1. Introduction
The use of surfactants in biocatalytic reactions has significantly contributed to advancements in science and various industries, especially in pharmaceutical and cosmetic applications. These compounds, which act at the interface between two immiscible phases, reduce surface and interfacial tension (typically between water and oil). Thus, they facilitate the formation of more effective reaction conditions [
1,
2]. Notably, certain surfactants can stabilize proteins at concentrations below the critical micelle concentration (CMC), the minimum concentration at which surfactants self-assemble into micelles. However, exceeding this concentration may lead to protein denaturation [
3].
Surfactants not only influence interfacial phenomena but also play a crucial role in emulsion formulation. In water–oil mixtures, surfactants act as emulsifiers, forming emulsions in which one phase is dispersed within the other. In drug technology, such emulsions are particularly beneficial for delivering active pharmaceutical ingredients (APIs) with hydrophilic or hydrophobic properties, and can be used for both external and internal administration [
4,
5]. However, a major limitation in the use of emulsions, especially in pharmaceutical and cosmetic applications, is their limited physical stability, which can lead to the separation of the aqueous and oil phases [
2]. One of the techniques for mitigating this phenomenon is to ensure proper emulsion preparation, particularly through effective mixing using appropriate equipment, such as a homogenizer [
4]. A key factor influencing emulsion stability is the selection of an optimal emulsifier. Sodium dodecyl sulfate (SDS), an anionic surfactant, is among the most commonly used compounds in this context. It is characterized by a bipolar structure, consisting of a hydrophilic head group and a hydrophobic tail [
6]. SDS is also well known for its high ability to form colloidal aggregates, which has led to its extensive use in both the biological processes and the pharmaceutical and food industries [
3,
7]. Sharma et al. [
7] investigated the interaction of SDS with butylated hydroxyanisole (BHA), a potential phenolic antioxidant with a broad range of pharmacological actions. Similarly, Liu et al. [
8] employed SDS to improve the bioavailability of luteolin, a flavonoid compound with diverse pharmacological effects. Additional studies have reported the use of SDS in pharmaceutical research, including investigations involving sulfanilamide and anti-HIV drugs [
9,
10].
Emulsion stabilization often requires the use of more than one agent. As an additional stabilizing agent in the emulsion, apart from the emulsifier described above, a gelling agent is often introduced. Carbomer, a cross-linked and pH-sensitive poly(acrylic acid) (PAA), is considered one of the most effective compounds for stabilizing biphasic systems. At neutral to alkaline pH, the chains of carbomer stretch, leading to the formation of a three-dimensional microgel network capable of entrapping and stably suspending dispersed particles [
11,
12]. These polymers exhibit high thickening efficiency across a broad pH range (4.5–11), typically with peak viscosity near pH 7 and a gradual decline above pH 9 [
13]. The pH ranges may vary depending on the composition of the gels. In the industry, carbomer is marketed under various trade names, including Carbopol
®. Acrylic acid polymers, such as carbomer, enable the formation of high-quality and high-viscosity gels, even at low concentrations of the gelling agent [
14]. In pharmaceutical formulations, carbomer is primarily used in gel formulation for buccal, transdermal, ocular, rectal, and nasal drug delivery systems [
15].
The wide use of emulsions containing surfactants and gelling agents in the pharmaceutical and cosmetics industry is not limited only to the formation of drug or cosmetic products. Biocatalysis is another important area in pharmaceutical science where multiphase systems are employed, as they provide an optimal medium for enzyme-catalyzed reactions [
16,
17,
18].
Candida antarctica lipase B (known as CALB) is one of the most frequently used lipases. Due to its lipolytic activity, it catalyzes the hydrolysis of triglycerides, with its catalytic activity enhanced at the oil-water interface [
19,
20,
21,
22]. Moreover, CALB undergoes a conformational change from closed to an open form through the movement of the polypeptide chain, known as the “lid” [
21,
23]. Verma et al. [
24] reported that surfactant can affect the surface activity of enzymes. It should be noted that CALB in its free form often exhibits low catalytic activity, resulting in a poor reaction performance [
21]. Therefore, immobilization on a suitable support is often used to enhance both the catalytic activity and stability of the enzyme. Exemplary applications of supports for enzyme immobilization include the studies presented in the literature [
17,
21]. Immobeads represent a notable group of commercially available supports, composed of matrices (such as polyacrylic, polystyrene, and polypropylene) and characteristic functional chemical groups [
21,
25,
26]. Immobilization onto Immobead supports can be achieved through ionic and nonionic interactions or via covalent bonding. Our previous studies demonstrated that CALB immobilized onto Immobead IB-D152 support, characterized by a polyacrylic matrix and carboxylic acid functional group, exhibits a higher level of lipolytic activity compared to its free form [
18,
21].
