Hybrid PK-P/Fe3O4 Catalyst Derived from Pumpkin Peel (Bio-Waste) for Synozol Red KHL Dye Oxidation Under Photo-Fenton Reaction
Abstract
1. Introduction
2. Results and Discussion
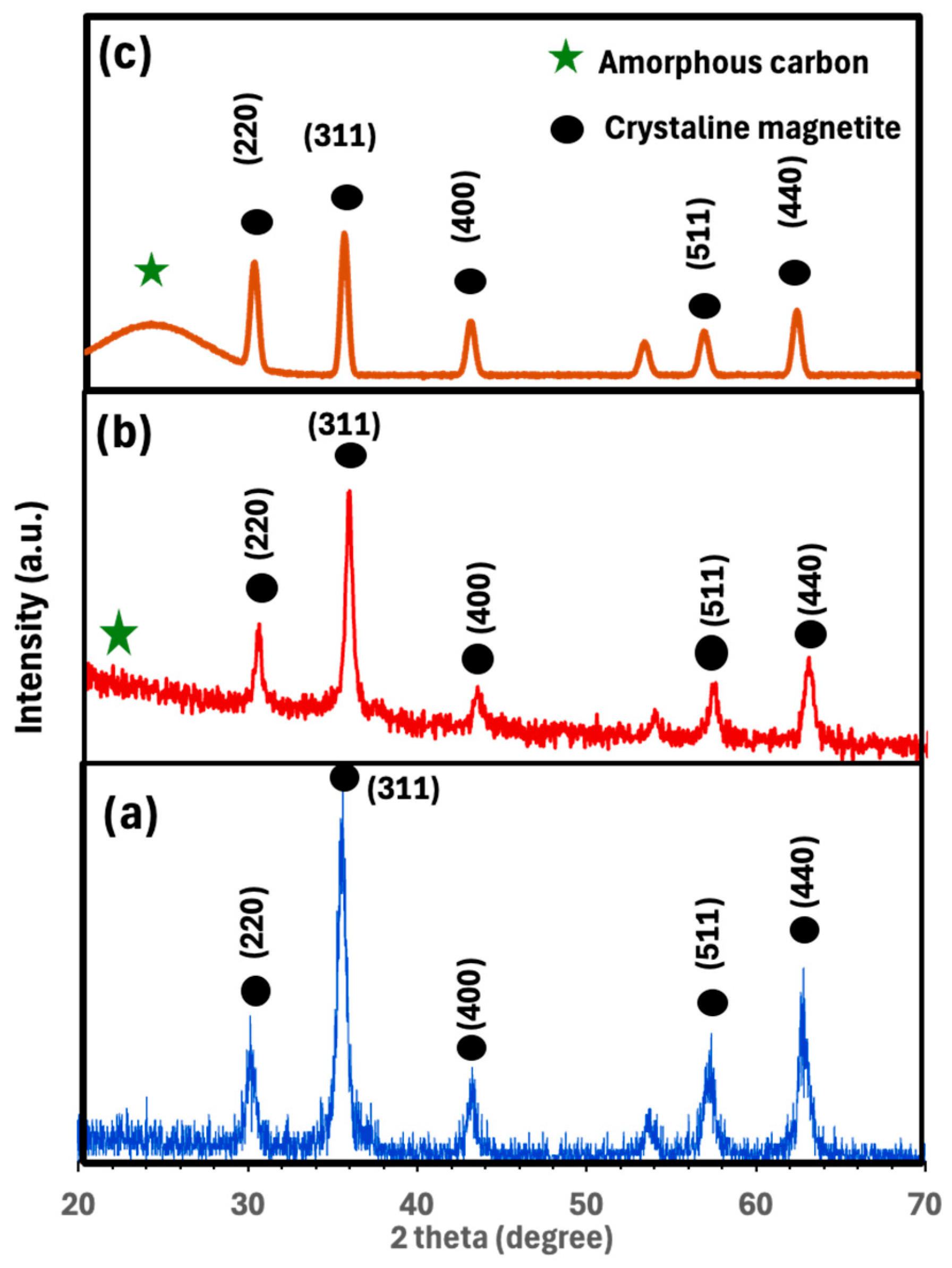
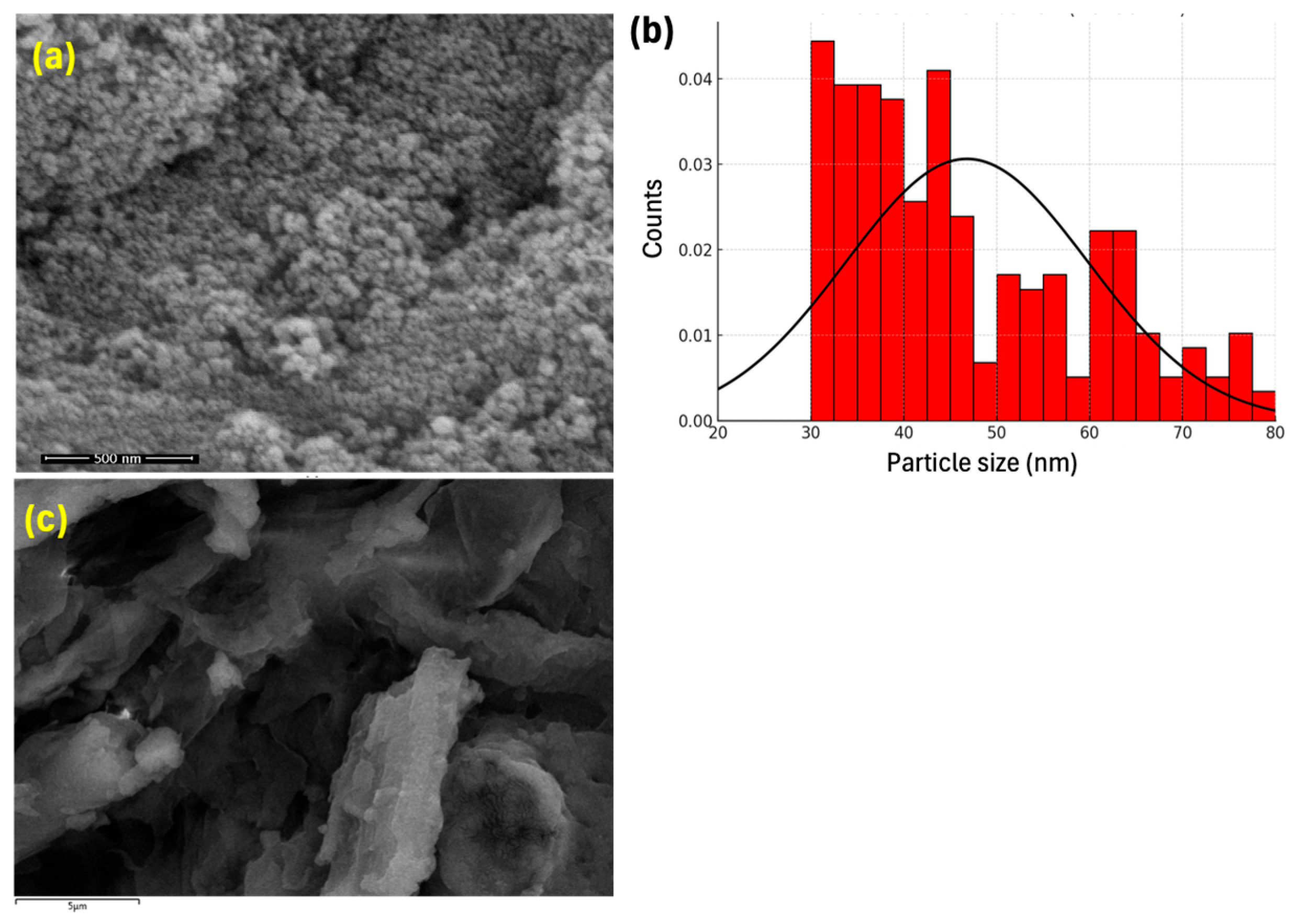
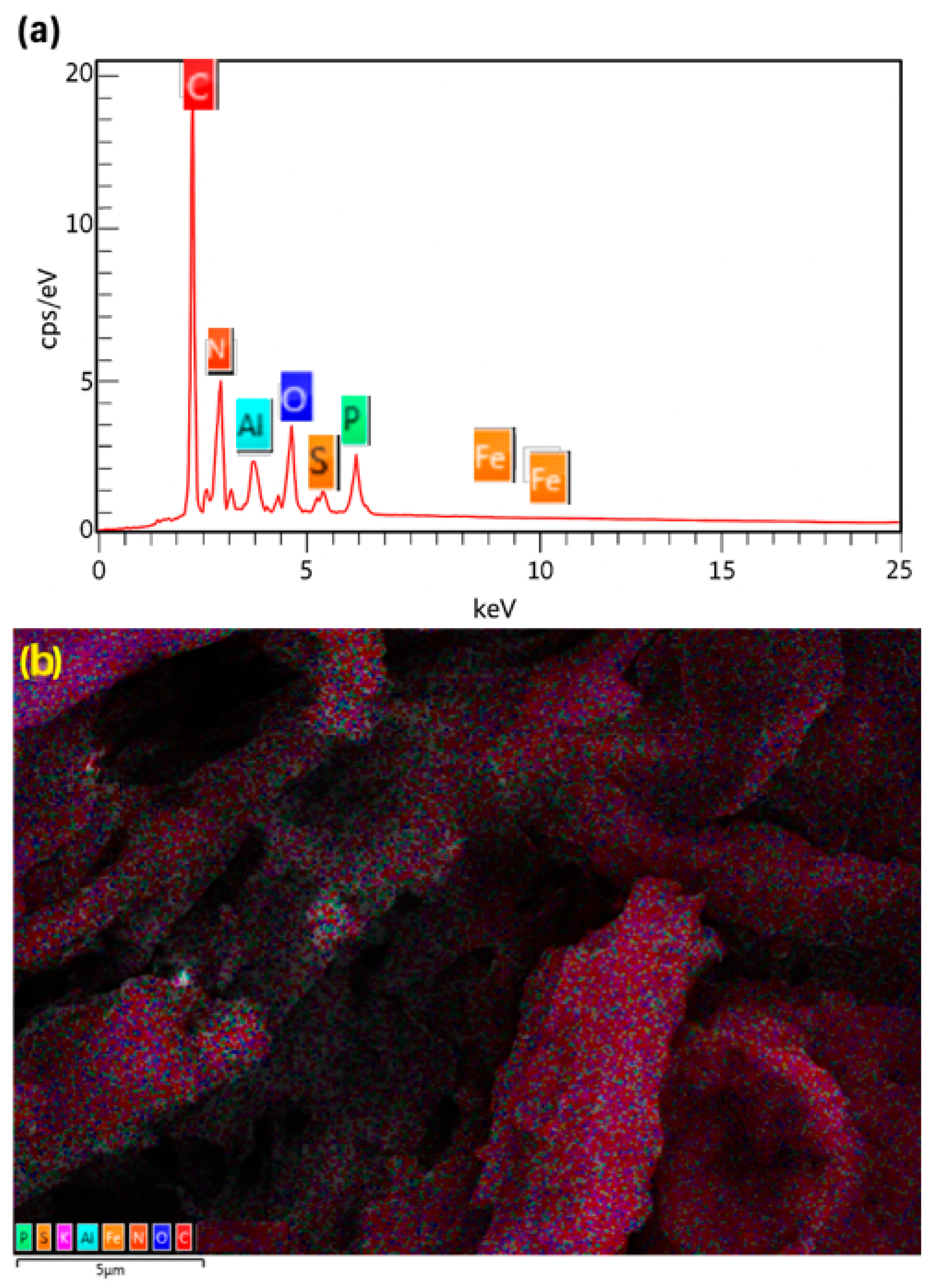
2.1. Catalyst Characterization
2.2. Dye-Containing Wastewater Oxidation
2.2.1. Effects of Different Treatment Systems
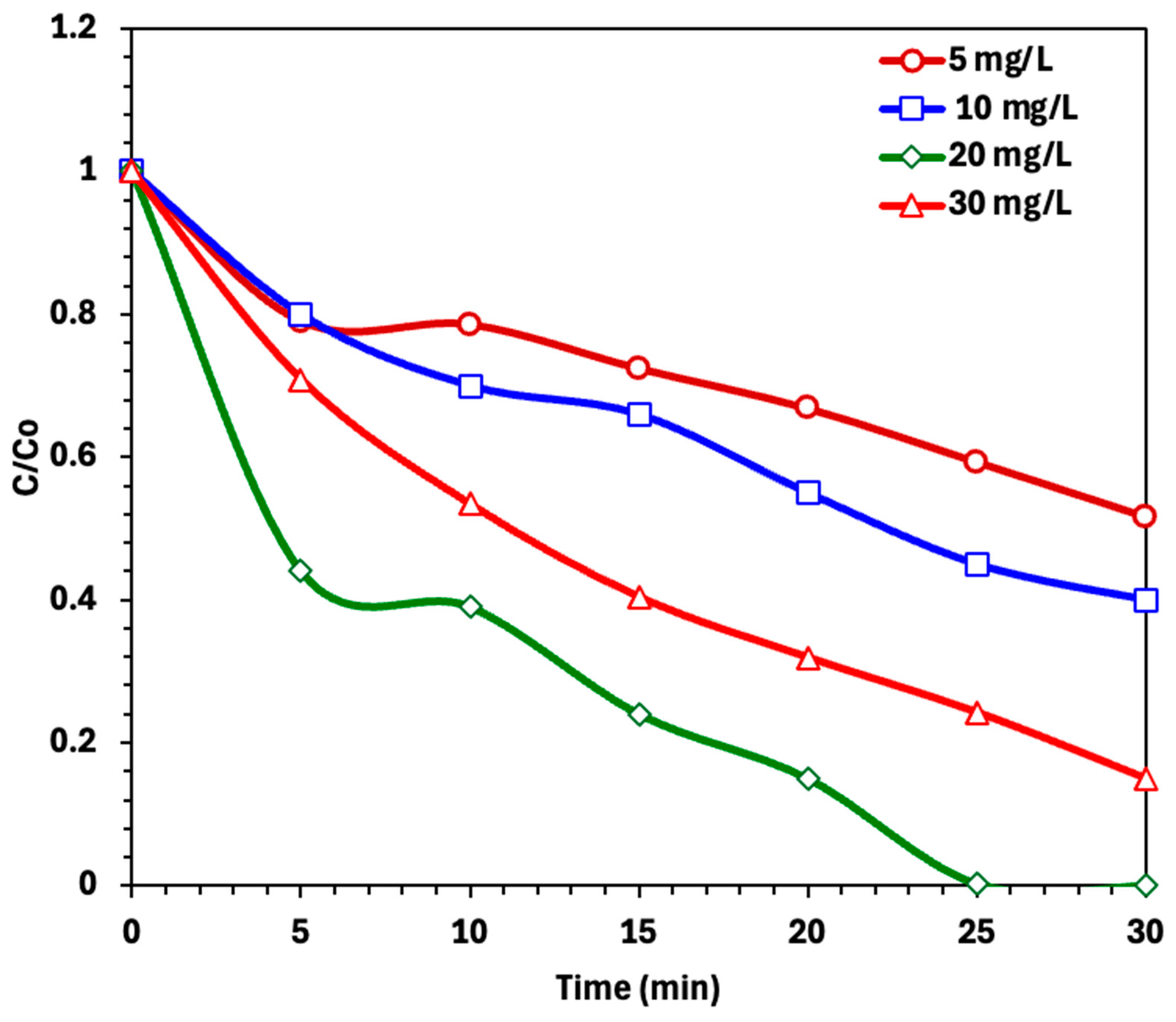
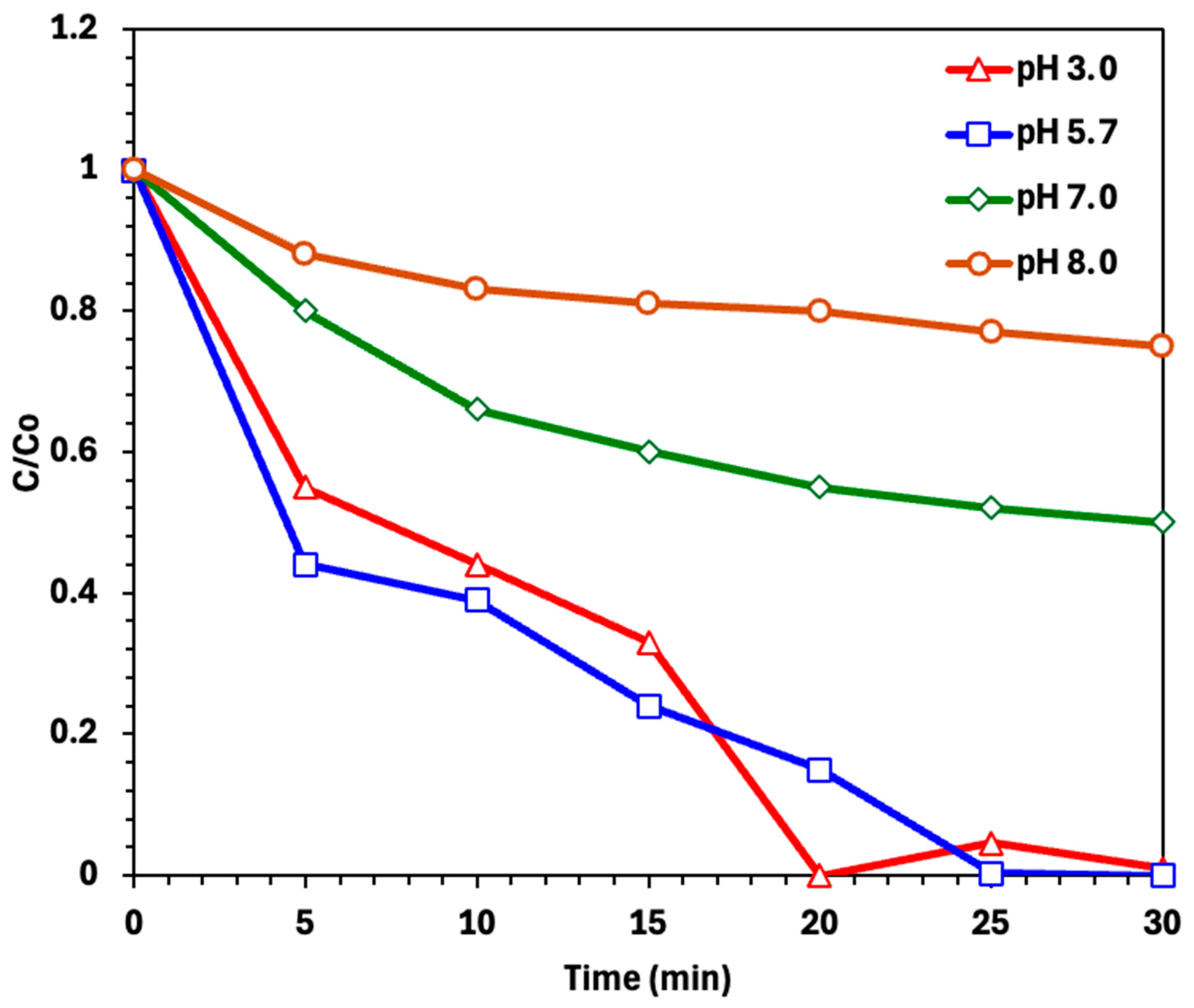
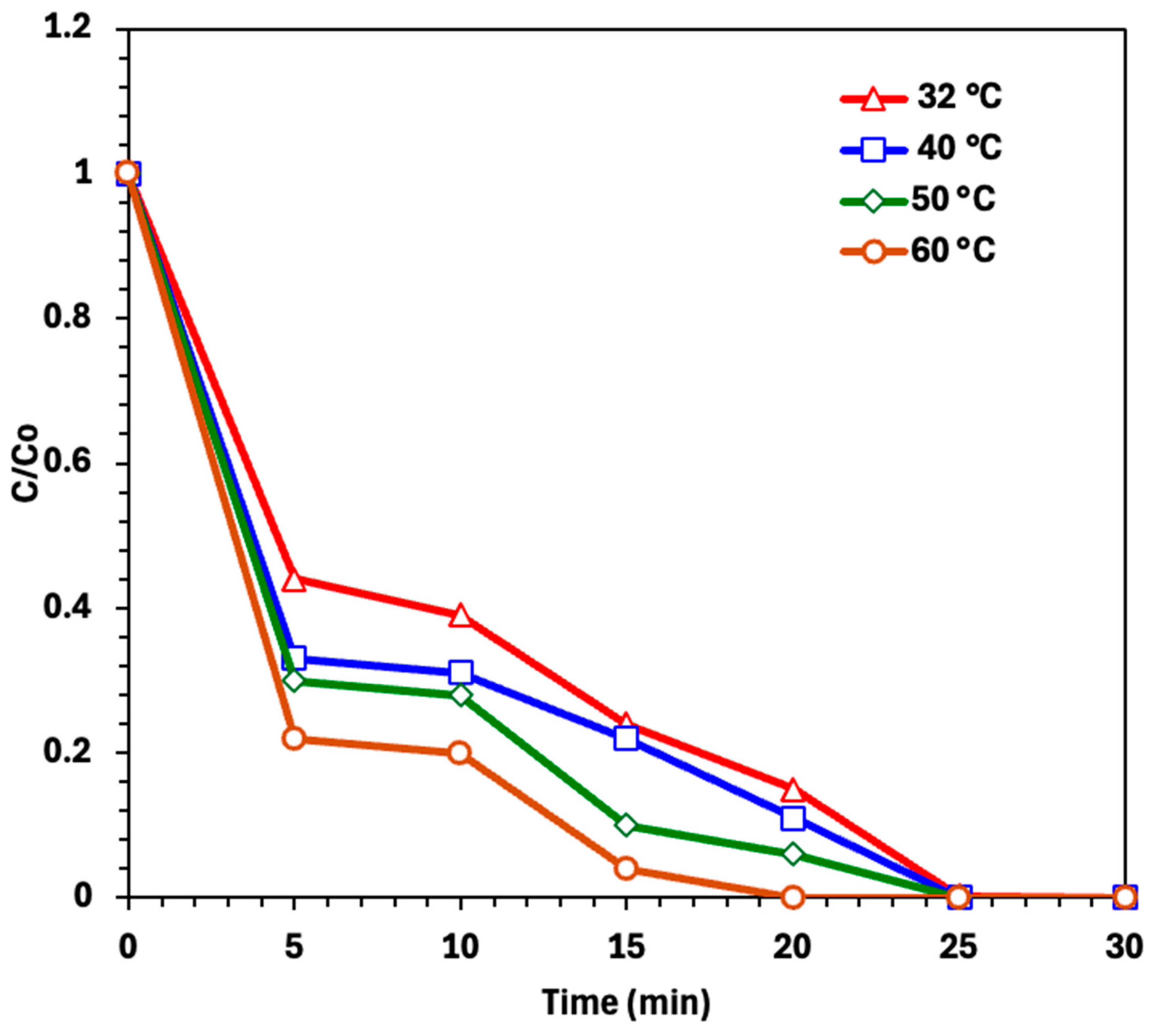
2.2.2. Effect of Photo-Fenton Operating Parameters
2.3. Comparative Investigation with Cited Literature
2.4. Oxidation Mechanism
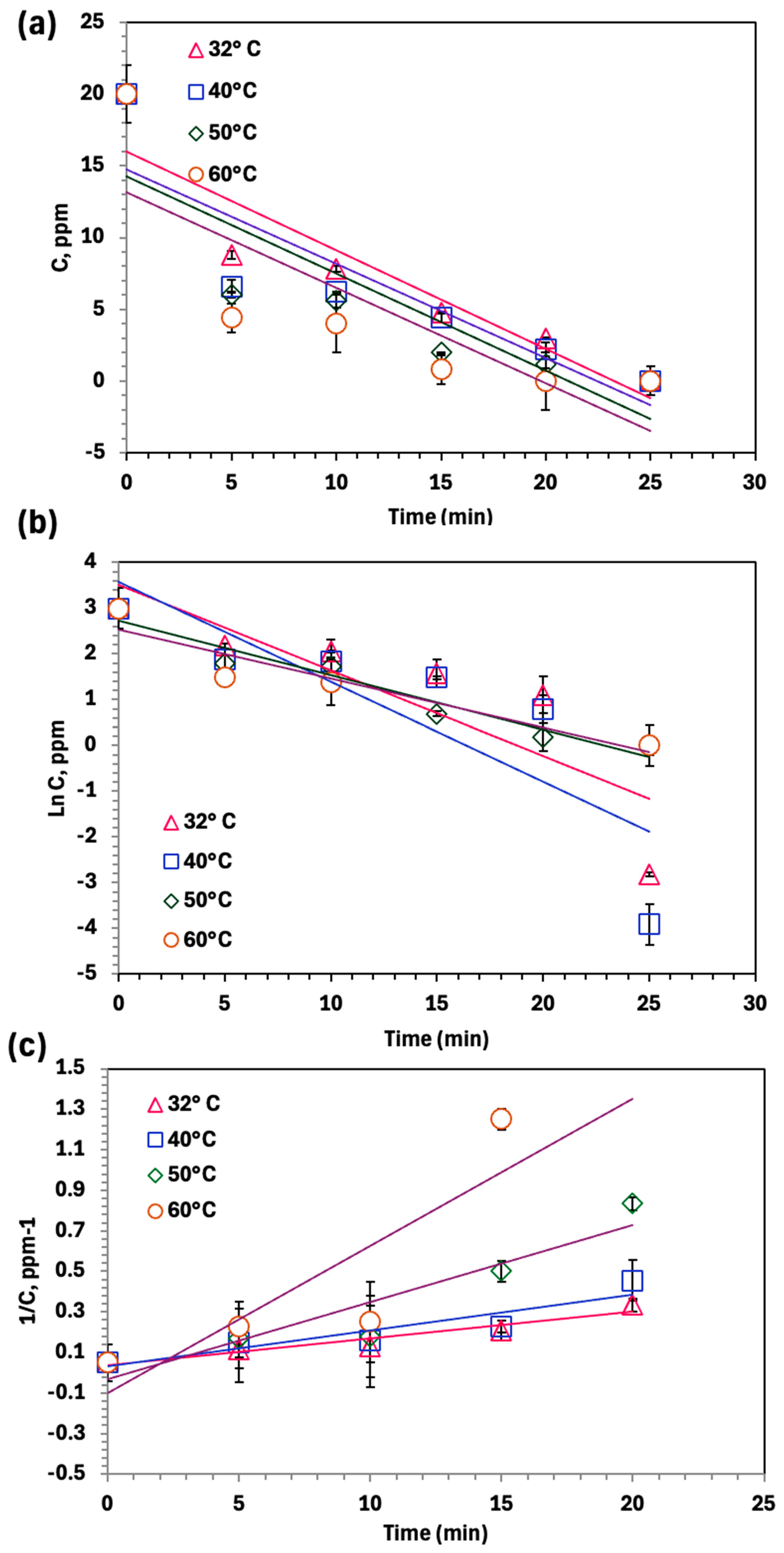
2.5. Oxidation Kinetics
2.6. Thermodynamic Investigation
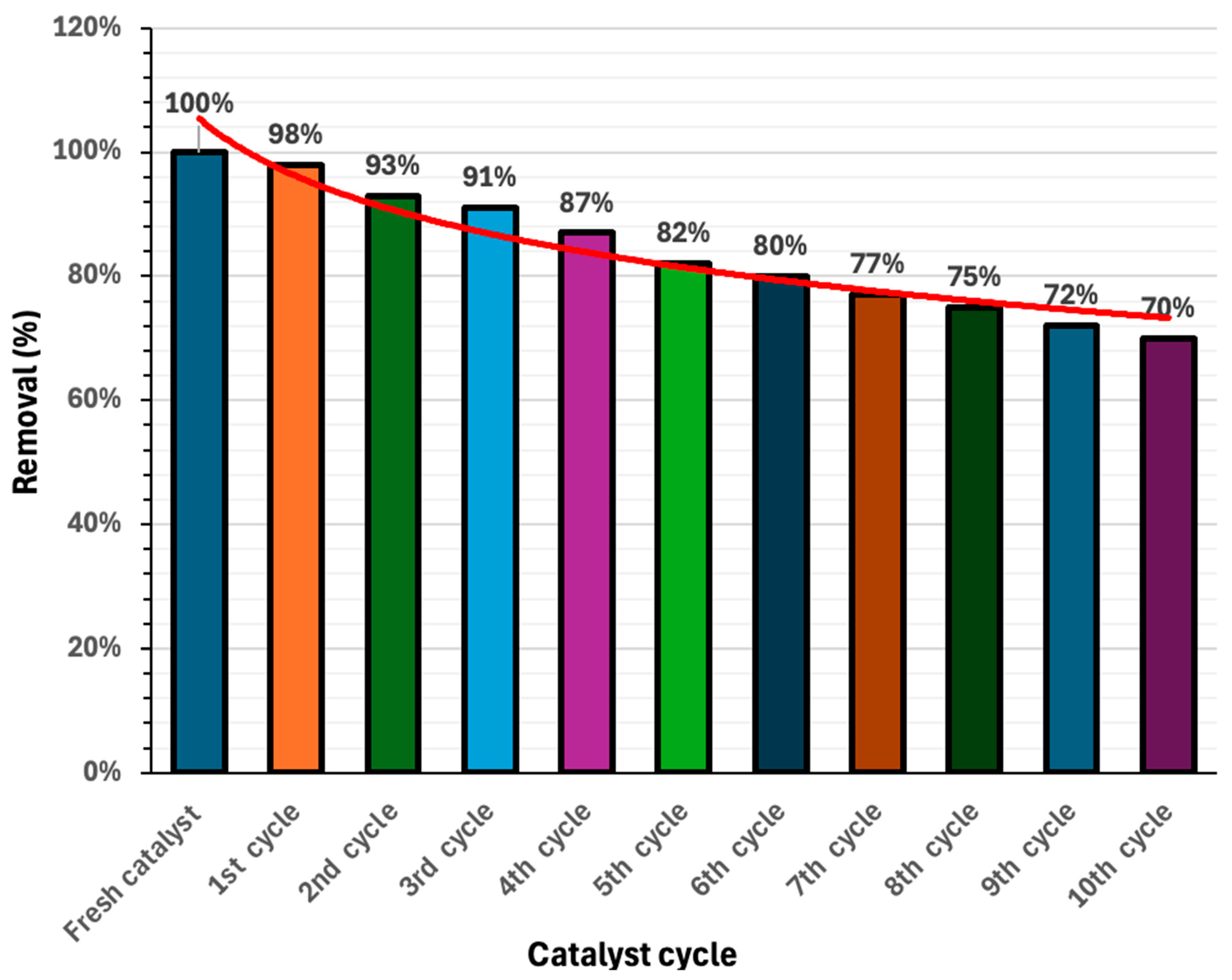
2.7. Reusability and Sustainability
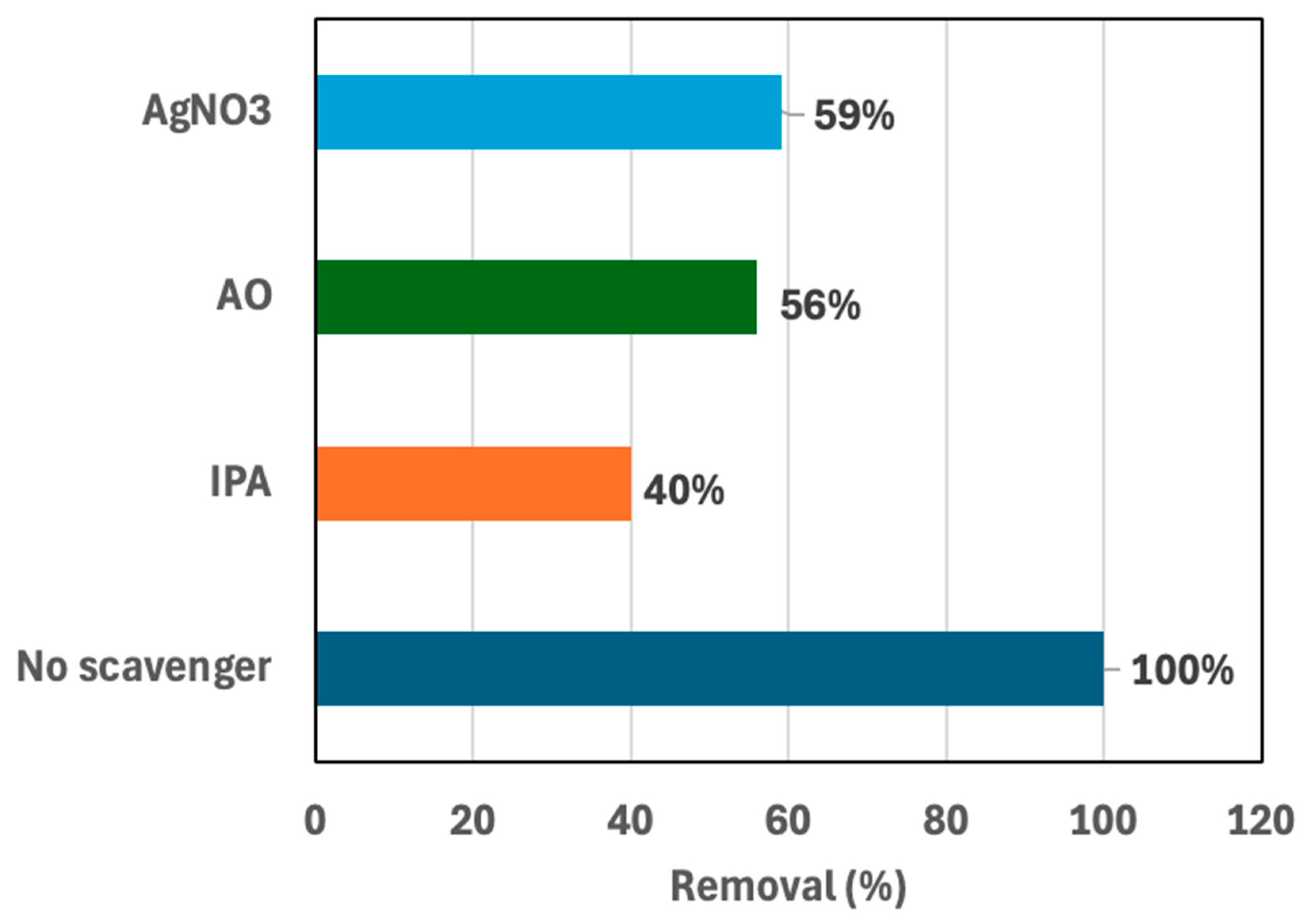
2.8. Radical Scavenging Test
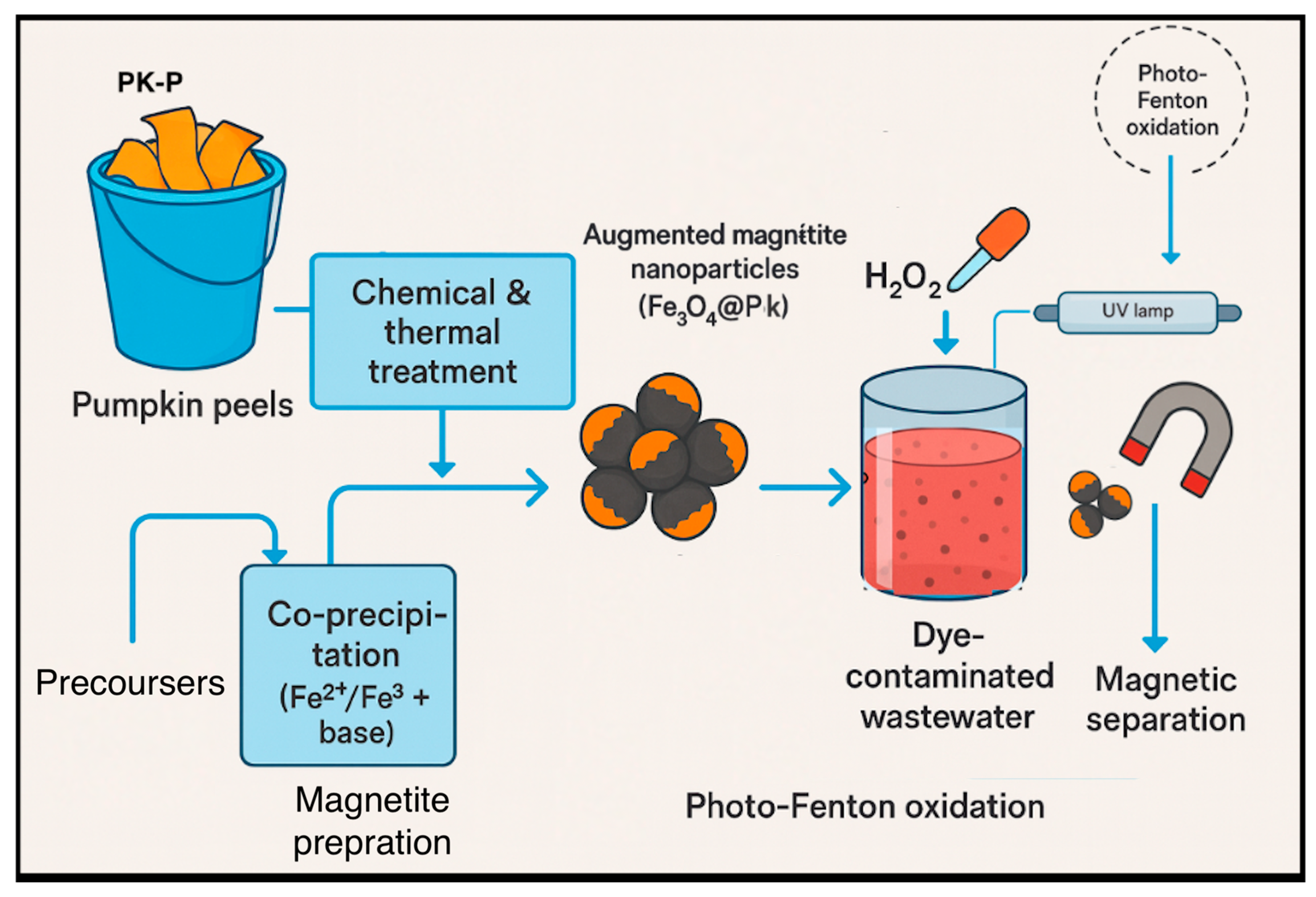
3. Materials and Methodology Investigation
3.1. Materials
3.2. Experimental Methodology
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bidira, F.; Al-Senani, G.M.; Bekele, E.A.; Garomsa, F.S.; Vigneshwaran, S.; Al-Qahtani, S.D.; Asaithambi, P. Synergistic pollutant removal from brewery wastewater using electrocoagulation-assisted pumpkin seed-activated carbon. Process Saf. Environ. Prot. 2025, 200, 107429. [Google Scholar] [CrossRef]
- Mangood, A.H.; Salama, E.S.; El Sayed, I.E.T.; Fouad, M.K.; Tony, M.A. Efficient Augmentation of Superparamagnetic Ferrite with Alum Sludge as a Sustainable Nanoadsorbent Matrix for Promoting Dye Removal: Preparation, Characterization and Application. Int. J. Environ. Res. 2025, 19, 1–23. [Google Scholar] [CrossRef]
