Investigating the Impact of Incorporating Alkali Metal Cations on the Properties of ZSM-5 Zeolites in the Methanol Conversion into Hydrocarbons
Abstract
1. Introduction
2. Results and Discussion
2.1. The Effect of Aluminum Content on the Catalytic Performance of Alkali Metal-Doped ZSM-5 Zeolite
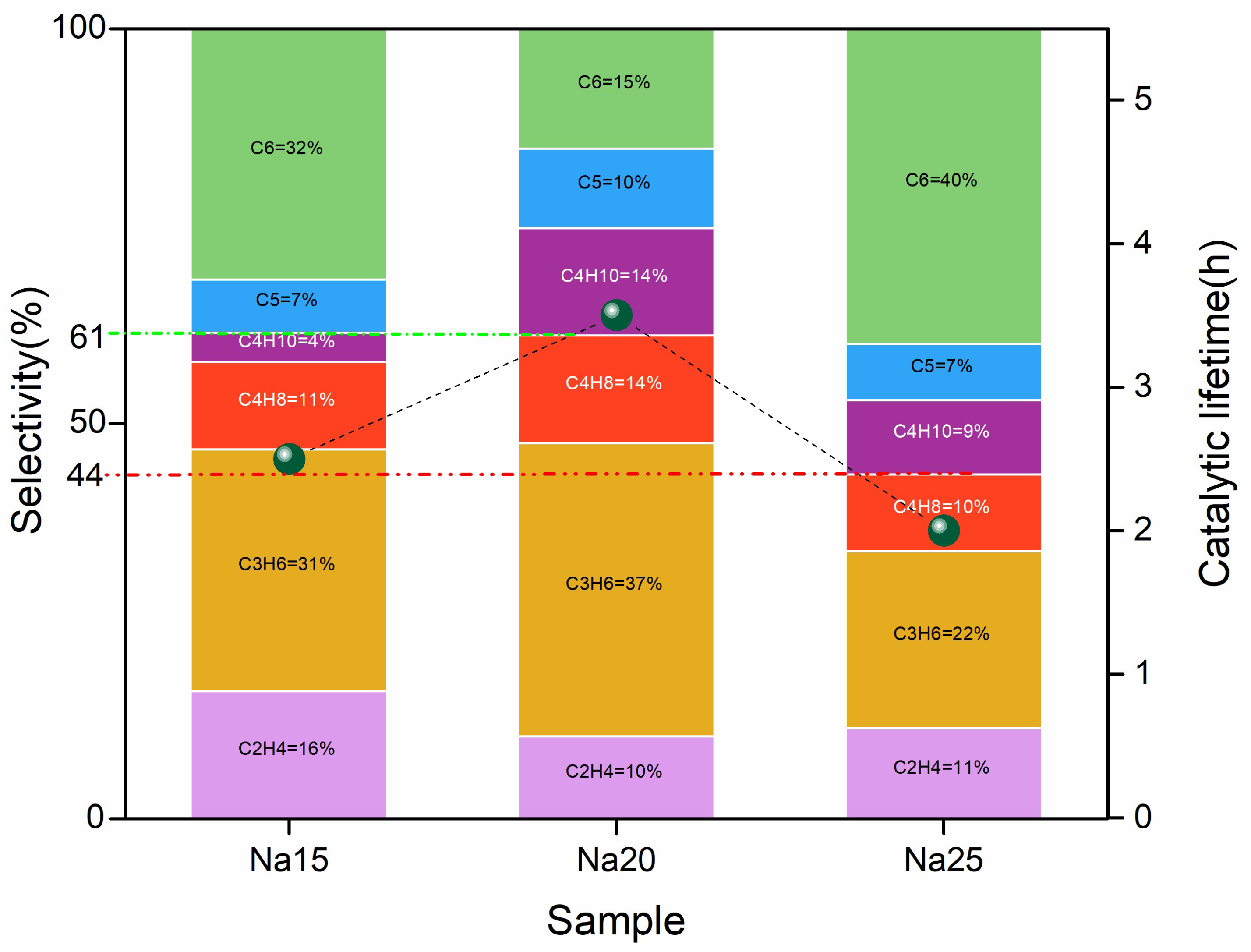
2.1.1. MTO Reaction Properties of Na-ZSM-5 Zeolites with Different Si/Al Ratios
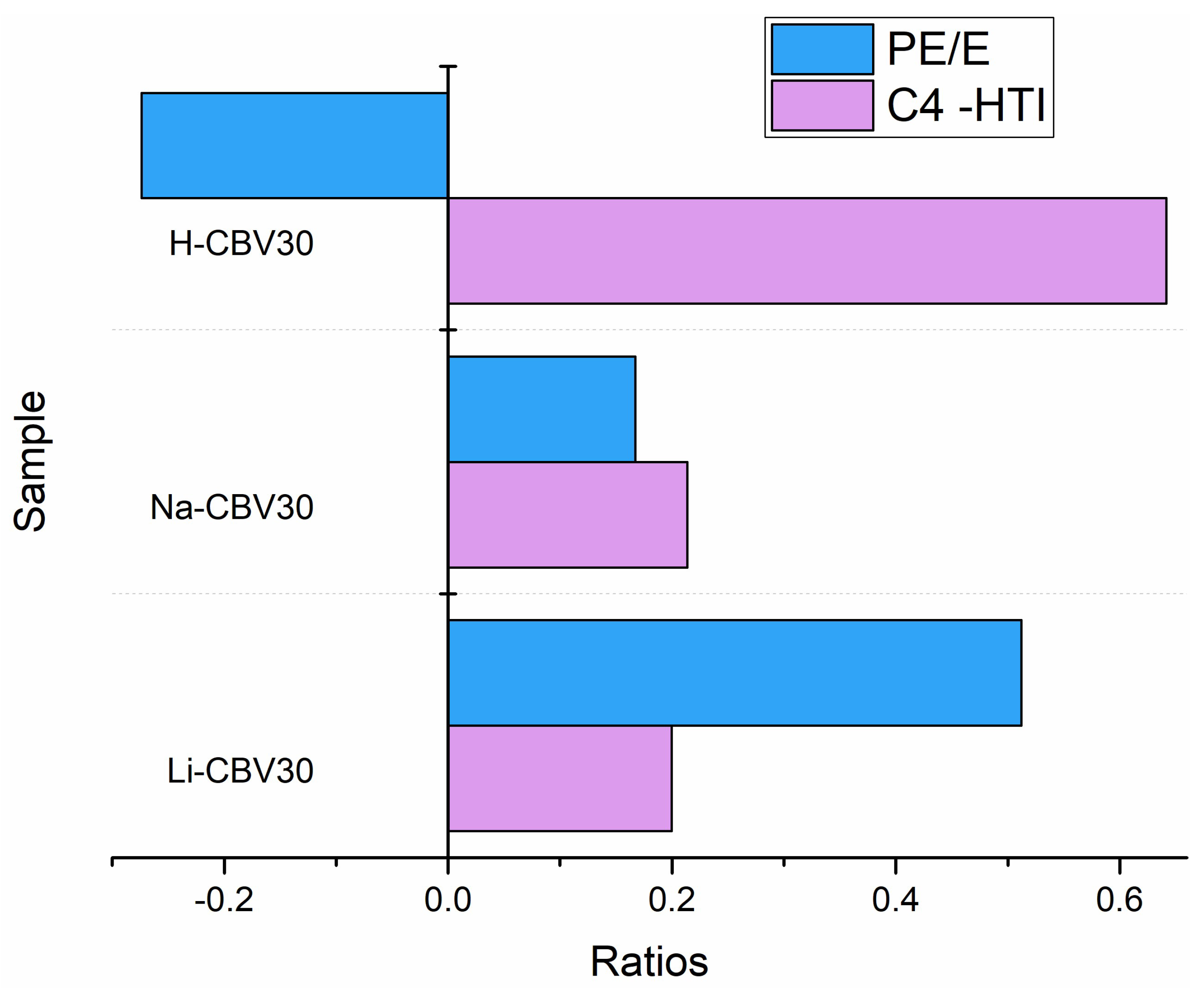
2.1.2. MTO Reaction Properties of Alkali Metal Ion-Loaded ZSM-5 Zeolite
2.2. The Effect of Different Alkali Metal Ions on the Physicochemical Properties of ZSM-5 Zeolite
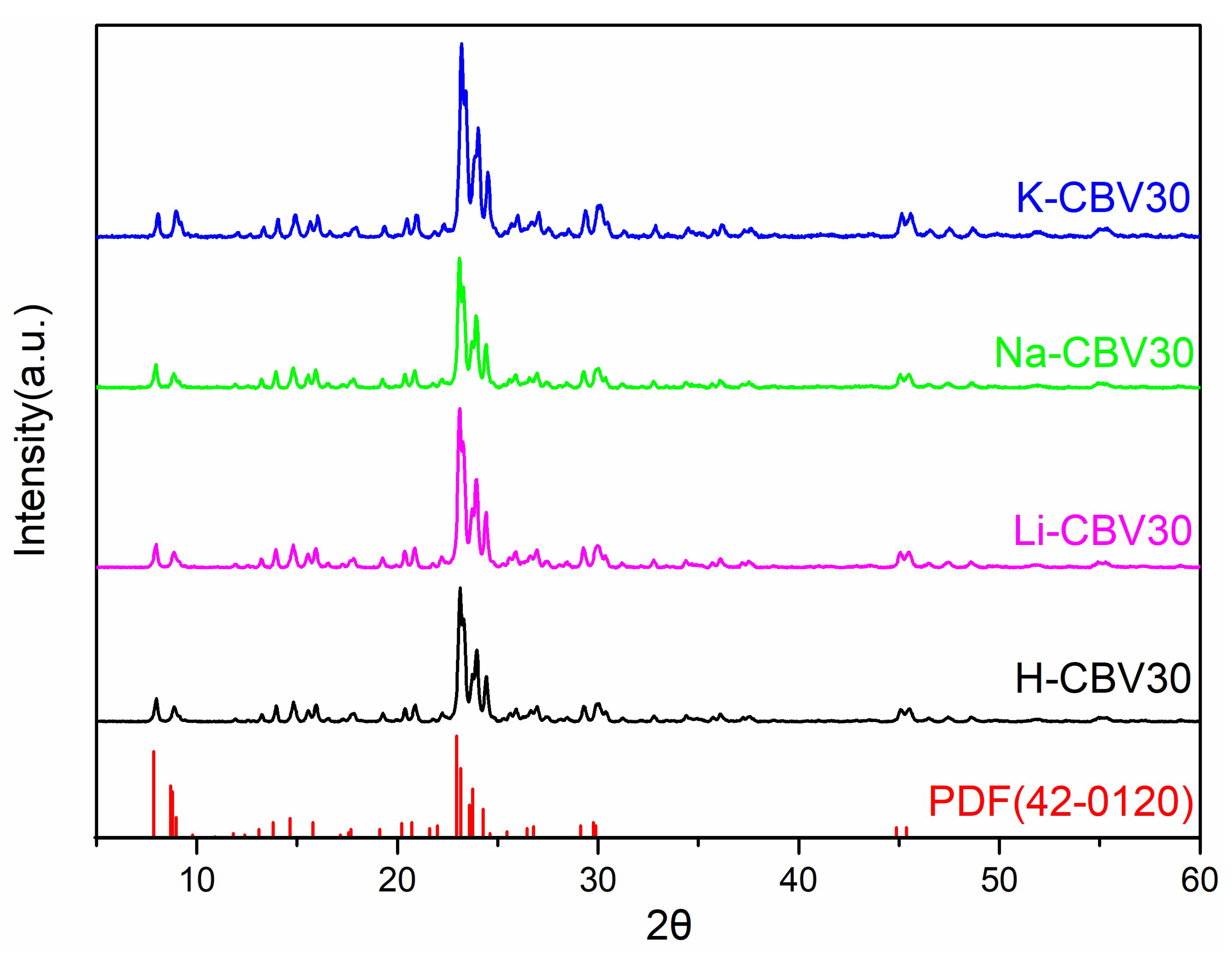
2.2.1. Effect on Zeolite Structure and Crystallinity
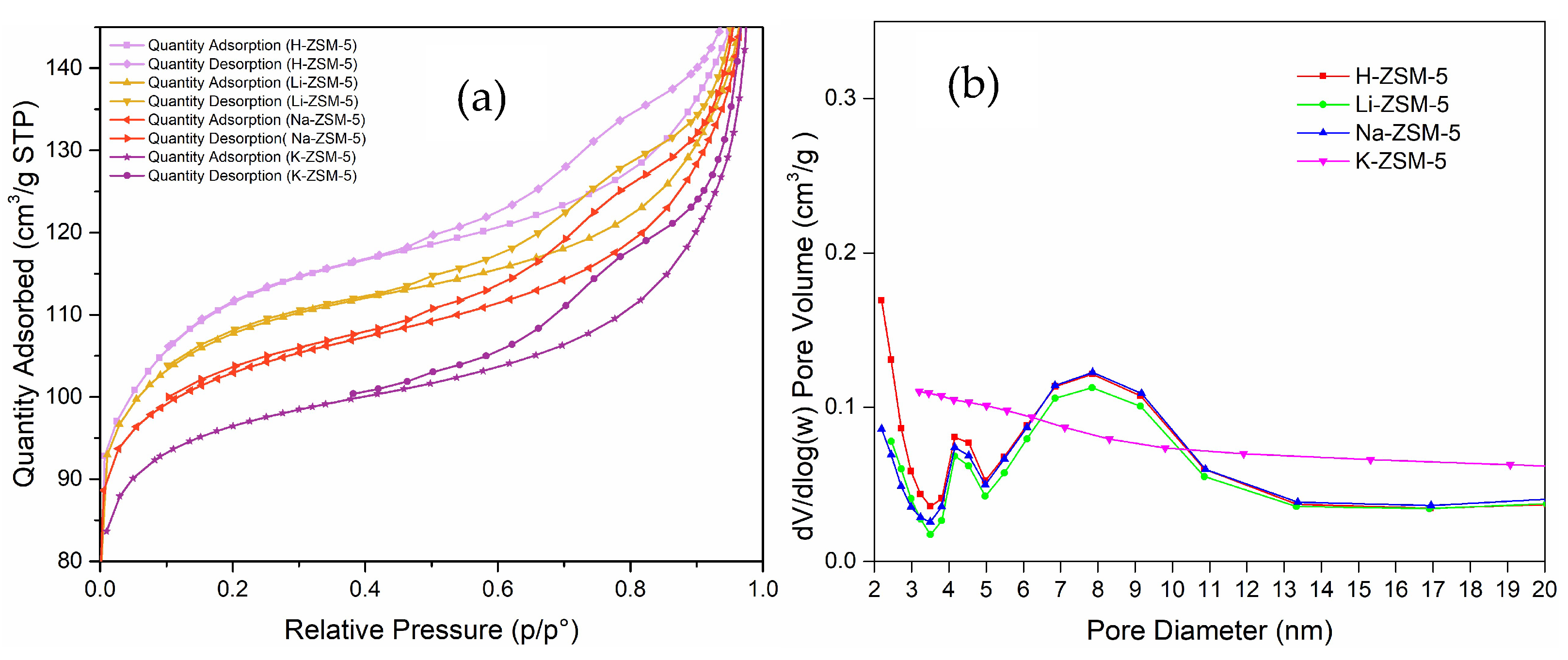
2.2.2. Effect on Pore Size and Surface Area
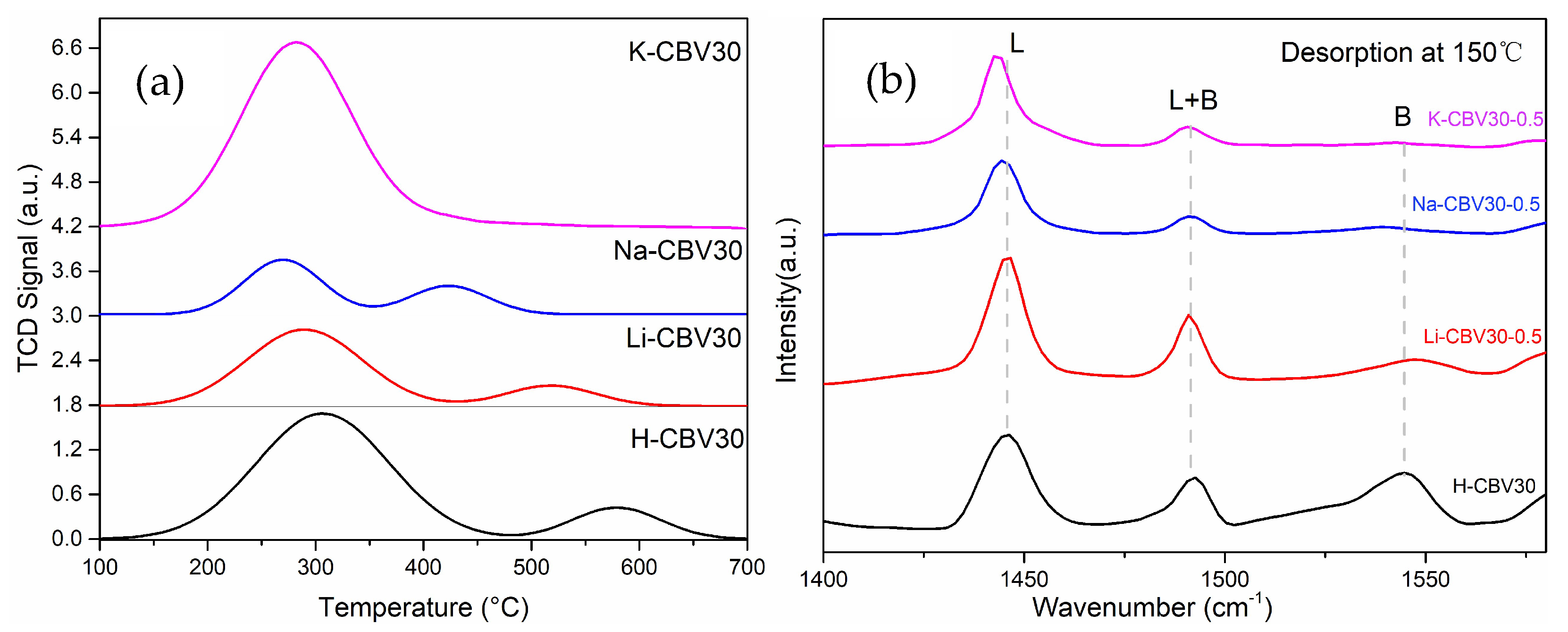
2.2.3. Effect on Zeolite Acidity
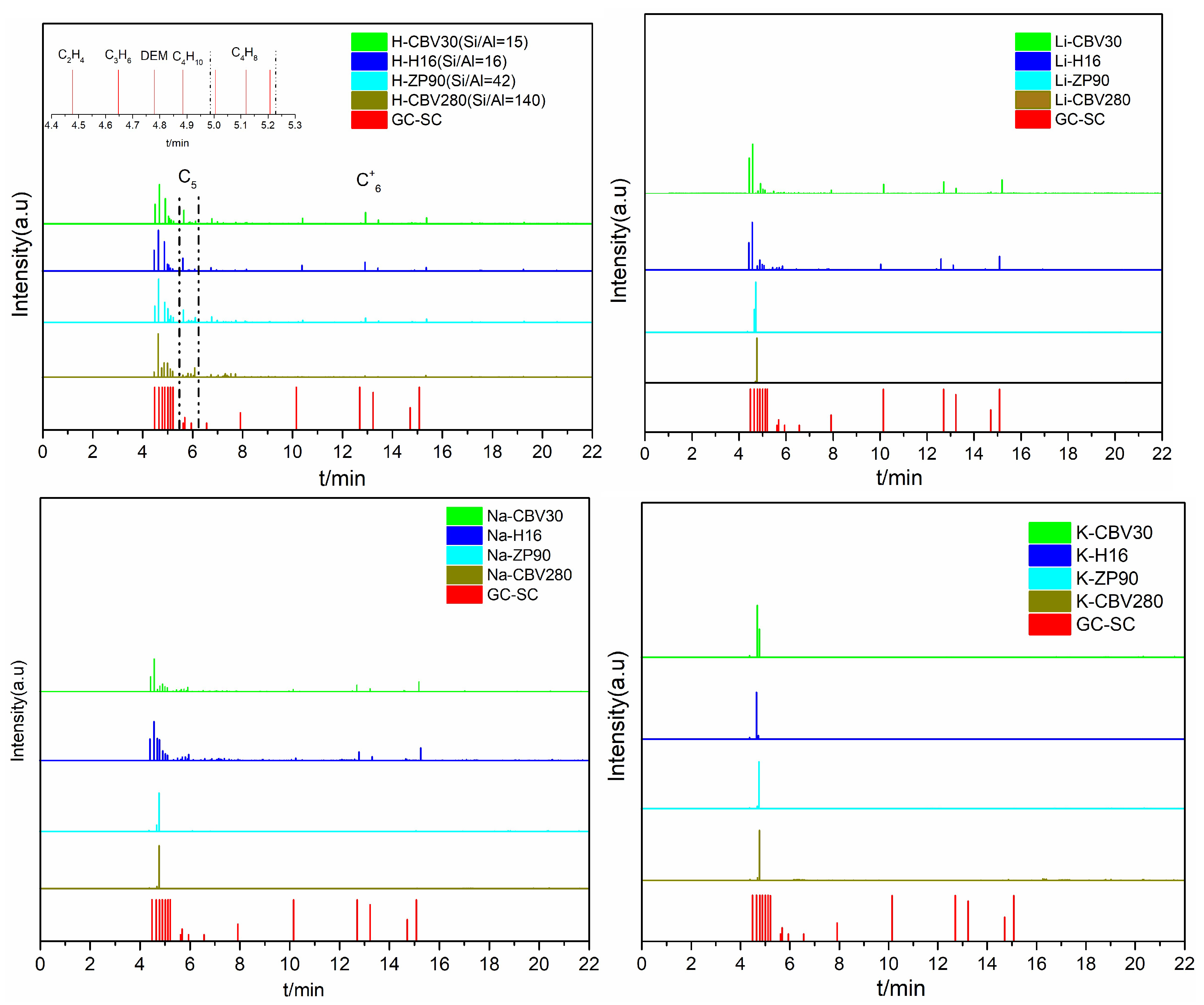
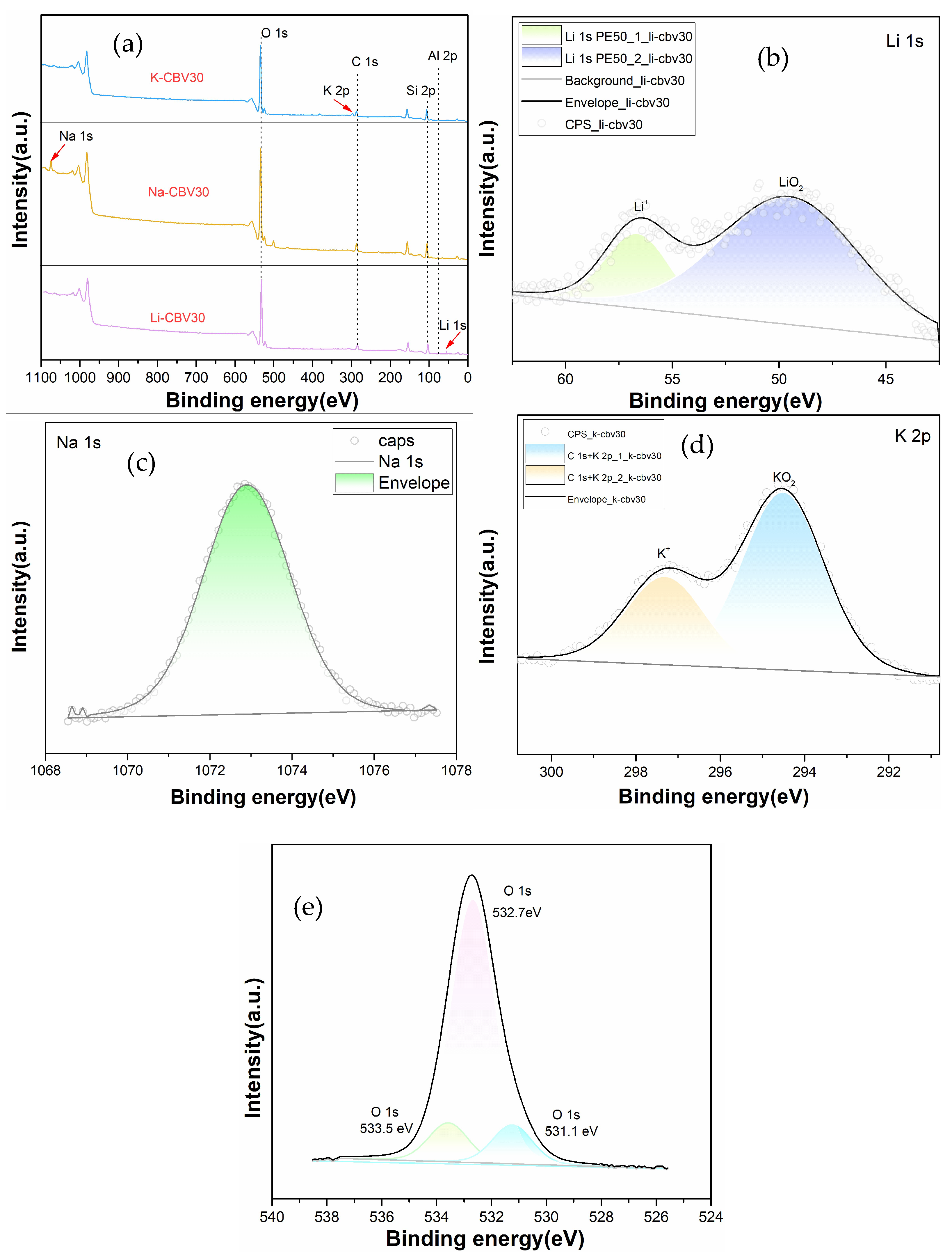
2.2.4. Speciation of Alkali Metal Ions in Zeolite Channels
2.3. Effect of Alkali Metal Loading on the Physicochemical Properties of Zeolites
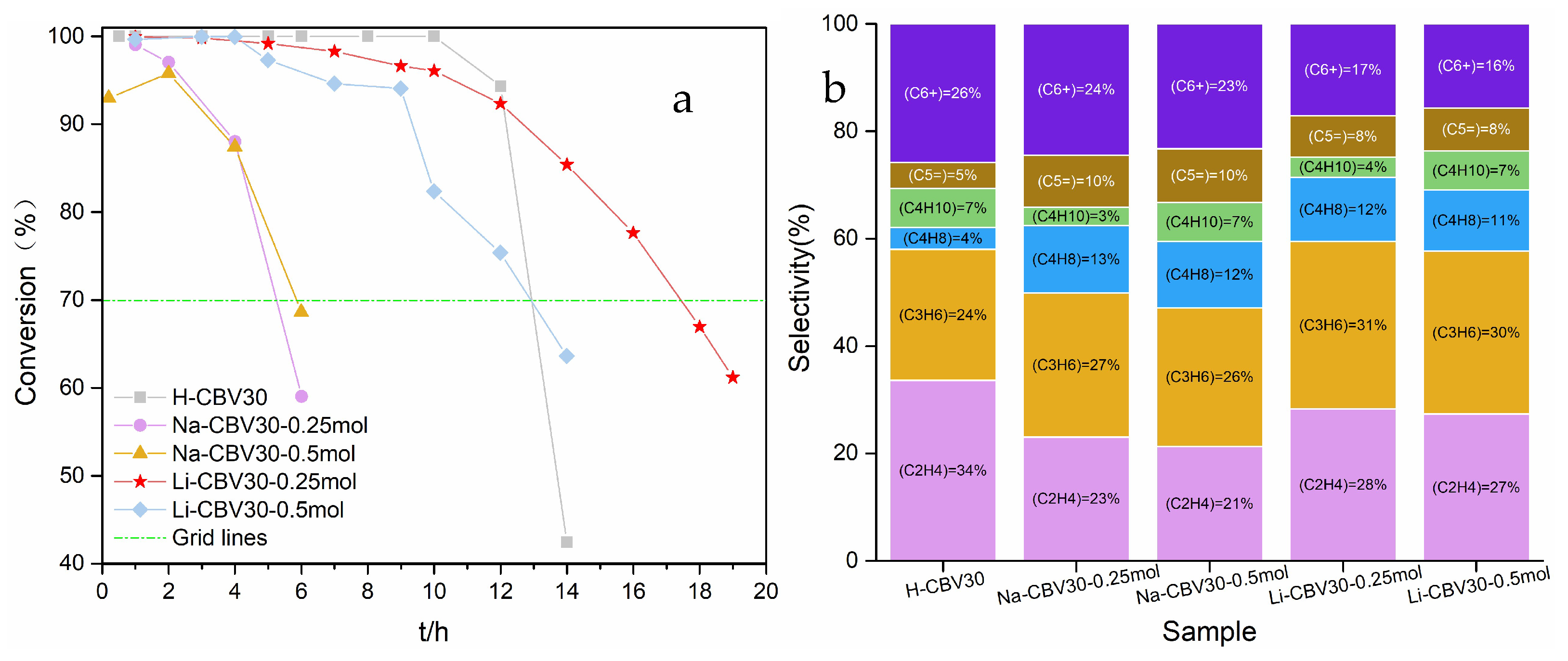
2.3.1. MTO Catalytic Performance After Optimizing Alkali Metal Loading
2.3.2. Properties of Li-ZSM-5 Zeolites with Different Loading Amounts
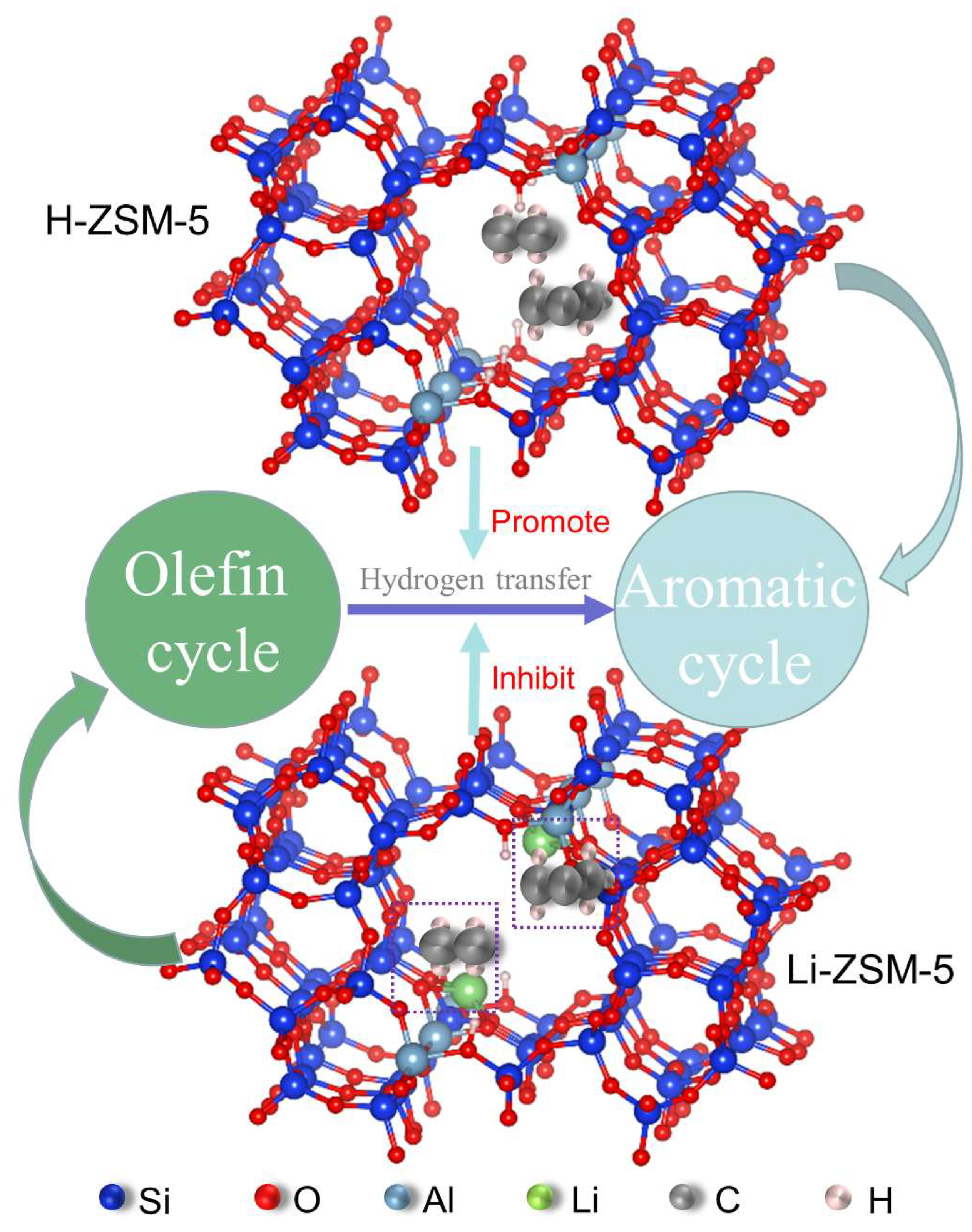
2.4. DFT Study on the Mechanism of Li+-Enhanced MTO
3. Experimental
3.1. Catalyst Preparation
3.1.1. Hydrothermal Synthesis of Na-ZSM-5 Zeolite
3.1.2. Cationic Exchange Method for Alkali Metal Ion-Loaded ZSM-5 Zeolite
3.2. Characterization
3.3. Methanol-to-Olefin (MTO)
3.4. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tabibian, S.S.; Sharifzadeh, M. Statistical and analytical investigation of methanol applications, production technologies, value-chain and economy with a special focus on renewable methanol. Renew. Sustain. Energy Rev. 2023, 179, 113281. [Google Scholar] [CrossRef]