Studies reported in the literature on lipase activity focus mainly on systems containing either a single surfactant or a mixture of two surfactants [
27,
28]. Only a limited number of studies explore the combined effect of surfactants and gelling agents, such as cross-linked poly(acrylic acid), on lipase activity. These types of studies usually concern enzymes other than lipases. For instance, enhanced protease performance was observed in a system composed of poly(acrylic acid) (PAA), sodium lauryl ether sulfate (SLES), and Ca
2+ cation [
29]. Additionally, in several studies, the impact of carbomer on enzyme activity (including lipase) has been investigated, revealing mixed results—ranging from increased activity to inhibitory effects. Notably, Carbopol 934-based gel caused immediate activation of
Mucor racemosus lipase LII, and a significant increase in enzyme activity [
30]. On the other hand, Carbopol
® was found to reduce the catalytic activity of trypsin [
31,
32]. Moreover, other studies have demonstrated that increasing concentrations of poly(acrylic acid) (PAA) can lead to a decrease in CALB catalytic activity [
33]. Recognizing a significant gap in the literature regarding the behavior of CALB in dual-stabilized emulsions, we designed a targeted experimental study. Specifically, we investigated the effect of the combination of SDS and carbomer (Carbopol
® Ultrez 10, cross-linked poly(acrylic acid) (PAA)) on the catalytic activity of CALB, in both free and immobilized forms. The selection of SDS as a stabilizer in the dual-stabilized emulsion system (with PAA) was based, among other factors, on its chemical structure as an anionic emulsifier, which can potentially interact with carbomer by electrostatic interactions. The research also included the selection of an appropriate support for CALB immobilization and an analysis of the effect of homogenization and SDS concentration, with or without the addition of carbomer at varying concentrations, on lipase activity. This approach fills an important gap in the current literature and may expand the use of dual-stabilized pharmaceutical formulations in biocatalysis, thus enabling the enhancement of the lipolytic activity of immobilized lipases. Furthermore, the presented approach may also be applied to other research areas, including drug delivery systems, food processing, and green synthesis, where stable emulsion systems are essential.
2. Results and Discussion
2.1. The Effect of Support Type on the Lipolytic Activity of CALB in Emulsion Stabilized with 5.0% SDS
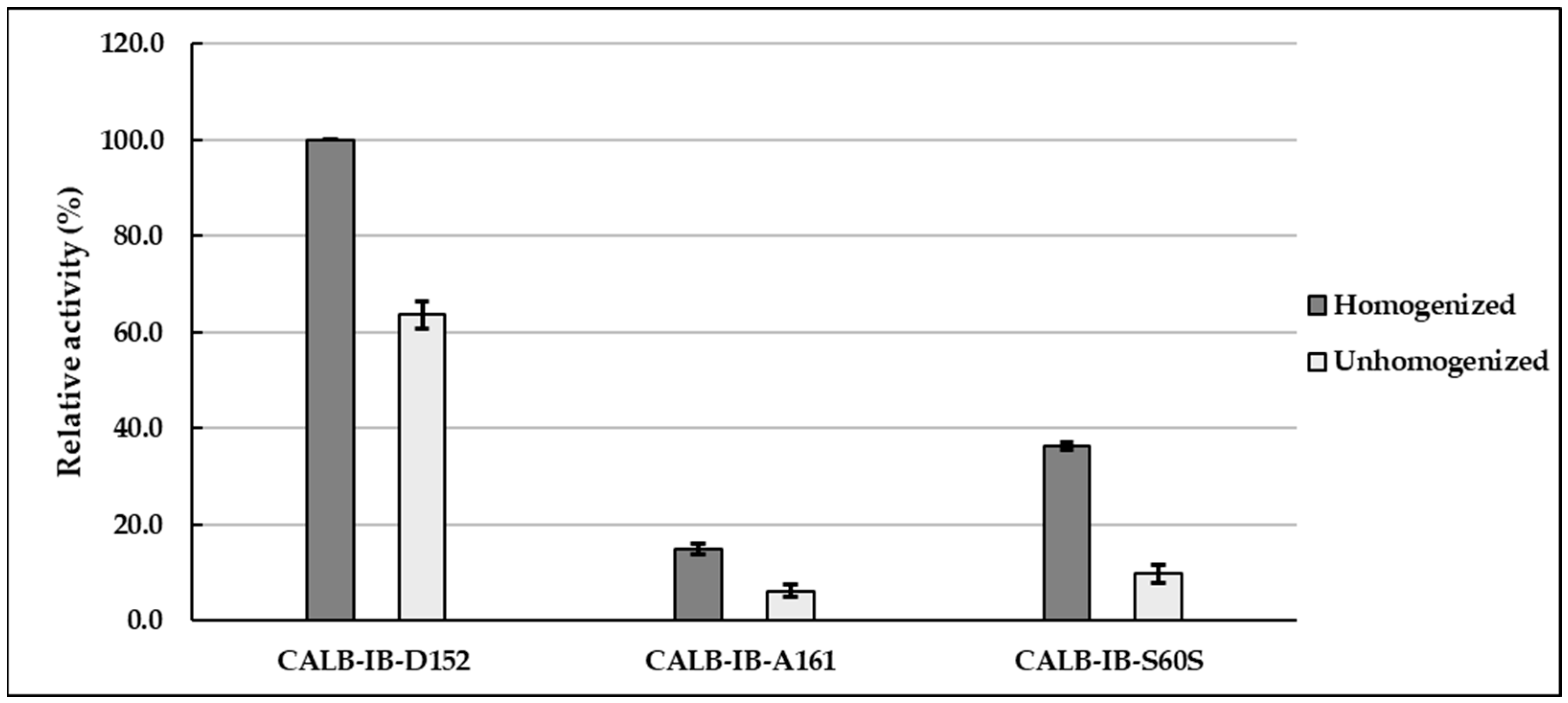
In the initial stage of the project, the lipolytic activity of CALB immobilized onto various supports differing in, among others, type of bond, matrix composition, functional groups, and particle size was assessed. Three supports were used in the study: (1) IB-D152, composed of a polyacrylic matrix with carboxylic acid functional groups, forming a cationic bond with lipase, particle size 350–700 µm; (2) IB-A161, consisting of a polystyrene matrix with quaternary ammonium-type functional groups, binding to lipase via a strong anionic bond, particle size 350–700 µm; (3) IB-S60S, composed of a super porous silica matrix with hydroxyl functional groups, binding to lipase via a nonionic bond, particle size 60–200 μm—data provided by the supplier, ChiralVision. Lipolytic activity was determined based on the hydrolysis of triglycerides in olive oil, catalyzed by immobilized CALB. The enzyme activity was tested in two types of emulsions: unhomogenized and homogenized, both containing 5.0% SDS. Based on the values of lipase activity (U), the relative activity (
