Synergistic Conversion and Catalytic Upgrading of Seaweed Biomass for Sustainable Bioenergy: Advances, Challenges, and Future Prospects
Abstract
1. Introduction
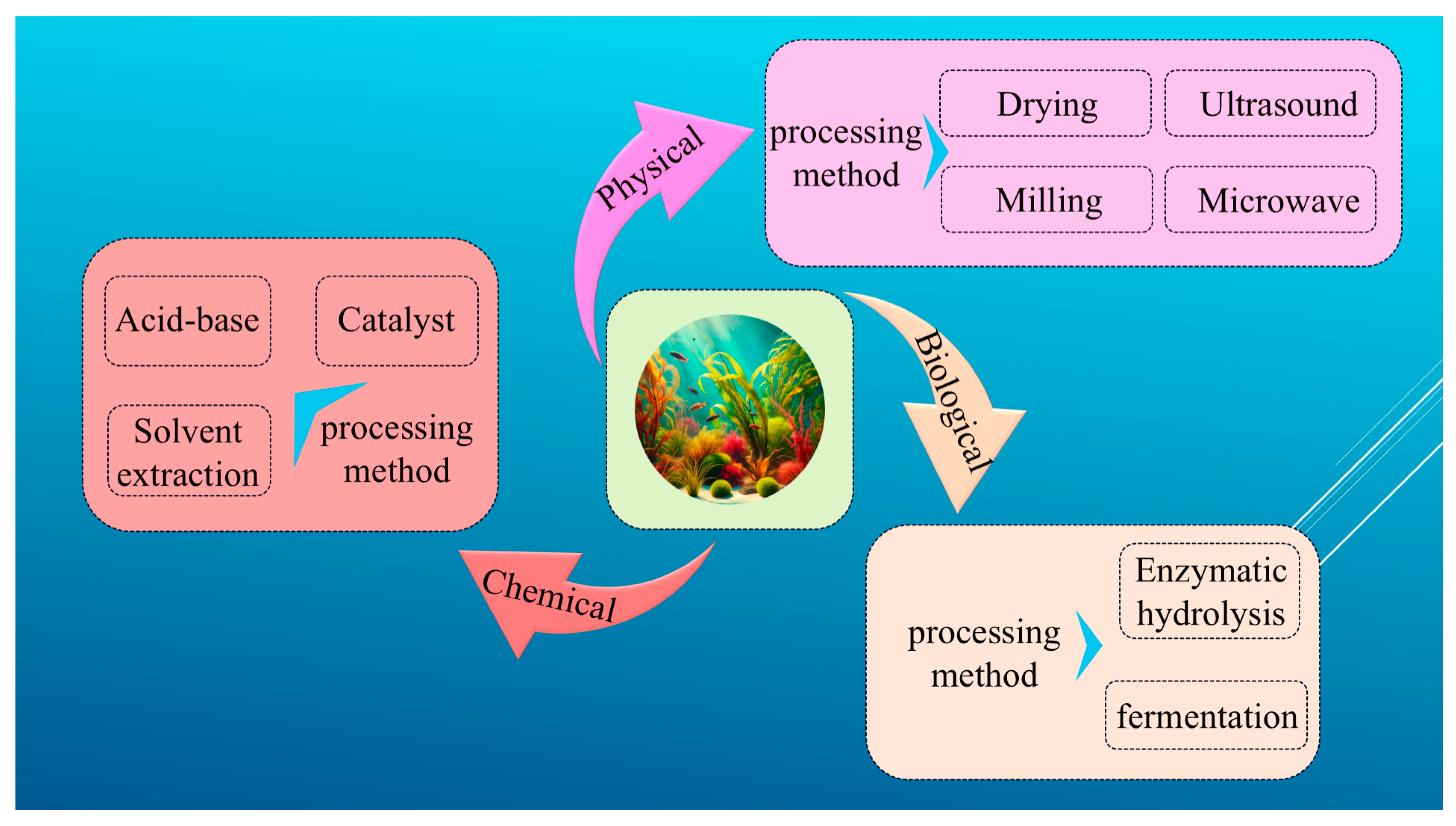
2. Algal Bioenergy Processing Methods
2.1. Algal Physical Processing Methods
2.2. Algal Chemical Processing Methods
2.3. Algal Bioprocessing Methods
3. Algal Biomass Upgrading Technologies for Enhanced Bioenergy Conversion
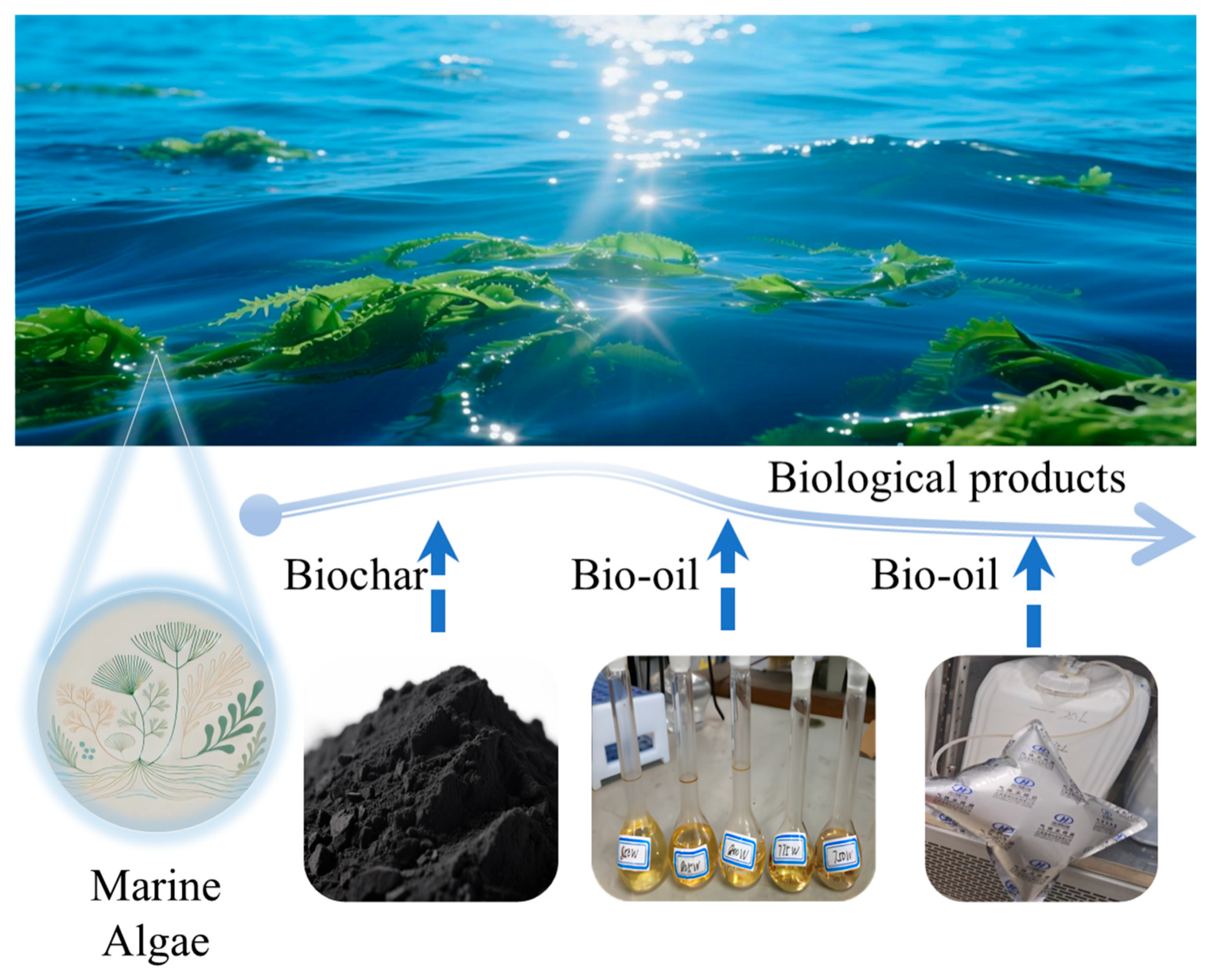
3.1. Advanced Valorization Pathways for Solid-Phase Products
3.2. Advanced Value-Added Approaches for Liquid-Phase Products
3.3. Advanced Value-Added Methods for Gas-Phase Products
4. Economic Assessment of Algal Biofuels
5. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.J. Energy access challenge and the role of fossil fuels in meeting electricity demand: Promoting renewable energy capacity for sustainable development. Geosci. Front. 2024, 15, 101873. [Google Scholar] [CrossRef]
- Narayanan, M. Marine algae biomass: A viable and renewable resource for biofuel production: A review. Algal Res. 2024, 82, 103687. [Google Scholar] [CrossRef]
- Abomohra, A.; El-Sheekh, M.; Hanelt, D. Screening of marine microalgae isolated from the hypersaline Bardawil lagoon for biodiesel feedstock. Renew. Energ. 2017, 101, 1266–1272. [Google Scholar] [CrossRef]
- Toth, G.B.; Hargrave, M.; Stedt, K.; Steinhagen, S.; Visch, W.; Pavia, H. Advances in Swedish Seaweed Aquaculture: Enhancing Biomass Production and Quality. Rev. Aquacult 2025, 17, e70031. [Google Scholar] [CrossRef]
- Hochman, G.; Palatnik, R.R. The Economics of Aquatic Plants: The Case of Algae and Duckweed. Annu. Rev. Resour. Econ. 2022, 14, 555–577. [Google Scholar] [CrossRef]
- Ortega, A.; Geraldi, N.R.; Alam, I.; Kamau, A.A.; Acinas, S.G.; Logares, R.; Gasol, J.M.; Massana, R.; Krause-Jensen, D.; Duarte, C.M. Important contribution of macroalgae to oceanic carbon sequestration. Nat. Geosci. 2019, 12, 748–754. [Google Scholar] [CrossRef]
- Chia, S.R.; Ong, H.C.; Chew, K.W.; Show, P.L.; Phang, S.M.; Ling, T.C.; Nagarajan, D.; Lee, D.J.; Chang, J.S. Sustainable approaches for algae utilisation in bioenergy production. Renew. Energ. 2018, 129, 838–852. [Google Scholar] [CrossRef]
- Wahlen, B.D.; Roni, M.S.; Cafferty, K.G.; Wendt, L.M.; Westover, T.L.; Stevens, D.M.; Newby, D.T. Managing variability in algal biomass production through drying and stabilization of feedstock blends. Algal Res. 2017, 24, 9–18. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, X.W.; Han, X.Q.; Li, G.; Yan, J.J. Using pre-drying technology to improve the exergetic efficiency of bioenergy utilization process with combustion: A case study of a power plant. Appl. Therm. Eng. 2017, 127, 1416–1426. [Google Scholar] [CrossRef]
- Lomartire, S.; Gonçalves, A.M.M. Novel Technologies for Seaweed Polysaccharides Extraction and Their Use in Food with Therapeutically Applications—A Review. Foods 2022, 11, 2654. [Google Scholar] [CrossRef]
- Lin, R.C.; Deng, C.; Ding, L.K.; Bose, A.; Murphy, J.D. Improving gaseous biofuel production from seaweed: The effect of hydrothermal pretreatment on energy efficiency. Energ. Convers. Manag. 2019, 196, 1385–1394. [Google Scholar] [CrossRef]
- Offei, F.; Mensah, M.; Thygesen, A.; Kemausuor, F. Seaweed Bioethanol Production: A Process Selection Review on Hydrolysis and Fermentation. Fermentation 2018, 4, 99. [Google Scholar] [CrossRef]
- Osman, M.E.H.; Abo-Shady, A.M.; Elshobary, M.E.; Abd El-Ghafar, M.O.; Hanelt, D.; Abomohra, A. Exploring the Prospects of Fermenting/Co-Fermenting Marine Biomass for Enhanced Bioethanol Production. Fermentation 2023, 9, 934. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Kyzas, G.Z. Progress in batch biosorption of heavy metals onto algae. J. Mol. Liq. 2015, 209, 77–86. [Google Scholar] [CrossRef]
- Vitova, M.; Bisova, K.; Kawano, S.; Zachleder, V. Accumulation of energy reserves in algae: From cell cycles to biotechnological applications. Biotechnol. Adv. 2015, 33, 1204–1218. [Google Scholar] [CrossRef]