- Kızıl, Y.; Benek, V.; Teğin, İ.; Önal, Y.; Erol, K.; Alacabey, I. Reactive Blue 19 Adsorption on Activated Carbon from Pumpkin (Cucurbita Pepo) Seed Waste: Kinetic, Isotherm and Thermodynamic Studies, Journal of Environmental Research. Eng. Manag. 2024, 80, 7–20. [Google Scholar] [CrossRef]
- Nour, M.M.; Tony, M.A.; Lee, W.H.; Nabwey, H.A. Opportunities of aluminum sludge dredging with soft magnetic conditioner for dewaterability enrichment. Sci. Rep. 2025, 15, 7796. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Zollinger, H. Color Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments; Wiley-VCH: Zurich, Switzerland, 2003. [Google Scholar]
- Daneshvar, N.; Aber, S.; Dorraji, M.S.; Khataee, A.R.; Rasoulifard, M.H. Photocatalytic degradation of the insecticide diazinon in the presence of prepared nanocrystalline ZnO powders under irradiation of UV-C light. Sep. Purif. Technol. 2007, 58, 91–98. [Google Scholar] [CrossRef]
- Ali, H. Biodegradation of synthetic dyes—A review. Water Air Soil. Pollut. 2010, 213, 251–273. [Google Scholar] [CrossRef]
- Raji, F.; Maghool, S.; Shayesteh, H.; Rahbar-Kelishami, A. Effective adsorptive removal of Pb2+ ions from aqueous solution using functionalized agri-waste biosorbent: New green mediation via Seidlitzia rosmarinus extract. Chemosphere 2024, 363, 142759. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Minocha, A.K. Conventional and non-conventional adsorbents for removal of pollutants from water-A review. Indian J. Chem. Technol. 2006, 13, 203–217. [Google Scholar]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewater: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Al-Kdasi, A.; Idris, A.; Saed, K.; Guan, C.T. Treatment of textile wastewater by advanced oxidation processes. Glob. Nest Int. J. 2004, 6, 222–230. [Google Scholar]
