1. Introduction
The decline in conventional oil reserves has intensified the need for efficient unconventional resource methods [
1]. Heavy and extra-heavy crude oils represent significant global deposits. However, their high viscosity and asphaltene content complicate extraction and pose environmental challenges [
2,
3,
4,
5]. Our present study examines heavy oil from the Ashalcha field (Russia) [
6].
Heavy oil exploitation requires enhanced oil recovery (EOR) techniques. Thermal EOR (ThEOR) is particularly effective for viscosity reduction [
7,
8,
9,
10,
11,
12]. Among ThEOR methods, in situ combustion (ISC) shows high promise due to its energy efficiency. ISC can break down heavy fractions such as asphaltenes and resins [
13,
14,
15]. ISC involves injecting an oxidizing gas (e.g., air) to create a propagating combustion front in the reservoir [
16]. This process relies on complex reactions and physical mechanisms. These include hydrocarbon oxidation and cracking, front displacement, gravity drainage, and fluid miscibility. The process also involves distillation of light hydrocarbons and formation of steam and hot water as secondary recovery agents. Oxidation occurs in three key regions: low-temperature oxidation (LTO, ~150–350 °C), fuel deposition (FD), and high-temperature oxidation (HTO, >350 °C) [
14,
16].
In the LTO region, molecular oxygen reacts with hydrocarbon components to form intermediate oxygenated species, including alcohols, aldehydes, ketones, carboxylic acids, and peroxides, while also producing some gaseous products such as CO, CO
2, CH
4, and C
2H
6 [
17]. In the FD region, these oxygenated products are further fully decomposed during pyrolysis to form deposited coke, which becomes a fuel in the HTO region. The HTO regime primarily involves the complete oxidation of fuel components (particularly coke) to carbon oxides and water, releasing substantial thermal energy [
18,
19]. This coke combustion process is considered the key reaction that results in producing carbon dioxide and is essential for the self-sustaining combustion front and propagation process.
ISC has favorable theoretical energy balances and utilizes only a minor proportion of in situ hydrocarbons for combustion. This establishes it as a cost-effective thermal energy delivery system. However, extensive commercial implementation has been limited by technical challenges [
20]. These challenges include combustion front instability, ignition difficulties, low combustion efficiency, and premature oxidant breakthrough. These issues have restricted successful field applications despite numerous pilot tests conducted since the conceptual development of ISC in the early 20th century. To address these limitations, research has increasingly focused on catalytic approaches, particularly transition metal catalysts extensively used throughout petroleum processing due to their unique electronic configurations and versatile redox properties that can significantly enhance oxidation kinetics and selectivity [
21,
22].
Heavy oil recovery catalysts can be classified into several categories: water-soluble, oil-soluble transition metal, amphiphilic, mineral-based/zeolitic, solid super acidic, and dispersed metal oxide nanoparticles [
23,
24,
25]. Oil-soluble organometallic catalysts show particularly promising results. They distribute homogeneously within the oil phase and maintain direct contact with hydrocarbon molecules. Water-soluble catalysts and dispersed metal oxide nanoparticles are effective in some aspects [
26]. However, they have drawbacks, including reduced ignition probability and pore blockage in oil wells. These issues arise from their low solubility and limited distribution in heavy oil environments. Ramirez-Garnica et al. reported significant improvements in combustion front propagation velocity and oil production efficiency using iron-, nickel-, molybdenum-, and cobalt-based organometallic precursors during Gulf-Mexican heavy oil combustion experiments [
27]. Similarly, our previous investigations demonstrated that manganese-based catalysts [
28,
29] substantially enhanced oxidation reaction rates and reduced activation energy requirements during thermal analysis of heavy oil. These catalysts showed particular efficacy in accelerating LTO reactions and promoting fuel deposition. However, challenges persist in optimizing catalyst performance in the HTO regime, which is critical for combustion front stability and propagation [
6]. This gap presents an opportunity to investigate alternative transition metal catalysts, particularly iron-based compounds, which may offer enhanced catalytic activity in this critical temperature range.
The selection of appropriate catalysts for ISC processes requires careful consideration of their efficiency, environmental compatibility, and economic viability. Recent research indicates that organometallic-ligated catalyst complexes exhibit advantageous characteristics, including thermal stability during decomposition, which explains our focus on properties more relevant to the iron acetylacetonate catalyst being studied in the present work, including cost-effectiveness, and excellent dispersibility in oil matrices [
30,
31]. Among transition metals, iron presents particularly compelling advantages for heavy oil oxidation, as its ability to readily cycle between Fe
2+ and Fe
3+ oxidation states facilitates electron transfer during oxidation reactions, while the oxide forms magnetite, demonstrate remarkable thermal stability at combustion temperatures that show superior activity in breaking carbon-carbon and carbon-hydrogen bonds, which is essential for complete hydrocarbon combustion [
32,
33].
In this study, we investigate the application of iron acetylacetonate (Fe(acac)
3) as a catalyst for ISC processes in heavy oil recovery. Iron acetylacetonate represents an ideal candidate compared to previously reported iron-based catalysts such as tallates [
34] and, nanoparticles [
24,
25]. It offers several advantages: remarkable oil solubility [
35], controlled thermal decomposition profile, and the ability to generate highly active iron oxide species under oxidative conditions [
36]. The catalyst forms active magnetite in situ at a specific temperature (~360 °C). This ensures that the catalyst is active precisely where and when it is needed to target combustion reactions critical for front stability.
