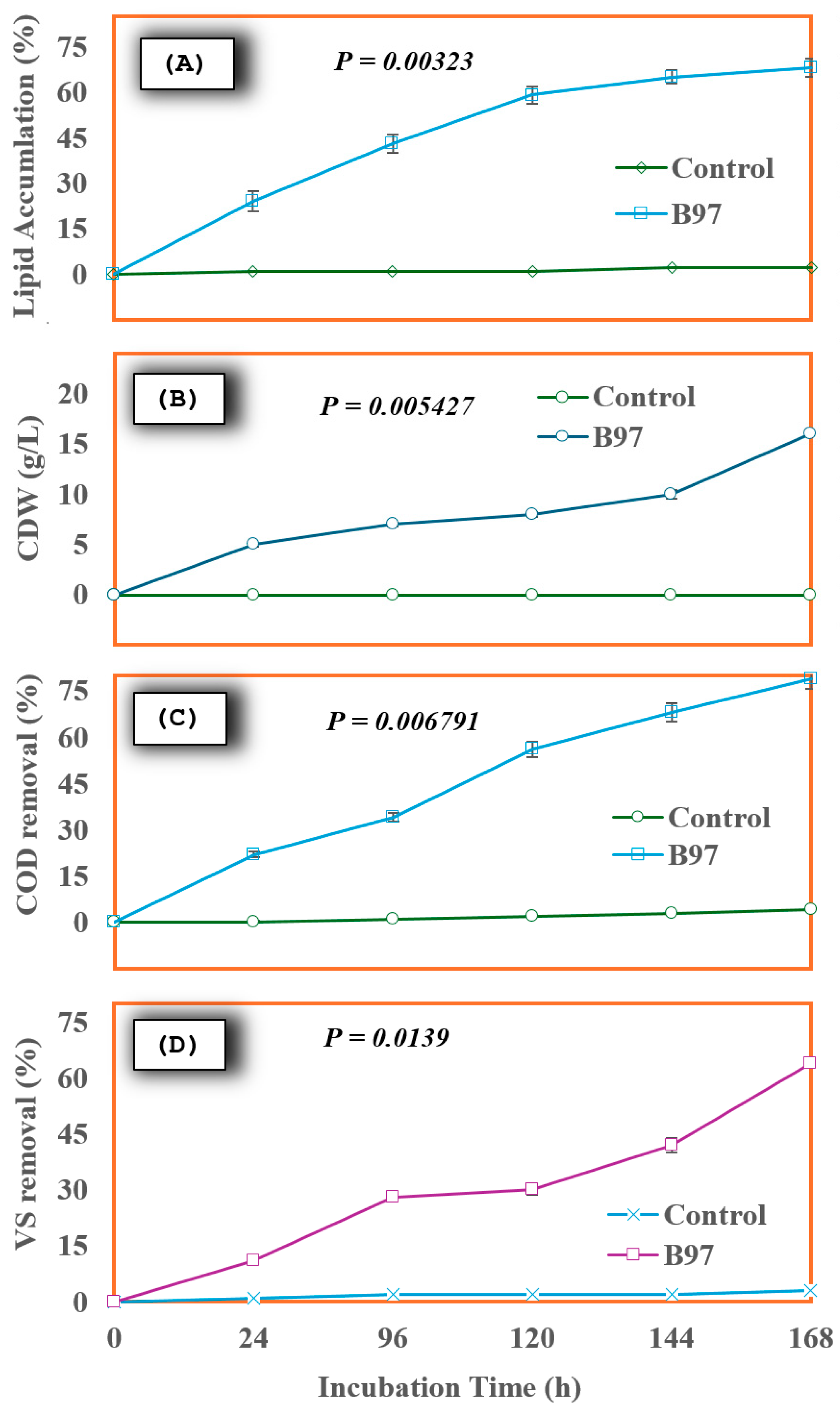
2.4.1. Effect on Lipid Accumulation Potential
2.4.2. Cell Dry Weight
2.4.3. COD Removal
2.4.4. VS Removal
2.5. Continuous-Shaking Aerobic Batch Reactor (CSABR) Performance
2.6. Lipid Profile Analysis Through FTIR Spectroscopy
2.7. Biodiesel Production (Transesterification of Extracted Lipids)
3. Methodology
3.1. Sampling of Wastewater Sludge and Physico-Chemical Characterization
3.2. Strain Isolation
3.3. Experimental Setup
3.4. Continuous-Shaking Aerobic Batch Reactor (CSABR)
3.5. Transesterification of Extracted Lipids
3.6. Analytical Procedures
3.6.1. Lipid Content
3.6.2. Lipid Analysis by FTIR Analysis
3.6.3. Post-Transesterified Lipid Analysis (FAMEs) by GC-MS
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Murtaza, G.; Zia, M.H. Wastewater Production, Treatment and Use in Pakistan; University of Agriculture: Faisalabad, Pakistan, 2012; p. 13. [Google Scholar]
- Manzoor, M.; Gul, I.; Iqrar, I.; Arshad, M. Solid Waste Management Practices in Pakistan; IGI Global Scientific Publishing: Hershey, PA, USA, 2020; pp. 248–269. [Google Scholar]
- Cavelius, P.; Engelhart-Straub, S.; Mehlmer, N.; Lercher, J.; Awad, D.; Brück, T. The Potential of Bio-fuels from First to Fourth Generation. PLoS Biol. 2023, 21, e3002063. [Google Scholar] [CrossRef]
- Hu, X.; Imran, M.; Wu, M.; Moon, H.C.; Liu, X. Alternative to Oil and Gas: Review of Economic Benefits and Potential of Wind Power in Pakistan. Math. Probl. Eng. 2020, 2020, e8884228. [Google Scholar] [CrossRef]
- Javed, M.S.; Raza, R.; Hassan, I.; Saeed, R.; Shaheen, N.; Iqbal, J.; Shaukat, S.F. The Energy Crisis in Pakistan: A Possible Solution via Biomass-Based Waste. J. Renew. Sustain. Energy 2016, 8, 043102. [Google Scholar] [CrossRef]
- Abdullah, B.; Muhammad, S.A.F.S.; Shokravi, Z.; Ismail, S.; Kassim, K.A.; Mahmood, A.N.; Aziz, M.M.A. Fourth Generation Biofuel: A Review on Risks and Mitigation Strategies. Renew. Sustain. Energy Rev. 2019, 107, 37–50. [Google Scholar] [CrossRef]
- Alsultan, A.G.; Asikin-Mijan, N.; Obeas, L.K.; Isalam, A.; Mansir, N.; Nassar, M.F.; Razali, S.Z.; Yunus, R.; Taufiq-Yap, Y.H. Biofuel and Biorefinery Technologies. In Biochar—Productive Technologies, Properties and Applications; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Lee, S.Y.; Sankaran, R.; Chew, K.W.; Tan, C.H.; Krishnamoorthy, R.; Chu, D.-T.; Show, P.-L. Waste to Bioenergy: A Review on the Recent Conversion Technologies. BMC Energy 2019, 1, 4. [Google Scholar] [CrossRef]
