2.1. Characterization Results
The X-ray diffraction results of the 5Ni/SBA-16 and 5Ni + (x = 0.5, 1, 2, 3 Gd)/SBA-16 samples showed different peaks in the structural composition of the catalysts. The typical peaks of SBA-16 are at low angles, that is, 2θ = 0.9–2.5° [
27]; however, as shown in
Figure 1, these peaks appear at a higher angle of 2θ = 3°, indicating a highly organized porous structure with a stable three-dimensional crystalline arrangement even after nickel loading. The diffraction peaks at 2θ = 37.2°, 43.2°, and 62.8° correspond to the 111, 200, and 220 crystal phases, respectively, demonstrating the presence of nickel in its oxidized form within the samples [
28]. Scherrer’s Equation (1) was applied to the catalysts promoted at ratios of x = 0, 2, and 3 wt% to observe the difference in the size of the NiO crystals. A significant decrease in the size of nickel oxide crystals was observed following the addition of a promoter. Specifically, the crystals in the sample without Gd measured 9.7–14 nm, whereas those in the sample with 0.5 wt% Gd measured 6.66–10.10 nm. Continuing to increase the Gd content caused further shrinkage until the crystals reached their minimum size of 6.5–7 nm, when the Gd content increased by 2%. However, when the Gd content increased to 3%, the crystals began to increase in size to 6.5–14.9 nm, as shown in
Table 1. This indicates that the addition of the promoter helped disperse the nickel better and spread it more widely on the surface of the SBA-16 support, which increased the active surface area for the DRM reaction owing to the absence of agglomeration in the particles during preparation or calcination. In contrast, for the catalyst promoted with 3 wt% Gd, the crystal size increased, demonstrating metal particle sintering as a result of heat treatment (calcination), leading to a lower distribution of active sites and thus a decrease in the activity and effectiveness of the catalyst, which might promote coking.
Scherer’s equation:
where
is the volume average diameter of the crystallite,
the wavelength, and
the instrumental line broadening found experimentally from sintered NiO samples.
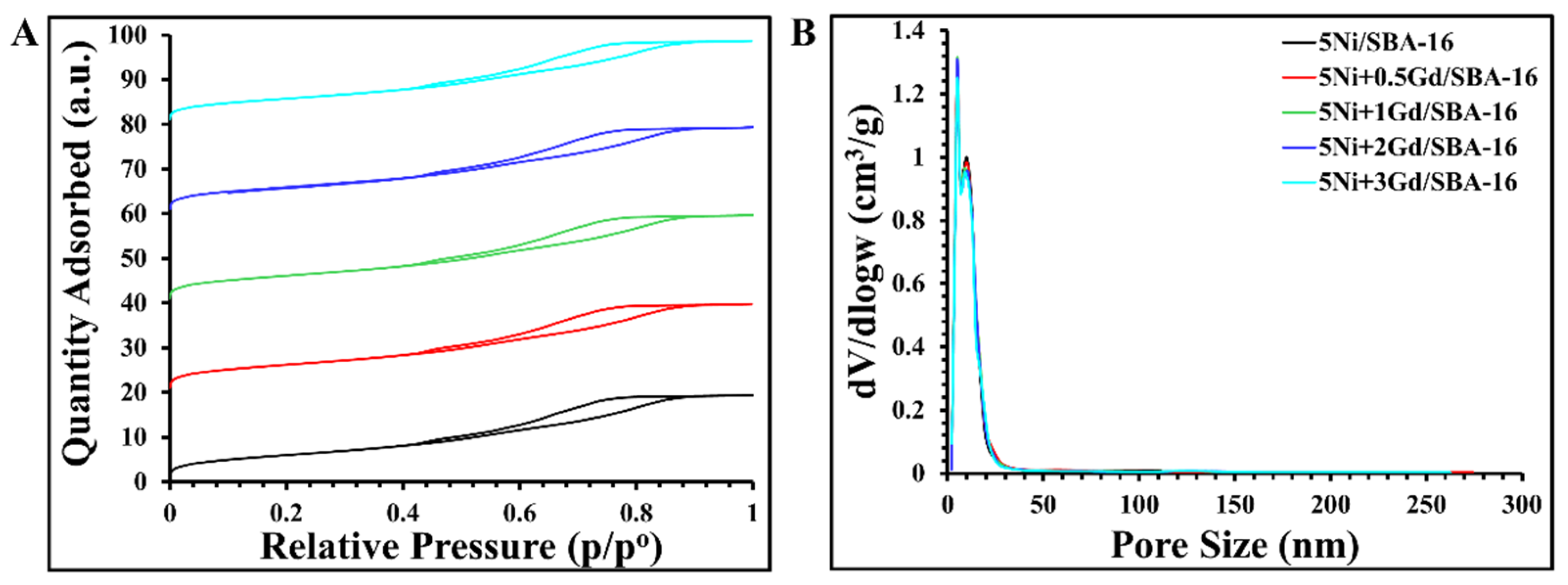
Surface area and porosity analysis showed an increase in relative pressure (p/p°) from 0.6 to 0.86, which was similar for all samples. However, the increase in the amount of adsorption with increasing promoter loading indicates an increase in the number of adsorption sites. The surface remained open because there was no sintering, resulting in a greater amount of nitrogen being adsorbed, as shown in
Figure 2A. The pore density peaked at a pore size of 48.8 nm with increasing catalyst loading. However, a decrease in the surface area, pore diameter, and pore volume is observed with increasing catalyst levels of 2–3 wt%, in contrast to catalysts loaded with 0.5–1 wt% Gd, where these values increase, as shown in
Table 2 (in comparison with SBA-16), indicating reduced agglomeration of nickel particles and improved dispersion on the support surface. Notably, the decrease in pore diameter and volume at high catalyst ratios is small and statistically insignificant, as the increase and decrease are approximately equal, indicating that the effectiveness of the catalyst in improving the pore structure is evident in
Figure 2B where bimodal pore size distribution falls within the mesopore region (2–50 nm).
