Microwave-Induced Deep Oxidation of Brilliant Green Using Carbon Nanotube-Supported Bismuth Ferrite
Abstract
1. Introduction
2. Results and Discussion
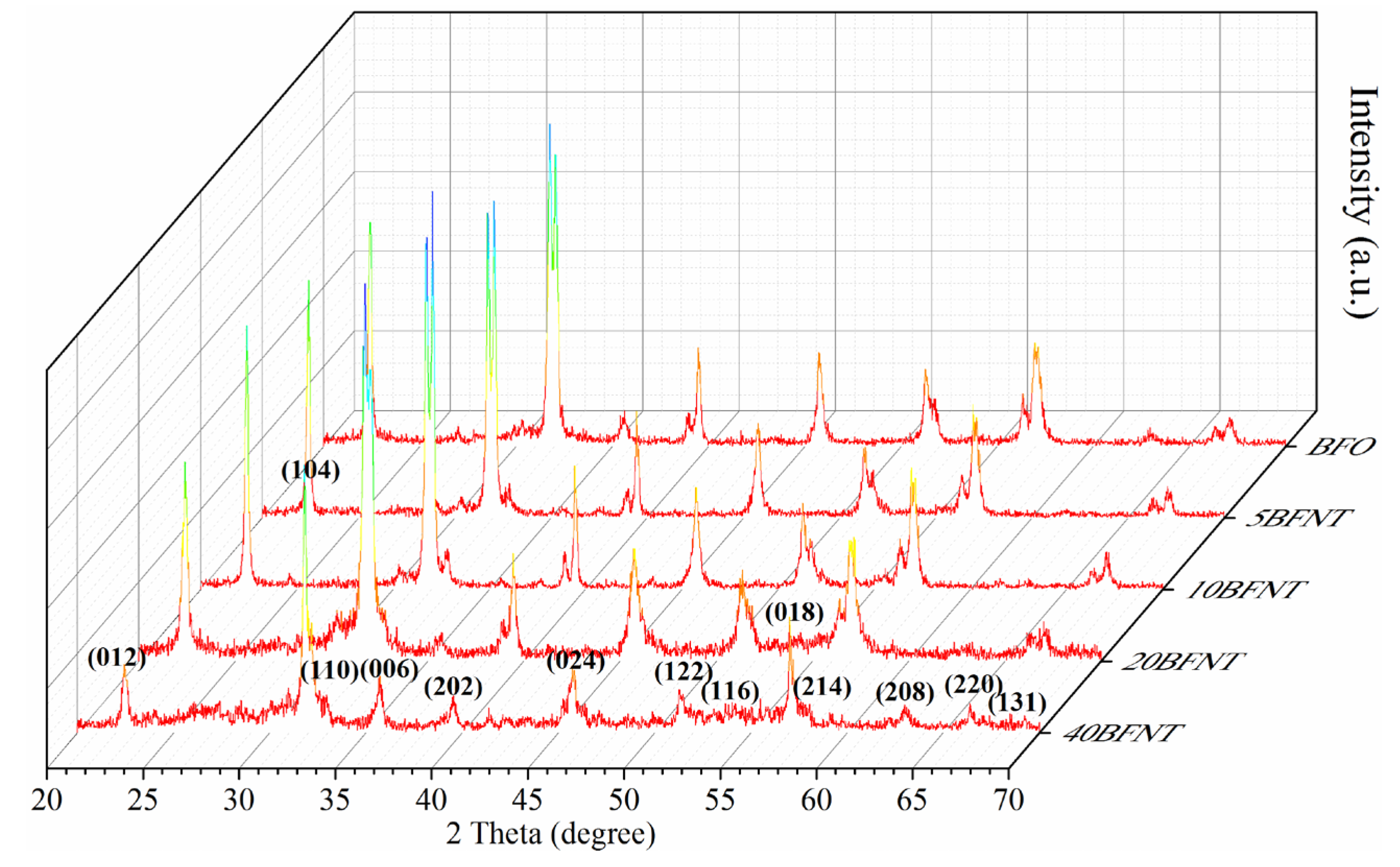
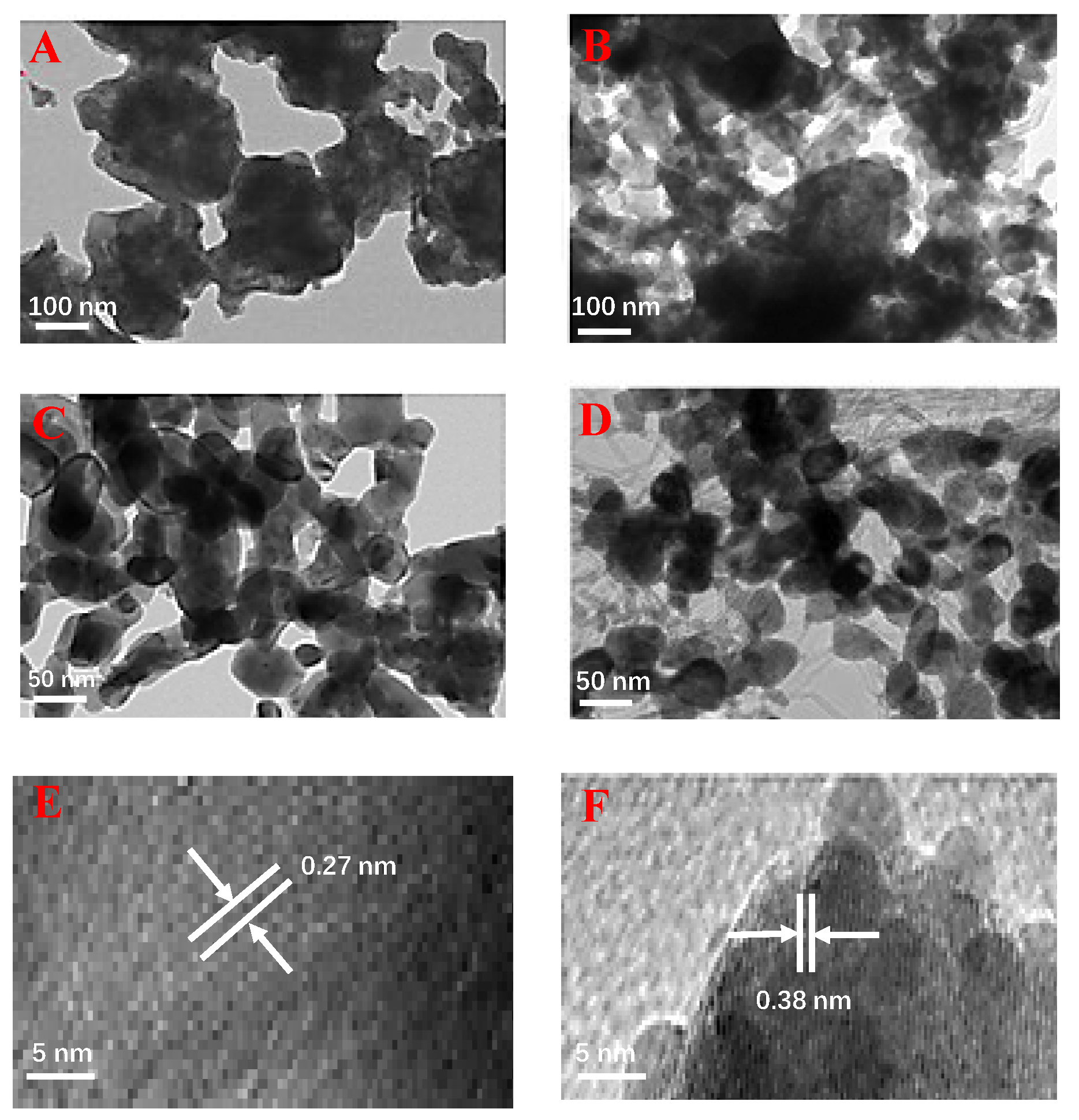
2.1. Characterization of the BFNT Catalyst
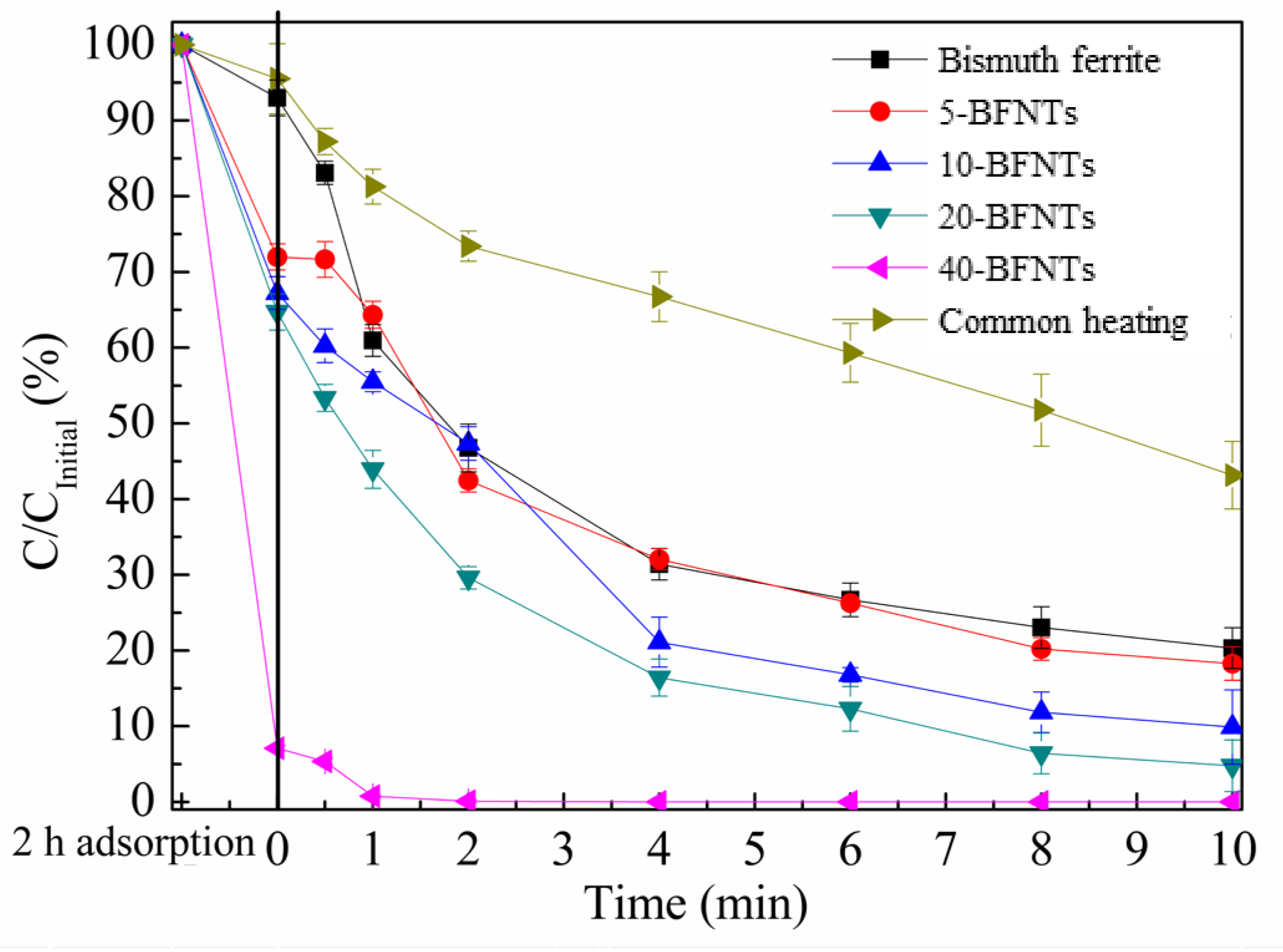
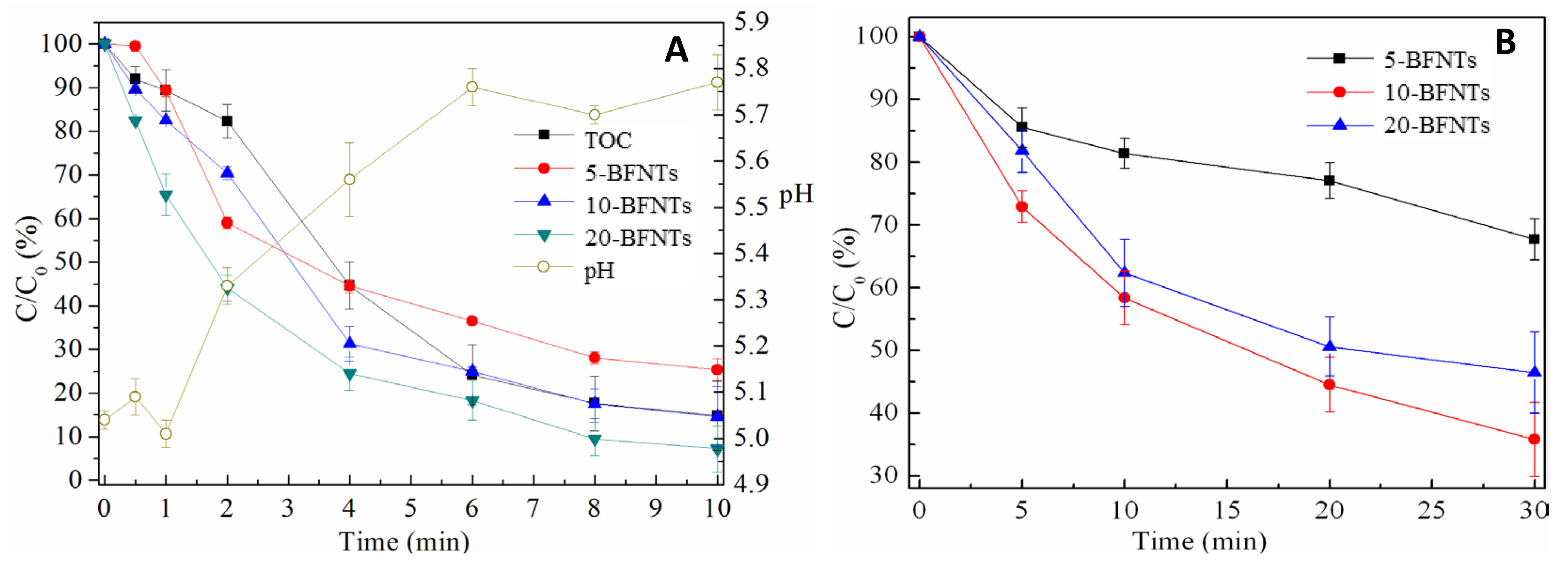
2.2. Degradation of BG
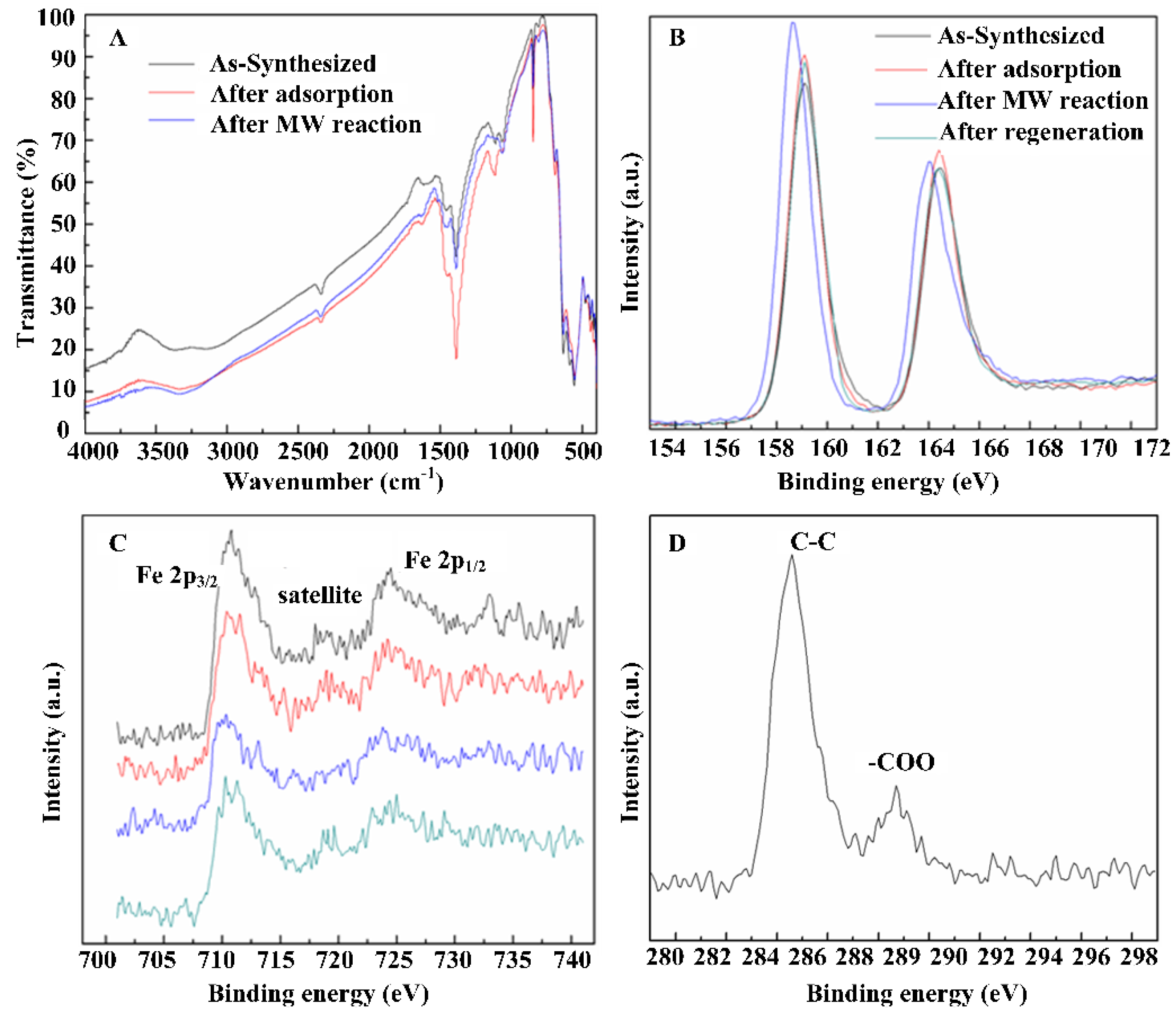
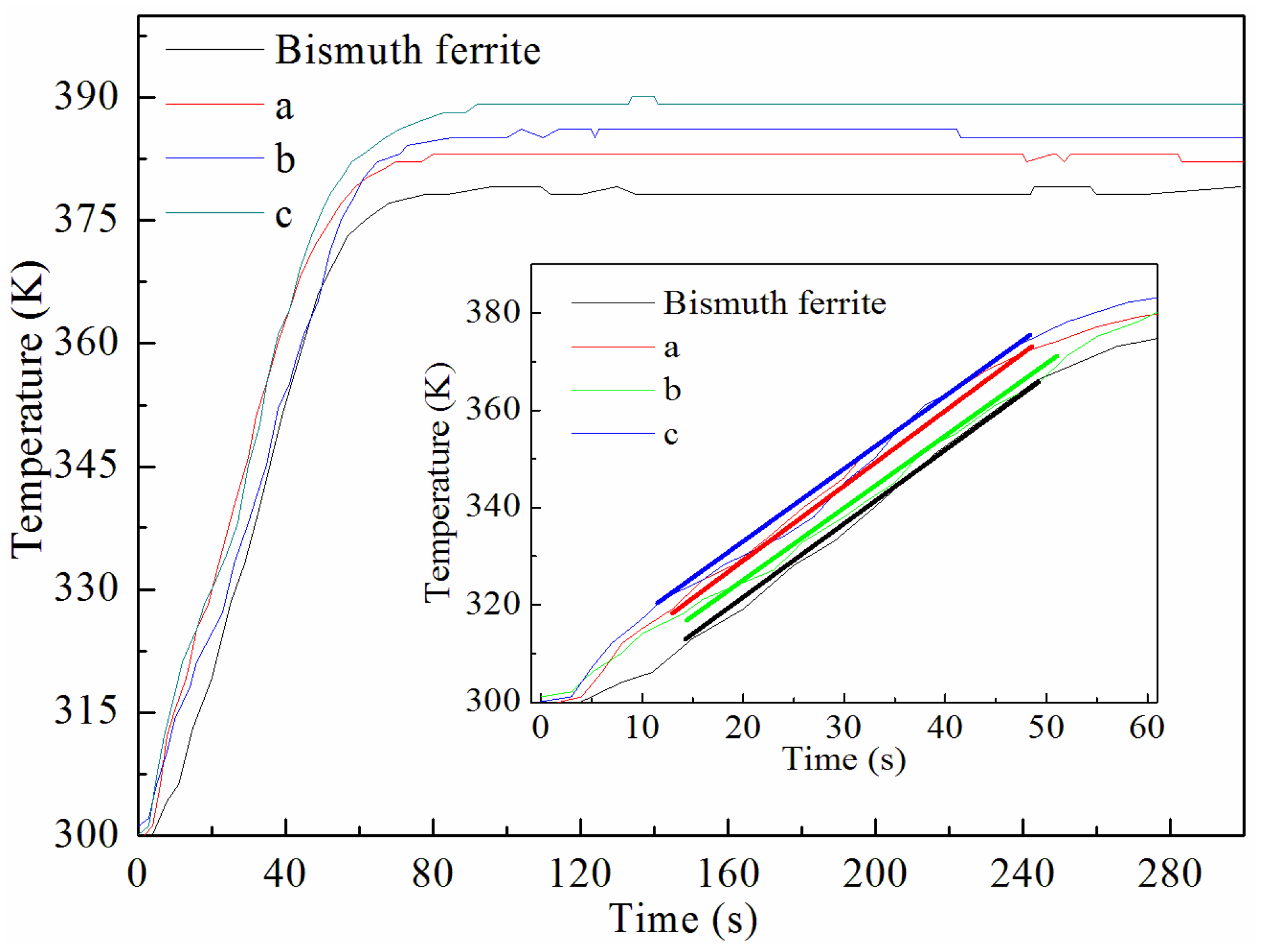
2.3. The Mechanism of the MW-Induced Reaction
2.4. Intermediates and Degradation Pathways
3. Materials and Methods
3.1. Materials
3.2. Preparation of BFNTs
3.3. Characterization Methods
3.4. MW-Induced Degradation of BG
3.5. Analysis Methods
3.6. Active Species Identification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, J.; Ye, W.; Xie, M.; Seo, D.H.; Luo, J.; Wan, Y.; Van der Bruggen, B. Environmental impacts and remediation of dye-containing wastewater. Nat. Rev. Earth Environ. 2023, 4, 785–803. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Zhang, Y.; van Bochove, B.; Mäkilä, E.; Seppälä, J.; Xu, W.; Willför, S.; Xu, C. Robust shape-retaining nanocellulose-based aerogels decorated with silver nanoparticles for fast continuous catalytic discoloration of organic dyes. Sep. Purif. Technol. 2020, 242, 116523. [Google Scholar] [CrossRef]
- Mandal, S.; Alankar, T.; Hughes, R.; Marpu, S.B.; Omary, M.A.; Shi, S.Q. Removal of hazardous dyes and waterborne pathogens using a nanoengineered bioadsorbent from hemp—Fabrication, characterization and performance investigation. Surf. Interfaces 2022, 29, 101797. [Google Scholar] [CrossRef]