- More, T.T.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Potential Use of Filamentous Fungi for Wastewater Sludge Treatment. Bioresour. Technol. 2010, 101, 7691–7700. [Google Scholar] [CrossRef]
- Patel, A.; Karageorgou, D.; Rova, E.; Katapodis, P.; Rova, U.; Christakopoulos, P.; Matsakas, L. An Overview of Potential Oleaginous Microorganisms and Their Role in Biodiesel and Omega-3 Fatty Acid-Based Industries. Microorganisms 2020, 8, E434. [Google Scholar] [CrossRef] [PubMed]
- Li, S.L.; Lin, Q.; Li, X.R.; Xu, H.; Yang, Y.X.; Qiao, D.R.; Cao, Y. Biodiversity of the Oleaginous Microorganisms in Tibetan Plateau. Braz. J. Microbiol. 2012, 43, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Powers-Fletcher, M.V.; Kendall, B.A.; Griffin, A.T.; Hanson, K.E. Filamentous Fungi. Microbiol. Spectr. 2016, 4, 311–341. [Google Scholar] [CrossRef]
- Hashem, A.H.; Abu-Elreesh, G.; El-Sheikh, H.H.; Suleiman, W.B. Isolation, Identification, and Statistical Optimization of a Psychrotolerant Mucor racemosus for Sustainable Lipid Production. Biomass Convers. Biorefin. 2022, 13, 3415–3426. [Google Scholar] [CrossRef]
- Cieślik, B.M.; Namieśnik, J.; Konieczka, P. Review of Sewage Sludge Management: Standards, Regulations and Analytical Methods. J. Clean. Prod. 2015, 90, 1–15. [Google Scholar] [CrossRef]
- Płonka, I.; Kudlek, E.; Pieczykolan, B. Municipal Sewage Sludge Disposal in the Republic of Poland. Appl. Sci. 2025, 15, 3375. [Google Scholar] [CrossRef]
- Kacprzak, M.; Neczaj, E.; Fijałkowski, K.; Grobelak, A.; Grosser, A.; Worwag, M.; Rorat, A.; Brattebo, H.; Almås, A.; Singh, B.R. Sewage Sludge Disposal Strategies for Sustainable Development. Environ. Res. 2017, 156, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A.; Edris, G.; Alalayah, W.M. Sludge Production from Municipal Wastewater Treatment in Sewage Treatment Plant. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 999–1006. [Google Scholar] [CrossRef]
- Tsapekos, P.; Kougias, P.G.; Kuthiala, S.; Angelidaki, I. Co-Digestion and Model Simulations of Source Separated Municipal Organic Waste with Cattle Manure under Batch and Continuously Stirred Tank Reactors. Energy Convers. Manag. 2018, 159, 1–6. [Google Scholar] [CrossRef]
