Electrochemical and Spectroelectrochemical Studies on Oxygen Reduction Mediated by Cu(II) Complexes Containing the Alkylamine Ligand N,N-Dimethylethylendiamine
Abstract
1. Introduction
2. Results and Discussion
2.1. Complexes’ Characterization
2.1.1. Solid-State Characterization
2.1.2. Characterization in Solution
2.2. Electrochemical Behavior of Cu-dmen Complexes
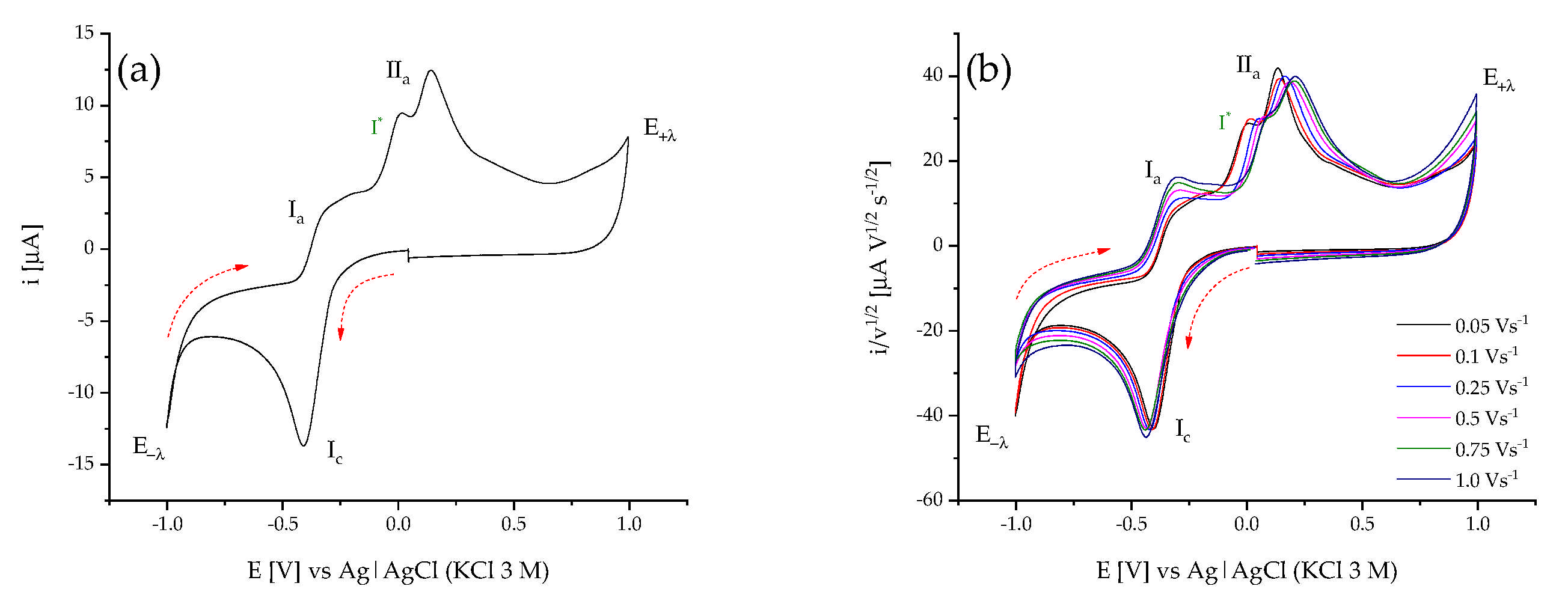
2.2.1. Cyclic Voltammetry for Bis(dmen) Complexes
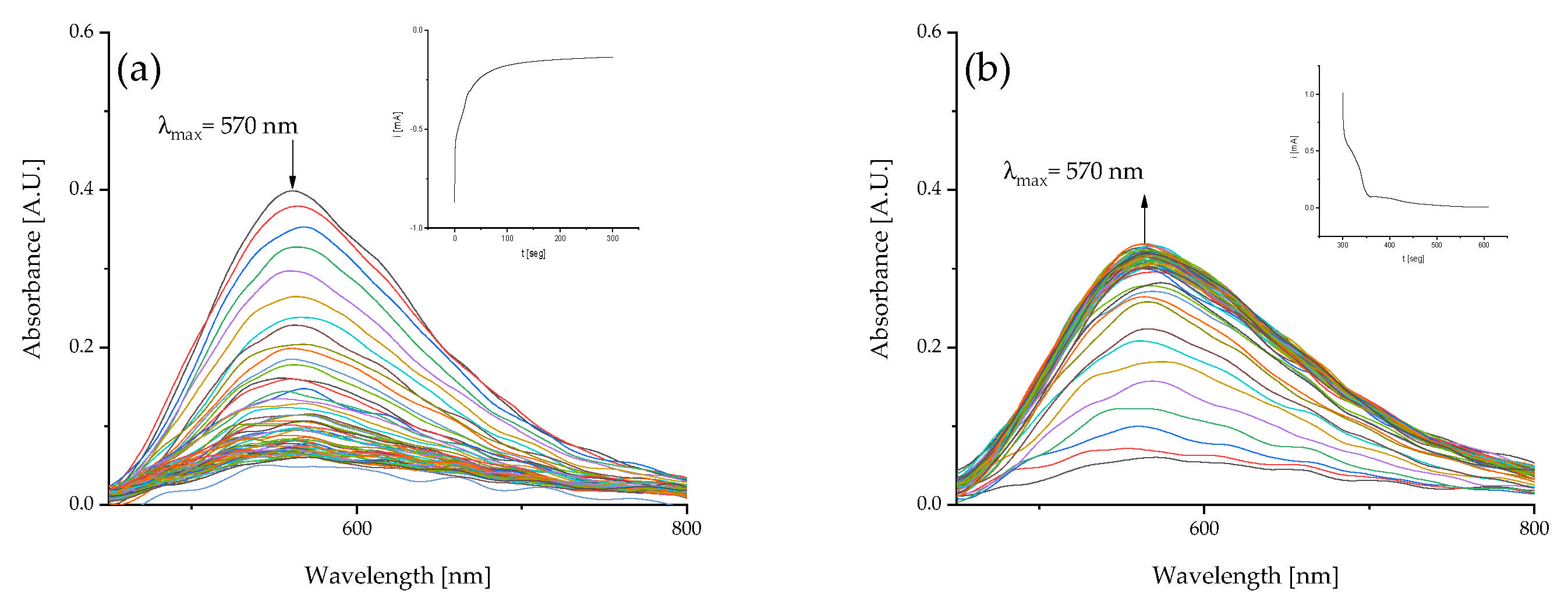
2.2.2. Spectroelectrochemical Characterization
2.2.3. Chronoamperometry
2.2.4. Proposed Mechanism
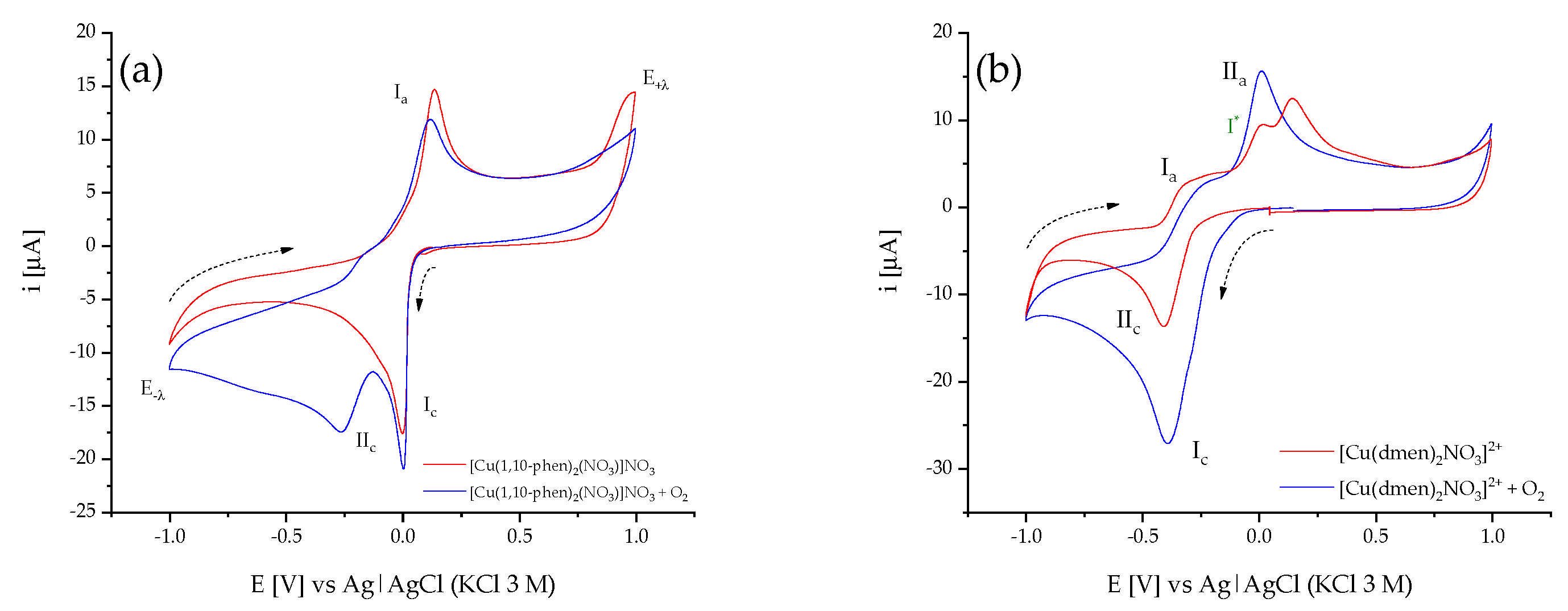
2.3. Electrochemical Characterization of Cu-1,10-Phen Complex
Cyclic Voltammetry for bis(1,10-phen)Cu complex
2.4. Electrochemical Studies in Presence of O2
2.4.1. Cyclic Voltammetry in Presence of O2
2.4.2. Spectroelectrochemical in Presence of O2
2.4.3. Proposed Mechanism for O2 Reduction
2.4.4. Foot of the Wave Analysis (FOWA) for O2 Reduction
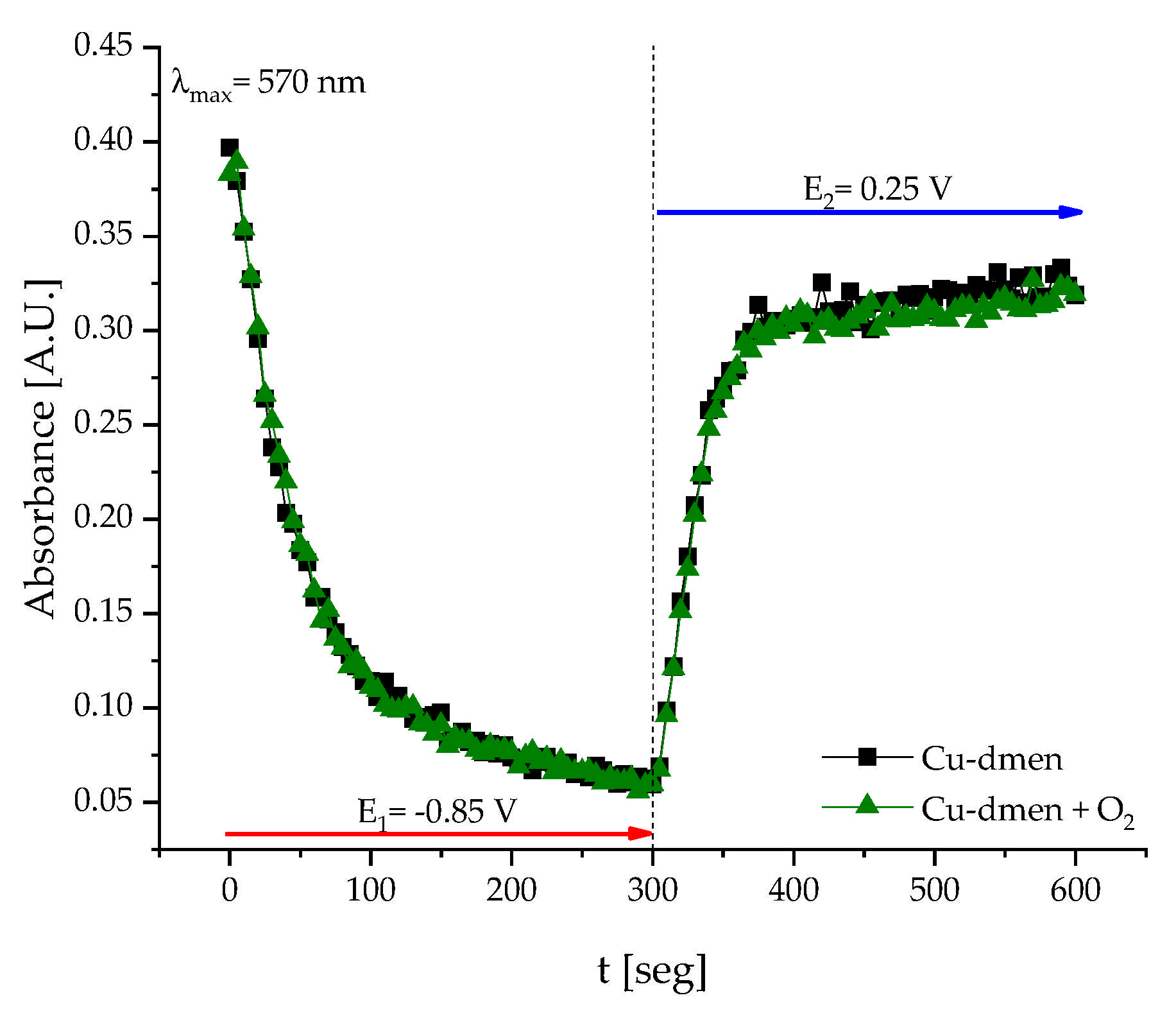
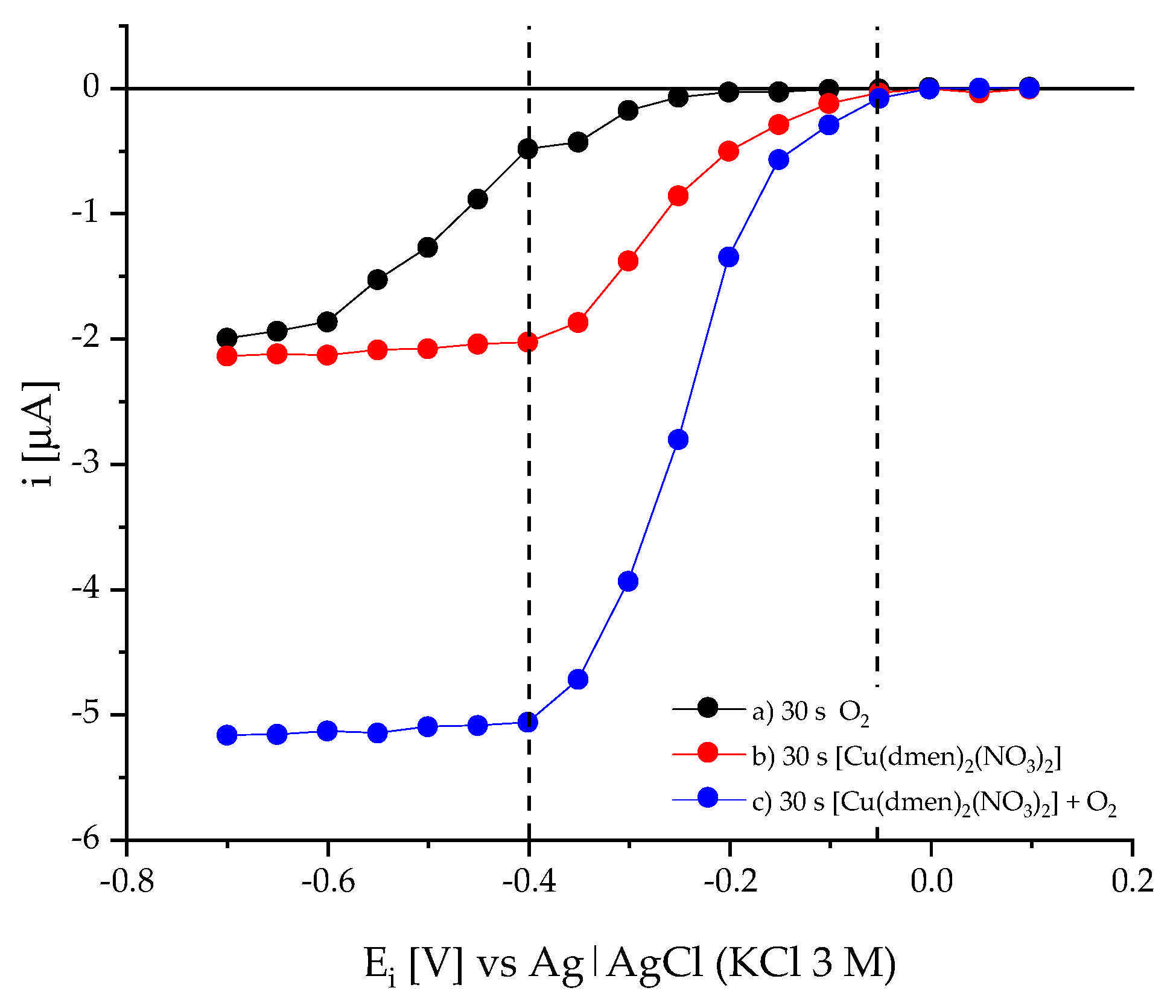
2.5. Chronoamperometry in Presence of O2
2.6. Electrochemical Impedance Spectroscopy Studies
3. Materials and Methods
3.1. Materials and Physical Measurements
3.2. Synthesis of Complexes
3.3. Single-Crystal XRD Analysis
3.4. Electrochemical Experiments
3.5. Spectroelectrochemical Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ew | Working electrode |
| EDC | Direct-current potential |
| EAC | Alternate-current potential |
| Epc | Cathodic peak potential |
| Epa | Anodic peak potential |
| CPE | Constant phase element |
| Ν | Scan rate |
| Τ | Perturbation time |
| ORR | Oxygen reduction reaction |
| FOWA | Foot of the wave analysis |
References
- Sueyoshi, T.; Mo, F.; Wang, D.D. Sustainable development of countries all over the world and the impact of renewable energy. Renew. Energy 2022, 184, 320–331. [Google Scholar] [CrossRef]
