Immobilization and Catalytic Properties of Limonene-1,2-Epoxide Hydrolase
Abstract
1. Introduction
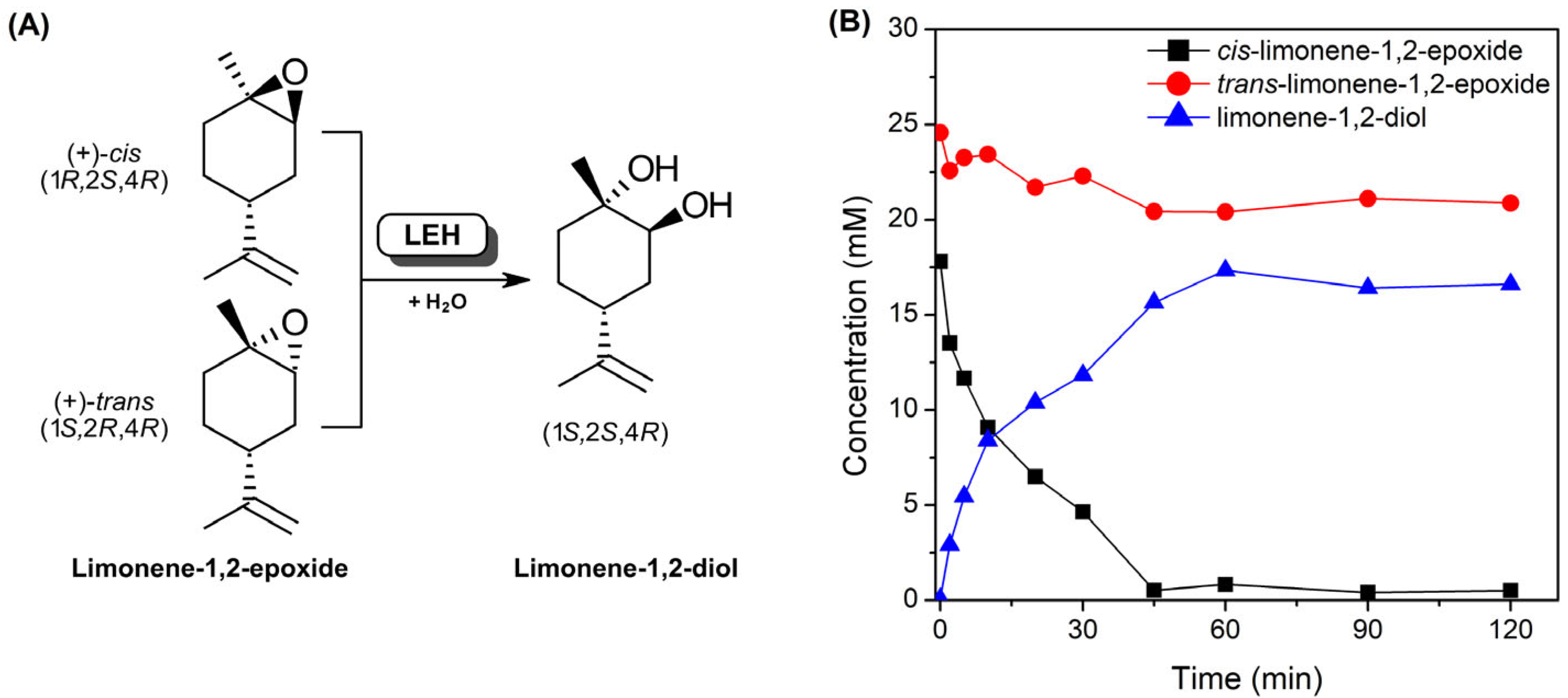
2. Results and Discussion
2.1. Expression and Purification of LEH
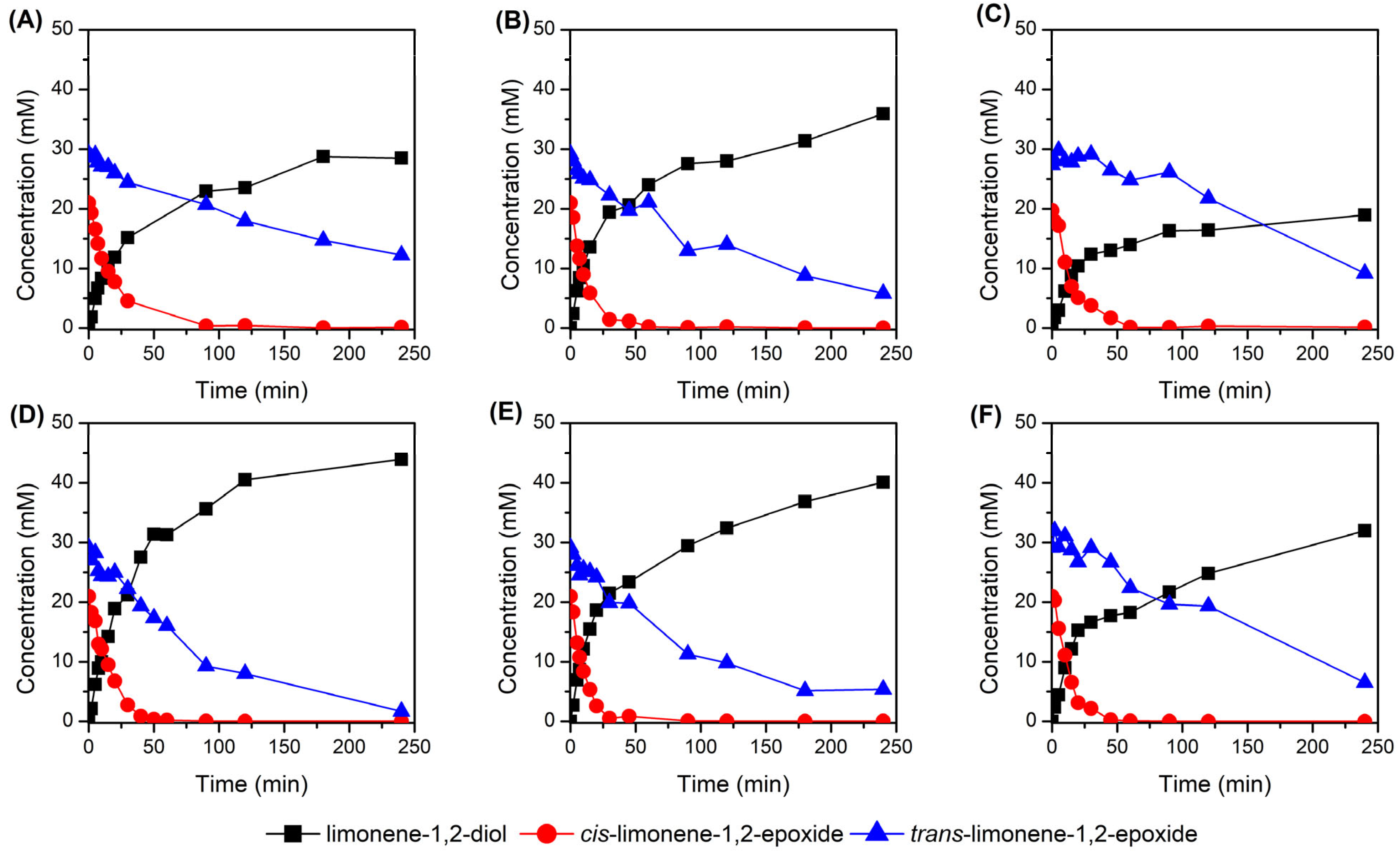
2.2. Immobilization of LEH on Different Carriers
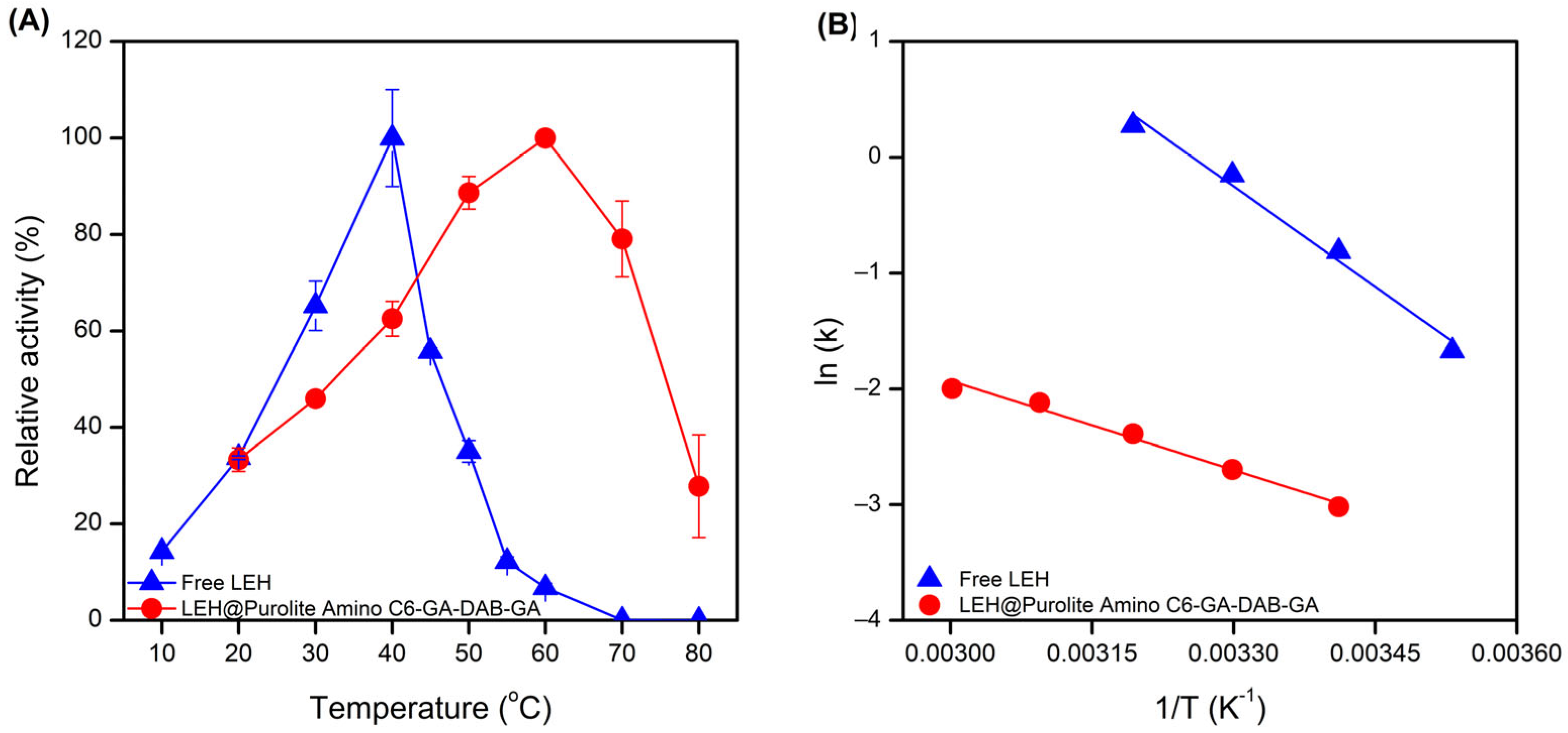
2.3. Temperature-Activity Profiles of Free and Immobilized LEH
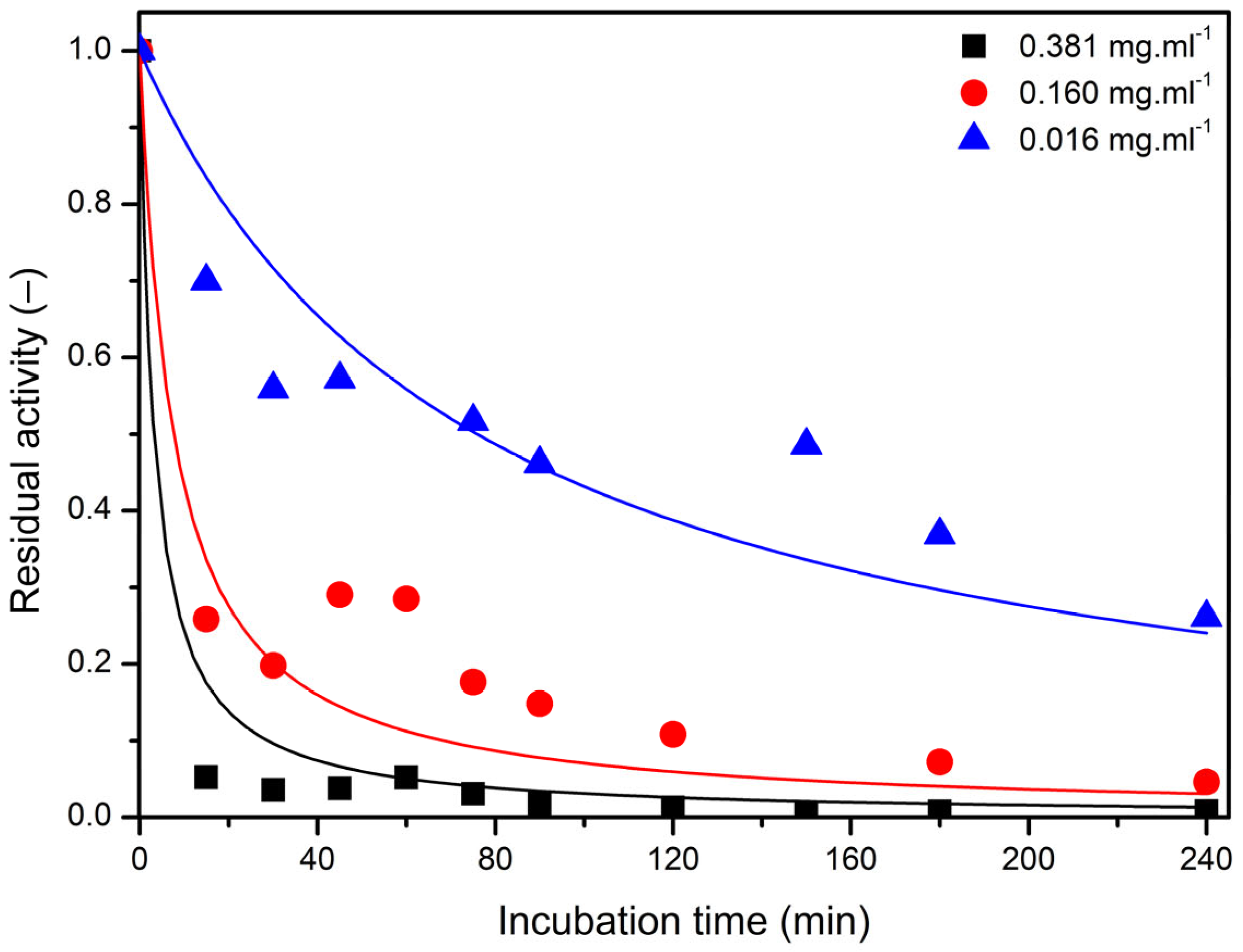
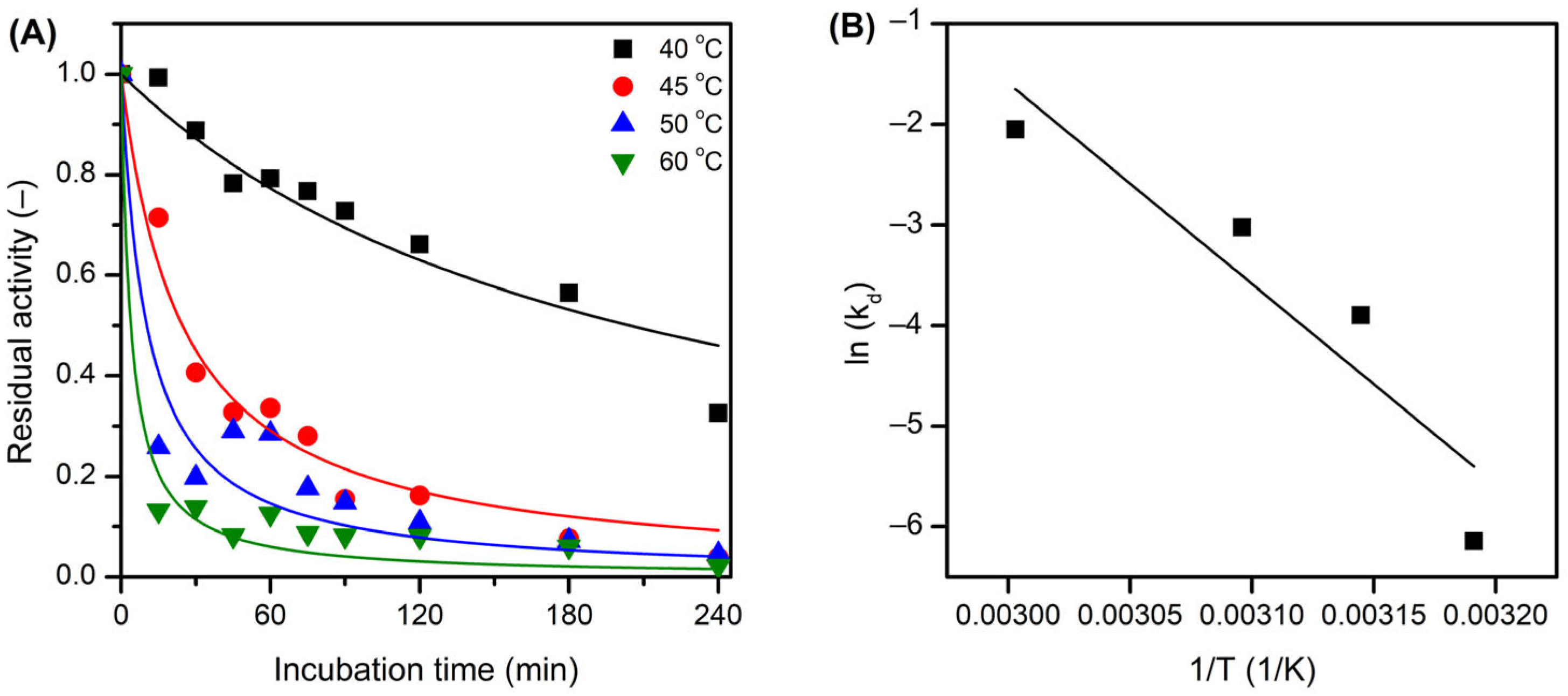
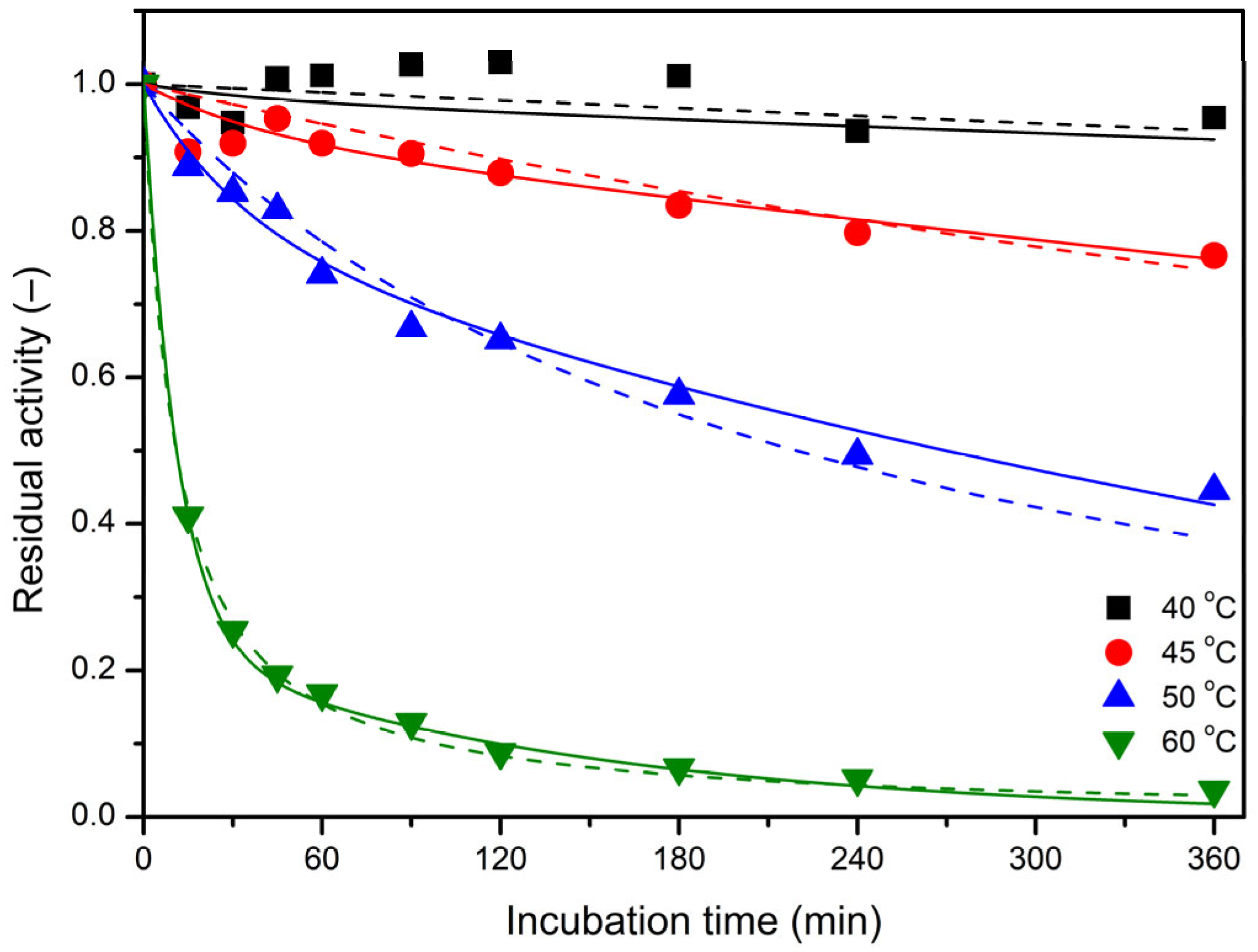
2.4. Thermal Inactivation of Free and Immobilized LEH
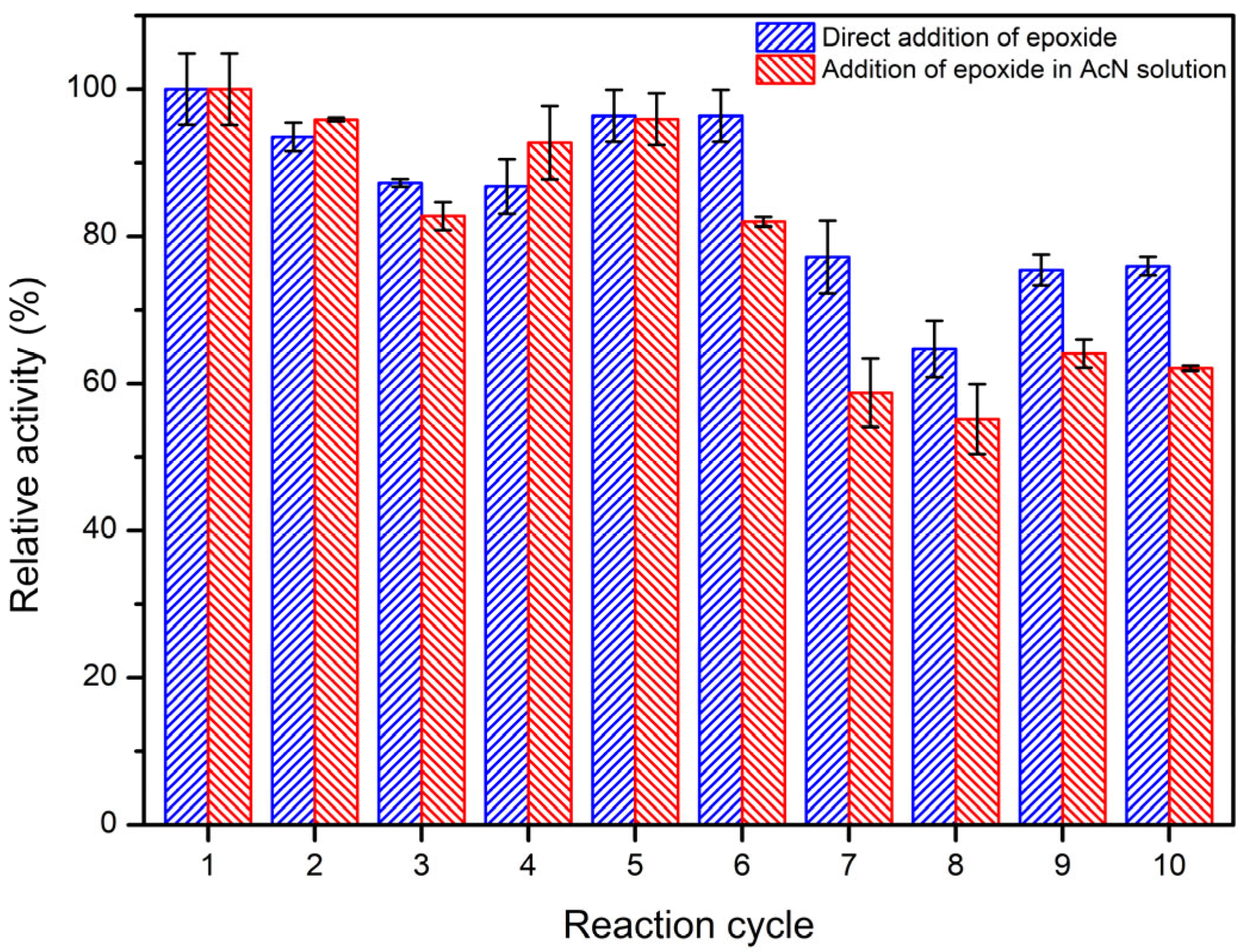
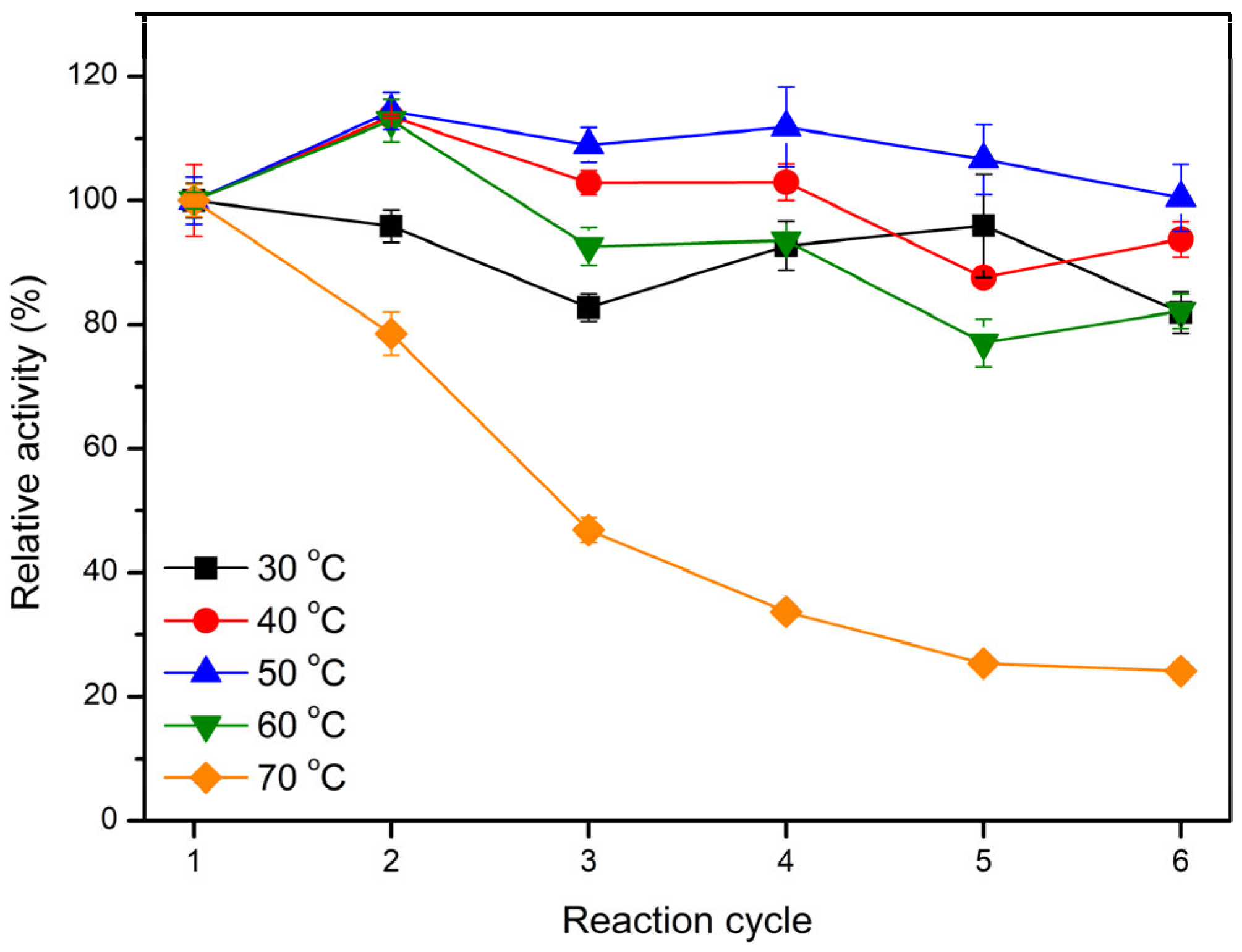
2.5. Operational Stability of Immobilized LEH
3. Materials and Methods
3.1. Materials
3.2. Cloning
3.3. Production and Purification of Limonene-1,2-Epoxide Hydrolase
3.4. Modification of Carriers
3.4.1. Modification of Epoxy Carriers with 1,4-Diaminobutane and Glutaraldehyde
3.4.2. Modification of Epoxy Carriers with IDA and Ni2+
3.4.3. Activation of Amino Carriers with Glutaraldehyde
3.4.4. Extension of Spacer Arms with 1,4-Diaminobutane and Glutaraldehyde
3.5. Immobilization of LEH
3.6. Enzyme Assay
3.7. Effect of Temperature on Free and Immobilized LEH
3.8. Thermal Stability of Free and Immobilized LEH
3.9. Operational Stability of Immobilized LEH
3.10. GC Analysis
3.11. Kinetic Analysis of the Enzyme Inactivation Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bučko, M.; Kaniaková, K.; Hronská, H.; Gemeiner, P.; Rosenberg, M. Epoxide Hydrolases: Multipotential Biocatalysts. Int. J. Mol. Sci. 2023, 24, 7334. [Google Scholar] [CrossRef]
- van der Werf, M.J.; Overkamp, K.M.; de Bont, J.A. Limonene-1,2-epoxide hydrolase from Rhodococcus erythropolis DCL14 belongs to a novel class of epoxide hydrolases. J. Bacteriol. 1998, 180, 5052–5057. [Google Scholar] [CrossRef]
- Arand, M.; Hallberg, B.M.; Zou, J.; Bergfors, T.; Oesch, F.; van der Werf, M.J.; de Bont, J.A.; Jones, T.A.; Mowbray, S.L. Structure of Rhodococcus erythropolis limonene-1,2-epoxide hydrolase reveals a novel active site. EMBO J. 2003, 22, 2583–2592. [Google Scholar] [CrossRef]
- van der Werf, M.J.; Swarts, H.J.; de Bont, J.A. Rhodococcus erythropolis DCL14 contains a novel degradation pathway for limonene. Appl. Environ. Microbiol. 1999, 65, 2092–2102. [Google Scholar] [CrossRef]
