Degradation of Tetracycline by Laccase–Mediator System Using Tea Polyphenols as Mediator
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Tetracycline Degradation Conditions with Lac–Mediator System
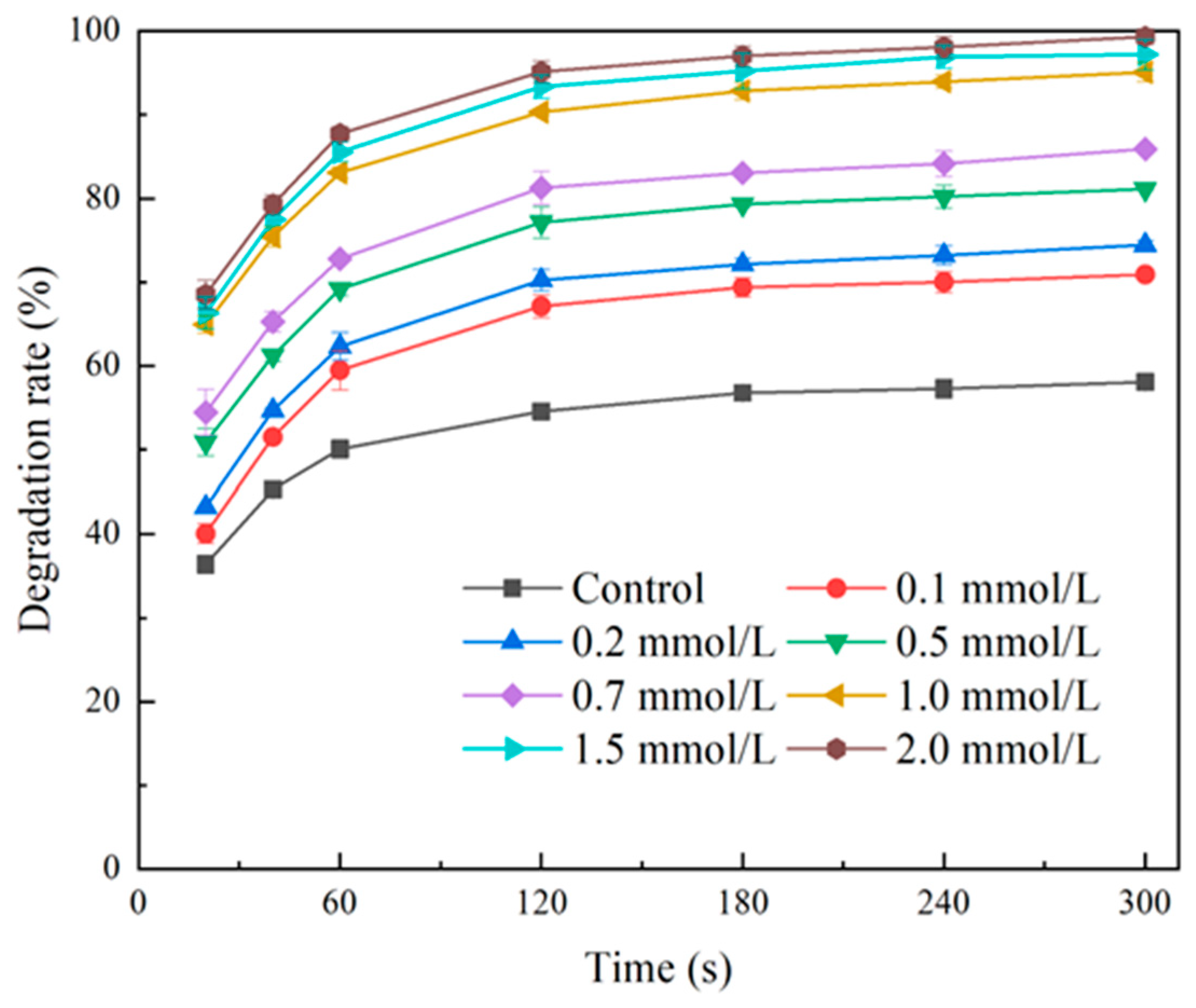
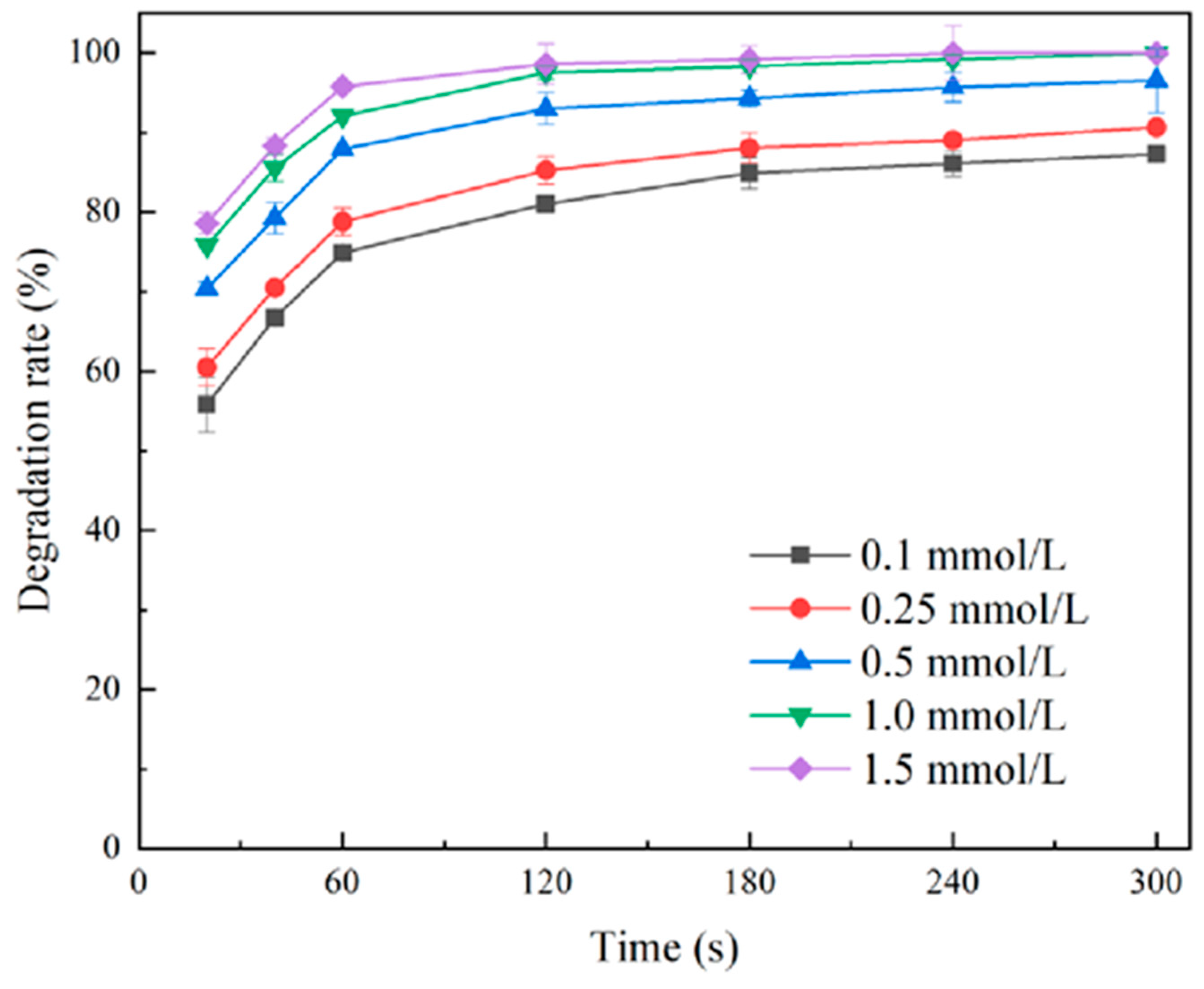
2.1.1. Effect of GTP Concentration
2.1.2. Effect of Laccase Concentration
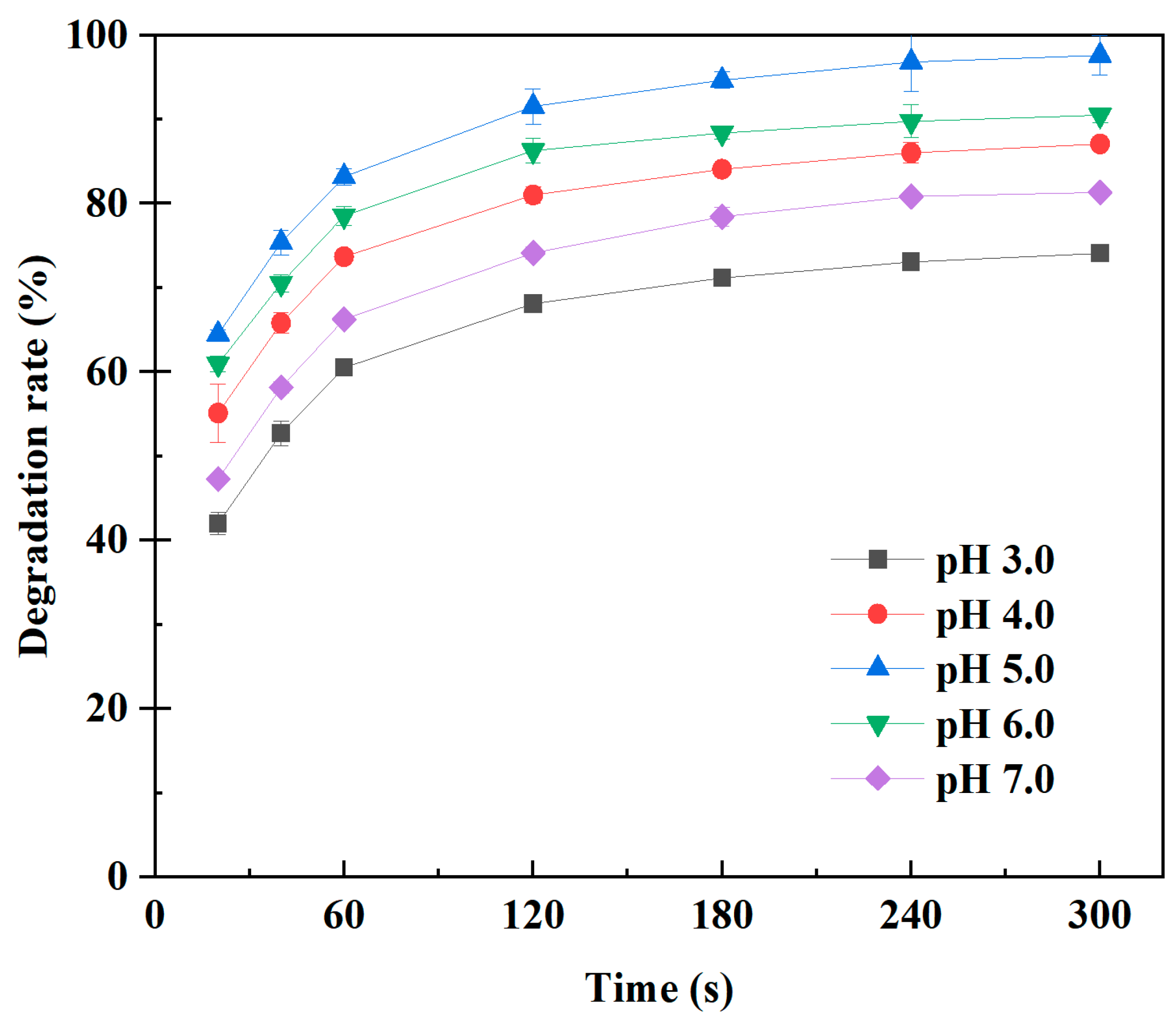
2.1.3. Effect of pH
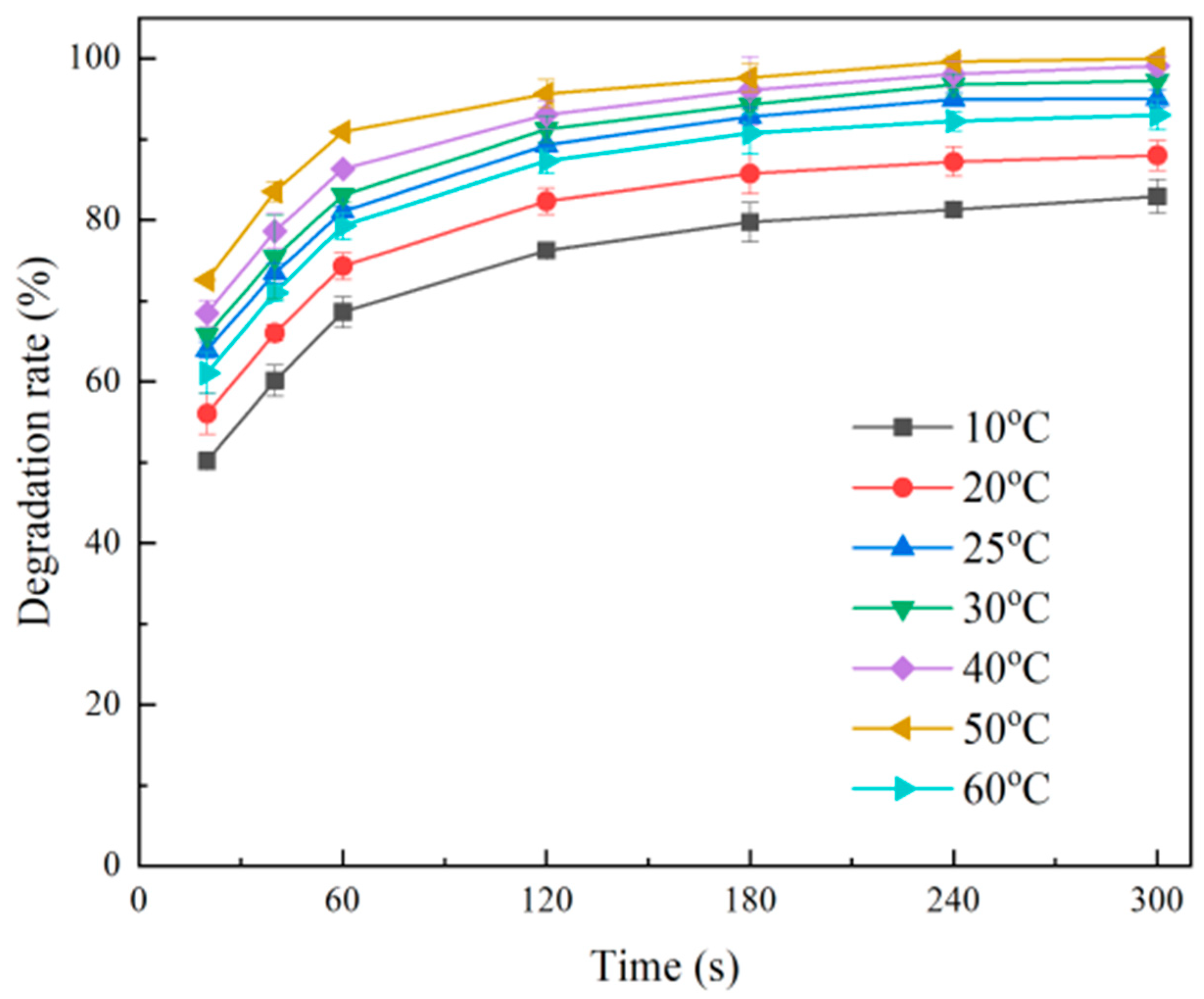
2.1.4. Effect of Temperature
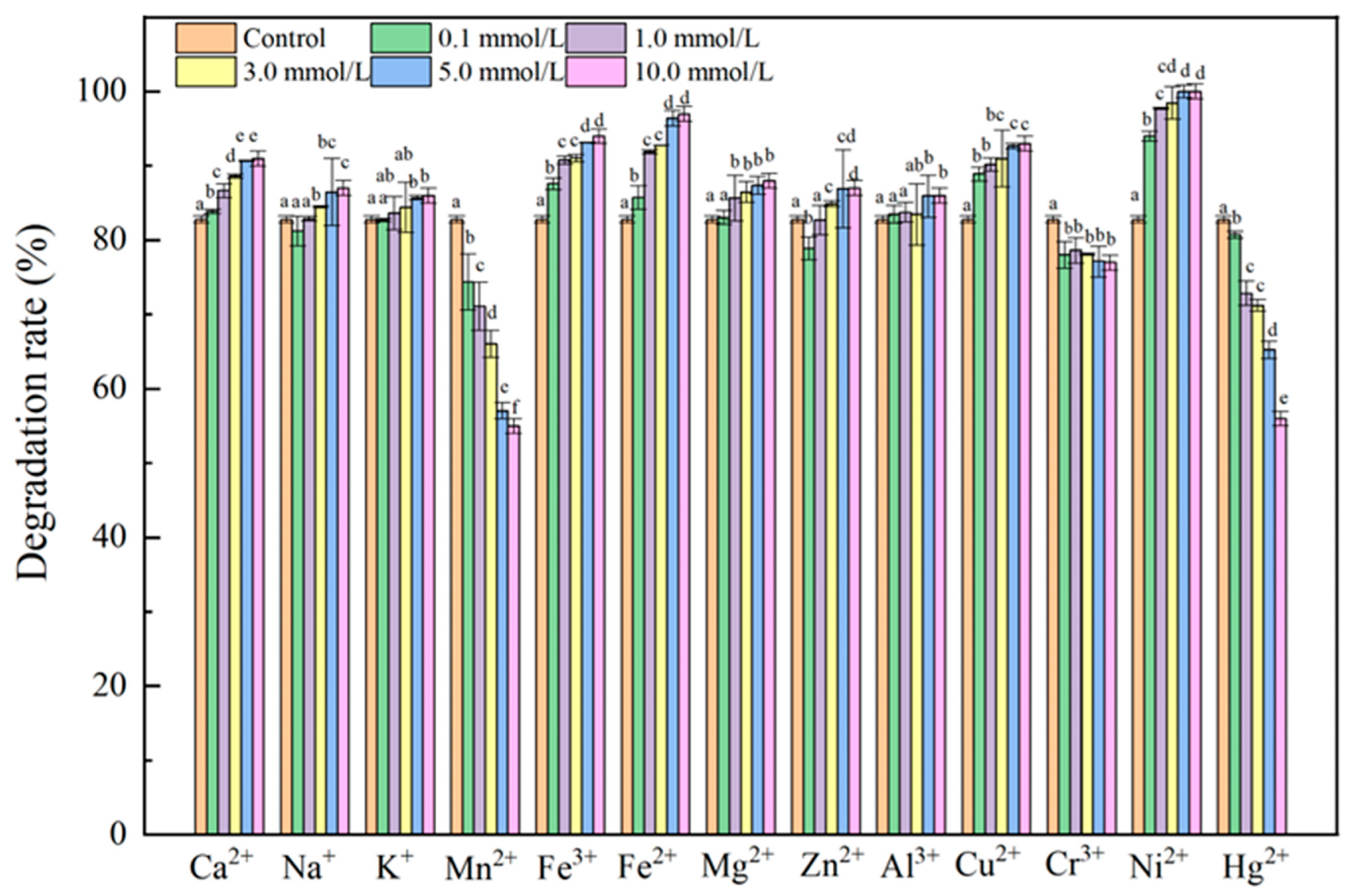
2.1.5. Effect of Different Metal Ions
2.2. The Functions of the Tea Polyphenol Components
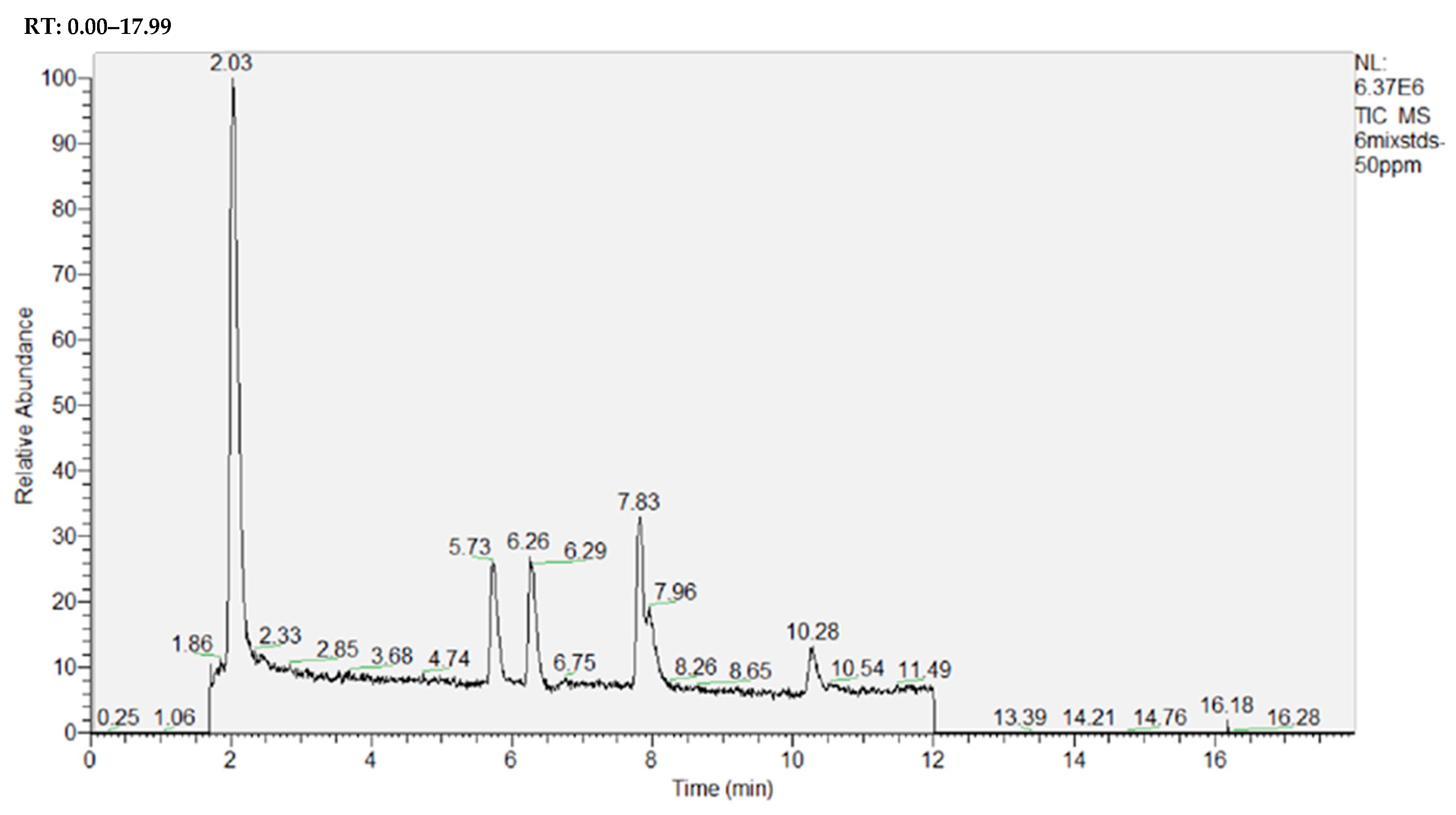
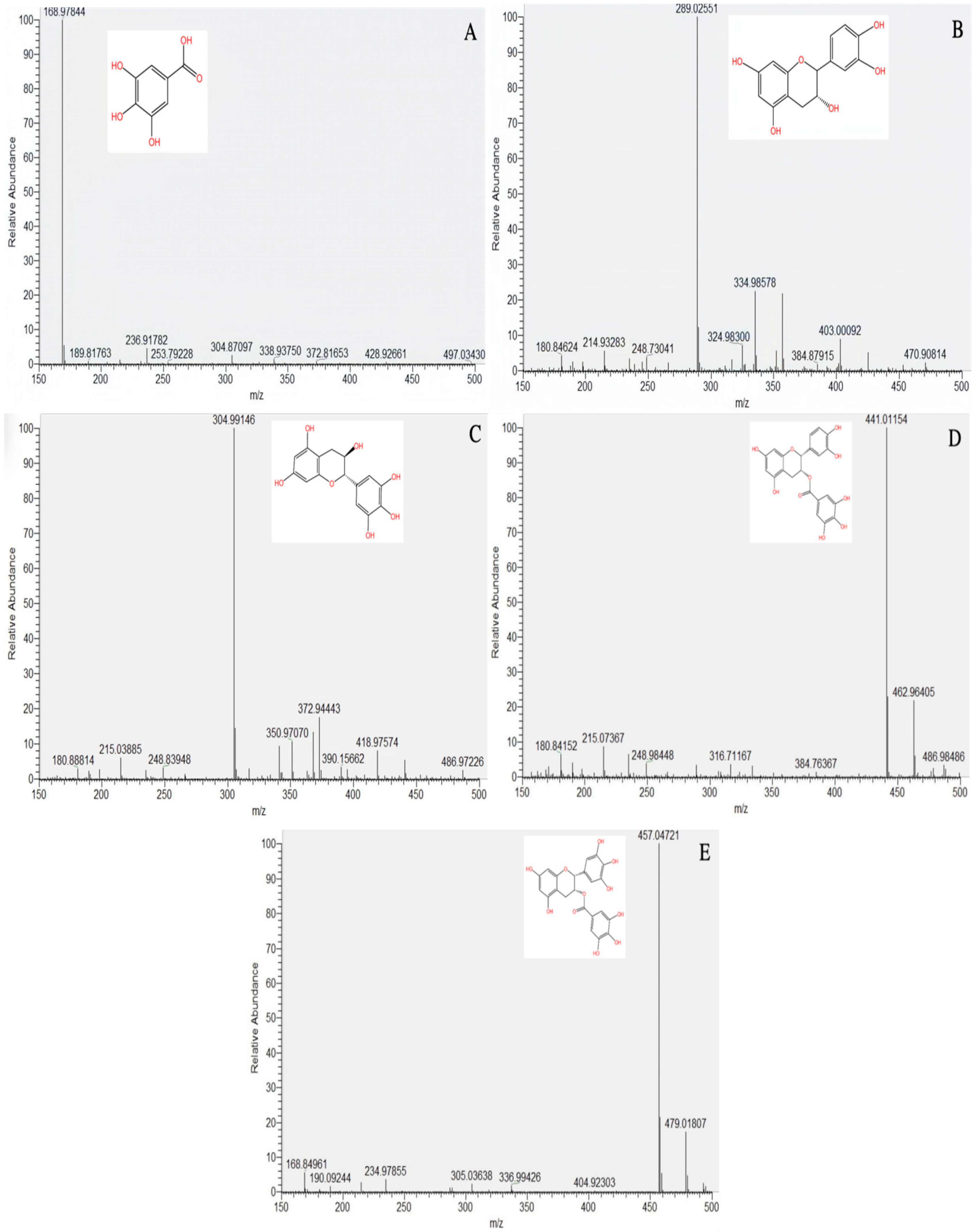
2.2.1. Main Tea Polyphenol Components
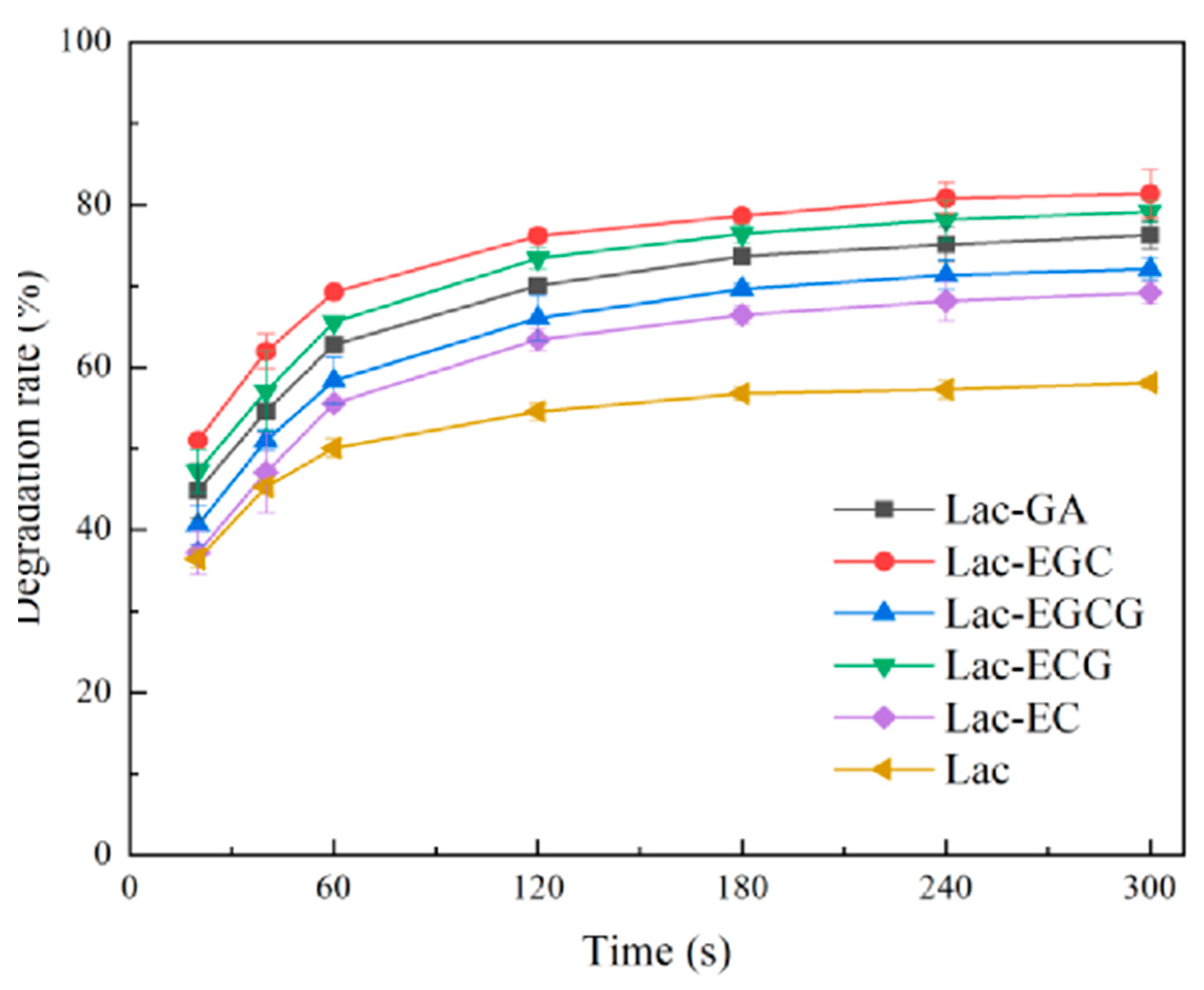
2.2.2. Mediator’s Effects on Tetracycline Degradation
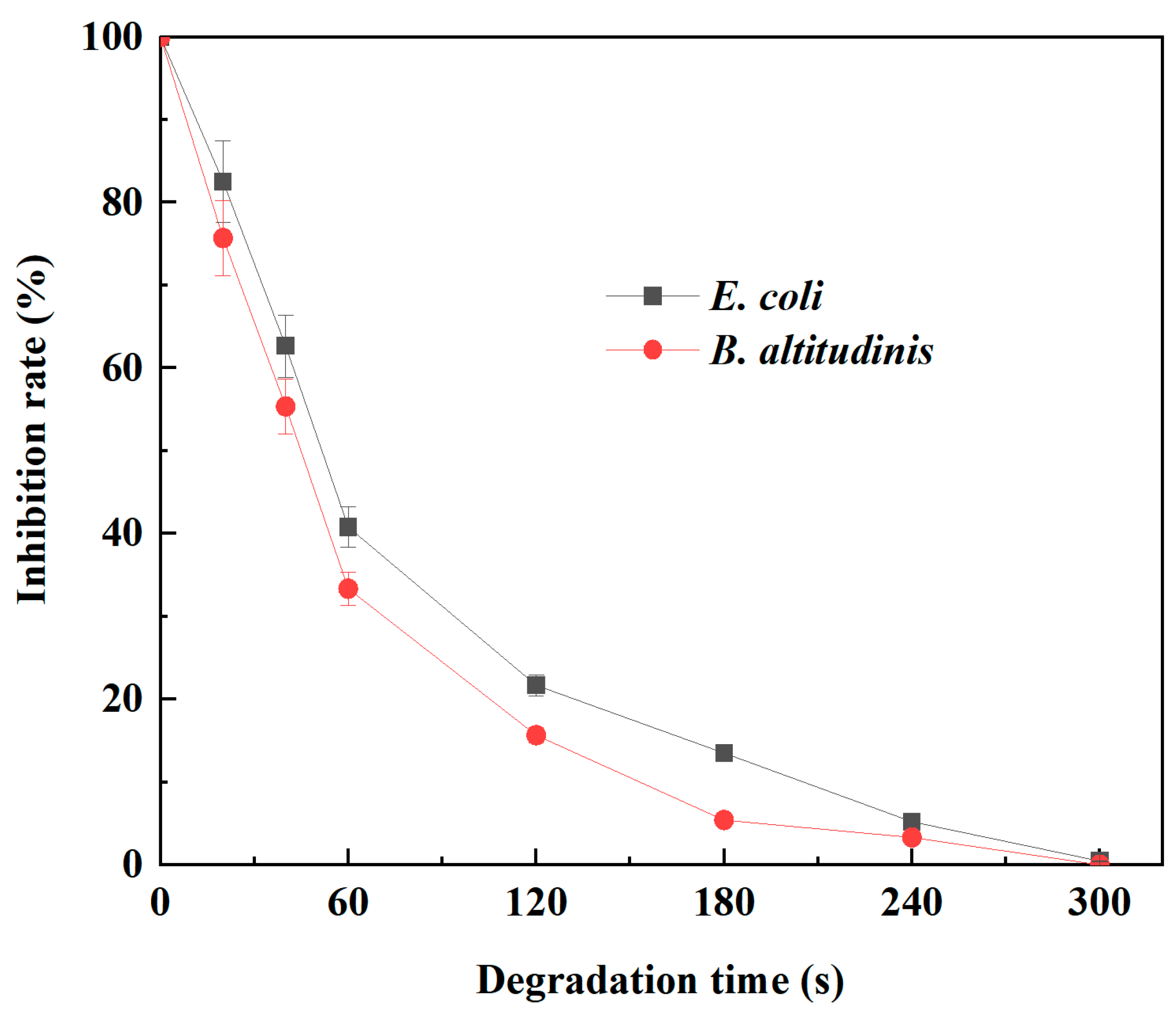
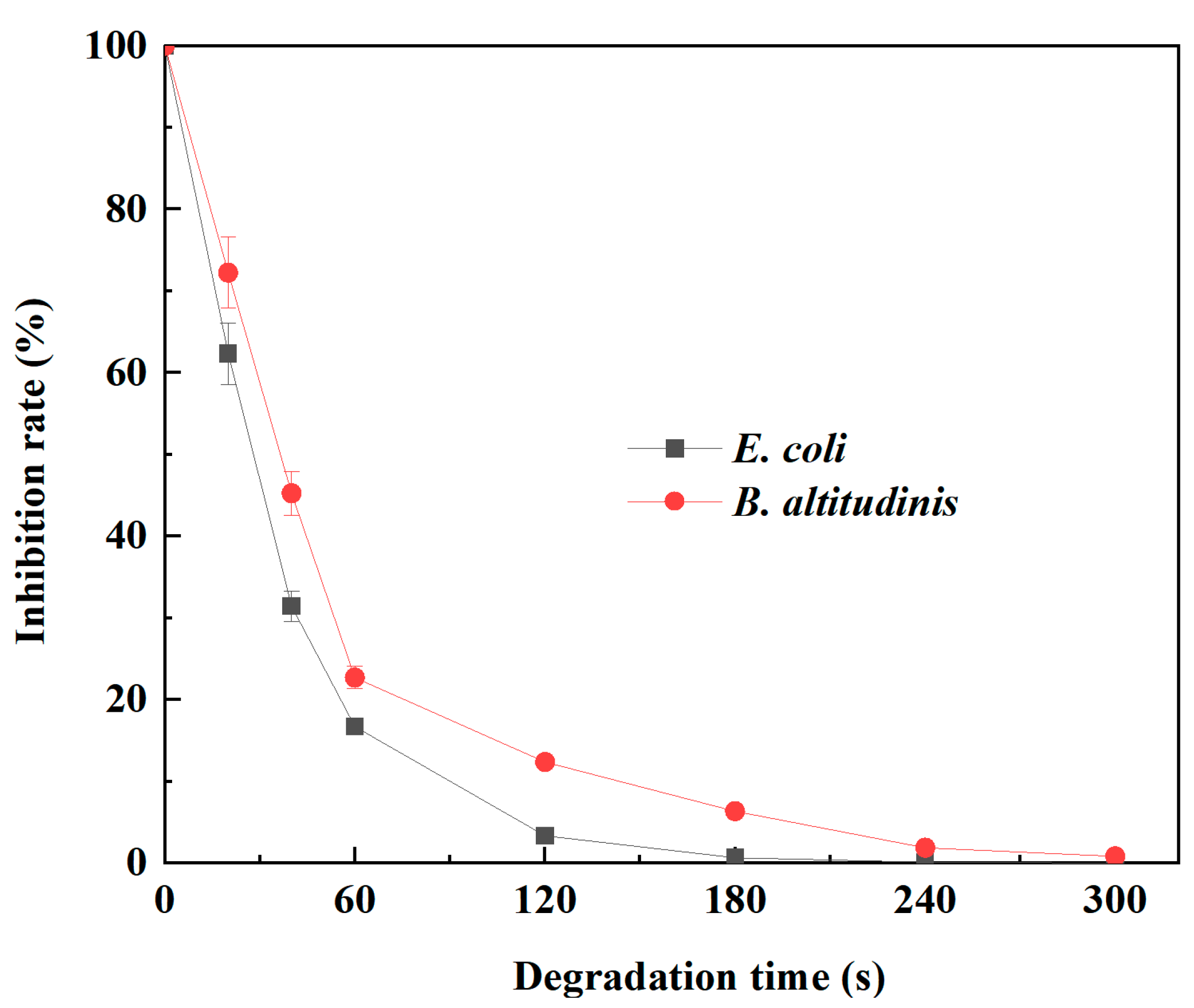
2.3. Antibacterial Activity of Tetracycline Degradation Products
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Extraction and Analysis of Polyphenols from Tea Residues
3.3. Optimization of Parameters for Tetracycline Degradation by Lac–Mediator System
3.4. Rate of Consumption of Tetracycline
3.5. Determination of Antibacterial Activity of Tetracycline Degradation Products
3.6. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Bei, Q.; Hassan, M.M.; Marimuthu, M.; Adade, S.Y.S.S.; Chen, Q.; Zareef, M. A Cu2+-modulated UCNPs@RBD sensor for sensitive detection of tetracyclines in food based on the spirolactam open-loop reaction. J. Food Compos. Anal. 2024, 133, 106374. [Google Scholar] [CrossRef]
- Leichtweis, J.; Vieira, Y.; Welter, N.; Silvestri, S.; Dotto, G.L.; Carissimi, E. A review of the occurrence, disposal, determination, toxicity and remediation technologies of the tetracycline antibiotic. Process Saf. Environ. 2022, 160, 25–40. [Google Scholar] [CrossRef]
- Amangelsin, Y.; Semenova, Y.; Dadar, M.; Aljofan, M.; Bjorklund, G. The impact of tetracycline pollution on the aquatic environment and removal strategies. Antibiotics 2023, 12, 440. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, H.; Xiong, P.; Zhu, Q.Q.; Liao, C.Y.; Jiang, G.B. Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: A review. Sci. Total Environ. 2021, 753, 141975. [Google Scholar] [CrossRef]