- Yua, X.-Z.; Wang, H. PEM Fuel Cell Fundamentals. In PEM Fuel Cell Electrocatalysts and Catalyst Layers, 1st ed.; Zhang, J., Ed.; Springer: Vancouver, BC, Canada, 2008; pp. 1–88. [Google Scholar]
- Gewirth, A.A.; Varnell, J.A.; DiAscro, A.M. Nonprecious Metal Catalysts for Oxygen Reduction in Heterogeneous Aqueous Systems. Chem. Rev. 2018, 118, 2313–2339. [Google Scholar] [CrossRef] [PubMed]
- Thorseth, M.A.; Tornow, C.E.; Tse, E.C.; Gewirth, A.A. Cu complexes that catalyze the oxygen reduction reaction. Cord. Chem. Rev. 2013, 257, 130–139. [Google Scholar] [CrossRef]
- Zagal, J.H.; Koper, M.T.M. Reactivity Descriptors for the Activity of Molecular MN4 Catalysts for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. Engl. 2016, 55, 14510–14521. [Google Scholar] [CrossRef]
- Fu, S.; Zhu, C.; Song, J.; Du, D.; Lin, Y. Metal-Organic Framework-Derived Non-Precious Metal Nanocatalysts for Oxygen Reduction Reaction. Adv. Energy Mater. 2017, 7, 1700363. [Google Scholar] [CrossRef]
- Gewirth, A.A.; Thorum, M.S. Electroreduction of Dioxygen for Fuel-Cell Applications: Materials and Challenges. Inorg. Chem. 2010, 49, 3557–3566. [Google Scholar] [CrossRef]
- Zhu, R.; Qin, Y.; Wu, T.; Ding, S.; Su, Y. Insights into local coordination environment of main group metal-nitrogen-carbon catalysts for enhanced oxygen reduction reaction. Appl. Surf. Sci. 2023, 631, 157581. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, G.; Wang, F.; Wei, P.; Liu, J. Bioinspired Transition-Metal Complexes as Electrocatalysts for the Oxygen Reduction Reaction. Chem. A Eur. J. 2019, 25, 3726–3739. [Google Scholar] [CrossRef]
