Catalyst Design and Engineering for Enhanced Microplastic Degradation and Upcycling—A Review
Abstract
1. Introduction
2. Difference Between MPs and Bulk Plastics
3. Microplastic Identification and Quantification

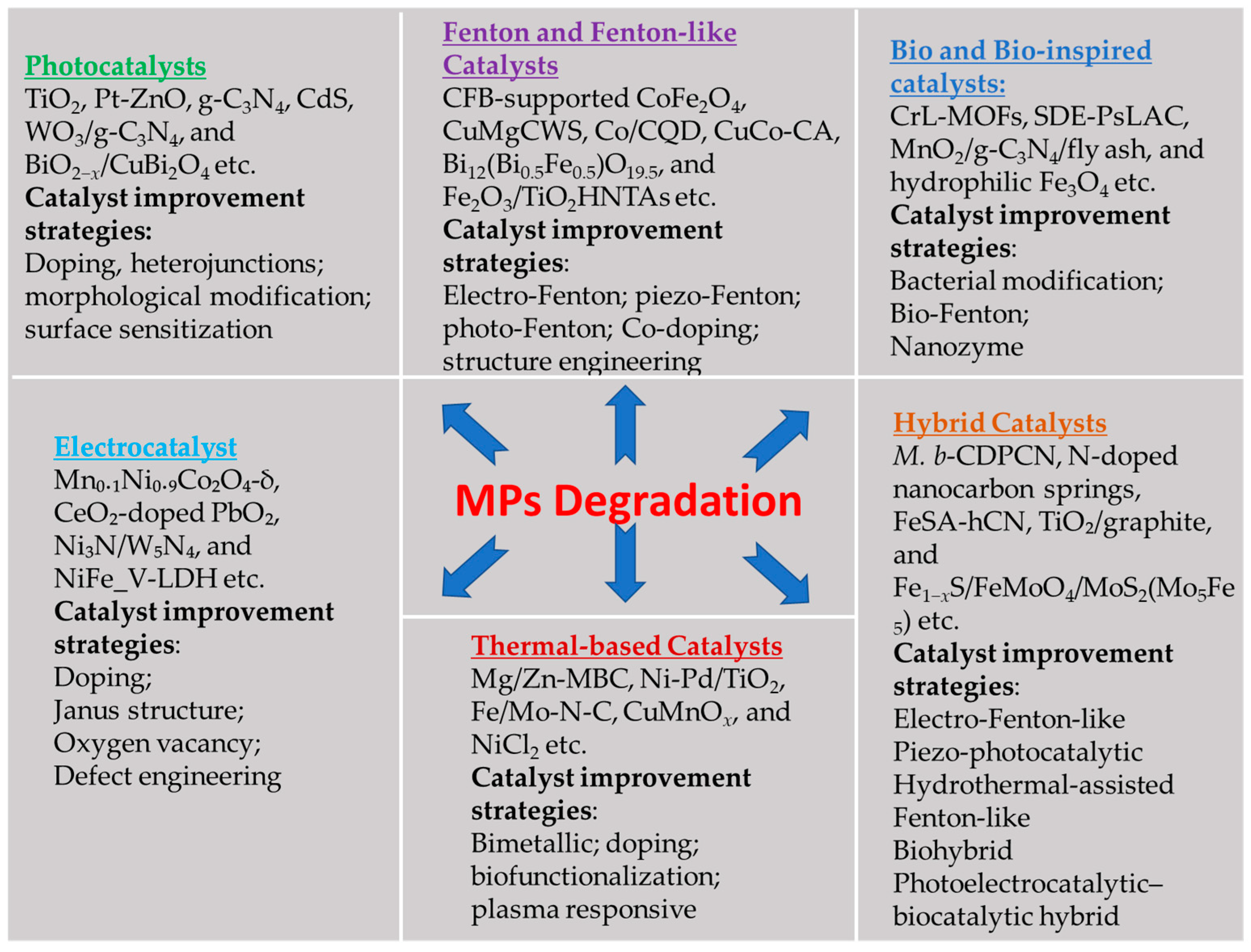
4. Enhanced Microplastic Degradation Using a Catalyst
4.1. Photocatalysts
4.2. Fenton and Fenton-like Catalysts
4.3. Thermal Catalytic Process
4.4. Bio and Bio-Inspired Catalysts
4.5. Electrocatalysts
4.6. Hybrid Catalysts Coupling Different Reaction Pathways
5. Challenges and Future Opportunities for Catalytic Microplastic Degradation and Upcycling
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Mohamed Nor, N.H.; Kooi, M.; Diepens, N.J.; Koelmans, A.A. Lifetime Accumulation of Microplastic in Children and Adults. Environ. Sci. Technol. 2021, 55, 5084–5096. [Google Scholar] [CrossRef]
- Thompson, R.C.; Courtene-Jones, W.; Boucher, J.; Pahl, S.; Raubenheimer, K.; Koelmans, A.A. Twenty Years of Microplastic Pollution Research—What Have We Learned? Science 2024, 386, eadl2746. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, U.; Sun, J.; Zhu, C.; Wu, M.; Zhao, B.; Gao, P. Plastic Recycling: A Review on Life Cycle, Methods, Misconceptions, and Techno—Economic Analysis. Adv. Sustain. Syst. 2023, 7, 2200471. [Google Scholar] [CrossRef]
- Sutkar, P.R.; Gadewar, R.D.; Dhulap, V.P. Recent Trends in Degradation of Microplastics in the Environment: A State-of-the-Art Review. J. Hazard. Mater. Adv. 2023, 11, 100343. [Google Scholar] [CrossRef]
- Puteri, M.N.; Gew, L.T.; Ong, H.C.; Ming, L.C. Technologies to Eliminate Microplastic from Water: Current Approaches and Future Prospects. Environ. Int. 2025, 199, 109397. [Google Scholar] [CrossRef]
- Welden, N.A.; Lusher, A. Microplastics. In Plastic Waste and Recycling; Elsevier: Amsterdam, The Netherlands, 2020; pp. 223–249. ISBN 9780128178805. [Google Scholar]
- Bermúdez, J.R.; Swarzenski, P.W. A Microplastic Size Classification Scheme Aligned with Universal Plankton Survey Methods. MethodsX 2021, 8, 101516. [Google Scholar] [CrossRef]
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a Consensus on the Definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef]
- Efimova, I.; Bagaeva, M.; Bagaev, A.; Kileso, A.; Chubarenko, I.P. Secondary Microplastics Generation in the Sea Swash Zone With Coarse Bottom Sediments: Laboratory Experiments. Front. Mar. Sci. 2018, 5, 313. [Google Scholar] [CrossRef]
- Gopalakrishnan, K.K.; Sivakumar, R.; Kashian, D. The Microplastics Cycle: An In-Depth Look at a Complex Topic. Appl. Sci. 2023, 13, 10999. [Google Scholar] [CrossRef]
- Hale, R.C.; Seeley, M.E.; La Guardia, M.J.; Mai, L.; Zeng, E.Y. A Global Perspective on Microplastics. J. Geophys. Res. Ocean. 2020, 125, e2018JC014719. [Google Scholar] [CrossRef]
- Mattsson, K.; Johnson, E.V.; Malmendal, A.; Linse, S.; Hansson, L.-A.; Cedervall, T. Brain Damage and Behavioural Disorders in Fish Induced by Plastic Nanoparticles Delivered through the Food Chain. Sci. Rep. 2017, 7, 11452. [Google Scholar] [CrossRef]
- Tiwari, M.; Rathod, T.D.; Ajmal, P.Y.; Bhangare, R.C.; Sahu, S.K. Distribution and Characterization of Microplastics in Beach Sand from Three Different Indian Coastal Environments. Mar. Pollut. Bull. 2019, 140, 262–273. [Google Scholar] [CrossRef]
- Nobre, C.R.; Santana, M.F.M.; Maluf, A.; Cortez, F.S.; Cesar, A.; Pereira, C.D.S.; Turra, A. Assessment of Microplastic Toxicity to Embryonic Development of the Sea Urchin Lytechinus Variegatus (Echinodermata: Echinoidea). Mar. Pollut. Bull. 2015, 92, 99–104. [Google Scholar] [CrossRef]
- Ali, S.S.; Alsharbaty, M.H.M.; Al-Tohamy, R.; Khalil, M.A.; Schagerl, M.; Al-Zahrani, M.; Sun, J. Microplastics as an Emerging Potential Threat: Toxicity, Life Cycle Assessment, and Management. Toxics 2024, 12, 909. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Sima, J.; Song, J.; Du, X.; Lou, F.; Zhu, Y.; Lei, J.; Huang, Q. Complete Degradation of Polystyrene Microplastics through Non-Thermal Plasma-Assisted Catalytic Oxidation. J. Hazard. Mater. 2024, 480, 136313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Hou, Y.; Wang, Y.; Wang, Q.; Shan, X.; Liu, J. Highly Efficient Low-Temperature Biodegradation of Polyethylene Microplastics by Using Cold-Active Laccase Cell-Surface Display System. Bioresour. Technol. 2023, 382, 129164. [Google Scholar] [CrossRef]
- Uheida, A.; Mejía, H.G.; Abdel-Rehim, M.; Hamd, W.; Dutta, J. Visible Light Photocatalytic Degradation of Polypropylene Microplastics in a Continuous Water Flow System. J. Hazard. Mater. 2021, 406, 124299. [Google Scholar] [CrossRef]
- Lin, J.; Hu, K.; Wang, Y.; Tian, W.; Hall, T.; Duan, X.; Sun, H.; Zhang, H.; Cortés, E.; Wang, S. Tandem Microplastic Degradation and Hydrogen Production by Hierarchical Carbon Nitride-Supported Single-Atom Iron Catalysts. Nat. Commun. 2024, 15, 8769. [Google Scholar] [CrossRef]
- Martín, A.J.; Mondelli, C.; Jaydev, S.D.; Pérez-Ramírez, J. Catalytic Processing of Plastic Waste on the Rise. Chem 2021, 7, 1487–1533. [Google Scholar] [CrossRef]
- Li, H.; Aguirre-Villegas, H.A.; Allen, R.D.; Bai, X.; Benson, C.H.; Beckham, G.T.; Bradshaw, S.L.; Brown, J.L.; Brown, R.C.; Cecon, V.S.; et al. Expanding Plastics Recycling Technologies: Chemical Aspects, Technology Status and Challenges. Green Chem. 2022, 24, 8899–9002. [Google Scholar] [CrossRef]
- Jehanno, C.; Alty, J.W.; Roosen, M.; De Meester, S.; Dove, A.P.; Chen, E.Y.-X.; Leibfarth, F.A.; Sardon, H. Critical Advances and Future Opportunities in Upcycling Commodity Polymers. Nature 2022, 603, 803–814. [Google Scholar] [CrossRef]
- Zhao, X.; Korey, M.; Li, K.; Copenhaver, K.; Tekinalp, H.; Celik, S.; Kalaitzidou, K.; Ruan, R.; Ragauskas, A.J.; Ozcan, S. Plastic Waste Upcycling toward a Circular Economy. Chem. Eng. J. 2022, 428, 131928. [Google Scholar] [CrossRef]
- Napper, I.E.; Davies, B.F.R.; Clifford, H.; Elvin, S.; Koldewey, H.J.; Mayewski, P.A.; Miner, K.R.; Potocki, M.; Elmore, A.C.; Gajurel, A.P.; et al. Reaching New Heights in Plastic Pollution—Preliminary Findings of Microplastics on Mount Everest. One Earth 2020, 3, 621–630. [Google Scholar] [CrossRef]