- Zhang, K.; Kim, W.J.; Park, A.H.A. Alkaline thermal treatment of seaweed for high-purity hydrogen production with carbon capture and storage potential. Nat. Commun. 2020, 11, 3783. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, X.M.; Han, X.X.; Liu, J.G. Combustion characteristics of seaweed biomass. 1. Combustion characteristics of Enteromorpha clathrata and Sargassum natans. Energ. Fuel 2009, 23, 5173–5178. [Google Scholar] [CrossRef]
- Maneein, S.; Milledge, J.J.; Nielsen, B.V.; Harvey, P.J. A Review of Seaweed Pre-Treatment Methods for Enhanced Biofuel Production by Anaerobic Digestion or Fermentation. Fermentation 2018, 4, 100. [Google Scholar] [CrossRef]
- Hu, Y.M.; Wang, S.; Wang, Q.; He, Z.X.; Abomohra, A.; Cao, B. Influence of torrefaction pretreatment on the pyrolysis characteristics of seaweed biomass. Cellulose 2019, 26, 8475–8487. [Google Scholar] [CrossRef]
- Adams, J.M.M.; Schmidt, A.; Gallagher, J.A. The impact of sample preparation of the macroalgae on the production of the biofuels bioethanol and biomethane. J. Appl. Phycol. 2015, 27, 985–991. [Google Scholar] [CrossRef]
- Tedesco, S.; Mac Lochlainn, D.; Olabi, A.G. Particle size reduction optimization of spp. biomass for enhanced methane production. Energy 2014, 76, 857–862. [Google Scholar] [CrossRef]
- Naseem, S.; Rizwan, M.; Durrani, A.I.; Munawar, A.; Gillani, S.R. Innovations in cell lysis strategies and efficient protein extraction from blue food (Seaweed). Sustain. Chem. Pharm. 2024, 39, 101586. [Google Scholar] [CrossRef]
- Wahidin, S.; Idris, A.; Yusof, N.M.; Kamis, N.H.H.; Shaleh, S.R.M. Optimization of the ionic liquid-microwave assisted one-step biodiesel production process from wet microalgal biomass. Energ. Convers. Manag. 2018, 171, 1397–1404. [Google Scholar] [CrossRef]
- Schultz-Jensen, N.; Thygesen, A.; Leipold, F.; Thomsen, S.T.; Roslander, C.; Lilholt, H.; Bjerre, A.B. Pretreatment of the macroalgae Chaetomorpha linum for the production of bioethanol—Comparison of five pretreatment technologies. Bioresour. Technol. 2013, 140, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Alavijeh, R.S.; Karimi, K.; Wijffels, R.H.; van den Berg, C.; Eppink, M. Combined bead milling and enzymatic hydrolysis for efficient fractionation of lipids, proteins, and carbohydrates of Chlorella vulgaris microalgae. Bioresour. Technol. 2020, 309, 123321. [Google Scholar] [CrossRef]
- Schwenzfeier, A.; Wierenga, P.A.; Gruppen, H. Isolation and characterization of soluble protein from the green microalgae Tetraselmis sp. Bioresour. Technol. 2011, 102, 9121–9127. [Google Scholar] [CrossRef]
- Postma, P.R.; Miron, T.L.; Olivieri, G.; Barbosa, M.J.; Wijffels, R.H.; Eppink, M.H.M. Mild disintegration of the green microalgae Chlorella vulgaris using bead milling. Bioresour. Technol. 2015, 184, 297–304. [Google Scholar] [CrossRef]
- Taleb, A.; Kandilian, R.; Touchard, R.; Montalescot, V.; Rinaldi, T.; Taha, S.; Takache, H.; Marchal, L.; Legrand, J.; Pruvost, J. Screening of freshwater and seawater microalgae strains in fully controlled photobioreactors for biodiesel production. Bioresour. Technol. 2016, 218, 480–490. [Google Scholar] [CrossRef]
- El-Mesery, H.S.; El-khawaga, S.E. Drying process on biomass: Evaluation of the drying performance and energy analysis of different dryers. Case Stud. Therm. Eng. 2022, 33, 101953. [Google Scholar] [CrossRef]
- Montingelli, M.E.; Benyounis, K.Y.; Stokes, J.; Olabi, A.G. Pretreatment of macroalgal biomass for biogas production. Energ. Convers. Manag. 2016, 108, 202–209. [Google Scholar] [CrossRef]
- Ha, G.-S.; El-Dalatony, M.M.; Kurade, M.B.; Salama, E.-S.; Basak, B.; Kang, D.; Roh, H.-S.; Lim, H.; Jeon, B.-H. Energy-efficient pretreatments for the enhanced conversion of microalgal biomass to biofuels. Bioresour. Technol. 2020, 309, 123333. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nižetić, S.; Ong, H.C.; Mofijur, M.; Ahmed, S.F.; Ashok, B.; Bui, V.T.V.; Chau, M.Q. Insight into the recent advances of microwave pretreatment technologies for the conversion of lignocellulosic biomass into sustainable biofuel. Chemosphere 2021, 281, 130878. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Rao, M. Trends in bioconversion of lignocellulose: Biofuels, platform chemicals & biorefinery concept. Prog. Energy Combust. Sci. 2012, 38, 522–550. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hosano, N.; Hosano, H. Recovering Microalgal Bioresources: A Review of Cell Disruption Methods and Extraction Technologies. Molecules 2022, 27, 2786. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, R.; Ang, W.M.R.; Chen, X.; Voigtmann, M.; Lau, R. Lipid releasing characteristics of microalgae species through continuous ultrasonication. Bioresour. Technol. 2014, 158, 7–11. [Google Scholar] [CrossRef]