- Muñoz-Becerra, K.; Zagal, J.H.; Venegas, R.; Recio, F.J. Strategies to improve the catalytic activity and stability of bioinspired Cu molecular catalysts for the ORR. Curr. Opin. Electrochem. 2022, 35, 101035. [Google Scholar] [CrossRef]
- Mano, N.; Soukharev, V.; Heller, A. A Laccase-Wiring Redox Hydrogel for Efficient Catalysis of O2 Electroreduction. J. Phys. Chem. B 2006, 110, 11180–11187. [Google Scholar] [CrossRef]
- Schweiger, H.; Vayner, E.; Anderson, A.B. Why Is There Such a Small Overpotential for O[sub 2] Electroreduction by Copper Laccase? Electrochem. Soc. 2005, 8, 585–588. [Google Scholar] [CrossRef]
- SFukuzumi, S.; Karlin, K.D. Kinetics and thermodynamics of formation and electron-transfer reactions of Cu–O2 and Cu2–O2 complexes. Cord. Chem. Rev. 2013, 257, 187–195. [Google Scholar] [CrossRef]
- Lewis, E.A.; Tolman, W.B. Reactivity of Dioxygen−Copper Systems. Chem. Rev. 2004, 104, 1047–1076. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Tada, A.; Suto, K.; Itaya, K. Adlayer Structures and Electrocatalytic Activity for O2 of Metallophthalocyanines on Au(111): In Situ Scanning Tunneling Microscopy Study. J. Phys. Chem. B 2003, 107, 5836–5843. [Google Scholar] [CrossRef]
- van der Ham, C.J.M.; Zwagerman, D.N.H.; Wu, L.; Hofmann, J.P.; Hetterscheid, D.G.H. A Heterogenized Copper Phenanthroline System to Catalyze the Oxygen Reduction Reaction. ChemElectroChem 2022, 9, e202101365. [Google Scholar] [CrossRef] [PubMed]
- McCrory, C.C.L.; Ottenwaelder, X.; Stack, T.D.P.; Chidsey, C.E.D. Kinetic and Mechanistic Studies of the Electrocatalytic Reduction of O2 to H2O with Mononuclear Cu Complexes of Substituted 1,10-Phenanthrolines. J. Phys. Chem. A 2007, 111, 12641–12650. [Google Scholar] [CrossRef] [PubMed]