- Zimmermann, L.; Göttlich, S.; Oehlmann, J.; Wagner, M.; Völker, C. What Are the Drivers of Microplastic Toxicity? Comparing the Toxicity of Plastic Chemicals and Particles to Daphnia Magna. Environ. Pollut. 2020, 267, 115392. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, J. The Chemical Behaviors of Microplastics in Marine Environment: A Review. Mar. Pollut. Bull. 2019, 142, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rafa, N.; Ahmed, B.; Zohora, F.; Bakya, J.; Ahmed, S.; Ahmed, S.F.; Mofijur, M.; Chowdhury, A.A.; Almomani, F. Microplastics as Carriers of Toxic Pollutants: Source, Transport, and Toxicological Effects. Environ. Pollut. 2024, 343, 123190. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Hu, B.; Wang, H. Analytical Methods for Microplastics in the Environment: A Review. Environ. Chem. Lett. 2023, 21, 383–401. [Google Scholar] [CrossRef]
- Wang, Z.-M.; Wagner, J.; Ghosal, S.; Bedi, G.; Wall, S. SEM/EDS and Optical Microscopy Analyses of Microplastics in Ocean Trawl and Fish Guts. Sci. Total Environ. 2017, 603–604, 616–626. [Google Scholar] [CrossRef]
- Ivleva, N.P.; Wiesheu, A.C.; Niessner, R. Microplastic in Aquatic Ecosystems. Angew. Chem. Int. Ed. 2017, 56, 1720–1739. [Google Scholar] [CrossRef]
- Primpke, S.; Lorenz, C.; Rascher-Friesenhausen, R.; Gerdts, G. An Automated Approach for Microplastics Analysis Using Focal Plane Array (FPA) FTIR Microscopy and Image Analysis. Anal. Methods 2017, 9, 1499–1511. [Google Scholar] [CrossRef]
- Araujo, C.F.; Nolasco, M.M.; Ribeiro, A.M.P.; Ribeiro-Claro, P.J.A. Identification of Microplastics Using Raman Spectroscopy: Latest Developments and Future Prospects. Water Res. 2018, 142, 426–440. [Google Scholar] [CrossRef]
- Batel, A.; Borchert, F.; Reinwald, H.; Erdinger, L.; Braunbeck, T. Microplastic Accumulation Patterns and Transfer of Benzo[a]Pyrene to Adult Zebrafish (Danio Rerio) Gills and Zebrafish Embryos. Environ. Pollut. 2018, 235, 918–930. [Google Scholar] [CrossRef]
- Matsuguma, Y.; Takada, H.; Kumata, H.; Kanke, H.; Sakurai, S.; Suzuki, T.; Itoh, M.; Okazaki, Y.; Boonyatumanond, R.; Zakaria, M.P.; et al. Microplastics in Sediment Cores from Asia and Africa as Indicators of Temporal Trends in Plastic Pollution. Arch. Environ. Contam. Toxicol. 2017, 73, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Peez, N.; Janiska, M.-C.; Imhof, W. The First Application of Quantitative 1H NMR Spectroscopy as a Simple and Fast Method of Identification and Quantification of Microplastic Particles (PE, PET, and PS). Anal. Bioanal. Chem. 2019, 411, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Richter, F.; Rein, G. The Role of Heat Transfer Limitations in Polymer Pyrolysis at the Microscale. Front. Mech. Eng. 2018, 4, 18. [Google Scholar] [CrossRef]
- Zeng, G.; Zhao, B.; Deng, C.; Zhu, C.; Cheng, M.M.-C.; Gao, P.-X. Microplastic Detection and Monitoring in Biological and Environmental Systems: A Mini Review of Techniques and Strategies. Int. J. High Speed Electron. Syst. 2026, 35, 2640002. [Google Scholar] [CrossRef]
- Cristoni, S.; Dusi, G.; Brambilla, P.; Albini, A.; Conti, M.; Brambilla, M.; Bruno, A.; Di Gaudio, F.; Ferlin, L.; Tazzari, V.; et al. SANIST: Optimization of a Technology for Compound Identification Based on the European Union Directive with Applications in Forensic, Pharmaceutical and Food Analyses. J. Mass Spectrom. 2017, 52, 16–21. [Google Scholar] [CrossRef]
- Elumalai, P.V.; Dhinesh, B.; Jayakar, J.; Nambiraj, M.; Hariharan, V. Effects of Antioxidants to Reduce the Harmful Pollutants from Diesel Engine Using Preheated Palm Oil–Diesel Blend. J. Therm. Anal. Calorim. 2022, 147, 2439–2453. [Google Scholar] [CrossRef]
- Maddison, C.; Sathish, C.I.; Lakshmi, D.; Wayne, O.; Palanisami, T. An Advanced Analytical Approach to Assess the Long-Term Degradation of Microplastics in the Marine Environment. npj Mater. Degrad. 2023, 7, 59. [Google Scholar] [CrossRef]
- Pfohl, P.; Wagner, M.; Meyer, L.; Domercq, P.; Praetorius, A.; Hüffer, T.; Hofmann, T.; Wohlleben, W. Environmental Degradation of Microplastics: How to Measure Fragmentation Rates to Secondary Micro- and Nanoplastic Fragments and Dissociation into Dissolved Organics. Environ. Sci. Technol. 2022, 56, 11323–11334. [Google Scholar] [CrossRef] [PubMed]
- Nene, A.; Sadeghzade, S.; Viaroli, S.; Yang, W.; Uchenna, U.P.; Kandwal, A.; Liu, X.; Somani, P.; Galluzzi, M. Recent Advances and Future Technologies in Nano-Microplastics Detection. Environ. Sci. Eur. 2025, 37, 7. [Google Scholar] [CrossRef]
- He, J.; Han, L.; Wang, F.; Ma, C.; Cai, Y.; Ma, W.; Xu, E.G.; Xing, B.; Yang, Z. Photocatalytic Strategy to Mitigate Microplastic Pollution in Aquatic Environments: Promising Catalysts, Efficiencies, Mechanisms, and Ecological Risks. Crit. Rev. Environ. Sci. Technol. 2022, 53, 504–526. [Google Scholar] [CrossRef]
- Wang, C.; Sun, R.; Huang, R.; Wang, H. Superior Fenton-like Degradation of Tetracycline by Iron Loaded Graphitic Carbon Derived from Microplastics: Synthesis, Catalytic Performance, and Mechanism. Sep. Purif. Technol. 2021, 270, 118773. [Google Scholar] [CrossRef]
- Ren, S.; Xu, X.; Zhu, Z.-S.; Yang, Y.; Tian, W.; Hu, K.; Zhong, S.; Yi, J.; Duan, X.; Wang, S. Catalytic Transformation of Microplastics to Functional Carbon for Catalytic Peroxymonosulfate Activation: Conversion Mechanism and Defect of Scavenging. Appl. Catal. B Environ. 2024, 342, 123410. [Google Scholar] [CrossRef]
- Hou, Q.; Zhen, M.; Qian, H.; Nie, Y.; Bai, X.; Xia, T.; Laiq Ur Rehman, M.; Li, Q.; Ju, M. Upcycling and Catalytic Degradation of Plastic Wastes. Cell Rep. Phys. Sci. 2021, 2, 100514. [Google Scholar] [CrossRef]
- Jeyaraj, J.; Baskaralingam, V.; Stalin, T.; Muthuvel, I. Mechanistic Vision on Polypropylene Microplastics Degradation by Solar Radiation Using TiO2 Nanoparticle as Photocatalyst. Environ. Res. 2023, 233, 116366. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, H.; Huo, P. Plastic Degradation and Conversion by Photocatalysis. In Plastic Degradation and Conversion by Photocatalysis (Volume 2): From Waste to Wealth; American Chemical Society: Washington, DC, USA, 2024; pp. 1–22. ISBN 9780841296091. [Google Scholar]