- Nour Manasik, M.; Tony, M.A.; Nabwey, H.A. Heterogeneous fenton oxidation with natural clay for textile levafix dark blue dye removal from aqueous effluent. Appl. Sci. 2023, 13, 8948. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Nabwey Hossam, A.; Maha, A.T. Dewatered Sludge Decorated with Nanoparticles for Alum Sludge Conditioning towards the Concept of “End-of-Waste”. Nanomaterials 2023, 13, 2903. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Bautista, P.; Mohedano, A.F.; Casas, J.A.; Zazo, J.A.; Rodriguez, J.J. An overview of the application of Fenton oxidation to industrial wastewaters. J. Chem. Technol. Biotechnol. 2008, 83, 1323–1338. [Google Scholar] [CrossRef]
- Deng, Y.; Englehardt, J.D. Treatment of landfill leachate by Fenton process. Water Res. 2006, 40, 3683–3694. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, J.; Xia, J.; Zhao, G.; Yang, Y.; Zhang, Z.; Li, J.; Wei, F.; Song, W. Biomass Cellulose-Derived Carbon Aerogel Supported Magnetite-Copper Bimetallic Heterogeneous Fenton-like Catalyst Towards the Boosting Redox Cycle of ≡Fe(III)/≡Fe(II). Nanomaterials 2025, 15, 614. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Recent advances in heterogeneous Fenton-like catalysis for water treatment. Chem. Eng. J. 2019, 373, 624–641. [Google Scholar]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef]
- Chen, Q.; Tan, X.; Liu, Y.; Liu, S.; Li, M.; Gu, Y.; Zhang, P.; Ye, S.; Yang, Z.; Yang, Y. Biomass-derived porous graphitic carbon materials for energy and environmental applications. J. Mater. Chem. A 2020, 8, 5773–5811. [Google Scholar] [CrossRef]
- Gillani, Q.F.; Bakbolat, B.; Tatykayev, B.; Sultanov, F.; Mentbayeva, A. Biomass-derived carbon materials for hydrogen storage: Structure-performance relationships and design strategies. J. Energy Storage 2025, 135, 118401. [Google Scholar] [CrossRef]
- Bai, S.; Tan, G.; Li, X.; Zhao, Q.; Meng, Y.; Wang, Y.; Zhang, Y.; Xiao, D. Pumpkin-derived porous carbon for supercapacitors with high performance. Chem. Asian J. 2016, 11, 1869–1873. [Google Scholar] [CrossRef] [PubMed]
