Banana (Musa sapientum) Waste-Derived Biochar–Magnetite Magnetic Composites for Acetaminophen Removal via Photochemical Fenton Oxidation
Abstract
1. Introduction
2. Results and Discussion
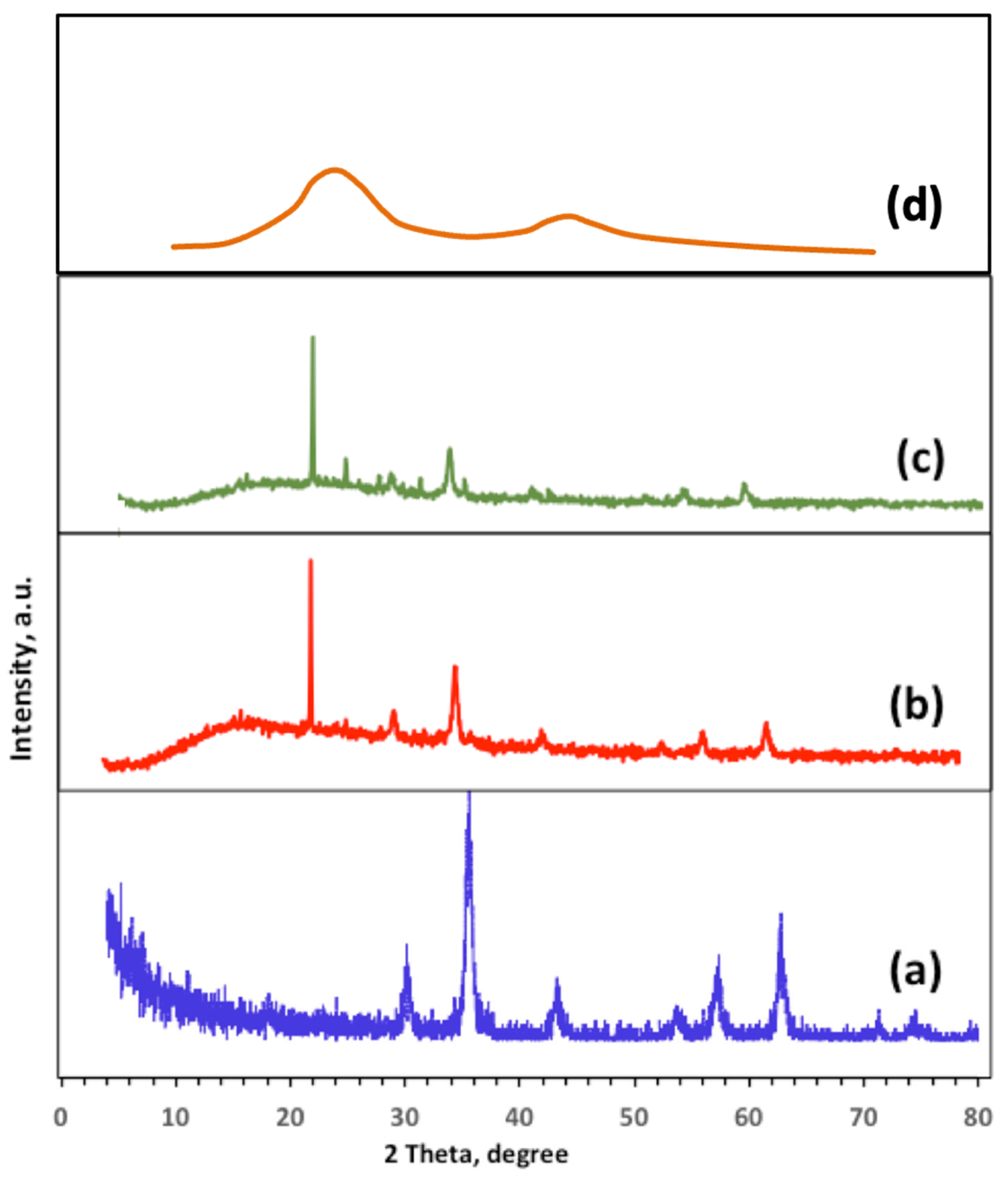
2.1. Structural and Morphological Characterization of Ban-Char500 Samples
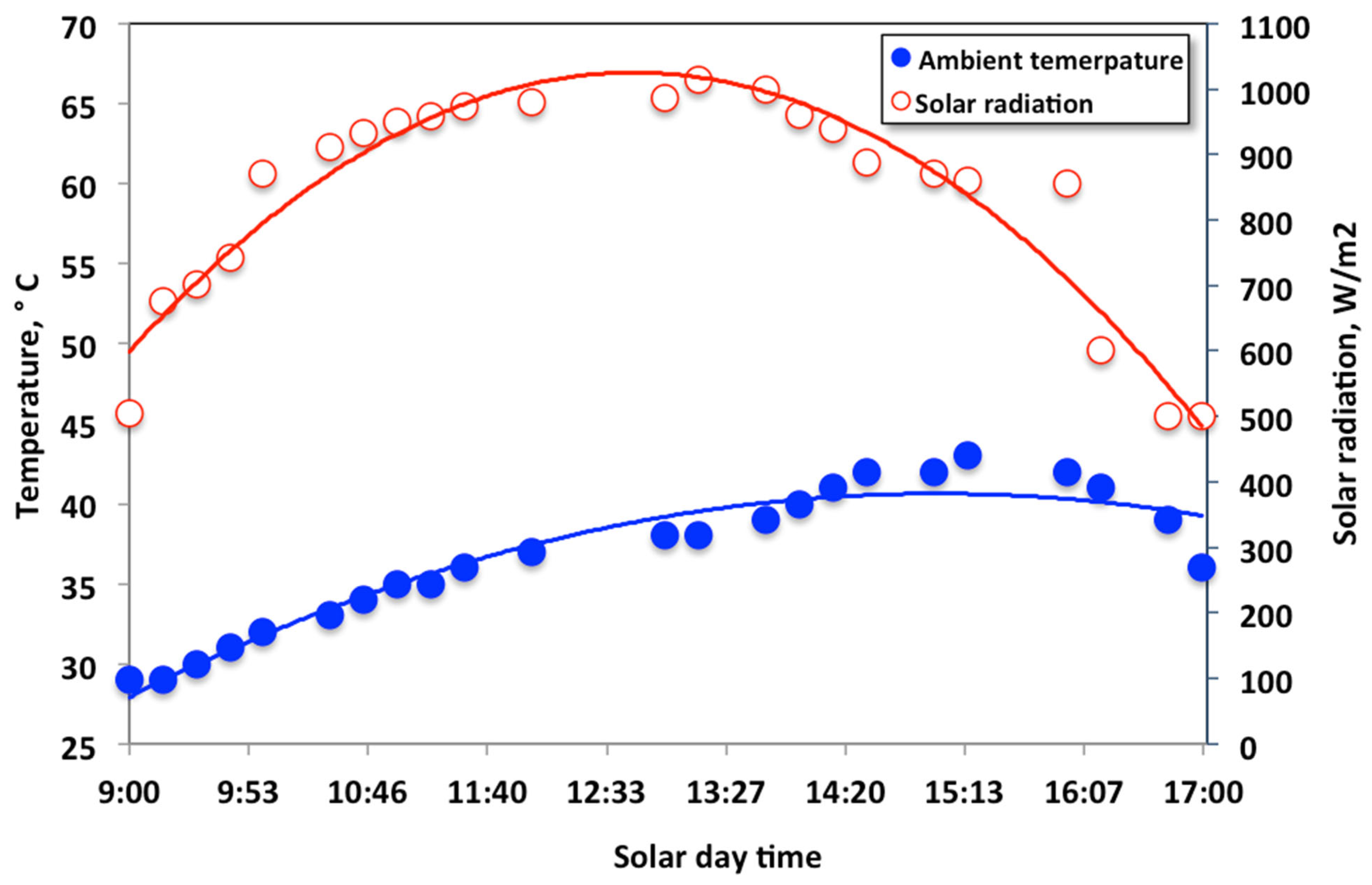
2.2. Solar Radiation and Collector Analysis
2.3. Solar-Fenton Oxidation
2.3.1. Comparison of Illumination Time for Various Oxidation and Adsorption Systems
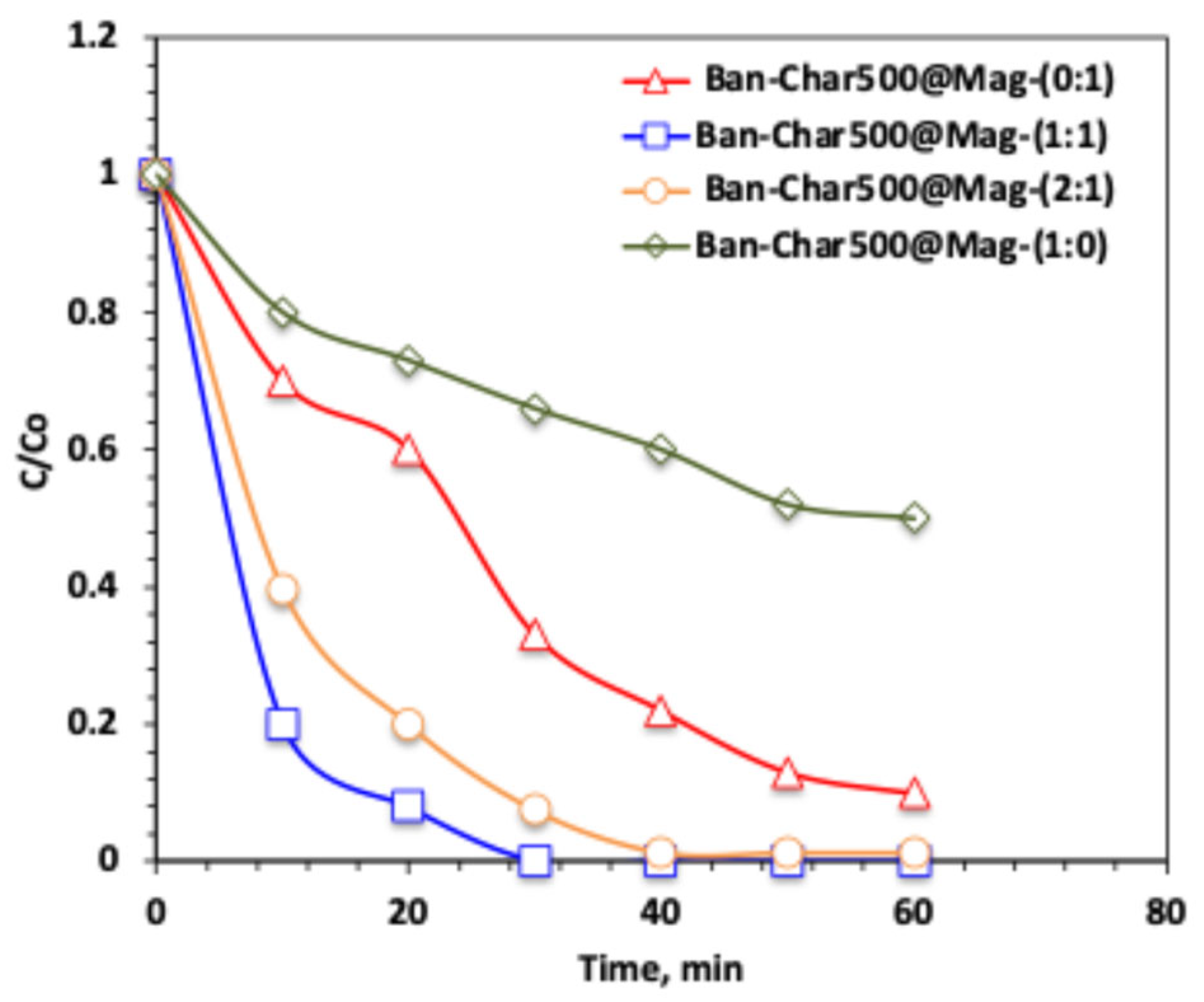
2.3.2. Effect of Char500@Mag Composite Type
2.3.3. Effect of Acetaminophen Loading
2.3.4. Catalyst Effect on Modified Solar-Fenton System
2.3.5. H2O2 Effect on Modified Solar-Fenton System
2.3.6. pH Effect on Modified Solar-Fenton System
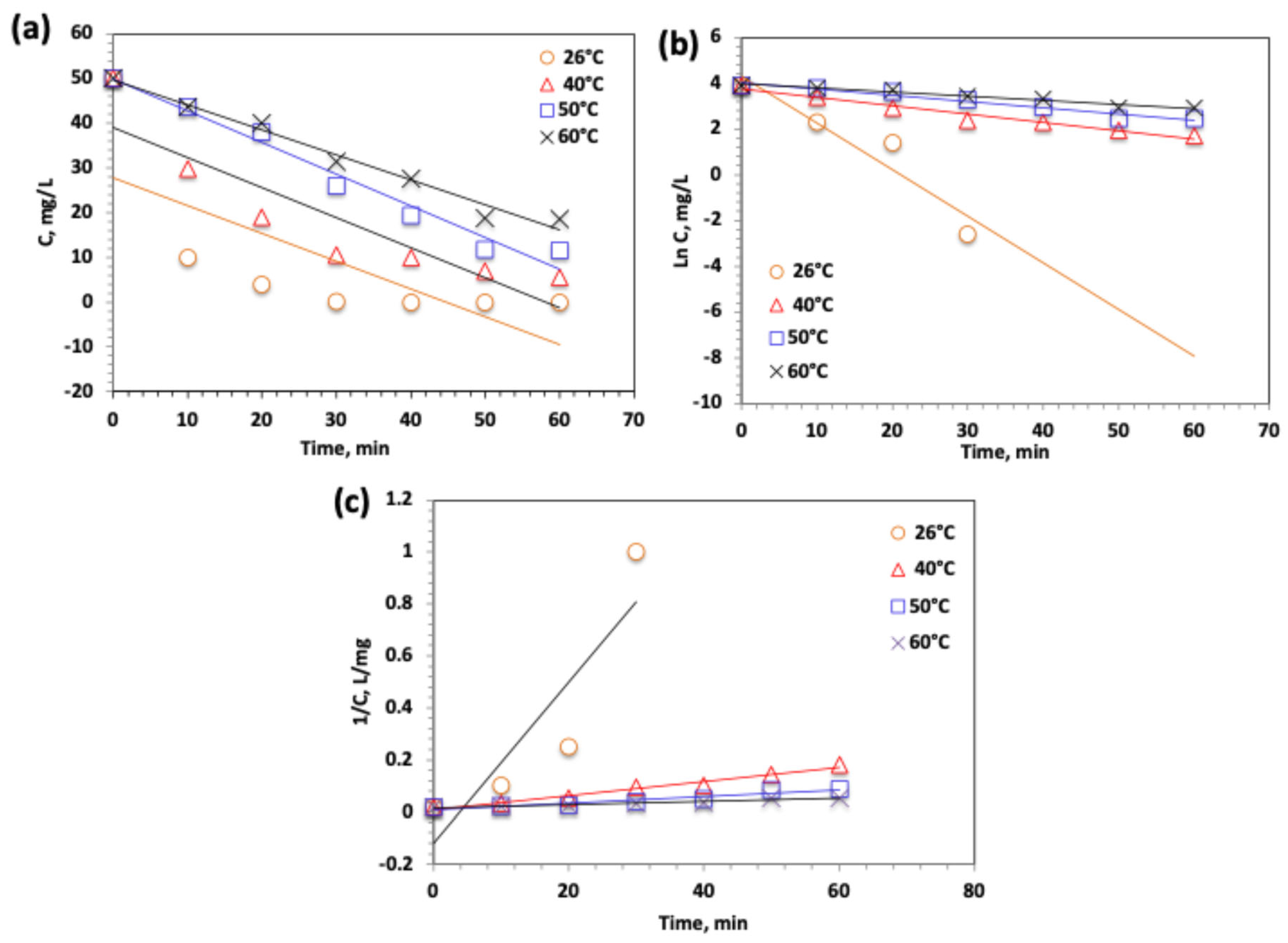
2.3.7. Temperature Effect on Modified Solar-Fenton System
2.3.8. Oxidation Kinetics
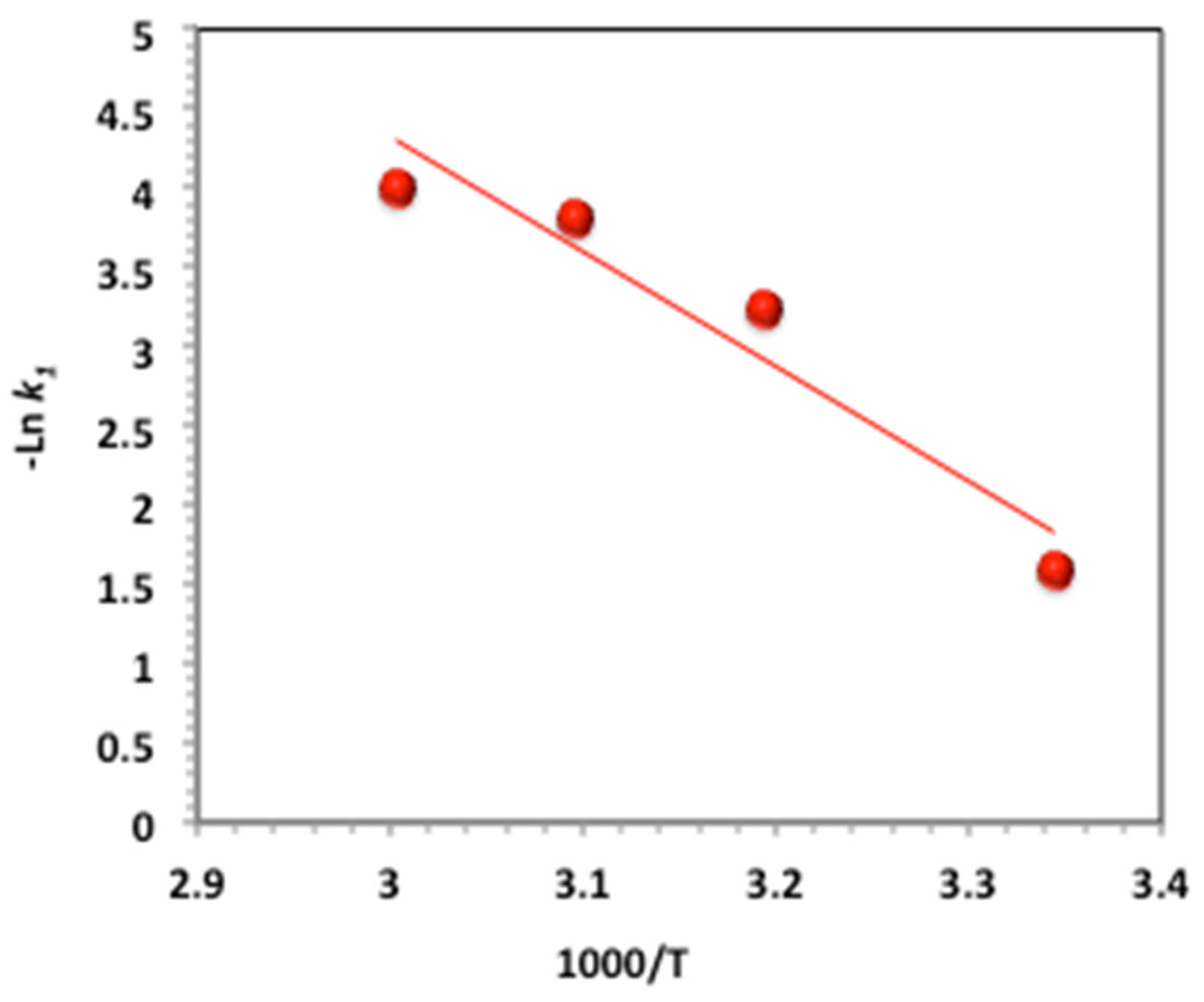
2.3.9. Activation Parameter Determination
2.3.10. Stability and Sustainability Assays
2.3.11. Study Compared with the Literature
3. Materials and Methods
3.1. Materials
3.2. Magnetic/Biochar from Musa Sapientum
3.3. Experimental Methodology and Analytical Determination
3.4. Ban-Char500-Mag Catalyst Characterizations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Basheer, A.A. Chemical chiral pollution: Impact on the society and science and need of the regulations in the 21st century. Chirality 2018, 30, 402–406. [Google Scholar] [CrossRef]
- Bashir, A.; Malik, L.A.; Ahad, S.; Manzoor, T.; Bhat, M.A.; Dar, G.N.; Pandith, A.H. Removal of heavy metal ions from aqueous system by ion–exchange and biosorption methods. Environ. Chem. Lett. 2019, 17, 729–754. [Google Scholar] [CrossRef]
- Bosio, G.N.; García Einschlag, F.S.; Carlos, L.; Mártire, D.O. Recent Advances in the Development of Novel Iron–Copper Bimetallic Photo Fenton Catalysts. Catalysts 2023, 13, 159. [Google Scholar] [CrossRef]
- Lai, Y.J.; Lee, D.J. Solid Mediator Z–Scheme Heterojunction Photocatalysis for Pollutant Oxidation in Water: Principles and Synthesis Perspectives. J. Taiwan Inst. Chem. Eng. 2021, 125, 88–114. [Google Scholar] [CrossRef]
- Zolfaghari, G.; Esmaili-Sari, A.; Anbia, M.; Younesi, H.; Ghasemian, M.B. A zinc oxide-coated nanoporous carbon adsorbent for lead removal from water: Optimization, equilibrium modeling, and kinetics studies. Int. J. Environ. Sci. Technol. 2013, 10, 325–340. [Google Scholar] [CrossRef][Green Version]