- Ahmadijokani, F.; Molavi, H.; Tajahmadi, S.; Rezakazemi, M.; Amini, M.; Kamkar, M.; Rojas, O.J.; Arjmand, M. Coordination chemistry of metal–organic frameworks: Detection, adsorption, and photodegradation of tetracycline antibiotics and beyond. Coordin. Chem. Rev. 2022, 464, 214562. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, H. Health risk from veterinary antimicrobial use in China′s food animal production and its reduction. Environ. Pollut. 2016, 219, 993–997. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Ying, G.G.; Pan, C.G.; Liu, Y.-S.; Zhao, J.-L. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Niu, J.; Wang, Y.; Ji, Y.; Zhang, Y. Solar photocatalytic abatement of tetracycline over phosphate oxoanion decorated Bi2WO6/polyimide composites. J. Hazard. Mater. 2021, 403, 123860. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ke, Y.; Zhu, Y.; Xu, M.; Chen, C.; Xie, S. Enrichment of tetracycline-degrading bacterial consortia: Microbial community succession and degradation characteristics and mechanism. J. Hazard. Mater. 2023, 448, 130984. [Google Scholar] [CrossRef]
- Dai, Y.; Peng, W.; Ji, Y.; Wei, J.; Che, J.; Huang, Y.; Huang, W.; Yang, W.; Xu, W. A self-powered photoelectrochemical aptasensor using 3D-carbon nitride and carbon-based metal-organic frameworks for high-sensitivity detection of tetracycline in milk and water. J. Food Sci. 2024, 89, 8022–8035. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.D.; Liu, J.Y.; Shangguan, W.F. A review on photocatalysis in antibiotic wastewater: Pollutant degradation and hydrogen production. Chin. J. Catal. 2020, 41, 1440–1450. [Google Scholar] [CrossRef]
- Philip, D.; Lakshmi, D.; Kumar, P.S.; Vo, D.V.N.; Kartik, A. Occurrence and removal of antibiotics from industrial wastewater. Environ. Chem. Lett. 2021, 19, 1477–1507. [Google Scholar] [CrossRef]
- Ouyang, Q.; Liu, Y.; Chen, Q.S.; Guo, Z.M.; Zhao, J.W.; Li, H.H.; Hu, W.W. Rapid and specific sensing of tetracycline in food using a novel upconversion aptasensor. Food Control 2017, 81, 156–163. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Hassan, M.M.; Rong, Y.W.; Liu, R.; Li, H.H.; Ouyang, Q.; Chen, Q.S. An upconversion nanosensor for rapid and sensitive detection of tetracycline in food based on magnetic-field-assisted separation. Food Chem. 2022, 373, 131497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yu, Y.; Li, Y.; Zhang, C.; Zhang, W.L.; Wang, L.F.; Niu, L.H. A novel BC/g-C3N4 porous hydrogel carrier used in intimately coupled photocatalysis and biodegradation system for efficient removal of tetracycline hydrochloride in water. Chemosphere 2023, 317, 137888. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Y.; Liu, G.; Fang, J. Study on a novel immobilized microbe pellets constructed with Alcaligenes sp. R3 and its ability to remove tetracycline. J. Environ. Chem. Eng. 2023, 11, 109378. [Google Scholar] [CrossRef]
- Liu, L.; Yin, Q.; Hou, Y.; Ma, R.; Li, Y.; Wang, Z.Y.; Yang, G.G.; Liu, Y.; Wang, H.L. Fungus reduces tetracycline-resistant genes in manure treatment by predation of bacteria. Sci. Total Environ. 2024, 906, 167462. [Google Scholar] [CrossRef]
- Phoon, B.L.; Ong, C.C.; Saheed, M.S.M.; Show, P.L.; Chang, J.S.; Ling, T.C.; Lam, S.S.; Juan, J.C. Conventional and emerging technologies for removal of antibiotics from wastewater. J. Hazard. Mater. 2020, 400, 122961. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Chen, J.L.; Hu, S.W.; Wang, H.L.; Jiang, W.; Chen, X.B. Facile construction of novel Bi2WO6/Ta3N5 Z-scheme heterojunction nanofibers for efficient degradation of harmful pharmaceutical pollutants. Chem. Eng. J. 2020, 402, 126165. [Google Scholar] [CrossRef]
- Kang, X.X.; Jia, X.G.; Kang, Z.Y.; Zhang, Y.C.; Zhang, D.B.; Wei, J.L.; Guo, A.H.; Ge, M.; He, Z.X. Activation of peroxydisulfate by black fungus derived N-doped biochar for tetracycline degradation via non-radical dominated oxidation pathway. Surf. Interfaces 2022, 31, 102007. [Google Scholar] [CrossRef]
- Chen, X.L.; Yang, Y.Y.; Ke, Y.C.; Chen, C.; Xie, S.G. A comprehensive review on biodegradation of tetracyclines: Current research progress and prospect. Sci. Total Environ. 2022, 814, 152852. [Google Scholar] [CrossRef]
- He, X.; Kai, T.; Ding, P. Heterojunction photocatalysts for degradation of the tetracycline antibiotic: A review. Environ. Chem. Lett. 2021, 19, 4563–4601. [Google Scholar] [CrossRef]
- Li, R.; Zhou, T.Y.; Khan, A.; Ling, Z.M.; Sharma, M.; Feng, P.Y.; Ali, G.; Saif, I.; Wang, H.Y.; Li, X.K. Feed-additive of bioengineering strain with surface displayed laccase degrades sulfadiazine in broiler manure and maintains intestinal flora structure. J. Hazard. Mater. 2021, 406, 124440. [Google Scholar] [CrossRef]
