Recent Advances in Transition Metal Dichalcogenide-Based Electrodes for Asymmetric Supercapacitors
Abstract
1. Introduction
2. TMD Electrode for Supercapacitors and Performance Evaluation
2.1. Advantages of TMD as a Pseudocapacitive Electrode
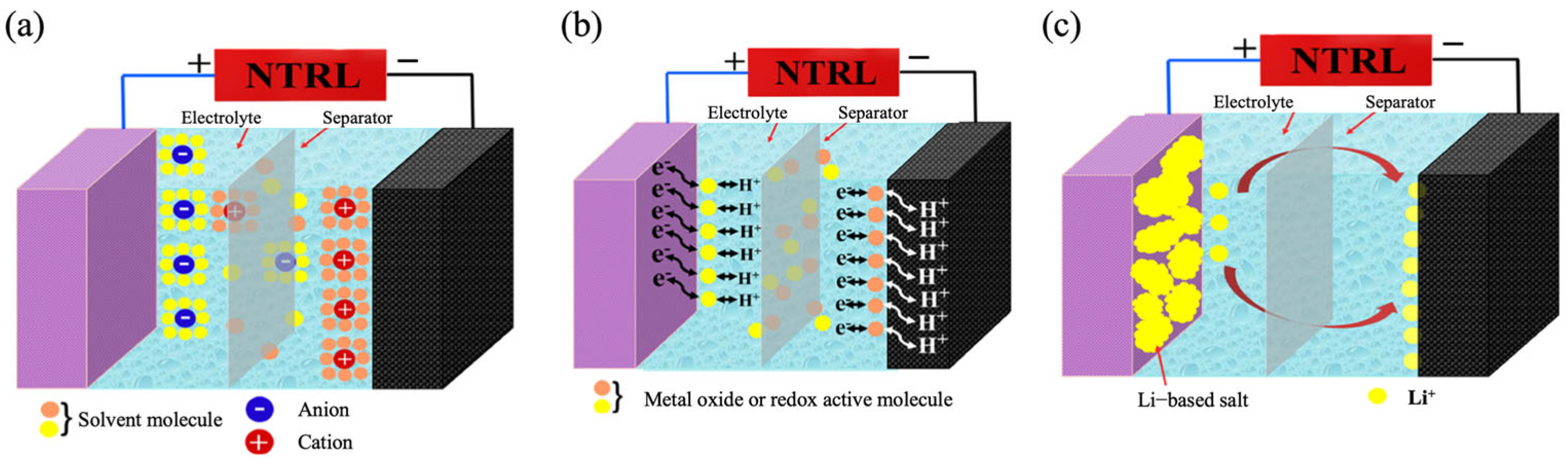
2.2. Basic Characteristics of Electrochemical on Asymmetric Supercapacitor
2.2.1. Cyclic Voltammetry
2.2.2. Linear Sweep Voltammetry
2.2.3. Galvanostatic Charge/Discharge
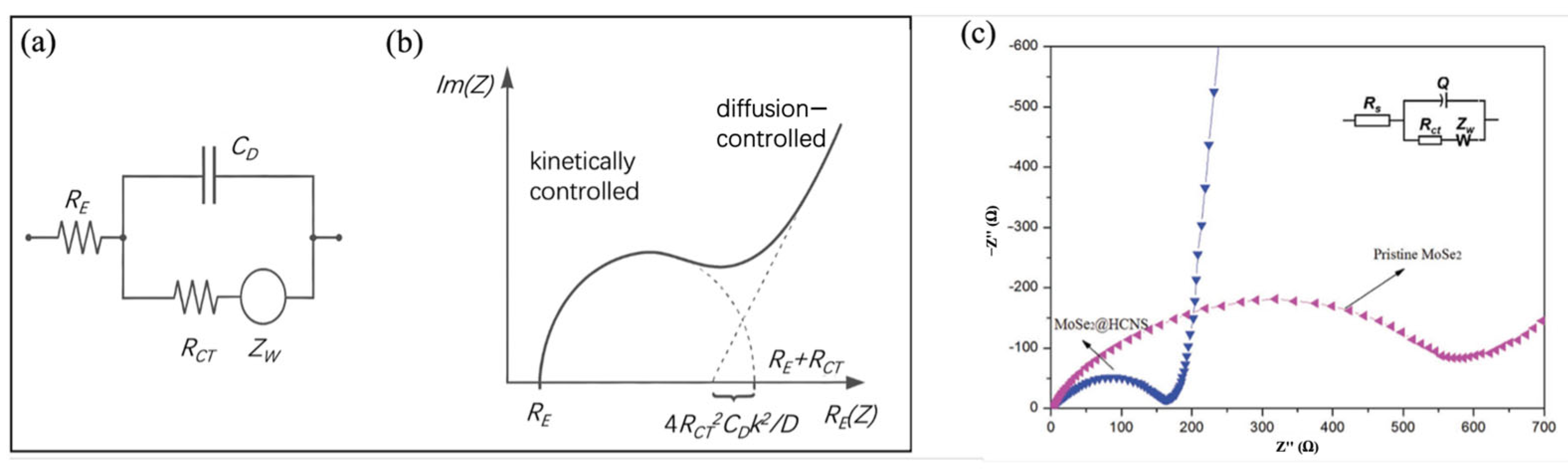
2.2.4. Electrochemical Impedance Spectroscopy
3. Transition Metal Dichalcogenide Electrodes
3.1. Electrocatalytic Contributions to the Performance of TMD Electrodes
3.2. Electronic Origins of Electrocatalytic Activity
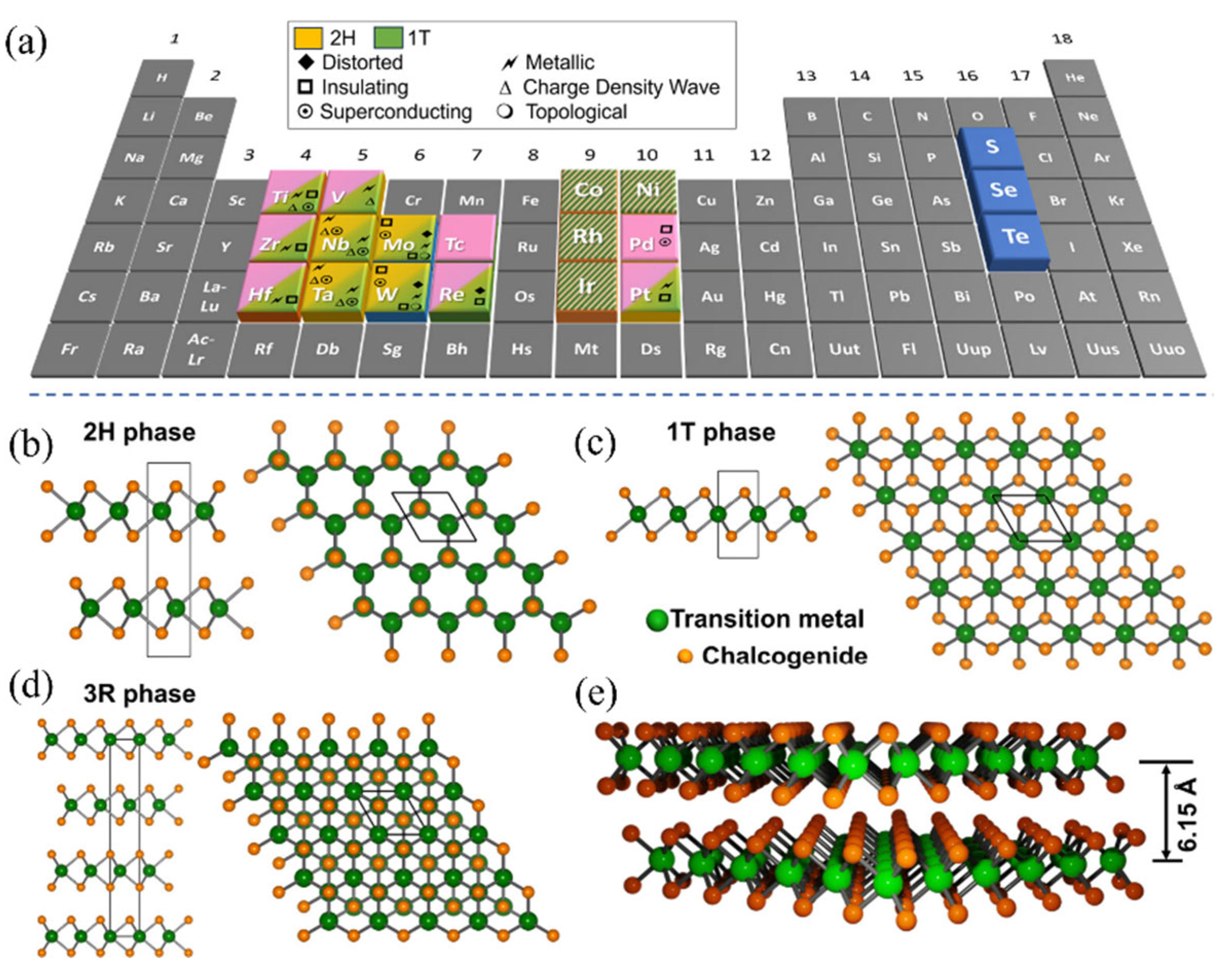
3.2.1. Phase Engineering
3.2.2. Electronic Structure
3.3. Design Principles for Asymmetric Supercapacitors
4. TMD-Based ASCs
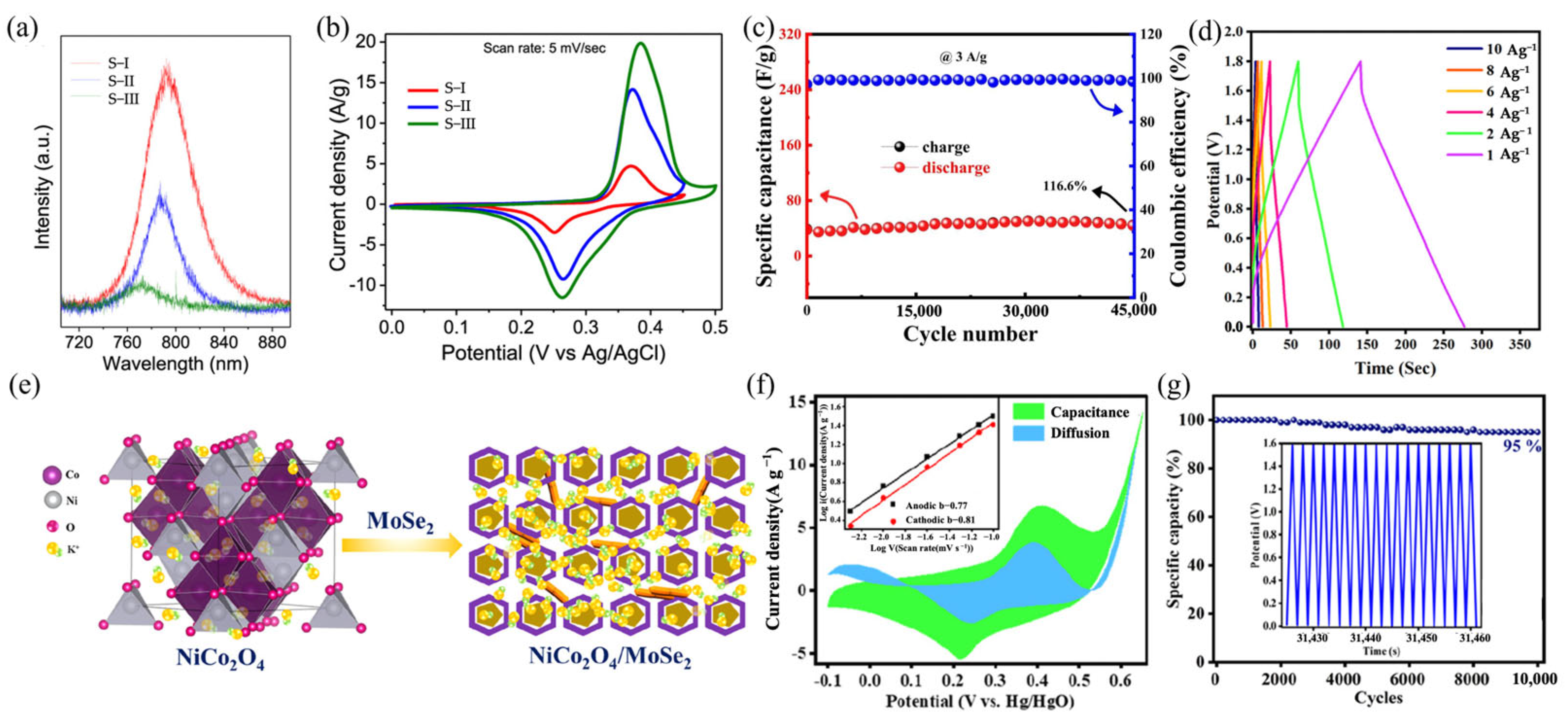
4.1. MoSe2-Based ASCs
4.2. WSe2-Based ASCs
4.3. MoS2-Based ASCs
4.4. WS2-Based ASCs
5. Conclusions and Prospects
- Improving catalytic activity by increasing active site density, ensuring that catalytic activity occurs across the entire basal plane of 2H-phase TMD nanosheets rather than being limited to edge sites.
- Preventing interlayer restacking of TMD nanosheets to improve electrode stability and electrical conductivity.
- Synthesizing well-designed heterostructures, such as vertically and laterally structured hetero lattices, to achieve specific applications.
- Exploring redox-additive electrolytes and interface-coupled reaction systems to extend the potential window and enable synergistic redox reactions at the electrode–electrolyte interface.
- Investigating the application of TMDs in asymmetric supercapacitors under real-world conditions, such as temperature variation and long-term mechanical stress, especially in miniaturized and flexible energy storage platforms.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| TMD | Transition metal dichalcogenide |
| ASC | Asymmetric supercapacitor |
| Sp.C | Specific capacitance |
| Pd | High power density |
| Ed | Energy density |
| 2D | Two-dimensional |
| SSA | Specific surface area |
| CNT | Carbon nanotube |
| EDLCs | Double-layer capacitors |
| HSCs | Hybrid supercapacitors |
| CV | Cyclic voltammetry |
| LSV | Linear sweep voltammetry |
| GCD | Galvanostatic charge/discharge |
| EIS | Electrochemical impedance spectroscopy |
| Rs | Inner resistance |
| Rct | Charge transfer resistance |
| CVD | Chemical vapor deposition |
| BET | Brunauer–Emmett–Teller |
| LDHs | Layered double hydroxides |
| NSA | Nanosheet array |
| PDOS | Projection density of states |
Note
| AC | activated carbon |
| GFs-25 | 25 mg of graphene flakes powder added to the composite electrode |
| MWCNTs | multiwalled carbon nanotubes |
| NSAs | nanosheet arrays |
| e-Ti3C2Tx | expanded MXene layers |
| rGO | reduced graphene oxide |
| Mo-3 | 3.0% molybdenum-doped |
| NF | nickel foam |
| Graphene-0.8 | the addition of graphene slurry is 0.8 g |
| RAE | redox-additive electrolyte, 0.35 M potassium ferrocyanide in 6 M NaOH |
| LDH | layered double hydroxide |
| LDH41 | the molar ratio of Ni2+ to Cr3+ is maintained at 4:1 |
| HCSs | hollow carbon spheres |
| EC | ethylene carbonate |
| DMC | dimethyl carbonate |
| N-3DG | 3D nitrogen-doped graphene featuring hierarchical porosity |
| 3D-IEMoS2@G | interlayer-enlarged MoS2/rGO integrated into a 3D networked structure |
| ASC | asymmetric supercapacitor |
| QSSASC | quasi-solid-state asymmetric supercapacitor |
| SSC | symmetric supercapacitor |
| QSSC | quasi-solid-state symmetric supercapacitor |
| BN | functionalized boron nitride |
| Mx-WS2-Hal | Ti3AlC2-decorated hierarchical structured WS2/halloysite |
| PANI | polyaniline |
| CNF | carbon nanofiber |
| Z8-800 | ZIF-8 subjected to pyrolysis treatment at 900 °C |
References
- Hassan, Q.; Viktor, P.; Al-Musawi, T.J.; Ali, B.M.; Algburi, S.; Alzoubi, H.M.; Al-Jiboory, A.K.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M. The renewable energy role in the global energy Transformations. Renew. Energy Focus 2024, 48, 100545. [Google Scholar] [CrossRef]
- Olabi, A.; Elsaid, K.; Obaideen, K.; Abdelkareem, M.A.; Rezk, H.; Wilberforce, T.; Maghrabie, H.M.; Sayed, E.T. Renewable energy systems: Comparisons, challenges and barriers, sustainability indicators, and the contribution to UN sustainable development goals. Int. J. Thermofluids 2023, 20, 100498. [Google Scholar] [CrossRef]
- Mlilo, N.; Brown, J.; Ahfock, T. Impact of intermittent renewable energy generation penetration on the power system networks—A review. Technol. Econ. Smart Grids Sustain. Energy 2021, 6, 25. [Google Scholar] [CrossRef]
- Njema, G.G.; Ouma, R.B.O.; Kibet, J.K. A review on the recent advances in battery development and energy storage technologies. J. Renew. Energy 2024, 2024, 2329261. [Google Scholar] [CrossRef]
- Das, S.; Kundu, A.; Kuila, T.; Murmu, N.C. Recent advancements on designing transition metal-based carbon-supported single atom catalysts for oxygen electrocatalysis: Miles to go for sustainable Zn-air batteries. Energy Storage Mater. 2023, 61, 102890. [Google Scholar] [CrossRef]
- Dutta, A.; Mitra, S.; Basak, M.; Banerjee, T. A comprehensive review on batteries and supercapacitors: Development and challenges since their inception. Energy Storage 2022, 5, e339. [Google Scholar] [CrossRef]