This research addresses the identified gap in HTO catalysis through three specific objectives. First, we aim to quantify the influence of Fe(acac)3 on the kinetic parameters (activation energy and pre-exponential factors) of both LTO and HTO regions during heavy oil oxidation. Second, we will characterize the structural and morphological evolution of iron species during thermal decomposition to identify the catalytically active phases responsible for enhanced oxidation. Third, we seek to establish mechanistic relationships between the iron oxide phases formed and their specific catalytic effects on oxidation pathways, with particular focus on HTO enhancement. By revealing how Fe(acac)3 transforms oxidation pathways and enhances combustion, we aim to advance more efficient, cost-effective, and environmentally friendly methods for heavy oil recovery through catalytically improved in situ combustion.
2. Results and Discussions
2.1. Thermal Behavior of Heavy Oil Oxidation: Catalytic Vs. Non-Catalytic Processes
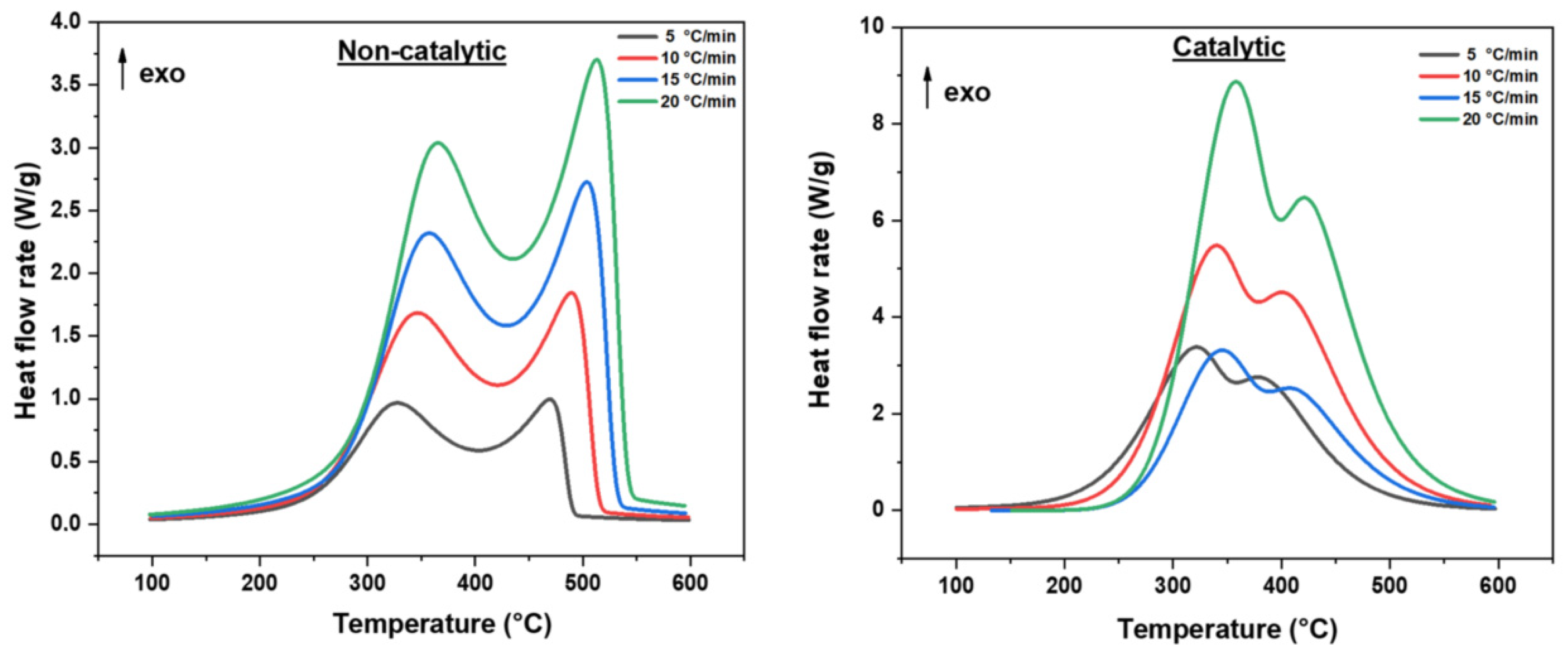
Figure 1 presents differential scanning calorimetry (DSC) profiles of heavy oil oxidation with and without iron acetylacetonate (III) catalyst at various heating rates (5, 10, 15, and 20 °C/min). The profiles show significant differences in oxidation behavior, particularly in the high-temperature oxidation (HTO) region.
Both catalytic and non-catalytic systems exhibit the characteristic two-stage oxidation process of heavy oils, with distinct exothermic peaks as shown in
Table 1.
However, the introduction of iron acetylacetonate (III) causes dramatic changes in the HTO region, which is critical for combustion front stabilization in practical applications.
In the catalytic system, this peak appears at 400–450 °C, representing a shift to lower temperatures across all heating rates. This temperature shift suggests that iron acetylacetonate (III) reduces the energy barrier for complete combustion reactions, facilitating earlier initiation of the HTO stage. Such catalytic activity is particularly beneficial for combustion front propagation, as it enables more efficient fuel consumption at lower temperatures [
37].