- Johansson, P.; Unge, T.; Cronin, A.; Arand, M.; Bergfors, T.; Jones, T.A.; Mowbray, S.L. Structure of an atypical epoxide hydrolase from Mycobacterium tuberculosis gives insights into its function. J. Mol. Biol. 2005, 351, 1048–1056. [Google Scholar] [CrossRef]
- Ferrandi, E.E.; Sayer, C.; Isupov, M.N.; Annovazzi, C.; Marchesi, C.; Iacobone, G.; Peng, X.; Bonch-Osmolovskaya, E.; Wohlgemuth, R.; Littlechild, J.A.; et al. Discovery and characterization of thermophilic limonene-1,2-epoxide hydrolases from hot spring metagenomic libraries. FEBS J. 2015, 282, 2879–2894. [Google Scholar] [CrossRef] [PubMed]
- Stojanovski, G.; Dobrijevic, D.; Hailes, H.C.; Ward, J.M. Identification and catalytic properties of new epoxide hydrolases from the genomic data of soil bacteria. Enzym. Microb. Technol. 2020, 139, 109592. [Google Scholar] [CrossRef] [PubMed]
- Hiraga, K.; Itoh, T.; Verma, D.; Wang, W.; Huang, C.; Ardolino, M.; Zhong, Y.-L.; Murphy, G. Directed Evolution to Reverse Epoxide Hydrolase Enantioselectivity for meso-3,4-Epoxytetrahydrofuran. ChemCatChem 2023, 15, e202300238. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, J.; Li, C.; Qu, G.; Yuan, B.; Chen, L.; Sun, Z. Engineering Limonene Epoxide Hydrolases for the Enantiocomplementary Synthesis of Chiral 1,3-Diols and Oxetanes. ACS Catal. 2025, 15, 9201–9209. [Google Scholar] [CrossRef]
- van der Werf, M.J.; Orru, R.V.A.; Overkamp, K.M.; Swarts, H.J.; Osprian, I.; Steinreiber, A.; de Bont, J.A.M.; Faber, K. Substrate specificity and stereospecificity of limonene-1,2-epoxide hydrolase from Rhodococcus erythropolis DCL14; an enzyme showing sequential and enantioconvergent substrate conversion. Appl. Microbiol. Biotechnol. 1999, 52, 380–385. [Google Scholar] [CrossRef]
- Zheng, H.; Reetz, M.T. Manipulating the Stereoselectivity of Limonene Epoxide Hydrolase by Directed Evolution Based on Iterative Saturation Mutagenesis. J. Am. Chem. Soc. 2010, 132, 15744–15751. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Lonsdale, R.; Li, G.; Reetz, M.T. Comparing Different Strategies in Directed Evolution of Enzyme Stereoselectivity: Single- versus Double-Code Saturation Mutagenesis. ChemBioChem 2016, 17, 1865–1872. [Google Scholar] [CrossRef]
- Sun, Z.; Lonsdale, R.; Wu, L.; Li, G.; Li, A.; Wang, J.; Zhou, J.; Reetz, M.T. Structure-Guided Triple-Code Saturation Mutagenesis: Efficient Tuning of the Stereoselectivity of an Epoxide Hydrolase. ACS Catal. 2016, 6, 1590–1597. [Google Scholar] [CrossRef]