- Beni, A.A.; Esmaeili, A. Biosorption, an efficient method for removing heavy metals from industrial effluents: A review. Environ. Technol. Innov. 2020, 17, 100503. [Google Scholar] [CrossRef]
- Muthusamy, S.; Charles, J. Metal–organic framework of nanostructured polypyrrole incorporated with TiO2 nanoparticles for supercapacitor electrode. J. Mater. Sci. Mater. Electron. 2021, 32, 7349–7365. [Google Scholar] [CrossRef]
- Ribeiro, E.; Plantard, G.; Teyssandier, F.; Maury, F.; Sadiki, N.; Chaumont, D.; Goetz, V. Activated-carbon/TiO2 composites preparation: An original grafting by milling approach for solar water treatment applications. J. Environ. Chem. Eng. 2020, 8, 104115. [Google Scholar] [CrossRef]
- Muthukannan, V.; Praveen, K.; Natesan, B. Fabrication and characterization of magnetite/reduced graphene oxide composite incurred from iron ore tailings for high performance application. Mater. Chem. Phys. 2015, 162, 400–407. [Google Scholar] [CrossRef]
- Zhang, M.H.; Dong, H.; Zhao, L.; Wang, D.-X.; Meng, D. A Review on Fenton Process for OrganicWastewater Treatment Based on Optimization Perspective. Sci. Total. Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef]
- Abdollahzadeh, H.; Fazlzadeh, M.; Afshin, S.; Arfaeinia, H.; Feizizadeh, A.; Poureshgh, Y.; Rashtbari, Y. Efficiency of activated carbon prepared from scrap tires magnetized by Fe3O4 nanoparticles: Characterisation and its application for removal of reactive blue19 from aquatic solutions. Int. J. Environ. Anal. Chem. 2020, 102, 1911–1925. [Google Scholar] [CrossRef]
- Tony, M.A.; Ali, I.A. Mechanistic implications of redox cycles solar reactions of recyclable layered double hydroxides nanoparticles for remazol brilliant abatement. Int. J. Environ. Sci. Technol. 2021, 19, 9843–9860. [Google Scholar] [CrossRef]
- Channei, D.; Thammaacheep, P.; Jannoey, P. Utilizing banana peel in conjunction with TiO2 photocatalyst for the efficient decolorization of malachite green. Chem. Phys. Impact 2024, 8, 100629. [Google Scholar] [CrossRef]
- Morales, A.E.; Mora, E.S.; Pal, U. Use of diffuse reflectance spectroscopy for optical characterization of un-supported nanostructures. Rev. Mex. Física 2007, 53, 18–22. [Google Scholar]
- Maroudas, A.; Pandis, P.K.; Chatzopoulou, A.; Davellas, L.-R.; Sourkouni, G.; Argirusis, C. Synergetic decolorization of azo dyes using ultrasounds, photocatalysis and photo-fenton reaction. Ultrason. Sonochemistry 2021, 71, 105367. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Yang, Y. Extending the use of dewatered alum sludge as a P-trapping material in effluent purification: Study on two separate water treatment sludges. J. Environ. Sci. Health Part A 2010, 45, 1234–1239. [Google Scholar] [CrossRef]