- Liang, S.; Yang, L.; Chen, H.; Yu, W.; Tao, S.; Yuan, S.; Xiao, K.; Hu, J.; Hou, H.; Liu, B.; et al. Phosphorus Recovery from Incinerated Sewage Sludge Ash (ISSA) and Reutilization of Residues for Sludge Pretreated by Different Conditioners. Resour. Conserv. Recycl. 2021, 169, 105524. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, X.; Zeng, J.; Chen, S. Feasibility of Filamentous Fungi for Biofuel Production Using Hydrolysate from Dilute Sulfuric Acid Pretreatment of Wheat Straw. Biotechnol. Biofuels 2012, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Mir-Tutusaus, J.A.; Parladé, E.; Llorca, M.; Villagrasa, M.; Barceló, D.; Rodriguez-Mozaz, S.; Martinez-Alonso, M.; Gaju, N.; Caminal, G.; Sarrà, M. Pharmaceuticals Removal and Microbial Community Assessment in a Continuous Fungal Treatment of Non-Sterile Real Hospital Wastewater after a Coagulation-Flocculation Pretreatment. Water Res. 2017, 116, 65–75. [Google Scholar] [CrossRef]
- Kumar, R.; Gaurav, K.; Kaur, R.; Bajaj, B.K. Enhanced Lipid Production by Oleaginous Yeast Rhodosporidium toruloides by Utilizing Hydrolysates of Sunflower Stalks Pretreated by Microwave-Assisted Dilute Acid or Alkali. Energy Convers. Manag. 2021, 235, 113981. [Google Scholar]
- Banerjee, A.; Sharma, R.; Chisti, Y.; Banerjee, U.C. Botryococcus braunii: A Renewable Source of Hydrocarbons and Other Chemicals. Crit. Rev. Biotechnol. 2002, 22, 245–279. [Google Scholar] [CrossRef]
- Takeda, H. Classification of Botryococcus braunii and Related Species. Prog. Ind. Microbiol. 1991, 29, 49–72. [Google Scholar]
- Wu, Z.; Zhu, Y.; Huang, W.; Zhang, C.; Li, T.; Zhang, Y.; Li, A. Evaluation of Flocculation Induced by pH Increase for Harvesting Microalgae and Reuse of Flocculated Medium. Bioresour. Technol. 2012, 110, 496–502. [Google Scholar] [CrossRef]
- Abeysiriwardana-Arachchige, I.S.A.; Seneviratne, S.I.; Ariyaratne, W.K.H. Biomass-Based Energy in Sri Lanka: Prospects and Challenges. Energy Rep. 2021, 7, 163–171. [Google Scholar]
- Mori, A.; Kamahara, H.; Yoshitake, T.; Takemura, Y. Evaluation of Energy Potential from Microalgae Cultivated in Municipal Wastewater. Appl. Energy 2015, 160, 627–636. [Google Scholar]
- Guldhe, A.; Singh, B.; Rawat, I.; Ramluckan, K.; Bux, F. Efficacy of Microalgae for Industrial Wastewater Treatment: A Review on Operating Conditions, Treatment Efficiency and Biomass Productivity. Rev. Environ. Sci. Biotechnol. 2017, 16, 717–726. [Google Scholar]