- Wang, X.; Zhu, Z.; Jiang, J.; Li, R.; Xiong, J. Preparation of Heterojunction C3N4/WO3 Photocatalyst for Degradation of Microplastics in Water. Chemosphere 2023, 337, 139206. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, Z.; Liu, H.; Li, Y.; Zhang, G. Efficient Photocatalytic Degradation of Microplastics by Constructing a Novel Z-Scheme Fe-Doped BiO2−x/BiOI Heterojunction with Full-Spectrum Response: Mechanistic Insights and Theory Calculations. J. Hazard. Mater. 2024, 480, 136080. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.; Jin, M.; Zhu, J.; Zhou, Q.; Fu, L.; Wu, W. Photocatalytic Technologies for Transformation and Degradation of Microplastics in the Environment: Current Achievements and Future Prospects. Catalysts 2023, 13, 846. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, X. Nanomaterial ZnO Synthesis and Its Photocatalytic Applications: A Review. Nanomaterials 2025, 15, 682. [Google Scholar] [CrossRef]
- Surana, M.; Pattanayak, D.S.; Yadav, V.; Singh, V.K.; Pal, D. An Insight Decipher on Photocatalytic Degradation of Microplastics: Mechanism, Limitations, and Future Outlook. Environ. Res. 2024, 247, 118268. [Google Scholar] [CrossRef]
- Kang, S.; Sun, T.; Ma, Y.; Du, M.; Gong, M.; Zhou, C.; Chai, Y.; Qiu, B. Artificial Photosynthesis Bringing New Vigor into Plastic Wastes. SmartMat 2023, 4, e1202. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, L.; Nekliudov, A. Construction of Loading g-C3N4/TiO2 on Waste Cotton-Based Activated Carbon S-Scheme Heterojunction for Enhanced Photocatalytic Degradation of Microplastics: Performance, DFT Calculation and Mechanism Study. Opt. Mater. 2024, 154, 115786. [Google Scholar] [CrossRef]
- Cao, B.; Wan, S.; Wang, Y.; Guo, H.; Ou, M.; Zhong, Q. Highly-Efficient Visible-Light-Driven Photocatalytic H2 Evolution Integrated with Microplastic Degradation over MXene/ZnxCd1-XS Photocatalyst. J. Colloid Interface Sci. 2022, 605, 311–319. [Google Scholar] [CrossRef]
- Maulana, D.A.; Ibadurrohman, M. Slamet Synthesis of Nano-Composite Ag/TiO2 for Polyethylene Microplastic Degradation Applications. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1011, 012054. [Google Scholar] [CrossRef]
- Tofa, T.S.; Ye, F.; Kunjali, K.L.; Dutta, J. Enhanced Visible Light Photodegradation of Microplastic Fragments with Plasmonic Platinum/Zinc Oxide Nanorod Photocatalysts. Catalysts 2019, 9, 819. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, H.; Li, Y.; Zhang, G. Efficient Photocatalytic Degradation of Polystyrene Microplastics in Water over Core–Shell BiO2−x/CuBi2O4 Heterojunction with Full Spectrum Light Response. J. Colloid Interface Sci. 2025, 686, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, D.; Wu, X.; Wu, D.; Su, N.; Wang, Y.; Chen, F.; Fu, C.; Wang, J.; Zhang, Q. Synergistic Dual-Defect Band Engineering for Highly Efficient Photocatalytic Degradation of Microplastics via Nb-Induced Oxygen Vacancies in SnO2 Quantum Dots. J. Mater. Chem. A 2025, 13, 4429–4443. [Google Scholar] [CrossRef]
- Quilumbaquin, W.; Castillo-Cabrera, G.X.; Borrero-González, L.J.; Mora, J.R.; Valle, V.; Debut, A.; Loor-Urgilés, L.D.; Espinoza-Montero, P.J. Photoelectrocatalytic Degradation of High-Density Polyethylene Microplastics on TiO2-Modified Boron-Doped Diamond Photoanode. iScience 2024, 27, 109192. [Google Scholar] [CrossRef]
- Lin, L.; Yi, J.; Wang, J.; Qian, Q.; Chen, Q.; Cao, C.; Zhou, W. Enhancing Microplastic Degradation through Synergistic Photocatalytic and Pretreatment Approaches. Langmuir 2024, 40, 22582–22590. [Google Scholar] [CrossRef]
- He, Y.; Rehman, A.U.; Xu, M.; Not, C.A.; Ng, A.M.C.; Djurišić, A.B. Photocatalytic Degradation of Different Types of Microplastics by TiOx/ZnO Tetrapod Photocatalysts. Heliyon 2023, 9, e22562. [Google Scholar] [CrossRef]
- Jiang, R.; Lu, G.; Dang, T.; Wang, M.; Liu, J.; Yan, Z.; Xie, H. Insight into the Degradation Process of Functional Groups Modified Polystyrene Microplastics with Dissolvable BiOBr-OH Semiconductor-Organic Framework. Chem. Eng. J. 2023, 470, 144401. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Hidalgo-Jiménez, J.; Sauvage, X.; Saito, K.; Guo, Q.; Edalati, K. Phase and Sulfur Vacancy Engineering in Cadmium Sulfide for Boosting Hydrogen Production from Catalytic Plastic Waste Photoconversion. Chem. Eng. J. 2025, 504, 158730. [Google Scholar] [CrossRef]
- Xiao, Y.; Tian, Y.; Xu, W.; Zhu, J. Photodegradation of Microplastics through Nanomaterials: Insights into Photocatalysts Modification and Detailed Mechanisms. Materials 2024, 17, 2755. [Google Scholar] [CrossRef]
- Zhou, G.; Xu, H.; Song, H.; Yi, J.; Wang, X.; Chen, Z.; Zhu, X. Photocatalysis toward Microplastics Conversion: A Critical Review. ACS Catal. 2024, 14, 8694–8719. [Google Scholar] [CrossRef]
- Gu, X.; Li, L.; Wu, Y.; Dong, W. Enhancement of Microplastics Degradation with MIL-101 Modified BiOI Photocatalyst under Light and Dark Alternated System. J. Environ. Chem. Eng. 2024, 12, 112958. [Google Scholar] [CrossRef]
- Meng, Z.; Sun, C.; Wang, C.; Wang, Z.; Deng, S.; Yang, H. Boosting Photocatalytic Capability by Dispersing TiO2 into Chitin Matrix for Polystyrene Microplastics Degradation. Appl. Surf. Sci. 2025, 711, 164104. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J. Multivalent Metal Catalysts in Fenton/Fenton-like Oxidation System: A Critical Review. Chem. Eng. J. 2023, 466, 143147. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Wang, J. Fenton/Fenton-like Processes with in-Situ Production of Hydrogen Peroxide/Hydroxyl Radical for Degradation of Emerging Contaminants: Advances and Prospects. J. Hazard. Mater. 2021, 404, 124191. [Google Scholar] [CrossRef]
- Meyerstein, D. Re-Examining Fenton and Fenton-like Reactions. Nat. Rev. Chem. 2021, 5, 595–597. [Google Scholar] [CrossRef]
- Xiao, J.; Guo, S.; Wang, D.; An, Q. Fenton—Like Reaction: Recent Advances and New Trends. Chem.—A Eur. J. 2024, 30, e202304337. [Google Scholar] [CrossRef] [PubMed]
- Zarghami Qaretapeh, M.; Kouchakipour, S.; Hosseinzadeh, M.; Dashtian, K. Cuttlefish Bone-Supported CoFe2O4 Nanoparticles Enhance Persulfate Fenton-like Process for the Degradation of Polystyrene Nanoplastics. Chem. Eng. J. 2024, 490, 151833. [Google Scholar] [CrossRef]
