Abstract
Leghemoglobin (LegH), a plant-derived hemoprotein, is engineered for the first time as a standalone biocatalyst for carbene N–H insertion. Through semi-rational design, the K65P mutation in the heme pocket significantly enhances catalytic efficiency. Under mild aqueous conditions (PBS buffer, 25 °C), the K65P variant achieves 92% yield in the model reaction between benzylamine and ethyl α-diazoacetate—surpassing wild-type LegH by >1.6-fold in initial reaction rate. The mutant also exhibits markedly improved thermostability. This work establishes engineered LegH as a high-performance, cofactor-free biocatalyst for C–N bond formation, providing a sustainable platform for synthesizing chiral amine derivatives. The catalytic proficiency and inherent stability of the K65P mutant demonstrate the potential of plant hemoproteins in non-natural carbene transfer reactions without requiring immobilization supports.
1. Introduction
The N–H insertion reaction is a crucial strategy for constructing C–N bonds in organic synthesis and plays a significant role in the synthesis of α-amino acid derivatives and nitrogen-containing heterocyclic drug intermediates [1,2]. The former participates in important physiological activities in living organisms, and the latter is a key raw material for antibacterial and antiviral drugs. The reaction efficiency directly affects the synthesis quality of related compounds [3,4]. Traditional chemical synthesis relies on transition metal catalysts such as Rh and Cu [5], but it has obvious drawbacks: heavy metal residues tend to accumulate in the environment, disrupting the ecological balance and threatening human health [6,7]; and insufficient control of chiral selectivity affects the purity and efficacy of products [8,9].
In contrast, biocatalysis uses enzymes as catalysts, which combines green sustainability with excellent stereoselectivity and is an important direction to replace traditional chemical catalysis [10,11,12]. Among them, the catalytic potential of heme proteins (such as cytochrome P450 and myoglobin) in carbene transfer reactions has been widely confirmed [13,14]. However, natural enzymes generally suffer from low catalytic efficiency and poor stability, which restricts their industrial applications [15,16]. Leghemoglobin (LegH) is a monomeric hemoprotein found in the root nodules of leguminous plants. Its structure features a single heme group embedded within a highly flexible and accessible pocket, which contributes to its remarkable structural plasticity. This flexibility allows for easier engineering of the active site through mutagenesis compared to more rigid hemoproteins like cytochrome P450s. For instance, neuroglobin has been engineered for carbene N–H insertions and quinoxalinone formation, and de novo designed carbene transferases have been developed for chemoselective N–H insertions. Additionally, Vitreoscilla hemoglobin has been engineered for the synthesis of unnatural α-amino acids, and myoglobin variants have been used in carbene and nitrene transfer reactions [14]. These examples illustrate the broad potential of engineered hemoproteins as metalloenzymes in abiotic catalysis. The active site of LegH contains key residues such as Tyr31, His62, Lys65, Leu66, Val104, and Phe45, which surround the heme group and can be modulated to enhance substrate binding and catalytic activity. The inherent adaptability of LegH’s active site makes it an ideal scaffold for rational and semi-rational design aimed at improving catalytic performance in non-natural reactions, such as carbene transfer. Figure 1 summarizes previously reported heme protein-catalyzed nitroarene reduction systems, including those based on cytochrome P450 [17], myoglobin [18], and other engineered hemoproteins [19,20,21].
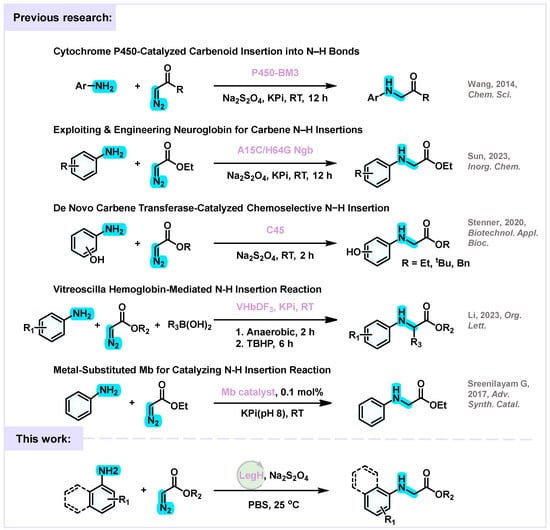
Figure 1.
Previous and current works on the reduction of nitroarenes catalyzed by heme proteins.
Leghemoglobin (LegH) is a specialized heme protein in the root nodules of leguminous plants. In addition to its function as an oxygen carrier, its structural plasticity makes it an ideal framework for enzyme molecular modification [21]. Compared with other heme proteins, LegH is easier to undergo site-directed mutagenesis and modification, facilitating the optimization of catalytic functions [22]. However, its catalytic potential in non-natural reactions has not been systematically explored. Based on this, we chose to modify LegH to catalyze N–H insertion reactions for the following reasons: From the perspective of LegH characteristics, its structural plasticity provides a basis for molecular modification. By adjusting amino acid residues, the active center can be optimized to enhance the binding and catalytic ability towards substrates (Scheme 1). The iron porphyrin structure in its active center is related to the carbene transfer process of the N–H insertion reaction, providing a potential structural basis for catalysis [23,24].
