Mechanistic Distinction Between Oxidative and Chlorination Transformations of Chloroperoxidase from Caldariomyces fumago Demonstrated by Dye Decolorization
Abstract
1. Introduction
2. Results
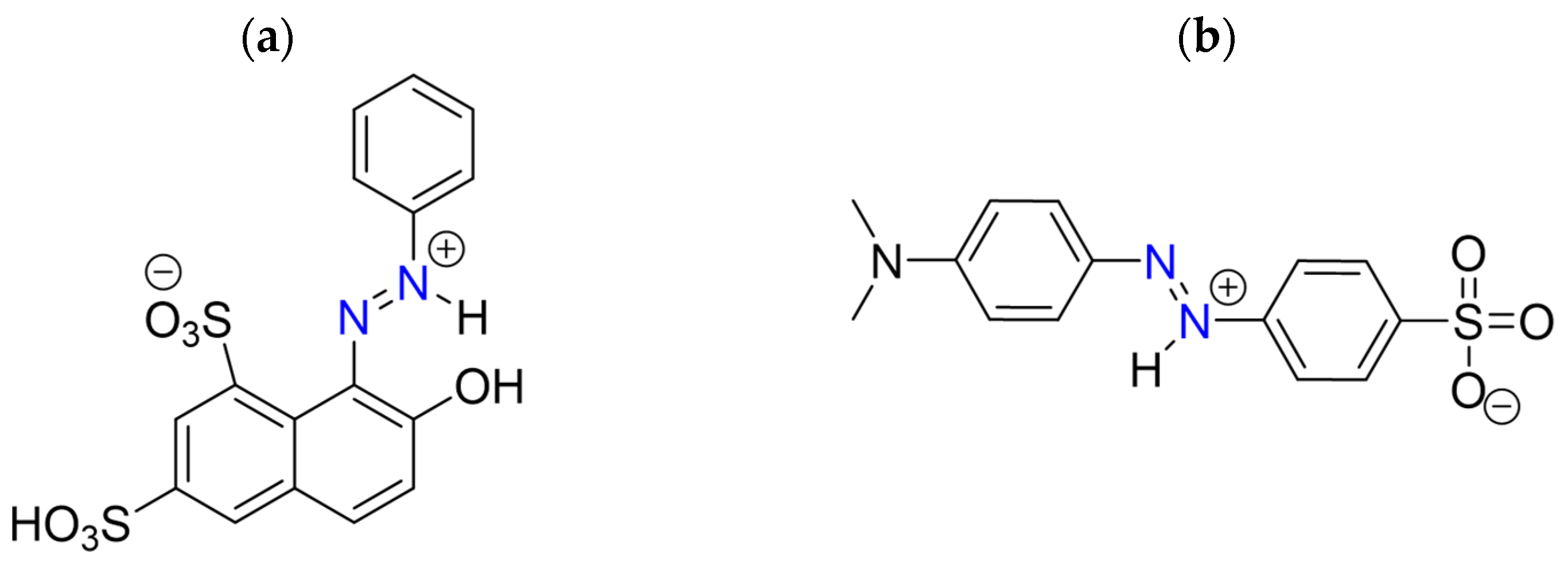
2.1. Optimizing Reaction Conditions for MO and OG Degradation
2.2. Halogen Dependence of the Decolorization of Azo Dyes by Chloroperoxidase
- Measured degradation efficiency at varying chloride and fluoride concentrations.
- Analyzed the degradation products of azo dyes with and without chloride ions using LC/MS.
- Examined azo dye degradation by hypochlorous acid (HClO) and analyzed the products with LC/MS.
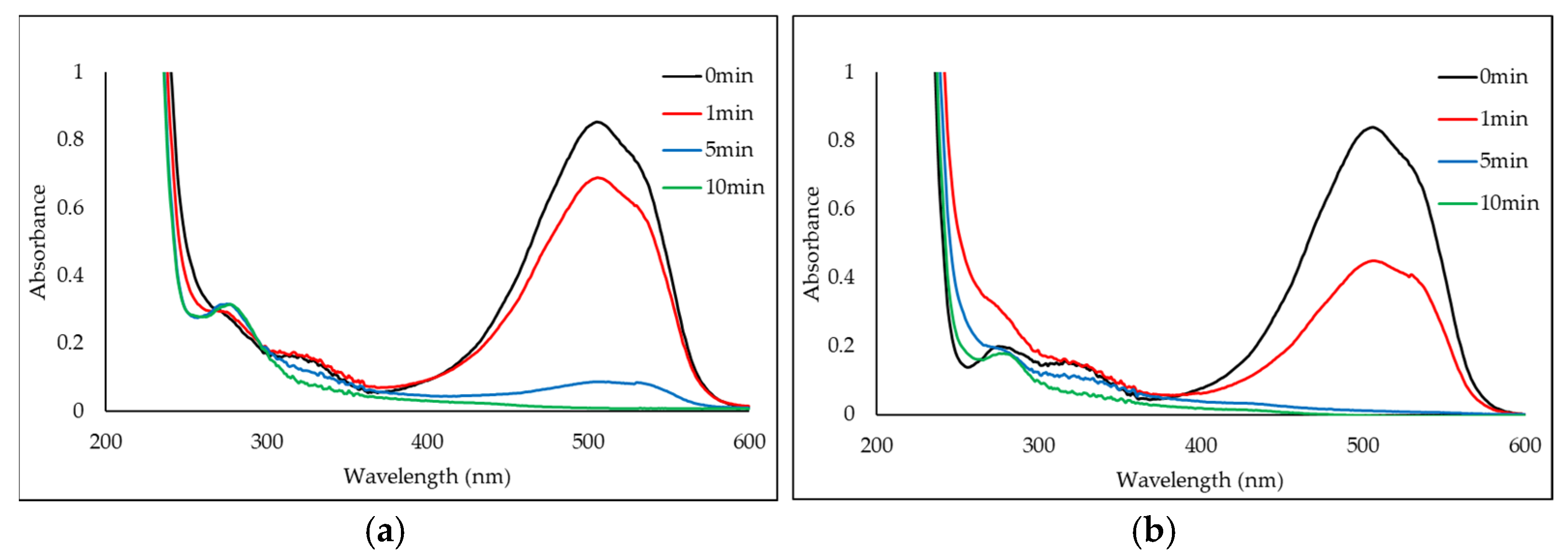
2.2.1. Degradation Efficiency with Chloride and Fluoride Ions
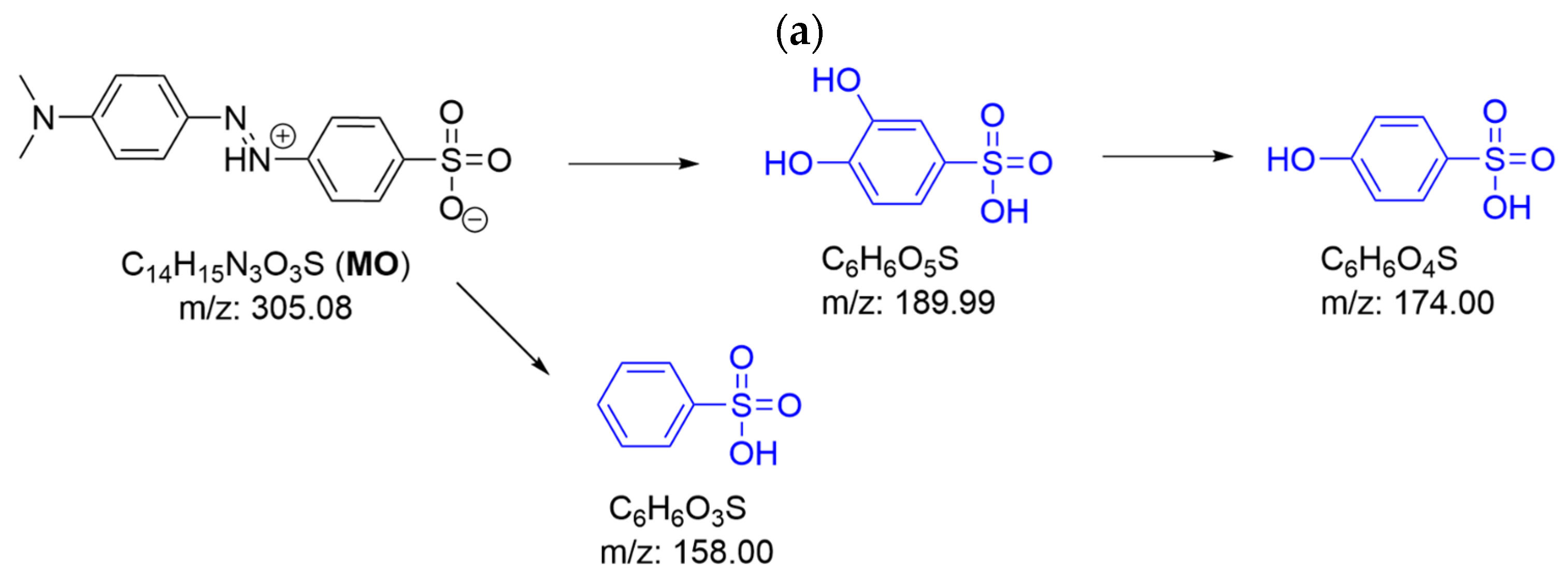
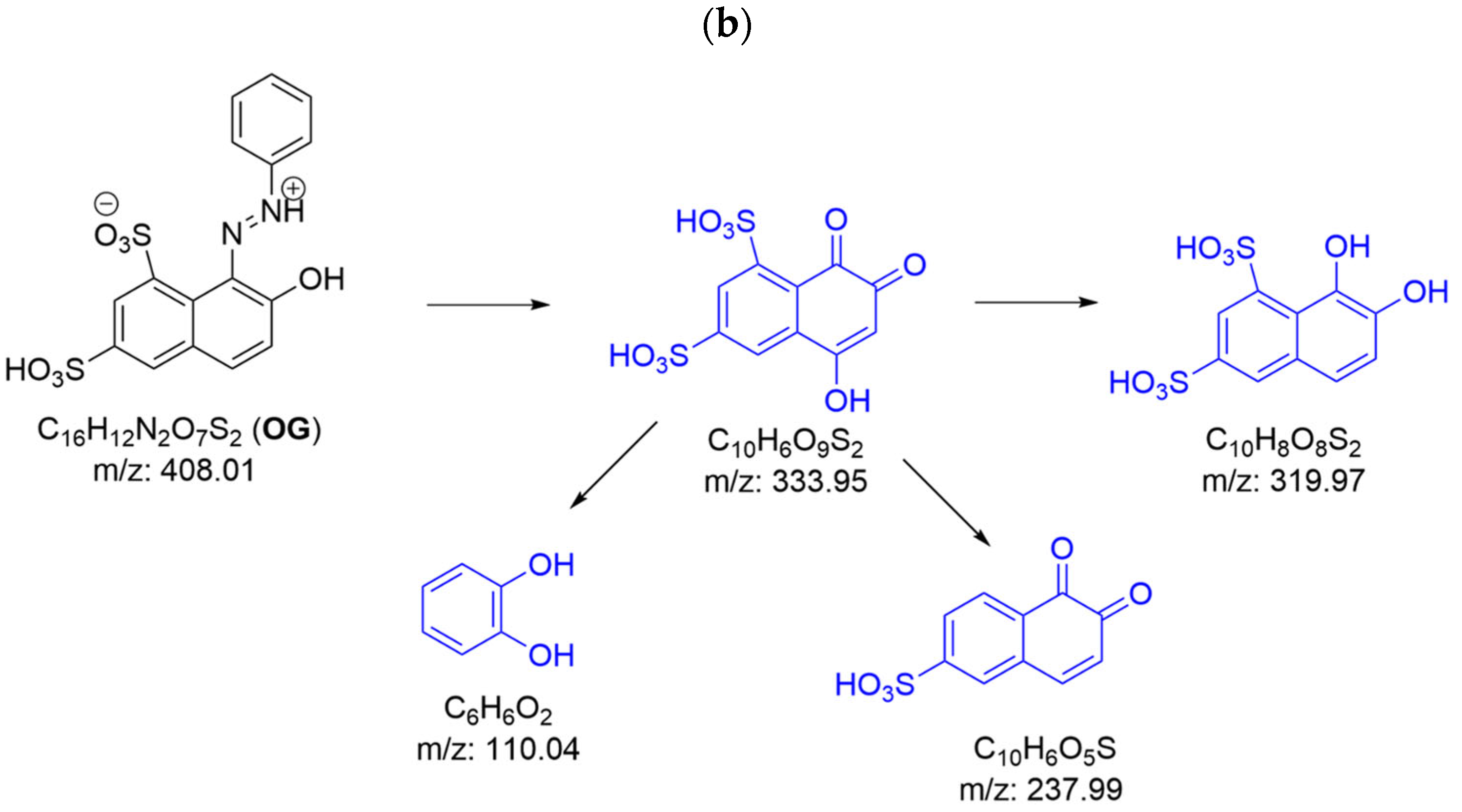
2.2.2. Degradation Products of Azo Dyes by CPO with and Without Chloride Ions by LC/MS
2.2.3. Azo Dye Decolorization Products with Hypochlorous Acid
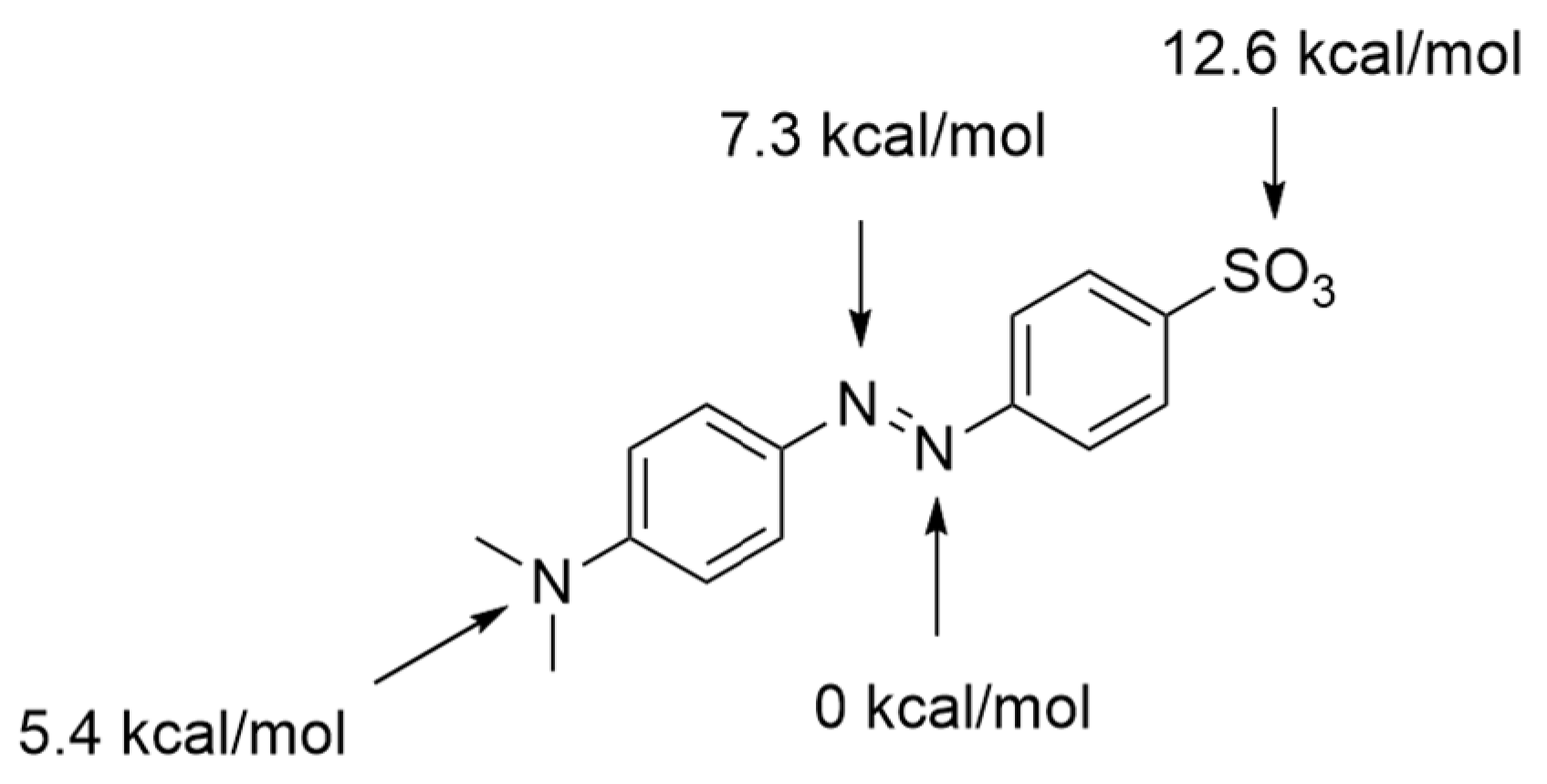
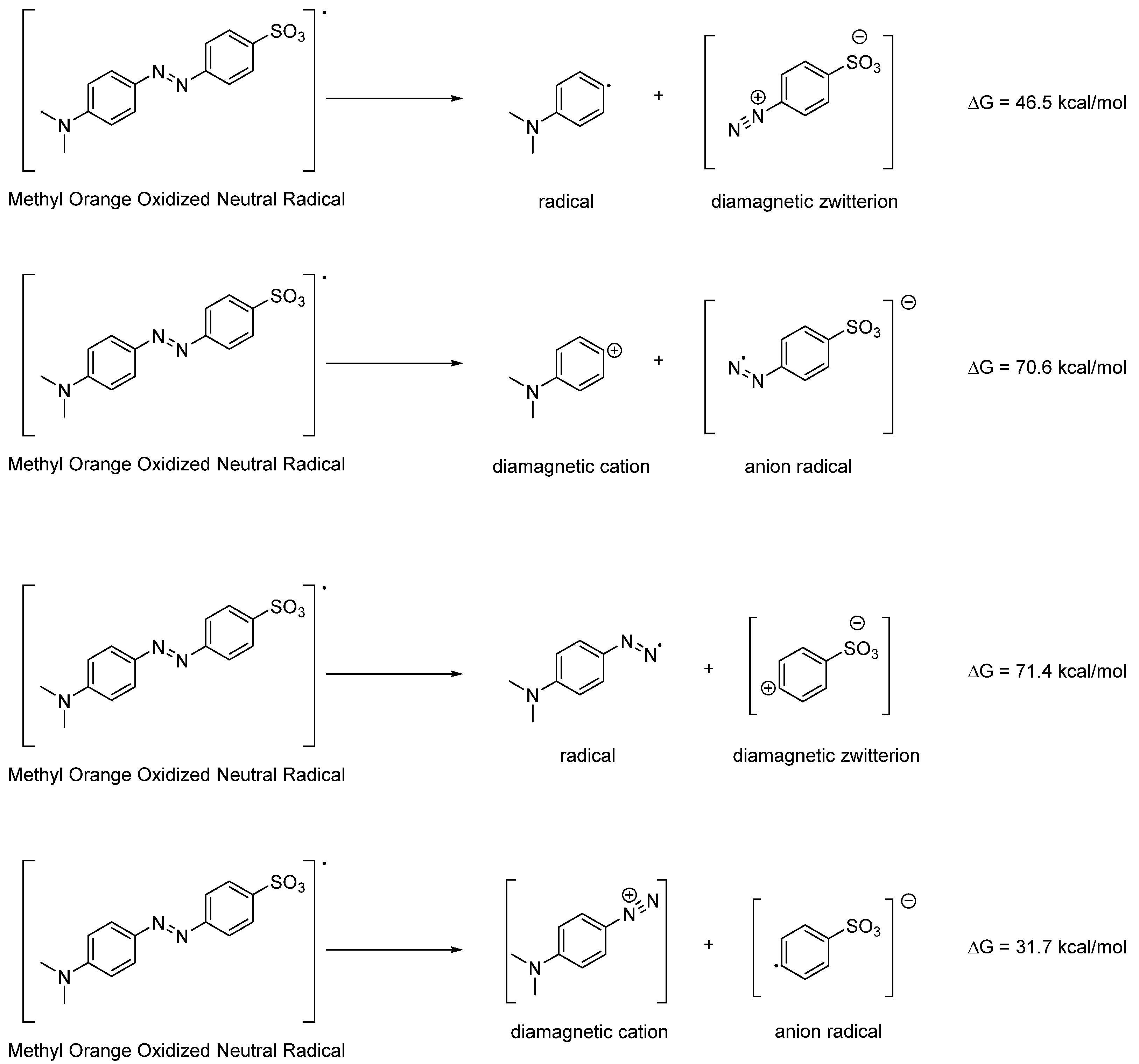
2.3. DFT Analysis of Reaction Products
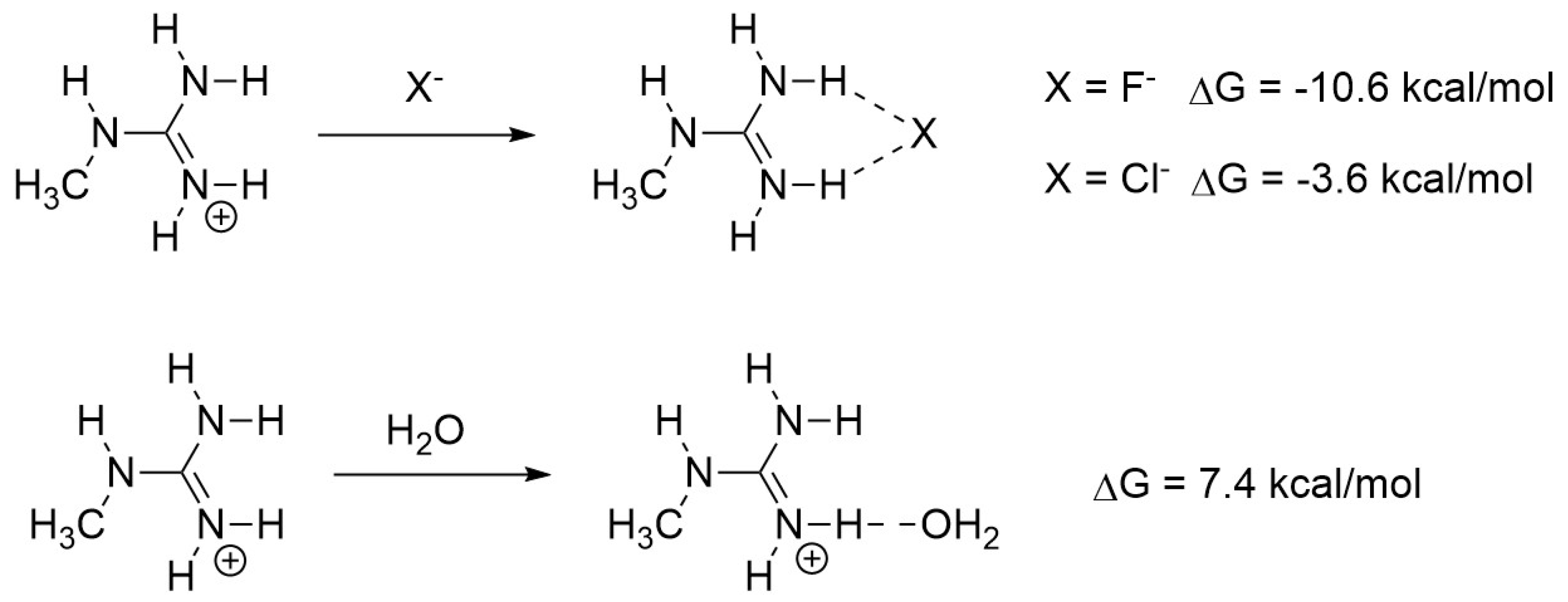
2.4. DFT Analysis of Anion Binding by an Arginine Side Chain
3. Discussion and Conclusions
4. Materials and Methods
4.1. Materials
4.2. Production of CPO
4.3. CPO Driven Degradation of Methyl Orange with and Without Anions (Cl−/F−)
4.4. Detection of ClO− via TMB Assay
4.5. CPO Quantification and Activity
4.6. Density Functional Theory Calculations
4.7. Liquid Chromatography–Mass Spectrometry
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edo, G.I.; Itoje-akpokiniovo, L.O.; Obasohan, P.; Ikpekoro, V.O.; Samuel, P.O.; Jikah, A.N.; Nosu, L.C.; Ekokotu, H.A.; Ugbune, U.; Oghroro, E.E.A.; et al. Impact of Environmental Pollution from Human Activities on Water, Air Quality and Climate Change. Ecol. Front. 2024, 44, 874–889. [Google Scholar] [CrossRef]
- Shetty, S.S.; Deepthi, D.; Harshitha, S.; Sonkusare, S.; Naik, P.B.; Kumari, N.S.; Madhyastha, H. Environmental Pollutants and Their Effects on Human Health. Heliyon 2023, 9, e19496. [Google Scholar] [CrossRef] [PubMed]
- Siddiqua, A.; Hahladakis, J.N.; Al-Attiya, W.A.K.A. An Overview of the Environmental Pollution and Health Effects Associated with Waste Landfilling and Open Dumping. Environ. Sci. Pollut. Res. 2022, 29, 58514–58536. [Google Scholar] [CrossRef]