- Gothwal, R.; Shashidhar, T. Antibiotic pollution in the environment: A review. Clean Soil Air Water 2015, 43, 479–489. [Google Scholar] [CrossRef]
- Tian, M.; He, X.M.; Feng, Y.Z.; Wang, W.T.; Chen, H.S.; Gong, M.; Liu, D.; Liu Clarke, J.H.; van Eerde, A. Pollution by antibiotics and antimicrobial resistance in livestock and poultry manure in China, and Countermeasures. Antibiotics 2021, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.Y.; Hu, X.T.; Xu, X.C.; Zhang, W.; Zou, X.B.; Shi, J.Y.; Zheng, K.Y.; Arslan, M. A portable test strip based on fluorescent europium-based metal-organic framework for rapid and visual detection of tetracycline in food samples. Food Chem. 2021, 354, 129501. [Google Scholar] [CrossRef]
- Liang, N.; Hu, X.T.; Zhang, X.N.; Li, W.T.; Guo, Z.A.; Huang, X.W.; Li, Z.H.; Zhang, R.J.; Shen, T.T.; Zou, X.B.; et al. Ratiometric sensing for ultratrace tetracycline using electrochemically active metal-organic frameworks as response signals. J. Agric. Food Chem. 2023, 71, 7584–7592. [Google Scholar] [CrossRef]
- Tan, H.; Kong, D.L.; Ma, Q.Y.; Li, Q.Q.; Zhou, Y.Q.; Jiang, X.; Wang, Z.Y.; Parales, R.E.; Ruan, Z.Y. Biodegradation of tetracycline antibiotics by the yeast strain Cutaneotrichosporon dermatis M503. Microorganisms 2022, 10, 565. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Chen, H.B.; Wang, Y.Z.; Yang, Z.L.; Zhang, H.Y. Efficient biodegradation of chlortetracycline in high concentration from strong-acidity pharmaceutical residue with degrading fungi. J. Hazard. Mater. 2022, 424, 127671. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Jin, Y.; Yan, B.J.; Jiang, Y.; Yang, S.G.; Song, T.H. Degradation of tetracycline by heat/peroxymonosulfate and ultrasound/peroxymonosulfate systems: Performance and kinetics. Water Sci. Technol. 2024, 89, 421–433. [Google Scholar] [CrossRef]
- Zhao, L.; Dong, Y.H.; Wang, H. Residues of veterinary antibiotics in manures from feedlot livestock in eight provinces of China. Sci. Total Environ. 2010, 408, 1069–1075. [Google Scholar] [CrossRef]
- Liang, N.; Hu, X.; Li, W.; Wang, Y.; Guo, Z.; Huang, X.; Li, Z.; Zhang, X.; Zhang, J.; Xiao, J. A dual-signal fluorescent sensor based on MoS2 and CdTe quantum dots for tetracycline detection in milk. Food Chem. 2022, 378, 132076. [Google Scholar] [CrossRef]