- Palanisamy, R.; Karuppiah, D.; Venkatesan, S.; Mani, S.; Kuppusamy, M.; Marimuthu, S.; Karuppanan, A.; Govindaraju, R.; Marimuthu, S.; Rengapillai, S.; et al. High-performance asymmetric supercapacitor fabricated with a novel MoS2/Fe2O3/Graphene composite electrode. Colloid Interface Sci. Commun. 2022, 46, 100573. [Google Scholar] [CrossRef]
- Yang, X.; Niu, H.; Jiang, H.; Wang, Q.; Qu, F. A high energy density all-solid-state asymmetric supercapacitor based on MoS2/graphene nanosheets and MnO2/graphene hybrid electrodes. J. Mater. Chem. A 2016, 4, 11264–11275. [Google Scholar] [CrossRef]
- Kumar, R.; Thangappan, R. Development of Asymmetric Electrodes of Robust MoS2/rGO Heterostructures with MnO2 Nanohybrid Composites for Cylindrical Type Energy Storage Devices. Energy Fuels 2024, 38, 11216–11232. [Google Scholar] [CrossRef]
- Sadavar, S.V.; Lee, S.; Park, S. Advancements in asymmetric supercapacitors: From historical milestones to challenges and future directions. Adv. Sci. 2024, 11, e2403172. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and mechanisms of asymmetric supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Robinson, J.A.; Schaak, R.E.; Sun, D.; Sun, Y.; Mallouk, T.E.; Terrones, M. Correction to transition metal dichalcogenides and beyond: Synthesis, properties, and applications of single- and few-layer nanosheets. Acc. Chem. Res. 2015, 48, 897. [Google Scholar] [CrossRef] [PubMed]
- Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O.V.; Kis, A. 2D transition metal dichalcogenides. Nat. Rev. Mater. 2017, 2, 17033. [Google Scholar] [CrossRef]
- Palchoudhury, S.; Ramasamy, K.; Han, J.; Chen, P.; Gupta, A. Transition metal chalcogenides for next-generation energy storage. Nanoscale Adv. 2023, 5, 2724–2742. [Google Scholar] [CrossRef]
- Khan, R.; Kalla, R.M.N.; Ramachandran, T.; Al-Sehemi, A.G.; Kumar, Y.A.; Somu, P.; Lee, J. Transition metal dichalcogenides for next-generation supercapacitors: Recent advances, challenges, and future perspectives. J. Alloys Compd. 2025, 1039, 182874. [Google Scholar] [CrossRef]
- Chen, J.-M.; Zhang, H.-L.; Peng, X.; Shao, X.; Chai, Y.-F.; Ma, M.; Li, Z.-L.; Liu, S.-D.; Ding, B. Heterostructure engineering of transition metal dichalcogenides for high-performance supercapacitors. Rare Met. 2025, 1–39. [Google Scholar] [CrossRef]
- Zoller, F.; Luxa, J.; Bein, T.; Fattakhova-Rohlfing, D.; Bouša, D.; Sofer, Z. Flexible freestanding MoS2-based composite paper for energy conversion and storage. Beilstein J. Nanotechnol. 2019, 10, 1488–1496. [Google Scholar] [CrossRef]
- Yu, X.; Ding, Y.; Sun, J. Design principles for 2D transition metal dichalcogenides toward lithium−sulfur batteries. iScience 2023, 26, 107489. [Google Scholar] [CrossRef]
- Piecha, D.; Szczerba, M.; Palowska, R.; Marzec, M.M.; Sokołowski, K.; Uchacz, T.; Liu, L.; Sulka, G.D.; Brzózka, A. Formation of 2H and 1T/2H MoSe2 via thermal selenization of electrodeposited Mo thin films and nanowires. Appl. Surf. Sci. 2025, 684, 161801. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Shaheen, M.; Ifseisi, A.A.; Aftab, S.; Ahmad, Z.; Siyal, S.H.; Iqbal, M.J. Transition metal dichalcogenide electrodes with interface engineering for high-performance hybrid supercapacitors. RSC Adv. 2023, 13, 18038–18044. [Google Scholar] [CrossRef]
- Chen, X.; Ding, J.; Jiang, J.; Zhuang, G.; Zhang, Z.; Yang, P. Preparation of a MoS2/carbon nanotube composite as an electrode material for high-performance supercapacitors. RSC Adv. 2018, 8, 29488–29494. [Google Scholar] [CrossRef] [PubMed]
- Kirubasankar, B.; Palanisamy, P.; Arunachalam, S.; Murugadoss, V.; Angaiah, S. 2D MoSe2-Ni(OH)2 nanohybrid as an efficient electrode material with high rate capability for asymmetric supercapacitor applications. Chem. Eng. J. 2019, 355, 881–890. [Google Scholar] [CrossRef]
- Su, H.; Niu, C.; Zhang, R.; Huang, M.; Li, Z. Construction of a Ni2P/NiSe2/MoSe2 hybrid for advanced supercapacitors. J. Alloys Compd. 2025, 1010, 178074. [Google Scholar] [CrossRef]
- Chen, H.; Hu, M.; Wang, X.; Xu, X.; Jing, P.; Liu, B.; Guo, R.; Zhang, J. Constructing Novel Ternary Heterostructure of CeP5O14/WP/WS2 to Enhance Catalytic Activity for Hydrogen Evolution in a Full pH Range. Small Struct. 2023, 4, 2300026. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Khizar, A.; Khan, S.; Hegazy, H.; Alahmari, A. Augmenting the electrochemical capability of TMDCs thin film electrodes via interface engineering for energy storage applications. Mater. Sci. Eng. B 2024, 310, 117757. [Google Scholar] [CrossRef]
- Raza, A.; Farid, A.; Yousaf, M.; Alfares, A.M.; Rasheed, A.; Khan, I.; Ghanem, M.A.; Mohammed, K.M. CVD based synthesis of binder free nanostructured MgS/MoS2@NiF electrode material for asymmetric supercapacitor applications. Electrochim. Acta 2024, 497, 144579. [Google Scholar] [CrossRef]
- Mohammadi, S.; Mousavi-Khoshdel, S.M. An experimental and computational study of graphene oxide functionalized with tris(hydroxymethyl)aminomethane as an electrode material for supercapacitors. Sci. Rep. 2023, 13, 16756. [Google Scholar] [CrossRef]
- De, S.; Acharya, S.; Maity, C.K.; Nayak, G.C. Boron Nitride/Ti3C2Tx MXene Nanosheet/WS2 Nanostructure Ternary Composites for All-Solid-State Flexible Asymmetric Supercapacitors. ACS Appl. Nano Mater. 2023, 6, 11175–11186. [Google Scholar] [CrossRef]