- Ye, J.; Li, N.; Gao, W.; Peng, W.; Peng, X.; Cheng, Z.; Yan, B.; Fu, Y.; Zhan, S.; Chen, G.; et al. Bimetal-Carbon Engineering for Polypropylene Conversion into Hydrocarbons and Ketones in a Fenton-like System. Appl. Catal. B Environ. Energy 2024, 358, 124411. [Google Scholar] [CrossRef]
- Camilli, E.; Pighin, A.F.; Copello, G.J.; Villanueva, M.E. Cobalt/Carbon Quantum Dots Core-Shell Nanoparticles as an Improved Catalyst for Fenton-like Reaction. Nano-Struct. Nano-Objects 2024, 37, 101097. [Google Scholar] [CrossRef]
- Ye, Q.; Xu, H.; Hunter, T.N.; Harbottle, D.; Kale, G.M.; Tillotson, M.R. Advanced Polystyrene Nanoplastic Remediation through Electro-Fenton Process: Degradation Mechanisms and Pathways. J. Environ. Chem. Eng. 2025, 13, 118907. [Google Scholar] [CrossRef]
- Wu, M.; Wang, R.; Miao, L.; Sun, P.; Zhou, B.; Xiong, Y.; Dong, X. Synergistically Piezocatalytic and Fenton-like Activation of H2O2 by a Ferroelectric Bi12(Bi0.5Fe0.5)O19.5 Catalyst to Boost Degradation of Polyethylene Terephthalate Microplastic (PET-MPs). J. Colloid Interface Sci. 2025, 682, 738–750. [Google Scholar] [CrossRef]
- Xue, Z.; Yu, X.; Ke, X.; Zhao, J. A Novel Route for Microplastic Mineralization: Visible-Light-Driven Heterogeneous Photocatalysis and Photothermal Fenton-like Reaction. Environ. Sci. Nano 2024, 11, 113–122. [Google Scholar] [CrossRef]
- Hu, K.; Zhou, P.; Yang, Y.; Hall, T.; Nie, G.; Yao, Y.; Duan, X.; Wang, S. Degradation of Microplastics by a Thermal Fenton Reaction. Acs Es&T Eng. 2022, 2, 110–120. [Google Scholar] [CrossRef]
- Zanaty, M.; Zaki, A.H.; El-Dek, S.I.; Abdelhamid, H.N. Zeolitic Imidazolate Framework@hydrogen Titanate Nanotubes for Efficient Adsorption and Catalytic Oxidation of Organic Dyes and Microplastics. J. Environ. Chem. Eng. 2024, 12, 112547. [Google Scholar] [CrossRef]
- Bule Možar, K.; Miloloža, M.; Martinjak, V.; Radovanović-Perić, F.; Bafti, A.; Ujević Bošnjak, M.; Markić, M.; Bolanča, T.; Cvetnić, M.; Kučić Grgić, D.; et al. Evaluation of Fenton, Photo-Fenton and Fenton-like Processes in Degradation of PE, PP, and PVC Microplastics. Water 2024, 16, 673. [Google Scholar] [CrossRef]
- Shen, M.; Song, B.; Zhou, C.; Hu, T.; Zeng, G.; Zhang, Y. Advanced Oxidation Processes for the Elimination of Microplastics from Aqueous Systems: Assessment of Efficiency, Perspectives and Limitations. Sci. Total Environ. 2022, 842, 156723. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Liu, Y.; Lou, X.; Zhang, Q.; Chen, J. Rational Design of Chemical Catalysis for Plastic Recycling. ACS Catal. 2022, 12, 4659–4679. [Google Scholar] [CrossRef]
- Wang, H.; Chen, H.; Wan, Q.; Zheng, Y.; Wan, Y.; Liu, X.; Song, X.; Ma, W.; Huo, P. Catalytic Degradation of Polyethylene Terephthalate Microplastics by Co-N/C@CeO2 Composite in Thermal-Assisted Activation PMS System: Process Mechanism and Toxicological Analysis. Chem. Eng. J. 2025, 514, 163192. [Google Scholar] [CrossRef]
- Wang, J.; Sun, C.; Huang, Q.-X.; Chi, Y.; Yan, J.-H. Adsorption and Thermal Degradation of Microplastics from Aqueous Solutions by Mg/Zn Modified Magnetic Biochars. J. Hazard. Mater. 2021, 419, 126486. [Google Scholar] [CrossRef]
- Nabgan, W.; Nabgan, B.; Tuan Abdullah, T.A.; Ikram, M.; Jadhav, A.H.; Jalil, A.A.; Ali, M.W. Highly Active Biphasic Anatase-Rutile Ni-Pd/TNPs Nanocatalyst for the Reforming and Cracking Reactions of Microplastic Waste Dissolved in Phenol. ACS Omega 2022, 7, 3324–3340. [Google Scholar] [CrossRef]
- Ma, S.; Tang, S.; Zhao, Y. Upcycling Polystyrene Microplastics to Fe/Mo-Doped Sponge-Carbon: Mo5+ Enhanced Electron Transfer for Boosting Fenton-like Performance. Chem. Eng. J. 2024, 498, 155460. [Google Scholar] [CrossRef]
- Sun, R.; Yang, J.; Huang, R.; Wang, C. Controlled Carbonization of Microplastics Loaded Nano Zero-Valent Iron for Catalytic Degradation of Tetracycline. Chemosphere 2022, 303, 135123. [Google Scholar] [CrossRef]
- Hao, Y.; Che, X.; Hou, X.; Shi, L.; Huang, L. Recent Advances in the Thermo-Catalytic Upcycling of Polyethylene Waste. ACS Appl. Polym. Mater. 2025, 7, 6597–6612. [Google Scholar] [CrossRef]
- Vo, T.-P.; Rintala, J.; Dai, L.; Oh, W.-D.; He, C. The Role of Ubiquitous Metal Ions in Degradation of Microplastics in Hot-Compressed Water. Water Res. 2023, 245, 120672. [Google Scholar] [CrossRef]
- Daligaux, V.; Richard, R.; Manero, M.-H. Deactivation and Regeneration of Zeolite Catalysts Used in Pyrolysis of Plastic Wastes—A Process and Analytical Review. Catalysts 2021, 11, 770. [Google Scholar] [CrossRef]
- Razali, N.A.; Wan Abdullah, W.R.; Mohd Zikir, N. Effect of Thermo-Photocatalytic Process Using Zinc Oxide on Degradation of Macro/Micro-Plastic in Aqueous Environment. J. Sustain. Sci. Manag. 2020, 15, 1–14. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, S.; Sun, B.; Han, Y.; Zhang, Z.; Shen, X.; Li, Z. Adsorption Efficiency and In-Situ Catalytic Thermal Degradation Behaviour of Microplastics from Water over Fe-Modified Lignin-Based Magnetic Biochar. Sep. Purif. Technol. 2025, 353, 128468. [Google Scholar] [CrossRef]
- Li, S.; Yang, Y.; Yang, S.; Zheng, H.; Zheng, Y.; M, J.; Nagarajan, D.; Varjani, S.; Chang, J.-S. Recent Advances in Biodegradation of Emerging Contaminants—Microplastics (MPs): Feasibility, Mechanism, and Future Prospects. Chemosphere 2023, 331, 138776. [Google Scholar] [CrossRef]
- Gao, W.; Xu, M.; Zhao, W.; Yang, X.; Xin, F.; Dong, W.; Jia, H.; Wu, X. Microbial Degradation of (Micro)Plastics: Mechanisms, Enhancements, and Future Directions. Fermentation 2024, 10, 441. [Google Scholar] [CrossRef]
- Thakur, B.; Singh, J.; Singh, J.; Angmo, D.; Vig, A.P. Biodegradation of Different Types of Microplastics: Molecular Mechanism and Degradation Efficiency. Sci. Total Environ. 2023, 877, 162912. [Google Scholar] [CrossRef]
- Du, H.; Xie, Y.; Wang, J. Microplastic Degradation Methods and Corresponding Degradation Mechanism: Research Status and Future Perspectives. J. Hazard. Mater. 2021, 418, 126377. [Google Scholar] [CrossRef]