The HTO peak morphology in the catalytic system also differs significantly. It appears broader and more pronounced relative to the LTO peak. This indicates a more sustained and robust combustion process. Such behavior is advantageous for front stability because it suggests prolonged reactions rather than short, intense oxidation bursts.
Furthermore, the response to increasing heating rates is more dramatic in the catalytic system’s HTO region. As heating rates increase from 5 to 20 °C/min, the HTO peak temperature increases more substantially in the catalytic system than in the non-catalytic one, suggesting that the catalytic effect becomes more pronounced under more rapid heating conditions. This heating rate sensitivity could have important implications for controlling combustion front propagation rates in field applications. The enhanced HTO behavior observed in the catalytic system strongly indicates that iron acetylacetonate (III) specifically targets the complete combustion mechanisms that dominate the HTO region. This selective catalytic effect on HTO reactions is particularly valuable for combustion processes where complete fuel utilization and stable combustion fronts are critical for operational success.
In the subsequent sections, we will further analyze this preferential catalytic effect on the HTO region through XRPD analysis of the catalyst at different temperatures and thermogravimetric data, providing mechanistic insights into how the iron species evolve during the oxidation process to specifically enhance HTO reactions.
2.2. Structural Characterization of Catalyst via XRPD and TG-DTG
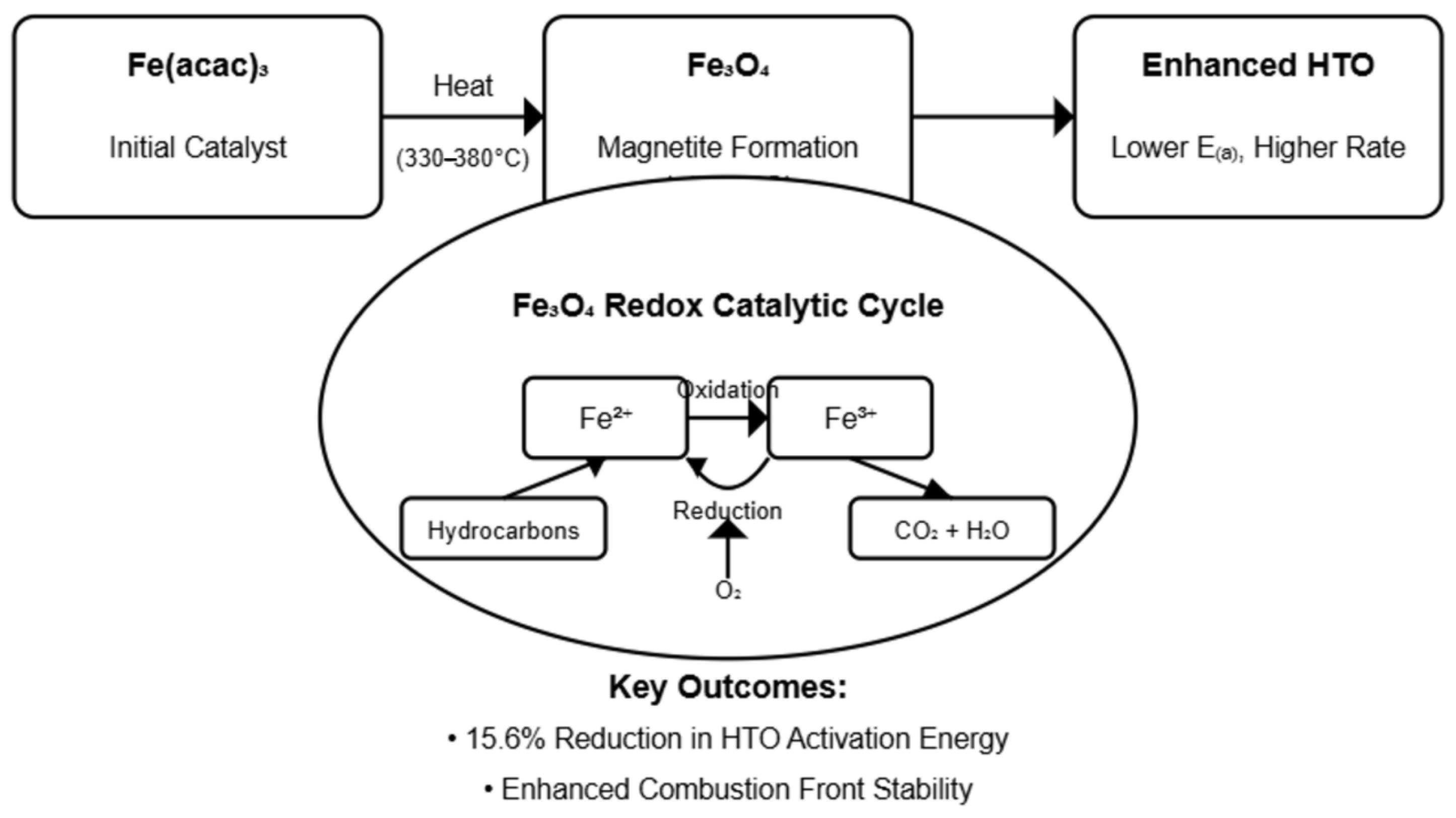
The thermal decomposition of iron (III) acetylacetonate was investigated using TG-DTG analysis, revealing a critical transformation in the catalyst structure that strategically precedes the HTO region of heavy oil oxidation (
Figure 2). A 15.1% mass loss occurs between 330 and 380 °C, with a prominent DTG peak at 360 °C. This represents the final decomposition stage that determines subsequent catalytic activity. It corresponds to the decomposition of remaining organic ligands and formation of iron oxide species (XRPD analysis confirms this transformation). The previous mass losses (27.4% and 37.0%) observed at lower temperatures are associated with the initial removal of coordinated and non-coordinated acetylacetonate ligands.