- Adane, T.; Adugna, A.T.; Alemayehu, E. Textile Industry Effluent Treatment Techniques. J. Chem. 2021, 2021, 5314404. [Google Scholar] [CrossRef]
- Shabbir, M. (Ed.) Textiles and Clothing, 1st ed.; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Zhang, R.; He, Q.; Huang, Y.; Wang, X. Spectroscopic and QM/MM Investigations of Chloroperoxidase Catalyzed Degradation of Orange G. Arch. Biochem. Biophys. 2016, 596, 1–9. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Tan, Y.; Jiang, Y.; Hu, M.; Li, S.; Zhai, Q. Rapid Decolorization of Anthraquinone and Triphenylmethane Dye Using Chloroperoxidase: Catalytic Mechanism, Analysis of Products and Degradation Route. Chem. Eng. J. 2014, 244, 9–18. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Jiang, Y.; Hu, M.; Li, S.; Zhai, Q. Highly Efficient Biodecolorization/Degradation of Congo Red and Alizarin Yellow R by Chloroperoxidase from Caldariomyces Fumago: Catalytic Mechanism and Degradation Pathway. Ind. Eng. Chem. Res. 2013, 52, 13572–13579. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, M.; Jiang, Y.; Hu, M.; Li, S.; Zhai, Q. Efficient Decolorization/Degradation of Aqueous Azo Dyes Using Buffered H2O2 Oxidation Catalyzed by a Dosage below Ppm Level of Chloroperoxidase. Chem. Eng. J. 2012, 191, 236–242. [Google Scholar] [CrossRef]
- El-Sayed, E.; Abd El-Aziz, E.; Othman, H.; Hassabo, A. Azo Dyes: Synthesis, Classification and Utilisation in Textile Industry. Egypt. J. Chem. 2024, 67, 87–97. [Google Scholar] [CrossRef]
- Chamorro, A.F.; Lerma, T.A.; Palencia, M. CTAB Surfactant Promotes Rapid, Efficient, and Simultaneous Removal of Cationic and Anionic Dyes through Adsorption on Glycerol/Citrate Polyester. Water 2024, 16, 1860. [Google Scholar] [CrossRef]
- Sonwani, R.K.; Swain, G.; Jaiswal, R.P.; Singh, R.S.; Rai, B.N. Moving Bed Biofilm Reactor with Immobilized Low-Density Polyethylene–Polypropylene for Congo Red Dye Removal. Environ. Technol. Innov. 2021, 23, 101558. [Google Scholar] [CrossRef]