Arel) was calculated. The results are presented in
Figure 1.
Analysis of the obtained lipolytic activity results revealed that the highest activity, both in homogenized and unhomogenized emulsions, was observed for CALB immobilized onto the IB-D152 support (Arel = 100.0 ± 0.0% in homogenized, and Arel = 63.6 ± 2.8% in unhomogenized). Lower activity values were received for the other two supports: IB-A161 (Arel = 15.0 ± 1.1% in homogenized, and Arel = 6.2 ± 1.3% in unhomogenized), and IB-S60S (Arel = 36.4 ± 0.7% in homogenized, and Arel = 9.8 ± 1.8% in unhomogenized). It should be emphasized that, regardless of the support tested, CALB exhibited higher activity in homogenized emulsions compared to unhomogenized systems (p < 0.05). We hypothesize that the utilization of an additional procedure, i.e., homogenization, may improve substrate availability for immobilized CALB, which acts at the oil-water interface, thereby affecting enzyme activity.
Regarding the amount of CALB immobilized onto the tested supports, as presented in our previous work [
21], the lipase loading was as follows: 25.2 mg/g of the IB-D152 support, 37.8 mg/g of the IB-A161 support, and 129.8 mg/g of the IB-S60S support. These differences in lipase loading may result from various factors, including the physical and chemical properties of the supports, and the type of bond involved (in this study: cationic and anionic—lower lipase loading, nonionic—higher loading). We hypothesize that ionic interactions between the catalytic protein and the supports (IB-D152 and IB-A161) may influence both the lipase loading and the activity of CALB. Although IB-S60S support enabled the highest value of CALB loading, it did not result in enhanced enzyme activity. Notably, the highest CALB activity in emulsions containing 5.0% SDS was observed on the IB-D152 support, despite its lowest lipase loading. Additionally, to compare the activity of CALB immobilized on the tested supports (per mg of lipase), the specific activity (U/mg lipase) was determined. CALB immobilized onto the IB-D152 support showed the highest specific activity (4.14 ± 0.20 U/mg of lipase). In contrast, CALB immobilized onto IB-A161 as well as IB-S60S supports demonstrated lower values (0.41 ± 0.05 U/mg of lipase and 0.29 ± 0.02 U/mg of lipase, respectively). For a more accurate comparison of the effects of the tested supports on lipase activity, enzyme loading on the supports should be at a similar level. The high catalytic activity of CALB immobilized onto the IB-D152 in emulsions may be attributed to the physical and chemical nature of the support and its ionic interactions with the catalytic protein. However, precise identification of the factors influencing the obtained differences in the lipolytic activity requires further investigation, including detailed experimental characterization of the morphology and surface chemistry of the supports. Similar relationships regarding lipolytic activity were described in our previous study [
21], in which we investigated immobilized CALB in emulsions containing gum arabic. We also noted high lipolytic activity (in peanut oil) of another lipase—Amano lipase A from
Aspergillus niger immobilized onto the IB-D152 support, as was described in our previous paper [
18]. Based on the obtained results of CALB lipolytic activity, the IB-D152 support as well as the homogenized emulsion systems were subjected to further steps of the experimental work.