- Gonçalves, A.L.; Pires, J.C.M.; Simões, M. A Review on the Use of Microalgal Consortia for Wastewater Treatment. Algal Res. 2017, 24, 403–415. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Chen, F. Growing Phototrophic Cells without Light. Biotechnol. Lett. 2006, 28, 607–616. [Google Scholar] [CrossRef]
- Das, D.M.; Rahman, M.H.; Arif, M.; Chowdhury, R. Prospect of Microalgal Biofuel Production Using Wastewater in Bangladesh: A Review. Int. J. Renew. Energy Res. 2018, 8, 1115–1126. [Google Scholar]
- Chisti, Y. Constraints to Commercialization of Algal Fuels. J. Biotechnol. 2013, 167, 201–214. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The Promising Future of Microalgae: Current Status, Challenges, and Optimization of a Sustainable and Renewable Industry for Biofuels, Feed, and Other Products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.E.; Senol, F.S.; Ercetin, T.; Kahraman, A.; Celep, F.; Akaydin, G.; Sener, B. Assessment of Antioxidant and Anticholinesterase Activities of Selected Turkish Salvia Species with Their Total Phenol and Flavonoid Contents. Ind. Crops Prod. 2013, 41, 21–30. [Google Scholar] [CrossRef]
- Tan, X.B.; Lam, M.K.; Uemura, Y.; Lim, J.W.; Wong, C.Y.; Lee, K.T. Cultivation of Microalgae for Biodiesel Production: A Review on Upstream and Downstream Processing. Chin. J. Chem. Eng. 2018, 26, 17–30. [Google Scholar] [CrossRef]
- Taleb, A.; Kandiah, M.; Tan, C.H.; Lim, J.W.; Ho, Y.C.; Ma, N.L.; Peng, W.; Show, P.L. Treatment of Palm Oil Mill Effluent Using Microalgae Cultivated in Palm Oil Mill Final Discharge. J. Water Process Eng. 2020, 33, 101055. [Google Scholar]
- Sathish, A.; Sims, R.C. Biodiesel from Mixed Culture Algae via a Wet Lipid Extraction Procedure. Bioresour. Technol. 2012, 118, 643–647. [Google Scholar] [CrossRef]
- Johnson, M.B.; Wen, Z. Development of an Attached Microalgal Growth System for Biofuel Production. Appl. Microbiol. Biotechnol. 2010, 85, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Kumar, R.R.; Singh, R.; Singh, R. Microalgal Consortium for Wastewater Treatment and Biomass Production. Int. J. Environ. Sci. Technol. 2014, 11, 253–260. [Google Scholar]
- Meng, X.; Yang, J.; Xu, X.; Zhang, L.; Nie, Q.; Xian, M. Biodiesel Production from Oleaginous Microorganisms. Renew. Energy 2009, 34, 1–5. [Google Scholar] [CrossRef]