- Li, W.; Cheng, C.; Zhao, J.; Song, Y.; Xue, C. Enhanced Azo Dye Removal through Sequential Ultrasound-Assisted-Treatment and Photocatalysis Using CdZnS. Angew. Chem. Int. Ed. 2025, 64, e202425508. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, C.; Liu, Z.J. Study on removal of four azo dyes from wastewater by ozonation, Advances in Energy Science and Equipment Engineering. In Proceedings of the International Conference on Energy Equipment Science and Engineering (ICEESE), Guangzhou, China, 30–31 May 2015; pp. 1241–1244. [Google Scholar]
- Zhong, Q.; Sun, Y.; Yang, S.; Xu, C.; Liu, Z.; Zhang, K.; Yan, S.; He, H. Anion Vacancies Triggered Spin Polarization Enables Efficient Piezocatalysis for Water Cleanup. Angew. Chem. 2025, 137, e202507265. [Google Scholar] [CrossRef]
- Soltani-Nezhad, F.; Saljooqi, A.; Mostafavi, A.; Shamspur, T. Synthesis of Fe3O4/CdS–ZnS nanostructure and its application for photocatalytic degradation of chlorpyrifos pesticide and brilliant green dye from aqueous solutions. Ecotoxicol. Environ. Saf. 2020, 189, 109886. [Google Scholar] [CrossRef]
- Qi, C.; Chen, H.; Xu, C.; Xu, Z.; Chen, H.; Yang, S.; Li, S.; He, H.; Sun, C. Synthesis and application of magnetic materials-barium ferrite nanomaterial as an effective microwave catalyst for degradation of brilliant green. Chemosphere 2020, 260, 127681. [Google Scholar] [CrossRef]
- Shang, X.; Liu, X.; Ma, X.; Zhang, Z.; Lin, C.; He, M.; Ouyang, W. Efficient degradation of chlorpyrifos and intermediate in soil by a novel microwave induced advanced oxidation process: A two-stage reaction. J. Hazard. Mater. 2024, 464, 133001. [Google Scholar] [CrossRef]
- Cipagauta-Díaz, S.; Estrella-González, A.; Navarrete-Magaña, M.; Gómez, R. N Doped-TiO2 Coupled to BiVO4 with High Performance in Photodegradation of Ofloxacin Antibiotic and Rhodamine B Dye Under Visible Light. Catal. Today 2022, 394–396, 445–457. [Google Scholar] [CrossRef]