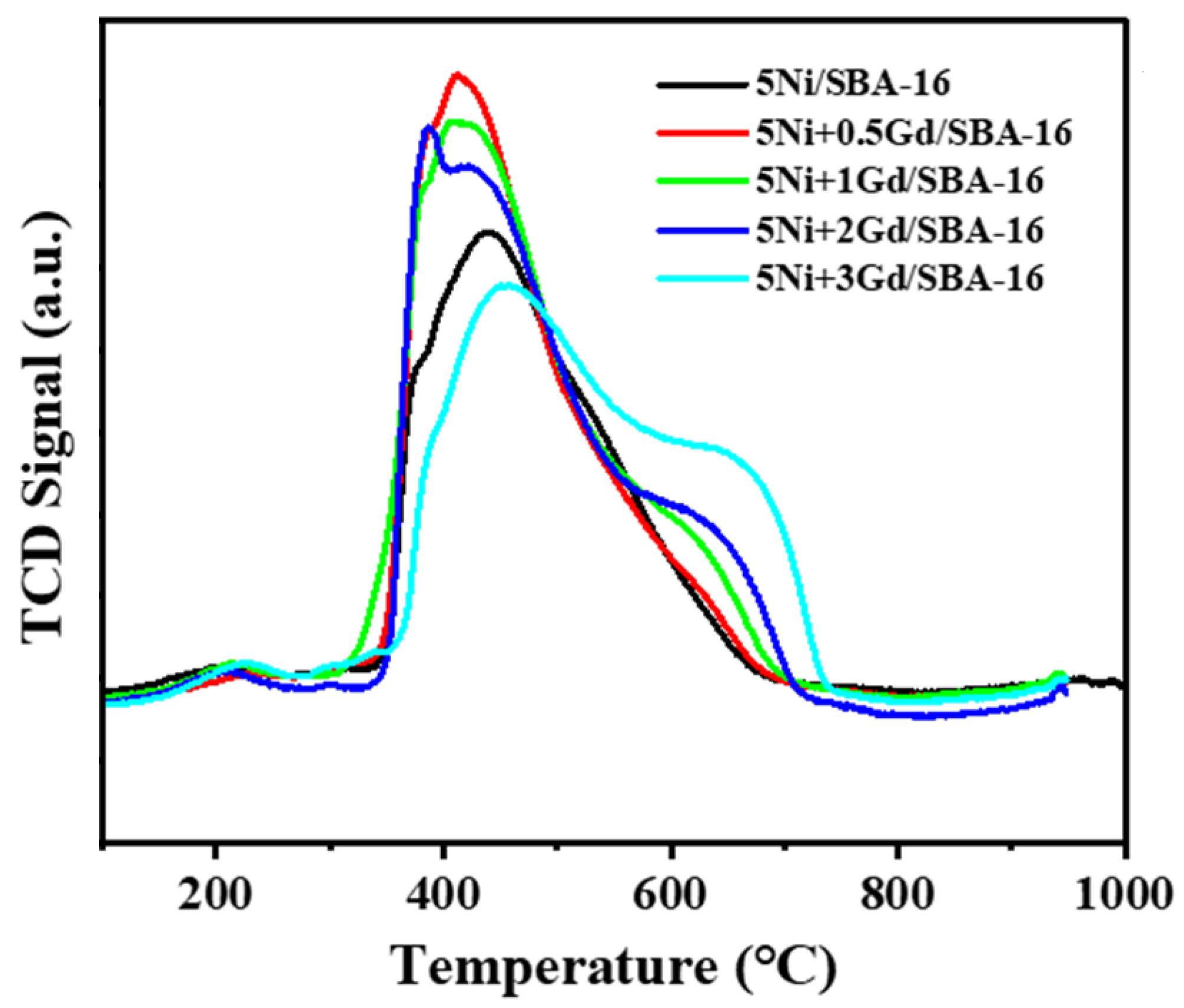
The analysis in
Figure 3 demonstrating H
2-temperature programmed reduction, reveals that the reduction peaks occur at varying temperatures, with the peak temperature decreasing gradually as the promoter content increases from 0.5 to 2 wt%. Gd promotion facilitates the reduction in Nio by increasing the number of active sites susceptible to reduction; however, the catalyst loaded with 3 wt% Gd requires a higher reduction temperature, as shown in
Table 3. Loading the catalyst with more than 2 wt% Gd may negatively influence the structure, with nickel agglomerating on the support, resulting in a compound that is both more stable and more resistant to reduction, potentially leading to undesirable outcomes. In addition, the catalyst loaded with the 2 wt% promoter exhibited the narrowest peak among the investigated catalysts. To further explore these findings, the TPR profiles were deconvoluted (
Figure S1), and it was evident that each catalyst had three deconvoluted peaks. The first peak had a maxima at 380, 385, 385, 385, and 435 °C for 5Ni/SBA-16, 5Ni+0.5Gd/SBA-16, 5Ni+1Gd/SBA-16, 5Ni+2Gd/SBA-16, and 5Ni+3Gd/SBA-16, respectively (
Table S1). This suggests that all the catalysts had approximately similar types of surface nickel oxide species, except 5Ni+3Gd/SBA-16, which showed a shift of nickel oxide species from the surface to the bulk. The second reduction peak temperatures were 430, 430, 435, 440, and 540 °C, indicating a similar behavior as that of the first reduction peak. The final reduction peaks were shifted to higher temperatures at 515, 515, 560, 570, and 665 °C for 5Ni/SBA-16, 5Ni+0.5Gd/SBA-16, 5Ni+1Gd/SBA-16, 5Ni+2Gd/SBA-16, and 5Ni+3Gd/SBA-16, respectively (
Table S1). These findings indicate that the third peak is mainly associated with nickel oxide bulk species having strong interactions with the support. The weakly bonded surface species and medium-strength nickel oxide species could play a significant role in influencing the catalytic performance of these catalysts. These results show that all nickel oxide surfaces and medium-strength species were reduced at approximately similar temperatures, suggesting a high degree of nickel homogeneity on the support [
29,
30].