- Sun, Z.; Lonsdale, R.; Kong, X.-D.; Xu, J.-H.; Zhou, J.; Reetz, M.T. Reshaping an Enzyme Binding Pocket for Enhanced and Inverted Stereoselectivity: Use of Smallest Amino Acid Alphabets in Directed Evolution. Angew. Chem. Int. Ed. 2015, 54, 12410–12415. [Google Scholar] [CrossRef]
- Arabnejad, H.; Bombino, E.; Colpa, D.I.; Jekel, P.A.; Trajkovic, M.; Wijma, H.J.; Janssen, D.B. Computational Design of Enantiocomplementary Epoxide Hydrolases for Asymmetric Synthesis of Aliphatic and Aromatic Diols. ChemBioChem 2020, 21, 1893–1904. [Google Scholar] [CrossRef]
- Li, J.-K.; Qu, G.; Li, X.; Tian, Y.; Cui, C.; Zhang, F.-G.; Zhang, W.; Ma, J.-A.; Reetz, M.T.; Sun, Z. Rational enzyme design for enabling biocatalytic Baldwin cyclization and asymmetric synthesis of chiral heterocycles. Nat. Commun. 2022, 13, 7813. [Google Scholar] [CrossRef]
- Xu, Z.-B.; Qu, J. Water-Promoted Kinetic Separation of trans- and cis-Limonene Oxides. Chin. J. Chem. 2012, 30, 1133–1136. [Google Scholar] [CrossRef]
- Ferrandi, E.E.; Marchesi, C.; Annovazzi, C.; Riva, S.; Monti, D.; Wohlgemuth, R. Efficient Epoxide Hydrolase Catalyzed Resolutions of (+)- and (−)-cis/trans-Limonene Oxides. ChemCatChem 2015, 7, 3171–3178. [Google Scholar] [CrossRef]
- Byrne, C.M.; Allen, S.D.; Lobkovsky, E.B.; Coates, G.W. Alternating Copolymerization of Limonene Oxide and Carbon Dioxide. J. Am. Chem. Soc. 2004, 126, 11404–11405. [Google Scholar] [CrossRef] [PubMed]
- Bailer, J.; Feth, S.; Bretschneider, F.; Rosenfeldt, S.; Drechsler, M.; Abetz, V.; Schmalz, H.; Greiner, A. Synthesis and self-assembly of biobased poly(limonene carbonate)-block-poly(cyclohexene carbonate) diblock copolymers prepared by sequential ring-opening copolymerization. Green Chem. 2019, 21, 2266–2272. [Google Scholar] [CrossRef]
- Zhang, D.; del Rio-Chanona, E.A.; Wagner, J.L.; Shah, N. Life cycle assessments of bio-based sustainable polylimonene carbonate production processes. Sustain. Prod. Consum. 2018, 14, 152–160. [Google Scholar] [CrossRef]
- Blair, M.; Tuck, K.L. A new diastereoselective entry to the (1S,4R)- and (1S,4S)-isomers of 4-isopropyl-1-methyl-2-cyclohexen-1-ol, aggregation pheromones of the ambrosia beetle Platypus quercivorus. Tetrahedron Asymmetry 2009, 20, 2149–2153. [Google Scholar] [CrossRef]
- Leung, A.E.; Blair, M.; Forsyth, C.M.; Tuck, K.L. Synthesis of the Proposed Structures of Prevezol C. Org. Lett. 2013, 15, 2198–2201. [Google Scholar] [CrossRef]