- Wei, R.; Wang, P.; Zhang, G.; Wang, N.; Zheng, T. Microwave-responsive catalysts for wastewater treatment: A review. Chem. Eng. J. 2020, 382, 122781. [Google Scholar] [CrossRef]
- Chen, J.; Xu, W.; Zhu, J.; Wang, X.; Zhou, J. Highly Effective Direct Decomposition of H2S by Microwave Catalysis on Core-Shell Mo2N-MoC@SiO2 Microwave Catalyst. Appl. Catal. B Environ. 2020, 268, 118454. [Google Scholar] [CrossRef]
- Xia, H.; Li, C.; Yang, G.; Shi, Z.; Jin, C.; He, W.; Xu, J.; Li, G. A review of microwave-assisted advanced oxidation processes for wastewater treatment. Chemosphere 2022, 287, 131981. [Google Scholar] [CrossRef]
- Ding, S.; Huang, W.; Yang, S.; Mao, D.; Yuan, J.; Dai, Y.; Kong, J.; Sun, C.; He, H.; Li, S.; et al. Degradation of Azo Dye Direct Black BN Based on Adsorption and Microwave-Induced Catalytic Reaction. Front. Environ. Sci. Eng. 2017, 12, 5. [Google Scholar] [CrossRef]
- Gole, V.L.; Priya, A. Microwave-Photocatalyzed Assisted Degradation of Brilliant Green Dye: A Batch to Continuous Approach. J. Water Process. Eng. 2017, 19, 101–105. [Google Scholar] [CrossRef]
- Chen, J.; Xue, S.; Song, Y.; Shen, M.; Zhang, Z.; Yuan, T.; Tian, F.; Dionysiou, D.D. Microwave-Induced Carbon Nanotubes Catalytic Degradation of Organic Pollutants in Aqueous Solution. J. Hazard. Mater. 2016, 310, 226–234. [Google Scholar] [CrossRef]
- Foong, S.Y.; Liew, R.K.; Yang, Y.; Cheng, Y.W.; Yek, P.N.Y.; Mahari, W.A.W.; Lee, X.Y.; Han, C.S.; Vo, D.-V.N.; Van Le, Q.; et al. Valorization of Biomass Waste to Engineered Activated Biochar by Microwave Pyrolysis: Progress, Challenges, and Future Directions. Chem. Eng. J. 2020, 389, 124401. [Google Scholar] [CrossRef]