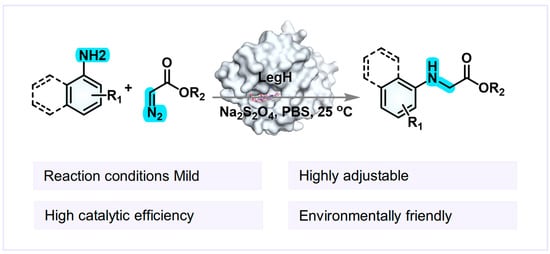
Scheme 1.
Schematic diagram of the carbene N–H insertion reaction catalyzed by LegH.
2. Results and Discussion
2.1. LegH Key Residue Mutations Enhance Carbene N–H Insertion Yields
We utilized various hemoproteins and enzymes (e.g., Hemin, BSA, LegH, etc.) as biocatalysts and sodium dithionite (Na2S2O4) as a reducing agent to conduct N–H insertion reactions with aniline and ethyl diazoacetate as substrates in phosphate-buffered solution (PBS, pH 7.4). It is evident that both WT-LegH and bovine hemoglobin (bHb) exhibited excellent catalytic activity toward the target reaction. However, compared with bHb, the monomeric protein structure of WT-LegH endows it with superior molecular modification potential-facilitating the engineering modification of its active site via approaches such as site-directed mutagenesis to achieve the directed enhancement in catalytic efficiency. Therefore, we analyzed the interactions between the carbene complex in the active cavity of WT-LegH and the aniline substrate through homology modeling and molecular docking, investigating the surrounding active residues [25,26]. From Figure 2, it can be observed that Tyr31, His62, Lys65, Leu66, Val104 and PHE45 are most directly involved with the substrate. Subsequently, we selected the three residues closest to the substrate, Tyr31, His62, and Lys65, to construct a mutation library using site-saturation mutagenesis [27,28]. After screening the mutant library, we were pleased to discover that the K65P mutant exhibited the highest catalytic activity (93 ± 1%), leading us to conduct further studies with K65P as the optimal biocatalyst for the N–H insertion reaction (optimal temperature, pH, and other details can be found in the Supplementary Materials (Figures S1–S12)).
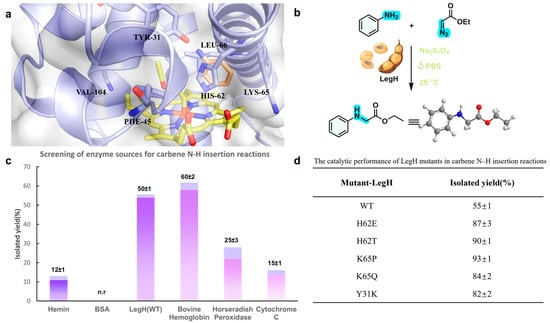
Figure 2.
Enzyme source screening and catalytic performance of LegH mutants in carbene N–H insertion reactions. (a) Screening of enzyme sources for carbene N-H insertion reactions, (b) Schematic diagram of the carbene N–H insertion reaction catalyzed by LegH, (c) Enzyme source screening and catalytic performance of LegH mutants in carbene N–H insertion reactions and (d) The catalytie performanee of LegH mutants in earbene N-H insertion reactions.
2.2. Efficient Carbene N–H Insertion Catalyzed by LegH
To gain comprehensive understanding, we conducted systematic reactions under optimized conditions using the biocatalyst LegH for its high selectivity and unique catalytic properties under mild conditions, with sodium dithionite (Na2S2O4) as the essential reductant. The results were highly promising: secondary amines 3a, 3b, and 3c exhibited excellent reactivity, achieving yields of 93%, 96%, and 95%, respectively. This high performance indicates strong affinity of the LegH active site for secondary amines and effective promotion of carbene N–H insertion [29,30]. Significantly, a sterically hindered derivative of 3f bearing an Ot-Bu/ester group still afforded a high yield of 99 ± 0.1%. This demonstrates the LegH-catalyzed system’s notable tolerance for bulky groups, implying sufficient active site flexibility to accommodate sterically demanding substrates while maintaining high catalytic efficiency. Representative substrates 3g, 3h, and 3i also showed good reactivity, with yields of 91 ± 0.6%, 94 ± 1.1%, and 82 ± 1.3%, respectively. Overall, yields for most tested amine substrates were predominantly ≥90%, highlighting the system’s broad adaptability. The turnover number (TON) for the K65P variant under optimized conditions was approximately 184, and the turnover frequency (TOF) was approximately 15.3 h−1. The system performs excellently with both common and sterically hindered amine substrates, providing a robust platform for synthesizing complex organic molecules, pharmaceuticals, and functional materials (Figure 3) [31,32].
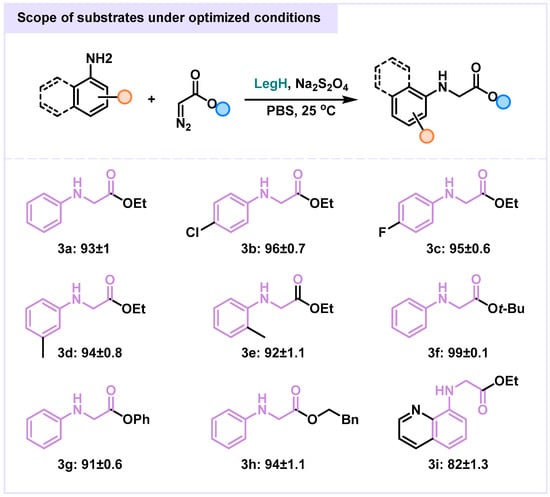
Figure 3.