- Dutt, S.; Verma, S.; Singh, A.; Mahajan, P.; Padha, B.; Ahmed, A.; Young, S.-J.; Gupta, V.; Agha, D.N.Q.; Arya, S. Flexible and highly stable textile-based symmetric supercapacitor comprising binder-free MnO2/rGO-CF nanocomposite electrodes. J. Electron. Mater. 2023, 52, 7447–7458. [Google Scholar] [CrossRef]
- Verma, S.; Mahajan, P.; Padha, B.; Ahmed, A.; Arya, S. Nanowires based solid-state asymmetric self-charging supercapacitor driven by PVA-ZnO-KOH flexible piezoelectric matrix. Electrochim. Acta 2023, 465, 142933. [Google Scholar] [CrossRef]
- Allagui, A.; Freeborn, T.J.; Elwakil, A.S.; Maundy, B.J. Reevaluation of performance of electric double-layer capacitors from constant-current charge/discharge and cyclic voltammetry. Sci. Rep. 2016, 6, 38568. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, Y.; Zhou, M.; Li, F.; Luo, H.; Zhang, W.; Ma, L.; Huang, Y. Metal-heteroatom-doped CoS/MXene nanohybrid for efficient supercapacitor electrode materials. Electrochim. Acta 2024, 483, 143955. [Google Scholar] [CrossRef]
- Guo, Z.; Yang, L.; Wang, W.; Cao, L.; Dong, B. Ultrathin VS2 nanoplate with in-plane and out-of-plane defects for an electrochemical supercapacitor with ultrahigh specific capacitance. J. Mater. Chem. A 2018, 6, 14681–14688. [Google Scholar] [CrossRef]
- Chatterjee, D.P.; Nandi, A.K. A review on the recent advances in hybrid supercapacitors. J. Mater. Chem. A 2021, 9, 15880–15918. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Aziz, U. Supercapattery: Merging of battery-supercapacitor electrodes for hybrid energy storage devices. J. Energy Storage 2022, 46, 103823. [Google Scholar] [CrossRef]
- Malavekar, D.; Pujari, S.; Jang, S.; Bachankar, S.; Kim, J.H. Recent Development on Transition Metal Oxides-Based Core–Shell Structures for Boosted Energy Density Supercapacitors. Small 2024, 20, 2312179. [Google Scholar] [CrossRef]
- Nandihalli, N. A Review of Nanocarbon-Based Anode Materials for Lithium-Ion Batteries. Crystals 2024, 14, 800. [Google Scholar] [CrossRef]
- Gregory, G.L.; Gao, H.; Liu, B.; Gao, X.; Rees, G.J.; Pasta, M.; Bruce, P.G.; Williams, C.K. Buffering Volume Change in Solid-State Battery Composite Cathodes with CO2-Derived Block Polycarbonate Ethers. J. Am. Chem. Soc. 2022, 144, 17477–17486. [Google Scholar] [CrossRef]
- Bongu, C.S.; Krishnan, M.R.; Soliman, A.; Arsalan, M.; Alsharaeh, E.H. Flexible and Freestanding MoS2/Graphene Composite for High-Performance Supercapacitors. ACS Omega 2023, 8, 36789–36800. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, Y.; Ma, Z.; Zhu, T.; Liu, L.; Zheng, J.; Gong, X. All-solid-state asymmetric supercapacitors with metal selenides electrodes and ionic conductive composites electrolytes. Adv. Funct. Mater. 2019, 29, 1904182. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Ono, K. Electrochemical impedance spectroscopy. Nat. Rev. Methods Primers 2021, 1, 41. [Google Scholar]
- Liu, L.; Zhang, X.; Liu, Y.; Gong, X. Electrochemical energy storage devices-batteries, Supercapacitors, and Battery-Supercapacitor Hybrid Devices. ACS Appl. Electron. Mater. 2025, 7, 2233–2270. [Google Scholar] [CrossRef]
- Appel, A.M.; Helm, M.L. Determining the Overpotential for a Molecular Electrocatalyst. ACS Catal. 2014, 4, 630–633. [Google Scholar] [CrossRef]
- Petrii, O.A.; Nazmutdinov, R.R.; Bronshtein, M.D.; Tsirlina, G.A. Life of the Tafel equation: Current understanding and prospects for the second century. Electrochim. Acta 2007, 52, 3493–3504. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.-J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Guo, H.; Liu, B.; Liang, M.; Lv, Z.; Adair, K.R.; Sun, X. Few-Layer MoSe2 Nanosheets with Expanded (002) Planes Confined in Hollow Carbon Nanospheres for Ultrahigh-Performance Na-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1707480. [Google Scholar] [CrossRef]
- Friebe, C.; Schubert, U.S. Development of Active Organic and Polymeric Materials for Batteries and Solar Cells: Introduction to Essential Characterization Techniques. Adv. Energy Mater. 2015, 5, 1500858. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Electrical Circuits. In Electrochemical Impedance Spectroscopy; Wiley: Hoboken, NJ, USA, 2008; pp. 61–72. [Google Scholar] [CrossRef]
- Mei, B.-A.; Munteshari, O.; Lau, J.; Dunn, B.; Pilon, L. Physical Interpretations of Nyquist Plots for EDLC Electrodes and Devices. J. Phys. Chem. C 2017, 122, 194–206. [Google Scholar] [CrossRef]
- Bauknecht, S.; Kowal, J.; Bozkaya, B.; Settelein, J.; Karden, E. Electrochemical Impedance Spectroscopy as an Analytical Tool for the Prediction of the Dynamic Charge Acceptance of Lead-Acid Batteries. Batteries 2022, 8, 66. [Google Scholar] [CrossRef]
- Orazem, M.E.; Pébère, N.; Tribollet, B. Enhanced Graphical Representation of Electrochemical Impedance Data. J. Electrochem. Soc. 2006, 153, B129–B136. [Google Scholar] [CrossRef]
- Muller, G.A.; Cook, J.B.; Kim, H.-S.; Tolbert, S.H.; Dunn, B. High performance pseudocapacitor based on 2D layered metal chalcogenide nanocrystals. Nano Lett. 2015, 15, 1911–1917. [Google Scholar] [CrossRef]
- Kumar, K.S.; Choudhary, N.; Jung, Y.; Thomas, J. Recent advances in two-dimensional nanomaterials for supercapacitor electrode applications. ACS Energy Lett. 2018, 3, 482–495. [Google Scholar] [CrossRef]
