Photocatalytic Remediation of Carcinogenic Polycyclic Aromatic Hydrocarbons (PAHs) Using UV/FeCl3 in Industrial Soil
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Samples Area
4.2. Analysis Methods
4.2.1. Photocatalysis Treatment Method and Extraction of PAHCs
4.2.2. Determination of PAHCs Using GCMSMS/TQD
4.2.3. Quality Standards for Analysis Methods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bolden, A.L.; Rochester, J.R.; Schultz, K.; Kwiatkowski, C.F. Polycyclic aromatic hydrocarbons and female reproductive health: A scoping review. Reprod. Toxicol. 2017, 73, 61–74. [Google Scholar] [CrossRef]
- Tran, L.H.; Drogui, P.; Mercier, G.; Blais, J.F. Comparison between Fenton oxidation process and electrochemical oxidation for PAH removal from an amphoteric surfactant solution. J. Appl. Electrochem. 2010, 40, 1493–1510. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review. Chem. Eng. J. 2016, 284, 582–598. [Google Scholar] [CrossRef]
- Zhong, P.; Zhang, X.; Yu, X.; Yang, S.; Yang, P.; Li, T.; Zhao, X.; Li, X. Soil photocatalysis revisited: Unveiling potential in organic contaminant remediation. Chem. Eng. J. 2025, 521, 166992. [Google Scholar] [CrossRef]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic aromatic hydrocarbons: Sources, toxicity, and remediation approaches. Front. Microbiol. 2020, 11, 562813. [Google Scholar] [CrossRef]
- Sahoo, S.; Acharya, R. An overview on recent developments in synthesis and molecular level structure of visible-light responsive g-C3N4 photocatalyst towards environmental remediation. Mater. Today Proc. 2021, 35, 150–155. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T., Jr. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Bouzaida, I.; Ferronato, C.; Chovelon, J.M.; Rammah, M.E.; Herrmann, J.M. Heterogeneous photocatalytic degradation of the anthraquinonic dye, Acid Blue 25 (AB25): A kinetic approach. J. Photochem. Photobiol. A Chem. 2004, 168, 23–30. [Google Scholar] [CrossRef]
- Jia, S.; Shu, X.; Song, H.; An, Z.; Xiang, X.; Zhang, J.; Zhu, Y.; He, J. Insights into Photocatalytic Selective Dehydrogenation of Ethanol over Au/Anatase–Rutile TiO2. Ind. Eng. Chem. Res. 2021, 60, 12282–12291. [Google Scholar] [CrossRef]
- Valenzuela, M.A.; Albiter, E.; Ríos-Bernÿ, O.; Córdova, I.; Flores, S.O. Photocatalytic reduction of organic compounds. J. Adv. Oxid. Technol. 2010, 13, 321–340. [Google Scholar] [CrossRef]
- Bastani, S.; Jalali Kandeloos, A.; Jalili, M.; Ghahari, M. Nanocomposites Based on Upconversion Nanoparticles. In Upconversion Nanoparticles (UCNPs) for Functional Applications; Springer Nature: Singapore, 2023; pp. 127–163. [Google Scholar]
- Assabane, A.; Ichou, Y.A.; Tahiri, H.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation of polycarboxylic benzoic acids in UV-irradiated aqueous suspensions of titania.: Identification of intermediates and reaction pathway of the photomineralization of trimellitic acid (1, 2, 4-benzene tricarboxylic acid). Appl. Catal. B Environ. 2000, 24, 71–87. [Google Scholar] [CrossRef]