Substrate Scope of LegH-Catalyzed Carbene N–H Insertion.
2.3. Plausible Mechanism of LegH-Catalyzed Carbene N–H Insertion Reaction
In the realm of modern chemical synthesis, the development of efficient and selective methods for C–N bond formation is of paramount importance due to the prevalence of C–N bonds in numerous bioactive molecules, pharmaceuticals, and functional materials. Among various strategies, carbene N–H insertion reactions have emerged as a powerful tool. To further elucidate the intrinsic mechanism of N–H insertion catalyzed by LegH, molecular docking analysis was employed [33,34]. The results indicate that the K65P mutant significantly optimizes the spatial conformation of the protein, markedly enhancing its substrate affinity. Compared to the wild-type LegH, the active site entrance of the K65P mutant is enlarged, providing additional space that facilitates the easier entry of the substrate aniline into the active site for reaction with the carbene intermediate, thereby improving catalytic efficiency. As depicted in the three-dimensional structure, conformational changes occur at the amino acid residues of the K65P mutant compared to the wild-type protein, shortening the distance between the substrate and the carbene intermediate and strengthening their binding to the protein active site. This rearrangement allows for a more precise positioning of the substrate and carbene intermediate within the active site, thereby enhancing catalytic efficiency [35,36]. The proposed mechanism is supported by molecular docking results (Figure 4) and the significantly improved catalytic performance of the K65P mutant, which aligns with the structural changes observed in the active site.
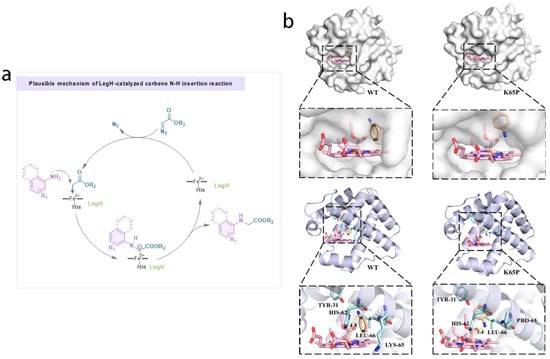
Figure 4.
Plausible mechanism of LegH-catalyzed carbene N–H insertion reaction. (a) Plausible mechanism of LegH-cstdyzed carbene N-H insertion reaction and (b) Molecular docking analysis for N–H insertion catalyzed by LegH.
3. Materials and Methods
3.1. Materials and Instruments
Amines, diazo compounds were purchased from Bide Pharmatech Ltd (Shanghai, China). and Energy Chemical company (Beijing, China). Other chemicals and reagents were purchased from commercial suppliers Shanghai Chemical Reagent Company (Shanghai, China) and used without any further purification, unless otherwise stated. Horseradish peroxidase, Hemoglobin from bovine blood, Cytochrome C was purchased from Shanghai Yuan Ye Biological Technology Company (Shanghai, China). Ni-NTA Superflow resin obtained from Beijing Solarbio Science & Technology Co., Ltd (Beijing, China). E. coli BL21(DE3) Competent Cell, Spin Miniprep, and Gel Extraction Kits were all obtained from Tiangen (Tianjin, China). Primers, genes and taq DNA Polymerase are obtained from Sangon Biotech (Shanghai, China). All reactions were carried out in oven-dried glassware with magnetic stirring. Silica gel chromatography purifications were carried out using AMD Silica Gel 60 230–400 mesh. Thin Layer Chromatography (TLC) and preparative TLC were carried out using Merck Millipore TLC silica gel 60 F254 glass plates. Proton nuclear magnetic resonance (1H NMR) spectra were recorded on a 400 MHz spectrometer (JEOL Company, Akishima, Japan) in CDCl3 or DMSO-d6, and carbon nuclear magnetic resonance (13C NMR) spectra were recorded on 100 MHz spectrometer (JEOL Company, Akishima, Japan) in CDCl3. Chemical shifts for protons are reported in parts per million downfield from tetramethylsilane (TMS) and are referenced to residual protium in the NMR solvent (CHCl3 = δ 7.26 ppm, DMSO-d6 = δ 2.50 ppm). Chemical shifts for carbon are reported in parts per million downfield from tetramethylsilane (TMS) and are referenced to the carbon resonances of the solvent residual peak (CDCl3 = δ 77.16 ppm). NMR data are presented as follows: chemical shift (δ ppm), multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br = broad), coupling constant in Hertz (Hz), integration. The experiments were performed triplicate, and all data were obtained based on the average values. Mass spectra were recorded on the Bruker MicrOTOF Q II and an Orbitrap Fusion Tribrid mass spectrometer (Thermo Scientific, San Jose, CA, USA) coupled with HESI ion source.