- Almuqati, N.S.; Aldawsari, A.M.; Alharbi, K.N.; González-Cortés, S.; Alotibi, M.F.; Alzaidi, F.; Dilworth, J.R.; Edwards, P.P. Catalytic production of light olefins: Perspective and prospective. Fuel 2024, 366, 131270. [Google Scholar] [CrossRef]
- Witoon, T.; Lapkeatseree, V.; Numpilai, T.; Cheng, C.K.; Limtrakul, J. CO2 hydrogenation to light olefins over mixed Fe–Co–K–Al oxides catalysts prepared via precipitation and reduction methods. Chem. Eng. J. 2022, 428, 131389. [Google Scholar] [CrossRef]
- Alabdullah, M.A.; Gomez, A.R.; Vittenet, J.; Bendjeriou-Sedjerari, A.; Xu, W.; Abba, I.A.; Gascon, J. A viewpoint on the refinery of the future: Catalyst and process challenges. ACS Catal. 2020, 10, 8131–8140. [Google Scholar] [CrossRef]
- Zhao, S.; Li, H.; Wang, B.; Yang, X.; Peng, Y.; Du, H.; Zhang, Y.; Han, D.; Li, Z. Recent advances on syngas conversion targeting light olefins. Fuel 2022, 321, 124124. [Google Scholar] [CrossRef]
- Tian, P.; Wei, Y.; Ye, M.; Liu, Z. Methanol to olefins (MTO): From fundamentals to commercialization. ACS Catal. 2015, 5, 1922–1938. [Google Scholar] [CrossRef]
- Van Bekkum, H.; Flanigen, E.M.; Jansen, J.C. Introduction to Zeolite Science and Practice, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 2001; pp. 248–249. [Google Scholar]
- Sun, H.; Zhang, Z.; Sun, H.; Yao, B.; Lou, C. Numerical Investigation of Exergy Loss of Ammonia Addition in Hydrocarbon Diffusion Flames. Entropy 2022, 24, 922. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhi, Y.; Han, J.; Zhang, W.; Wu, X.; Sun, T.; Wei, Y.; Liu, Z. Advances in Catalysis for Methanol-to-Olefins Conversion. Adv. Catal. 2017, 61, 37–122. [Google Scholar] [CrossRef]
- Boltz, M.; Losch, P.; Louis, B. A General Overview on the Methanol to Olefins Reaction: Recent Catalyst Developments. Adv. Chem. Lett. 2014, 1, 247–256. [Google Scholar] [CrossRef]