- Hao, X.; Suo, H.; Peng, H.; Xu, P.; Gao, X.; Du, S. Simulation and exploration of cavitation process during microalgae oil extracting with ultrasonic-assisted for hydrogen production. Int. J. Hydrog. Energy 2021, 46, 2890–2898. [Google Scholar] [CrossRef]
- Cheng, J.; Huang, R.; Yu, T.; Li, T.; Zhou, J.; Cen, K. Biodiesel production from lipids in wet microalgae with microwave irradiation and bio-crude production from algal residue through hydrothermal liquefaction. Bioresour. Technol. 2014, 151, 415–418. [Google Scholar] [CrossRef]
- Zheng, H.; Yin, J.; Gao, Z.; Huang, H.; Ji, X.; Dou, C. Disruption of Chlorella vulgaris Cells for the Release of Biodiesel-Producing Lipids: A Comparison of Grinding, Ultrasonication, Bead Milling, Enzymatic Lysis, and Microwaves. Appl. Biochem. Biotechnol. 2011, 164, 1215–1224. [Google Scholar] [CrossRef]
- Teh, Y.Y.; Lee, K.T.; Chen, W.-H.; Lin, S.-C.; Sheen, H.-K.; Tan, I.S. Dilute sulfuric acid hydrolysis of red macroalgae Eucheuma denticulatum with microwave-assisted heating for biochar production and sugar recovery. Bioresour. Technol. 2017, 246, 20–27. [Google Scholar] [CrossRef]
- Iqbal, J.; Theegala, C. Microwave assisted lipid extraction from microalgae using biodiesel as co-solvent. Algal Res. 2013, 2, 34–42. [Google Scholar] [CrossRef]
- Biller, P.; Friedman, C.; Ross, A.B. Hydrothermal microwave processing of microalgae as a pre-treatment and extraction technique for bio-fuels and bio-products. Bioresour. Technol. 2013, 136, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Milledge, J.J.; Heaven, S. A review of the harvesting of micro-algae for biofuel production. Rev. Environ. Sci. Bio/Technol. 2013, 12, 165–178. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, Y.; Liu, S.; Fan, L.; Zhou, N.; Peng, P.; Wang, Y.; Guo, F.; Min, M.; Cheng, Y.; et al. Fast microwave-assisted pyrolysis of wastes for biofuels production—A review. Bioresour. Technol. 2020, 297, 122480. [Google Scholar] [CrossRef]
- Kang, E.-k.; Lee, B.M.; Hwang, H.A.; Kim, J.-H. Analysis of mono-sugars obtained by acid hydrolysis of algae-based polysaccharides. J. Ind. Eng. Chem. 2012, 18, 1366–1369. [Google Scholar] [CrossRef]
- Laurens, L.M.L.; Nagle, N.; Davis, R.; Sweeney, N.; Van Wychen, S.; Lowell, A.; Pienkos, P.T. Acid-catalyzed algal biomass pretreatment for integrated lipid and carbohydrate-based biofuels production. Green. Chem. 2015, 17, 1145–1158. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Msagati, T.A.M.; Mamba, B.B. Acid mine drainage: Prevention, treatment options, and resource recovery: A review. J. Clean. Prod. 2017, 151, 475–493. [Google Scholar] [CrossRef]
- Lymperatou, A.; Engelsen, T.K.; Skiadas, I.V.; Gavala, H.N. Different pretreatments of beach-cast seaweed for biogas production. J. Clean. Prod. 2022, 362, 132277. [Google Scholar] [CrossRef]
- Ding, J.; Yang, S.; Meng, X.; Yoo, C.G.; Liang, L.; Hao, N.; Pu, Y.; Yu, C.; Ragauskas, A.J. Borate-assisted alkaline extraction of hemicellulose from switchgrass with enhanced structural stability and purity. Int. J. Biol. Macromol. 2025, 321, 146180. [Google Scholar] [CrossRef]
- Moyo, A.; Parbhakar-Fox, A.; Meffre, S.; Cooke, D.R. Alkaline industrial wastes—Characteristics, environmental risks, and potential for mine waste management. Environ. Pollut. 2023, 323, 121292. [Google Scholar] [CrossRef]
- Zhou, H.; Park, A.-H.A. Bio-energy with carbon capture and storage via alkaline thermal Treatment: Production of high purity H2 from wet wheat straw grass with CO2 capture. Appl. Energy 2020, 264, 114675. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, C.; Barreiro-Vescovo, S.; de Godos, I.; Fernandez, M.; Zouhayr, A.; Ballesteros, M. Biochemical methane potential of microalgae biomass using different microbial inocula. Biotechnol. Biofuels 2018, 11, 184. [Google Scholar] [CrossRef]