Strong interaction of NiO may refer to NiO, which resides in the channels of SBA-15 [
29]. After the addition of 0.5 wt% Gd, reduction peaks in the low temperature region are grown, which indicates the growing concentration of NiO, which is held by weak to medium-strength interaction over the support. Interestingly, upon increasing the loading of Gd further, the intensity of the lower temperature peaks is decreased, and the intensity of the high temperature peak is increased. It indicates the progressive shift in the moderate interaction of NiO with support into a strong interaction upon increasing loading of Gd from 0.5 to 3 wt%. The growing interaction between NiO and the support induces better dispersion of NiO over the support.
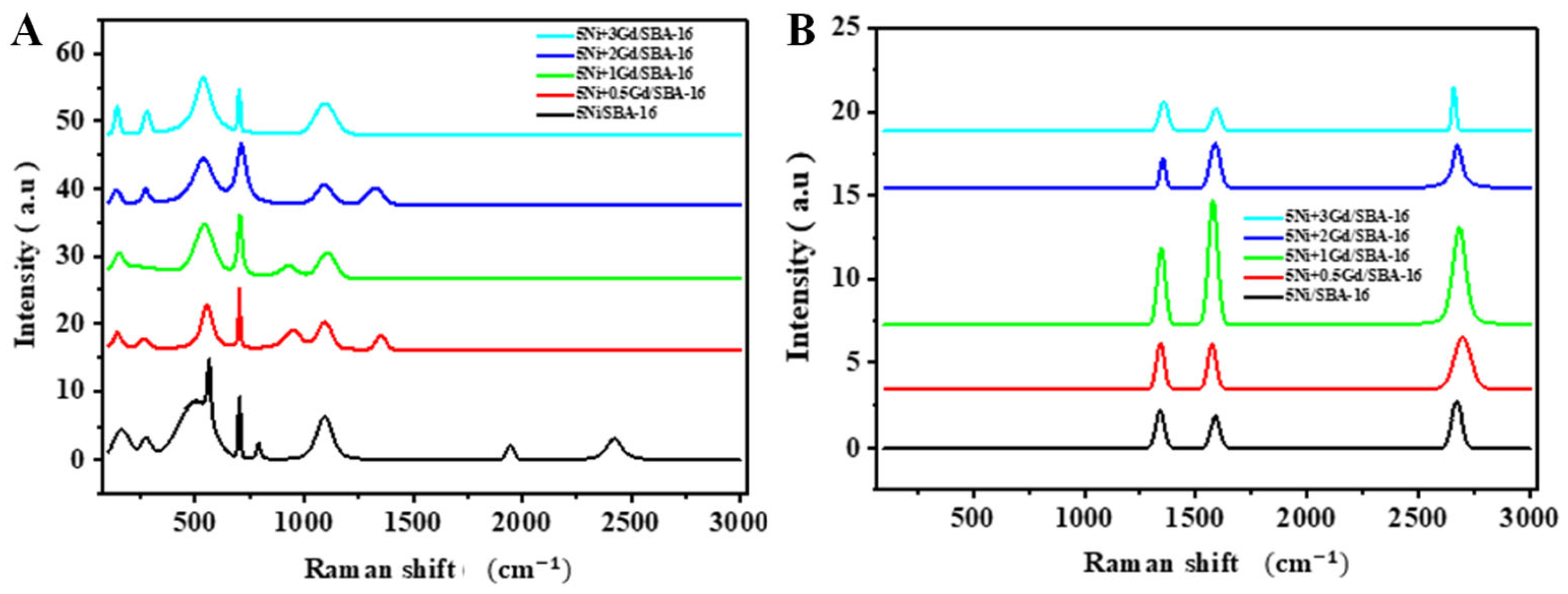
Raman spectroscopy results show peaks for the fresh catalysts in
Figure 4A, which indicate the reference structure of the catalyst. A peak at (143.7
shift indicates the presence of certain oxide phases such as TiO
2 and SiO
2, which are attributed to SBA-16 lattice vibrations, indicating that the support retains its porous structure after preparation [
31]. The peak at a 500
shift is attributed to the typical vibrations of NiO bonds, indicating its presence on the catalyst surface [
32]. Peaks appearing at the (≥1300
) shift indicate the presence of carbon in the catalysts [
33], which may be attributed to CO
2 adsorbed on the catalyst surface during calcination. It Is worth noting that the shift observed in the (1900, 2400
range was only seen in the promoter-free catalyst, suggesting that Gd was involved in altering the catalyst’s surface structure, possibly by eliminating impurities during calcination or by decreasing the surface’s capacity to adsorb CO
2, which could indicate improved catalyst stability prior to reaction. No distinct peaks related to gadolinium oxides were visible in the data, suggesting that the gadolinium was either evenly distributed across the catalyst surface or integrated into the SBA-16 support framework [
34]. In
Figure 4B, Raman spectroscopy shows three main peaks for the used catalysts: shift (1344, 1576, 2680