- Langerman, M.; Hetterscheid, D.G.H. Fast Oxygen Reduction Catalyzed by a Copper(II) Tris(2-pyridylmethyl)amine Complex through a Stepwise Mechanism. Angew. Chem. Int. Ed. Engl. 2019, 58, 12974–12978. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, G.-S.; Liu, H.-C.; Zhu, M.-J.; Xie, M.-Y.; Cen, M.-S.; Li, Q.-J.; Wang, T.-S.; Zhang, H.-X. Bidirectional O2 reduction/H2O oxidation boosted by a pentadentate pyridylalkylamine copper(II) complex. Electrochimica Acta 2023, 446, 142099. [Google Scholar] [CrossRef]
- He, F.; Mi, L.; Shen, Y.; Chen, X.; Yang, Y.; Mei, H.; Liu, S.; Mori, T.; Zhang, Y. Driving electrochemical oxygen reduction and hydrazine oxidation reaction by enzyme-inspired polymeric Cu(3,3′-diaminobenzidine) catalyst. J. Mater. Chem. A 2017, 5, 17413–17420. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Kotani, H.; Lucas, H.R.; Doi, K.; Suenobu, T.; Peterson, R.L.; Karlin, K.D. Mononuclear Copper Complex-Catalyzed Four-Electron Reduction of Oxygen. J. Am. Chem. Soc. 2010, 132, 6874–6875. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, Z.-L.; Peng, S.; Luo, Z.-L.; Che, Y.-T.; You, L.-Q.; Li, R.-R.; Zhang, H.-X. Catalytic oxygen reduction by pyridylalkylamine copper complexes bearing triazolylpyridines. Mol. Catal. 2024, 567, 114446. [Google Scholar] [CrossRef]
- Cai, C.X.; Xue, K.H.; Xu, X.Y.; Luo, Q.H. Electrocatalysis for the reduction of O2 and H2O2 based on complex of copper(II) with the tris(3-aminopropyl)amine and imidazole ligands. J. Appl. Electrochem. 1997, 27, 793–798. [Google Scholar] [CrossRef]
- Wang, M.; Xu, X.; Gao, J.; Jia, N.; Cheng, Y. Electrocatalytic reduction of O2 at pyrolytic graphite electrode modified by a novel copper(II) complex with 2-[bis(2-aminoethyl)amino]ethanol and imidazole ligands. Russ. J. Electrochem. 2006, 42, 878–881. [Google Scholar] [CrossRef]
- Fleming, G.; Shepherd, R. Infrared spectra and normal coordinate analysis of bis(ethylenediamine) and bis(trimethylenediamine) complexes with copper(II). Spectrochim. Acta Part A Mol. Spectrosc. 1987, 43, 1141–1146. [Google Scholar] [CrossRef]
