Synthesis of Glyceric Acid by Mixed-Metal Oxide-Supported AuPt Alloy Catalyst in Mild Conditions
Abstract
1. Introduction
2. Results
2.1. Texture and Morphology of Catalysts
2.1.1. XRD Analysis
2.1.2. BET Analysis
2.1.3. XPS Analysis
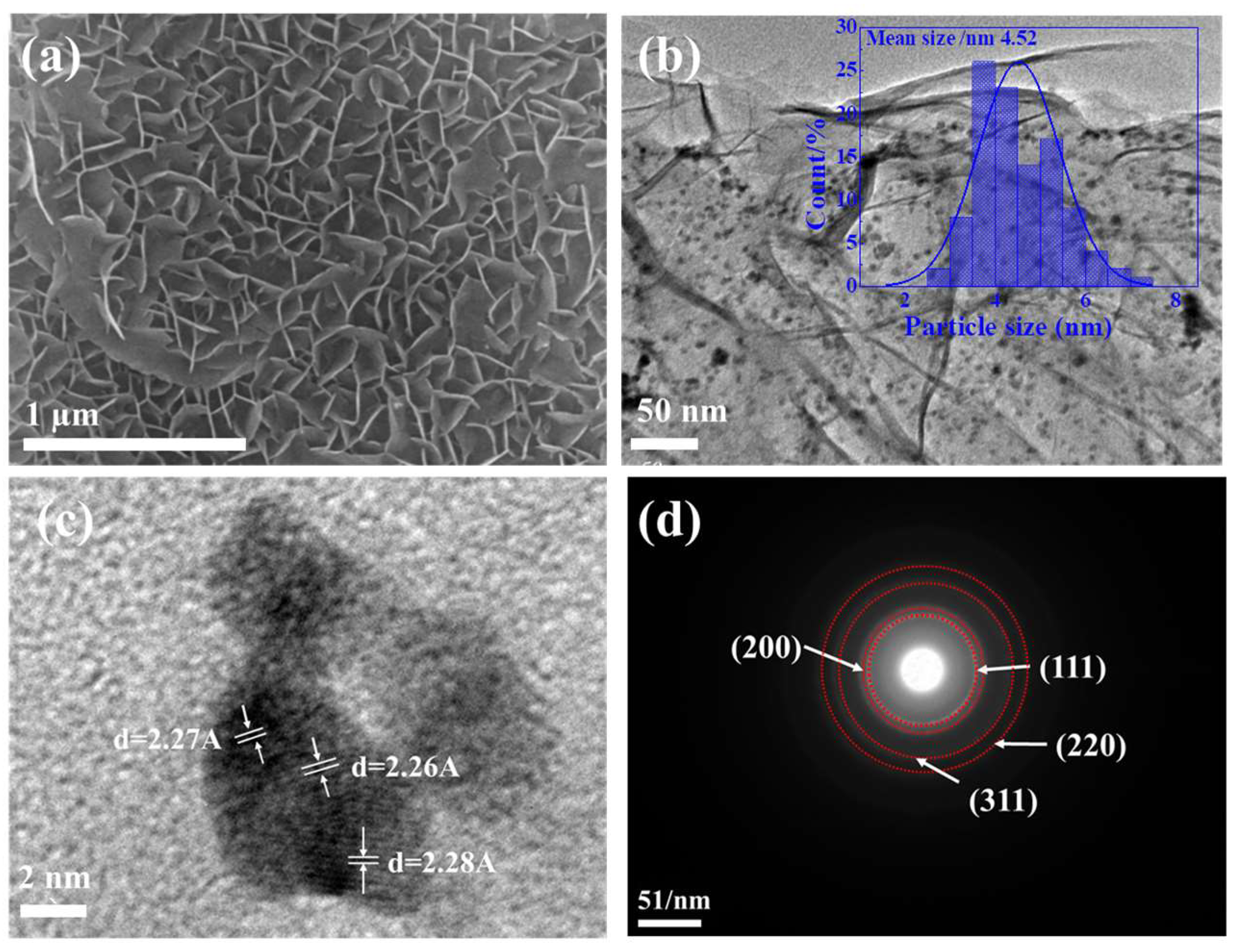
2.1.4. SEM-EDS and TEM Analysis
2.1.5. Support Basicity
2.2. Catalytic Screening Experiments
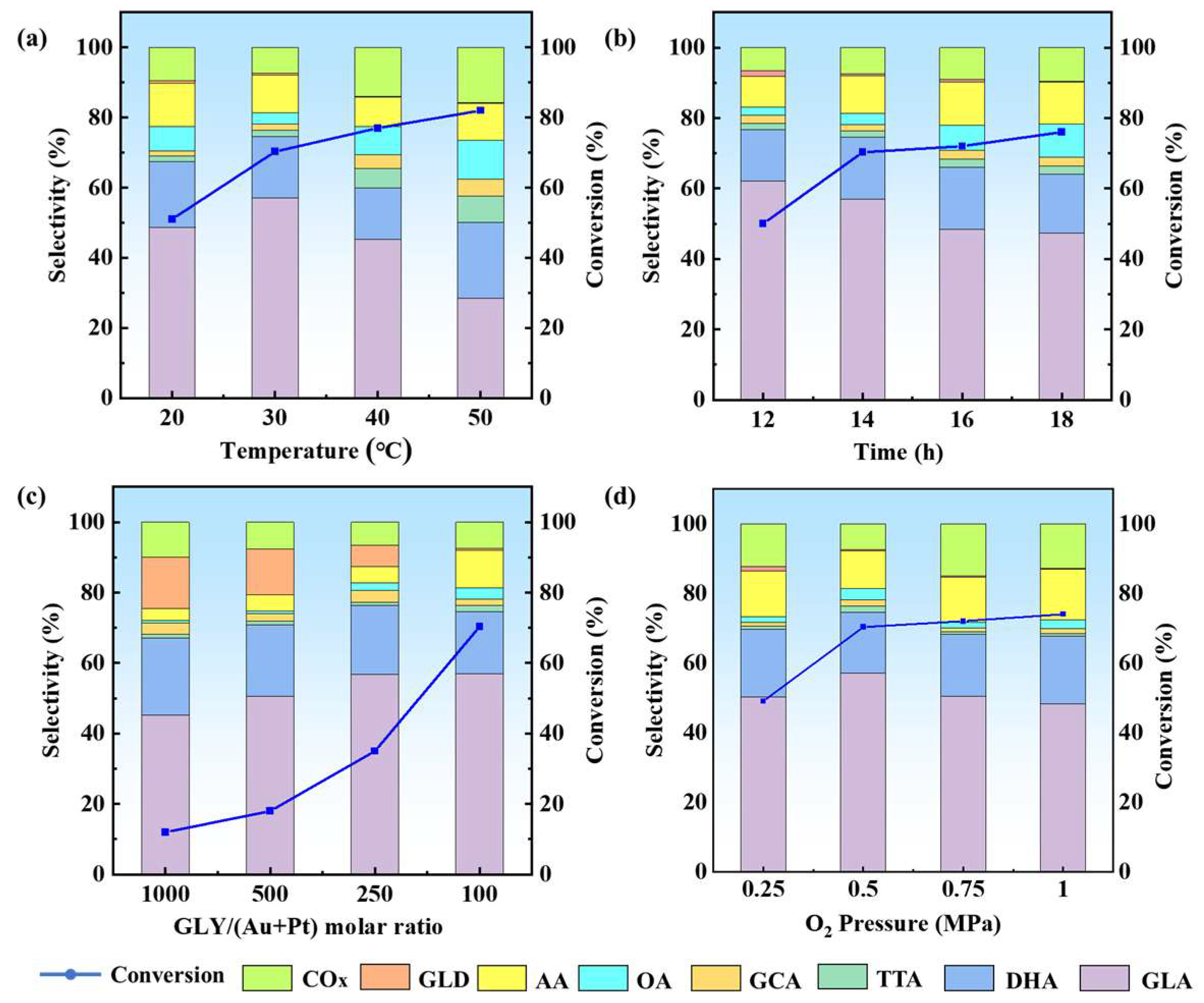
2.3. Optimization of Reaction Conditions
2.4. Response Surface Analysis Experiment
2.4.1. RSM Experiments and Predicted Model
2.4.2. Response Surface Analysis
2.4.3. Verification of Response Surface Model
2.5. Kinetic Study
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalysis and Analysis Procedures
3.5. Experimental Design and Mathematical Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ravikumar, Y.; Razack, S.A.; Ponpandian, L.N.; Zhang, G.; Yun, J.; Huang, J.; Lee, D.; Li, X.; Dou, Y.; Qi, X. Microbial hosts for production of D-arabitol: Current state-of-art and future prospects. Trends Food Sci. Technol. 2022, 120, 100–110. [Google Scholar] [CrossRef]
- Fokum, E.; Zabed, H.M.; Ravikumar, Y.; Elshobary, M.E.; Chandankere, R.; Zhang, Y.; Yun, J.; Qi, X. Co-fermentation of glycerol and sugars by Clostridium beijerinckii: Enhancing the biosynthesis of 1,3-propanediol. Food Biosci. 2021, 41, 101028. [Google Scholar] [CrossRef]
- Xiong, Z.; Liu, J.; Tian, Y.; Wang, Z.; Wang, X.; Shi, T.; Jin, W.; Yuan, L.; Gao, R. Structural and aggregation changes of silver carp myosin induced with alcohols: Effects of ethanol, 1,2-propanediol, and glycerol. Food Chem. 2024, 452, 139542. [Google Scholar] [CrossRef]
- Li, L.; Zhou, Y.; Huang, C.; Jian, L.; Lin, Z.; Lin, L.; Li, C.; Ye, Y. Insight into the influence of plant oils on the composition of diacylglycerol fabricated by glycerolysis and esterification. Ind. Crops Prod. 2023, 204, 117324. [Google Scholar] [CrossRef]
- Boasiako, T.A.; Hua, F.; YuQing, X.; Boateng, I.D.; Ma, Y. Enzymatic catalytic dynamics of lactic-acetic acid co-fermentation: Effect of cellulase on the physicochemical, phytochemicals, volatiles, and antioxidant activity of jujube puree extracts. Ind. Crops Prod. 2024, 222, 119590. [Google Scholar] [CrossRef]