- Tony, M.A.; Lin, L.-S. Iron Coated-Sand from Acid Mine Drainage Waste for Being a Catalytic Oxidant Towards Municipal Wastewater Remediation. Int. J. Environ. Res. 2021, 15, 191–201. [Google Scholar] [CrossRef]
- Wu, H.F.; Wang, J.P.; Duan, E.G.; Feng, Y.F.; Wan, Z.Y.; Wu, Y.X.; Lu, Y.Q. Study on the preparation of granular alum sludge adsorbent for phosphorus removal. Water Sci. Technol. 2019, 79, 2378–2386. [Google Scholar] [CrossRef]
- Abdou, K.A.; Mohammed, A.N.; Moselhy, W.; Farghali, A.A. Assessment of modified rice husk and sawdust as bio-adsorbent for heavy metals removal using nano particles in fish farm. Asian J. Anim. Vet. Adv. 2018, 13, 180–188. [Google Scholar] [CrossRef]
- Foroughi, M.; Chavoshi, S.; Bagheri, M.; Yetilmezsoy, K.; Samadi, M.T. Alum-based sludge (AbS) recycling for turbidity removal in drinking water treatment: An insight into statistical, technical, and health-related standpoints. J. Mater. Cycles Waste Manag. 2018, 20, 1999–2017. [Google Scholar] [CrossRef]
- Yin, Z.; Li, Y.; Song, T.; Bao, M.; Li, Y.; Lu, J.; Li, Y. Preparation of superhydrophobic magnetic sawdust for effective oil/water separation. J. Clean. Prod. 2020, 253, 120058. [Google Scholar] [CrossRef]
- Tony, M.A. Paradigms of homo/heterogonous Fenton systems incorporating ‘Solar Energy’ based on ‘Emerging Pollutants’ removal–challenges, advancements and visualized bibliometric analysis. Int. J. Environ. Anal. Chem. 2021, 103, 7877–7908. [Google Scholar] [CrossRef]
- Nabwey, H.A.; Tony, M.A. Thermal energy storage using a hybrid composite based on technical-grade paraffin-AP25 wax as a phase change material. Nanomaterials 2023, 13, 2635. [Google Scholar] [CrossRef]
- Hilder, M.; Winther-Jensen, O.; Winther-Jensen, B.; MacFarlane, D.R. Graphene/zinc nano-composites by electrochemical co-deposition. Phys. Chem. Chem. Phys. 2012, 14, 14034–14040. [Google Scholar] [CrossRef]
- Tauc, J. Amorphous and Liquid Semiconductors; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Kulkarni, S.A.; Sawadh, P.S.; Palei, P.K.; Kokate, K.K. Effect of synthesis route on the structural, optical and magnetic properties of Fe3O4 nanoparticles. Ceram. Int. 2014, 40, 1945–1949. [Google Scholar] [CrossRef]