) which indicates amorphous carbon (D band) due to defects in the carbon structure, regular graphitic carbon (G band), and accumulated carbon (2D band), respectively [
16,
33]. The disappearance of the original peaks of the catalyst structure after the DRM reaction and the emergence of only carbon spectral peaks suggest that the catalyst surface is entirely covered with carbon layers, thereby preventing Raman spectroscopy from detecting the original phases of the catalysts. The addition of a promoter at a rate of 2–3 wt% resulted in a decrease in the intensity of the carbon peaks, indicating reduced carbon deposition during the reaction. The data indicate a greater resistance of the catalyst to coke formation and improved stability under the reaction conditions, as illustrated in
Table 4.
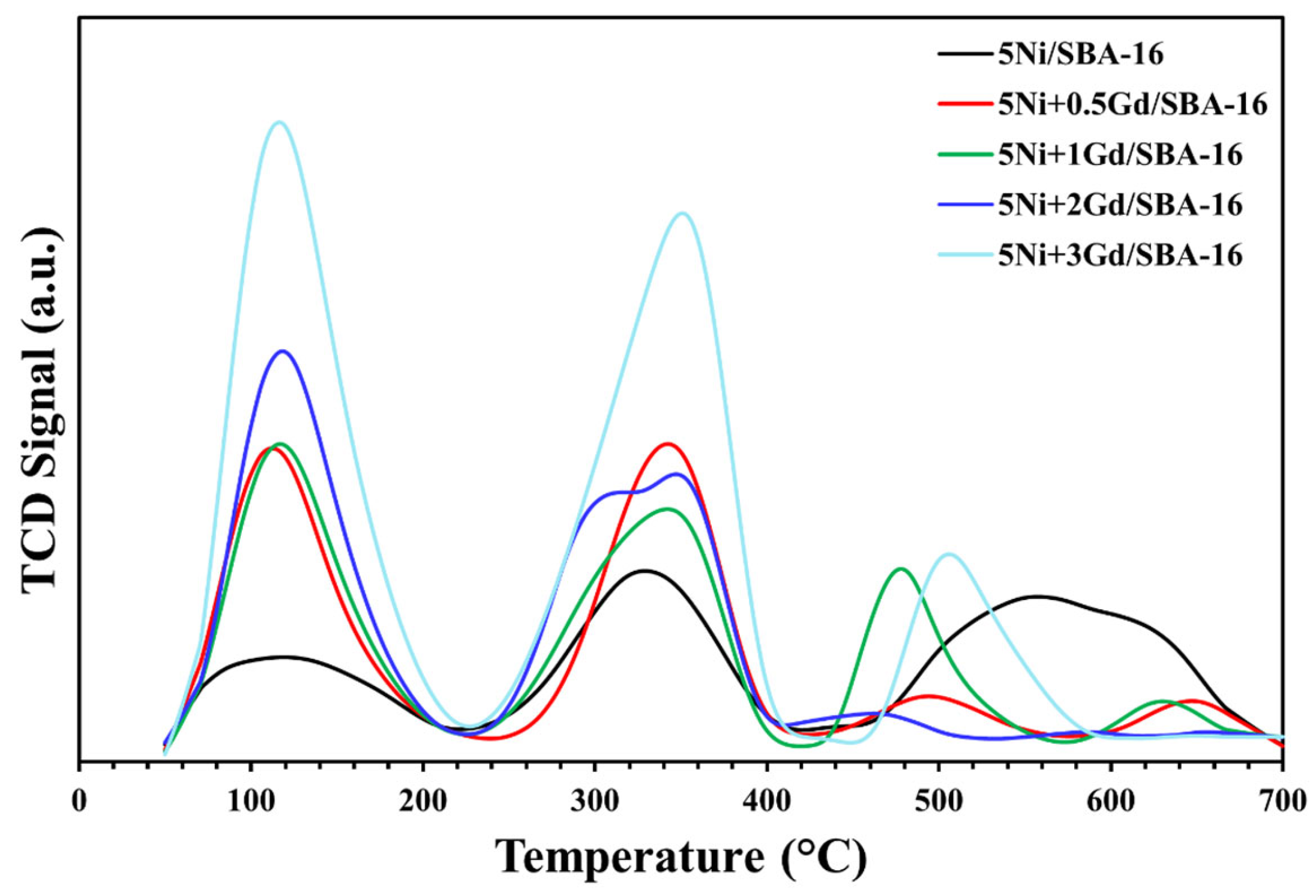
Temperature-programmed desorption profiles using ammonia (NH
3) as the probe gas, shown in
Figure 5, for fresh catalysts exhibited desorption peaks that could be divided into three sections. Section I represents desorption peaks within the temperature range of 60 and 210 °C, Section II contains peaks between 210 and 410 °C, while Section III comprises desorption peaks at temperatures above 410–700 °C. Sections I and II indicate desorption peaks associated with weak- and medium-strength acidic sites present on the surface of the catalysts, while Section III indicates strong acidic sites. In comparison with 5Ni/SBA-16, the Gd-promoted catalysts demonstrated enhanced acidic sites in all sections, especially in Sections I and II, indicating enhanced weak- and medium-strength acidic sites. These results indicate that the Gd promoter significantly influenced the acidic sites, which could potentially affect the activity results. Previous studies have shown that weak- and medium-strength acidic sites facilitate the adsorption and activation of methane molecules [
35]. Hence, it can be concluded that the Gd promoter contributed to improving the acidic sites favorable for methane activation, resulting in higher activity of these catalysts, as discussed in
Section 3.2.