- Hart, A. Circular economy: Closing the catalyst loop with metal reclamation from spent catalysts, industrial waste, waste shells and animal bones. Biomass Convers. Biorefinery 2021, 13, 11483–11498. [Google Scholar] [CrossRef]
- Ponnumsamy, V.K.; Al-Hazmi, H.E.; Shobana, S.; Dharmaraja, J.; Jadhav, D.A.; J, R.B.; Piechota, G.; Igliński, B.; Kumar, V.; Bhatnagar, A.; et al. A review on homogeneous and heterogeneous catalytic microalgal lipid extraction and transesterification for biofuel production. Chin. J. Catal. 2024, 59, 97–117. [Google Scholar] [CrossRef]
- Taghavi Dehaghani, M.S.; Esfandiari, Z.; Rostamabadi, H.; Nodeh, H.R. Application of amino acid-based natural deep eutectic solvents in extraction of different analytes: A review study. Trends Food Sci. Amp Technol. 2024, 147, 104448. [Google Scholar] [CrossRef]
- Ramluckan, K.; Moodley, K.G.; Bux, F. An evaluation of the efficacy of using selected solvents for the extraction of lipids from algal biomass by the soxhlet extraction method. Fuel 2014, 116, 103–108. [Google Scholar] [CrossRef]
- Santoro, I.; Nardi, M.; Benincasa, C.; Costanzo, P.; Giordano, G.; Procopio, A.; Sindona, G. Sustainable and Selective Extraction of Lipids and Bioactive Compounds from Microalgae. Molecules 2019, 24, 4347. [Google Scholar] [CrossRef]
- Choi, O.K.; Lee, J.W. CO2-triggered switchable solvent for lipid extraction from microalgal biomass. Sci. Total Environ. 2022, 819, 153084. [Google Scholar] [CrossRef] [PubMed]
- Anto, S.; Premalatha, M.; Mathimani, T. Tertiary amine as an efficient CO2 switchable solvent for extracting lipids from hypersaline microalgae. Chemosphere 2022, 288, 132442. [Google Scholar] [CrossRef]
- Braud, L.; McDonnell, K.; Murphy, F. Environmental life cycle assessment of algae systems: Critical review of modelling approaches. Renew. Sustain. Energy Rev. 2023, 179, 113218. [Google Scholar] [CrossRef]
- Malekzadeh, M.; Abedini Najafabadi, H.; Hakim, M.; Feilizadeh, M.; Vossoughi, M.; Rashtchian, D. Experimental study and thermodynamic modeling for determining the effect of non-polar solvent (hexane)/polar solvent (methanol) ratio and moisture content on the lipid extraction efficiency from Chlorella vulgaris. Bioresour. Technol. 2016, 201, 304–311. [Google Scholar] [CrossRef]
- Negahdar, L.; Delidovich, I.; Palkovits, R. Aqueous-phase hydrolysis of cellulose and hemicelluloses over molecular acidic catalysts: Insights into the kinetics and reaction mechanism. Appl. Catal. B Environ. 2016, 184, 285–298. [Google Scholar] [CrossRef]
- Yang, W.; Li, X.; Liu, S.; Feng, L. Direct hydrothermal liquefaction of undried macroalgae Enteromorpha prolifera using acid catalysts. Energ. Convers. Manag. 2014, 87, 938–945. [Google Scholar] [CrossRef]
- Kumar, P.; Sumpter, B.G.; Saito, T.; Davis, R.J. Importance of hydrogen bonding in base-catalyzed transesterification reactions with vicinal diols. J. Catal. 2024, 429, 115246. [Google Scholar] [CrossRef]
- Chamola, R.; Khan, M.F.; Raj, A.; Verma, M.; Jain, S. Response surface methodology based optimization of in situ transesterification of dry algae with methanol, H2SO4 and NaOH. Fuel 2019, 239, 511–520. [Google Scholar] [CrossRef]
- Delmiro, T.M.; Calixto, G.Q.; Viegas, C.V.; Melo, D.M.A.; Viana, G.A.C.M.; Mendes, L.B.B.; Braga, R.M. Renewable aromatic hydrocarbons from flash catalytic pyrolysis of Monoraphidium sp. lipid extract. Bioresour. Technol. Rep. 2021, 15, 100799. [Google Scholar] [CrossRef]
- Norouzi, O.; Tavasoli, A.; Jafarian, S.; Esmailpour, S. Catalytic upgrading of bio-products derived from pyrolysis of red macroalgae Gracilaria gracilis with a promising novel micro/mesoporous catalyst. Bioresour. Technol. 2017, 243, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Santillan-Jimenez, E.; Pace, R.; Marques, S.; Morgan, T.; McKelphin, C.; Mobley, J.; Crocker, M. Extraction, characterization, purification and catalytic upgrading of algae lipids to fuel-like hydrocarbons. Fuel 2016, 180, 668–678. [Google Scholar] [CrossRef]