- Leung, A.E.; Rubbiani, R.; Gasser, G.; Tuck, K.L. Enantioselective total syntheses of the proposed structures of prevezol B and evaluation of anti-cancer activity. Org. Biomol. Chem. 2014, 12, 8239–8246. [Google Scholar] [CrossRef] [PubMed]
- Alexandrino, T.D.; Moya, A.M.T.M.; de Medeiros, T.D.M.; Morari, J.; Velloso, L.A.; Leal, R.F.; Maróstica, M.R.; Pastore, G.M.; Cazarin, C.B.B.; Bicas, J.L. Anti-inflammatory effects of monoterpenoids in rats with TNBS-induced colitis. PharmaNutrition 2020, 14, 100240. [Google Scholar] [CrossRef]
- Lappas, C.M.; Lappas, N.T. d-Limonene modulates T lymphocyte activity and viability. Cell. Immunol. 2012, 279, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Leitão, S.G.; Martins, G.R.; Martínez-Fructuoso, L.; de Sousa Silva, D.; da Fonseca, T.S.; Castilho, C.V.V.; Baratto, L.C.; Alviano, D.S.; Alviano, C.S.; Leitão, G.G.; et al. Absolute Stereochemistry of Antifungal Limonene-1,2-diols from Lippia rubella. Rev. Bras. Farmacogn. 2020, 30, 537–543. [Google Scholar] [CrossRef]
- Karboune, S.; Archelas, A.; Furstoss, R.; Baratti, J. Immobilization of epoxide hydrolase from Aspergillus niger onto DEAE-cellulose: Enzymatic properties and application for the enantioselective resolution of a racemic epoxide. J. Mol. Catal. B Enzym. 2005, 32, 175–183. [Google Scholar] [CrossRef]
- Karboune, S.; Archelas, A.; Baratti, J.C. Free and immobilized Aspergillus niger epoxide hydrolase-catalyzed hydrolytic kinetic resolution of racemic p-chlorostyrene oxide in a neat organic solvent medium. Process Biochem. 2010, 45, 210–216. [Google Scholar] [CrossRef]
- Maritz, J.; Krieg, H.M.; Yeates, C.A.; Botes, A.L.; Breytenbach, J.C. Calcium alginate entrapment of the yeast Rhodosporidium toruloides for the kinetic resolution of 1,2-epoxyoctane. Biotechnol. Lett. 2003, 25, 1775–1781. [Google Scholar] [CrossRef]
- Bao, W.; Pan, H.; Zhang, Z.; Cheng, Y.; Xie, Z.; Zhang, J. Isolation of the stable strain Labrys sp. BK-8 for l(+)-tartaric acid production. J. Biosci. Bioeng. 2015, 119, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Bučko, M.; Vikartovská, A.; Lacík, I.; Kolláriková, G.; Gemeiner, P.; Pätoprstý, V.; Brygin, M. Immobilization of a whole-cell epoxide-hydrolyzing biocatalyst in sodium alginate−cellulose sulfate−poly(methylene-co-guanidine) capsules using a controlled encapsulation process. Enzym. Microb. Technol. 2005, 36, 118–126. [Google Scholar] [CrossRef]