- Sun, X.M.; Ren, L.J.; Bi, Z.Q.; Ji, X.J.; Zhao, Q.Y.; Huang, H. Adaptive Evolution of Microalgae Schizochytrium sp. under High Salinity Stress to Alleviate Oxidative Damage and Improve Lipid Biosynthesis. Bioresour. Technol. 2018, 267, 438–444. [Google Scholar] [CrossRef]
- Liu, J.; Huang, J.; Sun, Z.; Zhong, Y.; Jiang, Y.; Chen, F. Differential Lipid and Fatty Acid Profiles of Photoautotrophic and Heterotrophic Chlorella zofingiensis: Assessment of Algal Oils for Biodiesel Production. Bioresour. Technol. 2011, 102, 106–110. [Google Scholar] [CrossRef]
- Bellou, S.; Baeshen, M.N.; Elazzazy, A.M.; Aggeli, D.; Sayegh, F.; Aggelis, G. Microalgal Lipids Biochemistry and Biotechnological Perspectives. Biotechnol. Adv. 2014, 32, 1476–1493. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Lam, M.K.; Uemura, Y.; Lim, J.W.; Lee, K.T. Development of an Integrated Process for Sustainable Production of Biodiesel from Microalgae Cultivated in Palm Oil Mill Effluent (POME). Appl. Energy 2019, 241, 174–183. [Google Scholar]
- Wang, H.; Ji, B.; Wang, J.; Li, Y.; Liu, Y.; Wang, F.; Zhao, H. Pilot-Scale Extraction of Lipids and Proteins from Wet Microalgae with an Ionic Liquid. Environ. Technol. 2020, 41, 2731–2740. [Google Scholar]
- Kandasamy, S.; Arumugam, R.; Sundararaju, P.; Purushothaman, M.; Arunkumar, K. Synthesis of Fatty Acid Methyl Ester from Microalgae Using Marine Bacterium Pseudoalteromonas sp. as Whole-Cell Biocatalyst. Algal Res. 2021, 54, 102218. [Google Scholar]
- Mutanda, T.; Ramesh, D.; Karthikeyan, S.; Kumari, S.; Anandraj, A.; Bux, F. Bioprospecting for Hyper-Lipid Producing Microalgal Strains for Sustainable Biofuel Production. Bioresour. Technol. 2011, 102, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, C.; Mitra, M.; Bhayani, K.; Bharadwaj, S.V.V.; Ghosh, T.; Dubey, S.; Mishra, S. Abiotic Stresses as Tools for Metabolites in Microalgae. Bioresour. Technol. 2017, 244, 1216–1226. [Google Scholar] [CrossRef]
- Chang, H.X.; Huang, Y.; Fu, Q.; Liao, Q.; Zhu, X. Downstream Processing of Microalgae for Pigments, Protein and Carbohydrate in Industrial Application: A Review. Bioresour. Technol. 2018, 262, 89–97. [Google Scholar]
- Suali, E.; Sarbatly, R. Conversion of Microalgae to Biofuel. Renew. Sustain. Energy Rev. 2012, 16, 4316–4342. [Google Scholar] [CrossRef]
- Malibari, R.; Alyuz, B.; Aljundi, I.H.; Alqahtani, N.; Akram, F.; Alomar, A.; Alshehri, S.; Karaca, F. Wastewater Treatment Using Microalgae: A Review. Environ. Res. 2022, 203, 111762. [Google Scholar]