- Lin, J.; Ye, W.; Xie, M.; Seo, D.H.; Luo, J.; Wan, Y.; Van Der Bruggen, B. Environmental Impacts and Remediation of Dye-Containing Wastewater. Nat. Rev. Earth Environ. 2023, 4, 785–803. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A Critical Review on the Treatment of Dye-Containing Wastewater: Ecotoxicological and Health Concerns of Textile Dyes and Possible Remediation Approaches for Environmental Safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Chopra, L. Dye Waste: A Significant Environmental Hazard. Mater. Today Proc. 2022, 48, 1310–1315. [Google Scholar] [CrossRef]
- Simpson, J.B.; Sekela, J.J.; Carry, B.S.; Beaty, V.V.; Patel, S.; Redinbo, M.R. Diverse but Desolate Landscape of Gut Microbial Azoreductases: A Rationale for Idiopathic IBD Drug Response. Gut Microbes 2023, 15, 2203963. [Google Scholar] [CrossRef]
- Stingley, R.L.; Zou, W.; Heinze, T.M.; Chen, H.; Cerniglia, C.E. Metabolism of Azo Dyes by Human Skin Microbiota. J. Med. Microbiol. 2010, 59, 108–114. [Google Scholar] [CrossRef]
- Haque, M.M.; Hossen, M.N.; Rahman, A.; Roy, J.; Talukder, M.R.; Ahmed, M.; Ahiduzzaman, M.; Haque, M.A. Decolorization, Degradation and Detoxification of Mutagenic Dye Methyl Orange by Novel Biofilm Producing Plant Growth-Promoting Rhizobacteria. Chemosphere 2024, 346, 140568. [Google Scholar] [CrossRef]
- Donkadokula, N.Y.; Kola, A.K.; Naz, I.; Saroj, D. A Review on Advanced Physico-Chemical and Biological Textile Dye Wastewater Treatment Techniques. Rev. Environ. Sci. Biotechnol. 2020, 19, 543–560. [Google Scholar] [CrossRef]
- Selvaraj, V.; Swarna Karthika, T.; Mansiya, C.; Alagar, M. An over Review on Recently Developed Techniques, Mechanisms and Intermediate Involved in the Advanced Azo Dye Degradation for Industrial Applications. J. Mol. Struct. 2021, 1224, 129195. [Google Scholar] [CrossRef]
- Georgin, J.; Ramos, C.G.; De Oliveira, J.S.; Dehmani, Y.; El Messaoudi, N.; Meili, L.; Franco, D.S.P. A Critical Review of the Advances and Current Status of the Application of Adsorption in the Remediation of Micropollutants and Dyes Through the Use of Emerging Bio-Based Nanocomposites. Sustainability 2025, 17, 2012. [Google Scholar] [CrossRef]
- Saini, D.; Dumra, S.; Kumar, V.; Aggarwal, R.; Naziruddin, A.R.; Sonkar, S.K. Magnetic Graphene Nanosheets from Expired Iron-Supplemented Tablets for Hydroxyl-Mediated Photocatalytic Oxidation of Azo Dyes. ACS Appl. Nano Mater. 2023, 6, 1573–1581. [Google Scholar] [CrossRef]