The key findings can be summarized as follows: CALB immobilized onto the IB-D152 support exhibited the highest relative and specific activity values, despite the lowest lipase loading. Emulsion homogenization enhances the lipolytic activity of immobilized CALB.
2.2. The Effect of SDS Concentration on CALB Lipolytic Activity
To assess the effect of emulsifier concentration on the lipolytic activity of both free and immobilized CALB, homogenized oil-in-water (o/w) emulsions with varying amounts of emulsifier were applied. An anionic emulsifier, SDS, was used at concentrations of 2.5%, 5.0%, 7.5%, and 10.0% in the emulsion. The immobilized form of CALB was obtained by binding the free enzyme onto IB-D152 support. Lipolytic activity was assessed by measuring the hydrolysis of triglycerides in olive oil, catalyzed by CALB. The results of lipase activity (U) were used to calculate the relative activity (
Arel). The data are presented in
Figure 2.
Analysis of the results demonstrated that the highest immobilized CALB activity was observed in emulsions containing 5.0% and 7.5% of the anionic emulsifier—SDS (
Arel = 100.0 ± 0.0%, 104.44 ± 5.09 U/g of support, and
Arel = 98.0 ± 3.5%, respectively). At other tested concentrations, the activity was slightly lower (2.5% SDS gained
Arel = 89.5 ± 4.4%, while 10.0% SDS resulted in
Arel = 88.4 ± 7.6%, and enzyme activity was 92.22 ± 6.94 U/g of support). Statistically significant differences in catalytic activity (
p < 0.05) were observed between emulsions containing 2.5% SDS and 5.0% SDS and between 2.5% SDS and 7.5% SDS. These findings suggest that SDS concentrations of 5.0% and 7.5% are optimal for maximizing the activity of immobilized CALB in the tested emulsion systems. In contrast, the activity of free lipase remained relatively unaffected by changes in SDS concentration (within the tested range), with comparable relative activities: 2.5% SDS-
Arel = 16.5 ± 1.0%; 5.0% SDS-
Arel = 17.0 ± 3.7%; 7.5% SDS-
Arel = 16.6 ± 0.7%; and 10.0% SDS-
Arel = 16.0 ± 1.4%. It should be noted that at each concentration, the activity of free lipase was noticeably lower than that of its immobilized form (approximately five-fold lower). These results indicate the hyperactivation of CALB immobilized onto the IB-D152 support, compared to its free form. We hypothesize that the enhanced activity observed in the emulsion system may be attributed to several factors, including stabilization of the enzyme in a favorable conformation, reduced protein aggregation (due to low lipase loading), and localization at the oil-water interface, which enhances the substrate availability (for lipase). In contrast, the free form appears to be more sensitive to the denaturing effects of SDS, as suggested by its consistently low activity values. However, determining the factors influencing these phenomena requires further detailed experimental research. It has been reported [
34] that SDS-modified lipase exhibits significant conformational changes, including a decrease in α-helical and β-turns content, as well as the random coils, and an increase in β-sheet content, compared to the native enzyme. These structural modifications may contribute to enhanced catalytic activity. In the literature [
35,
36], the hyperactivation of lipases using the nonionic emulsifier Triton X-100 was also discussed. It was observed that hydrophobic interactions between lipase and Triton X-100, along with the surfactant’s influence on the enzyme’s active site, may play a key role in enhancing lipase activity. Additionally, a zwitterionic surfactant (calix[4]-L-proline derivative) has been shown to influence lipase hyperactivation. This compound protects the active site and enzyme conformation through electrostatic interaction and hydrogen bonds [
37].