- Mishra, A.; Medhi, K.; Malaviya, P. Bioremediation of Heavy Metal-Contaminated Soil Using Microalgae: Recent Advances and Future Outlook. Appl. Sci. 2021, 11, 10585. [Google Scholar]
- Hossain, M.F.; Hasan, M.R.; Islam, M.S.; Rahman, M.S. Bioremediation of Industrial Effluents by Microalgae: Current Status and Future Prospects. J. Environ. Manage. 2020, 276, 111321. [Google Scholar]
- Rajkumar, R.; Yaakob, Z.; Takriff, M.S. Potential of Microalgae and Bioremediation of Wastewater. Environ. Eng. Res. 2014, 19, 1–8. [Google Scholar]
- Olguín, E.J. Dual Purpose Microalgae-Bacteria-Based Systems That Treat Wastewater and Produce Biodiesel and Chemical Products within a Biorefinery. Biotechnol. Adv. 2012, 30, 1031–1046. [Google Scholar] [CrossRef]
- Muñoz, R.; Guieysse, B. Algal–Bacterial Processes for the Treatment of Hazardous Contaminants: A Review. Water Res. 2006, 40, 2799–2815. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and Wastewater Treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Christenson, L.; Sims, R. Production and Harvesting of Microalgae for Wastewater Treatment, Biofuels, and Bioproducts. Biotechnol. Adv. 2011, 29, 686–702. [Google Scholar] [CrossRef]
- Kumar, K.S.; Dahms, H.U.; Won, E.J.; Lee, J.S.; Shin, K.H. Microalgae—A Promising Tool for Heavy Metal Remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Min, M.; Li, Y.; Chen, P.; Chen, Y.; Liu, Y.; Wang, Y.; Ruan, R. Cultivation of Green Microalgae in Dairy Wastewater for Nutrient Removal and Biomass Production. Appl. Biochem. Biotechnol. 2010, 160, 1674–1684. [Google Scholar]
- Markou, G.; Georgakakis, D. Cultivation of Filamentous Cyanobacteria (Cyanospira sp.) in Agro-Industrial Wastes and Wastewaters: Growth and Biomass Quality Evaluation. Biomass Bioenergy 2011, 35, 4396–4405. [Google Scholar]
- Chinnasamy, S.; Bhatnagar, A.; Hunt, R.W.; Das, K.C. Microalgae Cultivation in a Wastewater Dominated by Carpet Mill Effluents for Biofuel Applications. Bioresour. Technol. 2010, 101, 3097–3105. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.F.; Chen, P.; Min, M.; Zhou, W.; Martinez, B.; Zhu, J.; Ruan, R. Characterization of a Microalga Chlorella sp. Well Adapted to Highly Concentrated Municipal Wastewater for Nutrient Removal and Biodiesel Production. Bioresour. Technol. 2011, 102, 5138–5144. [Google Scholar] [CrossRef]