In
Figure 6, the SEM images reveal a substantial modification in the surface morphology of the catalysts, which is ascribed to the variance in their chemical makeup resulting from the inclusion or exclusion of the Gd promoter. The catalyst without Gd in image A appears highly agglomerated on the surface, suggesting an uneven nickel distribution on the support surface, whereas image B shows more dispersed particles with better distribution, making them appear smoother, indicating that the addition of 0.5 wt% promoter enhanced particle dispersion. The surface in image C appears to be free of agglomerations; however, the particles are larger and more irregular in shape. The particles in image D exhibit a more uniform and even distribution, suggesting a potential benefit from incorporating the promoter at a concentration of 2 wt%. However, image E reflects significant surface roughness and severe sintering of the metal oxide due to the addition of 3 wt% promoter to the catalyst. Moreover, SEM-EDX profiles (
Figure S2) indicate that metal particles are well dispersed over the surface of the catalyst.
In
Figure 7, images of the promoter-free sample taken before the DMR reaction using transmission electron microscopy (TEM) revealed a clear agglomeration of NiO particles, which is depicted by the dark area on the support surface in image A. The evaluation of metal particle size is challenging because of excessive sintering. Moreover, the agglomeration of metal particles resulted in a decrease in the number of active sites on the surface, leading to carbon deposition and the formation of nanofibers (
Figure 7B) [
36,
37].
Figure 7B shows that the metal particles are surrounded by carbon and dispersed within the fibers as dark spots. On the contrary, the sample loaded with 2 wt% Gd promoter displayed an even distribution of nickel particles prior to the reaction as depicted in
Figure 7C. The metal nanoparticles range from 10 nm up to 150 nm showing a wide range of metal particles well dispersed over the surface of SBA-16. Highly dispersed Gd-promoted catalyst exhibited excellent stability under reaction conditions enabling the catalyst to sustain the reaction for an extended period as demonstrated in
Figure 7D, which displays metal particles retaining a spherical shape without any indication of carbon nanofibers [
35].
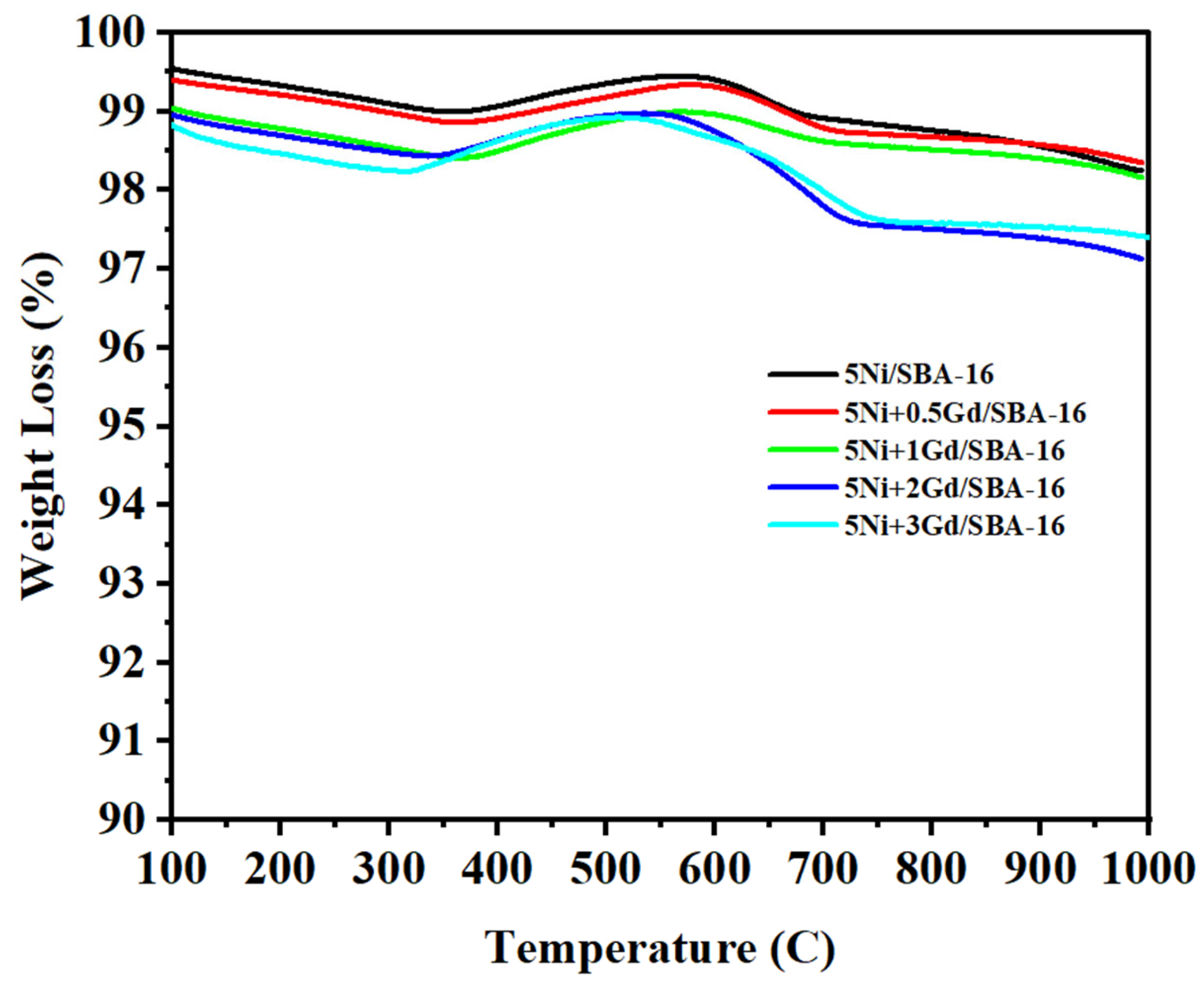
The amount of carbon deposited on the catalyst surface during the DMR reaction was determined by TGA
Figure 8. Mass loss at low temperatures (≤300 °C) is primarily caused by the removal of moisture [
28], whereas mass loss at high temperatures (≤650 °C) is due to the formation of graphitic carbon on the surface during the reaction [
37]. A slight increase in weight could be associated with the oxidation of metal nanoparticles. The TGA reveals a more stable thermal stability and composition for all catalysts, as the mass loss did not exceed 4% of the sample’s mass, signifying that the catalysts can endure high temperatures during the reaction.