- Katryniok, B.; Kimura, H.; Skrzyńska, E.; Girardon, J.-S.; Fongarland, P.; Capron, M.; Ducoulombier, R.; Mimura, N.; Paul, S.; Dumeignil, F. Selective catalytic oxidation of glycerol: Perspectives for high value chemicals. Green Chem. 2011, 13, 1960–1979. [Google Scholar] [CrossRef]
- Behr, A.; Eilting, J.; Irawadi, K.; Leschinski, J.; Lindner, F. Improved utilisation of renewable resources: New important derivatives of glycerol. Green Chem. 2008, 10, 13–30. [Google Scholar] [CrossRef]
- Behr, A.; Irawadi, K.A. Glycerin-Oxidation mit magnetisch abtrennbaren Nanokatalysatoren. Chem. Ing. Tech. 2015, 87, 1726–1732. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, Y.; Shi, J.; Ning, W.; Hou, Z. Selective glycerol oxidation using platinum nanoparticles supported on multi-walled carbon nanotubes and nitrogen-doped graphene hybrid. Chin. J. Catal. 2017, 38, 537–544. [Google Scholar] [CrossRef]
- Rodriguez, A.A.; Williams, C.T.; Monnier, J.R. Selective liquid-phase oxidation of glycerol over Au–Pd/C bimetallic catalysts prepared by electroless deposition. Appl. Catal. A Gen. 2014, 475, 161–168. [Google Scholar] [CrossRef]
- Olmos, C.M.; Chinchilla, L.E.; Rodrigues, E.G.; Delgado, J.J.; Hungría, A.B.; Blanco, G.; Pereira, M.F.R.; Órfão, J.J.M.; Calvino, J.J.; Chen, X. Synergistic effect of bimetallic Au-Pd supported on ceria-zirconia mixed oxide catalysts for selective oxidation of glycerol. Appl. Catal. B Environ. 2016, 197, 222–235. [Google Scholar] [CrossRef]
- Hamid, S.B.A.; Basiron, N.; Yehye, W.A.; Sudarsanam, P.; Bhargava, S.K. Nanoscale Pd-based catalysts for selective oxidation of glycerol with molecular oxygen: Structure–activity correlations. Polyhedron 2016, 120, 124–133. [Google Scholar] [CrossRef]
- Sadalage, P.S.; Dar, M.A.; Bhor, R.D.; Bhalerao, B.M.; Kamble, P.N.; Paiva-Santos, A.C.; Nimbalkar, M.S.; Sonawane, K.D.; Pai, K.; Patil, P.S.; et al. Optimization of biogenic synthesis of biocompatible platinum nanoparticles with catalytic, enzyme mimetic and antioxidant activities. Food Biosci. 2022, 50, 102024. [Google Scholar] [CrossRef]
- El Roz, A.; Fongarland, P.; Dumeignil, F.; Capron, M. Glycerol to Glyceraldehyde Oxidation Reaction Over Pt-Based Catalysts Under Base-Free Conditions. Front. Chem. 2019, 7, 156. [Google Scholar] [CrossRef]