- Vandana, T.U.; Tripathy, B.K.; Mishra, R.K.; Sharma, A.; Mohanty, K. A review on waste biomass-derived biochar: Production, characterisation, and advanced analytical techniques for pollutants assessment in water and wastewater. Process Saf. Environ. Prot. 2025, 201, 107505. [Google Scholar] [CrossRef]
- Leichtweis, M.G.; Molina, A.K.; Petropoulos, S.A.; Carocho, M.; Pires, T.C.S.P.; Dias, M.I.; Calhelha, R.; Oliveira, M.B.P.P.; Pereira, C.; Barros, L. Valorization of Pumpkin Peel as a Source of Bioactive Compounds: Optimization of Heat- and Ultrasound-Assisted Extraction. Molecules 2023, 28, 3168. [Google Scholar] [CrossRef]
- Añibarro-Ortega, M.; Pinela, J.; Ćirić, A.; Martins, V.; Rocha, F.; Soković, M.D.; Barata, A.M.; Carvalho, A.M.; Barros, L.; Ferreira, I.C.F.R. Valorisation of table tomato crop by-products: Phenolic profiles and in vitro antioxidant and antimicrobial activities. Food Bioprocess. Technol. 2020, 124, 307–319. [Google Scholar] [CrossRef]
- Salami, A.; Asefi, N.; Kenari, R.E.; Gharekhani, M. Extraction of pumpkin peel extract using supercritical CO2and subcritical water technology: Enhancing oxidative stability of canola oil. J. Food Sci. Technol. 2021, 58, 1101–1109. [Google Scholar] [CrossRef]
- Kang, S.F.; Liao, C.H.; Po, S.T. Decolorization of textile wastewater by photo-Fenton oxidation technology. Chemosphere 2000, 41, 1287–1294. [Google Scholar] [CrossRef]
- Gavril Rațu, R.N.; Stoica, F.; Lipșa, F.D.; Constantin, O.E.; Stănciuc, N.; Aprodu, I.; Râpeanu, G. Pumpkin and Pumpkin By-Products: A Comprehensive Overview of Phytochemicals, Extraction, Health Benefits, and Food Applications. Foods 2024, 13, 2694. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nour, M.M.; Tony, M.A.; Nabwey, H.A. Immobilization of magnetic nanoparticles on cellulosic wooden sawdust for competitive Nudrin elimination from environmental waters as a green strategy: Box–Behnken design optimization. Int. J. Environ. Res. Public. Health 2022, 19, 15397. [Google Scholar] [CrossRef]