- Li, L.; Chen, X.; Quan, X.; Qiu, F.; Zhang, X. Synthesis of CuOx/TiO2 Photocatalysts with Enhanced Photocatalytic Performance. ACS Omega 2023, 8, 2723–2732. [Google Scholar] [CrossRef]
- Baig, A.; Siddique, M.; Panchal, S. A review of visible-light-active zinc oxide photocatalysts for environmental application. Catalysts 2025, 15, 100. [Google Scholar] [CrossRef]
- Monteiro, F.C.; Guimaraes, I.D.L.; de Almeida Rodrigues, P.; de Pinho, J.V.D.A.; Conte-Junior, C.A. Degradation of PAHs using TiO2 as a semiconductor in the heterogeneous photocatalysis process: A systematic review. J. Photochem. Photobiol. A Chem. 2023, 437, 114497. [Google Scholar] [CrossRef]
- Ghamarpoor, R.; Fallah, A.; Jamshidi, M. A review of synthesis methods, modifications, and mechanisms of ZnO/TiO2-based photocatalysts for photodegradation of contaminants. ACS Omega 2024, 9, 25457–25492. [Google Scholar] [CrossRef] [PubMed]
- Ismael, M. Highly effective ruthenium-doped TiO2 nanoparticles photocatalyst for visible-light-driven photocatalytic hydrogen production. New J. Chem. 2019, 43, 9596–9605. [Google Scholar] [CrossRef]
- Ismael, M. A review and recent advances in solar-to-hydrogen energy conversion based on photocatalytic water splitting over doped-TiO2 nanoparticles. Sol. Energy 2020, 211, 522–546. [Google Scholar] [CrossRef]
- Gupta, B.; Melvin, A.A. TiO2/RGO composites: Its achievement and factors involved in hydrogen production. Renew. Sustain. Energy Rev. 2017, 76, 1384–1392. [Google Scholar] [CrossRef]
- Khalid, N.R.; Majid, A.; Tahir, M.B.; Niaz, N.A.; Khalid, S. Carbonaceous-TiO2 nanomaterials for photocatalytic degradation of pollutants: A review. Ceram. Int. 2017, 43, 14552–14571. [Google Scholar] [CrossRef]
- Devi, L.G.; Kavitha, R.J.A.S.S. A review on plasmonic metal⿿ TiO2 composite for generation, trapping, storing and dynamic vectorial transfer of photogenerated electrons across the Schottky junction in a photocatalytic system. Appl. Surf. Sci. 2016, 360, 601–622. [Google Scholar] [CrossRef]
- Tan, L.L.; Chai, S.P.; Mohamed, A.R. Synthesis and applications of graphene--based TiO2 photocatalysts. ChemSusChem 2012, 5, 1868–1882. [Google Scholar] [CrossRef] [PubMed]
- Papoulis, D. Halloysite based nanocomposites and photocatalysis: A Review. Appl. Clay Sci. 2019, 168, 164–174. [Google Scholar] [CrossRef]
- Li, C.; Zhu, N.; Yang, S.; He, X.; Zheng, S.; Sun, Z.; Dionysiou, D.D. A review of clay based photocatalysts: Role of phyllosilicate mineral in interfacial assembly, microstructure control and performance regulation. Chemosphere 2021, 273, 129723. [Google Scholar] [CrossRef]
- Saber, N.B.; Mezni, A.; Alrooqi, A.; Altalhi, T. A review of ternary nanostructures based noble metal/semiconductor for environmental and renewable energy applications. J. Mater. Res. Technol. 2020, 9, 15233–15262. [Google Scholar] [CrossRef]
- Sekar, P.; Bericat-Vadell, R.; Patehebieke, Y.; Broqvist, P.; Wallentin, C.J.; Gorlin, M.; Sá, J. Decoupling plasmonic hot carrier from thermal catalysis via electrode engineering. Nano Lett. 2024, 24, 8619–8625. [Google Scholar] [CrossRef]