- Skrzyńska, E.; El Roz, A.; Paul, S.; Capron, M.; Dumeignil, F. Glycerol Partial Oxidation over Pt/Al2O3 Catalysts Under Basic and Base-Free Conditions—Effect of the Particle Size. J. Am. Oil Chem. Soc. 2018, 96, 63–74. [Google Scholar] [CrossRef]
- Ning, X.; Li, Y.; Yu, H.; Peng, F.; Wang, H.; Yang, Y. Promoting role of bismuth and antimony on Pt catalysts for the selective oxidation of glycerol to dihydroxyacetone. J. Catal. 2016, 335, 95–104. [Google Scholar] [CrossRef]
- Xu, C.; Du, Y.; Li, C.; Yang, J.; Yang, G. Insight into effect of acid/base nature of supports on selectivity of glycerol oxidation over supported Au-Pt bimetallic catalysts. Appl. Catal. B Environ. 2015, 164, 334–343. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, W.; Zeng, S.; Su, D.; Meng, X.; Xiao, F. Mg-Al Mixed Oxides Supported Bimetallic Au-Pd Nanoparticles with Superior Catalytic Properties in Aerobic Oxidation of Benzyl Alcohol and Glycerol. Chin. J. Chem. 2012, 30, 2189–2197. [Google Scholar] [CrossRef]
- Shen, Y.; Li, Y.; Liu, H. Base-free aerobic oxidation of glycerol on TiO2-supported bimetallic Au–Pt catalysts. J. Energy Chem. 2015, 24, 669–673. [Google Scholar] [CrossRef]
- Villa, A.; Veith, G.M.; Prati, L. Selective Oxidation of Glycerol Under Acidic Conditions Using Gold Catalysts. Angew. Chem. Int. Ed. 2010, 49, 4499–4502. [Google Scholar] [CrossRef]
- Cherni, D.; Moussa, N.; Nsib, M.F.; Evangelisti, C.; Prati, L.; Villa, A. Base-free glycerol oxidation over N-TiO2 supported Au–Pt catalysts. React. Kinet. Mech. Catal. 2019, 128, 979–990. [Google Scholar] [CrossRef]
- Ke, Y.-H.; Yu, X.-M.; Wang, X.; Liu, H.; Yuan, H. Mofs-derived transition metal carbon-based compounds supported Au-Pt catalyst for the catalytic oxidation of glycerol to glyceric acid. Appl. Surf. Sci. 2025, 682, 161674. [Google Scholar] [CrossRef]
- Li, X.; Feng, B.; Zhang, H.; Liu, Y.; Xiao, M.; Huang, T.; Zhu, Q.; Song, H. Gold nanoparticle-catalyzed oxidative esterification of furfural: Enhancement by NaOH-etched γ-Al2O3 support. Fuel 2025, 380, 133140. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, C.; Zhou, C.; Rehman, A.U.; Pan, Z.; Adhikari, B.; Chen, L.; Ma, H.; Wang, Y.; Zhu, Z.; et al. Flame-catalytic infrared dry system for tomato continuous peeling. Food Bioprod. Process. 2024, 147, 124–139. [Google Scholar] [CrossRef]