- Lin, Y.C.; Zhuang, G.L.; Tasi, P.F.; Tseng, H.H. Removal of protein, histological dye and tetracycline from simulated bioindustrial wastewater with a dual pore size PPSU membrane. J. Hazard. Mater. 2022, 431, 128525. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, G.; Wang, H. Current state of sludge production, management, treatment and disposal in China. Water Res. 2015, 78, 60–73. [Google Scholar] [CrossRef]
- Shi, B.Q.; Zhang, X.N.; Li, W.T.; Liang, N.N.; Hu, X.T.; Xiao, J.B.; Wang, D.Y.; Zou, X.B.; Shi, J.Y. An intrinsic dual-emitting fluorescence sensing toward tetracycline with self-calibration model based on luminescent lanthanide-functionalized metal-organic frameworks. Food Chem. 2023, 400, 133995. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Tang, Y.W.; Wang, M.; Jin, C.Y.; Liu, J.Y.; Li, S.Y.; Li, Z.L.; Zhu, J.W. The enhanced peroxymonosulfate-assisted photocatalytic degradation of tetracycline under visible light by g-C3N4/Na-BiVO4 heterojunction catalyst and its mechanism. J. Environ. Chem. Eng. 2021, 9, 105524. [Google Scholar] [CrossRef]
- Saravanan, A.; Senthil Kumar, P.; Vo, D.-V.N.; Jeevanantham, S.; Karishma, S.; Yaashikaa, P.R. A review on catalytic-enzyme degradation of toxic environmental pollutants: Microbial enzymes. J. Hazard. Mater. 2021, 419, 126451. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.T.; Meng, F.P.; Zhang, B.; Xia, Y.F. Elimination of tetracyclines in seawater by laccase-mediator system. Chemosphere 2023, 333, 138916. [Google Scholar] [CrossRef]
- Lassouane, F.; Aït-Amar, H.; Amrani, S.; Rodriguez-Couto, S. A promising laccase immobilization approach for Bisphenol A removal from aqueous solutions. Bioresour. Technol. 2019, 271, 360–367. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Esmail, G.A.; Arasu, M.V. Effective degradation of tetracycline by manganese peroxidase producing Bacillus velezensis strain Al-Dhabi 140 from Saudi Arabia using fibrous-bed reactor. Chemosphere 2021, 268, 128726. [Google Scholar] [CrossRef]
- Wang, F.; Wang, M.T.; Wang, M.M.; Xu, L.; Qian, J.Y.; Guan, G.Q.; Xu, B.G. Clarification of sugarcane juice catalyzed by magnetic immobilized laccase intensified by alternating magnetic field. Foods 2025, 14, 444. [Google Scholar] [CrossRef]
- Shi, L.L.; Yu, H.B.; Dong, T.B.; Kong, W.; Ke, M.; Ma, F.Y.; Zhang, X.Y. Biochemical and molecular characterization of a novel laccase from selective lignin-degrading white-rot fungus Echinodontium taxodii 2538. Process Biochem. 2014, 49, 1097–1106. [Google Scholar] [CrossRef]