- Olsbye, U.; Svelle, S.; Bjørgen, M.; Beato, P.; Janssens, T.V.W.; Joensen, F.; Bordiga, S.; Lillerud, K.P. Conversion of methanol to hydrocarbons: How zeolite cavity and pore size controls product selectivity. Angew. Chem. Int. Ed. 2012, 51, 5810–5831. [Google Scholar] [CrossRef]
- Dahl, I.M.; Kolboe, S. On the reaction mechanism for hydrocarbon formation from methanol over SAPO-34: I. Isotopic labeling studies of the co-reaction of ethene and methanol. J. Catal. 1994, 149, 458–464. [Google Scholar] [CrossRef]
- Ilias, S.; Bhan, A. Mechanism of the catalytic conversion of methanol to hydrocarbons. ACS Catal. 2013, 3, 18–31. [Google Scholar] [CrossRef]
- Valecillos, J.; Epelde, E.; Albo, J.; Aguayo, A.T.; Bilbao, J.; Castaño, P. Slowing down the deactivation of H-ZSM-5 zeolite catalyst in the methanol-to-olefin (MTO) reaction by P or Zn modifications. Catal. Today 2020, 348, 243–256. [Google Scholar] [CrossRef]
- Xue, Y.; Li, J.; Wang, P.; Cui, X.; Zheng, H.; Niu, Y.; Dong, M.; Qin, Z.; Wang, J.; Fan, W. Regulating Al distribution of ZSM-5 by Sn incorporation for improving catalytic properties in methanol to olefins. Appl. Catal. B 2021, 280, 119391. [Google Scholar] [CrossRef]
- Yang, M.; Fan, D.; Wei, Y.; Tian, P.; Liu, Z. Recent progress in methanol-to-olefins (MTO) catalysts. Adv. Mater. 2019, 31, 1902181. [Google Scholar] [CrossRef]
- Chizallet, C.; Bouchy, C.; Larmier, K.; Pirngruber, G. Molecular views on mechanisms of Brønsted acid-catalyzed reactions in zeolites. Chem. Rev. 2023, 123, 6107–6196. [Google Scholar] [CrossRef] [PubMed]
- Haag, W.O.; Lago, R.M.; Weisz, P.B. The active site of acidic aluminosilicate catalysts. Nature 1984, 309, 589–591. [Google Scholar] [CrossRef]
- Mortier, W.J.; Sauer, J.; Lercher, J.A.; Noller, H. Bridging and terminal hydroxyls. A structural chemical and quantum chemical discussion. J. Phys. Chem. 1984, 88, 905–912. [Google Scholar] [CrossRef]