- Alshammari, H.; Alhumaimess, M.; Alotaibi, M.H.; Alshammar, A.S. Catalytic activity of bimetallic AuPd alloys supported MgO and MnO 2 nanostructures and their role in selective aerobic oxidation of alcohols. J. King Saud Univ. Sci. 2017, 29, 561–566. [Google Scholar] [CrossRef]
- Flynn, P.C.; Wanke, S.E. A model of supported metal catalyst sintering: II. Application of model. J. Catal. 1974, 34, 400–410. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Basu, P.; Bhanja, P.; Salam, N.; Dey, T.K.; Bhaumik, A.; Das, D.; Islam, S.M. Silver nanoparticles supported over Al2O3@Fe2O3 core-shell nanoparticles as an efficient catalyst for one-pot synthesis of 1,2,3-triazoles and acylation of benzyl alcohol. Mol. Catal. 2017, 439, 31–40. [Google Scholar] [CrossRef]
- Ou, T.-C.; Chang, F.-W.; Roselin, L.S. Production of hydrogen via partial oxidation of methanol over bimetallic Au–Cu/TiO2 catalysts. J. Mol. Catal. A Chem. 2008, 293, 8–16. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, P.; Liu, Y.; Pan, J.; Li, D.; Wang, B.; Feng, J. Support morphology effect on the selective oxidation of glycerol over AuPt/CeO2 catalysts. J. Catal. 2020, 385, 146–159. [Google Scholar] [CrossRef]
- Yan, H.; Zhao, M.; Zhou, X.; Zhao, S.; Li, S.; Zha, M.; Meng, F.; Feng, X.; Chen, X.; Liu, Y.; et al. Switchable Structure Promoted Leaching Free Atomically Dispersed Pt Catalyst for Low Carbon Biomass Polyol Oxidation. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Weng, X.; Liu, Y.; Wang, K.-K.; Feng, J.-J.; Yuan, J.; Wang, A.-J.; Xu, Q.-Q. Single-step aqueous synthesis of AuPt alloy nanodendrites with superior electrocatalytic activity for oxygen reduction and hydrogen evolution reaction. Int. J. Hydrogen Energy 2016, 41, 18193–18202. [Google Scholar] [CrossRef]
- Vazquez, P.; Pizzio, L.; Caceres, C.; Blanco, M.; Thomas, H.; Alesso, E.; Finkielsztein, L.; Lantano, B.; Moltrasio, G.; Aguirre, J. Silica-supported heteropolyacids as catalysts in alcohol dehydration reactions.pdf. J. Mol. Catal. A 2000, 161, 223–232. [Google Scholar] [CrossRef]