2.2. Catalytic Activity Results
The catalytic activity of the different catalysts at a reaction temperature of 800 °C, 42 L h
−1 g
cat−1 gas hour space velocity, and CH
4: CO
2: N
2 (3:3:1) gas feed are shown in
Figure 9. The unpromoted catalyst (5Ni/SBA-16) exhibited initial CH
4 and CO
2 conversion values of 62 and 73%, respectively. The Gd incorporation as a promoter enhanced the catalytic activity with initial CH
4 conversions of 62.5, 69.5, 69.5, and 66% for 5Ni+0.5Gd/SBA-16, 5Ni+1Gd/SBA-16, 5Ni+2Gd/SBA-16, and 5Ni+3Gd/SBA-16, respectively. Similarly, initial CO
2 conversions were found to be 74.5, 76, 77, and 79% for 5Ni+0.5Gd/SBA-16, 5Ni+3Gd/SBA-16, 5Ni+2Gd/SBA-16, and 5Ni+1Gd/SBA-16, respectively. All the catalysts have displayed good stability and the highest conversions of CH
4, and CO
2 were obtained for 5Ni+1Gd/SBA-16. Incorporating Gd to the catalyst modified its surface properties and the abundance of active elements necessary for methane activation, as well as enhanced CO
2 adsorption by dispersing nickel and improving surface basicity. This increased adsorption facilitated carbon gasification resulting from the reverse Boudouard reaction, and hence cleaning the catalyst surface, ultimately increasing H
2 production [
15]. The higher CO
2 conversions and H
2/CO ratios less than unity also indicated the occurrence of reverse water gas shift side reaction that consumed CO
2 to produce more CO. The H
2/CO ratio results also showed that the gadolinium-enriched catalysts led to an improvement and a higher production rate than the catalyst without a promoter. However, increasing the promoter concentration may cause a decline in performance, as in the sample to which 2 and 3 wt% Gd were added. A catalyst with 1 wt% Gd was the optimal ratio for increased H
2 production, balancing enhanced performance with the avoidance of negative effects associated with high concentrations.
The 1 wt% Gd (5Ni+1Gd/SBA-16) incorporation has played a significant role in enhancing the catalytic performance of this catalyst because Gd (a) promoted the amount of weak and moderate reducible NiO species over the surface of the catalyst (
Table S1), (b) enhanced NiO dispersion, (c) reduced active metal particle size, (d) increased specific surface area, (e) improved acidic sites, and (f) suppressed carbon formation and metal particle agglomeration.
The comparison of this work with previous reports (
Table 5) shows that the 5Ni+1Gd/SBA-16 catalyst outperforms several catalysts, including a noble metal (Ruthenium) based catalyst (1GdRuCeZr) [
38]. Under similar operating conditions, 5Ni+4Gd/YZr [
39] showed better catalytic performance than 5Ni+1Gd/SBA-16. Most of these catalysts either had a higher amount of active metal (Ni) or a lower space velocity, and even with lower space velocities, these catalysts [
40,
41,
42,
43,
44] remained less active than 5Ni+1Gd/SBA-16. These findings highlight the role of Gd promoter in enhancing the catalytic activity that could potentially be further tuned to test under industrial conditions.