- Xia, W.; Wang, J.; Wang, L.; Chen, Q.; Chen, K. Ethylene and propylene production from ethanol over Sr/ZSM-5 catalysts: A combined experimental and computational study. Appl. Catal. B 2021, 294, 120242. [Google Scholar] [CrossRef]
- Guisnet, M.; Costa, L.; Ribeiro, F.R. Prevention of zeolite deactivation by coking. J. Mol. Catal. A Chem. 2009, 305, 69–83. [Google Scholar] [CrossRef]
- Müller, S.; Liu, Y.; Kirchberger, F.M.; Tonigold, M.; Sanchez-Sanchez, M.; Lercher, J.A. Hydrogen transfer pathways during zeolite catalyzed methanol conversion to hydrocarbons. J. Am. Chem. Soc. 2016, 138, 15994–16003. [Google Scholar] [CrossRef]
- Setiabudi, H.D.; Jalil, A.A.; Triwahyono, S.; Kamarudin, N.H.N.; Jusoh, R. Ir/Pt-HZSM5 for n-pentane isomerization: Effect of Si/Al ratio and reaction optimization by response surface methodology. Chem. Eng. J. 2013, 217, 300–309. [Google Scholar] [CrossRef]
- Smit, B.; Maesen, T.L.M. Towards a molecular understanding of shape selectivity. Nature 2008, 451, 671–678. [Google Scholar] [CrossRef]
- Yaripour, F.; Shariatinia, Z.; Sahebdelfar, S.; Irandoukht, A. Effect of boron incorporation on the structure, products selectivities and lifetime of H-ZSM-5 nanocatalyst designed for application in methanol-to-olefins (MTO) reaction. Microporous Mesoporous Mater. 2015, 203, 41–53. [Google Scholar] [CrossRef]
- Jiang, X.; Su, X.; Bai, X.; Li, Y.; Yang, L.; Zhang, K.; Zhang, Y.; Liu, Y.; Wu, W. Conversion of methanol to light olefins over nanosized [Fe,Al]ZSM-5 zeolites: Influence of Fe incorporated into the framework on the acidity and catalytic performance. Microporous Mesoporous Mater. 2018, 263, 243–250. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, R.; Hou, H.; Zhou, Z.; Wang, X. Facile synthesis of an Mg-incorporated ZSM-5 zeolite from dual silicon sources and its application for conversion of methanol to olefins. ChemistrySelect 2021, 6, 7056–7061. [Google Scholar] [CrossRef]