- Carrettin, S.; McMorn, P.; Johnston, P.; Griffin, K.; Kiely, C.J.; Hutchings, G.J. Oxidation of glycerol using supported Pt, Pd and Au catalysts. Phys. Chem. Chem. Phys. 2003, 5, 1329–1336. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Xu, M.; Yang, Y.; Li, Y.; Liu, N.; Meng, X.; Chen, L.; Shi, S.; Wei, M. Glycerol aerobic oxidation to glyceric acid over Pt/hydrotalcite catalysts at room temperature. Sci. Bull. 2019, 64, 1764–1772. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, D.; Luo, J.; Pu, Y.; Li, F.; Xiao, F.; Zhao, N. The effects of calcination temperature of support on Au/CuO-ZrO2 catalysts for oxidation of glycerol to dihydroxyacetone. J. Colloid Interface Sci. 2020, 560, 130–137. [Google Scholar] [CrossRef]
- Du, C.X.; Han, D.L.; Song, Z.Q.; Chen, Y.C.; Chen, X.G.; Wang, X.Z. Calibration of contact parameters for complex shaped fruits based on discrete element method: The case of pod pepper (Capsicum annuum). Biosyst. Eng. 2023, 226, 43–54. [Google Scholar] [CrossRef]
- Chen, Z.L.; Ma, J.; Li, P.; Wen, B.; Wang, Y.; Ma, Y.H.; Huang, W.Y. Preparation of hypoglycemic anthocyanins from mulberry (Fructus mori) fruits by ultrahigh pressure extraction. Innov. Food Sci. Emerg. Technol. 2023, 84, 103255. [Google Scholar] [CrossRef]
- Betchem, G.; Dabbour, M.; Tuly, J.A.; Billong, L.F.; Ma, H.L. Optimization of fermentation conditions to improve the functional and structural characteristics of rapeseed meal with a mutant Bacillus subtilis species. Ind. Crops Prod. 2023, 205, 117424. [Google Scholar] [CrossRef]
- Johnson, N.A.N.; Ekumah, J.N.; Ma, Y.K.; Akpabli-Tsigbe, N.D.K.; Adade, S.; Manching, X.; Quaisie, J.; Kwaw, E.; Wang, C.C. Optimization of fermentation parameters for the production of a novel selenium enriched mulberry (Morus nigra) wine. LWT-Food Sci. Technol. 2023, 178, 114608. [Google Scholar] [CrossRef]
- Guo, X.; Yang, D.; Zuo, C.; Peng, Z.; Li, C.; Zhang, S. Catalysts, Process Optimization, and Kinetics for the Production of Methyl Acrylate over Vanadium Phosphorus Oxide Catalysts. Ind. Eng. Chem. Res. 2017, 56, 5860–5871. [Google Scholar] [CrossRef]
- Yang, Z.K.; Li, M.R.; Li, Y.X.; Li, Z.H.; Huang, X.W.; Wang, X.; Shi, J.Y.; Zou, X.B.; Zhai, X.D.; Povey, M.; et al. Improving properties of Litsea cubeba oil Pickering emulsion-loaded gelatin-based bio-nanocomposite film via optimizing blending ratio: Application for mango preservation. Food Hydrocoll. 2023, 145, 109052. [Google Scholar] [CrossRef]