- Yao, X.; Hong, X.; Liu, Y. Visible Mie resonances in dielectric hollow spheres: Principle, regulation, and applications. Responsive Mater. 2023, 1, e20230019. [Google Scholar] [CrossRef]
- Manjavacas, A.; Liu, J.G.; Kulkarni, V.; Nordlander, P. Plasmon-induced hot carriers in metallic nanoparticles. ACS Nano 2014, 8, 7630–7638. [Google Scholar] [CrossRef] [PubMed]
- Richardson, H.H.; Hickman, Z.N.; Govorov, A.O.; Thomas, A.C.; Zhang, W.; Kordesch, M.E. Thermooptical properties of gold nanoparticles embedded in ice: Characterization of heat generation and melting. Nano Lett. 2006, 6, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Baqais, A.; El-Saeid, M.H.; Alshabanat, M. UV-light-induced photocatalytic degradation of organic pesticides in agricultural soils with Fe2O3 and H2O2. J. Saudi Chem. Soc. 2024, 28, 101953. [Google Scholar] [CrossRef]
- De la Obra, I.; Esteban García, B.; García Sánchez, J.L.; Casas López, J.L.; Sánchez Pérez, J.A. Low cost UVA-LED as a radiation source for the photo-Fenton process: A new approach for micropollutant removal from urban wastewater. Photochem. Photobiol. Sci. 2017, 16, 72–78. [Google Scholar] [CrossRef]
- López-Vinent, N.; Cruz-Alcalde, A.; Gutiérrez, C.; Marco, P.; Giménez, J.; Esplugas, S. Micropollutant removal in real WW by photo-Fenton (circumneutral and acid pH) with BLB and LED lamps. Chem. Eng. J. 2020, 379, 122416. [Google Scholar] [CrossRef]
- Pliego, G.; Garcia-Muñoz, P.; Zazo, J.A.; Casas, J.A.; Rodriguez, J.J. Improving the Fenton process by visible LED irradiation. Environ. Sci. Pollut. Res. 2016, 23, 23449–23455. [Google Scholar] [CrossRef]
- Petryayeva, E.; Krull, U.J. Localized surface plasmon resonance: Nanostructures, bioassays and biosensing—A review. Anal. Chim. Acta 2011, 706, 8–24. [Google Scholar] [CrossRef]
- Zhu, L.; Gao, M.; Peh, C.K.; Ho, G.W. Solar-driven photothermal nanostructured materials designs and prerequisites for evaporation and catalysis applications. Mater. Horiz. 2018, 5, 323–343. [Google Scholar] [CrossRef]
- Li, S.; Miao, P.; Zhang, Y.; Wu, J.; Zhang, B.; Du, Y.; Han, X.; Sun, J.; Xu, P. Recent advances in plasmonic nanostructures for enhanced photocatalysis and electrocatalysis. Adv. Mater. 2021, 33, 2000086. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.G.; Sandhel, A.; Wood, T.E.; Ali, F.M.; Hoch, L.B.; Perovic, D.D.; Mims, C.A.; Ozin, G.A. Photomethanation of gaseous CO2 over Ru/Silicon nanowire catalysts with visible and near--infrared photons. Adv. Sci. 2014, 1, 1400001. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Bai, B.; Liu, J. Temperature-driven coupled transport of pollutants and suspended particles established by granular thermodynamics. Int. J. Heat Mass Transf. 2024, 228, 125645. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Y.; Guo, X.; Chen, Z.; Zhang, W.; Wang, Y.; Tang, X.; Zhang, Y.; Zhao, Y. Sulfur-doped g-C3N4/rGO porous nanosheets for highly efficient photocatalytic degradation of refractory contaminants. J. Mater. Sci. Technol. 2020, 41, 117–126. [Google Scholar] [CrossRef]