- Bučko, M.; Vikartovská, A.; Gemeiner, P.; Lacík, I.; Kolláriková, G.; Marison, I.W. Nocardia tartaricans cells immobilized in sodium alginate–cellulose sulfate–poly (methylene-co-guanidine)capsules: Mechanical resistance and operational stability. J. Chem. Technol. Biotechnol. 2006, 81, 500–504. [Google Scholar] [CrossRef]
- Cassimjee, K.E.; Kourist, R.; Lindberg, D.; Wittrup Larsen, M.; Thanh, N.H.; Widersten, M.; Bornscheuer, U.T.; Berglund, P. One-step enzyme extraction and immobilization for biocatalysis applications. Biotechnol. J. 2011, 6, 463–469. [Google Scholar] [CrossRef]
- Wang, Z.; Su, M.; Li, Y.; Wang, Y.; Su, Z. Production of tartaric acid using immobilized recominant cis-epoxysuccinate hydrolase. Biotechnol. Lett. 2017, 39, 1859–1863. [Google Scholar] [CrossRef]
- Yu, C.-Y.; Li, X.-F.; Lou, W.-Y.; Zong, M.-H. Cross-linked enzyme aggregates of Mung bean epoxide hydrolases: A highly active, stable and recyclable biocatalyst for asymmetric hydrolysis of epoxides. J. Biotechnol. 2013, 166, 12–19. [Google Scholar] [CrossRef]
- Yu, C.-Y.; Wei, P.; Li, X.-F.; Zong, M.-H.; Lou, W.-Y. Using Ionic Liquid in a Biphasic System to Improve Asymmetric Hydrolysis of Styrene Oxide Catalyzed by Cross-Linked Enzyme Aggregates (CLEAs) of Mung Bean Epoxide Hydrolases. Ind. Eng. Chem. Res. 2014, 53, 7923–7930. [Google Scholar] [CrossRef]
- Grulich, M.; Maršálek, J.; Kyslík, P.; Štěpánek, V.; Kotik, M. Production, enrichment and immobilization of a metagenome-derived epoxide hydrolase. Process Biochem. 2011, 46, 526–532. [Google Scholar] [CrossRef]
- Yildirim, D.; Tükel, S.S.; Alptekin, Ö.; Alagöz, D. Immobilized Aspergillus niger epoxide hydrolases: Cost-effective biocatalysts for the prepation of enantiopure styrene oxide, propylene oxide and epichlorohydrin. J. Mol. Catal. B Enzym. 2013, 88, 84–90. [Google Scholar] [CrossRef]
- Onur, H.; Tülek, A.; Aslan, E.S.; Binay, B.; Yildirim, D. A new highly enantioselective stable epoxide hydrolase from Hypsibius dujardini: Expression in Pichia pastoris and immobilization in ZIF-8 for asymmetric hydrolysis of racemic styrene oxide. Biochem. Eng. J. 2022, 189, 108726. [Google Scholar] [CrossRef]