- Roy, S.; Joseph, A.; Zhang, X.; Bhattacharyya, S.; Puthirath, A.B.; Biswas, A.; Tiwary, C.S.; Vajtai, R.; Ajayan, P.M. Engineered two-dimensional transition metal dichalcogenides for energy conversion and storage. Chem. Rev. 2024, 124, 9376–9456. [Google Scholar] [CrossRef] [PubMed]
- Han, S.A.; Bhatia, R.; Kim, S.-W. Synthesis, properties and potential applications of two-dimensional transition metal dichalcogenides. Nano Converg. 2015, 2, 17. [Google Scholar] [CrossRef]
- Xu, X.; Wang, X.; Yu, S.; Wang, C.; Liu, G.; Li, H.; Yang, J.; Li, J.; Sun, T.; Hai, X.; et al. Two-Dimensional Topological Platinum Telluride Superstructures with Periodic Tellurium Vacancies for Efficient and Robust Catalysis. ACS Nano 2024, 18, 32635–32649. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, Y.; Ji, Q.; Li, T.; Xu, C.; Qi, C.; He, H.; Yang, S.; Li, S.; Yan, S.; et al. Understanding spatial effects of tetrahedral and octahedral cobalt cations on peroxymonosulfate activation for efficient pollution degradation. Appl. Catal. B Environ. 2021, 291, 120072. [Google Scholar] [CrossRef]
- Lu, B.; Chen, B.; Wang, D.; Li, C.; Gao, R.; Liu, Y.; Mao, R.; Yang, J.; Zhou, G. Engineering the interfacial orientation of MoS2/Co9S8 bidirectional catalysts with highly exposed active sites for reversible Li-CO2 batteries. Proc. Natl. Acad. Sci. USA 2023, 120, e2216933120. [Google Scholar] [CrossRef]
- Hao, C.; Li, X.; Huang, H.; Ge, L.; Fu, Z.; Lu, Y.; Wang, Y.; Zhang, S.; Cheng, Z. Simultaneous Activation of Different Coordination Sites in Single-Phase FeCoMo3O8 for the Oxygen Evolution Reaction. ACS Energy Lett. 2023, 8, 4506–4513. [Google Scholar] [CrossRef]
- Ma, Y.; Leng, D.; Zhang, X.; Fu, J.; Pi, C.; Zheng, Y.; Gao, B.; Li, X.; Li, N.; Chu, P.K.; et al. Enhanced Activities in Alkaline Hydrogen and Oxygen Evolution Reactions on MoS2 Electrocatalysts by In-Plane Sulfur Defects Coupled with Transition Metal Doping. Small 2022, 18, e2203173. [Google Scholar] [CrossRef]
- Tang, Q. Enhanced 1T’-Phase Stabilization and Chemical Reactivity in a MoTe2 Monolayer through Contact with a 2D Ca2N Electride. Chemphyschem 2019, 20, 595–601. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, D.; Yin, Z.; Yan, Q.; Zhang, H. Graphene and Graphene-Based Materials for Energy Storage Applications. Small 2014, 10, 3480–3498. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]
- Yun, Q.; Li, L.; Hu, Z.; Lu, Q.; Chen, B.; Zhang, H. Layered Transition Metal Dichalcogenide-Based Nanomaterials for Electrochemical Energy Storage. Adv. Mater. 2020, 32, e1903826. [Google Scholar] [CrossRef]
- Pan, F.; Li, Z.; Yao, S.; Liu, J.; Wei, Z.; Chen, X.; Xie, Y.; Du, F. Combined intercalation and space-charge mechanism enabled high-capacity, ultrafast and long-lifespan sodium-ion storage for chalcogenide anodes. Energy Environ. Sci. 2025, 18, 1856–1866. [Google Scholar] [CrossRef]
- Godínez-Salomón, J.F.; Ospina-Acevedo, F.; Albiter, L.A.; Bailey, K.O.; Naymik, Z.G.; Mendoza-Cruz, R.; Balbuena, P.B.; Rhodes, C.P. Titanium Substitution Effects on the Structure, Activity, and Stability of Nanoscale Ruthenium Oxide Oxygen Evolution Electrocatalysts: Experimental and Computational Study. ACS Appl. Nano Mater. 2022, 5, 11752–11775. [Google Scholar] [CrossRef]
- Splendiani, A.; Sun, L.; Zhang, Y.; Li, T.; Kim, J.; Chim, C.-Y.; Galli, G.; Wang, F. Emerging Photoluminescence in Monolayer MoS2. Nano Lett. 2010, 10, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Acerce, M.; Voiry, D.; Chhowalla, M. Metallic 1T phase MoS2 nanosheets as supercapacitor electrode materials. Nat. Nanotechnol. 2015, 10, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Eda, G.; Yamaguchi, H.; Voiry, D.; Fujita, T.; Chen, M.; Chhowalla, M. Photoluminescence from Chemically Exfoliated MoS2. Nano Lett. 2011, 11, 5111–5116. [Google Scholar] [CrossRef]
- Fang, Y.; Pan, J.; He, J.; Luo, R.; Wang, D.; Che, X.; Bu, K.; Zhao, W.; Liu, P.; Mu, G.; et al. Structure re-determination and superconductivity observation of bulk 1T MoS2. Angew. Chem. Int. Ed. 2018, 57, 1232–1235. [Google Scholar] [CrossRef]
- Choudhary, N.; Li, C.; Chung, H.-S.; Moore, J.; Thomas, J.; Jung, Y. High-performance one-body core/shell nanowire supercapacitor enabled by conformal growth of capacitive 2D WS2 layers. ACS Nano 2016, 10, 10726–10735. [Google Scholar] [CrossRef]
- Soon, J.M.; Loh, K.P. Electrochemical double-layer capacitance of MoS2 nanowall films. Electrochem. Solid-State Lett. 2007, 10, A250–A254. [Google Scholar] [CrossRef]
- Hou, W.; Sun, Y.; Zhang, Y.; Wang, T.; Wu, L.; Du, Y.; Zhong, W. Mixed-dimensional heterostructure of few-layer MXene based vertical aligned MoS2 nanosheets for enhanced supercapacitor performance. J. Alloys Compd. 2021, 859, 157797. [Google Scholar] [CrossRef]
- Tan, Y.; Luo, F.; Zhu, M.; Xu, X.; Ye, Y.; Li, B.; Wang, G.; Luo, W.; Zheng, X.; Wu, N.; et al. Controllable 2H-to-1T’ phase transition in few-layer MoTe2. Nanoscale 2018, 10, 19964–19971. [Google Scholar] [CrossRef] [PubMed]
- El Garah, M.; Bertolazzi, S.; Ippolito, S.; Eredia, M.; Janica, I.; Melinte, G.; Ersen, O.; Marletta, G.; Ciesielski, A.; Samorì, P. MoS2 nanosheets via electrochemical lithium-ion intercalation under ambient conditions. FlatChem 2018, 9, 33–39. [Google Scholar] [CrossRef]