- Hong, Y.; Wu, Y.-R. Acidolysis as a biorefinery approach to producing advanced bioenergy from macroalgal biomass: A state-of-the-art review. Bioresour. Technol. 2020, 318, 124080. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, C. Catalytic Thermochemical Conversion of Algae and Upgrading of Algal Oil for the Production of High-Grade Liquid Fuel: A Review. Catalysts 2020, 10, 145. [Google Scholar] [CrossRef]
- Gonçalves, M.C.P.; Romanelli, J.P.; Cansian, A.B.M.; Pucci, E.F.Q.; Guimarães, J.R.; Tardioli, P.W.; Saville, B.A. A review on the production and recovery of sugars from lignocellulosics for use in the synthesis of bioproducts. Ind. Crop Prod. 2022, 186, 115213. [Google Scholar] [CrossRef]
- Ge, L.; Wang, P.; Mou, H. Study on saccharification techniques of seaweed wastes for the transformation of ethanol. Renew. Energ. 2011, 36, 84–89. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cho, J.M.; Chang, Y.K.; Oh, Y.-K. Cell disruption and lipid extraction for microalgal biorefineries: A review. Bioresour. Technol. 2017, 244, 1317–1328. [Google Scholar] [CrossRef]
- Wu, C.; Xiao, Y.; Lin, W.; Li, J.; Zhang, S.; Zhu, J.; Rong, J. Aqueous enzymatic process for cell wall degradation and lipid extraction from Nannochloropsis sp. Bioresour. Technol. 2017, 223, 312–316. [Google Scholar] [CrossRef]
- Demuez, M.; González-Fernández, C.; Ballesteros, M. Algicidal microorganisms and secreted algicides: New tools to induce microalgal cell disruption. Biotechnol. Adv. 2015, 33, 1615–1625. [Google Scholar] [CrossRef]
- Tan, I.S.; Lee, K.T. Solid acid catalysts pretreatment and enzymatic hydrolysis of macroalgae cellulosic residue for the production of bioethanol. Carbohydr. Polym. 2015, 124, 311–321. [Google Scholar] [CrossRef]
- Singh, R.; Jain, R.; Soni, P.; Santos-Villalobos, S.d.l.; Chattaraj, S.; Roy, D.; Mitra, D.; Gaur, A. Graphing the Green route: Enzymatic hydrolysis in sustainable decomposition. Curr. Res. Microb. Sci. 2024, 7, 100281. [Google Scholar] [CrossRef]
- Tabacof, A.; Calado, V.; Pereira, N. The Macroalga Kappaphycus alvarezii as a Potential Raw Material for Fermentation Processes within the Biorefinery Concept: Challenges and Perspectives. Fermentation 2024, 10, 283. [Google Scholar] [CrossRef]
- Montingelli, M.E.; Tedesco, S.; Olabi, A.G. Biogas production from algal biomass: A review. Renew. Sustain. Energy Rev. 2015, 43, 961–972. [Google Scholar] [CrossRef]
- Kusmiyati, K.; Hadiyanto, H.; Fudholi, A. Treatment updates of microalgae biomass for bioethanol production: A comparative study. J. Clean. Prod. 2023, 383, 135236. [Google Scholar] [CrossRef]
- Ding, L.; Cheng, J.; Lin, R.; Deng, C.; Zhou, J.; Murphy, J.D. Improving biohydrogen and biomethane co-production via two-stage dark fermentation and anaerobic digestion of the pretreated seaweed Laminaria digitata. J. Clean. Prod. 2020, 251, 119666. [Google Scholar] [CrossRef]
- Lee, J.y.; Li, P.; Lee, J.; Ryu, H.J.; Oh, K.K. Ethanol production from Saccharina japonica using an optimized extremely low acid pretreatment followed by simultaneous saccharification and fermentation. Bioresour. Technol. 2013, 127, 119–125. [Google Scholar] [CrossRef]
- Bello, M.; Modu, A.K.; Boryo, D.E.A.; Ranganathan, P. Sustainable algal biorefinery: A review on current perspective on technical maturity, supply infrastructure, business and industrial opportunities. J. Environ. Manag. 2024, 370, 122208. [Google Scholar] [CrossRef]
- Sundaram, T.; Kamalesh, R.; Saravanan, A.; Yaashikaa, P.R.; Vickram, A.S.; Teena, R.A.; Thiruvengadam, S. Harnessing algal biomass for renewable energy and biofuel production: Current strategies and future insights. Int. J. Biol. Macromol. 2025, 319, 145487. [Google Scholar] [CrossRef]
- Xia, A.; Jacob, A.; Tabassum, M.R.; Herrmann, C.; Murphy, J.D. Production of hydrogen, ethanol and volatile fatty acids through co-fermentation of macro- and micro-algae. Bioresour. Technol. 2016, 205, 118–125. [Google Scholar] [CrossRef]
- Chen, B.; Gu, Z.; Wu, M.; Ma, Z.; Lim, H.R.; Khoo, K.S.; Show, P.L. Advancement pathway of biochar resources from macroalgae biomass: A review. Biomass Bioenergy 2022, 167, 106650. [Google Scholar] [CrossRef]
- Dang, B.-T.; Ramaraj, R.; Huynh, K.-P.-H.; Le, M.-V.; Tomoaki, I.; Pham, T.-T.; Hoang Luan, V.; Thi Le Na, P.; Tran, D.P.H. Current application of seaweed waste for composting and biochar: A review. Bioresour. Technol. 2023, 375, 128830. [Google Scholar] [CrossRef] [PubMed]