2.3. Response Surface Methodology Approach (RSM)
The Central Composite Design (CCD) methodology was employed, in this study, the experimental variables, namely space velocity, reaction temperature, and the CH4/CO2 molar ratio, were systematically varied within predefined ranges. These factors were normalized into standardized, dimensionless values spanning from −1 to +1, facilitating uniformity in statistical modeling and response surface analysis. The midpoint of each range represents the central level of the corresponding factor, while deviations from these midpoints define the extent of variation considered. This normalization enhances the interpretability of the regression coefficients and ensures consistency in the construction of the response surface. The overall central point, derived from the mean values of all variables, acts as a key reference for identifying potential curvature in the system’s response, thereby enabling a more accurate assessment of the interactions and effects of the experimental parameters on the outcome variable.
In this investigation, a quadratic model was used while preserving model simplicity and interpretability. Although higher-order models offer greater flexibility, they typically demand a larger number of experimental runs to achieve statistical reliability and may introduce unwarranted complexity. This added complexity can elevate the risk of overfitting, potentially compromising the model’s generalizability without yielding substantial improvements in predictive performance.
The predicted response variable
modeled as a function of the input factors
,
, that can be expressed in either coded (standardized) or real values. The regression equation takes the form of a second-order polynomial, incorporating multiple components: the intercept
), linear (
) that describe the main effect of each individual factor, quadratic terms (
) capturing the nonlinear impact or curvature, interaction terms (
) accounting for the collective impact of simultaneous variation in two variables, and
represents the residual variability which cannot be explained by the model [
47]. The model’s accuracy is evaluated through ANOVA [
48].
Table 6 presents the actual and coded values of the studied factors.
2.6. Simulation Using Design-Expert Software
In this section, 3D presentation of the models in Equations (3)–(5) are shown in
Figure 10,
Figure 11 and
Figure 12. The response variable’s models at their expected levels with each element can be seen in these charts.
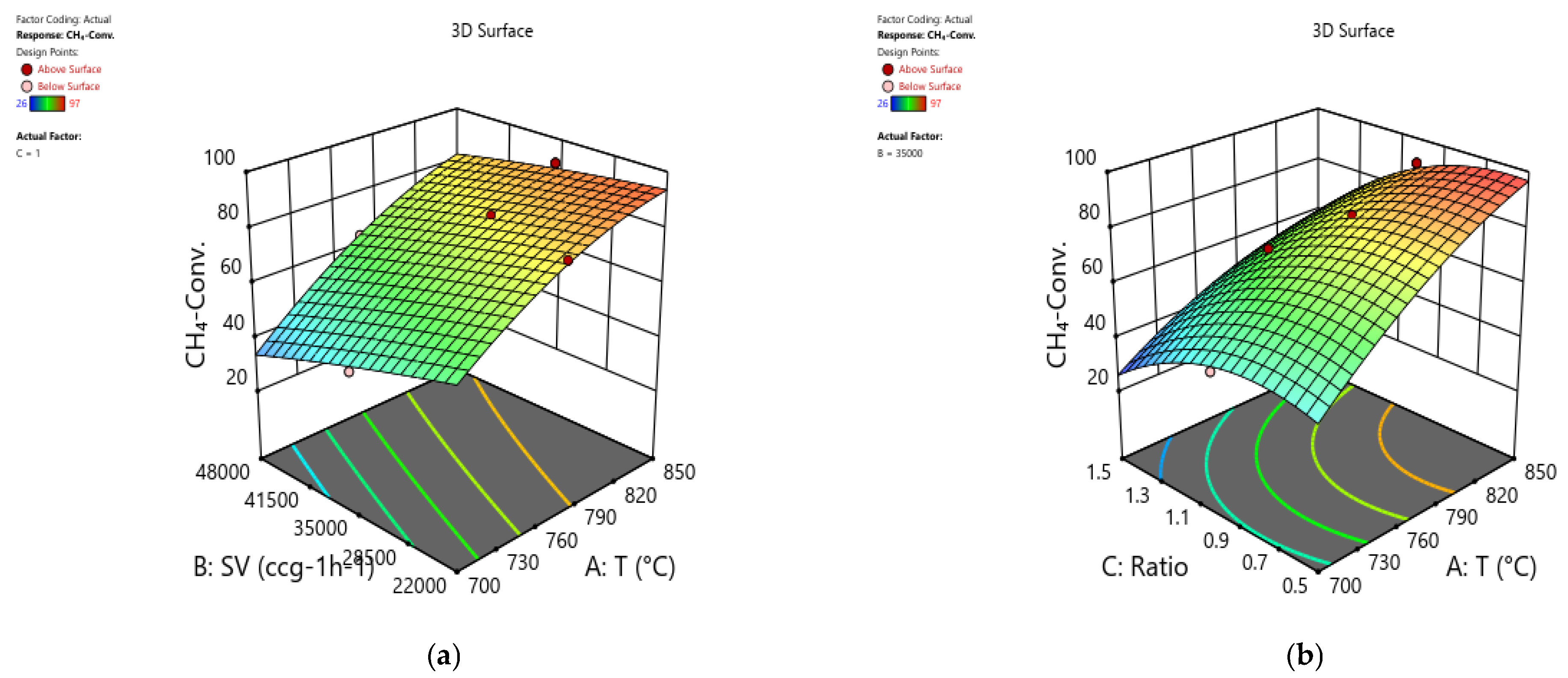
Figure 10 displays the impact of temperature, space velocity, and CH
4/CO
2 ratio on CH
4 conversion in 3D response surface plots.