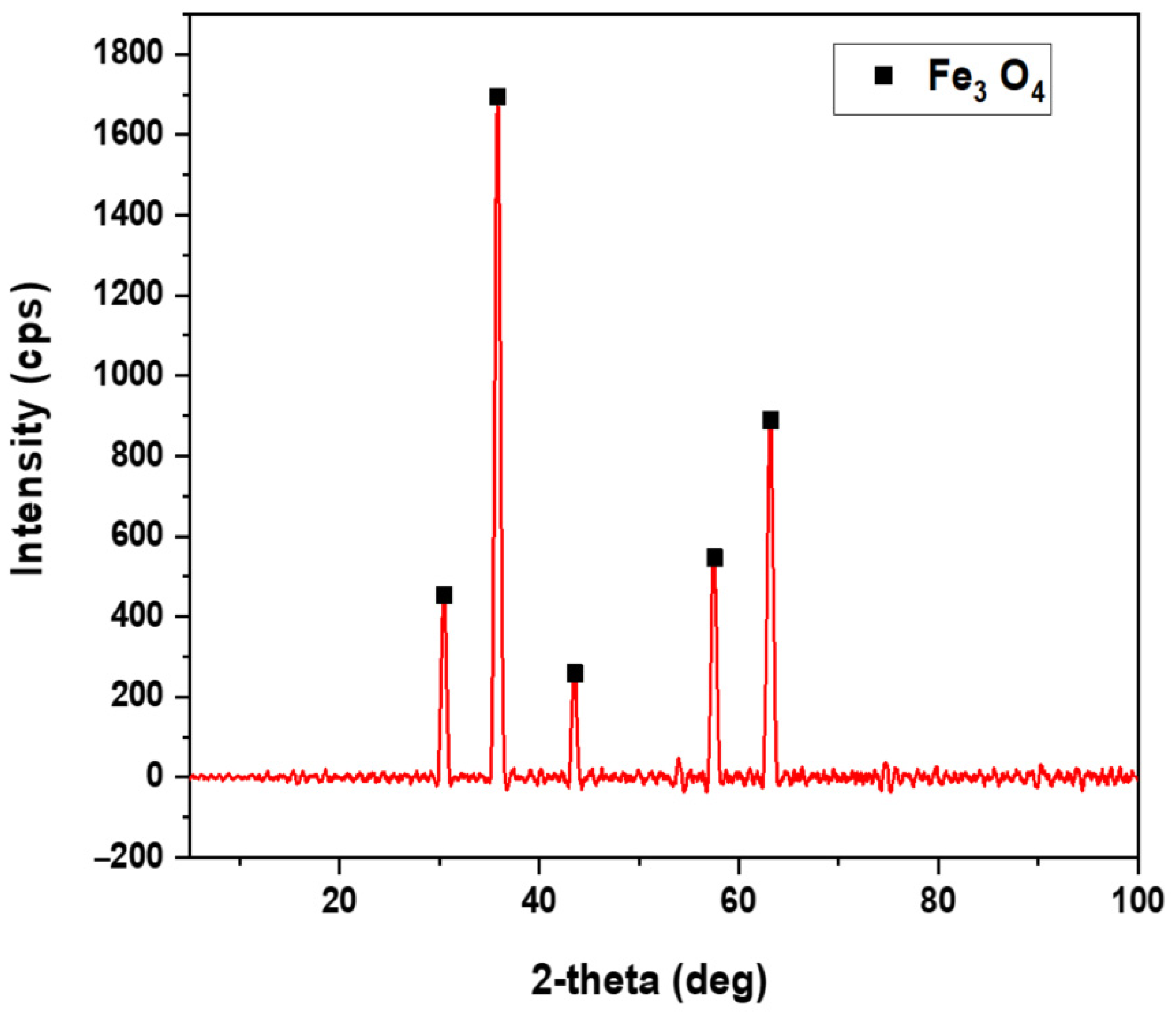
Figure 3 presents XRPD analysis of Fe(acac)
3 heated to 360 °C. This temperature corresponds to the mass loss and DTG peak observed in
Figure 2. The analysis confirms the formation of crystalline magnetite at this temperature. The results show magnetite (Fe
3O
4) formation with its characteristic cubic spinel structure. Well-defined diffraction peaks appear at 2θ values of 30.1°, 35.5° (highest intensity), 43.1°, 57.0°, and 62.6°. The peak sharpness and intensity indicate complete crystallization into highly ordered magnetite. No precursor remnants or competing iron oxide phases are detectable.
The temperature at which this transformation represents a synergy with the oxidation process, as the catalyst achieves its fully active Fe3O4 configuration exactly at the boundary of the HTO reactions (400–550 °C). This synchronization effectively facilitates maximum reactivity within the system for enhanced catalytic performance exactly when high-temperature combustion mechanisms become dominant. Magnetite’s exceptional catalytic properties derive from its unique crystallographic architecture, wherein Fe2+ and Fe3+ ions occupy specific octahedral and tetrahedral sites within the spinel lattice. This distinctive mixed-valence arrangement creates a robust electron transfer network that significantly accelerates oxidation kinetics by facilitating oxygen activation and promoting complete combustion pathways that characterize efficient HTO processes.