- Zhou, Y.; Santos, S.; Shamzhy, M.; Marinova, M.; Blanchenet, A.M.; Kolyagin, Y.G.; Simon, P.; Trentesaux, M.; Sharna, S.; Ersen, O.; et al. Liquid metals for boosting stability of zeolite catalysts in the conversion of methanol to hydrocarbons. Nat. Commun. 2024, 15, 2228. [Google Scholar] [CrossRef] [PubMed]
- Dyballa, M.; Obenaus, U.; Blum, M.; Dai, W. Alkali metal ion exchanged ZSM-5 catalysts: On acidity and methanol-to-olefin performance. Catal. Sci. Technol. 2018, 8, 4440–4449. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, H.; Yan, W. Effect of alkali metal cations modification on the acid/basic properties and catalytic activity of ZSM-5 in cracking of supercritical n-dodecane. Fuel 2019, 243, 155–161. [Google Scholar] [CrossRef]
- Auepattana-aumrung, C.; Suriye, K.; Jongsomjit, B.; Panpranot, J.; Praserthdam, P. Inhibition effect of Na+ form in ZSM-5 zeolite on hydrogen transfer reaction via 1-butene cracking. Catal. Today 2020, 358, 237–245. [Google Scholar] [CrossRef]
- Nagy, J.B.; Bodart, P.; Collette, H.; El Hage-Al Asswad, J.; Gabelica, Z.; Aiello, R.; Nastro, A.; Pellegrino, C. Aluminium distribution and cation location in various M-ZSM-5-type zeolites (M = Li, Na, K, Rb, Cs, NH4). Zeolites 1988, 8, 209–220. [Google Scholar] [CrossRef]
- Yuanyuan, S.; Li, Z.; Zhou, X.; Li, G.; Tan, M.; Ao, S.; Sun, W.; Chen, H. Mesoporous zeolite ZSM-5 confined Cu nanoclusters for efficient selective catalytic reduction of NOx by NH3. Appl. Catal. B 2024, 346, 123747. [Google Scholar] [CrossRef]
- ASTM D5758-01(2021); Test Method for Determination of Relative Crystallinity of Zeolite ZSM-5 by X-Ray Diffraction. The World Trade Organization Technical Barriers to Trade (TBT) Committee: Geneva, Switzerland, 2021. [CrossRef]