- Rincon, I.; Hidalgo, T.; Armani, G.; Rojas, S.; Horcajada, P. Enzyme_Metal—Organic Framework Composites as Novel Approach for Microplastic Degradation. ChemSusChem 2024, 17, e202301350. [Google Scholar] [CrossRef]
- Zandieh, M.; Liu, J. Removal and Degradation of Microplastics Using the Magnetic and Nanozyme Activities of Bare Iron Oxide Nanoaggregates. Angew. Chem. 2022, 134, e202212013. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.; Chen, Z.; Yu, Z.; Xue, J.; Luan, T.; Chen, S.; Zhou, S. Mechanisms of Polystyrene Microplastic Degradation by the Microbially Driven Fenton Reaction. Water Res. 2022, 223, 118979. [Google Scholar] [CrossRef]
- Zhang, A.; Hou, Y.; Hou, S.; Wang, Y.; Wang, Q. Enhancement of Microplastics Degradation Efficiency: Microbial Laccase-Driven Radical Chemical Coupling Catalysis. Chem. Eng. J. 2025, 507, 160579. [Google Scholar] [CrossRef]
- Li, X.; Li, G.; Wang, J.; Li, X.; Yang, Y.; Song, D. Elucidating Polyethylene Microplastic Degradation Mechanisms and Metabolic Pathways via Iron-Enhanced Microbiota Dynamics in Marine Sediments. J. Hazard. Mater. 2024, 466, 133655. [Google Scholar] [CrossRef]
- Wan, P.; Chen, G.; Fan, J.; Tan, W.; Li, X.; Chen, L.; Li, K. Modulating Ion Migration Realizes Both Enhanced and Long-Term-Stable Nanozyme Activity for Efficient Microplastic Degradation. Chem. Sci. 2025, 16, 15955–15963. [Google Scholar] [CrossRef]
- Ren, J.; Meng, Y.; Wang, Z.; Xie, G. Degradation of Microplastics by Microbial in Combination with a Micromotor. ACS Sustain. Chem. Eng. 2025, 13, 4018–4027. [Google Scholar] [CrossRef]
- Huang, Q.-S.; Chen, S.-Q.; Zhao, X.-M.; Song, L.-J.; Deng, Y.-M.; Xu, K.-W.; Yan, Z.-F.; Wu, J. Enhanced Degradation of Polyethylene Terephthalate (PET) Microplastics by an Engineered Stenotrophomonas Pavanii in the Presence of Biofilm. Sci. Total Environ. 2024, 955, 177129. [Google Scholar] [CrossRef]
- Shao, D.; Zhao, W.; Ji, S.; Yang, C.; Zhang, J.; Guo, R.; Zhang, B.; Lyu, W.; Feng, J.; Xu, H.; et al. Joule Heat Assisting Electrochemical Degradation of Polyethylene Microplastics Melted on Anode. Appl. Catal. B Environ. Energy 2024, 357, 124281. [Google Scholar] [CrossRef]
- Mao, Y.; Fan, S.; Li, X.; Shi, J.; Wang, M.; Niu, Z.; Chen, G. Trash to Treasure: Electrocatalytic Upcycling of Polyethylene Terephthalate (PET) Microplastic to Value-Added Products by Mn0.1Ni0.9Co2O4-δ RSFs Spinel. J. Hazard. Mater. 2023, 457, 131743. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Liu, Z.; Wang, B.; Tao, M.; Ji, H.; Xiang, X.; Fu, Z.; Liao, L.; Liao, P.; Chen, R. Effective Degradation of Polystyrene Microplastics by Ti/La/Co-Sb-SnO2 Anodes: Enhanced Electrocatalytic Stability and Electrode Lifespan. Sci. Total Environ. 2024, 922, 171002. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, B.; Liu, Z.; Yang, H.; Chen, Z.; Sun, X. La-Doped Ti/Sb-SnO2 Electrode Enhanced Removal of Microplastics by Advanced Electrocatalysis Oxidation Process (AEOP) Strategy. Desalination Water Treat. 2024, 320, 100622. [Google Scholar] [CrossRef]
- Ning, Z.; Duan, X.; Li, Y.; Zhao, X.; Chang, L. Degradation of Polyvinyl Chloride Microplastics via Electrochemical Oxidation with a CeO2–PbO2 Anode. J. Clean. Prod. 2023, 432, 139668. [Google Scholar] [CrossRef]
- Ma, F.; Wang, S.; Gong, X.; Liu, X.; Wang, Z.; Wang, P.; Liu, Y.; Cheng, H.; Dai, Y.; Zheng, Z.; et al. Highly Efficient Electrocatalytic Hydrogen Evolution Coupled with Upcycling of Microplastics in Seawater Enabled via Ni3N/W5N4 Janus Nanostructures. Appl. Catal. B Environ. 2022, 307, 121198. [Google Scholar] [CrossRef]
- Kuila, S.K.; Dhanda, A.; Samal, B.; Ghangrekar, M.M.; Dubey, B.K.; Kundu, T.K. Defect Engineered 2D Graphitic Carbon Nitride for Photochemical, (Bio)Electrochemical, and Microplastic Remediation Advancements. ACS Es&T Water 2024, 4, 4283–4298. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, H.; Xu, Q.; Dong, M.; Yang, J.; Yang, W.; Feng, Y.; Su, Z.-M. Vacancy-Rich NiFe-LDH/Carbon Paper as a Novel Self-Supporting Electrode for the Electro-Fenton Degradation of Polyvinyl Chloride Microplastics. J. Hazard. Mater. 2025, 485, 136797. [Google Scholar] [CrossRef]
- Miranda Zoppas, F.; Sacco, N.; Soffietti, J.; Devard, A.; Akhter, F.; Marchesini, F.A. Catalytic Approaches for the Removal of Microplastics from Water: Recent Advances and Future Opportunities. Chem. Eng. J. Adv. 2023, 16, 100529. [Google Scholar] [CrossRef]
- Ye, J.; Chen, Y.; Gao, C.; Wang, C.; Hu, A.; Dong, G.; Chen, Z.; Zhou, S.; Xiong, Y. Sustainable Conversion of Microplastics to Methane with Ultrahigh Selectivity by a Biotic–Abiotic Hybrid Photocatalytic System. Angew. Chem. 2022, 134, e202213244. [Google Scholar] [CrossRef]
- Kang, J.; Zhou, L.; Duan, X.; Sun, H.; Ao, Z.; Wang, S. Degradation of Cosmetic Microplastics via Functionalized Carbon Nanosprings. Matter 2019, 1, 745–758. [Google Scholar] [CrossRef]
- Miao, F.; Liu, Y.; Gao, M.; Yu, X.; Xiao, P.; Wang, M.; Wang, S.; Wang, X. Degradation of Polyvinyl Chloride Microplastics via an Electro-Fenton-like System with a TiO2/Graphite Cathode. J. Hazard. Mater. 2020, 399, 123023. [Google Scholar] [CrossRef]
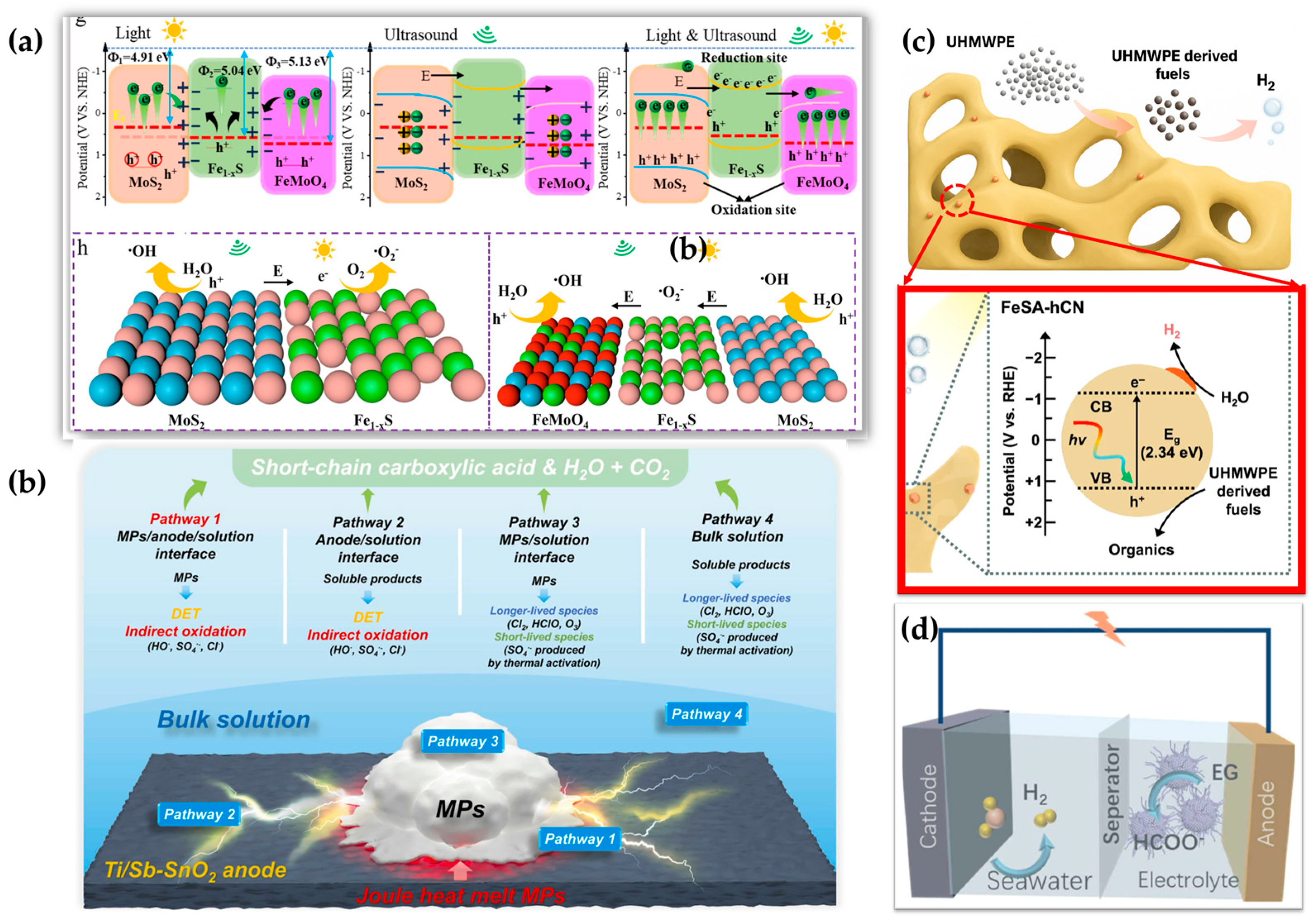
- Lu, Y.; Dong, Y.; Liu, W.; Jin, Q.; Lin, H. Piezo-Photocatalytic Enhanced Microplastic Degradation on Hetero-Interpenetrated Fe1−xS/FeMoO4/ MoS2 by Producing H2O2 and Self-Fenton Action. Chem. Eng. J. 2025, 508, 160935. [Google Scholar] [CrossRef]
- Kim, J.; Jang, J.; Hilberath, T.; Hollmann, F.; Park, C.B. Photoelectrocatalytic Biosynthesis Fuelled by Microplastics. Nat. Synth. 2022, 1, 776–786. [Google Scholar] [CrossRef]
- Feng, Z.-Y.; Jiang, J.-C.; Jin, B.; Meng, L.-Y. Hydrogen Evolution Coupled with Microplastic Upcycling in Seawater via Solvent-Free Plasma F-Functionalized CoNi Alloy Catalysts. J. Alloys Compd. 2024, 1008, 176593. [Google Scholar] [CrossRef]
- Efremenko, E.N.; Lyagin, I.V.; Maslova, O.V.; Senko, O.V.; Stepanov, N.A.; Aslanli, A.G.G. Catalytic Degradation of Microplastics. Russ. Chem. Rev. 2023, 92, RCR5069. [Google Scholar] [CrossRef]