- Saeed, M.; Alwadai, N.; Farhat, L.B.; Baig, A.; Nabgan, W.; Iqbal, M. Co3O4-Bi2O3 heterojunction: An effective photocatalyst for photodegradation of rhodamine B dye. Arab. J. Chem. 2022, 15, 103732. [Google Scholar] [CrossRef]
- Quiroga, J.M.; Riaza, A.; Manzano, M.A. Chemical degradation of PCB in the contaminated soils slurry: Direct Fenton oxidation and desorption combined with the photo-Fenton process. J. Environ. Sci. Health Part A 2009, 44, 1120–1126. [Google Scholar] [CrossRef]
- Xu, C.; Dong, D.; Meng, X.; Su, X.; Zheng, X.; Li, Y. Photolysis of polycyclic aromatic hydrocarbons on soil surfaces under UV irradiation. J. Environ. Sci. 2013, 25, 569–575. [Google Scholar] [CrossRef]
- Huang, J.; Tu, Z.; Teng, X.; Tang, X.; Fouda, M.M.; Abdel-Rahman, H.S.; Qu, R. The effect of soil organic matter on the photodegradation of polycyclic aromatic hydrocarbons: Experimental analysis and excited-state theory calculations. J. Environ. Chem. Eng. 2025, 13, 117891. [Google Scholar] [CrossRef]
- Khataee, A.; Salahpour, F.; Fathinia, M.; Seyyedi, B.; Vahid, B. Iron rich laterite soil with mesoporous structure for heterogeneous Fenton-like degradation of an azo dye under visible light. J. Ind. Eng. Chem. 2015, 26, 129–135. [Google Scholar] [CrossRef]
- Hodaifa, G.; Agabo García, C.; Borja, R. Study of Catalysts’ Influence on Photocatalysis/Photodegradation of Olive Oil Mill Wastewater. Determ. Optim. Work. Conditions. Catal. 2020, 10, 554. [Google Scholar]
- Lucas, M.S.; Beltrán-Heredia, J.; Sanchez-Martin, J.; Garcia, J.; Peres, J.A. Treatment of high strength olive mill wastewater by Fenton’s reagent and aerobic biological process. J. Environ. Sci. Health Part A 2013, 48, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Nieto, L.M.; Hodaifa, G.; Rodríguez, S.; Giménez, J.A.; Ochando, J. Degradation of organic matter in olive-oil mill wastewater through homogeneous Fenton-like reaction. Chem. Eng. J. 2011, 173, 503–510. [Google Scholar] [CrossRef]
- Hodaifa, G.; Ochando-Pulido, J.M.; Rodriguez-Vives, S.; Martinez-Ferez, A. Optimization of continuous reactor at pilot scale for olive-oil mill wastewater treatment by Fenton-like process. Chem. Eng. J. 2013, 220, 117–124. [Google Scholar] [CrossRef]
- García, C.A.; Hodaifa, G. Real olive oil mill wastewater treatment by photo-Fenton system using artificial ultraviolet light lamps. J. Clean. Prod. 2017, 162, 743–753. [Google Scholar] [CrossRef]
- Zhang, L.; Li, P.; Gong, Z.; Li, X. Photocatalytic degradation of polycyclic aromatic hydrocarbons on soil surfaces using TiO2 under UV light. J. Hazard. Mater. 2008, 158, 478–484. [Google Scholar] [CrossRef]
- Zhao, X.; Qin, L.; Gatheru Waigi, M.; Cheng, P.; Yang, B.; Wang, J.; Ling, W. Removal of bound PAH residues in contaminated soils by Fenton oxidation. Catalysts 2019, 9, 619. [Google Scholar] [CrossRef]