Thermal stability analysis further reveals that once formed at 360 °C, the magnetite phase demonstrates remarkable thermal resilience throughout the entire HTO temperature window, as evidenced by the pronounced plateau in the TG curve above this critical temperature. This sustained structural integrity ensures consistent catalytic performance even under the extreme thermal conditions of high-temperature combustion. The direct correlation between crystalline Fe
3O
4 formation at 360 °C and the enhanced HTO performance in DSC analysis (
Figure 1) establishes a definitive structure activity relationship where magnetite catalyzes combustion through multiple mechanisms by providing redox-active Fe
2+/Fe
3+ sites for electron transfer, reducing activation energy barriers, improving fuel utilization, and enhancing combustion front stability [
38]. This transformation explains the characteristic shift in HTO peak temperature in catalytic profiles compared to the non-catalytic, with the catalyst already transformed into its optimally active Fe
3O
4 configuration, enabling combustion initiation at lower temperatures [
34], resulting in the observed temperature shift and increased exothermic intensity, suggesting that controlled preheating to 360 °C could maximize catalytic efficiency by maintaining the active magnetite phase throughout the HTO process, potentially yielding significant improvements in combustion efficiency and process stability.
The influence of reservoir pressure on this process should be considered for field applications. Elevated pressure is expected to slightly increase the decomposition temperature of Fe(acac)
3 due to the suppression of gaseous ligand release. However, even with this shift, the formation of the active magnetite phase would still occur strategically before the HTO regime. Furthermore, the magnetite (Fe
3O
4) phase is known to be remarkably stable under the high-temperature, steam-rich conditions of in situ combustion, as evidenced by its persistent catalytic activity in similar high-pressure processes like catalytic aquathermolysis [
6,
36].
2.3. Kinetic Analysis of Heavy Oil Oxidation
The Kissinger isoconversional method was employed to determine the kinetic parameters of heavy oil oxidation in both the LTO and HTO regions, with and without the iron acetylacetonate (III) catalyst.
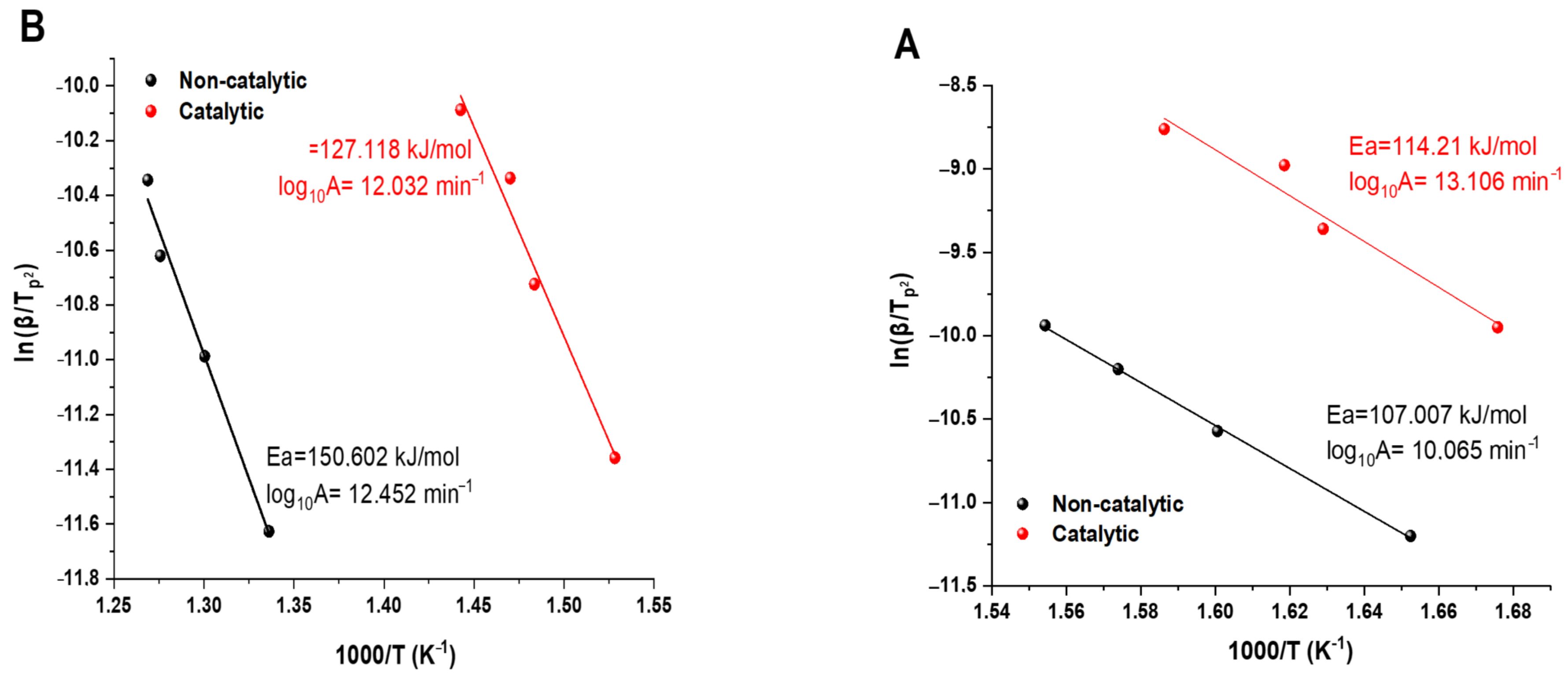
Figure 3 presents the Kissinger plots for the LTO and HTO regions, respectively, from which activation energies (Ea) and pre-exponential factors (A) were derived [
39].