- Zandieh, M.; Griffiths, E.; Waldie, A.; Li, S.; Honek, J.; Rezanezhad, F.; Van Cappellen, P.; Liu, J. Catalytic and Biocatalytic Degradation of Microplastics. Exploration 2024, 4, 20230018. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Y.; Mo, A.; Jiang, J.; Liang, Y.; Cao, X.; He, D. Removal of Microplastics in Water: Technology Progress and Green Strategies. Green Anal. Chem. 2022, 3, 100042. [Google Scholar] [CrossRef]
- Hettiarachchi, H.; Meegoda, J.N. Microplastic Pollution Prevention: The Need for Robust Policy Interventions to Close the Loopholes in Current Waste Management Practices. Int. J. Environ. Res. Public Health 2023, 20, 6434. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Redondo-Hasselerharm, P.E.; Nor, N.H.M.; de Ruijter, V.N.; Mintenig, S.M.; Kooi, M. Risk Assessment of Microplastic Particles. Nat. Rev. Mater. 2022, 7, 138–152. [Google Scholar] [CrossRef]
- Xayachak, T.; Haque, N.; Lau, D.; Pramanik, B.K. The Missing Link: A Systematic Review of Microplastics and Its Neglected Role in Life-Cycle Assessment. Sci. Total Environ. 2024, 954, 176513. [Google Scholar] [CrossRef]
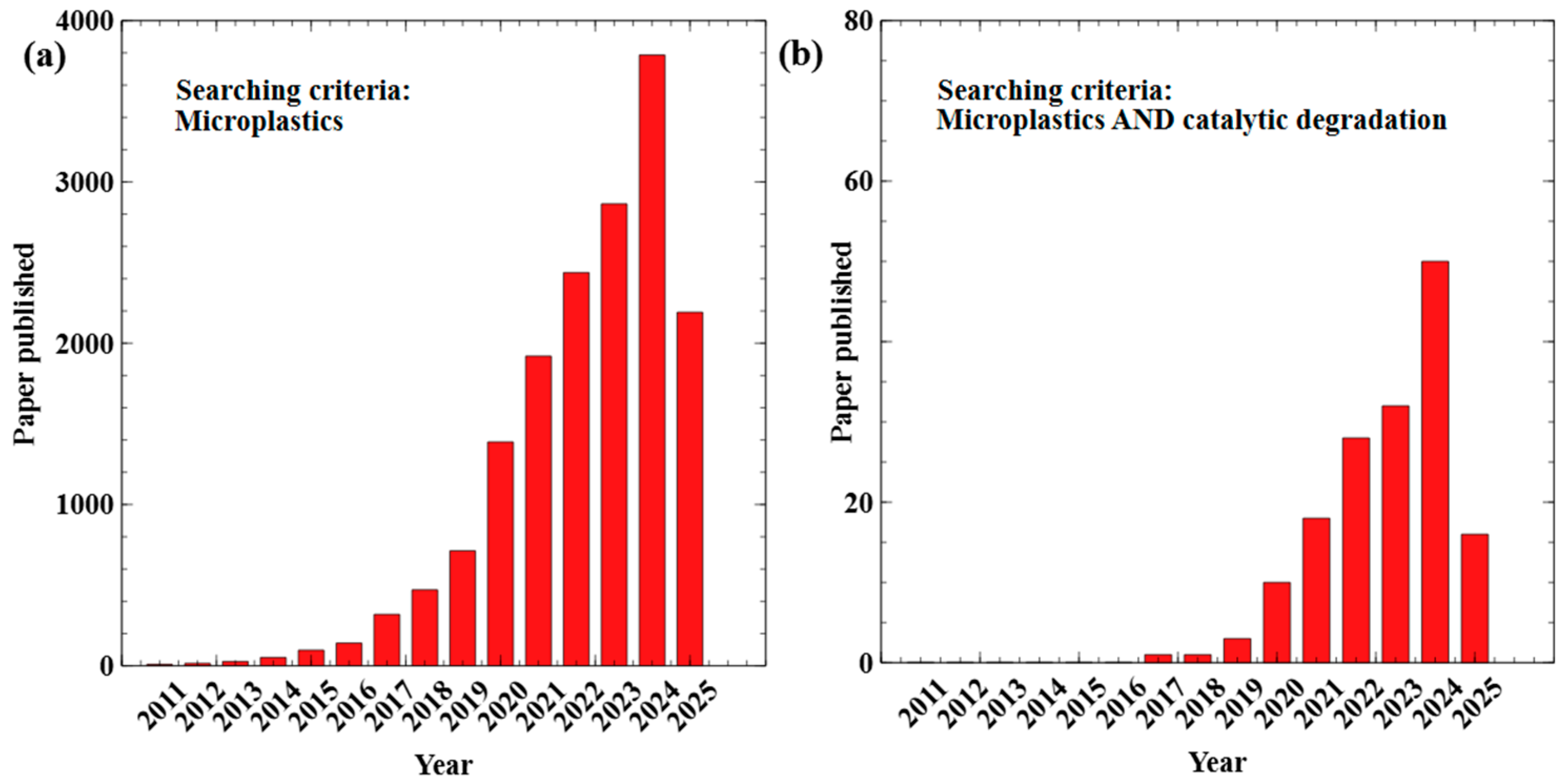
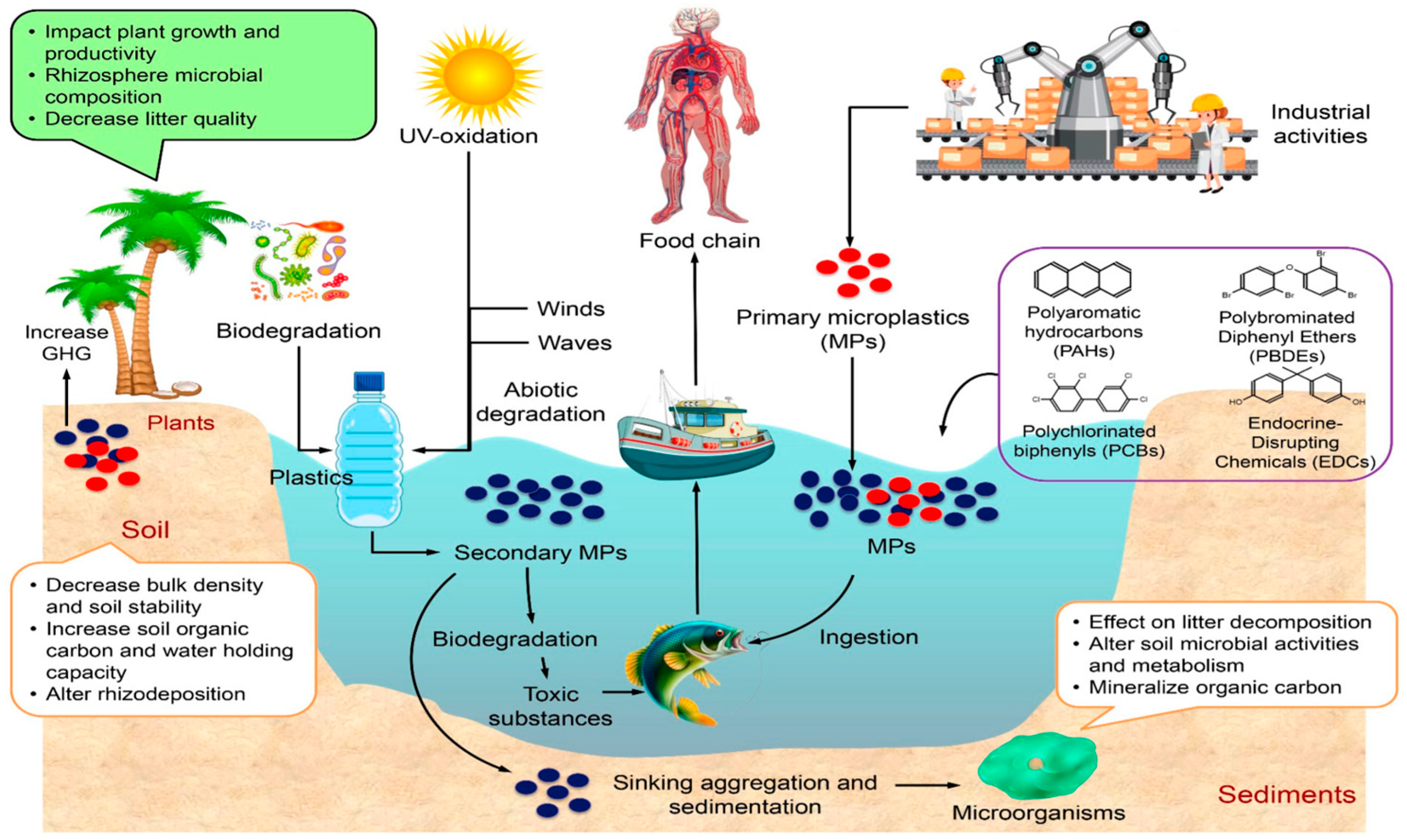

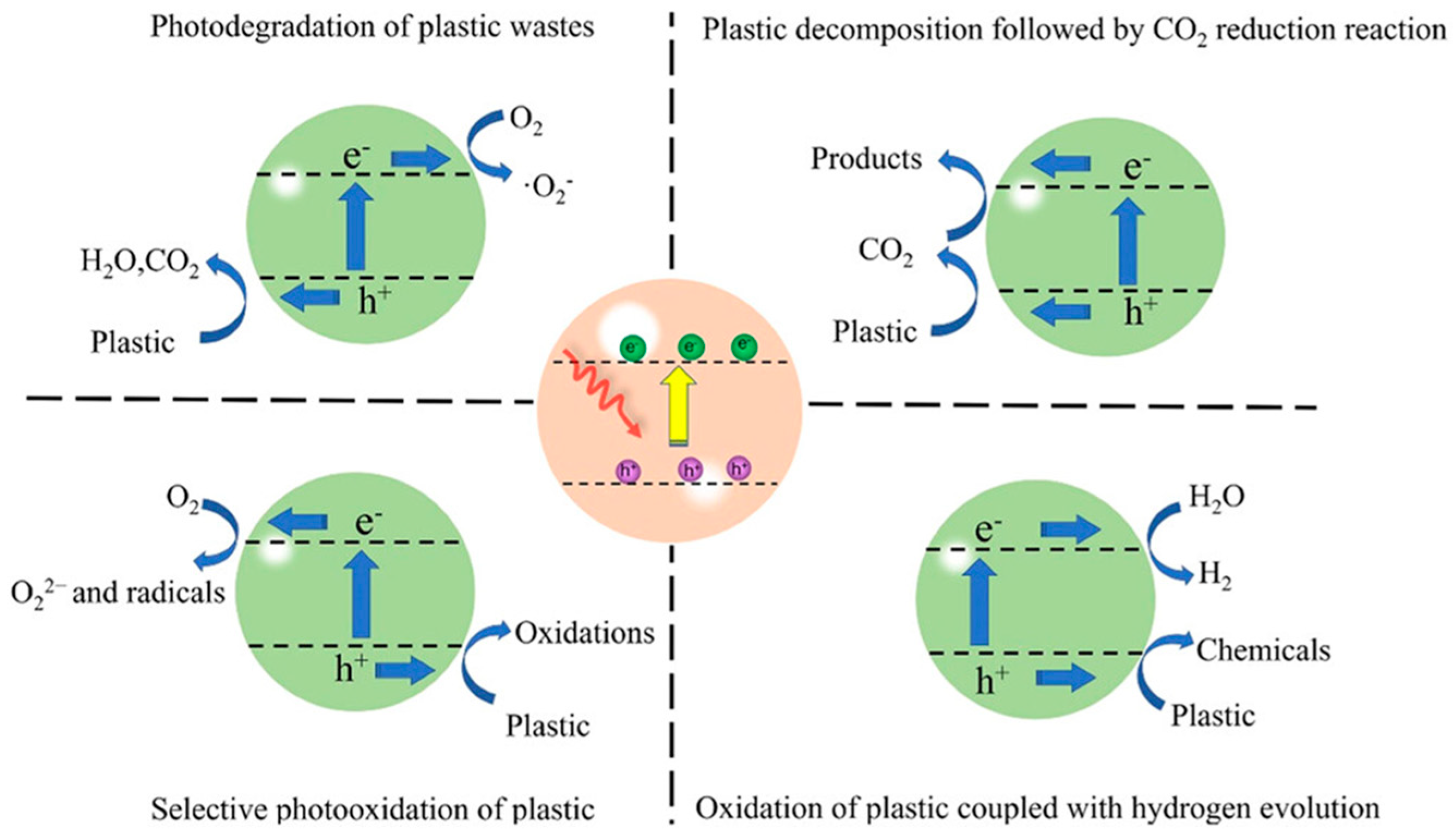

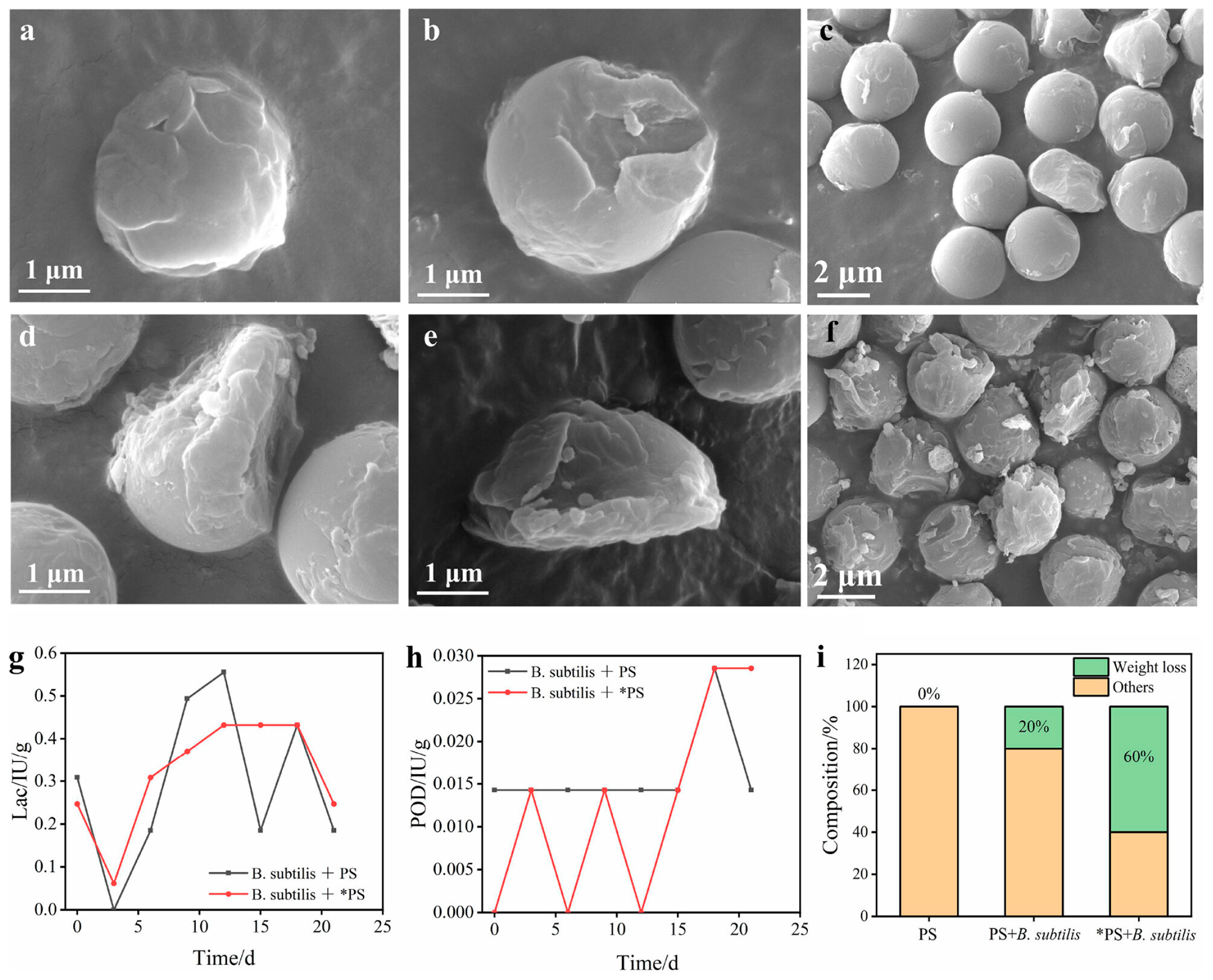
| MPs Size Categories | Size Range | Size References |
|---|---|---|
| Nanoplastic | 0.001–1 µm | Virus, DNA |
| Microplastic | 1–1000 µm | Bacteria |
| Human Hair | ||
| Mesoplastic | 1–10 mm | Pencil Tip |
| Macroplastic | >1 cm | Golf Ball |
| Category | Bulk Plastic | Microplastic |
|---|---|---|
| Origin | Intentionally manufactured | MPs are either produced at that size (primary microplastics) or result from the fragmentation of larger plastic waste (secondary microplastics) through aging, weathering, and fragmentation |
| Size | >5 mm | <5 mm, no lower limit yet |
| Shape | Varied shapes can be controlled | Fragments, fibers, films, foams, and microbeads. The shapes can be broadly categorized as regular (spherical, cylindrical, etc.) or irregular |
| Polymer type | Single type or with controlled polymer types | MPs can be composed of a wide variety of polymer types as a mixture. The most common include polyethylene (PE), polypropylene (PP), polystyrene (PS), polyethylene terephthalate (PET), and polyvinyl chloride (PVC). Other polymers like polyamide (PA), polyurethane (PU), polycarbonate (PC), and polyester (PES) are also found in microplastics. The specific polymers found in microplastics can vary depending on the source and location |
| Surface | Low surface-to-volume ratio | Higher surface area-to-volume ratio; higher surface energy. Higher surface charge due to ion absorption. May include cracks, pits, and other surface features due to UV radiation, mechanical abrasion, and chemical degradation |
| Crystallinity | Standard and uniform | Weathering and aging processes can significantly alter the crystallinity of microplastics |
| Surface chemistry | Relatively clean | Absorbed substances from the environment, including pollutants (organic, inorganic), nutrients, and microorganisms, leading to changes in their surface properties and behavior. Presence of specific functional groups on the surface (e.g., hydroxyl, carboxyl) |
| Method | Advantages | Limitations | Particle Size Best Suited For | Typical Pretreatment Steps |
|---|---|---|---|---|
| Optical Microscopy | Rapid visualization; low cost; simple operation | Limited resolution; cannot identify polymer type | >100 µm | Filtration/sieving; density separation |
| SEM/EDS | Very high resolution; reveals surface structure and aggregation | Cannot identify polymer type; expensive instrumentation | <100 µm | Filtration/digestion → drying → conductive coating (Au/C) or wet-mode imaging |
| µ-FTIR/FPA-FTIR | Enables polymer identification; FPA allows batch imaging/statistics; high throughput | Low sensitivity for nanoplastics; easily disturbed by environmental fouling | >20 µm | Filtration onto IR-transparent substrates; H2O2 or enzymatic digestion |
| Micro-Raman | High spatial resolution (sub-micron detection); less affected by water | Strong fluorescence background; long acquisition time; high instrument cost | <20 µm | Clean low-fluorescence filters; filtration |
| 1H NMR | Rich quantitative information: polymer composition, degradation pathways, additives | Requires large sample amounts; expensive; not suitable for routine monitoring | <300 µm | Bulk sample concentration; solvent extraction |
| Py-GC/MS (incl. TED-GC/MS) | Accurate qualitative/quantitative identification of polymers in mixed or weathered samples; improved throughput | Destructive method; no morphological information | Not theoretically limited; practically constrained by heat and mass transfer | Drying; homogenization; removal of salts/water |
| TGA | Characterizes thermal degradation behavior; combined with FTIR/MS, gives composition and quantification | Overlapping thermal peaks; interference from organics or minerals | Not theoretically limited; practically constrained by heat and mass transfer | Drying; homogenization; removal of inorganic/organic matter |
| Catalyst | MP Type | Size | Condition | Products | Quantification Method | Efficiency | Ref. |
|---|---|---|---|---|---|---|---|
| Fe-doped BiO2−x/BiOI heterojunction | PET | NA | Xenon lamp (200–1000 nm, 500 W) in water | CO2 and H2O | Degradation efficiency by FT-IR characterization | 95.3% removal for tetracycline in 60 min | [54] |
| g-C3N4/TiO2/WCT-AC | PE | 0.15 mm | 500 W xenon lamp irradiation for 200 h in water at 25 °C with 600 rpm | Not reported | Weight loss | 67.58% removal in 200 h | [59] |
| C3N4/WO3 | PET | 0.45 μm | 300 W xenon lamp at 25 °C in water | H2: 14.21 mM and formate, methanol, acetic acid, and ethanol | Weight loss was measured | 14.21 mM H2 production rate | [53] |
| MXene/ZnxCd1−xS | PET solution | NA | 300 W Xenon lamp, reaction in 50 mL PET solution | 14.17 mmol·g−1·h−1 H2 generation rate. Glycolate, acetate, ethanol, etc. | H NMR spectroscopy | Not reported | [60] |
| Ag/TiO2 nano-composites | PE | 100−250 μm | UV lamp irradiation at 2000 rpm | Not reported | Weight loss | 100% in 90 min for 125−200 μm | [61] |