- Zou, S.-P.; Wang, Z.-C.; Qin, C.; Zheng, Y.-G. Covalent immobilization of Agrobacterium radiobacter epoxide hydrolase on ethylenediamine functionalised epoxy supports for biocatalytical synthesis of (R)-epichlorohydrin. Biotechnol. Lett. 2016, 38, 1579–1585. [Google Scholar] [CrossRef]
- Li, F.-L.; Zheng, Y.-C.; Li, H.; Chen, F.-F.; Yu, H.-L.; Xu, J.-H. Preparing β-blocker (R)-Nifenalol based on enantioconvergent synthesis of (R)-p-nitrophenylglycols in continuous packed bed reactor with epoxide hydrolase. Tetrahedron 2019, 75, 1706–1710. [Google Scholar] [CrossRef]
- Kamble, M.; Salvi, H.; Yadav, G.D. Preparation of amino-functionalized silica supports for immobilization of epoxide hydrolase and cutinase: Characterization and applications. J. Porous Mater. 2020, 27, 1559–1567. [Google Scholar] [CrossRef]
- Freitas, A.I.; Domingues, L.; Aguiar, T.Q. Tag-mediated single-step purification and immobilization of recombinant proteins toward protein-engineered advanced materials. J. Adv. Res. 2022, 36, 249–264. [Google Scholar] [CrossRef]
- Fichan, I.; Larroche, C.; Gros, J.B. Water Solubility, Vapor Pressure, and Activity Coefficients of Terpenes and Terpenoids. J. Chem. Eng. Data 1999, 44, 56–62. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Berenguer-Murcia, Á.; Carballares, D.; Morellon-Sterling, R.; Fernandez-Lafuente, R. Stabilization of enzymes via immobilization: Multipoint covalent attachment and other stabilization strategies. Biotechnol. Adv. 2021, 52, 107821. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, O.; Torres, R.; Ortiz, C.; Berenguer-Murcia, Á.; Rodrigues, R.C.; Fernandez-Lafuente, R. Heterofunctional Supports in Enzyme Immobilization: From Traditional Immobilization Protocols to Opportunities in Tuning Enzyme Properties. Biomacromolecules 2013, 14, 2433–2462. [Google Scholar] [CrossRef] [PubMed]
- Robescu, M.S.; Bavaro, T. A Comprehensive Guide to Enzyme Immobilization: All You Need to Know. Molecules 2025, 30, 939. [Google Scholar] [CrossRef]
- Yildirim, D.; Tükel, S.S.; Alagöz, D.; Alptekin, Ö. Preparative-scale kinetic resolution of racemic styrene oxide by immobilized epoxide hydrolase. Enzym. Microb. Technol. 2011, 49, 555–559. [Google Scholar] [CrossRef]
- Wijma, H.J.; Floor, R.J.; Jekel, P.A.; Baker, D.; Marrink, S.J.; Janssen, D.B. Computationally designed libraries for rapid enzyme stabilization. Protein Eng. Des. Sel. 2014, 27, 49–58. [Google Scholar] [CrossRef]