- Zhang, Q.; Mei, L.; Cao, X.; Tang, Y.; Zeng, Z. Intercalation and exfoliation chemistries of transition metal dichalcogenides. J. Mater. Chem. A 2020, 8, 15417–15444. [Google Scholar] [CrossRef]
- Naz, R.; Imtiaz, M.; Liu, Q.; Yao, L.; Abbas, W.; Li, T.; Zada, I.; Yuan, Y.; Chen, W.; Gu, J. Highly defective 1T-MoS2 nanosheets on 3D reduced graphene oxide networks for supercapacitors. Carbon 2019, 152, 697–703. [Google Scholar] [CrossRef]
- Chen, C.-A.; Lee, C.-L.; Yang, P.-K.; Tsai, D.-S.; Lee, C.-P. Active Site Engineering on Two-Dimensional-Layered Transition Metal Dichalcogenides for Electrochemical Energy Applications: A Mini-Review. Catalysts 2021, 11, 151. [Google Scholar] [CrossRef]
- Sokolikova, M.S.; Sherrell, P.C.; Palczynski, P.; Bemmer, V.L.; Mattevi, C. Direct solution-phase synthesis of 1T’ WSe2 nanosheets. Nat. Commun. 2019, 10, 712. [Google Scholar] [CrossRef]
- Sokolikova, M.S.; Mattevi, C. Direct synthesis of metastable phases of 2D transition metal dichalcogenides. Chem. Soc. Rev. 2020, 49, 3952–3980. [Google Scholar] [CrossRef]
- Yi, L.; Nie, K.; Li, B.; Zhang, Y.; Hu, C.; Hao, X.; Wang, Z.; Qu, X.; Liu, Z.; Huang, W. Tailoring Copper Single-Atoms-Stabilized Metastable Transition-Metal-Dichalcogenides for Sustainable Hydrogen Production. Angew. Chem. Int. Ed. Engl. 2025, 64, e202414701. [Google Scholar] [CrossRef]
- Romanov, R.I.; Zabrosaev, I.V.; Kozodaev, M.G.; Yakubovsky, D.I.; Tatmyshevskiy, M.K.; Timofeev, A.A.; Doroshina, N.V.; Novikov, S.M.; Volkov, V.S.; Markeev, A.M. Stabilization of the Nano-Sized 1T Phase through Rhenium Doping in the Metal-Organic CVD MoS2 Films. ACS Omega 2023, 8, 16579–16586. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Li, B.; Peng, W.; Wang, C.; Li, K.; Zhu, Y.; Mei, Y. A palladium doped 1T-phase molybdenum disulfide–black phosphorene two-dimensional van der Waals heterostructure for visible-light enhanced electrocatalytic hydrogen evolution. Nanoscale 2021, 13, 5892–5900. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Tang, Q. Modulating the Electronic Structure and In-Plane Activity of 2D-TMDs (MoS2, TaS2, NbS2) Monolayers by Interfacial Engineering. J. Phys. Chem. C 2020, 124, 8822–8833. [Google Scholar] [CrossRef]
- Behera, S.K.; Ramamurthy, P.C. Assessing the accurate exchange-correlation functional in van der Waals TMD Materials AB2 (A = Nb, Ta and B = S, Se, Te). ChemRxiv 2024. [Google Scholar] [CrossRef]
- Pakhira, S.; Upadhyay, S.N. Efficient electrocatalytic H2 evolution mediated by 2D Janus MoSSe transition metal dichalcogenide. Sustain. Energy Fuels 2022, 6, 1733–1752. [Google Scholar] [CrossRef]
- Dimakis, N.; Vadodaria, O.; Ruiz, K.; Gupta, S. Molybdenum disulfide monolayer electronic structure information as explored using density functional theory and quantum theory of atoms in molecules. Appl. Surf. Sci. 2021, 555, 149545. [Google Scholar] [CrossRef]
- Son, E.; Lee, S.; Seo, J.; Kim, U.; Kim, S.H.; Baik, J.M.; Han, Y.-K.; Park, H. Engineering the Local Atomic Configuration in 2H TMDs for Efficient Electrocatalytic Hydrogen Evolution. ACS Nano 2023, 17, 10817–10826. [Google Scholar] [CrossRef]
- Koley, S. Intercalation in 2H-TaSe2 for modulation of electronic properties and electrochemical energy storage. Phys. B Condens. Matter 2023, 669, 415312. [Google Scholar] [CrossRef]
- Crane, M.J.; Lim, M.B.; Zhou, X.; Pauzauskie, P.J. Rapid synthesis of transition metal dichalcogenide–carbon aerogel composites for supercapacitor electrodes. Microsyst. Nanoeng. 2017, 3, 17032. [Google Scholar] [CrossRef]
- Prabhu, P.; Jose, V.; Lee, J.-M. Design strategies for development of TMD-based heterostructures in electrochemical energy systems. Matter 2020, 2, 526–553. [Google Scholar] [CrossRef]
- Ge, Y.; Jalili, R.; Wang, C.; Zheng, T.; Chao, Y.; Wallace, G.G. A robust free-standing MoS2/poly(3,4-ethylenedioxythiophene): Poly(styrenesulfonate) film for supercapacitor applications. Electrochim. Acta 2017, 235, 348–355. [Google Scholar] [CrossRef]
- Gu, M.; Rao, A.M.; Zhou, J.; Lu, B. Molecular modulation strategies for two-dimensional transition metal dichalcogenide-based high-performance electrodes for metal-ion batteries. Chem. Sci. 2024, 15, 2323–2350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Tang, Y.; Liu, H.; Ji, H.; Jiang, C.; Zhang, J.; Zhang, X.; Lee, C.-S. Uniform incorporation of flocculent molybdenum disulfide nanostructure into three-dimensional porous graphene as an anode for high-performance lithium-ion batteries and hybrid supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 4691–4699. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, Z.; Shakir, I.; Xu, Y.; Lu, H. Rational synthesis of carbon shell coated polyaniline/MoS2 monolayer composites for high-performance supercapacitors. Nano Res. 2016, 9, 951–962. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Lu, A.-Y.; Zhu, H.; Xiao, J.; Chuu, C.-P.; Han, Y.; Chiu, M.-H.; Cheng, C.-C.; Yang, C.-W.; Wei, K.-H.; Yang, Y.; et al. Janus monolayers of transition metal dichalcogenides. Nat. Nanotechnol. 2017, 12, 744–749. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, J.; Han, G.; Li, M.; Ji, Q.; Ma, D.; Zhang, Y.; Li, C.; Lang, X.; Zhang, Y.; et al. Chemical vapor deposition of monolayer WS2 nanosheets on Au foils toward direct application in hydrogen evolution. Nano Res. 2015, 8, 2881–2890. [Google Scholar] [CrossRef]