| Pt/ZnO nanorods | LDPE | 50 μm | 50 W dichroic halogen lamp, 175 h | Not reported | Carbonyl index (CI) and vinyl index (VI) calculation through FTIR | 13% and 15% increase for CI and VI with Pt compared to ZnO only | [62] |
| Core–shell BiO2−x/CuBi2O4 heterojunction | PS and PE | 4 μm | Full-spectrum light sources (300 W, Xenon lamp | Benzoic acid, ethylbenzene, and styrene | FTIR was used to quantify the carbonyl content | Severe damage to the surface PS after 15 d of full-spectrum light irradiation compared to BiO2−x and CuBi2O4 alone | [63] |
| Nb-doped SnO2 quantum dot | PE | 350 μm | Visible light from an 8 W LED (400–800 nm) and a 200 W Xe lamp (380–1100 nm) | CO2 and H2O with HC intermediates | Weight loss | 28.9% weight loss after 7 h | [64] |
| TiO2-modified boron-doped diamond (BDD/TiO2) | HDPE | 250 μm | 6.89 mA cm−2 current density and UV light in aqueous media | Organic compounds such as aldehydes and ketones | FTIR was used to quantify the carbonyl content | 89.91 ± 0.08% of HDPE MPs in a 10-h | [65] |
| BOC-S, BOC-N, and BiOCl photocatalysts | PET | 37 μm | 180 °C for 12 h. 300 W xenon lamp | CO2 | Weight loss | 44.33% degradation of PET MPs within 5 h | [66] |
| TiOx/ZnO tetrapod | PE and PES microfibers | 100 μm | 365 nm UV light at room temperature | Not reported | Weight loss | Complete mass loss of PE and PES under UV illumination for 480 h and 624 h | [67] |
| BiOBr-OH semiconductor–organic framework | PS | 5 μm | 250 W Xe lamp for 72 h | Monomeric molecules and multiple molecular complexes | Weight loss, filter with 1 μm filter paper | 7.31% mass loss after 72 h | [68] |
| S vacancy-rich CdS | PET | ~500 μm | Simulated solar irradiation 6 h | H2, Terephthalic acid, Ethylene glycol, Formic acid | Mass loss and GC | 23-fold increase in H2 production compared to commercial CdS | [69] |
| BiOI-MOF composite | PE | 230 ± 90 μm | 500 W Xenon lamp for 6 h | Alcohols, lipids, carboxylic acids, long-chain alkane | ATR-FTIR for carbonyl | CI decreased to 0.127 in 6 h | [72] |
| TiO2-anchored chitin sponge | PS | 1 μm | 60 W lamp (λ = 365 nm), UV light | 2-Butanone, 3,3-dimethyl cyclohexanone | UV-vis dye-assisted quantification | 58.4% in 6 h | [73] |
| Catalyst | Plastic Type | Size | Mechanism | Condition | Products | Quantification Method | Efficiency | Ref. |
|---|---|---|---|---|---|---|---|---|
| Cuttlefish bone-supported CoFe2O4 nanoparticles | PS | 70 nm | Fenton-like | 100 rpm and 25 °C, APS dosage (0.25–1.25 g/L) | Not reported | TOC analyzer | 88.27% removal in 30 min | [78] |
| CuMg co-doped carbonized wood sponge catalysts CuMgCWS | PP | ~50 μm | Electro-Fenton | Hydrothermal at 160 °C for 14 h | Hydrocarbons and ketones | Weight loss, GCMS | 80 wt% selectivity to hydrocarbons and ketones | [79] |
| Cobalt/carbon quantum dots core–shell nanoparticles | PP | <25 μm | Fenton-like | 4.0 mL of hydrogen peroxide 35% v/v | Not reported | Weight loss | 9.6% degradation in 24 h | [80] |
| Copper–cobalt–carbon aerogel (CuCo-CA) | PS | ~119 nm | Fenton | Current: 20 mA; initial pH: 7.0; electrolyte: 0.05 M | Acetophenone, benzoic acid, esters, aldehydes, and alcohols | FTIR, UV-Vis, and direct infusion MS | 94.8 % removal efficiency in 6 h | [81] |
| Ferroelectric Bi12(Bi0.5Fe0.5)O19.5 | PET | 500–600 μm | Piezo-Fenton | Ultrasound treatment (40 kHz, 120 W), RT | Low-toxicity intermediates | Weight loss, HPLC, LC-MS | 28.9% removal rate in 72 h | [82] |
| α-Fe2O3 nanoflower on hierarchical TiO2 | PS | 310 nm | Photo-Fenton | A Hg lamp (365 nm, 0.5 W cm−2), 75 °C | Carboxylic acids or carboxylates, CO2 | 1H NMR, GC | Nearly 100% degradation in 4 h at 75 °C | [83] |
| Zeolitic imidazolate framework@hydrogen titanate nanotubes (HTNT@ZIF-67) | Toothpaste MPs | ~300 μm | Fenton | 1 mL, 30% H2O2 addition | Not reported | HPLC-MS and weight loss | 97% removal efficiency in 3 h | [85] |
| Catalyst | MP Type | Size | Mechanism | Condition | Products | Quantification Method | Efficiency | Ref. |
|---|---|---|---|---|---|---|---|---|
| Hopcalite-(CuMnOx) | PS | 200 µm | Plasma-assisted thermal oxidation | Plasma (20.6 kV, 8.6 kHz), 79% N2 and 21% O2 | CO2 | Weight loss and micro-GC | 98.7% PS-MP conversion to CO2 in 60 min | [19] |
| Mg/Zn -MBC (Mo-doped carbon sponge) | PS | 1.0 μm | Pyrolysis | 500 °C for 10 min | Aromatics in the range of C6−C9 | MP weight concentration | 94.81% MBC only 98.75% Mg-MBC 99.46% Zn-MBC | [90] |
| Anatase-Rutile Ni-Pd/TNPs | Mixed MPs made from waste plastic blending | ≤5 mm2 | Reforming and cracking | 500–700 °C, N2, phenol-dissolved MPs as feed | H2 and liquid fuels | GC-MS, FTIR, GC-FID, and GC-TCD | H2 yield (93%) and phenol conversion (77%) at 700 °C | [91] |
| NiCl2 | HDPE blended to small size | Not reported | Pyrolysis | 800 °C, 3 h, N2 | Functional carbon | TGA-DSC | 33.4% carbon yield with 15:1 catalyst to HDPE ratio | [49] |
| Co-N/C@CeO2 composite | PET | ~4 µm | Thermal Fenton | PMS (5 mM) + Co-N/C@CeO2 (0.5 g/L) + H2O2 (1 mL), T = 55–65 °C | HC intermediates, CO2 + H2O | Mass loss; GC-MS; UPLC-MS | 92.3% PET MP degradation at 55 °C with PMS + H2O2 (vs. 52.3% without H2O2) | [89] |
| Fe3+, Al3+, Cu2+, Zn2+ | PE (spheres and fragments), PA (fibers), PP (fragments) | 150–500 µm | Hydrothermal degradation | 180–300 °C, 30 min (10–85 bar) | Olefins, paraffins, ethanol, glycols, nanoplastics | Weight loss, SCOD, TOC, GC-MS, Py-GC-MS, FTIR | PA: >95% in Fe3+, 92% in Al3+ at 300 °C; PE: ~17–25%; PP: ~13% in Fe3+ at 300 °C | [95] |
| Zeolite catalysts | PE, PP, PS, PET, PVC | Pellets (~3 mm) or powders (<1 mm) | Pyrolysis | 300–600 °C, often ~500 °C, 0.1–0.8 MPa; 15–120 min | Olefins, aromatics, gasoline/diesel paraffins, waxes, H2, CH4, C2–C4 | GC-MS, TGA, FTIR | Liquid oil yield: 80–90% | [96] |
| ZnO nanoparticles (<50 nm) | PP | Macro: 100 mm2; micro: 25 mm2 | Thermo-photocatalytic | UV-C (254 nm, 11 W), 1–3 g/L ZnO, 35–50 °C, 6 h, air bubbling (1.6 L/min) | Nanoplastic | Weight loss measurement | 7.89% weight loss in 6 h | [97] |
| Fe-MBC | PS | 100 nm | Pyrolysis | 550 °C, 10 min, N2 atmosphere | Styrene (74.6%), benzene, toluene, ethylbenzene, α-methylstyrene | UV-Vis (224 nm) for PS conc. GC-MS | Removal efficiency ≈ 99% (initial) | [98] |