Studies have shown that ionic surfactants, such as SDS, can bind to enzymes via electrostatic attraction and hydrophobic interactions. At low concentrations, SDS molecules interact with positively charged amino acid residues (lysine, arginine, and histidine) and hydrophobic regions of the protein. Among these, hydrophobic interactions are likely more significant than electrostatic ones. At higher concentrations, SDS binds cooperatively, forming clusters on the protein surface. Electrostatic interactions are strongly influenced by pH and ionic strength. SDS has been found to significantly inhibit lipase activity, which results from the binding of SDS to hydrophobic fragments of lipase [
38]. Ozyilmaz and Eski [
37] described SDS as a well-known effective surfactant in protein denaturation due to its ability to unfold the protein’s secondary and tertiary structures. They observed complete inhibition of lipase activity in the presence of SDS, likely because the substrate and surfactant compete for the enzyme’s active site. Additionally, enzyme activity decreased with increasing SDS concentration. In contrast, Vaidya et al. [
34] indicated that lower surfactant concentrations can lead to lipase hyperactivation. They noticed that SDS-modified lipase exhibited a substantially higher activity compared to the free form, as well as that SDS bound to the enzyme via electrostatic interaction. Additionally, the literature also highlights that surfactants enhance the water–oil interfacial area through emulsification, thereby increasing the rate of lipase-catalyzed reactions [
39].
Emulsifiers are used to stabilize emulsions, which is a common practice in pharmaceutical technology. In the context of catalytic applications, emulsifiers may contribute to improved substrate availability within an emulsion system composed of olive oil and water. It should be noted that emulsifiers may also interact with lipase as a protein molecule, and potentially influence its catalytic activity. Therefore, the effect of SDS should be considered both in terms of pharmaceutical technology (emulsion stabilization) and catalysis (its impact on the catalytic protein molecule within the emulsion and substrate availability).
The key experimental results can be summarized as follows: immobilized CALB showed significantly higher activity at 5.0% and 7.5% SDS compared to 2.5% SDS (p < 0.05). Additionally, it exhibited hyperactivation in all tested emulsions. In contrast, the activity of the free enzyme was not significantly dependent on changes in SDS concentration. Based on the experimental data, 5.0% SDS was selected as the optimal concentration for further studies involving CALB.
2.3. The Effect of Carbomer Addition to an Emulsion Stabilized with 5.0% SDS, on the Lipolytic Activity of CALB
To investigate the impact of carbomer (cross-linked poly(acrylic acid) (PAA), Carbopol® Ultrez 10), used as a secondary emulsion-stabilizing agent, on the lipolytic activity of immobilized CALB, carbomer at a concentration of 0.1% was added as an additional excipient to emulsions containing 5.0% SDS. The activity of immobilized CALB was evaluated in emulsion systems both with and without the addition of carbomer. Enzyme activity (U) was determined in the lipase-catalyzed hydrolysis of triglycerides in olive oil (expressed as relative activity—Arel). Analysis of the data indicates that the addition of 0.1% carbomer led to an increase in the activity of immobilized CALB (enzyme activity was 124.44 ± 5.09 U/g of support), compared to the emulsion system without carbomer (enzyme activity was 104.44 ± 5.09 U/g of support) (p < 0.05). This increase in activity suggests a beneficial effect of carbomer on the biocatalyst’s activity within the emulsion system. As mentioned in the introduction, studies described in the literature regarding the effect of carbomer on lipase activity report mixed results, ranging from an increase in activity to an inhibitory effect. It is believed that the increased viscosity of the aqueous phase in the emulsion, caused by the addition of carbomer, contributes to improved emulsion stability. In addition, from a biocatalytic perspective, viscosity may also influence the substrate availability to the enzyme. We hypothesize that electrostatic interactions between the negatively charged sulfate groups of SDS and the carboxyl groups of carbomer, as well as interactions with the polyacrylic support containing carboxylic acid functional groups, may contribute to the observed enhancement in lipase activity. Furthermore, the possibility of direct interactions between lipase and carbomer or lipase and SDS should be considered, as they may influence the stabilization of the enzyme’s conformation or its activity at the oil-water interface. When explaining the mechanism behind the enhanced activity, potential changes in ionic strength or pH should also be taken into account. Additional experimental studies are required to determine the reasons for the increased lipase activity in the presence of carbomer.