- Pretshc, E.; Bühlmann, P.; Badertscher, M. Structure Determination of Organic Compounds, 4th ed.; Springer: Berlin/Heidelberg, 2009. [Google Scholar]
- Larkin, P.J. IR and Raman Spectra–Structure Correlations: Characteristic Group Frecuencies. In Infrared and Raman Spectroscopy, 2nd ed.; John Fedor: Stamford, CT, USA, 2018; pp. 85–134. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordinatio Compounds Part B, 6th ed.; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Masternak, J.; Zienkiewicz-Machnik, M.; Łakomska, I.; Hodorowicz, M.; Kazimierczuk, K.; Nosek, M.; Majkowska-Młynarczyk, A.; Wietrzyk, J.; Barszcz, B. Synthesis and Structure of Novel Copper(II) Complexes with N,O- or N,N-Donors as Radical Scavengers and a Functional Model of the Active Sites in Metalloenzymes. Int. J. Mol. Sci. 2021, 22, 7286. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, B.; Bhadbhade, M.M. Non-Thermochromic Bis(N,N-dimethylethylenediamine)copper(II) Dinitrate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1995, 51, 2016–2018. [Google Scholar] [CrossRef]
- Willery, M.; Julliard, P.-G.; Molton, F.; Thomas, F.; Fortage, J.; Costentin, C.; Collomb, M.-N. Mechanism of Electrochemical Proton Reduction Catalyzed by a Cobalt Tetraaza Schiff Base Macrocyclic Complex: Ligand Protonation and/or Influence of the Chloro Ligand. ACS Catal. 2024, 14, 11352–11365. [Google Scholar] [CrossRef]
- Cukman, D.; Pravdic, V. An Investigation of the Electrochemical Reactions of a Nickel Cyanide Complex at Mercury Electrode by Cyclic Voltammetry. Croat. Chem. Acta 1978, 51, 243–247. [Google Scholar]
- Bruch, Q.J.; Malakar, S.; Goldman, A.S.; Miller, A.J.M. Mechanisms of Electrochemical N2 Splitting by a Molybdenum Pincer Complex. Inorg. Chem. 2022, 61, 2307–2318. [Google Scholar] [CrossRef]