- Tony, M.A.; Lin, L.-S. Iron recovery from acid mine drainage sludge as a Fenton source for municipal wastewater treatment. Int. J. Environ. Anal. Chem. 2020, 100, 1071–1083. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Tsoutsa, E.K.; Kyzas, G.Z.; Katsoyiannis, I.A. Sustainable use of low-cost adsorbents prepared from waste fruit peels for the removal of selected reactive and basic dyes found in wastewaters. Environ. Sci. Pollut. Res. Int. 2024, 31, 14662–14689. [Google Scholar] [CrossRef] [PubMed]
- Yun Wang, Xiao-hui Xu, Juan Han, Yong-sheng Yan, Separation/enrichment of trace tetracycline antibiotics in water by [Bmim]BF4–(NH4)2SO4 aqueous two-phase solvent sublation. Desalination 2011, 266, 114–118. [CrossRef]
- Shamshad, J.; Ur Rehman, R. Innovative approaches to sustainable wastewater treatment: A comprehensive exploration of conventional and emerging technologies. Environ. Sci. Adv. 2025, 4, 189–222. [Google Scholar] [CrossRef]
- Zhang, T. Sustainable wastewater treatment and the circular economy. Water 2025, 17, 335. [Google Scholar] [CrossRef]
- Hung, Y.-T. Advances in biotechnologies for water and wastewater treatment. Water 2025, 17, 509. [Google Scholar] [CrossRef]
- Lee, C.; Lin, L.-S.; Martínez-Huitle, C.A.; Pensini, E. Towards decentralized and sustainable water and wastewater treatment technologies. Sci. Rep. 2025, 15, 14331. [Google Scholar] [CrossRef]
- Islam, F.A.S. Advanced wastewater treatment technologies in addressing future water scarcity through resource recovery and reuse. J. Eng. Res. Rep. 2025, 27, 370–398. [Google Scholar] [CrossRef]