- Hodges, G.; Smith, J.R.L.; Oakes, J. Metalloporphyrin-Catalysed Oxidation of Azonaphthol Dyes: The Mechanism of Oxidative Bleaching by Oxoiron(IV) Porphyrins in Aqueous Solution. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1997; pp. 653–662. [Google Scholar] [CrossRef]
- Singh, R.L.; Singh, P.K.; Singh, R.P. Enzymatic Decolorization and Degradation of Azo Dyes—A Review. Int. Biodeterior. Biodegrad. 2015, 104, 21–31. [Google Scholar] [CrossRef]
- Alneyadi, A.; Shah, I.; AbuQamar, S.; Ashraf, S. Differential Degradation and Detoxification of an Aromatic Pollutant by Two Different Peroxidases. Biomolecules 2017, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Velusamy, K.; Periyasamy, S.; Kumar, P.S.; Femina Carolin, C.; Jayaraj, T.; Gokulakrishnan, M.; Keerthana, P. Transformation of Aqueous Methyl Orange to Green Metabolites Using Bacterial Strains Isolated from Textile Industry Effluent. Environ. Technol. Innov. 2022, 25, 102126. [Google Scholar] [CrossRef]
- Bharatbhai Vishani, D.; Shrivastav, A. Enzymatic Decolorization and Degradation of Azo Dyes. In Development in Wastewater Treatment Research and Processes; Elsevier: Amsterdam, The Netherlands, 2022; pp. 419–432. [Google Scholar]
- Weerasinghe, K.; De Silva, S.M.; Abeyrathna, H.; Cooray, A.T.; Walpita, J. Sequential Anaerobic/Aerobic Methods in Dye Elimination. In Advanced Removal Techniques for Dye-Containing Wastewaters; Muthu, S.S., Khadir, A., Eds.; Sustainable Textiles: Production, Processing, Manufacturing & Chemistry; Springer: Singapore, 2021. [Google Scholar]
- Ali, S.S.; Al-Tohamy, R.; Sun, J. Performance of Meyerozyma Caribbica as a Novel Manganese Peroxidase-Producing Yeast Inhabiting Wood-Feeding Termite Gut Symbionts for Azo Dye Decolorization and Detoxification. Sci. Total Environ. 2022, 806, 150665. [Google Scholar] [CrossRef]
- Dixit, S.; Garg, S. Enzymatic Degradation of Sulphonated Azo Dye Using Purified Azoreductase from Facultative Klebsiella Pneumoniae. Folia Microbiol. 2021, 66, 79–85. [Google Scholar] [CrossRef]
- Singh, G.B.; Vinayak, A.; Mudgal, G.; Kesari, K.K. Azo Dye Bioremediation: An Interdisciplinary Path to Sustainable Fashion. Environ. Technol. Innov. 2024, 36, 103832. [Google Scholar] [CrossRef]