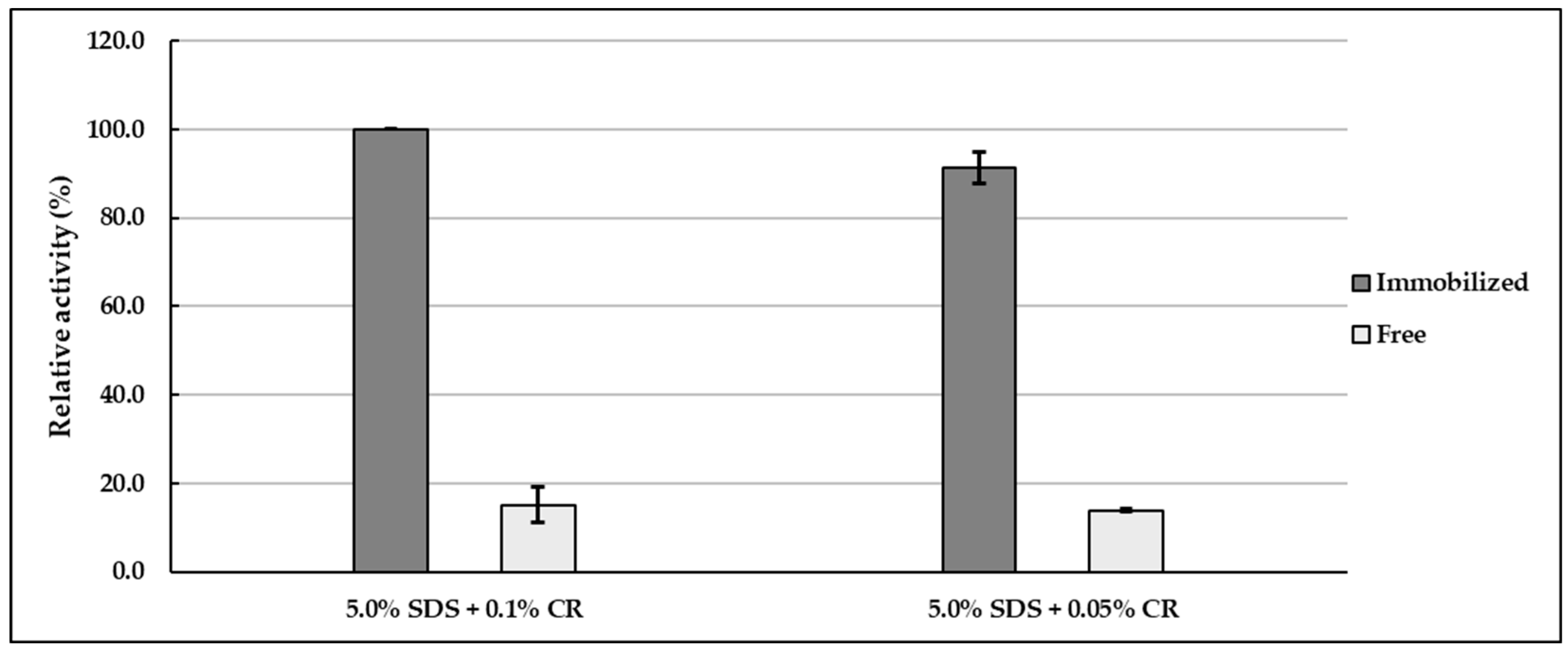
In the next stage of the project, the activity of both immobilized and free lipases was tested in homogenized emulsion systems containing carbomer (labeled as CR), at concentrations of 0.1% and 0.05%, along with a 5.0% addition of anionic emulsifier (SDS) (
Figure 3). Analysis of the data indicates that the activity of immobilized CALB was significantly higher in the emulsion containing 0.1% carbomer (
Arel = 100.0 ± 0.0%, 124.44 ± 5.09 U/g of support), compared to the emulsion with 0.05% carbomer (
Arel = 91.4 ± 3.6%) (
p < 0.05). In contrast, the catalytic activity of free CALB remained somewhat unchanged between the two systems, with a comparable relative activity observed in the emulsions containing 0.1% carbomer (
Arel = 15.1 ± 3.9%) and 0.05% carbomer (
Arel = 13.8 ± 0.3%). These findings suggest that increasing the concentration of carbomer (within the tested range of 0.05–0.1%) in emulsion systems containing 5.0% SDS has a statistically significant effect on the enhancement of immobilized CALB activity. However, no statistically significant difference was observed in the activity of immobilized CALB between the emulsion containing 5.0% SDS and the same emulsion with the addition of 0.05% carbomer (
p > 0.05).
It should be emphasized that the activity of immobilized CALB (expressed as relative activity) in emulsions containing either 0.1% or 0.05% carbomer was circa six-fold higher than the activity of free lipase tested in an emulsion of the same composition. These results may indicate a positive effect of the lipase immobilization process on the IB-D152 support, leading to enhanced lipolytic activity. The immobilized enzyme exhibited hyperactivation, i.e., a higher catalytic activity than the free form of lipase. Similar findings (hyperactivation of CALB; activity retention of 178.0%) were described in our previous study [
21], where CALB was also immobilized onto the IB-D152 support, although the emulsion was stabilized using a different emulsifier—gum arabic.