- Akpabli-Tsigbe, N.D.K.; Ma, Y.K.; Ekumah, J.N.; Osabutey, J.; Hu, J.; Xu, M.Q.; Johnson, N.A.N.; Quaisie, J. Two-step optimization of solid-state fermentation conditions of heilong48 soybean variety for maximum chlorogenic acid extraction yield with improved antioxidant activity. Ind. Crops Prod. 2021, 168, 113565. [Google Scholar] [CrossRef]
- El-Mesery, H.S.; Adelusi, O.A.; Ghashi, S.; Njobeh, P.B.; Hu, Z.C.; Kun, W. Effects of storage conditions and packaging materials on the postharvest quality of fresh Chinese tomatoes and the optimization of the tomatoes’ physiochemical properties using machine learning techniques. LWT-Food Sci. Technol. 2024, 201, 116280. [Google Scholar] [CrossRef]
- Tang, S.X.; Xia, Z.L.; Gu, J.N.; Wang, W.B.; Huang, Z.D.; Zhang, W.H. High-precision apple recognition and localization method based on RGB-D and improved SOLOv2 instance segmentation. Front. Sustain. Food Syst. 2024, 8, 1403872. [Google Scholar] [CrossRef]
- Motta, D.; Trujillo, F.J.S.; Dimitratos, N.; Villa, A.; Prati, L. An investigation on AuPt and AuPt-Bi on granular carbon as catalysts for the oxidation of glycerol under continuous flow conditions. Catal. Today 2018, 308, 50–57. [Google Scholar] [CrossRef]
- Evans, C.D.; Kondrat, S.A.; Smith, P.J.; Manning, T.D.; Miedziak, P.J.; Brett, G.L.; Armstrong, R.D.; Bartley, J.K.; Taylor, S.H.; Rosseinsky, M.J.; et al. The preparation of large surface area lanthanum based perovskite supports for AuPt nanoparticles: Tuning the glycerol oxidation reaction pathway by switching the perovskite B site. Faraday Discuss. 2016, 188, 427–450. [Google Scholar] [CrossRef]
- Yang, P.; Pan, J.; Liu, Y.; Zhang, X.; Feng, J.; Hong, S.; Li, D. Insight into the Role of Unsaturated Coordination O2c-Ti5c-O2c Sites on Selective Glycerol Oxidation over AuPt/TiO2 Catalysts. ACS Catal. 2018, 9, 188–199. [Google Scholar] [CrossRef]

| Binding Energy (eV) | Ratio a | Au Loading b | Pt Loading b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Au0 | Auδ+ | Pt0 | Pt2+ | |||||||||
| 4f7/2 | 4f5/2 | 4f7/2 | 4f5/2 | 4f7/2 | 4f5/2 | 4f7/2 | 4f5/2 | |||||
| Fresh Au1.5Pt1.5/MgO-Al2O3 | 83.6 | 87.1 | 85.4 | 88.9 | 71.1 | 74.5 | 72.8 | 76.2 | 0.84 | 0.87 | 1.37 | 1.41 |
| Spent Au1.5Pt1.5/MgO-Al2O3 | 83.3 | 86.8 | 85.1 | 88.6 | 71.4 | 74.8 | 73.1 | 76.5 | 0.81 | 0.86 | 1.35 | 1.38 |
| Catalysts | GLY Conversion (%) | Product Selectivity (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| GLA | DHA | GLD | TTA | GCA | OA | AA | COx | ||