- Lee, C.; Anson, F.C. Electron Exchange Between bis(1,10-Phenanthroline)copper(1+) Adsorbed on Graphite and bis(1,10-Phenanthroline)copper(2+) in Solution. Inorg. Chem. 1984, 23, 837–844. [Google Scholar] [CrossRef]
- Lei, Y.; Anson, F.C. Mechanistic aspects of the electroreduction of dioxygen as catalyzed by copper-phenanthroline complexes adsorbed on graphite electrodes. Inorg. Chem. 1994, 33, 5003–5009. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, X.-Q.; Liu, J.-C.; Pu, Y.-L.; Ou-Yang, Q.-R.; Lu, D.-Y.; Zhang, H.-X. Oxygen reduction mediated by tetrapyridylalkylamine Cu(II) complexes with diimines. Electrochim. Acta 2024, 477, 143758. [Google Scholar] [CrossRef]
- Wang, V.C.-C.; Johnson, B.A. Interpreting the Electrocatalytic Voltammetry of Homogeneous Catalysts by the Foot of the Wave Analysis and Its Wider Implications. ACS Catal. 2019, 9, 7109–7123. [Google Scholar] [CrossRef]
- Langerman, M.; van Langevelde, P.H.; van de Vijver, J.J.; Siegler, M.A.; Hetterscheid, D.G.H. Scaling Relation between the Reduction Potential of Copper Catalysts and the Turnover Frequency for the Oxygen and Hydrogen Peroxide Reduction Reactions. Inorg. Chem. 2023, 62, 19593–19602. [Google Scholar] [CrossRef] [PubMed]
- Thorseth, M.A.; Letko, C.S.; Tse, E.C.M.; Rauchfuss, T.B.; Gewirth, A.A. Ligand Effects on the Overpotential for Dioxygen Reduction by Tris(2-pyridylmethyl)amine Derivatives. Inorg. Chem. 2013, 52, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Jüttner, K. Electrochemical impedance spectroscopy (EIS) of corrosion processes on inhomogeneous surfaces. Electrochimica Acta 1990, 35, 1501–1508. [Google Scholar] [CrossRef]
- Jorcin, J.-B.; Orazem, M.E.; Pébère, N.; Tribollet, B. CPE analysis by local electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1473–1479. [Google Scholar] [CrossRef]