- Taherizadeh, A.; Harpf, A.; Simon, A.; Choi, J.; Richter, H.; Voigt, I.; Stelter, M. Thermochemical study of the structural stability of low-silicate CHA zeolite crystals. Results Chem. 2022, 4, 100466. [Google Scholar] [CrossRef]
- Qi, R.; Fu, T.; Wan, W.; Li, Z. Pore fabrication of nano-ZSM-5 zeolite by internal desilication and its influence on the methanol to hydrocarbon reaction. Fuel Process. Technol. 2017, 155, 191–199. [Google Scholar] [CrossRef]
- Matadamas, J.; Alférez, R.; López, R.; Román, G.; Kornhauser, I.; Rojas, F. Advanced and delayed filling or emptying of pore entities by vapor sorption or liquid intrusion in simulated porous networks. Colloids Surf. A Physicochem. Eng. Asp. 2016, 496, 39–51. [Google Scholar] [CrossRef]
- Kutarov, V.V.; Robens, E.; Tarasevich, Y.I.; Aksenenko, E.V. Adsorption hysteresis at low relative pressures. Theor. Exp. Chem. 2011, 47, 163–168. [Google Scholar] [CrossRef]
- Fu, T.; Chang, J.; Shao, J.; Li, Z. Fabrication of a nano-sized ZSM-5 zeolite with intercrystalline mesopores for conversion of methanol to gasoline. J. Energy Chem. 2017, 26, 139–146. [Google Scholar] [CrossRef]
- Miyamoto, T.; Katada, N.; Kim, J.H.; Niwa, M. Acidic property of MFI-type gallosilicate determined by temperature-programmed desorption of ammonia. J. Phys. Chem. B 1998, 102, 6738–6745. [Google Scholar] [CrossRef]
- Chen, K.; Wu, X.; Zhao, J.; Zhao, H.; Li, A.; Zhang, Q.; Xia, T.; Liu, P.; Meng, B.; Song, W.; et al. Organic-free modulation of the framework Al distribution in ZSM-5 zeolite by magnesium participated synthesis and its impact on the catalytic cracking reaction of alkanes. J. Catal. 2022, 413, 735–750. [Google Scholar] [CrossRef]
- Yarulina, I.; De Wispelaere, K.; Bailleul, S.; Goetze, J.; Radersma, M.; Abou-Hamad, E.; Vollmer, I.; Goesten, M.; Mezari, B.; Hensen, E.J.M.; et al. Structure–performance descriptors and the role of Lewis acidity in the methanol-to-propylene process. Nat. Chem. 2018, 10, 804–812. [Google Scholar] [CrossRef]