- Amarasekara, A.; Tanzim, F.S.; Asmatulu, E. Briquetting and carbonization of naturally grown algae biomass for low-cost fuel and activated carbon production. Fuel 2017, 208, 612–617. [Google Scholar] [CrossRef]
- Skoglund, N.; Werner, K.; Nylund, G.M.; Pavia, H.; Albers, E.; Broström, M. Combustion of seaweed—A fuel design strategy. Fuel Process. Technol. 2017, 165, 155–161. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, F.; Wang, H.; Ho, S.-H. The magic of algae-based biochar: Advantages, preparation, and applications. Bioengineered 2023, 14, 2252157. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Adewuyi, Y.G.; Shi, S.; Chen, H.; Li, Y.; Liu, D.; Liu, Y. Removal of gaseous Hg0 using novel seaweed biomass-based activated carbon. Chem. Eng. J. 2019, 366, 41–49. [Google Scholar] [CrossRef]
- Kostas, E.T.; Williams, O.S.A.; Duran-Jimenez, G.; Tapper, A.J.; Cooper, M.; Meehan, R.; Robinson, J.P. Microwave pyrolysis of Laminaria digitata to produce unique seaweed-derived bio-oils. Biomass Bioenergy 2019, 125, 41–49. [Google Scholar] [CrossRef]
- Zainal, N.A.; Zulkifli, N.W.M.; Gulzar, M.; Masjuki, H.H. A review on the chemistry, production, and technological potential of bio-based lubricants. Renew. Sustain. Energy Rev. 2018, 82, 80–102. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Prussi, M.; Buffi, M.; Rizzo, A.M.; Pari, L. Review and experimental study on pyrolysis and hydrothermal liquefaction of microalgae for biofuel production. Appl. Energy 2017, 185, 963–972. [Google Scholar] [CrossRef]
- Anastasakis, K.; Ross, A.B. Hydrothermal liquefaction of the brown macro-alga Laminaria Saccharina: Effect of reaction conditions on product distribution and composition. Bioresour. Technol. 2011, 102, 4876–4883. [Google Scholar] [CrossRef]
- Felix, C.; Ubando, A.; Madrazo, C.; Gue, I.H.; Sutanto, S.; Tran-Nguyen, P.L.; Go, A.W.; Ju, Y.-H.; Culaba, A.; Chang, J.-S.; et al. Non-catalytic in-situ (trans) esterification of lipids in wet microalgae Chlorella vulgaris under subcritical conditions for the synthesis of fatty acid methyl esters. Appl. Energy 2019, 248, 526–537. [Google Scholar] [CrossRef]
- El-Hefnawy, M.E.; Alhayyani, S.; Ismail, A.; El-Sherbiny, M.; Al-Harbi, M.; Abomohra, A.; Sakran, M.; Zidan, N. Integrated approach for enhanced crude bio-oil yield from microalgae cultivated on the aqueous phase of hydrothermal co-liquefaction with agar-free seaweed residues. J. Clean. Prod. 2023, 392, 136286. [Google Scholar] [CrossRef]
- El-Hefnawy, M.E.; Alhayyani, S.; El-Sherbiny, M.M.; Abomohra, A.E.-F.; Al-Harbi, M. Endogenous bioethanol production by solid-state prefermentation for enhanced crude bio-oil recovery through integrated hydrothermal liquefaction of seaweeds. J. Clean. Prod. 2022, 355, 131811. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Shu, C.M.; Sarangi, P.K.; Shadangi, K.P.; Rakshit, S.; Kennedy, J.F.; Gupta, V.K.; Sharma, M. Catalytic hydrodeoxygenation of bio-oil and model compounds—Choice of catalysts, and mechanisms. Renew. Sust. Energ. Rev. 2023, 187, 113700. [Google Scholar] [CrossRef]
- Soni, V.K.; Sharma, P.R.; Choudhary, G.; Pandey, S.; Sharma, R.K. Ni/Co-Natural Clay as Green Catalysts for Microalgae Oil to Diesel-Grade Hydrocarbons Conversion. Acs Sustain. Chem. Eng. 2017, 5, 5351–5359. [Google Scholar] [CrossRef]
- Mudassir, M.A.; Batool, M.; Kousar, S.; Makarem, M.A.; Razia, E.T.; Meshksar, M.; Murtaza, M.; Tariq, K.; Din, M.A.U.; Bodlah, M.A.; et al. Plasma-assisted hydrodeoxygenation of bio-oils. Fuel Process. Technol. 2023, 250, 107872. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, G.L.; Miao, C.X. Green and renewable bio-diesel produce from oil hydrodeoxygenation: Strategies for catalyst development and mechanism. Renew. Sust. Energ. Rev. 2019, 101, 568–589. [Google Scholar] [CrossRef]
- Liu, R.; Rahman, M.M.; Sarker, M.; Chai, M.; Li, C.; Cai, J. A review on the catalytic pyrolysis of biomass for the bio-oil production with ZSM-5: Focus on structure. Fuel Process. Technol. 2020, 199, 106301. [Google Scholar] [CrossRef]