In this study, the following equipment was used in the experimental process: an analytical balance (Sartorius Corporate, model: PRACTUM-124-ICN, Gottingen, Germany), UV-Vis spectrophotometer (Shimadzu Corporation, model: UV-2700) (Shimadzu Company, Kyoto, Japan), constant-temperature incubator (New Brunswick, model: INNOVA-43) (Eppendorf Company, Mississauga, ON, Canada). biochemical incubator (Shanghai Yiheng Scientific Instruments Co., Ltd., model: LRH-70, Shanghai, China), pH meter (model: HI2221-02, HANNA company in Italy), protein/nucleic acid electrophoresis system (Bio-Rad Laboratories, model: 552BR, Hercules, CA, USA), power supply for electrophoresis (Bio-Rad Laboratories, model: 164-5052, Hercules, CA, USA), gel documentation system (Bio-Rad Laboratories, model: BR-720), PCR thermal cycler (Bio-Rad Laboratories, model: C1000, CA, USA), large-capacity refrigerated centrifuge (Beckman Coulter, model: J6-MI), high-speed refrigerated centrifuge (Beckman Coulter, model: AVANTI-J26XPI, Brea, CA, USA), benchtop refrigerated centrifuge (Sigma-Aldrich, model: 3K-15, St. Louis, MO, USA), laminar flow hood (Suzhou Antai Airtech Co., Ltd., model: SW-CJ-1FD, Suzhou, China), ultrasonic cell disruptor (Ningbo Scientz Biotechnology Co., Ltd., model: SCIENTZ-II-D, Ningbo, China), ultra-low temperature freezer (Zhongke Meiling Cryogenics Co., Ltd., model: DW-HL780, Hefei, China), autoclave (Shanghai Shen’an Medical Instrument Co., Ltd., model: LDZM-60KCS-II, Shanghai, China), circulating water vacuum pump (Shanghai Qiuzuo Scientific Instruments Co., Ltd., model: SHZ-D, Shanghai, China), NMR spectrometer (Varian, model: Inova-500, Agilent Technologies, Inc., Santa Clara, CA, USA), rotary evaporator (Shanghai Yarong Biochemical Instrument Factory, model: RE52CS, Shanghai, China), benchtop refrigerated centrifuge (Hunan Xiangli Scientific Instruments Co., Ltd., Hunan, China), digital constant-temperature magnetic stirrer (Jintan Dadi Automation Instrument Co., Ltd., Jintan, China), fume hood (Beijing Senleipu Laboratory Equipment Co., Ltd., Beijing, China), ultrasonic cleaner (Kunshan Ultrasonic Instrument Co., Ltd., model: KQ-250DE, Kunshan, China), analytical balance (Kunshan Jutian Instrument Equipment Co., Ltd., model: JJ224BC, Kunshan, China), electronic balance (Kunshan Jutian Instrument Equipment Co., Ltd., model: BSA223S-CW, Kunshan, China), constant-temperature water bath (Beijing Shuiguangming Medical Instrument Co., Ltd., Beijing, China), constant-temperature shaking incubator (Harbin Donglian Electronic Technology Development Co., Ltd., Harbin, China).
3.2. Experimental Procedures for the Heterologous Expression of Soybean Hemoglobin
3.2.1. Construction of the LegH Expression Vector
The coding sequence of soybean leghemoglobin (LegH) was retrieved from NCBI. A 6 × His tag (HHHHHH) was added at the C-terminus to facilitate protein purification. The gene was codon-optimized for Escherichia coli and inserted between the BamHI and XhoI sites of pET-28a (+). The resulting plasmid, pET-28a (+)-LegH-His6, was synthesized by Sangon Biotech (Shanghai, China).
3.2.2. Transformation of the LegH Plasmid
Competent E. coli BL21(DE3) cells stored at –80 °C were thawed on ice for 1 min. Four microlitres of the LegH plasmid were added, gently mixed, and incubated on ice for 30 min. Cells were heat-shocked at 42 °C for 60 s, then immediately chilled on ice for 2–3 min. Nine hundred microlitres of antibiotic-free LB medium were added, and the mixture was incubated at 37 °C and 180 rpm for 60 min to allow for expression of the kanamycin resistance gene. The culture was centrifuged at 3000 rpm for 3 min. After discarding 950 µL of the supernatant, the pellet was gently resuspended in the remaining medium and spread on LB agar plates containing 50 µg mL−1 kanamycin. Plates were inverted and incubated at 37 °C for 12–15 h. A single colony was inoculated into 1 mL LB-kanamycin medium and grown at 37 °C, 180 rpm for 12–16 h. Six hundred microlitres of the culture were mixed with an equal volume of 60% glycerol and stored at –20 °C.
3.2.3. Colony PCR Confirmation of Positive Clones
Primers used for colony PCR are listed in Table 1.

Table 1.
Colony PCR primers.
Single colonies were picked with a sterile loop and transferred directly into PCR tubes. The reaction mixture (Table 2) was prepared on ice and amplified using the following program: 98 °C for 15 min; 35 cycles of 98 °C for 30 s, 62 °C for 30 s, and 72 °C for 2 min; final extension at 72 °C for 5 min.

Table 2.
Colony PCR reaction system.