- Hsu, C.H.; Mansfeld, F. Technical Note: Concerning the Conversion of the Constant Phase Element Parameter Y into a Capacitance. Corrosion 2001, 57, 747–748. [Google Scholar] [CrossRef]
- Morad, M. An electrochemical study on the inhibiting action of some organic phosphonium compounds on the corrosion of mild steel in aerated acid solutions. Corros. Sci. 2000, 42, 1307–1326. [Google Scholar] [CrossRef]
- Monticelli, C.; Balbo, A.; Esvan, J.; Chiavari, C.; Martini, C.; Zanotto, F.; Marvelli, L.; Robbiola, L. Evaluation of 2-(salicylideneimino) thiophenol and other Schiff bases as bronze corrosion inhibitors by electrochemical techniques and surface analysis. Corros. Sci. 2019, 148, 144–158. [Google Scholar] [CrossRef]
- Clark, R.C.; Reid, J.S. The analytical calculation of absorption in multifaceted crystals. Acta Crystallogr. Sect. A 1995, 51, 887–897. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.; McCabe, P.; Pidcock, E.; Wood, P.A. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]




| Catalyst | Cond [pH/Electrode] | E1/2 CuII|CuI | TOF [s−1] | Reference |
|---|---|---|---|---|
| [Cu(dmen)2(CH3COO)2] | aqueous solution, glassy carbon | −0.326 V vs. Ag|AgCl | 4.8 × 103 | this work |
| [Cu(dmen)2(NO3)2] | aqueous solution, glassy carbon | −0.32 V vs. Ag|AgCl | 1.3 × 104 | this work |
| [Cu(dmen)2Cl2] | aqueous solution, glassy carbon | −0.324 V vs. Ag|AgCl | 5.3 × 103 | this work |
| [Cu(1,10-phen)2(NO3)2]NO3 | aqueous solution, glassy carbon | N.R. | N.R. | this work |
| [Cu(tmpa)(L)]2+ | pH 7.0 PB, glassy carbon | 0.002 V vs. Ag|AgCl | 1.8 × 106 | [18] |
| Cu-phthalocyanine | Au(111) electrode | N.R. | N.R. | [15] |
| [Cu(1,10-phen)] | pH 7.0 PB, Au electrode | N.R. | N.R. | [16] |
| [Cu(tpmen)](ClO4)2 | pH 7.0 Pb, glassy carbon | 0.002 V vs. Ag|AgCl | 6.5 × 104 | [19] |