In the LTO region, the non-catalytic heavy oil oxidation exhibited an activation energy of 107.0 kJ/mol with a pre-exponential factor of log
10A = 10.1 min
−1. Upon addition of the iron acetylacetonate (III) catalyst, a slight increase in activation energy to 114.2 kJ/mol was observed, accompanied by a significant increase in the pre-exponential factor to log
10A = 13.1 min
−1. This improvement is primarily attributed to enhanced miscibility and collision frequency from effective Fe(acac)
3 dispersion. Similar effects have been observed in prior combustion studies [
40]. While minor changes cannot be excluded, miscibility effects are the predominant factor.
The increased activation energy in the catalytic system during LTO indicates that the catalyst raises the energy barrier for initiating low-temperature oxidation reactions. However, this is compensated by the substantially higher pre-exponential factor, which is related to the frequency of molecular collisions and the proportion of properly oriented molecules during collision. This suggests that the catalyst primarily enhances LTO reactions by increasing the collision efficiency rather than by lowering the activation energy threshold.
The most significant catalytic effect occurred in the HTO region (
Figure 4B). The activation energy decreased substantially from 150.6 kJ/mol (non-catalytic) to 127.1 kJ/mol (catalytic), representing a 23.5 kJ/mol reduction. Concurrently, the pre-exponential factor slightly decreased from log
10A = 12.5 min
−1 to log
10A = 12.0 min
−1.
This significant reduction in activation energy for the HTO region matches perfectly with our previous observations from DSC and XRPD analyses, confirming that the magnetite (Fe
3O
4) formed at temperatures around 360 °C is, in fact, highly effective at catalyzing high-temperature combustion reactions. The lower activation energy facilitates the initiation and propagation of complete oxidation reactions at lower temperatures than would be required without the presence of the catalyst [
6].
The divergent effects of the catalyst on LTO and HTO kinetics provide valuable insights into the reaction mechanisms. In the LTO region, the catalyst appears to modify the reaction pathway without significantly altering the energy barrier, primarily enhancing the frequency of successful molecular interactions through better orientation or proximity effects. Conversely, in the HTO region, the catalyst alters the reaction energetics by providing an alternative reaction pathway with a lower activation energy barrier. This is characteristic of true catalytic behavior, where the catalyst participates directly in the reaction mechanism, possibly through the alternating oxidation states of iron (Fe
2+/Fe
3+) within the magnetite structure, facilitating electron transfer during combustion reactions. The substantial reduction in HTO activation energy suggests that magnetite provides an alternative reaction pathway. Ex situ XRPD analysis confirms magnetite formation at 360 °C. The catalytic enhancement during active HTO strongly indicates involvement of the Fe
2+/Fe
3+ redox cycle. This cycle is well-documented for facilitating electron transfer in hydrocarbon oxidation reactions [
32,
38,
41]. Therefore, the proposed mechanism in
Figure 5 correlates the identified active phase with the kinetic results.
The significant decrease in HTO activation energy has profound implications for combustion front stability, allowing reactions to proceed more readily at lower temperatures and ensuring more complete fuel utilization. These kinetic analyses provide quantitative evidence for the preferential catalytic enhancement of the HTO region by iron acetylacetonate (III), aligning with thermal analysis and structural characterization. This substantial reduction in activation energy represents a significant advancement for improving the efficiency and stability of thermal recovery operations, such as in situ combustion.