After amplification, 5 µL of 6× DNA loading buffer were added to each sample. PCR products were analysed by 1% agarose gel electrophoresis. Colonies yielding the expected band were cultured and stored at –20 °C in 30% glycerol.
3.2.4. Sequencing Confirmation
A 500 µL aliquot of the glycerol stock from Section 3.2.2 was cultured at 37 °C and 180 rpm for 6 h. The sample was sent for Sanger sequencing. The remaining culture was mixed with an equal volume of 60% glycerol and stored at –20 °C. Chromatograms were aligned with the reference sequence using BioEdit.
3.2.5. Induction of LegH Expression
A glycerol stock of the verified recombinant strain was cultured at 37 °C and 180 rpm for 12–15 h. Two millilitres of this pre-culture were used to inoculate 1 L LB medium containing 50 µg mL−1 kanamycin. Cells were grown at 37 °C to an OD600 of 0.6–0.8. IPTG and 5-aminolevulinic acid (5-ALA) were added to final concentrations of 0.05 mM and 0.2 mM, respectively. The culture was then shifted to 25 °C and 160 rpm for 30 h.
3.2.6. Purification of LegH
Cell Harvest and Lysis
Cells were harvested by centrifugation at 4000 rpm and 4 °C for 30 min. The pellet was resuspended in ice-cold PBS. Suspensions were lysed on ice with a 6 mm probe using 2 s pulses and 3 s pauses at 45% amplitude for 20 min per cycle; three cycles were applied. The lysate was centrifuged at 12,000 rpm and 4 °C for 30 min. The supernatant was collected for purification.
Ni-NTA Affinity Chromatography
The Ni-NTA column was first washed with two column volumes (CV) of ultra-pure water and then equilibrated with 3 CV of PBS. The clarified lysate was loaded onto the column and allowed to flow through by gravity. The flow-through was collected and re-loaded two additional times. After binding, the column was washed with 4 CV of PBS, followed by 2 CV of 20 mM imidazole in PBS to remove non-specific proteins. Bound LegH was eluted with 2 CV of 200 mM imidazole in PBS. The column was regenerated with 1 M imidazole, washed with 5 CV of PBS, and stored in 20% ethanol at 4 °C.
Desalting and Concentration
The eluted protein was applied to a Sephadex G-25 desalting column pre-equilibrated with 5 CV of PBS and 5 CV of deionised water. The red fraction was collected and concentrated using a 10 kDa cut-off centrifugal filter at 4000 rpm and 4 °C. The column was washed with 3 CV of PBS and 3 CV of deionised water and stored in 20% ethanol at 4 °C.
3.2.7. SDS-PAGE Analysis
A 12% separating gel and a 5% stacking gel were prepared. Twenty micrograms of protein were mixed with 5× loading buffer, heated at 95 °C for 10 min, cooled, and centrifuged at 5000 rpm for 1 min. Samples and 5 µL protein marker were loaded onto the gel. Electrophoresis was run at 90 V until the dye front entered the separating gel and then at 120 V for 1 h. Gels were stained with Coomassie Brilliant Blue R-250 for 30 min and destained overnight.
3.2.8. Protein Concentration Determination
One microlitre of purified LegH was measured with a NanoDrop at 280 nm. Three independent readings were averaged. The total yield was calculated from the concentration and the final volume.
3.2.9. UV-Vis Spectroscopy and RZ Value
Purified LegH was scanned from 200 to 800 nm using a UV-2700 spectrophotometer with a 1 nm slit width and a scan speed of 100 nm s−1. PBS was used for baseline correction. The RZ value was defined as A404/A275; a higher RZ indicates higher purity.
3.2.10. Optimisation of Expression Conditions
Induction Temperature
Cultures were grown as described in Section 3.2.5 until OD600 0.6–0.8. IPTG (0.05 mM) and 5-ALA (0.2 mM) were added, and cultures were shifted to 20, 25, 30, 35, or 37 °C at 190 rpm for 30 h. Protein yield and purity were assessed by SDS-PAGE and NanoDrop as in Section 3.2.7 and Section 3.2.8.
IPTG-Induced Concentration
Cultures were grown to OD600 0.8–1.0. 5-ALA was added to 0.2 mM, followed by IPTG at 0.05, 0.1, 0.2, 0.3, 0.5, or 0.8 mM. Cultures were incubated at 30 °C and 190 rpm for 30 h and analysed as above.
5-ALA Concentration
Cultures were grown to OD600 0.8–1.0. IPTG was added to 0.10 mM, followed by 5-ALA at 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.8, or 1.0 mM. Cultures were incubated at 30 °C and 190 rpm for 30 h and analysed as above.