- Rossi, S.; Capson-Tojo, G.; Sànchez-Zurano, A.; Carecci, D.; Batstone, D.J.; Acìén-Fernandez, G.F.; Ficara, E. Recent advances and challenges in mechanistic modelling of photosynthetic processes for wastewater treatment. Water Res. 2025, 278, 123216. [Google Scholar] [CrossRef]
- Chiang, P.C.; Chang, E.E.; Liang, C.H. NOM characteristics and treatabilities of ozonation processes. Chemosphere 2002, 46, 929–936. [Google Scholar] [CrossRef]
- Daneshvar, N.; Salari, D.; Khataee, A.R. Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J. Photochem. Photobiol. A: Chem. 2004, 162, 317–322. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. Utilization of agro-industrial and municipal waste materials as potential adsorbents for water treatment—A review. Chem. Eng. J. 2010, 157, 277–296. [Google Scholar] [CrossRef]
- Thiam, A.; Salazar, R.; Brillas, E.; Sirés, I. In-situ dosage of Fe2+ catalyst using natural pyrite for thiamphenicol mineralization by photoelectro-Fenton process. J. Environ. Manag. 2020, 270, 110835. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The Materials Project—A Materials Genome Approach to Accelerating Materials Innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef]
- Wu, C.-C.; Chen, D.H. Spontaneous Synthesis of Gold Nanoparticles on Gum-Arabic–Modified Magnetic Nanoparticles. Nanoscale Res. Lett. 2012, 7, 182. [Google Scholar] [CrossRef]
- Donadel, K.; Felisberto, M.D.; Laranjeira, M.C. Preparation and characterization of hydroxyapatite-coated iron oxide particles by spray-drying technique. Acad Bras Cienc 2009, 81, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Haddad, M.; Regti, A.; Laamari, M.R.; Mamouni, R.; Saffaj, N. Use of Fenton reagent as advanced oxidative process for remov-ing textile dyes from aqueous solutions. J. Mater. Environ. Sci. 2014, 5, 667–674. [Google Scholar]