- Ullrich, R.; Hofrichter, M. Enzymatic Hydroxylation of Aromatic Compounds. Cell. Mol. Life Sci. 2007, 64, 271–293. [Google Scholar] [CrossRef]
- Sundaramoorthy, M.; Terner, J.; Poulos, T.L. The Crystal Structure of Chloroperoxidase: A Heme Peroxidase–Cytochrome P450 Functional Hybrid. Structure 1995, 3, 1367–1378. [Google Scholar] [CrossRef]
- Bhandari, Y.; Sajwan, H.; Pandita, P.; Koteswara Rao, V. Chloroperoxidase Applications in Chemical Synthesis of Industrial Relevance. Biocatal. Biotransformation 2023, 41, 403–420. [Google Scholar] [CrossRef]
- Kühnel, K.; Blankenfeldt, W.; Terner, J.; Schlichting, I. Crystal Structures of Chloroperoxidase with Its Bound Substrates and Complexed with Formate, Acetate, and Nitrate. J. Biol. Chem. 2006, 281, 23990–23998. [Google Scholar] [CrossRef]
- He, J.; Zhang, Y.; Yuan, Q.; Liang, H. Catalytic Activity and Application of Immobilized Chloroperoxidase by Biometric Magnetic Nanoparticles. Ind. Eng. Chem. Res. 2019, 58, 3555–3560. [Google Scholar] [CrossRef]
- Ahmad, R.; Rizaldo, S.; Shaner, S.E.; Kissel, D.S.; Stone, K.L. Immobilization of a Bienzymatic System via Crosslinking to a Metal-Organic Framework. Catalysts 2022, 12, 969. [Google Scholar] [CrossRef]
- Ahmad, R.; Shanahan, J.; Rizaldo, S.; Kissel, D.S.; Stone, K.L. Co-Immobilization of an Enzyme System on a Metal-Organic Framework to Produce a More Effective Biocatalyst. Catalysts 2020, 10, 499. [Google Scholar] [CrossRef]
- Ang, D.L.; Hoque, M.Z.; Hossain, M.A.; Guerriero, G.; Berni, R.; Hausman, J.-F.; Bokhari, S.A.; Bridge, W.J.; Siddiqui, K.S. Computational Analysis of Thermal Adaptation in Extremophilic Chitinases: The Achilles’ Heel in Protein Structure and Industrial Utilization. Molecules 2021, 26, 707. [Google Scholar] [CrossRef]
- Hakamada, Y.; Hatada, Y.; Ozawa, T.; Ozaki, K.; Kobayashi, T.; Ito, S. Identification of Thermostabilizing Residues in a Bacillus Alkaline Cellulase by Construction of Chimeras from Mesophilic and Thermostable Enzymes and Site-Directed Mutagenesis. FEMS Microbiol. Lett. 2001, 195, 67–72. [Google Scholar] [CrossRef]
- Meghlaoui, F.Z.; Merouani, S.; Hamdaoui, O.; Alghyamah, A.; Bouhelassa, M.; Ashokkumar, M. Fe(III)-catalyzed Degradation of Persistent Textile Dyes by Chlorine at Slightly Acidic Conditions: The Crucial Role of Cl2●− Radical in the Degradation Process and Impacts of Mineral and Organic Competitors. Asia-Pac. J. Chem. Eng. 2021, 16, e2553. [Google Scholar] [CrossRef]