| Au3/MgO-Al2O3 | 14.0 ± 1.3 | 15.1 ± 1.7 | 56.5 ± 2.7 | 0.1 ± 0.01 | 0.4 ± 0.01 | 6.2 ± 1.0 | 2.3 ± 0.1 | 11.1 ± 1.4 | 8.3 ± 1.1 |
| Pt3/MgO-Al2O3 | 43.0 ± 1.0 | 55.1 ± 1.4 | 11.6 ± 1.5 | 0.1 ± 0.03 | 1.6 ± 0.07 | 1.5 ± 0.2 | 2.2 ± 1.0 | 15.8 ± 1.7 | 12.1 ± 1.0 |
| Au1Pt2/MgO-Al2O3 | 46.0 ± 1.2 | 50.1 ± 1.6 | 11.2 ± 1.8 | 0.1 ± 0.01 | 2.3 ± 0.3 | 1.2 ± 0.01 | 5.7 ± 1.0 | 19.8 ± 1.5 | 9.6 ± 1.0 |
| Au1.5Pt1.5/MgO-Al2O3 | 50.0 ± 2.0 | 61.8 ± 2.3 | 19.0 ± 2.7 | 0.2 ± 0.02 | 1.0 ± 0.05 | 1.4 ± 0.04 | 1.5 ± 0.2 | 7.7 ± 1.2 | 7.4 ± 1.1 |
| Au2Pt1/MgO-Al2O3 | 34.0 ± 2.2 | 40.6 ± 1.5 | 31.9 ± 1.6 | 0.2 ± 0.03 | 1.9 ± 0.1 | 1.4 ± 0.05 | 4.5 ± 1.0 | 8.5 ± 1.0 | 11.0 ± 2.0 |
| Au1.5Pt1.5/ZnO-Al2O3 | 14.2 ± 1.3 | 48.7 ± 1.1 | 28.3 ± 1.9 | 0.1 ± 0.01 | 0.8 ± 0.04 | 6.2 ± 0.9 | 5.1 ± 1.20 | 0.5 ± 0.01 | 10.3 ± 1.0 |
| 0Au1.5Pt1.5/MgO-Al2O3 | 37.0 ± 1.9 | 33.0 ± 1.6 | 29.3 ± 2.1 | 0.2 ± 0.01 | 2.6 ± 0.3 | 2.1 ± 0.1 | 6.8 ± 1.1 | 20.0 ± 2.0 | 6.0 ± 0.8 |
| MgO-Al2O3 | 3.9 ± 1.5 | 36.6 ± 1.9 | 19.8 ± 1.7 | 0.2 ± 0.03 | 0.4 ± 0.01 | 11.6 ± 1.1 | 1.0 ± 0.1 | 13.5 ± 1.2 | 16.9 ± 1.7 |
| Reaction conditions: 30 mL 0.1 M GLY, 30 °C, reaction time = 8 h, GLY/(AuxPty) = 100 mol/mol, = 0.5 MPa, stirring speed = 500 rpm. | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Jin, J.; Jin, A.; Li, S.; Chen, X.; Hu, T.; Shen, L.; Yin, H. Synthesis of Glyceric Acid by Mixed-Metal Oxide-Supported AuPt Alloy Catalyst in Mild Conditions. Catalysts 2025, 15, 963. https://doi.org/10.3390/catal15100963
Wang Z, Jin J, Jin A, Li S, Chen X, Hu T, Shen L, Yin H. Synthesis of Glyceric Acid by Mixed-Metal Oxide-Supported AuPt Alloy Catalyst in Mild Conditions. Catalysts. 2025; 15(10):963. https://doi.org/10.3390/catal15100963
Chicago/Turabian StyleWang, Zhiqing, Jianchuan Jin, Aiqian Jin, Shiyu Li, Xinyue Chen, Tongjie Hu, Lingqin Shen, and Hengbo Yin. 2025. "Synthesis of Glyceric Acid by Mixed-Metal Oxide-Supported AuPt Alloy Catalyst in Mild Conditions" Catalysts 15, no. 10: 963. https://doi.org/10.3390/catal15100963
APA StyleWang, Z., Jin, J., Jin, A., Li, S., Chen, X., Hu, T., Shen, L., & Yin, H. (2025). Synthesis of Glyceric Acid by Mixed-Metal Oxide-Supported AuPt Alloy Catalyst in Mild Conditions. Catalysts, 15(10), 963. https://doi.org/10.3390/catal15100963