- Ye, G.; Gong, Y.; Lin, J.; Li, B.; He, Y.; Pantelides, S.T.; Zhou, W.; Vajtai, R.; Ajayan, P.M. Defects engineered monolayer MoS2 for improved hydrogen evolution reaction. Nano Lett. 2016, 16, 1097–1103. [Google Scholar] [CrossRef]
- Wang, H.; Kong, D.; Johanes, P.; Cha, J.J.; Zheng, G.; Yan, K.; Liu, N.; Cui, Y. MoSe2 and WSe2 nanofilms with vertically aligned molecular layers on curved and rough surfaces. Nano Lett. 2013, 13, 3426–3433. [Google Scholar] [CrossRef]
- Kong, D.; Wang, H.; Cha, J.J.; Pasta, M.; Koski, K.J.; Yao, J.; Cui, Y. Synthesis of MoS2 and MoSe2 films with vertically aligned layers. Nano Lett. 2013, 13, 1341–1347. [Google Scholar] [CrossRef]
- Yang, Y.; Fei, H.; Ruan, G.; Xiang, C.; Tour, J.M. Edge-oriented MoS2 nanoporous films as flexible electrodes for hydrogen evolution reactions and supercapacitor devices. Adv. Mater. 2014, 26, 8163–8168. [Google Scholar] [CrossRef]
- Geim, A.K.; Grigorieva, I.V. Van der Waals heterostructures. Nature 2013, 499, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Mishchenko, A.; Carvalho, A.; Neto, A.H.C. 2D materials and van der Waals heterostructures. Science 2016, 353, aac9439. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Pakhira, S.; Fujisawa, K.; Wang, X.; Iyiola, O.O.; López, N.P.; Elías, A.L.; Rajukumar, L.P.; Zhou, C.; Kabius, B.; et al. Low-temperature Synthesis of Heterostructures of Transition Metal Dichalcogenide Alloys (WxMo1–xS2) and Graphene with Superior Catalytic Performance for Hydrogen Evolution. ACS Nano 2017, 11, 5103–5112. [Google Scholar] [CrossRef] [PubMed]
- Balasingam, S.K.; Lee, J.S.; Jun, Y. Few-layered MoSe2 nanosheets as an advanced electrode material for supercapacitors. Dalton Trans. 2015, 44, 15491–15498. [Google Scholar] [CrossRef]
- Gao, Y.-P.; Huang, K.-J.; Shuai, H.-L.; Liu, L. Synthesis of sphere-feature molybdenum selenide with enhanced electrochemical performance for supercapacitor. Mater. Lett. 2017, 209, 319–322. [Google Scholar] [CrossRef]
- Firmiano, E.G.d.S.; Rabelo, A.C.; Dalmaschio, C.J.; Pinheiro, A.N.; Pereira, E.C.; Schreiner, W.H.; Leite, E.R. Supercapacitor Electrodes Obtained by Directly Bonding 2D MoS2 on Reduced Graphene Oxide. Adv. Energy Mater. 2014, 4, 1301380. [Google Scholar] [CrossRef]
- Zhao, L.; Hong, C.; Lin, L.; Wu, H.; Su, Y.; Zhang, X.; Liu, A. Controllable nanoscale engineering of vertically aligned MoS2 ultrathin nanosheets by nitrogen doping of 3D graphene hydrogel for improved electrocatalytic hydrogen evolution. Carbon 2017, 116, 223–231. [Google Scholar] [CrossRef]
- Wang, H.; Gao, Z.; Shi, H.; Cui, X.; Jia, J.; Xu, H. Reinforcing the assembly of NiV-LDH and MoS2 with PANI as a coupling agent to form a ternary composite of NiV-LDH/PANI/MoS2 for enhanced supercapacitor performance. J. Electroanal. Chem. 2023, 951, 117933. [Google Scholar] [CrossRef]
- Kumar, R.; Thangappan, R. Significant Electrode of Supercapattery Devices: The Induced Charge Storage Capability of MoSe2/rGO Nanosheets Bedecked by MnO2 Nanorod Composites. Langmuir 2025, 41, 9753–9768. [Google Scholar] [CrossRef]
- Masanta, S.; Nayak, C.; Maitra, S.; Rudra, S.; Chowdhury, D.; Raha, S.; Pradhan, M.; Satpati, B.; Pal, P.; Singha, A. Engineering Multifunctionality in MoSe2 Nanostructures Via Strategic Mn Doping for Electrochemical Energy Storage and Photosensing. ACS Appl. Nano Mater. 2023, 6, 5479–5492. [Google Scholar] [CrossRef]
- Yesuraj, J.; Kim, J.; Yang, R.; Kim, K. Dual Functionality of Dichalcogenide-Supported Pentagon Core–Hexagon Ring-Structured NiCo2O4 Nanoplates: An Effective Hybridization for Tuning of a Diffused- to a Surface-Controlled Process and Boosting of CO2 Electrocatalysis. ACS Appl. Energy Mater. 2022, 5, 10149–10164. [Google Scholar] [CrossRef]
- Singh, D.; Singh, A.; Ojha, S.K.; Ojha, A.K. Facile synthesis of layered 2H-WSe2 nanosheets for asymmetric supercapacitor device application. Synth. Met. 2023, 293, 117263. [Google Scholar] [CrossRef]
- Singh, D.; Ojha, S.K.; Maurya, A.; Preitschopf, T.; Fischer, I.; Ojha, A.K. Controlled synthesis of 2H-WSe2@rGO nanocomposites: An efficient electrode material for high performance asymmetric supercapacitor device application. J. Alloys Compd. 2023, 968, 171828. [Google Scholar] [CrossRef]
- Singh, D.; Maurya, A.; Ojha, S.K.; Preitschopf, T.; Fischer, I.; Ojha, A.K. An improved energy storage performance of Mo doped WSe2@rGO electrode for asymmetric supercapacitor device application. Mater. Chem. Phys. 2025, 340, 130823. [Google Scholar] [CrossRef]
- Kaur, J.; Sharma, S.; Chand, P.; Arya, A.; Sharma, A. Synergetic effect driven WO3/MoS2 nanocomposite based electrode for high-efficiency supercapacitors. Electrochim. Acta 2025, 523, 145965. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, Y.; Xie, Y.; Yao, F.; Gao, X.; Bai, H.; Zhang, K.; Liu, R.; Yue, H. A novel Co9S8–MoS2 nanosheet arrays@HCSs with superior capacitive and cycling performance for hybrid supercapacitor. J. Power Sources 2024, 605, 234537. [Google Scholar] [CrossRef]
- Raza, A.; Farid, A.; Rasheed, A.; Yousaf, M.; Ayub, N.; Khan, I.; Ghanem, M.A.; Marken, F. Chemical vapor deposition-based synthesis of cost-effective binder-free nanostructured Ag/MoS2/Ni–F electrode material for portable energy storage devices. Mater. Chem. Phys. 2024, 325, 129623. [Google Scholar] [CrossRef]