- Ikram, M.; Naeem, M.; Zahoor, M.; Hanafiah, M.; Oyekanmi, A.; Ullah, R.; Farraj, D.; Elshikh, M.; Zekker, I.; Gulfam, N. Biological Degradation of the Azo Dye Basic Orange 2 by Escherichia Coli: A Sustainable and Ecofriendly Approach for the Treatment of Textile Wastewater. Water 2022, 14, 2063. [Google Scholar] [CrossRef]
- Harris, D.C. Quantitative Chemical Analysis, 8th ed.; W. H. Freeman: New York, NY, USA, 2010. [Google Scholar]
- Holčapek, M.; Jandera, P.; Fischer, J.; Prokeš, B. Analytical Monitoring of the Production of Biodiesel by High-Performance Liquid Chromatography with Various Detection Methods. J. Chromatogr. A 1999, 858, 13–31. [Google Scholar] [CrossRef]
- López, J.F.; Grimalt, J.O. Phenyl- and Cyclopentylimino Derivatization for Double Bond Location in Unsaturated C37-C40 Alkenones by GC-MS. J. Am. Soc. Mass. Spectrom. 2004, 15, 1161–1172. [Google Scholar] [CrossRef]
- Banerjee, P.; Kemmler, E.; Dunkel, M.; Preissner, R. ProTox 3.0: A Webserver for the Prediction of Toxicity of Chemicals. Nucleic Acids Res. 2024, 52, W513–W520. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A Webserver for the Prediction of Toxicity of Chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.S.; He, X.; Li, S.L.; Truhlar, D.G. MN15: A Kohn–Sham Global-Hybrid Exchange–Correlation Density Functional with Broad Accuracy for Multi-Reference and Single-Reference Systems and Noncovalent Interactions. Chem. Sci. 2016, 7, 5032–5051. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Shibata, H.; Sato, K.; Mizuguchi, J. Crystal Structure of Methyl Orange Derivatives and Their Electronic Spectra. J. Imaging Sci. Technol. 2009, 53, 50302-1–50302-7. [Google Scholar] [CrossRef]
- Ojala, W.H.; Lu, L.K.; Albers, K.E.; Gleason, W.B.; Richardson, T.I.; Lovrien, R.E.; Sudbeck, E.A. Intermolecular Interactions of Sulfonated Azo Dyes: Crystal Structures of the Diammonium, Dilithium, Magnesium and Calcium Salts of 7-Hydroxy-8-(Phenylazo)-1,3-Naphthalenedisulfonic Acid (Orange G). Acta Crystallogr. B Struct. Sci. 1994, 50, 684–694. [Google Scholar] [CrossRef]