- Sydney, E.B.; de Carvalho, J.C.; Sydney, A.C.N.; Soccol, C.R. Microalgae for Biodiesel Production and Other Applications: A Review. Renew. Sustain. Energy Rev. 2011, 15, 303–310. [Google Scholar]
- Li, Y.; Horsman, M.; Wu, N.; Lan, C.Q.; Dubois-Calero, N. Biofuels from Microalgae. Biotechnol. Prog. 2008, 24, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L.; Owende, P. Biofuels from Microalgae—A Review of Technologies for Production, Processing, and Extractions of Biofuels and Co-Products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
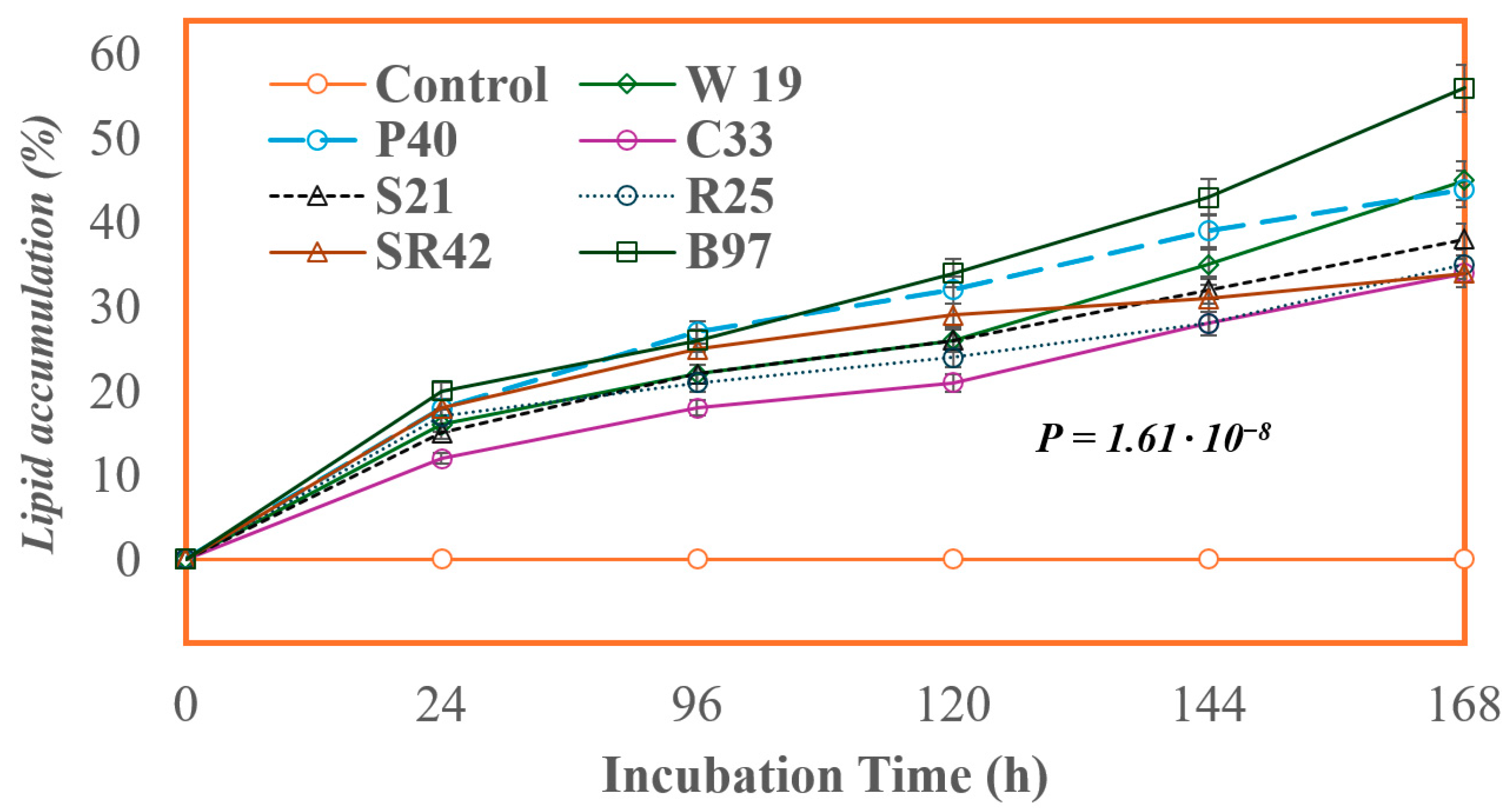
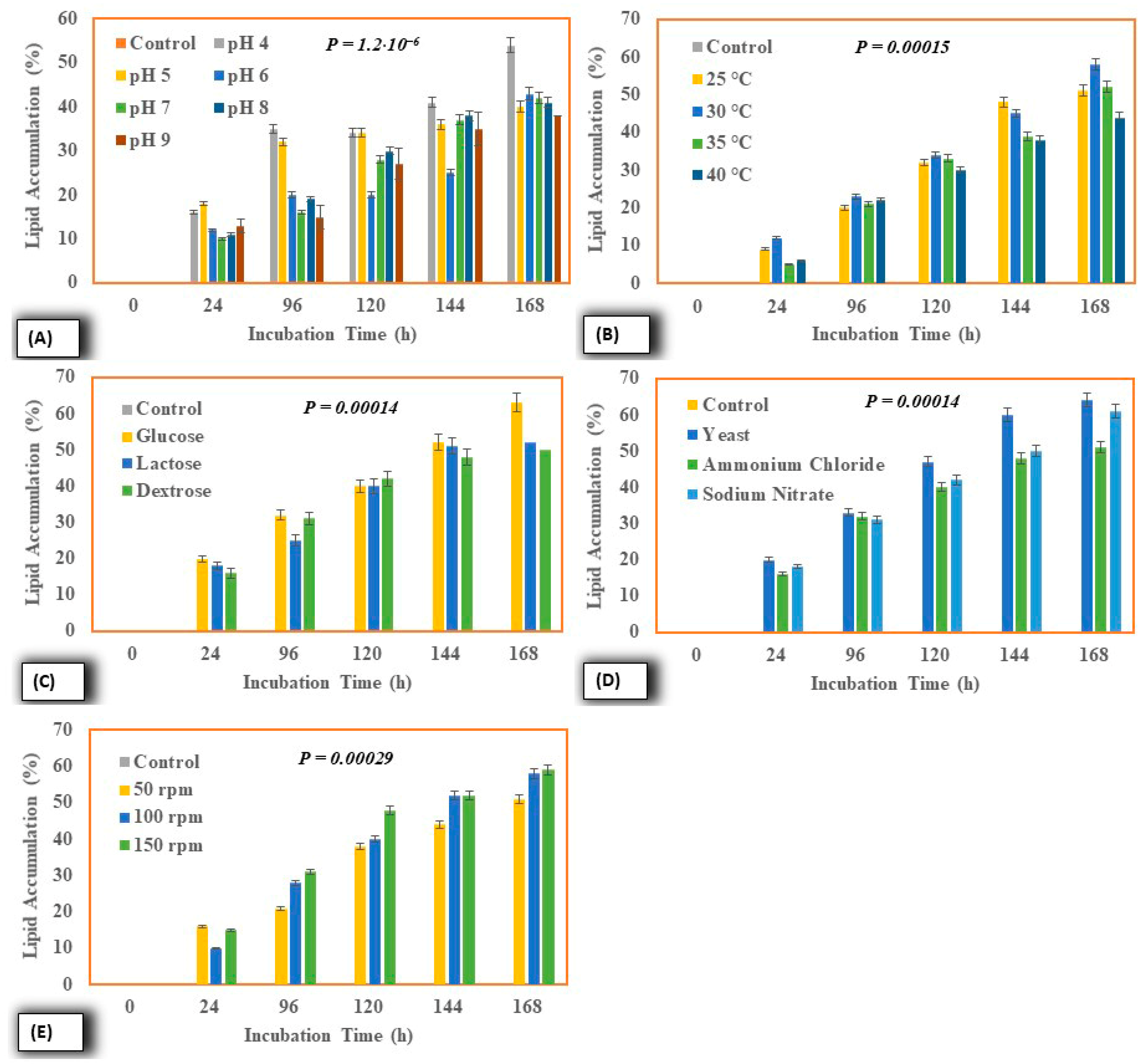
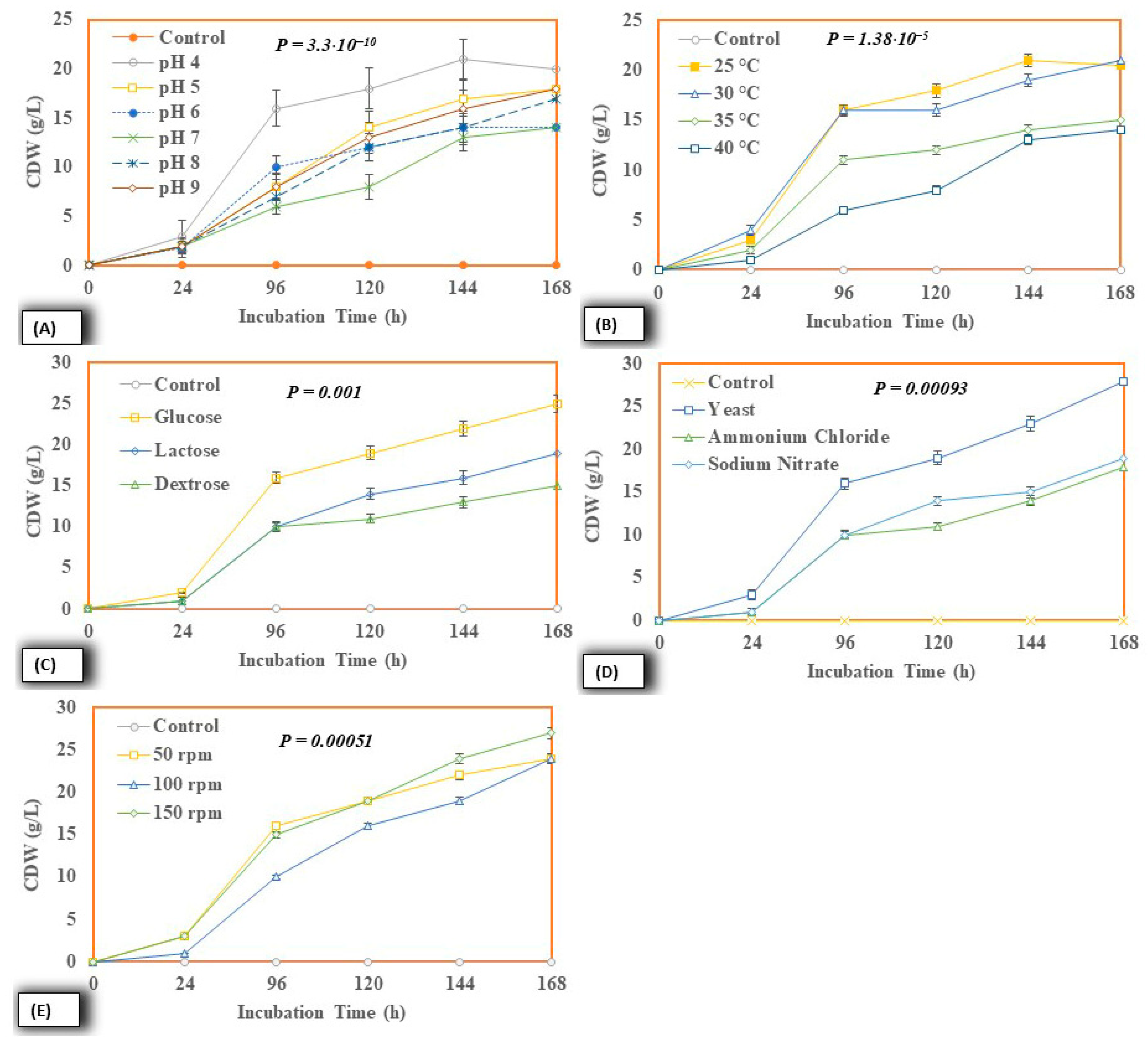
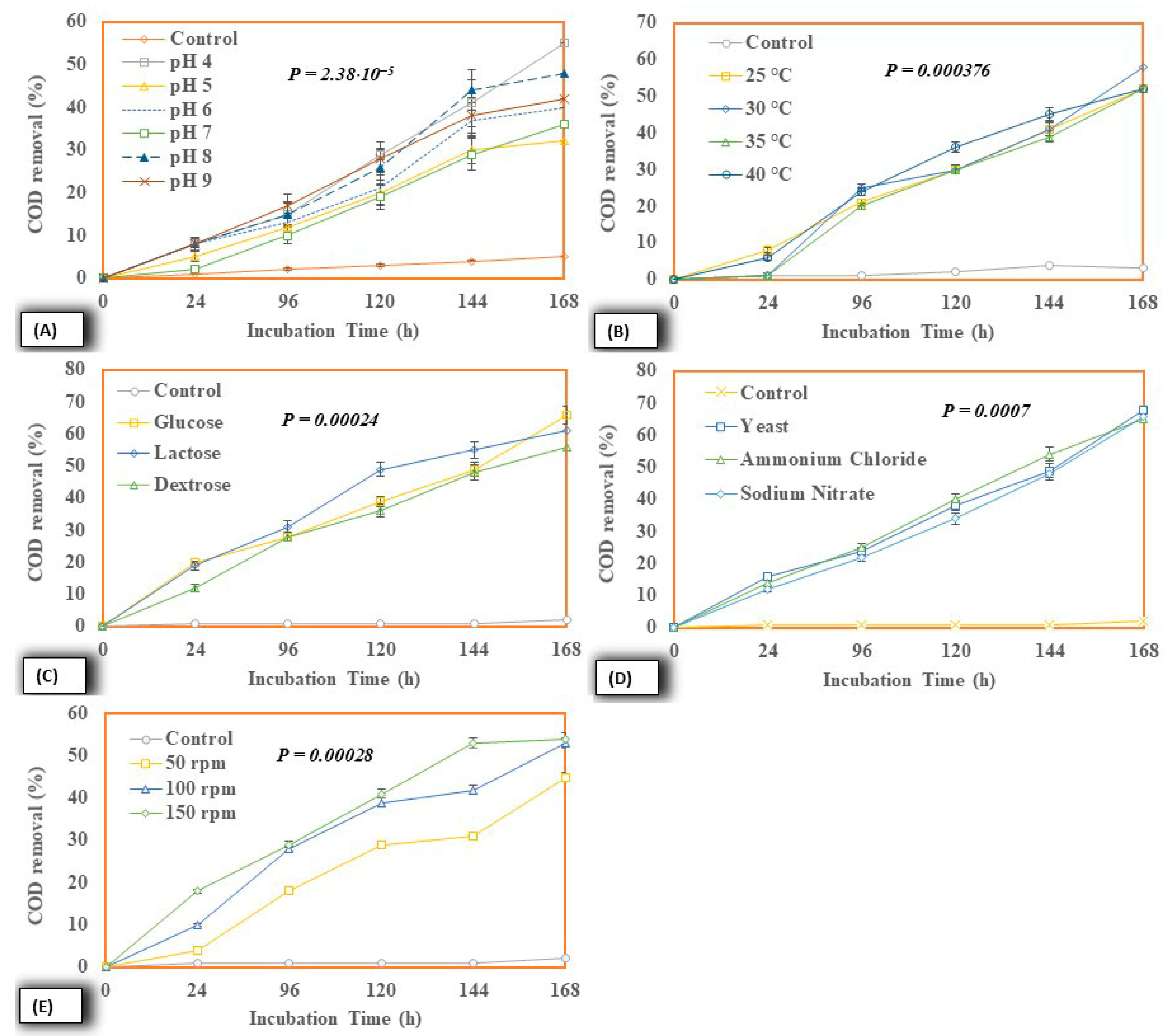

| S. No. | Liquid Sludge | Results |
|---|---|---|
| 1. | Total dissolved solids (mg/L) | 815 |
| 2. | Total suspended solids (mg/L) | 300 |
| 3. | Total solids % | 3.5 |
| 4. | Fixed solids % | 0.1 |
| 5. | Volatile solids % | 3.4 |
| 6. | COD (mg/L) | 6400 |
| 7. | Total nitrogen % | 0.19 |
| 8. | Total carbon % | 52.52 |
| 9. | pH | 6.64 |
| 10. | EC (µs/cm) | 700 |
| 11. | Color | Light brownish-grey that turns brown over time |
| 12. | Moisture % | 97 |
| S. No. | Metal | Concentration (mg/L) |
|---|---|---|
| 1. | Arsenic (As) | 0.01 |
| 2. | Cadmium (Cd) | 16.73 |
| 3. | Cobalt (Co) | 0.2 |
| 4. | Chromium (Cr) | 0.01 |
| 5. | Copper (Cu) | 0.72 |
| 6. | Potassium (K) | 21.21 |
| 7. | Nikel (Ni) | 0.01 |
| 8. | Lead (Pb) | 0.02 |
| Source | Label | Form | Color | Elevation | Margin | Possible Genera |
|---|---|---|---|---|---|---|
| Bread | B97 | Filamentous Irregular Rhizoid | White, brown, black/grey | Umbonate Convex Fluffy | Diffused/spreading outwards | Rhizopus (Rhizopus oryzae) |
| Bread | P40 | Filamentous Irregular Rhizoid | White, green, brown, black | Umbonate Convex | Filiform Entire | Mucor |
| Citrus | C33 | Filamentous Irregular Circular | White, black, brown, green | Raised Convex | Filiform Lobate Undulate | Aspergillus/Penicillium |
| Apple | W19 | Irregular | White, brown | Flat Convex | Filiform | Alternaria/Cladosporium |
| Soil | S21 | Filamentous | Green | Raised | Filiform | Mucor |
| Rocks | R25 | Filamentous Rhizoid | Green | Raised | Filiform | Rhizopus/Mucor |
| Sweets | SR42 | Filamentous Irregular Rhizoid | Dark green, white | Convex | Filiform | Aspergillus/Penicillium |