- Amdouni, W.; Fricaudet, M.; Otoničar, M.; Garcia, V.; Fusil, S.; Kreisel, J.; Maghraoui-Meherzi, H.; Dkhil, B. BiFeO3 Nanoparticles: The “Holy-Grail” of Piezo-Photocatalysts? Adv. Mater. 2023, 35, 2301841. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Liu, Y.; Li, F.; Chu, J.; Chen, K. Superparamagnetic cobalt-ferrite-modified carbon nanotubes using a facile method. Mater. Sci. Eng. B-Adv. Funct. Solid-State Mater. 2010, 166, 132–134. [Google Scholar] [CrossRef]
- Park, Y.-K.; Chung, K.-H.; Park, I.-S.; Kim, S.-C.; Kim, S.-J.; Jung, S.-C. Photocatalytic degradation of 1,4-dioxane using liquid phase plasma on visible light photocatalysts. J. Hazard. Mater. 2020, 399, 123087. [Google Scholar] [CrossRef] [PubMed]
- Soltani, T.; Lee, B.-K. Novel and facile synthesis of Ba-doped BiFeO3 nanoparticles and enhancement of their magnetic and photocatalytic activities for complete degradation of benzene in aqueous solution. J. Hazard. Mater. 2016, 316, 122–133. [Google Scholar] [CrossRef]
- Aykaç, A.; Gergeroglu, H.; Beşli, B.; Akkaş, E.Ö.; Yavaş, A.; Güler, S.; Güneş, F.; Erol, M. An Overview on Recent Progress of Metal Oxide/Graphene/CNTs-Based Nanobiosensors. Nanoscale Res. Lett. 2021, 16, 65. [Google Scholar] [CrossRef]
- Mallakpour, S.; Khadem, E. Carbon nanotube–metal oxide nanocomposites: Fabrication, properties and applications. Chem. Eng. J. 2016, 302, 344–367. [Google Scholar] [CrossRef]
- Chen, X.; Liu, H.; Hu, D.; Liu, H.; Ma, W. Recent Advances in Carbon Nanotubes-Based Microwave Absorbing Composites. Ceram. Int. 2021, 47, 23749–23761. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, W.; Liang, Q.; Huang, J.; Shao, B.; Liu, Y.; Liu, Y.; He, Q.; Wu, T.; Gong, J.; et al. Microwave-assisted high-efficiency degradation of methyl orange by using CuFe2O4/CNT catalysts and insight into degradation mechanism. Environ. Sci. Pollut. Res. 2021, 28, 42683–42693. [Google Scholar] [CrossRef]