- Chalella Mazzocato, M.; Jacquier, J.-C. Recent Advances and Perspectives on Food-Grade Immobilisation Systems for Enzymes. Foods 2024, 13, 2127. [Google Scholar] [CrossRef]
- Zhang, C.; You, S.; Zhang, J.; Qi, W.; Su, R.; He, Z. An effective in-situ method for laccase immobilization: Excellent activity, effective antibiotic removal rate and low potential ecological risk for degradation products. Bioresour. Technol. 2020, 308, 123271. [Google Scholar] [CrossRef]
- Zhou, Q.Z.K.; Chen, X.D. Effects of temperature and pH on the catalytic activity of the immobilized β-galactosidase from Kluyveromyces lactis. Biochem. Eng. J. 2001, 9, 33–40. [Google Scholar] [CrossRef]
- Tiarsa, E.R.; Yandri, Y.; Suhartati, T.; Satria, H.; Irawan, B.; Hadi, S. The Stability Improvement of Aspergillus fumigatus α-Amylase by Immobilization onto Chitin-Bentonite Hybrid. Biochem. Res. Int. 2022, 2022, 5692438. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef]
- Fleming, P.J.; Fleming, K.G. HullRad: Fast Calculations of Folded and Disordered Protein and Nucleic Acid Hydrodynamic Properties. Biophys. J. 2018, 114, 856–869. [Google Scholar] [CrossRef] [PubMed]
- Barbirato, F.; Verdoes, J.C.; de Bont, J.A.M.; van der Werf, M.J. The Rhodococcus erythropolis DCL14 limonene-1,2-epoxide hydrolase gene encodes an enzyme belonging to a novel class of epoxide hydrolases. FEBS Lett. 1998, 438, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Petrovičová, T.; Markošová, K.; Hegyi, Z.; Smonou, I.; Rosenberg, M.; Rebroš, M. Co-Immobilization of Ketoreductase and Glucose Dehydrogenase. Catalysts 2018, 8, 168. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Babich, L.; Hartog, A.F.; van der Horst, M.A.; Wever, R. Continuous-Flow Reactor-Based Enzymatic Synthesis of Phosphorylated Compounds on a Large Scale. Chem. A Eur. J. 2012, 18, 6604–6609. [Google Scholar] [CrossRef]
- Varga, V.; Štefuca, V.; Mihálová, L.; Levarski, Z.; Struhárňanská, E.; Blaško, J.; Kubinec, R.; Farkaš, P.; Sitkey, V.; Turňa, J.; et al. Recombinant Enzymatic Redox Systems for Preparation of Aroma Compounds by Biotransformation. Front. Microbiol. 2021, 12, 684640. [Google Scholar] [CrossRef] [PubMed]
- Vrábel, P.; Polakovič, M.; Štefuca, V.; Báleš, V. Analysis of mechanism and kinetics of thermal inactivation of enzymes: Evaluation of multitemperature data applied to inactivation of yeast invertase. Enzym. Microb. Technol. 1997, 20, 348–354. [Google Scholar] [CrossRef]