- Youssef, N.S.; Shaban, A.; Ibrahim, F.A.; Mahmoud, A.S. Degradation of methyl orange using Fenton catalytic reaction. Egy. J. Pet. 2016, 25, 317–321. [Google Scholar] [CrossRef]
- Xu, H.; Lu, G. On-line spectrophotometric method for decolourizing reaction kinetics of reactive black 5 by Fenton oxidation. Asian J. Chem. 2013, 25, 7989–7992. [Google Scholar] [CrossRef]
- Thabet, R.H.; Fouad, M.K.; El Sherbiny, S.A.; Tony, M.A. Construction of a hetero-junction recyclable composite photocatalyst from aluminum-based waste/magnetite for efficient carbamate insecticide oxidation. Environ. Sci. Water Res. Technol. 2022, 8, 1874–1894. [Google Scholar] [CrossRef]






| Type of Process/ Catalyst | Removal Efficiency | Operating Conditions | Oxidation Time | Reference |
|---|---|---|---|---|
| Heterogeneous photo-Fenton/Fe3O4 nanoparticles | 80% | pH 3–4, UV irradiation, H2O2 100 mg/L, catalyst 0.5 g/L | 60–90 min | [35] |
| Photocatalysis/Fe-doped TiO2 | 86% | pH 5.0–7.0, UV light, catalyst 0.5 g/L | 120 min | [42] |
| Photocatalysis/ZnO nanoparticles | 83% | pH 6.0, solar/UV light, catalyst 1.0 g/L | 90–120 min | [43] |
| Adsorption + Fenton/activated carbon/Fe3+ composite | 81–94% | pH 3.0, H2O2 50–80 mg/L, catalyst 1.0 g/L | 40–70 min | [44] |
| Biomass-derived photo-Fenton/pumpkin peel–Magnetite (PK-P/Fe3O4) | 100% | pH 5.7, H2O2 100 mg/L, UV irradiation, catalyst 10 mg/L | 25 min | Current work |
| Model and Equation * | Model Parameters | Values | |||
|---|---|---|---|---|---|
| T, °C | |||||
| 32 °C | 40 °C | 50 °C | 60 °C | ||
| 0th-order model | k0 (min−1) | 0.68 | 0.65 | 0.67 | 0.66 |
| t1/2 (min) | 14.70 | 15.38 | 14.92 | 15.15 | |
| R2 | 0.85 | 0.78 | 0.58 | 0.73 | |
| 1st-order model | k1 (min−1) | 0.064 | 0.0211 | 0.015 | 0.0103 |
| t1/2 (min) | 67.28 | 46.2 | 32.84 | 10.82 | |
| R2 | 0.93 | 0.96 | 0.95 | 0.87 | |
| 2nd-order model | k2 (L·mg−1·min−1) | 0.0123 | 0.0382 | 0.0185 | 0.0177 |