- Pourabdollahi, H.; Zarei, A.R. Synthesis of Carbon Nanotubes on Cerium-Substituted Barium Ferrite Substrate by Chemical Vapor Deposition for Preparation of a Microwave Absorbing Nanocomposite. Iran. J. Chem. Chem. Eng.-Int. Engl. Ed. 2020, 39, 1. [Google Scholar] [CrossRef]
- Yuan, Y.; Wei, S.; Liang, Y.; Wang, B.; Wang, Y.; Xin, W.; Wang, X.; Zhang, Y. Solvothermal assisted synthesis of CoFe2O4/CNTs nanocomposite and their enhanced microwave absorbing properties. J. Alloy. Compd. 2021, 867, 159040. [Google Scholar] [CrossRef]
- Sun, C.; Sun, K.N. Synthesis and characterization of LiZn ferrite-coated CNT via a sol-gel method. Adv. Compos. Lett. 2006, 15, 169–172. [Google Scholar]
- Soni, S.K.; Thomas, B.; Kar, V.R. A comprehensive review on CNTs and CNT-reinforced composites: Syntheses, characteristics and applications. Mater. Today Commun. 2020, 25, 101546. [Google Scholar] [CrossRef]
- Rao, G.V.S.; Rao, C.N.R.; Ferraro, J.R. Infrared and electronic spectra of rare earth perovskites: Ortho-Chromites, -manganites and -ferrites. Appl. Spectrosc. 1970, 24, 436–445. [Google Scholar] [CrossRef]
- Farhadi, S.; Siadatnasab, F. Perovskite-Type LaFeO3 Nanoparticles Prepared by Thermal Decomposition of the La[Fe(CN)6]·5H2O Complex: A New Reusable Catalyst for Rapid and Efficient Reduction of Aromatic Nitro Compounds to Arylamines with Propan-2-ol Under Microwave Irradiation. J. Mol. Catal. A Chem. 2011, 339, 108–116. [Google Scholar] [CrossRef]
- Beg, S.; Al-Alas, A.; Al-Areqi, N.A. Study on Structural and Electrical Properties of Layered Perovskite-Type Oxide-Ion Conductor. Mater. Chem. Phys. 2010, 124, 305–311. [Google Scholar] [CrossRef]