- Amir, M.; Baykal, A.; Güner, S.; Güngüneş, H.; Sözeri, H. Magneto-optical investigation and hyperfine interactions of copper substituted Fe3O4 nanoparticles. Ceram. Int. 2016, 42, 5650–5658. [Google Scholar] [CrossRef]
- Bachan, N.; Asha, A.; Jeyarani, W.J.; Kumar, D.A.; Shyla, J.M. A Comparative investigation on the structural, optical and electrical properties of SiO2–Fe3O4 core–shell nanostructures with their single components. Acta Metall. Sin. (Engl. Lett.) 2015, 28, 1317–1325. [Google Scholar] [CrossRef]
- Ge, J.; Zhang, Q.; Zhang, T.; Yin, Y. Core–satellite nanocomposite catalysts protected by a porous silica shell: Controllable reactivity, high stability, and magnetic recyclability. Angew. Chem. 2008, 120, 9056–9060. [Google Scholar] [CrossRef]
- Ahangaran, F.; Hassanzadeh, A.; Nouri, S. Surface modification of Fe3O4@SiO2 microsphere by silane coupling agent. Int. Nano Lett. 2013, 3, 23. [Google Scholar] [CrossRef]
- Zhang, H.; Niu, Z.; Liu, Z.; Wen, Z.; Li, W.; Wang, X.; Wu, W. Equilibrium, kinetic and thermodynamic studies of adsorption of Th (IV) from aqueous solution onto kaolin. J. Radioanal. Nucl. Chem. 2015, 303, 87–97. [Google Scholar] [CrossRef]
- Aroke, U.O.; Abdulkarim, A.; Ogubunka, R.O. Fourier-transform infrared characterization of kaolin, granite, bentonite and barite. ATBU J. Environ. Technol. 2013, 6, 42–53. [Google Scholar]
- Chang, C.-F.; Wu, Y.-L.; Hou, S.-S. Preparation and characterization of superparamagnetic nanocomposites of aluminosilicate/silica/magnetite. Colloids Surf. A Physicochem. Eng. Asp. 2009, 336, 159–166. [Google Scholar] [CrossRef]
- Chen, C.-W. Magnetism and Metallurgy of Soft Magnetic Materials; Courier Corporation: Chelmsford, MA, USA, 2013. [Google Scholar]
- James, R.D. Magnetic alloys break the rules. Nature 2015, 521, 298–299. [Google Scholar] [CrossRef]
- Guo, S.; Dan, Z.; Duan, N.; Chen, G.; Gao, W.; Zhao, W. Zn (II), Pb (II), and Cd (II) adsorption from aqueous solution by magnetic silica gel: Preparation, characterization, and adsorption. Environ. Sci. Pollut. Res. 2018, 25, 30938–30948. [Google Scholar] [CrossRef]
- Raut-Jadhav, S.; Pinjari, D.V.; Saini, D.R.; Sonawane, S.H.; Pandit, A.B. Intensification of degradation of methomyl (carbamate group pesticide) by using the combination of ultrasonic cavitation and process intensifying additives. Ultrason. Sonochem. 2016, 31, 135–142. [Google Scholar] [CrossRef]
- Nichela, D.A.; Berkovic, A.M.; Costante, M.R.; Juliarena, M.P.; Einschlag, F.S.G. Nitrobenzene degradation in Fenton-like systems using Cu (II) as catalyst. Comparison between Cu (II)-and Fe (III)-based systems. Chem. Eng. J. 2013, 228, 1148–1157. [Google Scholar] [CrossRef]