- Zhang, L.H.; Li, P.J.; Gong, Z.Q.; Oni, A.A. Photochemical behavior of benzo [a] pyrene on soil surfaces under UV light irradiation. J. Environ. Sci. 2006, 18, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Ormad, M.P.; Mosteo, R.; Ibarz, C.; Ovelleiro, J.L. Multivariate approach to the photo-Fenton process applied to the degradation of winery wastewaters. Appl. Catal. B Environ. 2006, 66, 58–63. [Google Scholar] [CrossRef]
- Martens, D.A.; Frankenberger, W.T., Jr. Enhanced degradation of polycyclic aromatic hydrocarbons in soil treated with an advanced oxidative process—Fenton’s reagent. Soil Sediment Contam. 1995, 4, 175–190. [Google Scholar] [CrossRef]
- Ziolli, R.L.; Jardim, W.F. Photocatalytic decomposition of seawater-soluble crude-oil fractions using high surface area colloid nanoparticles of TiO2. J. Photochem. Photobiol. A Chem. 2002, 147, 205–212. [Google Scholar] [CrossRef]
- Silva, M.J.; Soares, S.A.; Santos, I.D.; Pepe, I.M.; Teixeira, L.R.; Pereira, L.G.; Silva, L.B.; Celino, J.J. Optimization of the photocatalytic degradation process of aromatic organic compounds applied to mangrove sediment. Heliyon 2020, 6, e05163. [Google Scholar] [CrossRef]
- Oliveira, L.C.; Fabris, J.D.; Pereira, M.C. Iron oxides and their applications in catalytic processes: A review. Quim. Nova 2013, 36, 123–130. [Google Scholar] [CrossRef]
- Casanova Monteiro, F.; Caetano, E.H.; de Jesus Cubas, P.; Pupin, A.V.; Monteiro, J.F.; Fujiwara, S.T. Bi2Fe4O9 in pellet form is an alternative in the wastewater treatment process. J. Environ. Sci. Health Part A 2020, 55, 677–685. [Google Scholar] [CrossRef]
- de Jesus Cubas, P.; Semkiw, A.W.; Monteiro, F.C.; Los Weinert, P.; Monteiro, J.F.; Fujiwara, S.T. Synthesis of CuCr2O4 by self-combustion method and photocatalytic activity in the degradation of Azo Dye with visible light. J. Photochem. Photobiol. A Chem. 2020, 401, 112797. [Google Scholar]
- Aleboyeh, A.; Aleboyeh, H.; Moussa, Y. “Critical” effect of hydrogen peroxide in photochemical oxidative decolorization of dyes: Acid Orange 8, Acid Blue 74 and Methyl Orange. Dye. Pigment. 2003, 57, 67–75. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y.; Wan, J.; Gong, X.; Zhu, Y. Degradation of atrazine by a novel Fenton-like process and assessment the influence on the treated soil. J. Hazard. Mater. 2016, 312, 184–191. [Google Scholar] [CrossRef]
- Li, F.B.; Li, X.Z.; Liu, C.S.; Li, X.M.; Liu, T.X. Effect of oxalate on photodegradation of Bisphenol A at the interface of different iron oxides. Ind. Eng. Chem. Res. 2007, 46, 781–787. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.S.; Li, F.B.; Liu, C.P.; Liang, J.B. Photodegradation of polycyclic aromatic hydrocarbon pyrene by iron oxide in solid phase. J. Hazard. Mater. 2009, 162, 716–723. [Google Scholar] [CrossRef]
- Gupta, H.; Gupta, B. Photocatalytic degradation of polycyclic aromatic hydrocarbon benzo [a] pyrene by iron oxides and identification of degradation products. Chemosphere 2015, 138, 924–931. [Google Scholar] [CrossRef]