- Nazar, M.; Xu, Q.; Zahoor; Ullah, M.W.; Khan, N.A.; Iqbal, B.; Zhu, D.C. Integrated laccase delignification with improved lignocellulose recalcitrance for enhancing enzymatic saccharification of ensiled rice straw. Ind. Crop. Prod. 2023, 202, 116987. [Google Scholar] [CrossRef]
- Suda, T.; Hata, T.; Kawai, S.; Okamura, H.; Nishida, T. Treatment of tetracycline antibiotics by laccase in the presence of 1-hydroxybenzotriazole. Bioresour. Technol. 2012, 103, 498–501. [Google Scholar] [CrossRef]
- Lou, Q.; Wu, Y.X.; Ding, H.J.; Zhang, B.H.; Zhang, W.H.; Zhang, Y.; Han, L.; Liu, M.T.; He, T.; Zhong, J.Y. Degradation of sulfonamides in aquaculture wastewater by laccase-syringaldehyde mediator system: Response surface optimization, degradation kinetics, and degradation pathway. J. Hazard. Mater. 2022, 432, 128647. [Google Scholar] [CrossRef]
- Jinga, L.I.; Tudose, M.; Ionita, P. Laccase-TEMPO as an efficient system for doxorubicin removal from wastewaters. Int. J. Environ. Res. Public Health 2022, 19, 6645. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.T.; Li, C.; Wang, S.; Song, X.Q. Green tea (Camellia sinensis): A review of its phytochemistry, pharmacology, and toxicology. Molecules 2022, 27, 3909. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Gandhi, S.; Sharma, R.; Kaur, L.; Pal, M.; Deswal, G.; Chopra, B.; Grewal, A.S.; Dhingra, A.K. Extraction, phytochemistry & pharmacological potential of Camellia sinensis: A comprehensive review. Nat. Prod. J. 2024, 14, 21. [Google Scholar] [CrossRef]
- Jiao, Y.J.; Cai, M.; Zhang, X.; Feng, Z.; Zhang, Q.Z.; Li, L.L.; Jin, G.; Fan, S.S.; Lu, L.T. Impact of spreading time on flavor quality in Duyun Maojian summer green tea. LWT Food Sci. Technol. 2024, 214, 117103. [Google Scholar] [CrossRef]
- Tao, M.; Guo, W.L.; Liang, J.; Liu, Z.Q. Unraveling the key cooked off-flavor compounds in thermally sterilized green tea beverages, and masking effect of tea raw material baking. Food Chem. 2025, 464, 141671. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Hai, F.I.; Kang, J.; Leusch, F.D.L.; Roddick, F.; Magram, S.F.; Price, W.E.; Nghiem, L.D. Enhancement of trace organic contaminant degradation by crude enzyme extract from Trametes versicolor culture: Effect of mediator type and concentration. J. Taiwan Inst. Chem. E 2014, 45, 1855–1862. [Google Scholar] [CrossRef]