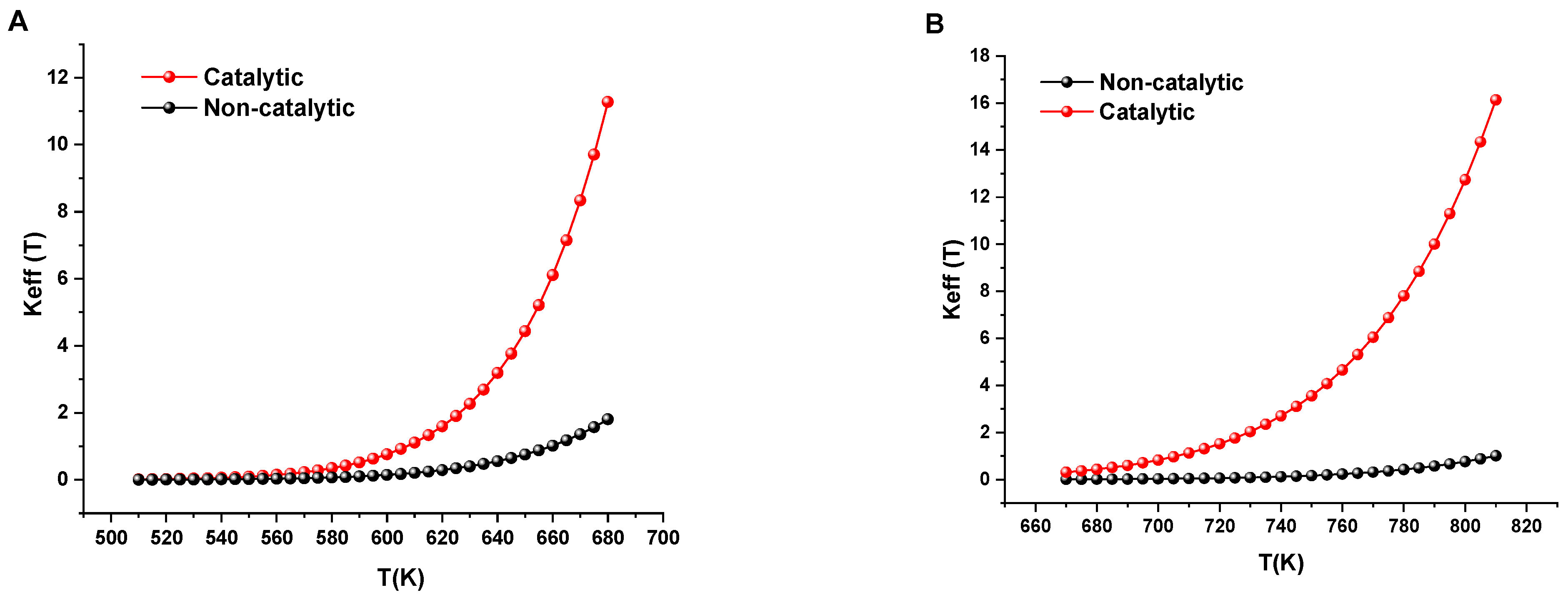
Our findings reveal the practical impact of these catalytic enhancements when examining the reaction rate effective constants for LTO and HTO, as illustrated in
Figure 4. The significant difference in reaction rates between catalytic and non-catalytic systems shown in
Figure 4 warrants more detailed analysis. In both LTO (a) and HTO (b) regions, we observe substantial catalytic enhancement, but with distinct mechanistic implications.
For the LTO region (
Figure 5A), the catalytic system exhibits reaction rates approximately 5–6 times higher than the non-catalytic system at equivalent temperatures. This enhancement begins at relatively low temperatures (~540 K) and increases progressively with temperature. The exponential divergence between catalytic and non-catalytic curves suggests that even before complete transformation to magnetite, the iron species present (likely iron oxyhydroxides or partially decomposed Fe(acac)
3) provide active sites that facilitate electron transfer during initial oxidation stages.
The HTO region (
Figure 5B) demonstrates even more dramatic catalytic effects, with reaction rates 8–10 times greater than the non-catalytic system near 800 K. This exceptional enhancement correlates directly with our structural characterization showing complete formation of crystalline magnetite at 360 °C (~633 K). The rate constant curves begin to diverge dramatically precisely at temperatures where magnetite becomes the dominant iron phase, confirming our proposed structure-activity relationship. Most notably, at equivalent reaction rates (for example, Keff = 2), the catalytic system achieves comparable oxidation at temperatures approximately 60–70 K lower than the non-catalytic system, a significant energy saving for field applications.
The practical implications of these kinetic findings are substantial. The accelerated oxidation rates in both regions suggest that catalyst-enhanced combustion would maintain more stable combustion fronts with more complete fuel utilization. The lower temperature requirements for achieving equivalent oxidation rates would translate to reduced energy inputs during field operations. Furthermore, the steep slope of the catalytic rate curve in the HTO region indicates that precise temperature control could enable fine-tuning of reaction rates, potentially allowing operators to optimize combustion front propagation velocities according to specific reservoir conditions.
These rate constant profiles provide quantitative confirmation of the proposed catalytic mechanism wherein magnetite’s mixed-valence Fe
2+/Fe
3+ configuration creates redox cycles that dramatically enhance oxidation efficiency. The results further suggest that pre-activation strategies targeting magnetite formation before reaching HTO temperatures could maximize catalytic performance in practical applications. Moreover, while
Figure 6 illustrates the role of crystalline magnetite in HTO, it is plausible that intermediate iron species, such as oxyhydroxide formed during the initial stages of Fe(acac)
3 decomposition, contribute to the enhanced reactivity observed in the LTO region by facilitating initial oxygen activation.
3. Experimental Section
3.1. Materials and Reagents
The heavy crude oil investigated in this study was obtained from the Ashalcha oilfield (Volga-Ural basin, Republic of Tatarstan, Russian Federation). The detailed compositional and physical properties of this high-viscosity petroleum fraction are summarized in
Table 2.
3.2. Iron (III) Acetylacetonate Catalyst Synthesis
Iron (III) acetylacetonate (Fe(acac)3) was synthesized through a multi-step procedure to ensure high purity and reproducibility. Iron (III) acetylacetonate was synthesized through a multi-step procedure to ensure high purity and reproducibility. This approach maintains consistency across our research on various metal acetylacetonate catalysts. The synthesis utilized 7.5 g of anhydrous iron (III) chloride (FeCl3), 15 mL of acetylacetone, a 20% potassium hydroxide (KOH) solution, and acetone as the recrystallization solvent.
Initially, FeCl3 was dissolved in approximately 100 mL of distilled water under continuous magnetic stirring, forming a homogeneous orange-brown solution. A 20% KOH solution was then added dropwise while monitoring the pH, which was adjusted to 8 to facilitate the quantitative precipitation of ferric oxyhydroxide. The resulting precipitate was collected by vacuum filtration, thoroughly washed to remove residual chloride ions, and partially dried under vacuum.