- Liu, C.; Luo, J.; Dong, H.; Zhao, Z.; Xu, C.; Abuelgasim, S.; Abdalazeez, A.; Wang, W.; Chen, D.; Tang, Q. Hydrogen-rich syngas production from biomass char by chemical looping gasification with Fe/Ca-based oxygen carrier. Sep. Purif. Technol. 2022, 300, 121912. [Google Scholar] [CrossRef]
- Du, Y.; Shi, T.; Guo, S.; Li, H.; Qin, Y.; Wang, Y.; He, C.; Wei, Y. Unraveling the intrinsic mechanism behind the retention of arsenic in the co-gasification of coal and sewage sludge: Focus on the role of Ca and Fe compounds. J. Hazard. Mater. 2024, 470, 134211. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Pérez, P.; Contreras, R. Reactivity of the carbon–carbon double bond towards nucleophilic additions: A DFT analysis. Tetrahedron 2004, 60, 6585–6591. [Google Scholar] [CrossRef]
- Zhang, Y.; Louis, B. Tailoring structure and acidity of ZSM-5 zeolite by algae carbon modification: Promoting effect in the MTO reaction. Microporous Mesoporous Mater. 2023, 350, 112431. [Google Scholar] [CrossRef]
- Xing, B.; Ma, J.; Li, R.; Jiao, H. Location, distribution and acidity of Al substitution in ZSM-5 with different Si/Al ratios—A periodic DFT computation. Catal. Sci. Technol. 2017, 7, 5694–5708. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
| Sample | Loaded Metal Cation Solution Concentration (mol/L) a | Target Cation Loading (wt%) b | BET Surface Area (m2/g) c | Relative Crystallinity d |
|---|---|---|---|---|
| H-CBV30 | / | / | 359 | 100% |
| Li-CBV30 | 0.5 | 0.43 | 341 | 122% |
| Na-CBV30 | 0.5 | 1.48 | 325 | 97% |
| K-CBV30 | 0.5 | 3.89 | 306 | 157% |
| Sample | NH3-TPD (μmol/g) a | Py-IR (μmol/g) b | ||||
|---|---|---|---|---|---|---|
| Weak Acid Content | Temperature (°C) | Strong Acid Content | Temperature (°C) | Amount of B Acid | Amount of L Acid | |