| Area % | FAMS (Esters) Formed | Possible Corresponding Lipid | No of Carbon Atoms: Double Bond | Compound Category |
|---|---|---|---|---|
| 0.22 | Methyl tetradecanoate | Myristic acid | C14:0 | SFA |
| 7.67 | Hexadecanoic acid, methyl ester | Palmitic acid | C16:0 | SFA |
| Pentadecanoic acid, 14-methyl, methyl ester | 14-Methylpentadecanoic acid | C15:0 | SFA (branched) | |
| 0.11 | Cis-10-heptadecenoic acid, methyl ester | Cis-10-heptadecenoic acid | C17:1 | MUFA |
| 38.69 | 9-Octadecenoic acid, methyl ester | Oleic acid | C18:1 | MUFA |
| 1.22 | Methyl stearate | Stearic acid | C19:0 | SFA |
| 4.99 | Oleic acid | Oleic acid | C18:0 | MUFA |
| 1.43 | Linoleic acid ethyl ester | - | C18:2 | PUFA |
| 1.63 | Ethyl oleate | - | C20:2 | UFA |
| 0.41 | 11,14-Eicosadienoic acid, methyl ester | Arachidic acid | C20:2 | PUFA |
| 9.00 | 13-docosenoic acid, methyl ester | C23:2 | MUFA | |
| 5.71 | Erucic acid | Erucic acid | C22:1 | MUFA |
| 0.63 | Tetracosanoic acid, methyl ester | C24:0 | SFA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, H.; Anjum, M.; Noor, B.; Qadeer, S.; Nawaz, R.; Khalid, A.; Saleem, A.R.; Kabeer, B.; Idris, A.M.; Sohail, M.T.; et al. Sustainable Biofuel Production from Sludge by Oleaginous Fungi: Effect of Process Variables on Lipid Accumulation. Catalysts 2025, 15, 1009. https://doi.org/10.3390/catal15111009
Ullah H, Anjum M, Noor B, Qadeer S, Nawaz R, Khalid A, Saleem AR, Kabeer B, Idris AM, Sohail MT, et al. Sustainable Biofuel Production from Sludge by Oleaginous Fungi: Effect of Process Variables on Lipid Accumulation. Catalysts. 2025; 15(11):1009. https://doi.org/10.3390/catal15111009
Chicago/Turabian StyleUllah, Habib, Muzammil Anjum, Bushra Noor, Samia Qadeer, Rab Nawaz, Azeem Khalid, Aansa Rukaya Saleem, Bilal Kabeer, Abubakr M. Idris, Muhammad Tayyab Sohail, and et al. 2025. "Sustainable Biofuel Production from Sludge by Oleaginous Fungi: Effect of Process Variables on Lipid Accumulation" Catalysts 15, no. 11: 1009. https://doi.org/10.3390/catal15111009
APA StyleUllah, H., Anjum, M., Noor, B., Qadeer, S., Nawaz, R., Khalid, A., Saleem, A. R., Kabeer, B., Idris, A. M., Sohail, M. T., & Rao, Z. (2025). Sustainable Biofuel Production from Sludge by Oleaginous Fungi: Effect of Process Variables on Lipid Accumulation. Catalysts, 15(11), 1009. https://doi.org/10.3390/catal15111009