| Carrier—Modification | Immobilization Yield (%) | Retained Activity (%) | Immobilized Preparation Activity (U·g−1) |
|---|---|---|---|
| Purolite Epoxy | 99.2 | 0.07 | 0.7 |
| Immobead | 98.5 | 0.04 | 0.5 |
| Purolite Epoxy-IDA-Ni2+ | 99.4 | 0.79 | 2.1 |
| Purolite Epoxy-DAB-GA | 99.0 | 2.19 | 15.0 |
| Immobead-DAB-GA | 100.0 | 0.61 | 4.2 |
| Purolite Amino C2-GA | 99.6 | 6.02 | 41.3 |
| Purolite Amino C6-GA | 100.0 | 5.74 | 45.5 |
| Purolite Amino C2-GA-DAB-GA | 99.5 | 7.57 | 51.9 |
| Purolite Amino C6-GA-DAB-GA | 99.5 | 8.83 | 60.5 |
| Purolite Amino C2-GA-DAB-GA-DAB-GA | 99.1 | 6.37 | 30.6 |
| Purolite Amino C6-GA-DAB-GA-DAB-GA | 99.1 | 6.54 | 44.7 |
| Carrier | Carrier Type | Modification/Activation | Designation |
|---|---|---|---|
| Purolite LifetechTM ECR8209M | Epoxy Methacrylate | - * | Purolite Epoxy |
| DAB-GA | Purolite Epoxy-DAB-GA | ||
| IDA-Ni2+ | Purolite Epoxy-IDA-Ni2+ | ||
| Immobead 150P | Epoxy Methacrylate | - | Immobead |
| DAB-GA | Immobead-DAB-GA | ||
| Purolite LifetechTM ECR8309M | Amino C2 Methacrylate | GA | Purolite Amino C2-GA |
| GA-DAB-GA | Purolite Amino C2-GA-DAB-GA | ||
| GA-DAB-GA-DAB-GA | Purolite Amino C2-GA-DAB-GA-DAB-GA | ||
| Purolite LifetechTM ECR8409M | Amino C6 Methacrylate | GA | Purolite Amino C6-GA |
| GA-DAB-GA | Purolite Amino C6-GA-DAB-GA | ||
| GA-DAB-GA-DAB-GA | Purolite Amino C6-GA-DAB-GA-DAB-GA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaniaková, K.; Hronská, H.; Petrovičová, T.; Rebroš, M.; Rosenberg, M.; Štefuca, V. Immobilization and Catalytic Properties of Limonene-1,2-Epoxide Hydrolase. Catalysts 2025, 15, 986. https://doi.org/10.3390/catal15100986
Kaniaková K, Hronská H, Petrovičová T, Rebroš M, Rosenberg M, Štefuca V. Immobilization and Catalytic Properties of Limonene-1,2-Epoxide Hydrolase. Catalysts. 2025; 15(10):986. https://doi.org/10.3390/catal15100986
Chicago/Turabian StyleKaniaková, Katarína, Helena Hronská, Tatiana Petrovičová, Martin Rebroš, Michal Rosenberg, and Vladimír Štefuca. 2025. "Immobilization and Catalytic Properties of Limonene-1,2-Epoxide Hydrolase" Catalysts 15, no. 10: 986. https://doi.org/10.3390/catal15100986
APA StyleKaniaková, K., Hronská, H., Petrovičová, T., Rebroš, M., Rosenberg, M., & Štefuca, V. (2025). Immobilization and Catalytic Properties of Limonene-1,2-Epoxide Hydrolase. Catalysts, 15(10), 986. https://doi.org/10.3390/catal15100986