- Galadima, A.; Muraza, O. Hydrothermal liquefaction of algae and bio-oil upgrading into liquid fuels: Role of heterogeneous catalysts. Renew. Sust. Energ. Rev. 2018, 81, 1037–1048. [Google Scholar] [CrossRef]
- Hu, Y.; Li, J.; Wang, S.; Xu, L.; Barati, B.; Cao, B.; Wang, H.; Xie, K.; Wang, Q. Catalytic fast hydropyrolysis of seaweed biomass with different zeolite catalysts to produce high-grade bio-oil. Process Saf. Environ. Prot. 2021, 146, 69–76. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, B.; Fan, Y.; Liu, M.; Xu, Q.; Huang, Y.; Zhang, H. Optimizing invasive plant biomass valorization: Deep eutectic solvents pre-treatment coupled with ZSM-5 catalyzed fast pyrolysis for superior bio-oil quality. Bioresour. Technol. 2024, 400, 130652. [Google Scholar] [CrossRef]
- Zhang, B.; Zhong, S.Y.; Fang, J.; Gao, X.H.; Wu, S.L.; Xu, Q.; Zhang, H.Y. Valorization of water hyacinth biomass for bio-oil production using a novel combinatorial approach of UV/HO advanced oxidation process pretreatment and catalytic fast pyrolysis over ZSM-5. Biomass Bioenerg. 2023, 175, 106890. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.S.; Liu, Y.G.; Tang, S.S.; Chen, G.H.; Zhang, R.Q.; Tang, X.Q. Coke formation on the surface of Ni/HZSM-5 and Ni-Cu/HZSM-5 catalysts during bio-oil hydrodeoxygenation. Fuel 2017, 189, 23–31. [Google Scholar] [CrossRef]
- Cordero-Lanzac, T.; Palos, R.; Hita, I.; Arandes, J.M.; Rodríguez-Mirasol, J.; Cordero, T.; Bilbao, J.; Castaño, P. Revealing the pathways of catalyst deactivation by coke during the hydrodeoxygenation of raw bio-oil. Appl. Catal. B-Environ. 2018, 239, 513–524. [Google Scholar] [CrossRef]
- Gao, X.Y.; Ge, Z.Y.; Zhu, G.F.; Wang, Z.Y.; Ashok, J.; Kawi, S. Anti-Coking and Anti-Sintering Ni/AlO Catalysts in the Dry Reforming of Methane: Recent Progress and Prospects. Catalysts 2021, 11, 1003. [Google Scholar] [CrossRef]
- Wang, X.; Guo, S.J.; Song, P.Y.; Xu, L.F.; Zhang, X.; Shen, B.X. Synthesis and catalytic performance of hierarchically porous catalysts during pyrolysis of lipids to produce liquid hydrocarbons: A review. Appl. Catal. A-Gen. 2024, 677, 119704. [Google Scholar] [CrossRef]
- Liu, L.; Hong, D.K.; Guo, X. Insight into CaO addition on coking resistance of Ni surface for sorption enhanced methane steam reforming: A density functional study. Appl. Surf. Sci. 2019, 475, 887–895. [Google Scholar] [CrossRef]
- Li, Y.; Luo, H.; Qin, L.; Wang, Z.; Wang, L.; Liu, Y.-Q. Improving anti-coking and sulfur-resisting performance of Rh-based lanthanum zirconate pyrochlore catalysts for diesel reforming through partial substitution with alkali earth metals. J. Anal. Appl. Pyrolysis 2023, 169, 105865. [Google Scholar] [CrossRef]
- Show, K.Y.; Yan, Y.G.; Ling, M.; Ye, G.X.; Li, T.; Lee, D.J. Hydrogen production from algal biomass—Advances, challenges and prospects. Bioresour. Technol. 2018, 257, 290–300. [Google Scholar] [CrossRef]
- Thakur, N.; Salama, E.S.; Sharma, M.; Sharma, P.; Sharma, D.; Li, X. Efficient utilization and management of seaweed biomass for biogas production. Mater. Today Sustain. 2022, 18, 100120. [Google Scholar] [CrossRef]
- Tabassum, M.R.; Xia, A.; Murphy, J.D. Potential of seaweed as a feedstock for renewable gaseous fuel production in Ireland. Renew. Sust. Energ. Rev. 2017, 68, 136–146. [Google Scholar] [CrossRef]
- Tabassum, M.R.; Xia, A.; Murphy, J.D. Biomethane production from various segments of brown seaweed. Energ. Convers. Manag. 2018, 174, 855–862. [Google Scholar] [CrossRef]
- Czyrnek-Delêtre, M.M.; Rocca, S.; Agostini, A.; Giuntoli, J.; Murphy, J.D. Life cycle assessment of seaweed biomethane, generated from seaweed sourced from integrated multi-trophic aquaculture in temperate oceanic climates. Appl. Energy 2017, 196, 34–50. [Google Scholar] [CrossRef]
- Gnansounou, E.; Raman, J.K. Life cycle assessment of algae biodiesel and its co-products. Appl. Energy 2016, 161, 300–308. [Google Scholar] [CrossRef]
- Jingura, R.M.; Matengaifa, R. Optimization of biogas production by anaerobic digestion for sustainable energy development in Zimbabwe. Renew. Sustain. Energy Rev. 2009, 13, 1116–1120. [Google Scholar] [CrossRef]