- Singh, C.; Chaudhary, R.; Gandhi, K. Preliminary study on optimization of pH, oxidant and catalyst dose for high COD content: Solar parabolic trough collector. Iran. J. Environ. Health Sci. Eng. 2013, 10, 13. [Google Scholar] [CrossRef]
- Shanmugam, B.K.; Easwaran, S.N.; Mohanakrishnan, A.S.; Kalyanaraman, C.; Mahadevan, S. Biodegradation of tannery dye effluent using Fenton’s reagent and bacterial consortium: A biocalorimetric investigation. J. Environ. Manag. 2019, 242, 106–113. [Google Scholar] [CrossRef]
- Toosi, F.S.; Hosseiny, M.; Joghataei, A.; Toosi, F.S. The application of SiO2 nanoparticles for anionic dye removal from aqueous solution. Arch. Hyg. Sci. 2017, 6, 136–144. [Google Scholar] [CrossRef]
- Buthiyappan, A.; Raman, A.A.A.; Daud, W.M.A.W. Development of an advanced chemical oxidation wastewater treatment system for the batik industry in Malaysia. RSC Adv. 2016, 6, 25222–25241. [Google Scholar] [CrossRef]
- Ahmadi, M.; Behin, J.; Mahnam, A.R. Kinetics and thermodynamics of peroxydisulfate oxidation of Reactive Yellow 84. J. Saudi Chem. Soc. 2016, 20, 644–650. [Google Scholar] [CrossRef]
- Do, Q.C.; Kim, D.-G.; Ko, S.-O. Catalytic activity enhancement of a Fe3O4@SiO2 yolk-shell structure for oxidative degradation of acetaminophen by decoration with copper. J. Clean. Prod. 2018, 172, 1243–1253. [Google Scholar] [CrossRef]
- Huang, X.; Nan, Z. Formation of octahedron-shaped ZnFe2O4/SiO2 with yolk–shell structure. J. Phys. Chem. Solids 2020, 141, 109410. [Google Scholar] [CrossRef]
- Phan, T.T.N.; Nikoloski, A.N.; Bahri, P.A.; Li, D. Facile fabrication of perovskite-incorporated hierarchically mesoporous/macroporous silica for efficient photoassisted-Fenton degradation of dye. Appl. Surf. Sci. 2019, 491, 488–496. [Google Scholar] [CrossRef]
- Nour, M.M.; Tony, M.A.; Nabwey, H.A. Heterogeneous fenton oxidation with natural clay for textile levafix dark blue dye removal from aqueous effluent. Appl. Sci. 2023, 13, 8948. [Google Scholar] [CrossRef]
- Hassan, E.A.; Tony, M.A.; Nabwey, H.A.; Awad, M.M. Potential of the Biomass Waste Originating from Saccharum officinarum as a Fenton Precursor for the Efficient Oxidation of Azo Dye from an Aqueous Stream. Processes 2023, 11, 1394. [Google Scholar] [CrossRef]
- Ye, Z.; Schukraft, G.E.; L’Hermitte, A.; Xiong, Y.; Brillas, E.; Petit, C.; Sirés, I. Mechanism and stability of an Fe-based 2D MOF during the photoelectro-Fenton treatment of organic micropollutants under UVA and visible light irradiation. Water Res. 2020, 184, 115986. [Google Scholar] [CrossRef]