3.3. Experimental Procedure for the LegH-Catalyzed Carbene N-H Insertion Reaction
3.3.1. Experimental Method
Aniline and a series of its derivatives (1 mmol), ethyl diazoacetate (1 mmol), enzyme (protein content: 15 mg), sodium dithionite (25 mg), and deionized water (10 mL) were mixed in a 25 mL round-bottom flask. The reaction mixture was kept at 25 °C for 12 h. After the reaction was completed, LegH was removed by centrifugation (4500 rpm, 5 min), and the mixture was extracted with dichloromethane. The organic phase was dried over anhydrous sodium sulfate, and the volatile substances were removed by concentration under reduced pressure. The residue was purified by column chromatography with ethyl acetate/petroleum ether (1/8), and the obtained product was characterized by proton nuclear magnetic resonance spectroscopy. All experiments were carried out in triplicate, with the final results presented as average values. The residue was purified by column chromatography, and the obtained product was first characterized by 1H NMR spectroscopy; yields were further determined via 1H NMR using 1,3,5-trimethoxybenzene as an internal standard. In addition, 13C NMR and ESI MS analyses are available in the SI.
3.3.2. Reaction Condition Optimization
Reaction temperature, time, and catalyst loading strongly influence the efficiency of the hemoglobin-Cu3(PO4)2 nanoflower-catalyzed N–H insertion of carbenes. Each parameter was optimized in turn.
Reaction Time
Generally, to investigate the effect of reaction time on catalytic yield, we examined the impact of time on the yield of the LegH-catalyzed carbene N–H insertion reaction under the following conditions: aniline (1 mmol), ethyl diazoacetate (1 mmol), deionized water (10 mL), catalyst (protein content: 15 mg), and sodium dithionite (25 mg) at 25 °C, by varying the reaction time as the single variable.
Reaction Temperature
Temperature is a critical parameter governing enzymatic reaction progression. In this study, we systematically examined the effect of reaction temperature on catalytic yield. The optimization study was conducted under the following fixed conditions: aniline (1 mmol), ethyl diazoacetate (1 mmol), deionized water (10 mL), catalyst (protein content: 15 mg), sodium dithionite (25 mg), and a reaction time of 6 h. The reaction temperature was varied across nine levels (0 °C, 5 °C, 10 °C, 15 °C, 20 °C, 25 °C, 30 °C, 35 °C and 40 °C) to identify the optimal temperature for the LegH-catalyzed carbene N–H insertion reaction.
Catalyst Loading
Catalyst loading is a key factor influencing enzymatic reaction kinetics. Generally, higher catalyst concentrations accelerate reaction rates when other parameters remain constant (e.g., substrate concentration, reaction time and temperature). However, excessive catalyst may increase viscosity and compromise reaction efficiency. Therefore, to evaluate and optimize catalyst loading, we conducted experiments under the following fixed conditions: aniline (1 mmol), ethyl diazoacetate (1 mmol), deionized water (10 mL), sodium dithionite (25 mg), at 25 °C for 6 h. The catalyst (with consistent protein content of 10%) was added at eleven different loadings: 0.5 mg, 1.0 mg, 1.5 mg, 2.0 mg, 2.5 mg, 3.0 mg, 4.0 mg, 6.0 mg, 8.0 mg, 10.0 mg and 15.0 mg.
4. Conclusions
This study demonstrates for the first time the successful engineering of leghemoglobin (LegH) as a highly efficient, cofactor-independent biocatalyst for carbene N–H insertion reactions. Through semi-rational design and site-saturation mutagenesis, the K65P mutation within the heme pocket was identified as a key substitution that significantly enhances catalytic performance. The K65P variant achieved a remarkable 92% yield in the model reaction between benzylamine and ethyl α-diazoacetate under mild aqueous conditions (PBS buffer, 25 °C), outperforming the wild-type enzyme by more than 1.6-fold in initial reaction rate. Moreover, the mutant exhibited superior thermostability, further underscoring its practical utility. Molecular docking analyses revealed that the K65P mutation induces favorable conformational changes at the active site, enlarging the substrate entrance and optimizing the spatial arrangement for improved substrate binding and carbene intermediate positioning. This structural remodeling underpins the enhanced catalytic efficiency and broad substrate scope, including sterically hindered amines, with most substrates yielding ≥90% product. This work establishes engineered LegH as a robust and sustainable platform for C–N bond formation, offering an eco-friendly alternative to traditional metal-based catalysts. The high catalytic proficiency, operational simplicity, and inherent stability of the K65P variant highlight the untapped potential of plant hemoproteins in non-natural carbene transfer reactions, paving the way for their application in the synthesis of valuable chiral amines and pharmaceutical intermediates.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal15100950/s1, Figures S1–S9: Data related to the expression of LegH at different induction stages; Figures S10–S12: Optimal temperature, pH, and other details; Table S1–S2: Legh single-point saturation mutation primers and mutant strains; The 1H NMR data of the products; The 13C NMR data and ESI MS analyses.
Author Contributions
Conceptualization, M.G.; methodology, X.Z. and Z.W.; writing—review and editing, H.Z. and M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science and Technology Development Plan Project of Jilin Province, China, grant number YDZJ202301ZYTS356.
Data Availability Statement
The data presented in this study are available on request from the corresponding author share their research data.