The semi-dried ferric oxyhydroxide was subsequently dispersed in 15 mL of acetylacetone and stirred vigorously. Within 30 min, the reaction medium transitioned to a dark-red color, signifying the successful complexation and formation of Fe(acac)3. The crude product was recovered by filtration as a red powder and subjected to double recrystallization from ethanol, yielding high-purity dark-red crystalline Fe(acac)3.
3.3. Sample Preparation for Thermal Analysis
To facilitate comparative assessment of catalytic effects on heavy oil oxidation behavior, two distinct sample formulations were prepared:
Control sample (non-catalytic): A homogeneous dispersion of heavy crude oil (10.0 wt%) thoroughly mixed with standardized quartz sand (90.0 wt%, 43–64 µm particle size). Quartz sand serves as an inert mixing matrix that resembles reservoir porous media. The 10 wt% oil to 90 wt% sand ratio reflects typical regional reservoir saturations. This ratio provides reliable dispersion and reproducible thermal behavior in similar investigations [
43].
Catalytic sample: Identical composition to the control sample with additional iron (III) acetylacetonate catalyst (2.0 wt%). The catalyst was pre-mixed with the oil phase before combining with sand.
Both control and catalytic samples (oil, oil-catalyst mixtures) were stirred with the sand mechanically and gentlye heated (~60 °C) to ensure complete dissolution and homogeneous distribution. The Fe(acac)
3 concentration of 2.0 wt% aligns with protocols in previous research work, shown to be optimal for catalytic enhancement and magnetite formation. Higher catalyst dosages may further accelerate transformation but may introduce diffusion limitations or economic inefficiencies [
44].
3.4. Thermal Analysis Methodology
Thermo-analytical characterization of heavy oil oxidation was conducted using simultaneous differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) on a Netzsch STA 449 F1 Jupiter instrument. Experiments were performed across a temperature range of 30–600 °C under oxidative conditions with airflow controlled (50 mL · min−1). To enable comprehensive kinetic analysis, measurements were executed at multiple heating rates (5, 10, 15, and 20 °C · min−1). Data acquisition and mathematical processing employed specialized software, including Proteus Analysis v5.2.1, NETZSCH Peak Separation (version 2010.09), and NETZSCH Thermokinetics 3.1 (version 06.08.2014).
3.5. Kinetic Analysis Approach
The oxidation of heavy petroleum constituents represents a multifaceted process involving numerous concurrent and sequential reactions within a heterogeneous system, further complicated by mass and heat transfer phenomena. Based on established methodologies, the reaction rate can be expressed by the fundamental relationship:
where
α denotes the conversion degree (determinable via DSC peak integration),
b represents the reaction order, and
PO2 corresponds to the oxygen partial pressure.
The temperature-dependent rate constant
k(
T) follows the classical Arrhenius relationship:
Previous research [
19,
45] has established that petroleum oxidation processes typically exhibit first-order kinetic behavior (
b = 1) with respect to both hydrocarbon concentration and oxygen partial pressure. Given the specific experimental configuration utilized (micro sample in a large furnace with high gas flow), a constant oxygen partial pressure can be assumed throughout the measurement, simplifying the kinetic expression to the following:
where
The Kissinger isoconversional method was selected for the determination of kinetic parameters due to its advantages in eliminating baseline selection and peak profile analysis requirements. This method enables direct calculation of activation energies from peak temperatures measured at different heating rates according to the following:
where
β is the heating rate,
represents the peak temperature,
A is the pre-exponential factor,
Ea corresponds to the activation energy, and
R is the universal gas constant.
4. Conclusions
This study demonstrates the catalytic efficiency of iron (III) acetylacetonate in heavy oil oxidation with several advantages, such as its synchronized thermal activation. Unlike catalysts that are active upon injection or over a broad range, Fe(acac)
3 decomposes into crystalline magnetite at ~360 °C, where the active phase specifically enhances High-Temperature Oxidation (HTO) reactions. Thermal decomposition analysis confirmed the formation of crystalline magnetite (Fe
3O
4) at 360 °C, with X-ray diffraction validating its structure. Kinetic analysis using Kissinger’s isoconversional method revealed a 15.6% reduction in activation energy (from 150.6 kJ/mol to 127.1 kJ/mol) for high-temperature oxidation (HTO) reactions, with minimal changes in the pre-exponential factor. Differential scanning calorimetry showed a marked increase in exothermic responses, nearly doubling the maximum heat flow in catalytic systems. This enhanced reactivity is attributed to the Fe
2+/Fe
3+ redox couple in magnetite, which facilitates electron transfer during oxidation [
41]. Thermogravimetric analysis further confirmed the exceptional thermal stability of the magnetite phase throughout the HTO temperature range.
These findings have critical implications for in situ combustion processes, suggesting that pre-activating the catalyst at approximately 360 °C could maximize its efficiency in field applications. The preferential enhancement of HTO reactions marks a significant advancement in catalyst design for thermal recovery operations. Future research should focus on field-scale implementation, optimizing catalyst concentration, exploring synergistic effects with other transition metals, and computational modeling of reaction kinetics. This work paves the way for more efficient heavy oil recovery strategies through catalytically enhanced in situ combustion technology, including practical aspects such as injection methods and an economic assessment of the catalyst impact on the overall recovery process.