- Hassan, E.A.; Tony, M.A.; Nabwey, H.A.; Awad, M.M. Management of Agricultural Water Containing Acetimidothioic Acid Pesticide through Catalytic Oxidation to Facilitate Reclaimed Water Recycling for Sustainable Food Production. Processes 2023, 11, 792. [Google Scholar] [CrossRef]
- Shoorangiz, M.; Nikoo, M.R.; Salari, M.; Rakhshandehroo, G.R.; Sadegh, M. Optimized electro-Fenton process with sacrificial stainless steel anode for degradation/mineralization of ciprofloxacin. Process Saf. Environ. Prot. 2019, 132, 340–350. [Google Scholar] [CrossRef]
- Thiam, A.; Salazar, R.; Brillas, E.; Sirés, I. In-situ dosage of Fe2+ catalyst using natural pyrite for thiamphenicol mineralization by photoelectro-Fenton process. J. Environ. Manag. 2020, 270, 110835. [Google Scholar] [CrossRef]
- Soliman, E.M.; Ahmed, S.A.; Fadl, A.A. Adsorptive removal of oil spill from sea water surface using magnetic wood sawdust as a novel nano-composite synthesized via microwave approach. J. Environ. Health 2020, 18, 79–90. [Google Scholar] [CrossRef]
- Srinivasakannan, C.; Bakar, M.Z.A. Production of activated carbon from rubber wood sawdust. Biomass Bioenergy 2004, 27, 89–96. [Google Scholar] [CrossRef]
- Sadeghi, M.; Mehdinejad, M.H.; Mengelizadeh, N.; Mahdavi, Y.; Pourzamani, H.; Hajizadeh, Y.; Zare, M.R. Degradation of diclofenac by heterogeneous electro-Fenton process using magnetic single-walled carbon nanotubes as a catalyst. J. Water Process Eng. 2019, 31, 100852. [Google Scholar] [CrossRef]
- Tamuly, C.; Saikia, I.; Hazarika, M.; Das, M. Reduction of aromatic nitro compounds catalyzed by Biogenic CuO nanoparticles. RSC Adv. 2014, 4, 53229–53236. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, X.; Liu, Y.; Ma, Y.; Li, T.; Lin, Y.; Xie, T.; Dong, S. Photo-Fenton degradation of emerging pollutants over Fe-POM nanoparticle/porous and ultrathin g-C3N4 nanosheet with rich nitrogen defect: Degradation mechanism, pathways, and products toxicity assessment. Appl. Catal. B Environ. 2020, 278, 119349. [Google Scholar] [CrossRef]
- Collins, D.; Luxton, T.; Kumar, N.; Shah, S.; Walker, V.K.; Shah, V. Assessing the impact of copper and zinc oxide nanoparticles on soil: A field study. PLoS ONE 2012, 7, e42663. [Google Scholar] [CrossRef]