Acknowledgments
The authors are grateful for the support of the Science and Technology Development Plan Project of Jilin Province. We thank the reviewers and editors for their careful review of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Durán, R.; Herrera, B. Theoretical Study of the Mechanism of Catalytic Enanteoselective N-H and O-H Insertion Reactions. J. Phys. Chem. A 2019, 124, 2–11. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Y.; Song, Q.; Li, L.; Zanoni, G.; Liu, S.; Xiang, M.; Anderson, E.A.; Bi, X. Chemoselective carbene insertion into the N−H bonds of NH3·H2O. Nat. Commun. 2022, 13, 7649–7662. [Google Scholar] [CrossRef]
- Sharma, A.; Vaid, H.; Kotwal, R.; Mughal, Z.N.; Gurubrahamam, R. Ramani Gurubrahamam. Rhodium (II)-Catalyzed Alkynyl Carbene Insertion into N–H Bonds. Org. Lett. 2024, 26, 4887–4892. [Google Scholar] [CrossRef]
- Kumar, D.; Rao Kuram, M. Copper-Catalyzed Chemoselective Divergent Carbene Insertion into the N−H bonds of Tryptamines. Adv. Synth. Catal. 2023, 365, 3935–3941. [Google Scholar] [CrossRef]
- Tang, M.; Jiao, X.; He, D.; Zhao, J.-X.; Liu, P.; Li, C.-T. Rh(II)/Pd(0) Dual Catalysis: Carbenoid N-H Insertion/Allylation Cascade Reaction to Construct Highly Functionalized and Polysubstituted Pyrrolidines. Molecules 2024, 29, 5880–5889. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, T.; Van Hecke, K.; Cazin, C.S.; Nolan, S.P. [Cu(NHC)(OR)] (R = C(CF3)3) complexes for N–H and S–H bond activation and as pre-catalysts in the Chan–Evans–Lam reaction. Dalton Trans. 2025, 4, 1329–1333. [Google Scholar] [CrossRef]
- Burtoloso, A.C.B.; Santiago, J.V.; Bernardim, B.; Talero, A.G. Advances in the Enantioselective Metal-catalyzed N-H and O-H Insertion Reactions with Diazocarbonyl Compounds. Curr. Org. Synth. 2015, 12, 650–659. [Google Scholar] [CrossRef]
- So, S.S.; Mattson, A.E. Urea Activation of α-Nitrodiazoesters: An Organocatalytic Approach to N–H Insertion Reactions. J. Am. Chem. Soc. 2022, 134, 8798–8801. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.Y.; Zhu, S.F.; Zhou, Q.L. Chiral proton-transfer shuttle catalysts for carbene insertion reactions. Org. Biomol. Chem. 2018, 16, 3087–3094. [Google Scholar] [CrossRef]
- Jintao, H.; Noor, N.D.M.; Kamarudin, N.H.A.; Ali, M.S.M. Review of enzyme catalysis in sustainable biofuel production: A comparative study of waste oils and alternative feedstocks. Int. J. Green. Energy 2025, 10, 1080–1088. [Google Scholar] [CrossRef]
- Mondal, D.; Snodgrass, H.M.; Gomez, C.A.; Lewis, J.C. Non-Native Site-Selective Enzyme Catalysis. Chem. Rev. 2025, 123, 10381–10431. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhai, S.; Zhan, X.; Siu, S.W. Data-driven revolution of enzyme catalysis from the perspective of reactions, pathways, and enzymes. Cell Rep. Phys. Sci. 2025, 6, 102466–102476. [Google Scholar] [CrossRef]
- Yang, Y.; Arnold, F.H. Navigating the Unnatural Reaction Space: Directed Evolution of Heme Proteins for Selective Carbene and Nitrene Transfer. Acc. Chem. Res. 2021, 54, 1209–1225. [Google Scholar] [CrossRef]
- Sreenilayam, G.; Moore, E.J.; Steck, V.; Fasan, R. Metal Substitution Modulates the Reactivity and Extends the Reaction Scope of Myoglobin Carbene Transfer Catalysts. Adv. Synth. Catal. 2021, 359, 2076–2089. [Google Scholar] [CrossRef]
- Razavi, B.S.; Blagodatskaya, E.; Kuzyakov, Y. Temperature selects for static soil enzyme systems to maintain high catalytic efficiency. Soil. Biol. Biochem. 2016, 97, 15–22. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, Z.; Xie, W.; Yu, Y.; Ning, C.; Yuan, M.; Mou, H. Improving catalytic efficiency and maximum activity at low pH of Aspergillus neoniger phytase using rational design. Int. J. Biol. Macromol. 2019, 131, 1117–1124. [Google Scholar] [CrossRef]
- Zeynizadeh, B.; Mohammad Aminzadeh, F.; Mousavi, H. Chemoselective reduction of nitroarenes, N-acetylation of arylamines, and one-pot reductive acetylation of nitroarenes using carbon-supported palladium catalytic system in water. Res. Chem. Intermed. 2021, 47, 3289–3312. [Google Scholar] [CrossRef]
- Ferlin, F.; Cappelletti, M.; Vivani, R.; Pica, M.; Piermatti, O.; Vaccaro, L. Au@zirconium-phosphonate nanoparticles as an effective catalytic system for the chemoselective and switchable reduction of nitroarenes. Green. Chem. 2019, 21, 614–626. [Google Scholar] [CrossRef]