- Eker, G.; Şengül, B.; Cindoruk, S.S. Performance evaluation of diethylamine to the removal of polycyclic aromatic hydrocarbons (PAHs) from polluted soils with sunlight. Polycycl. Aromat. Compd. 2021, 41, 306–318. [Google Scholar] [CrossRef]
- Jia, H.; Li, L.; Chen, H.; Zhao, Y.; Li, X.; Wang, C. Exchangeable cations-mediated photodegradation of polycyclic aromatic hydrocarbons (PAHs) on smectite surface under visible light. J. Hazard. Mater. 2015, 287, 16–23. [Google Scholar] [CrossRef]
- Dong, Y.; Liang, X.; Krumholz, L.R.; Philp, R.P.; Butler, E.C. The relative contributions of abiotic and microbial processes to the transformation of tetrachloroethylene and trichloroethylene in anaerobic microcosms. Environ. Sci. Technol. 2009, 43, 690–697. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.; Chen, Z.; Li, X.; Li, P. Photodegradation of pyrene on soil surfaces under UV light irradiation. J. Hazard. Mater. 2010, 173, 168–172. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.; Jia, H.; Lichtfouse, E.; Sharma, V.K. Abiotic transformation of polycyclic aromatic hydrocarbons via interaction with soil components: A systematic review. Crit. Rev. Environ. Sci. Technol. 2023, 53, 676–699. [Google Scholar] [CrossRef]
- De Luca, A.; Dantas, R.F.; Esplugas, S. Study of Fe (III)-NTA chelates stability for applicability in photo-Fenton at neutral pH. Appl. Catal. B Environ. 2015, 179, 372–379. [Google Scholar] [CrossRef]
- De Luca, A. Fenton and Photo-Fenton Like at Neutral pH for the Removal of Emerging Contaminants in Water and Wastewater Effluents; Universitat de Barcelona: Barcelona, Spain, 2016. [Google Scholar]
- Sonwani, R.K.; Giri, B.S.; Geed, S.R.; Sharma, A.; Singh, R.S.; Rai, B.N. Combination of UV-Fenton oxidation process with biological technique for treatment of polycyclic aromatic hydrocarbons using Pseudomonas pseudoalcaligenes NRSS3 isolated from petroleum contaminated site. Indian J. Exp. Biol. (IJEB) 2018, 56, 460–469. [Google Scholar]
- Manian, R. Bioremediation of polycyclic aromatic hydrocarbons contaminated soils: Recent progress, perspectives and challenges. Environ. Monit. Assess. 2023, 195, 1441. [Google Scholar] [CrossRef]
- Han, D.; Wan, J.; Ma, Y.; Wang, Y.; Li, Y.; Li, D.; Guan, Z. New insights into the role of organic chelating agents in Fe (II) activated persulfate processes. Chem. Eng. J. 2015, 269, 425–433. [Google Scholar] [CrossRef]
- Yang, X.; Cai, H.; Bao, M.; Yu, J.; Lu, J.; Li, Y. Highly Efficient Photocatalytic Remediation of Simulated Polycyclic Aromatic Hydrocarbons (PAHs) Contaminated Wastewater under Visible Light Irradiation by Graphene Oxide Enwrapped Ag3PO4 Composite. Chin. J. Chem. 2017, 35, 1549–1558. [Google Scholar] [CrossRef]
- Hussain, A.; Al-Barakah, F.N.; Al-Sewailem, M.; El-Saeid, M.H.; Waqar, M.; Ahmad, M. Oxidative photodegradation of pyrene and fluoranthene by Fe-based and Zn-based Fenton reagents. Sustainability 2017, 9, 870. [Google Scholar] [CrossRef]
- Karaca, G. Spatial distribution of polycyclic aromatic hydrocarbon (PAH) concentrations in soils from Bursa, Turkey. Arch. Environ. Contam. Toxicol. 2016, 70, 406–417. [Google Scholar] [CrossRef]