- Sharma, M.; Pershaanaa, M.; Singh, A.K.; Kasi, R.; Subramaniam, R.T.; Deb, P. ZnNi2O4/WS2 Nanoflake-Based Electrodes for Quasi-Solid-State Asymmetric Supercapacitors. ACS Appl. Nano Mater. 2024, 7, 23592–23603. [Google Scholar] [CrossRef]
- Vidhya, M.S.; Yuvakkumar, R.; Ravi, G.; Saravanakumar, B.; Velauthapillai, D. Asymmetric polyhedron structured NiSe2@MoSe2 device for use as a supercapacitor. Nanoscale Adv. 2021, 3, 4207–4215. [Google Scholar] [CrossRef]
- He, X.; Sun, H.; Li, Z.; Song, J.; Li, H.; Wang, C.; Niu, Y.; Jiang, J. Simultaneously Enhanced Energy Harvesting and Storage Performance Achieved by 3D Mix-Phase MoSe2—NiSe/NF. Adv. Funct. Mater. 2023, 34, 2307835. [Google Scholar] [CrossRef]
- Karade, S.S.; Nimbalkar, A.S.; Eum, J.-H.; Kim, H. Lichen-like anchoring of MoSe2 on functionalized multiwalled carbon nanotubes: An efficient electrode for asymmetric supercapacitors. RSC Adv. 2020, 10, 40092–40105. [Google Scholar] [CrossRef]
- Shui, J.; Bai, B.; Jiang, X.; Du, P. High-performance MoSe2/rGO composites based on interface and phase engineering for all-solid-state symmetric supercapacitors. Electrochim. Acta 2023, 469, 143257. [Google Scholar] [CrossRef]
- Jana, B.; Nath, R.; SenGupta, J.; Singha, A.; Das, K. Development of three-dimensional MoSe2/graphene-flake composite toward high-performance symmetric supercapacitor: An experimental and theoretical study. J. Energy Storage 2025, 120, 116354. [Google Scholar] [CrossRef]
- Macherla, N.; Nerella, M.; Koutavarapu, R.; Shim, J. MoSe2 nanosheets anchored on Ti3C2 MXene hybrid nanostructure for boosting electrochemical performance of supercapacitor. Mater. Chem. Phys. 2025, 339, 130765. [Google Scholar] [CrossRef]
- Patel, A.B.; Vaghasiya, J.V.; Chauhan, P.; Sumesh, C.K.; Patel, V.; Soni, S.S.; Patel, K.D.; Garg, P.; Solanki, G.K.; Pathak, V.M. Synergistic 2D MoSe2@WSe2 nanohybrid heterostructure toward superior hydrogen evolution and flexible supercapacitor. Nanoscale 2022, 14, 6636–6647. [Google Scholar] [CrossRef] [PubMed]
- Tomar, A.; Choudhary, N.; Malik, G.; Chandra, R. WSe2 Nanoflakes on Graphite Sheets for Flexible Symmetric Supercapacitors. ACS Appl. Nano Mater. 2024, 7, 26111–26125. [Google Scholar] [CrossRef]
- Pazhamalai, P.; Krishnamoorthy, K.; Manoharan, S.; Kim, S.J. High energy symmetric supercapacitor based on mechanically delaminated few-layered MoS2 sheets in organic electrolyte. J. Alloys Compd. 2019, 771, 803–809. [Google Scholar] [CrossRef]
- Chauhan, P.; Late, D.J.; Patel, V.; Sahatiya, P.; Sumesh, C. Hierarchical NiCo-LDH@MoS2/CuS composite as efficient trifunctional electrocatalyst for overall water splitting and asymmetric supercapacitor. Electrochim. Acta 2023, 469, 143197. [Google Scholar] [CrossRef]
- Panchal, K.; Sharma, K.S.; Bhakar, K.; Rajpurohit, N.A.; Kumar, D. Harnessing the potential of biomimetic-designed asteraceae flower-structured MoS2/Ni3S4@NiCr-LDH41 for high-performance symmetric and asymmetric supercapacitors. Energy Fuels 2024, 38, 19048–19063. [Google Scholar] [CrossRef]
- Jhanjhariya, N.; Lata, S. Potential window optimization to upgrade the performance of the designed triad MoS2/MWCNT/PPy as an asymmetric supercapacitor device. J. Energy Storage 2024, 82, 110577. [Google Scholar] [CrossRef]
- Zhan, C.; Liu, W.; Hu, M.; Liang, Q.; Yu, X.; Shen, Y.; Lv, R.; Kang, F.; Huang, Z.-H. High-performance sodium-ion hybrid capacitors based on an interlayer-expanded MoS2/rGO composite: Surpassing the performance of lithium-ion capacitors in a uniform system. NPG Asia Mater. 2018, 10, 775–787. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, B.; Chen, T.; Shi, C.; Dong, X.; Tao, X. Structural regulation of 1T-MoS2/Graphene composite materials for high-performance lithium-ion capacitors. J. Energy Storage 2024, 102, 114178. [Google Scholar] [CrossRef]
- Raza, A.; Rasheed, A.; Farid, A.; Yousaf, M.; Ayub, N.; Khan, I. Synthesis of binder-free nanofibers ZnS/MoS2/NiF electrode material for asymmetric supercapacitor applications. J. Energy Storage 2024, 84, 110811. [Google Scholar] [CrossRef]
- Mashkoor, F.; Mashkoor, R.; Shoeb, M.; Anwer, A.H.; Jeong, H.; Jeong, C. Freestanding WS2-MWCNT nanocomposite for electrochemical detection of contaminants of emerging concern-perfluorooctanoic acid “a forever chemical” and supercapacitor applications. ACS Sustain. Chem. Eng. 2023, 11, 13306–13319. [Google Scholar] [CrossRef]
- Sharma, M.; Singh, S.P.; Deb, P. Out-of-plane CuSe/WS2 heterostructure array as a high-performance electrode material in ammonium ion supercapacitor in harsh environment of extreme temperature. Adv. Funct. Mater. 2025, 35, 2424005. [Google Scholar] [CrossRef]
- Mashkoor, F.; Adnan, S.M.; Shoeb, M.; Jeong, C. Waste-to-wealth strategy: Ti3AlC2-MAX-supported WS2/halloysite nanocomposite for the removal of nickel metal ions from wastewater with machine learning simulation and subsequent application in supercapacitors. ACS Sustain. Chem. Eng. 2024, 12, 6547–6563. [Google Scholar] [CrossRef]
- Feng, T.; Mo, Y.; Lv, S.; Liu, G. Heterolayered 2D nanohybrids of graphene-WS2 nanosheets: Enabling enhanced supercapacitive performance of polyaniline. Energy Fuels 2023, 37, 6266–6275. [Google Scholar] [CrossRef]