- Antony, R.; Marimuthu, R.; Murugavel, R. Bimetallic Nanoparticles Anchored on Core-Shell Support as an Easily Recoverable and Reusable Catalytic System for Efficient Nitroarene Reduction. ACS Omega 2019, 4, 9241–9250. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, W.; Fu, X.; Chen, G.; Meng, S.; Chen, S. Ultra-low content of Pt modified CdS nanorods: Preparation, characterization, and application for photocatalytic selective oxidation of aromatic alcohols and reduction of nitroarenes in one reaction system. J. Hazard. Mater. 2019, 360, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Xie, L.; You, K.; Chen, W. High-level secretory production of leghemoglobin and myoglobin in Escherichia coli through inserting signal peptides. Food Biosci. 2023, 56, 103356–103367. [Google Scholar] [CrossRef]
- Shao, Y.; Xue, C.; Liu, W.; Zuo, S.; Wei, P.; Huang, L.; Lian, J.; Xu, Z. High-level secretory production of leghemoglobin in Pichia pastoris through enhanced globin expression and heme biosynthesis. Bioresour. Technol. 2022, 363, 127884–127894. [Google Scholar] [CrossRef]
- Sun, L.J.; Wang, H.; Xu, J.K.; Gao, S.Q.; Wen, G.B.; Lin, Y.W. Exploiting and Engineering Neuroglobin for Catalyzing Carbene N–H Insertions and the Formation of Quinoxalinones. Inorg. Chem. 2023, 62, 16294–16298. [Google Scholar] [CrossRef]
- Stenner, R.; Anderson, J.L.R. Chemoselective N-H insertion catalyzed by a de novo carbene transferase. Biotechnol. Appl. Biochem. 2023, 67, 527–535. [Google Scholar] [CrossRef]
- Li, F.; Xu, Y.; Xu, Y.; Xie, H.; Wu, J.; Wang, C.; Li, Z.; Wang, Z.; Wang, L. Engineering of Dual-Function Vitreoscilla Hemoglobin: A One-Pot Strategy for the Synthesis of Unnatural α-Amino Acids. Org. Lett. 2023, 25, 7115–7119. [Google Scholar] [CrossRef]
- Balhara, R.; Chatterjee, R.; Jindal, G. Mechanism and stereoselectivity in metal and enzyme catalyzed carbene insertion into X–H and C(sp2)–H bonds. Chem. Soc. Rev. 2024, 53, 11004–11044. [Google Scholar] [CrossRef] [PubMed]
- Siriboe, M.G.; Vargas, D.A.; Fasan, R. Dehaloperoxidase Catalyzed Stereoselective Synthesis of Cyclopropanol Esters. J. Org. Chem. 2023, 88, 7630–7640. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, L.; Su, H.; Liu, Y. Dioxygen Activation and Nδ,Nε-Dihydroxylation Mechanism Involved in the Formation of N-Nitrosourea Pharmacophore in Streptozotocin Catalyzed by Nonheme Diiron Enzyme SznF. Inorg. Chem. 2022, 61, 15721–15734. [Google Scholar] [CrossRef]
- Musiani, F.; Broll, V.; Evangelisti, E.; Ciurli, S. The model structure of the copper-dependent ammonia monooxygenase. J. Biol. Inorg. Chem. 2020, 25, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhou, T.-P.; Shen, Y.; HuJieyu, J.; Tang, Z.; Han, R.; Xu, G.; Schwaneberg, U.; Wang, B.; Ni, Y.; et al. Rational Engineering of Self-Sufficient P450s to Boost Catalytic Efficiency of Carbene-Mediated C–S Bond Formation. ACS Catal. 2020, 25, 995–1007. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Kamble, P.; Mundhe, P.; Jindal, M.; Thakur, P.; Bajaj, P. Multifaceted personality and roles of heme enzymes in industrial biotechnology. 3 Biotech 2023, 13, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Nam, D.; Tinoco, A.; Shen, Z.; Adukure, R.D.; Sreenilayam, G.; Khare, S.D.; Fasan, R. Enantioselective Synthesis of α-Trifluoromethyl Amines via Biocatalytic N–H Bond Insertion with Acceptor-Acceptor Carbene Donors. J. Am. Chem. Soc. 2022, 144, 2590–2602. [Google Scholar] [CrossRef] [PubMed]
- Natoli, S.N.; Hartwig, J.F. Noble-Metal Substitution in Hemoproteins: An Emerging Strategy for Abiological Catalysis. Acc. Chem. Res. 2019, 52, 326–335. [Google Scholar] [CrossRef]
- Gong, Z.; Wang, G.; Shao, S.; Wang, M.; Lu, K.; Gao, S. Co-degradation of Coexisting Pollutants Methylparaben (Mediators) and Amlodipine in Enzyme-Mediator Systems: Insight into the Mediating Mechanism. J. Hazard. Mater. 2012, 423, 127112–127122. [Google Scholar] [CrossRef]
- Steck, V.; Carminati, D.M.; Johnson, N.R.; Fasan, R. Enantioselective Synthesis of Chiral Amines via Biocatalytic Carbene N–H Insertion. ACS Catal. 2020, 10, 10967–10977. [Google Scholar] [CrossRef]
- Lather, J.; George, J. Improving Enzyme Catalytic Efficiency by Co-operative Vibrational Strong Coupling of Water. J. Phys. Chem. Lett. 2020, 12, 379–384. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).