- Alvaro, M.; Atienzar, P.; de la Cruz, P.; Delgado, J.L.; Garcia, H.; Langa, F. Sidewall Functionalization of Single-Walled Carbon Nanotubes with Nitrile Imines. Electron Transfer from the Substituent to the Carbon Nanotube. J. Phys. Chem. B 2004, 108, 12691–12697. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, H.; Qing, Q.; Yang, Y.; Li, Q.; Liu, Z.; Guo, X.; Du, Z. Effect of chemical oxidation on the structure of single-walled carbon nanotubes. J. Phys. Chem. B 2003, 107, 3712–3718. [Google Scholar] [CrossRef]
- Fruth, V.; Popa, M.; Calderon-Moreno, J.; Anghel, E.; Berger, D.; Gartner, M.; Anastasescu, M.; Osiceanu, P.; Zaharescu, M. Chemical Solution Deposition and Characterization of BiFeO3 Thin Films. J. Eur. Ceram. Soc. 2007, 27, 4417–4420. [Google Scholar] [CrossRef]
- Mills, P.; Sullivan, J.L. A study of the core level electrons in iron and its 3 oxides by means of X-ray photoelectron spectroscopy. J. Phys. D Appl. Phys. 1983, 16, 723–732. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X Ray Photoelectron Spectroscopy, 2nd ed; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Zhang, Z.; Zhong, X.; Liao, H.; Wang, F.; Wang, J.; Zhou, Y. Composition depth profiles of Bi3.15Nd0.85Ti3O12 thin films studied by X-ray photoelectron spectroscopy. Appl. Surf. Sci. 2011, 257, 7461–7465. [Google Scholar] [CrossRef]
- Jitianu, A.; Cacciaguerra, T.; Benoit, R.; Delpeux, S.; Béguin, F.; Bonnamy, S. Synthesis and characterization of carbon nanotubes–TiO2 nanocomposites. Carbon 2004, 42, 1147–1151. [Google Scholar] [CrossRef]
- He, H.; Yang, S.; Yu, K.; Ju, Y.; Sun, C.; Wang, L. Microwave Induced Catalytic Degradation of Crystal Violet in Nano-Nickel Dioxide Suspensions. J. Hazard. Mater. 2010, 173, 393–400. [Google Scholar] [CrossRef]
- Palma, V.; Barba, D.; Cortese, M.; Martino, M.; Renda, S.; Meloni, E. Microwaves and heterogeneous catalysis: A review on selected catalytic processes. Catalysts 2020, 10, 246. [Google Scholar] [CrossRef]