| t1/2 (min) | 4.065 | 1.31 | 2.70 | 2.82 | |
| R2 | 0.88 | 0.86 | 0.51 | 0.79 | |
| Temperature, °C | Ln k2 | Ea, kJ mol−1 | ΔG′, kJ mol−1 | ΔH′, kJ mol−1 | ΔS′, J mol−1 |
|---|---|---|---|---|---|
| 32 | −2.74 | 43.22 | 80.06 | 40.74 | −131.51 |
| 40 | −3.85 | 86.82 | 40.63 | −147.5 | |
| 50 | −4.199 | 90.59 | 40.54 | −154.95 | |
| 60 | −4.57 | 94.52 | 40.46 | −162.35 |
| Cycle | Removal (%) | k1 (min−1) | R2 |
|---|---|---|---|
| Fresh use | 100 | 0.064 | 0.93 |
| 1st cycle | 98 | 0.056 | 0.94 |
| 2nd cycle | 93 | 0.053 | 0.91 |
| 3rd cycle | 91 | 0.046 | 0.92 |
| 4th cycle | 87 | 0.040 | 0.90 |
| 5th cycle | 82 | 0.034 | 0.87 |
| 6th cycle | 80 | 0.030 | 0.86 |
| 7th cycle | 77 | 0.027 | 0.91 |
| 8th cycle | 75 | 0.026 | 0.93 |
| 9th cycle | 72 | 0.022 | 0.88 |
| 10th cycle | 70 | 0.018 | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nour, M.M.; Tony, M.A.; Fouad, M.K.; Nabwey, H.A. Hybrid PK-P/Fe3O4 Catalyst Derived from Pumpkin Peel (Bio-Waste) for Synozol Red KHL Dye Oxidation Under Photo-Fenton Reaction. Catalysts 2025, 15, 977. https://doi.org/10.3390/catal15100977
Nour MM, Tony MA, Fouad MK, Nabwey HA. Hybrid PK-P/Fe3O4 Catalyst Derived from Pumpkin Peel (Bio-Waste) for Synozol Red KHL Dye Oxidation Under Photo-Fenton Reaction. Catalysts. 2025; 15(10):977. https://doi.org/10.3390/catal15100977
Chicago/Turabian StyleNour, M. M., Maha A. Tony, Mai K. Fouad, and Hossam A. Nabwey. 2025. "Hybrid PK-P/Fe3O4 Catalyst Derived from Pumpkin Peel (Bio-Waste) for Synozol Red KHL Dye Oxidation Under Photo-Fenton Reaction" Catalysts 15, no. 10: 977. https://doi.org/10.3390/catal15100977
APA StyleNour, M. M., Tony, M. A., Fouad, M. K., & Nabwey, H. A. (2025). Hybrid PK-P/Fe3O4 Catalyst Derived from Pumpkin Peel (Bio-Waste) for Synozol Red KHL Dye Oxidation Under Photo-Fenton Reaction. Catalysts, 15(10), 977. https://doi.org/10.3390/catal15100977