| H-CBV30 | 260 | 306 | 43 | 578 | 431 | 360 |
| Li-CBV30 | 137 | 290 | 35 | 518 | 146 | 346 |
| Na-CBV30 | 66 | 271 | 29 | 424 | 46 | 260 |
| K-CBV30 | 303 | 282 | 0 | / | 23 | 314 |
| Sample | Target Cation Loading (wt%) a | BET Surface Area (m2/g) b | Relative Crystallinity c | NH3-TPD (μmol/g) d | Py-IR (μmol/g) e | ||||
|---|---|---|---|---|---|---|---|---|---|
| Weak Acid Content | Temperature (°C) | Strong Acid Content | Temperature (°C) | Amount of B Acid | Amount of L Acid | ||||
| Li-CBV30-0.25 mol/L | 0.4 | 345 | 123% | 187 | 296 | 33 | 523 | 146 | 346 |
| Li-CBV30-0.5 mol/L | 0.43 | 341 | 122% | 137 | 289 | 28 | 518 | 116 | 434 |
| Li-CBV30-1 mol/L | 0.64 | 313 | 120% | 167 | 300 | 40 | 534 | 59 | 379 |
| Model | E-C2H4 (eV) | E-C3H6 (eV) |
|---|---|---|
| H-ZSM-5 | −0.68 | −0.86 |
| Li-ZSM-5 | −0.87 | −1.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, S.; Yang, J.; Louis, B. Investigating the Impact of Incorporating Alkali Metal Cations on the Properties of ZSM-5 Zeolites in the Methanol Conversion into Hydrocarbons. Catalysts 2025, 15, 987. https://doi.org/10.3390/catal15100987
Dong S, Yang J, Louis B. Investigating the Impact of Incorporating Alkali Metal Cations on the Properties of ZSM-5 Zeolites in the Methanol Conversion into Hydrocarbons. Catalysts. 2025; 15(10):987. https://doi.org/10.3390/catal15100987
Chicago/Turabian StyleDong, Senlin, Jie Yang, and Benoit Louis. 2025. "Investigating the Impact of Incorporating Alkali Metal Cations on the Properties of ZSM-5 Zeolites in the Methanol Conversion into Hydrocarbons" Catalysts 15, no. 10: 987. https://doi.org/10.3390/catal15100987
APA StyleDong, S., Yang, J., & Louis, B. (2025). Investigating the Impact of Incorporating Alkali Metal Cations on the Properties of ZSM-5 Zeolites in the Methanol Conversion into Hydrocarbons. Catalysts, 15(10), 987. https://doi.org/10.3390/catal15100987