- Skitchenko, R.K.; Usoltsev, D.; Uspenskaya, M.; Kajava, A.V.; Guskov, A. Census of Halide-Binding Sites in Protein Structures. Bioinformatics 2020, 36, 3064–3071. [Google Scholar] [CrossRef]
- Sauer, K.; Yano, J.; Yachandra, V.K. X-Ray Spectroscopy of the Mn4Ca Cluster in the Water-Oxidation Complex of Photosystem II. Photosynth. Res. 2005, 85, 73–86. [Google Scholar] [CrossRef]
- Olesen, K.; Andréasson, L.-E. The Function of the Chloride Ion in Photosynthetic Oxygen Evolution. Biochemistry 2003, 42, 2025–2035. [Google Scholar] [CrossRef]
- Heyda, J.; Hrobárik, T.; Jungwirth, P. Ion-Specific Interactions between Halides and Basic Amino Acids in Water. J. Phys. Chem. A 2009, 113, 1969–1975. [Google Scholar] [CrossRef] [PubMed]
- Hager, L.P.; Morris, D.R.; Brown, F.S.; Eberwein, H. Chloroperoxidase. J. Biol. Chem. 1966, 241, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.L.; Poulos, T.L. Ligand Binding and Structural Perturbations in Cytochrome c Peroxidase. A Crystallographic Study. J. Biol. Chem. 1990, 265, 2588–2595. [Google Scholar] [CrossRef] [PubMed]
- Stone, K.L.; Behan, R.K.; Green, M.T. X-Ray Absorption Spectroscopy of Chloroperoxidase Compound I: Insight into the Reactive Intermediate of P450 Chemistry. Proc. Natl. Acad. Sci. USA 2005, 102, 16563–16565. [Google Scholar] [CrossRef]
- Stone, K.L.; Behan, R.K.; Green, M.T. Resonance Raman Spectroscopy of Chloroperoxidase Compound II Provides Direct Evidence for the Existence of an Iron(IV)–Hydroxide. Proc. Natl. Acad. Sci. USA 2006, 103, 12307–12310. [Google Scholar] [CrossRef]
- Stone, K.L.; Borovik, A. Lessons from Nature: Unraveling Biological CH Bond Activation. Curr. Opin. Chem. Biol. 2009, 13, 114–118. [Google Scholar] [CrossRef]
- Stone, K.L. Exploring the Role of the Axial Ligand in Thiolate-Ligated Heme Enzymes: Spectroscopy of High-Valent Iron Intermediates of Chloroperoxidase. Ph.D. Dissertation, Pennsylvania State University, State College, PA, USA, 2008. Available online: https://etda.libraries.psu.edu/catalog/8224 (accessed on 20 August 2025).
- Guo, Y.; Ma, Q.; Cao, F.; Zhao, Q.; Ji, X. Colorimetric Detection of Hypochlorite in Tap Water Based on the Oxidation of 3,3′,5,5′-Tetramethyl Benzidine. Anal. Methods 2015, 7, 4055–4058. [Google Scholar] [CrossRef]