- de la Hoz, A.; Díaz-Ortiz, Á.; Moreno, A. Microwaves in Organic Synthesis. Thermal and Non-Thermal Microwave Effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [CrossRef]
- Chen, H.; Yang, S.; Yu, K.; Ju, Y.; Sun, C. Effective Photocatalytic Degradation of Atrazine over Titania-Coated Carbon Nanotubes (CNTs) Coupled with Microwave Energy. J. Phys. Chem. A 2011, 115, 3034–3041. [Google Scholar] [CrossRef]
- Kishimoto, F.; Yoshioka, T.; Ishibashi, R.; Yamada, H.; Muraoka, K.; Taniguchi, H.; Wakihara, T.; Takanabe, K. Direct microwave energy input on a single cation for outstanding selective catalysis. Sci. Adv. 2023, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, M.; Shiga, T.; Shiomi, J. Effects of defects on thermoelectric properties of carbon nanotubes. Phys. Rev. B 2017, 95, 155405. [Google Scholar] [CrossRef]
- Clark, S.J.; Robertson, J. Band Gap and Schottky Barrier Heights of Multiferroic BiFeO3. Appl. Phys. Lett. 2007, 90, 132903. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Y.; Jiang, B.; Hu, S.; Wang, Y.; Zhang, C.; Liu, F.; Zhang, Y.; Li, G. Self-catalyst degradation of amoxicillin in alkaline condition driven by superoxide radical. Chem. Eng. J. 2023, 477, 146942. [Google Scholar] [CrossRef]
- Quan, X.; Zhang, Y.; Chen, S.; Zhao, Y.; Yang, F. Generation of hydroxyl radical in aqueous solution by microwave energy using activated carbon as catalyst and its potential in removal of persistent organic substances. J. Mol. Catal. A Chem. 2007, 263, 216–222. [Google Scholar] [CrossRef]
- Luo, W.; Zhu, L.; Wang, N.; Tang, H.; Cao, M.; She, Y. Efficient Removal of Organic Pollutants with Magnetic Nanoscaled BiFeO3 as a Reusable Heterogeneous Fenton-Like Catalyst. Environ. Sci. Technol. 2010, 44, 1786–1791. [Google Scholar] [CrossRef]