- Midander, K.; Cronholm, P.; Karlsson, H.L.; Elihn, K.; Mo, L.; Leygraf, C.; Wallinder, I.O. Surface characteristics, copper release, and toxicity of nano- and micrometer-sized copper and copper(II) oxide particles: A cross-disciplinary study. Small 2009, 5, 389–399. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der Grösseundderinneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Nachr. Ges. Wiss. Göttingen 1918, 26, 98–100. [Google Scholar]
- Nguyen, T.T.; Huynh, K.A.; Padungthon, S.; Pranudta, A.; Amonpattaratkit, P.; Tran, L.B.; Phan, P.T.; Nguyen, N.H. Synthesis of natural flowerlike iron-alum oxide with special interaction of Fe-Si-Al oxides as an effective catalyst for heterogeneous Fenton process. J. Environ. Chem. Eng. 2021, 9, 105732. [Google Scholar] [CrossRef]
- Molina, C.B.; Sanz-Santos, E.; Boukhemkhem, A.; Bedia, J.; Belver, C.; Rodriguez, J.J. Removal of emerging pollutants in aqueous phase by heterogeneous Fenton and photo-Fenton with Fe2O3-TiO2-clay heterostructures. Environ. Sci. Pollut. Res. 2020, 27, 38434. [Google Scholar] [CrossRef]
- Droguett, C.; Salazar, R.; Brillas, E.; Sirés, I.; Carlesi, C.; Marco, J.F.; Thiam, A. Treatment of antibiotic cephalexin by heterogeneous electrochemical Fenton-based processes using chalcopyrite as sustainable catalyst. Sci. Total Environ. 2020, 740, 140154. [Google Scholar] [CrossRef] [PubMed]
- Kalantary, R.R.; Farzadkia, M.; Kermani, M.; Rahmatinia, M. Heterogeneous electro-Fenton process by Nano-Fe3O4 for catalytic degradation of amoxicillin: Process optimization using response surface methodology. J. Environ. Chem. Eng. 2018, 6, 4644. [Google Scholar] [CrossRef]












| Kinetic Model | Parameters | Values | |||
|---|---|---|---|---|---|
| T, °C | |||||
| 26 °C | 40 °C | 50 °C | 60 °C | ||
| Zero-order ( | k0 (min−1) | 0.62 | 0.67 | 0.71 | 0.56 |
| t1/2 (min) | 40.32 | 37.314 | 35.21 | 44.64 | |
| R2 | 0.53 | 0.81 | 0.95 | 0.96 | |
| Pseudo-First-order ) | k1 (min−1) | 0.204 | 0.039 | 0.022 | 0.018 |
| t1/2 (min) | 38.5 | 31.5 | 17.76 | 3.39 | |
| R2 | 0.95 | 0.96 | 0.95 | 0.96 | |
| Pseudo-Second-order () | k2 (L.mg−1.min−1) × 10−2 | 0.4009 | 0.0027 | 0.0013 | 0.0006 |
| t1/2 (min) | 0.049 | 7.41 | 15.38538 | 33.34 | |
| R2 | 0.62 | 0.67 | 0.71 | 0.56 | |
| Parameter | T, °C | |||
|---|---|---|---|---|
| 26 °C | 40 °C | 50 °C | 60 °C | |
| ∆G′ (kJ/mol) | 77.18 | 85.22 | 89.57 | 92.98 |
| ∆H′ (kJ/mol) | 57.71 | 57.60 | 57.51 | 57.43 |
| ∆S′ (J/mol K) | −65.11 | −88.26 | −99.23 | −106.75 |
| Ea (kJ/mol) | 60.20 | |||
| Fenton’s Reaction Type/Catalyst * | Pharmaceutical Category | Oxidation (%) | Operating Conditions | Ref. |