- Wen, X.; Jia, Y.; Li, J. Enzymatic degradation of tetracycline and oxytetracycline by crude manganese peroxidase prepared from Phanerochaete chrysosporium. J. Hazard. Mater. 2010, 177, 924–928. [Google Scholar] [CrossRef]
- Cvancarová, M.; Moeder, M.; Filipová, A.; Cajthaml, T. Biotransformation of fluoroquinolone antibiotics by ligninolytic fungi—Metabolites, enzymes and residual antibacterial activity. Chemosphere 2015, 136, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.S.; Vieira, N.; da Luz, J.M.R.; de Cássia, S.; Silva, M.; Cardoso, W.S.; Kasuya, M.C.M. Production of fungal enzymes in Macaúba Coconut and enzymatic degradation of textile dye. Biocatal. Agric. Biotechnol. 2020, 26, 101651. [Google Scholar] [CrossRef]
- Weng, S.S.; Liu, S.M.; Lai, H.T. Application parameters of laccase-mediator systems for treatment of sulfonamide antibiotics. Bioresour. Technol. 2013, 141, 152–159. [Google Scholar] [CrossRef]
- Shams, S.; Ahmad, W.; Memon, H.; Wei, Y.; Yuan, Q.P.; Liang, H. Facile synthesis of laccase mimic Cu/H3BTC MOF for efficient dye degradation and detection of phenolic pollutants. RSC Adv. 2019, 9, 40845–40854. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.J.; Wu, Y.X.; Zou, B.C.; Lou, Q.; Zhang, W.H.; Zhong, J.Y.; Lu, L.; Dai, G.F. Simultaneous removal and degradation characteristics of sulfonamide, tetracycline, and quinolone antibiotics by laccase-mediated oxidation coupled with soil adsorption. J. Hazard. Mater. 2016, 307, 350–358. [Google Scholar] [CrossRef]
- Han, Z.W.; Wang, H.R.; Zheng, J.; Wang, S.S.; Yu, S.Y.; Lu, L. Ultrafast synthesis of laccase-copper phosphate hybrid nanoflowers for efficient degradation of tetracycline antibiotics. Environ. Res. 2023, 216, 114690. [Google Scholar] [CrossRef]
- Ouyang, B.B.; Xu, W.; Zhang, W.L.; Guang, C.; Mu, W.M. Efficient removal of sulfonamides and tetracyclines residues by the laccase-mediator system employing a novel laccase from Lysinibacillus fusiformis. J. Environ. Chem. Eng. 2022, 10, 108809. [Google Scholar] [CrossRef]
- Kiehne, A.; Engelhardt, U.H. Thermospray-LC-MS analysis of various groups of polyphenols in tea. I. Catechins, flavonol O-glycosides and flavone C-glycosides. Z. Lebensm.-Unters. Forsch. 1996, 202, 48–54. [Google Scholar] [CrossRef]