- Ago, H.; Kugler, T.; Cacialli, F.; Salaneck, W.R.; Shaffer, M.S.P.; Windle, A.H.; Friend, R.H. Work Functions and Surface Functional Groups of Multiwall Carbon Nanotubes. J. Phys. Chem. B 1999, 103, 8116–8121. [Google Scholar] [CrossRef]
- Jin, R.; Gao, W.; Chen, J.; Zeng, H.; Zhang, F.; Liu, Z.; Guan, N. Photocatalytic reduction of nitrate ion in drinking water by using metal-loaded MgTiO3-TiO2 composite semiconductor catalyst. J. Photochem. Photobiol. A 2004, 162, 585–590. [Google Scholar] [CrossRef]
- Li, P.; Zhang, L.; Wang, W.; Su, J.; Feng, L. Rapid Catalytic Microwave Method to Damage Microcystis aeruginosa with FeCl3-Loaded Active Carbon. Environ. Sci. Technol. 2011, 45, 4521–4526. [Google Scholar] [CrossRef]

| CNT Mass (%) | Surface Area (m2 g−1) |
|---|---|
| 0 | 5.9 ± 0.1 |
| 5 | 6.3 ± 0.1 |
| 10 | 12.1 ± 0.1 |
| 20 | 16.3 ± 0.2 |
| 40 | 82.9 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Chen, H.; Xue, Y.; Zhong, Q.; Yang, S. Microwave-Induced Deep Oxidation of Brilliant Green Using Carbon Nanotube-Supported Bismuth Ferrite. Catalysts 2025, 15, 964. https://doi.org/10.3390/catal15100964
Liu H, Chen H, Xue Y, Zhong Q, Yang S. Microwave-Induced Deep Oxidation of Brilliant Green Using Carbon Nanotube-Supported Bismuth Ferrite. Catalysts. 2025; 15(10):964. https://doi.org/10.3390/catal15100964
Chicago/Turabian StyleLiu, Haoran, Hongzhe Chen, Yan Xue, Qiang Zhong, and Shaogui Yang. 2025. "Microwave-Induced Deep Oxidation of Brilliant Green Using Carbon Nanotube-Supported Bismuth Ferrite" Catalysts 15, no. 10: 964. https://doi.org/10.3390/catal15100964
APA StyleLiu, H., Chen, H., Xue, Y., Zhong, Q., & Yang, S. (2025). Microwave-Induced Deep Oxidation of Brilliant Green Using Carbon Nanotube-Supported Bismuth Ferrite. Catalysts, 15(10), 964. https://doi.org/10.3390/catal15100964