- Sundriyal, S.; Shrivastav, V.; Tiwari, U.K.; Deep, A. WS2/carbon composites and nanoporous carbon structures derived from zeolitic imidazole framework for asymmetrical supercapacitors. Energy Fuels 2021, 35, 15133–15142. [Google Scholar] [CrossRef]

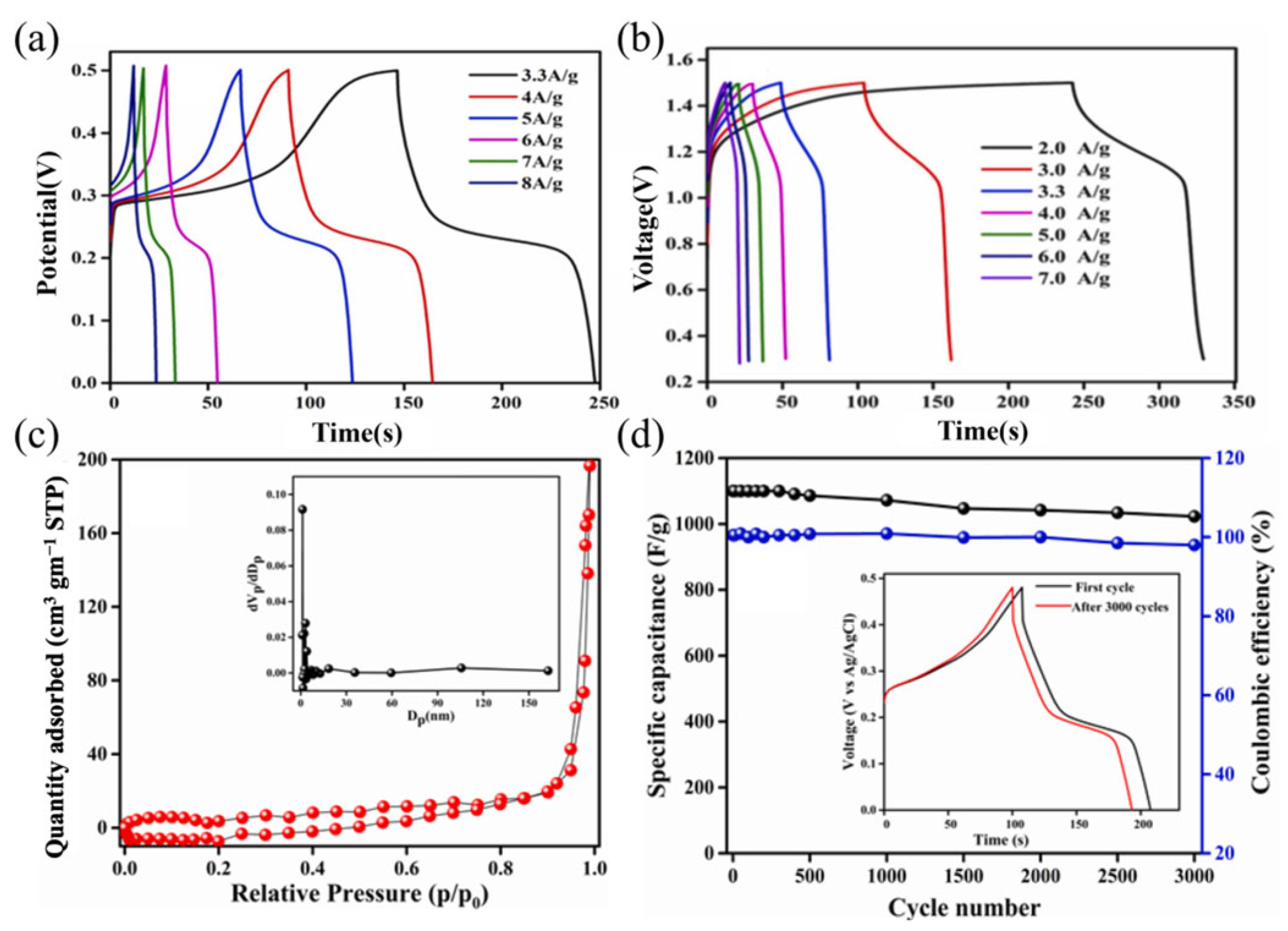
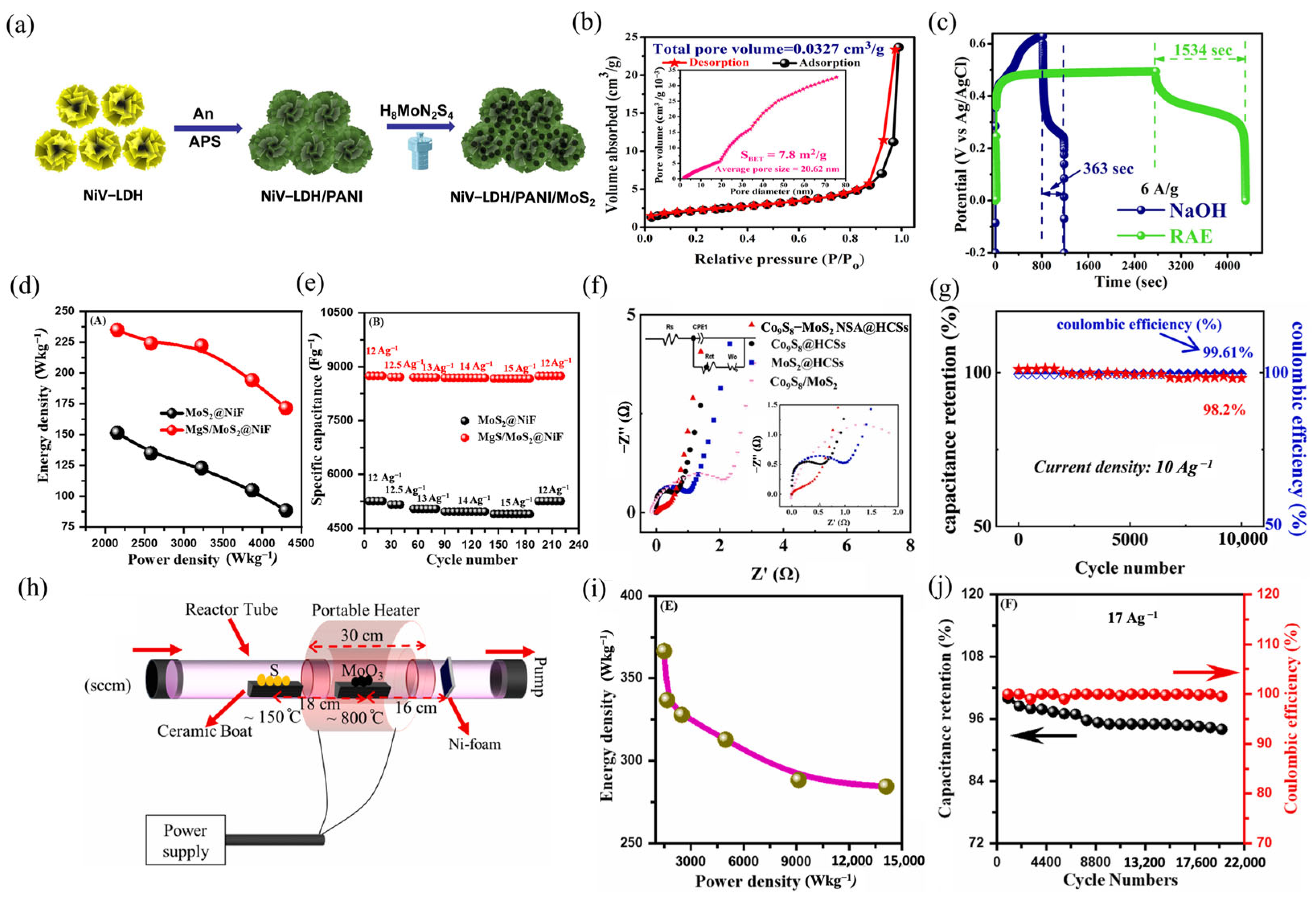
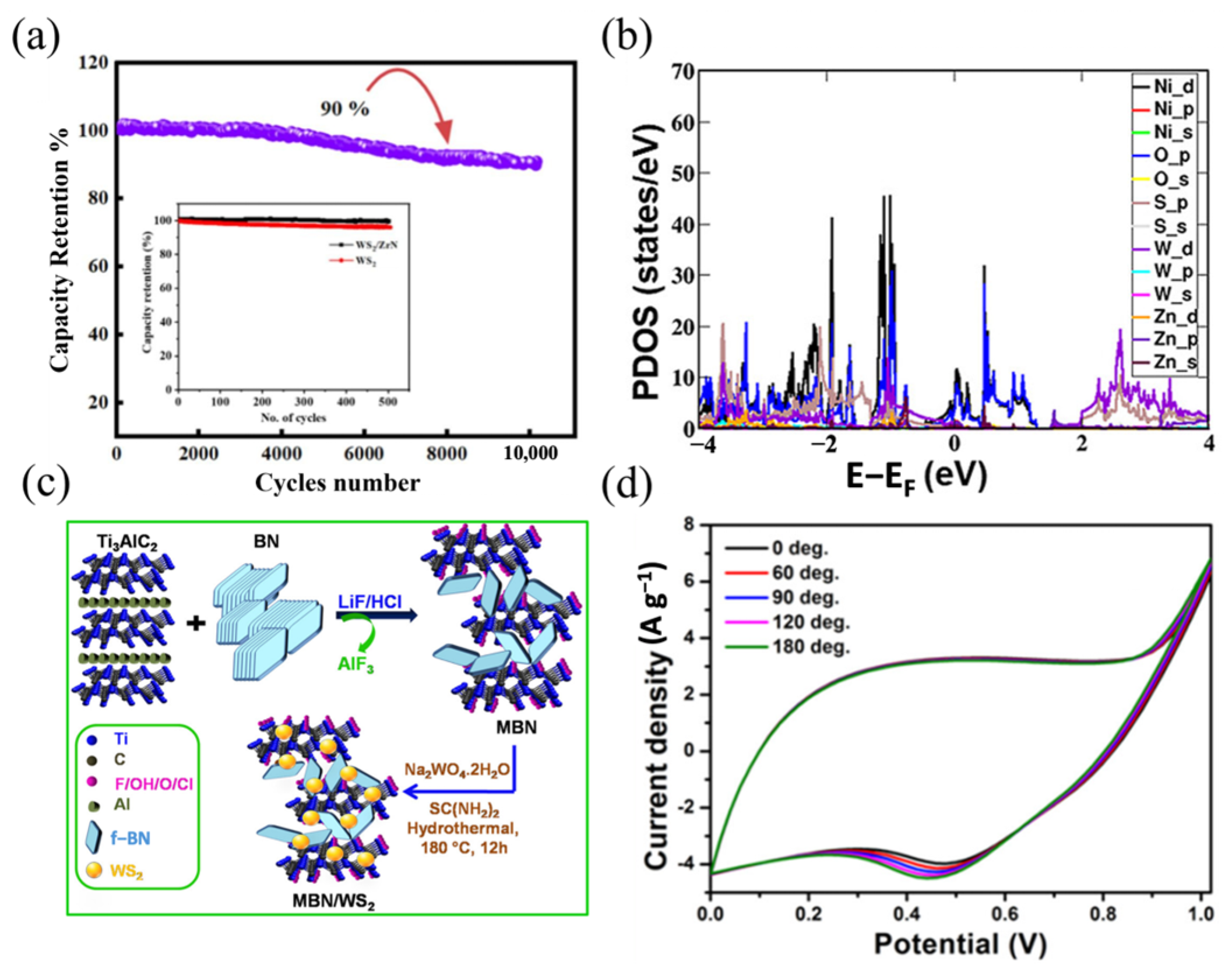
| Supercapacitor | Electrolyte | Potential Window | Current Density | Specific Capacitance of the Device | Energy Density | Power Density | Retention | Ref. |
|---|---|---|---|---|---|---|---|---|
| V | A g−1 | F g−1 | Wh kg−1 | W kg−1 | % (cycles) | |||
| MoSe2-Ni(OH)2//AC | 6 M KOH | 1.6 | 1 | 124 | 43 | 817 | 85 (5000) | [22] |
| Ni2P/NiSe2/MoSe2//AC | 4 M KOH | 1.6 | 0.5 | 66.1 | 23.5 | 400 | 116.6 (45,000) | [23] |
| NiSe2@MoSe2//AC | 1 M KOH | 1.5 | 1 | 305 | 79 | 738 | 87.35 (5000) | [121] |
| MoSe2@NiSe/NF//AC | 0.4 M Fe(CN)63− /Fe(CN)64− | 1.6 | 1 | 150.9 C g−1 | 54 | 806 | 92.8 (50,000) | [122] |
| Mn-doped MoSe2//AC | 3 M KOH | 1.2 | 1 | 112 | 22.25 | 1400 | 90 (5000) | [112] |
| MoSe2/MWCNT//MnO2 | 1 M LiCl | 1.6 | 1.5 | 112 | 35.6 | 964 | 80 (2000) | [123] |
| NiCo2O4/MoSe2//AC | PVA/KOH | 1.6 | 1 | 121.25 | 68.9 | 1280 | 95 (10,000) | [113] |
| MnO2/MoSe2/rGO//AC | PVA/H2SO4 | 1.8 | 1 | 85.1 | 30.2 | 807 | 80 (10,000) | [111] |
| MoSe2/rGO//MoSe2/rGO | PVA/KOH | 1 | 0.3 | 35.1 | 4.88 | 150 | 83.1 (10,000) | [124] |
| MoSe2-GFs-25//MoSe2-GFs-25 | PVA/KOH | 0.6 | 1 | 243 | 48.7 | 600 | 78 (13,000) | [125] |
| MoSe2/e-Ti3C2Tx//MoSe2/e-Ti3C2Tx | 1 M KOH | 1 | 1 | 93 C g−1 | 12.92 | 1001.02 | 80 (5000) | [126] |
| MoSe2/MWCNT//MoSe2/MWCNT | 1 M LiCl | 1 | 3 | 129 | 17.9 | 1500 | - | [123] |
| MoSe2@WSe2//MoSe2@WSe2 | PVA/H2SO4 | 1 | 1 | - | 14.44 | 397 | 91.94 (10,000) | [127] |
| WSe2//AC | PVA/KOH | 1.5 | 2 | 81.6 | 25.5 | 1111 | 77 (10,000) | [114] |
| WSe2@rGO//AC | PVA/KOH | 1.5 | 2 | 145 | 51.5 | 2133.3 | 82 (3000) | [115] |
| WSe2-Mo-3@rGO//AC | PVA/KOH | 1.6 | 2 | 194 | 70 | 1706 | 87 (3000) | [117] |
| WSe2@graphite//WSe2@graphite | 1 M HCl | 1.5 | 4 mA cm−2 | 88 mF cm−2 | 27.5 μWh cm−2 | 3000 μW cm−2 | 75.36 (5000) | [128] |
| MoS2//MoS2 | 0.5 M TEABF4 | 3 | 0.75 | 14.75 | 18.43 | 1125 | 91.2 (5000) | [129] |
| NiCo-LDH@MoS2CuS//AC | PVA/KOH | 1.6 | 1 | 46.66 mAh g−1 | 152.6 | 539.9 | 90.05 (7000) | [130] |
| MoS2/Ni3S4@NiCr-LDH41//AC | 6 M KOH | 1.6 | 1 | 71.37 | 25.37 | 800 | 85.01 (10,000) | [131] |
| NiV-LDH/PANI/MoS2//AC | 3 M KOH | 1.2 | 6 | 182.5 | 36.51 | 3600 | 78.84 (8000) | [110] |
| MoS2/MWCNTs/polypyrrole//AC | 1 M H2SO4 | 1.2 | 5 mV s−1 | 633.33 | 93.33 | 240.17 | - | [132] |
| N-3DG//3D-IEMoS2@G | 1 M NaClO4 in EC/DMC | 1–4.3 | - | - | 140 | 630 | 99 (10,000) | [133] |
| 1T-MoS2/Graphene-0.8//AC | 1 M LiPF6 | 1–4 | 5 | 93 | 235.4 | 249.6 | 89.9 (2000) | [134] |
| MoS2/Fe2O3/Graphene//AC | 3 M KOH | 1.5 | 1 | 150.1 | 46.8 | 750 | 77 (10,000) | [7] |
| WO3/MoS2//AC | RAE | 1.9 | 10 | 182.15 | 84.72 | 7624.8 | - | [117] |
| ZnS/MoS2/NF//AC | 2 M KOH | 1.72 | 1 | 494 | 203 | 860 | 97 (5000) | [135] |
| MgS/MoS2@NF//AC | 2 M KOH | 1.72 | 4 | 701 | 288 | 3440 | 95 (10,000) | [26] |
| Co9S8-MoS2 NSA@HCSs//HCSs | 6 M KOH | 1.6 | 1 | 198 | 45.6 | 770.4 | 98.2 (10,000) | [118] |
| Ag/MoS2/NF//AC | 2 M KOH | 1.66 | 1.8 | 957 | 366 | 1494 | 94 (20,000) | [119] |
| WS2/ZrN//AC | 1 M KOH | 1.6 | - | 200 | 76 | 4325 | 90 (10,000) | [25] |
| WS2-MWCNT//WS2-MWCNT | PVA/H2SO4 | 1.2 | 1 | 275 | 46.15 | 500 | 89.14 (10,000) | [136] |
| ZnNi2O4/WS2//AC (ASC) | KOH | 1.6 | 1 | 171.3 | 61.6 | 1236.5 | 68.4 (3000) | [120] |
| ZnNi2O4/WS2//AC (QSSASC) | PVA/KOH | 1.6 | 1 | 56.8 | 20.4 | 921.2 | 97.2 (3000) | [120] |
| CuSe/WS2//CuSe/WS2 (SSC) | 1 M (NH4)2SO4 | 1.5 | 1 | 100 | 31.3 | 750 | 73.2 (5000) | [137] |
| CuSe/WS2//CuSe/WS2 (QSSC) | PVA/(NH4)2SO4 | 1.6 | 1 | 135.6 | 48.2 | 800 | 80 (5000) | [137] |
| WS2/Ti3C2Tx/BN//Ti3C2Tx | PVA/KOH/KI gel | 1 | 1 | 140 | 19.4 | 997.7 | 84 (10,000) | [28] |
| Mx-WS2-Hal@Ni-Adsorbed// Mx-WS2-Hal@Ni-Adsorbed | 1 M Na2SO4 | 1.7 | 1.75 | 251.86 | 59.47 | 583 | 90 (10,000) | [138] |
| PANI/Graphene/WS2// PANI/Graphene/WS2 | 1 M H2SO4 | 1 | 1 | 71.7 | 9.96 | 250.04 | 71.6 (10,000) | [139] |
| WS2/Z8-800//Z8-800 | 1 M H2SO4 | 1.4 | 1 | 88 | 25 | 801 | 78 (3000) | [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, T.; Li, Y.; Lai, C.W.; Xiang, P.; Badruddin, I.A.; Dhiman, P.; Kumar, A. Recent Advances in Transition Metal Dichalcogenide-Based Electrodes for Asymmetric Supercapacitors. Catalysts 2025, 15, 945. https://doi.org/10.3390/catal15100945
Gao T, Li Y, Lai CW, Xiang P, Badruddin IA, Dhiman P, Kumar A. Recent Advances in Transition Metal Dichalcogenide-Based Electrodes for Asymmetric Supercapacitors. Catalysts. 2025; 15(10):945. https://doi.org/10.3390/catal15100945
Chicago/Turabian StyleGao, Tianyi, Yue Li, Chin Wei Lai, Ping Xiang, Irfan Anjum Badruddin, Pooja Dhiman, and Amit Kumar. 2025. "Recent Advances in Transition Metal Dichalcogenide-Based Electrodes for Asymmetric Supercapacitors" Catalysts 15, no. 10: 945. https://doi.org/10.3390/catal15100945
APA StyleGao, T., Li, Y., Lai, C. W., Xiang, P., Badruddin, I. A., Dhiman, P., & Kumar, A. (2025). Recent Advances in Transition Metal Dichalcogenide-Based Electrodes for Asymmetric Supercapacitors. Catalysts, 15(10), 945. https://doi.org/10.3390/catal15100945