- Griffin, B.W.; Ashley, P.L. Evidence for a Radical Mechanism of Halogenation of Monochlorodimedone Catalyzed by Chloroperoxidase. Arch. Biochem. Biophys. 1984, 233, 188–196. [Google Scholar] [CrossRef]






| LD50 | Est. LD50 b | Est. Tox. Class b | |
|---|---|---|---|
| Compound | (mg/kg) a | (mg/kg) | |
| Methyl orange | 60 | 60 | 3 |
| Orange G | -- | 8000 | 6 |
| Proposed Product c | |||
| 4-hydroxybenzenesulfonic acid | 3486 d | 1800 | 4 |
| Benzenesulfonic acid | 1175 | 1100 | 4 |
| 3,4-dihydroxybenzenesulfonic acid | -- | 3200 | 4 |
| Catechol | 300 | 100 | 3 |
| 6,7-dihydroxy-1,3-naphthalenedisulfonic acid | -- | 2000 | 4 |
| 5-hydroxy-7,8-dioxo-1,3-naphthalenedisulfonic acid | -- | 1000 | 4 |
| 5,6-dioxonaphthalenesulfonic acid | -- | 260 | 3 |
| 3-chloro-4-ethyl-benzenesulfonic acid | -- | 1900 | 3 |
| 4-ethyl-benzenesulfonic acid | -- | 1900 | 4 |
| 2,4-dichloro-3-hydroxy-dimethylamine | -- | 480 | 4 |
| Dimethylamine | 951 | 951 | 4 |
| 4-dimethylaminophenol | -- | 565 | 4 |
| Phenol | 100 | 270 | 3 |
| benzene | >2000 | 930 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paz-Ramirez, N.; Redwinski, J.; Cranswick, M.A.; Grice, K.A.; Stone, K.L. Mechanistic Distinction Between Oxidative and Chlorination Transformations of Chloroperoxidase from Caldariomyces fumago Demonstrated by Dye Decolorization. Catalysts 2025, 15, 965. https://doi.org/10.3390/catal15100965
Paz-Ramirez N, Redwinski J, Cranswick MA, Grice KA, Stone KL. Mechanistic Distinction Between Oxidative and Chlorination Transformations of Chloroperoxidase from Caldariomyces fumago Demonstrated by Dye Decolorization. Catalysts. 2025; 15(10):965. https://doi.org/10.3390/catal15100965
Chicago/Turabian StylePaz-Ramirez, Norman, Jacob Redwinski, Matthew A. Cranswick, Kyle A. Grice, and Kari L. Stone. 2025. "Mechanistic Distinction Between Oxidative and Chlorination Transformations of Chloroperoxidase from Caldariomyces fumago Demonstrated by Dye Decolorization" Catalysts 15, no. 10: 965. https://doi.org/10.3390/catal15100965
APA StylePaz-Ramirez, N., Redwinski, J., Cranswick, M. A., Grice, K. A., & Stone, K. L. (2025). Mechanistic Distinction Between Oxidative and Chlorination Transformations of Chloroperoxidase from Caldariomyces fumago Demonstrated by Dye Decolorization. Catalysts, 15(10), 965. https://doi.org/10.3390/catal15100965