As mentioned above, anionic surfactants typically induce conformational changes in enzymes that impair their activity and stability (e.g., protein denaturation). However, in the case of Lipex
® with SDS at alkaline pH, the surfactant does not inactivate the lipase but instead enhances its lipolytic activity by opening its “lid”. It has been emphasized that, in some cases, SDS may have a protective effect. However, in mixtures of SDS with nonionic detergents, it is often the nonionic component that fulfils this role, rescuing the protein folding from the SDS-unfolded state [
40]. De Melo et al. [
41] demonstrated that SDS increased the activity of fungal lipolytic enzyme (
TaLip) by up to five-fold. The interaction with SDS may prevent enzyme aggregation, thus preserving the integrity and stability of its monomeric form. Rasmussen et al. [
42] highlighted that the interactions of TIL (
Thermomyces lanuginosus lipase) and SDS are pH-sensitive. At pH 8.0, the enzyme forms a dimer with the SDS hemi-micelle, while avoiding the core–shell structure, and retaining its activity. Electrostatic interactions play a key role, although the mechanisms of interaction are complex. In the literature [
43], interactions between anionic SDS surfactant and poly(acrylic acid) modified with hydrophobic sidechains (mimicking the polymer Carbopol
®) have been described. These surfactant-polymer interactions, as noted, are not well understood and are difficult to study experimentally. Moreover, it has been noticed that the sorption of SDS within the polymer film is weak. As the SDS concentration increases, a stable structure within the film is formed. Xie et al. [
11] reported that the electrostatic repulsive force between fatty alcohol polyoxyethylene ether sodium sulfate (AES) and poly(acrylic acid)-based rheology modifiers can influence the polymer chain elongation. However, this repulsive force may also cause polymer chain curling.
The key experimental findings can be summarized as follows: the addition of 0.1% carbomer to emulsions stabilized with 5.0% SDS significantly increased the activity of immobilized CALB (p < 0.05). Increasing the carbomer concentration from 0.05% to 0.1% resulted in enhanced activity of immobilized CALB (p < 0.05), whereas the activity of the free enzyme remained largely unchanged and lower than that of the immobilized form.
2.4. Effect of SDS Concentration on CALB Activity in Carbomer-Stabilized Emulsion
The lipolytic activity of CALB was assessed in homogenized emulsion systems containing SDS at concentrations of 2.5%, 5.0%, 7.5%, and 10.0%. Each emulsion was stabilized by the addition of 0.1% carbomer. The enzyme activity (U) results were expressed as relative activity (
Arel). The activity of both free and lipase immobilized onto an IB-D152 support was tested. The results are shown in
Figure 4.
Analysis of the data indicated that the highest catalytic activity of immobilized CALB was achieved in emulsion systems containing 5.0% SDS and 0.1% carbomer (124.44 ± 5.09 U/g of support,
Arel = 100.0 ± 0.0%), as well as 2.5% SDS and 0.1% carbomer (121.11 ± 1.92 U/g of support,
Arel = 97.4 ± 4.4%). In contrast, emulsions with higher concentrations of SDS exhibited a substantial decrease in the activity of immobilized CALB:
Arel = 78.6 ± 1.8% for 7.5% SDS and
Arel = 54.5 ± 2.1% for 10.0% SDS. These results indicate that increasing the emulsifier concentrations above 5.0% (in the presence of 0.1% carbomer) negatively affects lipolytic activity. A similar trend was observed for free lipase, where the highest activity was noticed in the emulsions containing 5.0% SDS (
Arel = 15.1 ± 3.9%) and 2.5% SDS (
Arel = 14.8 ± 0.7%), both with 0.1% carbomer. Importantly, in emulsions containing 7.5% and 10.0% SDS with 0.1% carbomer, no catalytic activity of free lipase was recorded. This may suggest a potential inhibitory effect at higher surfactant concentrations. Notably, in emulsions stabilized solely with SDS (single stabilization), free lipase exhibited approximately 16.0% of its activity (expressed as relative activity) at SDS concentrations of 7.5% and 10.0% (
Figure 2). In summary, the results indicate a concentration-dependent effect of SDS on CALB activity in emulsions containing 0.1% carbomer. Immobilized CALB exhibited maximum activity at SDS concentrations of 2.5% and 5.0%, whereas a decrease in lipase activity was observed at higher SDS concentrations. However, free lipase showed significantly lower activity compared to the immobilized form. Furthermore, no catalytic activity of free CALB was observed at higher SDS concentrations.