Figure 10a presents a relation between CH
4 conversion, space velocity, and temperature at fixed CH
4/CO
2 ratio of 1. The response surface signifies that CH
4 conversion increases as temperature rises and SV decreases.
Figure 10b shows that increasing temperature and decreasing CH
4/CO
2 ratio significantly enhance CH
4 conversion at fixed SV of 35,000 mL h
−1 g
cat−1. In both cases, CH
4 conversion improves from 26.3% at 700 °C to 97.5% at 850 °C, with temperature employing the most significant impact on its variation.
Figure 11 depicts the effects of temperature, GHSV, and CH
4/CO
2 ratio on the variation of CO
2 conversion through three-dimensional response surface plots. In
Figure 11a, where CH
4/CO
2 is fixed at the center range, one, an increase in temperature and a decrease in SV result in a rise in CO
2 conversion as follows from 39.84% at 700 °C to 95.67% at 850 °C. Similarly,
Figure 11b shows that as both temperature and CH
4/CO
2 ratio increase, leading to a higher CO
2 Conversion change to follow the same range with SV fixed at 35,000 cc g
−1 h
−1.
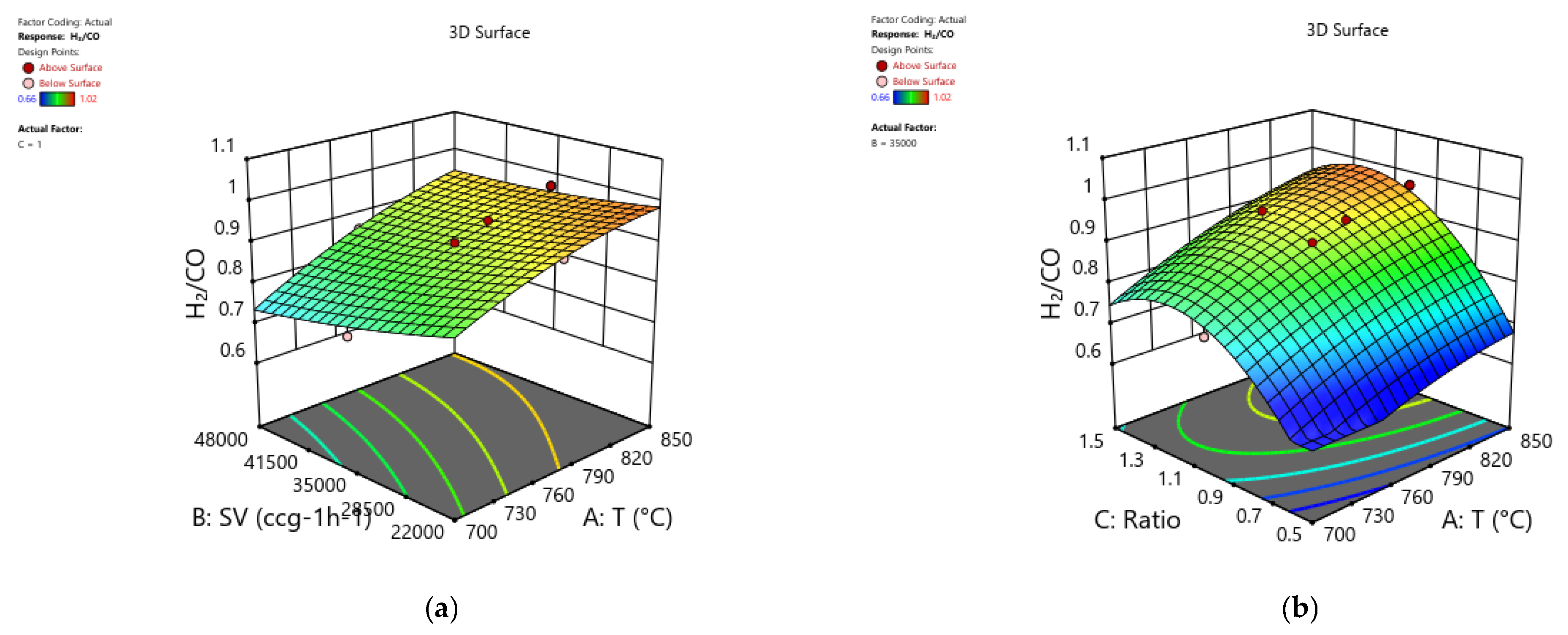
The strong impacts of temperature, GHSV, and CH
4/CO
2 ratio on H
2/CO are shown in 3D response surface plots in
Figure 12, which concludes the discussion.
Figure 12a shows that, with CH
4/CO
2 set at 1, the gas hourly space velocity (GHSV) increases H
2/CO from 0.66 at 700 °C to a maximum of 1.02 at 850 °C as a function of temperature. At SV = 35,000 cc g
−1 h
−1,
Figure 12b demonstrates that a larger H
2/CO is achieved by raising both the temperature and the CH
4/CO
2 ratio, within the same range of values.
Figures S4–S6 exhibit a two-dimensional representation of all these relations, and original units are used for all these factors.