- El-Saeid, M.H.; Al-Turki, A.M.; Nadeem, M.E.; Hassanin, A.S.; Al-Wabel, M.I. Photolysis degradation of polyaromatic hydrocarbons (PAHs) on surface sandy soil. Environ. Sci. Pollut. Res. 2015, 22, 9603–9616. [Google Scholar] [CrossRef]
- Konieczka, P.; Namieśnik, J. Estimating uncertainty in analytical procedures based on chromatographic techniques. J. Chromatogr. A 2010, 1217, 882–891. [Google Scholar] [CrossRef]
- Adeniji, A.O.; Okoh, O.O.; Okoh, A.I. Analytical methods for the determination of the distribution of total petroleum hydrocarbons in the water and sediment of aquatic systems: A review. J. Chem. 2017, 2017, 5178937. [Google Scholar] [CrossRef]
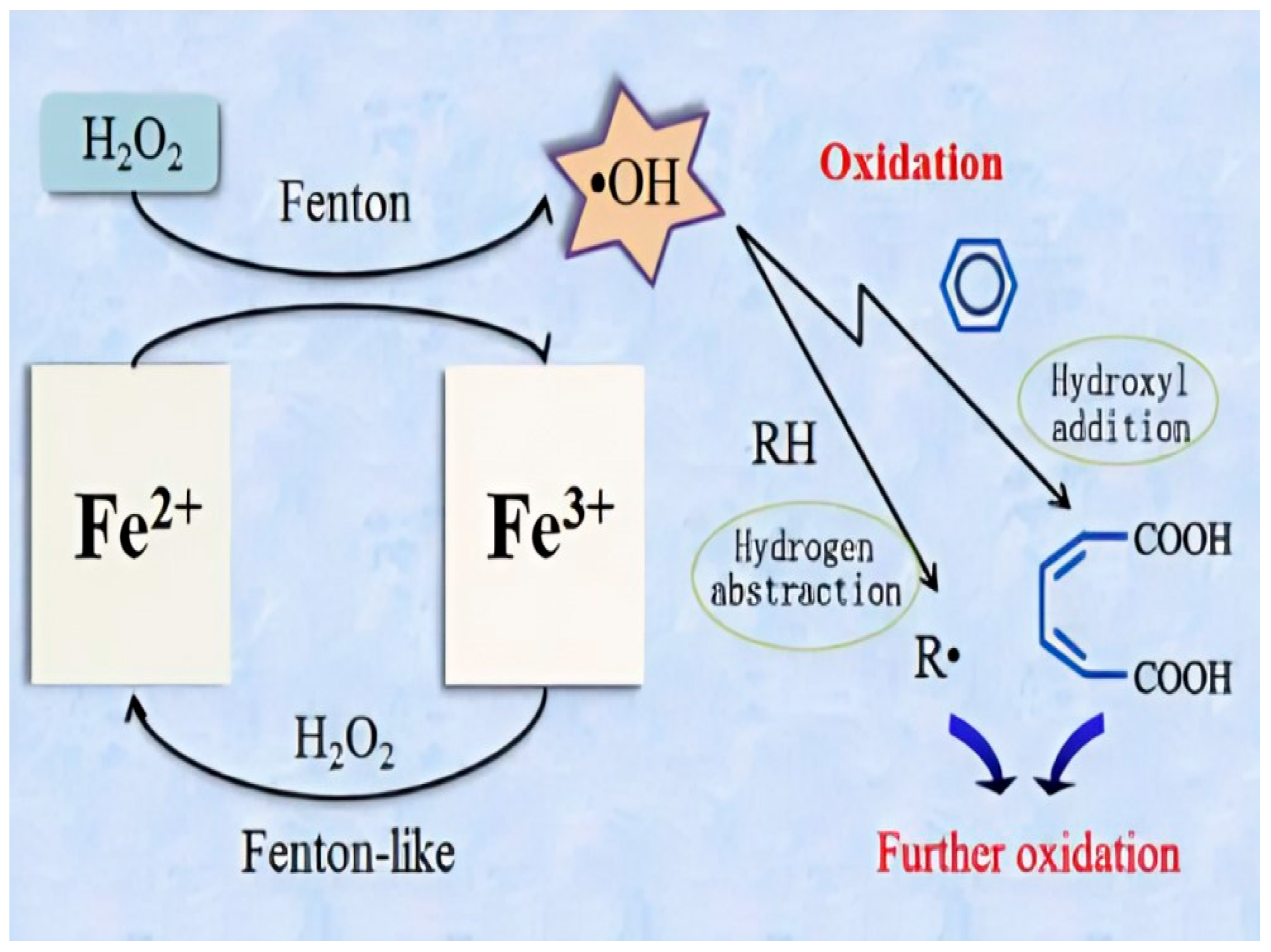
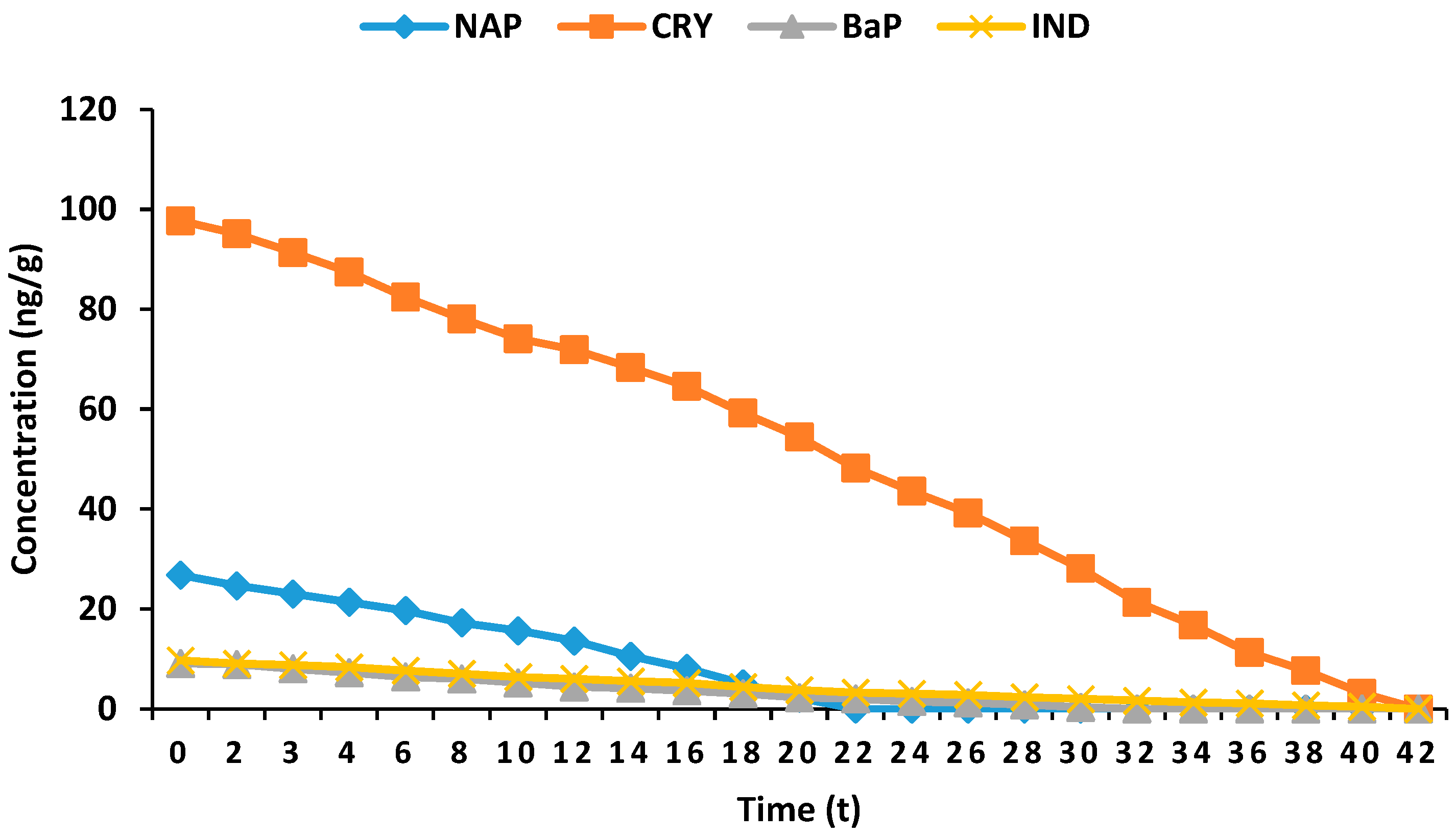

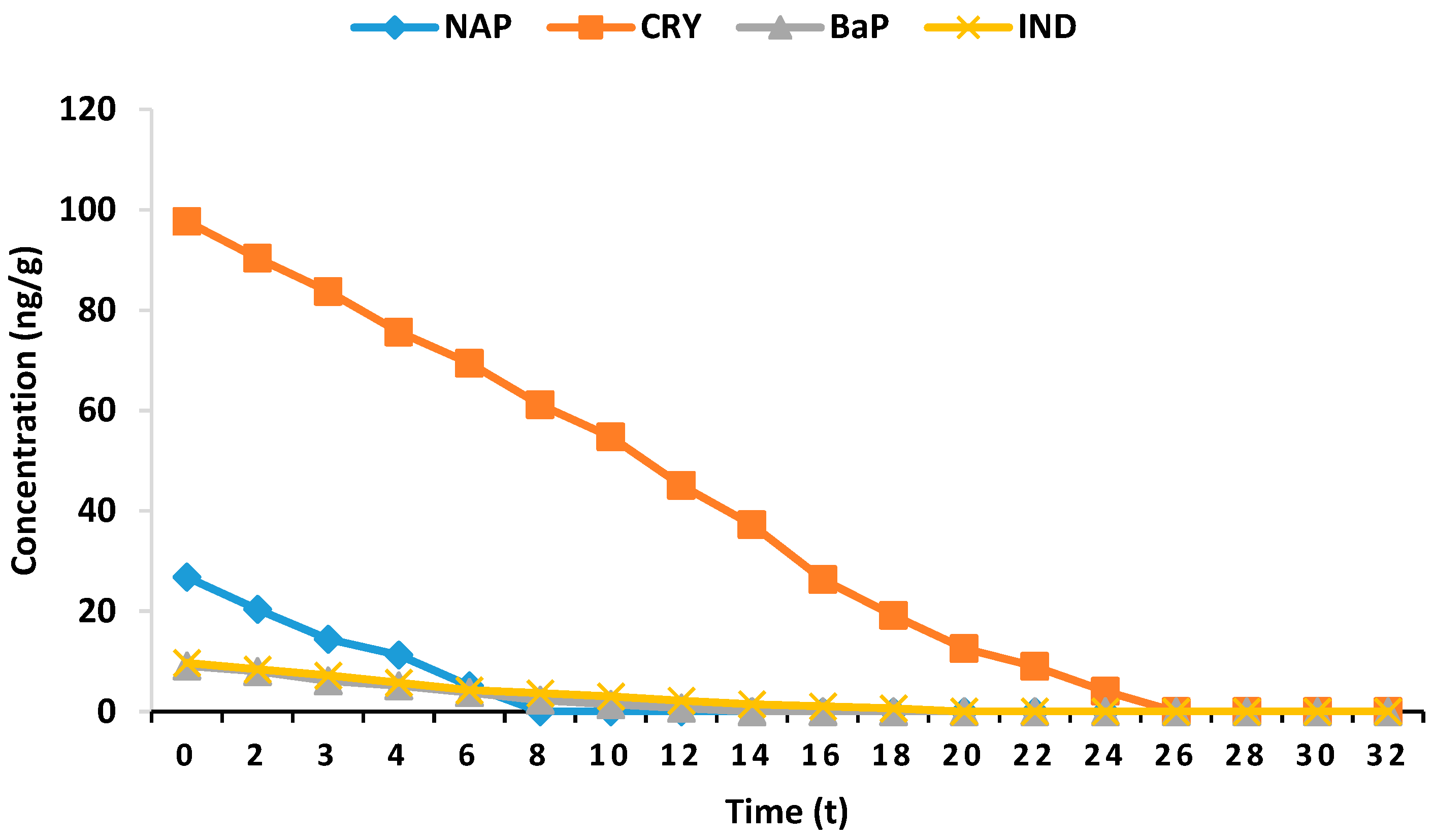
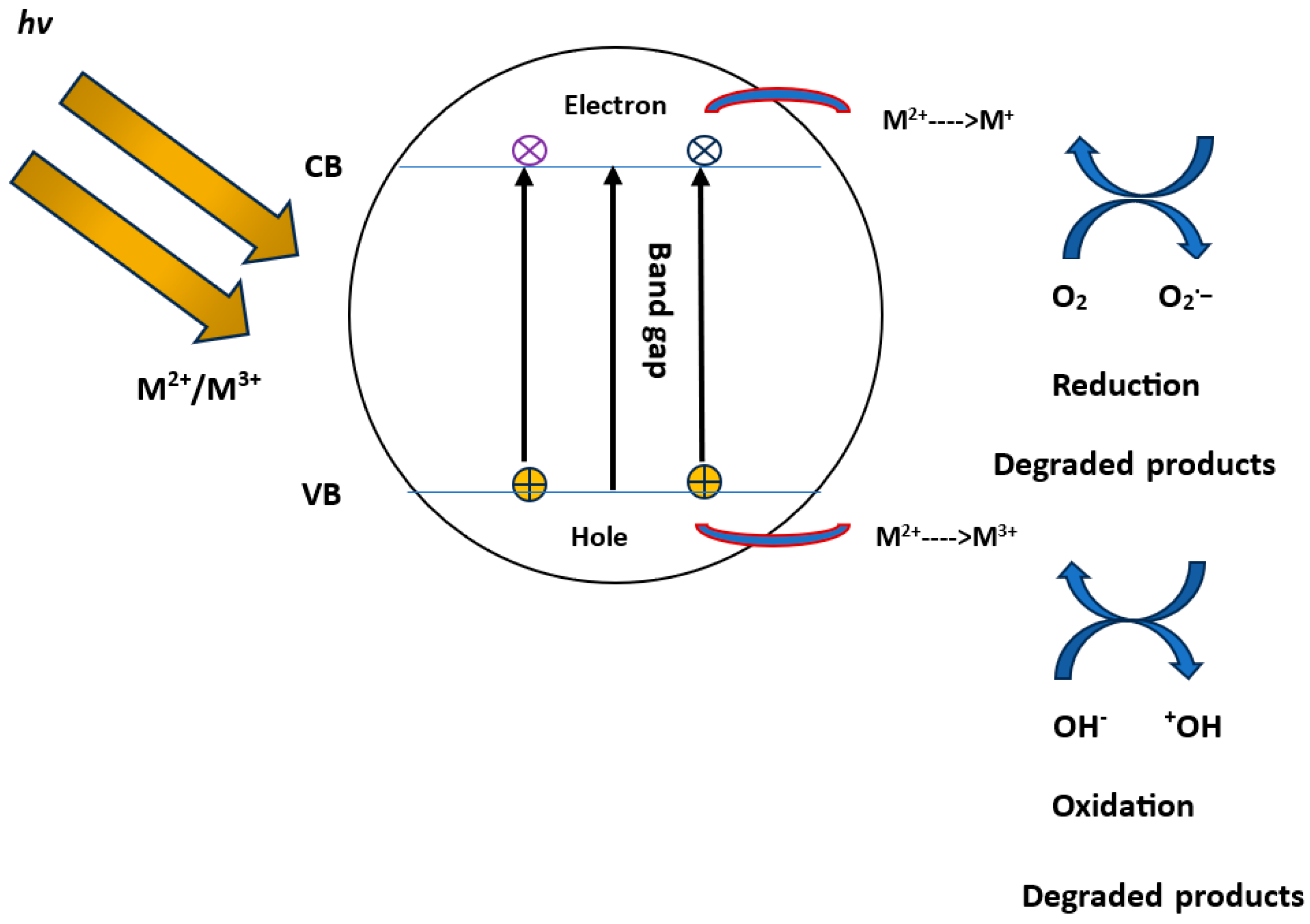
| Time (h) | PAHCs (ng/g) | |||
|---|---|---|---|---|
| NAP | CRY | BaP | IND | |
| 0 | 26.8 ± 1.87 | 97.7 ± 0.7 | 9.1 ± 0.7 | 9.7 ± 0.4 |
| 2 | 24.66 ± 2.08 | 95.11 ± 2.03 | 8.91 ± 0.42 | 9.06 ± 0.34 |
| 3 | 23.02 ± 3.56 | 91.34 ± 2.15 | 8.03 ± 0.51 | 8.78 ± 1.58 |
| 4 | 21.37 ± 3.22 | 87.44 ± 1.46 | 7.25 ± 1.33 | 8.35 ± 1.17 |
| 6 | 19.68 ± 1.44 | 82.41 ± 1.46 | 6.39 ± 1.22 | 7.66 ± 1.02 |
| 8 | 17.23 ± 1.71 | 78.11 ± 1.06 | 6.01 ± 1.04 | 7.04 ± 1.22 |
| 10 | 15.66 ± 1.16 | 74.12 ± 2.19 | 5.24 ± 1.12 | 6.38 ± 1.06 |
| 12 | 13.63 ± 1.23 | 71.88 ± 2.71 | 4.41 ± 1.78 | 6.02 ± 1.14 |
| 14 | 10.55 ± 1.43 | 68.33 ± 2.27 | 4.13 ± 0.62 | 5.55 ± 0.72 |
| 16 | 8.12 ± 0.82 | 64.57 ± 2.01 | 3.61 ± 0.44 | 5.17 ± 0.23 |
| 18 | 5.03 ± 0.57 | 59.22 ± 2.46 | 3.04 ± 0.32 | 4.39 ± 0.61 |
| 20 | 2.17 ± 0.36 | 54.46 ± 1.23 | 2.27 ± 0.41 | 3.77 ± 0.81 |
| 22 | Not detected | 48.22 ± 1.16 | 2.02 ± 0.61 | 3.27 ± 0.31 |
| 24 | Not detected | 43.57 ± 0.81 | 1.48 ± 0.27 | 3.06 ± 0.56 |
| 26 | Not detected | 39.23 ± 2.26 | 1.21 ± 0.37 | 2.81 ± 0.71 |
| 28 | Not detected | 33.66 ± 2.39 | 0.83 ± 0.17 | 2.35 ± 0.44 |
| 30 | Not detected | 28.11 ± 2.28 | 0.33 ± 0.11 | 2.02 ± 0.22 |
| 32 | Not detected | 21.33 ± 2.03 | Not detected | 1.64 ± 0.45 |
| 34 | Not detected | 16.78 ± 1.35 | Not detected | 1.29 ± 0.33 |
| 36 | Not detected | 11.28 ± 1.17 | Not detected | 1.11 ± 0.22 |
| 38 | Not detected | 7.66 ± 1.34 | Not detected | 0.71 ± 0.28 |
| 40 | Not detected | 3.14 ± 0.22 | Not detected | 0.46 ± 0.11 |
| 42 | Not detected | Not detected | Not detected | Not detected |
| Time (h) | PAHCs (ng/g) | |||
|---|---|---|---|---|
| NAP | CRY | BaP | IND | |
| 0 | 26.8 ± 1.87 | 97.7 ± 0.7 | 9.1 ± 0.7 | 9.7 ± 0.4 |
| 2 | 22.11 ± 1.26 | 93.55 ± 3.39 | 8.69 ± 1.11 | 9.04 ± 0.75 |
| 3 | 19.51 ± 1.03 | 89.22 ± 3.09 | 7.28 ± 0.77 | 8.11 ± 0.49 |
| 4 | 16.29 ± 0.89 | 85.37 ± 3.27 | 6.39 ± 0.57 | 7.02 ± 0.41 |
| 6 | 11.44 ± 1.37 | 81.88 ± 1.66 | 5.22 ± 0.61 | 6.17 ± 0.36 |
| 8 | 7.78 ± 1.71 | 75.60 ± 1.89 | 4.18 ± 0.45 | 5.01 ± 0.19 |
| 10 | 4.66 ± 1.02 | 70.19 ± 2.52 | 3.66 ± 0.33 | 4.07 ± 0.42 |
| 12 | Not detected | 64.77 ± 2.92 | 2.71 ± 0.29 | 3.22 ± 0.61 |