As emphasized, the activity of immobilized CALB in an emulsion containing 5.0% SDS and 0.1% carbomer was significantly higher than in the emulsion with 5.0% SDS alone (124.44 ± 5.09 vs. 104.44 ± 5.09 U/g of support;
p < 0.05). A similar activity profile was observed in the emulsion with 2.5% SDS and 0.1% carbomer (121.11 ± 1.92 U/g of support), compared to 2.5% SDS alone (93.33 ± 3.33 U/g of support), with the difference statistically significant (
p < 0.05). Notably, in both emulsions containing 2.5% SDS and 5.0% SDS with 0.1% carbomer, immobilized CALB demonstrated hyperactivation in relation to its free form, with activity approximately six-fold higher. These findings indicate that the dual-stabilization strategy, based on the addition of 0.1% of carbomer to the emulsion containing either 2.5% or 5.0% SDS, can significantly enhance the catalytic activity of immobilized CALB. However, it should be noted that the activity of immobilized CALB in the emulsion system with 10.0% SDS and 0.1% carbomer was lower (67.78 ± 3.85 U/g of support) than in the corresponding system without carbomer (92.22 ± 6.94 U/g of support). This suggests a strong dependence of immobilized CALB activity on SDS concentration in the presence of carbomer (
Table 1). Moreover, these results demonstrate that carbomer may contribute to either a significant increase or a decrease in immobilized CALB activity, depending on the SDS concentration. Therefore, among the tested pharmaceutical emulsion formulations, systems containing 2.5% or 5.0% of SDS and 0.1% carbomer exhibited the most promising and optimal catalytic properties.
Otzen et al. [
44] established that the most reliable model for SDS/protein interactions is the core–shell model, in which partially unfolded polypeptide chains decorate the surfaces of surfactant micelles. The formation of the complex is reversible under neutral to alkaline pH. The addition of a nonionic surfactant facilitates the recovery of the folded protein. Vishnyakov et al. [
43] pointed out that while SDS absorption by the Carbopol
® polymer film is weak, the electrostatic interactions between sulfate anions and counterions within the film are sufficient to cause significant layering. As SDS net concentration (
csn) increases, the surfactant predominantly remains in the aqueous phase instead of being absorbed by the polymer. It has also been found that SDS strongly adsorbs at hydrophobic interfaces.
The data presented in this study demonstrate a highly useful effect of combining the anionic emulsifier—SDS (at an appropriate concentration)—with 0.1% carbomer to enhance the catalytic activity of immobilized CALB in emulsion formulations. Few studies in the literature address the use of the emulsifier described in our work (SDS) and the carbomer additive as an effective approach for improving immobilized CALB activity in the proposed pharmaceutical formulation. This highlights the novelty of our research. Therefore, the results presented here constitute an important contribution to research involving the application of emulsion-based pharmaceutical formulation aimed at enhancing biocatalyst activity. The results of the dual-stabilized emulsion catalytic systems discussed in this work demonstrate that the combination of SDS and carbomer (at appropriate concentrations) effectively enhances the activity of immobilized CALB. These findings are consistent with the study objectives and offer a practical approach for designing pharmaceutical emulsion formulations with improved biocatalytic performance. Moreover, the results may also be valuable in other research areas. Potential applications include drug delivery systems, food processing, or green synthesis, where stable emulsion systems are essential.
4. Conclusions
Numerous studies on lipase activity reported in the literature focus mainly on systems containing either a single surfactant or a mixture of two surfactants. However, the combined effect of surfactant and a gelling agent, such as cross-linked poly(acrylic acid), on lipase activity has not been thoroughly investigated. To address this gap, we conducted experimental studies to assess the utility of a dual-stabilized system approach in lipase-catalyzed reactions. In this study, we investigated the effect of an emulsion system stabilized by an anionic surfactant—SDS, and a gelling agent—carbomer (Carbopol® Ultrez 10, cross-linked poly(acrylic acid)) on the activity of CALB, in both free and immobilized forms. The results showed that the activity of immobilized CALB in the emulsion system containing 5.0% SDS and 0.1% carbomer was significantly higher than in the emulsion with 5.0% SDS alone (124.44 ± 5.09 vs. 104.44 ± 5.09 U/g of support). Likewise, the emulsion with 2.5% SDS and 0.1% carbomer exhibited noticeably higher activity of immobilized CALB (121.11 ± 1.92 U/g of support) compared to 2.5% SDS alone (93.33 ± 3.33 U/g of support), with the difference statistically significant (p < 0.05). In contrast, the activity of immobilized CALB in the emulsion system with 10.0% SDS and 0.1% carbomer was lower (67.78 ± 3.85 U/g of support) than in the corresponding system containing 10.0% SDS alone, without carbomer additive (92.22 ± 6.94 U/g of support). Importantly, in the emulsions containing 2.5% SDS and 5.0% SDS with the addition of 0.1% carbomer, immobilized CALB exhibited hyperactivation, with activity exceeding that of the free form by circa six-fold. The presented results suggest that carbomer may contribute to either a significant increase or a decrease in immobilized CALB activity, depending on the SDS concentration. Therefore, using the dual-stabilization strategy that includes the addition of 0.1% of carbomer to the emulsion and either 2.5% or 5.0% SDS, the catalytic activity of immobilized CALB can be enhanced. This approach fills an important gap in the literature. The dual-stabilized pharmaceutical formulations strategy demonstrates strong potential in the development of emulsion systems for efficient enzyme activity enhancement in biocatalytic applications.