| Catalyst | Plastic Type | Size | Condition | Products | Quantification Method | Efficiency | Ref. |
|---|---|---|---|---|---|---|---|
| Candida rugosa lipase (CrL) immobilization in metal–organic frameworks (CrL-MOFs) | Bis-(hydroxyethyl) terephthalate (BHET) as model compound | NA | Water, 25 °C, 1 bar | H2BDC | High-performance liquid chromatography (HPLC) | 37 %, 24 h, 3 mg of degraded BHET per g of enzyme | [103] |
| Hydrophilic bare Fe3O4 nanoaggregates | HDPE, PP, PVC, PS, and PET | 20−800 μm | 130−260 °C, autoclave | Not reported | UV-Vis and weight measuring | 100 % degradation—close to their melting temperature | [104] |
| Shewanella putrefaciens 200 | PS | 1.20−1.30 mm | 25 °C and pH: 7.0 in water solution | Benzene ring derivatives | Weight loss was measured by an analytical balance | Weight loss of 6.1 ± 0.6% in 14 days | [105] |
| Manganese oxide free radical-modified SDE-PsLAC, E. coli BL21 | PE | 500–1500 μm | 37 °C or 15 °C for 192 h | Aromatics, aliphatics, alcohols, and esters | Weight loss | 91.2% at 37 °C and 52.4% at 15 °C within 192 h | [106] |
| Iron-enhanced microbiota | PE | 3–5 mm2 piece from commercial plastic bag | 30 to 60 days of cultivation at 37 °C | Heneicosane, octadecane, pentadecane, and 4,6-dimethyl dodecane | Weight loss | 12.38% weight loss in 60 days compared to 10.44% for samples without iron added | [107] |
| MnO2/g-C3N4/fly ash (MCNF) | PS, PE | 5 μm | RT with H2O2 addition | Not reported | Weight loss | PS degradation 60% in 24 days; 66% PE degradation in 50 days | [109] |
| Engineered S. pavanii with DuraPETase | PET | 500 μm | 30 °C and 150 rpm | TPA, MHET, BHET | HPLC | 38.04 μM product generation after 30-day incubation at 30 °C | [111] |
| Mn-doped iron phosphate (LFMP) | Polyamide 6, HDPE, and pp | 0.5 to 4.5 mm | 25 °C or 180 °C for 8 h in autoclave | CO2, H2O2, and inorganic small molecules | Weight loss | 91.5% at 180 °C for 8 h. Three times higher than that of undoped LFP | [108] |
| Catalyst | Plastic Type | Size | Condition | Products | Quantification Method | Efficiency | Ref. |
|---|---|---|---|---|---|---|---|
| Mn0.1Ni0.9Co2O4-δ rod-shaped fiber (RSF) spinel catalyst | PET | <500 μm | 5 mV s−1, 1 M KOH (pH = 14), 0.17 M ethylene glycol | H2 and formate | NMR spectrometer | EG to formate with >95% Faradaic efficiency at 1.42 V vs. RHE | [112] |
| Ti/La/Co-Sb-SnO2 anode | PS | 150 μm | 0.5 mol/L H2SO4 LSV: scanning rate: 1.0 mV s−1, V: 0 to 2.5 V | Alcohols, monocarboxylic acids, dicarboxylic acids, esters, ethers, and aldehydes | Weighing method and Py-GCMS | 28% removal in 3 h | [113] |
| Ni3N/W5N4 janus | PET flakes | <500 μm | Scan rate of 5 mV s−1 | H2 and HCOOH | NMR spectrometer | ~85% Faradaic efficiency | [116] |
| Vacancy-rich NiFe-LDH/carbon paper | PVC | 74–147 μm | 10 to 100 mV s−1 | H2O2 | Ion chromatography (IC) and GC-MS | ~76% selectivity for H2O2 | [118] |
| CeO2-modified PbO2 anode | PVC | Not reported | T: 20–100 °C, 10–60 mA/cm2, pH (3–11), PVC-MPs (50–150 mg/L), and Na2SO4 (10–90 mM) | H2O and CO2 | Weight loss and HPLC-MS | 38.67% weight loss in 6 h. 16.67% increase compared to pristine PbO2 anode | [115] |
| Catalyst | Plastic Type | Mechanism | Size | Condition | Products | Quantification Method | Efficiency | Ref. |
|---|---|---|---|---|---|---|---|---|
| FeSA-hCN (single-atom Fe on porous carbon nitride) | UHMWPE | Tandem MP degradation + H2 evolution | <180 μm | Simulated solar irradiation in aqueous suspension, pH 7 | 64% carboxylic acid selec; H2: 42 µmol/h | Mass loss, HPLC | Near 100% PE degradation | [22] |
| TiO2/graphite (TiO2/C) cathode | PVC | Electro-Fenton | 74–147 µm | −0.7 V vs. Ag/AgCl using Na2SO4 as supporting electrolyte | CO2, H2O, and Cl− | Dechlorination efficiency through ion chromatography | ~75% dechlorination efficiency in 6 h | [115] |
| Methanosarcina barkeri (M. b) and carbon dot-functionalized polymeric carbon nitrides | Poly(lactic acid), PE, PS, and PUR | Photo-biological | ≤0.04 cm2 | 395 ± 5 nm ultraviolet, 35 ± 2 °C | 100 % CH4 | Not reported | CH4 yield 7.24 ± 0.40 mmol g−1 | [120] |
| Magnetic N-doped nanocarbon springs | MPs from cosmetic pastes | Integrated carbocatalytic oxidation and hydrothermal (HT) | ≥0.45 μm | Peroxymonosulfate (PMS) added to an autoclave with water | CO2 and H2O | Filtration through a 0.45-μm membrane. Mass loss measurement and HPLC | 44% of MPs decompositions in 8 h | [121] |
| Fe1−xS/FeMoO4/MoS2 | PS | Piezo-photo-Fenton | 0.55−12.5 μm | Ultrasonic cleaner (at 120 W, 40 kHz) equipped with LED irradiation (24 W) at RT | Benzoic acid and phenylacetic acid | Centrifugation for calculating the weight loss | 58.46% of PS-MPs in 30 h | [123] |
| Zr-doped hematite (α-Fe2O3) photoanode | PET | Photo-biological | <2 mm | A mixed condition | Formate and acetate | Quantitative 1H NMR and HPLC | High faradaic efficiency (>90%) | [124] |
| F-functionalized CoNi-alloy catalyst | PET | Bifunctional | Not reported | 50 to 300 mV s−1 | H2 and formate | 1H NMR | 90.7% faradaic efficiency at 1.48 V | [125] |
| Ti/Sb-SnO2 and carbon felt | PE, PP, PS, PVC, PLA, and PET | Thermal-electro | ~400 μm | 20 mA·cm−2 current density, Na2SO4 electrolyte | Oxygen-containing species, H2O and CO2 | Weight loss and GC/MS | 99% degradation of PE MPs in 6 h | [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Zeng, G.; Gao, P.-X. Catalyst Design and Engineering for Enhanced Microplastic Degradation and Upcycling—A Review. Catalysts 2025, 15, 984. https://doi.org/10.3390/catal15100984
Zhu C, Zeng G, Gao P-X. Catalyst Design and Engineering for Enhanced Microplastic Degradation and Upcycling—A Review. Catalysts. 2025; 15(10):984. https://doi.org/10.3390/catal15100984
Chicago/Turabian StyleZhu, Chunxiang, Ge Zeng, and Pu-Xian Gao. 2025. "Catalyst Design and Engineering for Enhanced Microplastic Degradation and Upcycling—A Review" Catalysts 15, no. 10: 984. https://doi.org/10.3390/catal15100984
APA StyleZhu, C., Zeng, G., & Gao, P.-X. (2025). Catalyst Design and Engineering for Enhanced Microplastic Degradation and Upcycling—A Review. Catalysts, 15(10), 984. https://doi.org/10.3390/catal15100984