- Mehmood, M.A.; Amin, M.; Haider, M.N.; Malik, S.; Malik, H.A.; Alam, M.A.; Xu, J.; Alessa, A.H.; Khan, A.Z.; Boopathy, R. Wastewater-Grown Algal Biomass as Carbon-neutral, Renewable, and Low Water Footprint Feedstock for Clean Energy and Bioplastics. Curr. Pollut. Rep. 2024, 10, 172–188. [Google Scholar] [CrossRef]
| Seaweed Type | Polysaccharides | Monosaccharides/Monosaccharide Derivatives | Disadvantages |
|---|---|---|---|
| Pyrolysis | Medium-low temperature rapid pyrolysis | Rapid conversion of macroalgae to bio-oil, gaseous fuels, and char products; high energy density with demonstrated industrialization potential | High energy consumption; requires post-treatment refining of bio-oil to improve fuel quality |
| Anaerobic Fermentation | Sugar-to-bioethanol fermentation | Mature technology enabling organic wastewater treatment with biogas co-generation (biomethane); low-cost and eco-friendly operations | Slow kinetics; inefficient decomposition of low-carbohydrate substrates; high sensitivity to environmental conditions |
| Enzymatic Hydrolysis | Enzymatic pretreatment/cellulase hydrolysis | High-efficiency monosaccharide extraction for bioethanol synthesis; superior bioconversion rates; minimal environmental pollution | Prohibitive enzyme costs; necessity for activity-enhancing additives; limited substrate versatility |
| Bioelectrochemical | Microbial fuel cells/electrochemical hydrolysis | Concurrent organic matter degradation and electricity generation; viability for small-scale research and integrated energy systems | Low technological readiness; suboptimal electricity conversion efficiency; microbial consortia instability under environmental perturbations |
| Liquefaction | High-temperature/pressure liquefaction | High-yield liquid bio-oil production with elevated energy density; direct applicability as drop-in fuel | Demanding high-pressure apparatus; operational harshness; complex product profiles requiring energy-intensive purification |
| Hydrothermal Carbonization | Subcritical hydrothermal carbonization | Moderate operational temperatures yielding stable biochar; reduced reactor corrosion risks | Lower conversion efficiency than pyrolysis; extended reaction durations needed for optimal output |
| Seaweed Type | Efficiency | Cost | Sustainability |
|---|---|---|---|
| Pyrolysis | High energy density bio-oil yield | High (energy-intensive process, catalyst cost) | Medium (handles diverse feedstock, but requires product upgrading) |
| Anaerobic Fermentation | Medium (slow kinetics, substrate-dependent) | Low (mature, low-operational cost technology) | High (waste reduction, biogas production) |
| Enzymatic Hydrolysis | High bioconversion rate for target sugars | High (cost of specific enzymes, additives) | High (mild conditions, minimal pollution) |
| Bioelectrochemical | Low (current technology readiness level) | Very High (complex system, expensive materials) | High (direct energy recovery from organics) |
| Liquefaction | High yield of liquid product | High (high-pressure/temperature reactor cost) | Medium (utilizes wet biomass, but harsh conditions) |
| Hydrothermal Carbonization | Medium (lower conversion efficiency than pyrolysis) | Medium (moderate conditions, but extended durations) | High (converts wet feedstock, produces stable biochar) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Zhang, S.; Xian, S. Synergistic Conversion and Catalytic Upgrading of Seaweed Biomass for Sustainable Bioenergy: Advances, Challenges, and Future Prospects. Catalysts 2025, 15, 1008. https://doi.org/10.3390/catal15111008
Xu Q, Zhang S, Xian S. Synergistic Conversion and Catalytic Upgrading of Seaweed Biomass for Sustainable Bioenergy: Advances, Challenges, and Future Prospects. Catalysts. 2025; 15(11):1008. https://doi.org/10.3390/catal15111008
Chicago/Turabian StyleXu, Qing, Shenwei Zhang, and Shengxian Xian. 2025. "Synergistic Conversion and Catalytic Upgrading of Seaweed Biomass for Sustainable Bioenergy: Advances, Challenges, and Future Prospects" Catalysts 15, no. 11: 1008. https://doi.org/10.3390/catal15111008
APA StyleXu, Q., Zhang, S., & Xian, S. (2025). Synergistic Conversion and Catalytic Upgrading of Seaweed Biomass for Sustainable Bioenergy: Advances, Challenges, and Future Prospects. Catalysts, 15(11), 1008. https://doi.org/10.3390/catal15111008