| [Cu(pmea)(L)]2+ | pH 7.0 PB, glassy carbon | 0.162 V vs. Ag|AgCl | 1.4 × 103 | [38] |
| [Cu(tren)(L)]2+ | rotating electrode glassy carbon | N.R. | N.R. | [39] |
| [Cu(tren)(ImH)](ClO4)2 | pH 7.0 PB, pyrolytic graphite | N.R. | N.R. | [24] |
| [Cu(trpn)(ImH)](ClO4)2 | pH 6.4 Britton–Robinson, pyrolytic graphite | N.R. | N.R. | [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zúñiga, O.M.; Mendoza, A.; Cruz-Ramírez, M.; Ramírez-Palma, L.G.; Rebolledo-Chávez, J.P.F.; Ortiz-Frade, L. Electrochemical and Spectroelectrochemical Studies on Oxygen Reduction Mediated by Cu(II) Complexes Containing the Alkylamine Ligand N,N-Dimethylethylendiamine. Catalysts 2025, 15, 951. https://doi.org/10.3390/catal15100951
Zúñiga OM, Mendoza A, Cruz-Ramírez M, Ramírez-Palma LG, Rebolledo-Chávez JPF, Ortiz-Frade L. Electrochemical and Spectroelectrochemical Studies on Oxygen Reduction Mediated by Cu(II) Complexes Containing the Alkylamine Ligand N,N-Dimethylethylendiamine. Catalysts. 2025; 15(10):951. https://doi.org/10.3390/catal15100951
Chicago/Turabian StyleZúñiga, Omar Monsalvo, Angel Mendoza, Marisela Cruz-Ramírez, Lillian G. Ramírez-Palma, Juan Pablo F. Rebolledo-Chávez, and Luis Ortiz-Frade. 2025. "Electrochemical and Spectroelectrochemical Studies on Oxygen Reduction Mediated by Cu(II) Complexes Containing the Alkylamine Ligand N,N-Dimethylethylendiamine" Catalysts 15, no. 10: 951. https://doi.org/10.3390/catal15100951
APA StyleZúñiga, O. M., Mendoza, A., Cruz-Ramírez, M., Ramírez-Palma, L. G., Rebolledo-Chávez, J. P. F., & Ortiz-Frade, L. (2025). Electrochemical and Spectroelectrochemical Studies on Oxygen Reduction Mediated by Cu(II) Complexes Containing the Alkylamine Ligand N,N-Dimethylethylendiamine. Catalysts, 15(10), 951. https://doi.org/10.3390/catal15100951