- Das, A.; Datta, S.; Mukherjee, S.; Bose, S.; Ghosh, S.; Dhar, P. Evaluation of antioxidative, antibacterial and probiotic growth stimulatory activities of Sesamum indicum honey containing phenolic compounds and lignans. LWT Food Sci. Technol. 2015, 61, 244–250. [Google Scholar] [CrossRef]

| Component | GA | EGC | EGCG | EC | ECG |
|---|---|---|---|---|---|
| Content (mg/kg) | 117.40 ± 3.51 | 85.40 ± 2.61 | 9.20 ± 0.29 | 20.80 ± 0.63 | 7.20 ± 0.29 |
| Concentration (µmol/L) | 77.11 ± 3.31 | 31.03 ± 1.10 | 2.03 ± 0.07 | 8.03 ± 0.12 | 3.02 ± 0.09 |
| Degradation rate of laccase alone (%) | 58.08 ± 2.30 | ||||
| Degradation rates of different laccase–mediator systems (%) | 76.25 ± 3.05 | 81.35 ± 3.22 | 70.10 ± 2.79 | 69.15 ± 2.97 | 79.15 ± 3.38 |
| Calculated contribution (%/µM) | 0.23 ± 0.01 a | 0.75 ± 0.03 b | 6.01 ± 0.24 c | 0.018 ± 0.001 d | 7.02 ± 0.29 e |
| Laccase Source | Mediator | Tetracycline Concentration | Time | Degradation Efficiency | Refs. |
|---|---|---|---|---|---|
| Trametes versicolor | HBT | 10−4 mol/L | 1 h | 100% | [44] |
| Trametes versicolor | SA | 50 mg/L | 3 h | 100% | [57] |
| Bacillus amyloliquefaciens | ACE | 50 mg/L | 1 h | 86% | [58] |
| Lysinibacillus fusiformis | ABTS | 100 mg/L | 6 h | 100% | [59] |
| P. simulans | GTP | 50 mg/L | 5 min | 100% | This study |
| P. simulans | ECG | 50 mg/L | 5 min | 100% | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Zhang, S.; Xu, H.; Ma, A.; Zhuang, G.; Huo, S.; Zou, B.; Qian, J.; Guan, G.; Wang, F. Degradation of Tetracycline by Laccase–Mediator System Using Tea Polyphenols as Mediator. Catalysts 2025, 15, 952. https://doi.org/10.3390/catal15100952
Xu L, Zhang S, Xu H, Ma A, Zhuang G, Huo S, Zou B, Qian J, Guan G, Wang F. Degradation of Tetracycline by Laccase–Mediator System Using Tea Polyphenols as Mediator. Catalysts. 2025; 15(10):952. https://doi.org/10.3390/catal15100952
Chicago/Turabian StyleXu, Ling, Shuang Zhang, Hui Xu, Anzhou Ma, Guoqiang Zhuang, Shuhao Huo, Bin Zou, Jingya Qian, Guoqiang Guan, and Feng Wang. 2025. "Degradation of Tetracycline by Laccase–Mediator System Using Tea Polyphenols as Mediator" Catalysts 15, no. 10: 952. https://doi.org/10.3390/catal15100952
APA StyleXu, L., Zhang, S., Xu, H., Ma, A., Zhuang, G., Huo, S., Zou, B., Qian, J., Guan, G., & Wang, F. (2025). Degradation of Tetracycline by Laccase–Mediator System Using Tea Polyphenols as Mediator. Catalysts, 15(10), 952. https://doi.org/10.3390/catal15100952