|---|---|---|---|---|
| Electro/UV/Fenton/MSWCNTs–FeCl2 | Anti-Inflammatory | 71% | pH: 5, MSWCNTs–FeCl2: 0.05 M, oxidation time: 2 h | [55] |
| UV/Fenton (Fe/Cu/TiO2) | Anti-Inflammatory | 41% | Cu/Fe: 1 g/L, oxidation time: 1 h | [56] |
| Dark Fenton/Fe-POM | Antibiotics | 97% | Fe-POM: 1 g/L, oxidation time: 0.2 h | [57] |
| Electro/UV/Fenton/CuFeS2 | Antibiotics | 100% | pH: 3.0, CuFeS2: 0.05 g/L, oxidation time: 0.25 h | [58] |
| Dark Fenton/MOF-Fe | Antilipemic agent | 62% | pH: 5.1, MOF-Fe: 0.05 g/L, oxidation time: 4 h | [59] |
| Electro/UV/Fenton/ZVI/C | Lipid-regulating | 94% | pH: 6.0, ZVI: 0.05 M, oxidation time: 1 h | [59] |
| Dark/Fe/C | Antibiotics | 95% | pH: 6.0, Fe/C: 1.0 g/L, oxidation time: 0.2 h | [60] |
| Electro/UV/Fenton/Fe | Antibiotics | 98% | pH: 3.0, Fe: 0.01 M, oxidation time: 1 h | [61] |
| Electro/UV/Fenton/Fe/C | Antibiotics | 70% | pH: 5.0, Fe/C: 0.05 M, oxidation time: 2 h | [62] |
| Electro/UV/Fenton/FeS2 | Antibiotics | 99% | pH: 3.0, FeS2: 0.05 M, oxidation time: 0.3 h | [63] |
| Electro/UV/Fenton/Fe2O3 | Antibiotics | 98% | pH: 3.0, Fe2O3: 0.01 M, oxidation time: 1 h | [64] |
| Electro/Fenton/FeS2 | Antibiotic | 86% | pH: 4.0, FeS2: 0.02 M, oxidation time: 6 h | [49] |
| Solar-Fenton/Ban-Char500@Mag-(1:1) | Antibiotic | 92% | pH: 3.0, Ban-Char500@Mag-(1:1): 10 mg/L, oxidation time: 0.5 h | Current work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nour, M.M.; Tony, M.A.; Fouad, M.K.; Nabwey, H.A. Banana (Musa sapientum) Waste-Derived Biochar–Magnetite Magnetic Composites for Acetaminophen Removal via Photochemical Fenton Oxidation. Catalysts 2025, 15, 955. https://doi.org/10.3390/catal15100955
Nour MM, Tony MA, Fouad MK, Nabwey HA. Banana (Musa sapientum) Waste-Derived Biochar–Magnetite Magnetic Composites for Acetaminophen Removal via Photochemical Fenton Oxidation. Catalysts. 2025; 15(10):955. https://doi.org/10.3390/catal15100955
Chicago/Turabian StyleNour, Manasik M., Maha A. Tony, Mai Kamal Fouad, and Hossam A. Nabwey. 2025. "Banana (Musa sapientum) Waste-Derived Biochar–Magnetite Magnetic Composites for Acetaminophen Removal via Photochemical Fenton Oxidation" Catalysts 15, no. 10: 955. https://doi.org/10.3390/catal15100955
APA StyleNour, M. M., Tony, M. A., Fouad, M. K., & Nabwey, H. A. (2025). Banana (Musa sapientum) Waste-Derived Biochar–Magnetite Magnetic Composites for Acetaminophen Removal via Photochemical Fenton Oxidation. Catalysts, 15(10), 955. https://doi.org/10.3390/catal15100955