| 14 | Not detected | 57.08 ± 1.71 | 1.84 ± 0.26 | 2.51 ± 0.26 |
| 16 | Not detected | 48.66 ± 2.38 | 1.27 ± 0.21 | 2.17 ± 0.31 |
| 18 | Not detected | 39.11 ± 2.04 | 0.73 ± 0.06 | 1.73 ± 0.42 |
| 20 | Not detected | 30.28 ± 1.33 | 0.23 ± 0.11 | 1.02 ± 0.16 |
| 22 | Not detected | 23.79 ± 1.88 | Not detected | 0.61 ± 0.09 |
| 24 | Not detected | 16.33 ± 2.04 | Not detected | 0.37 ± 0.06 |
| 26 | Not detected | 10.29 ± 1.44 | Not detected | Not detected |
| 28 | Not detected | 7.46 ± 1.37 | Not detected | Not detected |
| 30 | Not detected | 2.41 ± 1.03 | Not detected | Not detected |
| 32 | Not detected | Not detected | Not detected | Not detected |
| Time (h) | PAHCs | |||
|---|---|---|---|---|
| NAP | CRY | BaP | IND | |
| 0 | 26.8 ± 1.87 | 97.7 ± 0.7 | 9.1 ± 0.7 | 9.7 ± 0.4 |
| 2 | 20.38 ± 0.78 | 90.39 ± 2.06 | 8.02 ± 1.24 | 8.41 ± 1.51 |
| 3 | 14.45 ± 1.19 | 83.77 ± 2.59 | 6.11 ± 0.69 | 7.21 ± 0.66 |
| 4 | 11.27 ± 0.93 | 75.66 ± 2.68 | 5.15 ± 0.72 | 5.77 ± 0.49 |
| 6 | 5.16 ± 0.82 | 69.44 ± 1.55 | 3.78 ± 0.44 | 4.26 ± 0.78 |
| 8 | Not detected | 61.18 ± 1.21 | 2.27 ± 0.67 | 3.66 ± 0.48 |
| 10 | Not detected | 54.77 ± 2.36 | 1.44 ± 0.57 | 3.02 ± 0.17 |
| 12 | Not detected | 45.08 ± 2.11 | 0.71 ± 0.23 | 2.18 ± 0.15 |
| 14 | Not detected | 37.23 ± 1.58 | Not detected | 1.45 ± 0.28 |
| 16 | Not detected | 26.34 ± 2.17 | Not detected | 1.04 ± 0.19 |
| 18 | Not detected | 19.18 ± 0.84 | Not detected | 0.65 ± 0.12 |
| 20 | Not detected | 12.56 ± 1.12 | Not detected | Not detected |
| 22 | Not detected | 8.99 ± 1.04 | Not detected | Not detected |
| 24 | Not detected | 4.05 ± 0.68 | Not detected | Not detected |
| 26 | Not detected | Not detected | Not detected | Not detected |
| 28 | Not detected | Not detected | Not detected | Not detected |
| 30 | Not detected | Not detected | Not detected | Not detected |
| 32 | Not detected | Not detected | Not detected | Not detected |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
EL-Saeid, M.H.; Alghamdi, A.G.; Alasmary, Z.; Al-Bugami, T.M. Photocatalytic Remediation of Carcinogenic Polycyclic Aromatic Hydrocarbons (PAHs) Using UV/FeCl3 in Industrial Soil. Catalysts 2025, 15, 956. https://doi.org/10.3390/catal15100956
EL-Saeid MH, Alghamdi AG, Alasmary Z, Al-Bugami TM. Photocatalytic Remediation of Carcinogenic Polycyclic Aromatic Hydrocarbons (PAHs) Using UV/FeCl3 in Industrial Soil. Catalysts. 2025; 15(10):956. https://doi.org/10.3390/catal15100956
Chicago/Turabian StyleEL-Saeid, Mohamed Hamza, Abdulaziz G. Alghamdi, Zafer Alasmary, and Thawab M. Al-Bugami. 2025. "Photocatalytic Remediation of Carcinogenic Polycyclic Aromatic Hydrocarbons (PAHs) Using UV/FeCl3 in Industrial Soil" Catalysts 15, no. 10: 956. https://doi.org/10.3390/catal15100956
APA StyleEL-Saeid, M. H., Alghamdi, A. G., Alasmary, Z., & Al-Bugami, T. M. (2025). Photocatalytic Remediation of Carcinogenic Polycyclic Aromatic Hydrocarbons (PAHs) Using UV/FeCl3 in Industrial Soil. Catalysts, 15(10), 956. https://doi.org/10.3390/catal15100956








