Emulsion Systems Stabilized with Nonionic Emulsifier and Cross-Linked Polyacrylic Acid: A Promising Strategy to Enhance the Activity of Immobilized CALB
Abstract
1. Introduction
2. Results and Discussion
2.1. The Influence of Kolliphor® CS 20 Concentration on Immobilized CALB Lipolytic Activity
2.2. The Influence of Carbopol® Ultrez 10 Added to the Emulsion on the Lipolytic Activity of Immobilized CALB
2.3. Influence of Kolliphor® CS 20 Concentration on Carbopol® Ultrez 10 Stabilized Emulsion
2.4. The Effect of Emulsifier Polyoxyethylene Chain Length and Emulsifier Mixture on the Lipolytic Activity of CALB
3. Materials and Methods
3.1. Materials and Equipment
3.2. Methods
3.2.1. Immobilization of Lipase B from Candida antarctica onto IB-D152 Support
3.2.2. Preparation of Formulations with Emulsifiers
Preparation of Formulation with Carbopol® Ultrez 10
Preparation of Formulation Without Carbopol® Ultrez 10
3.2.3. Evaluation of the Lipolytic Activity of Immobilized Lipase B from Candida antarctica
- Relative activity (Arel)—the ratio between the activity of the sample and the maximum activity of the sample under the test conditions (within the scope of a given experiment);
- Usample—the activity of the sample under the test conditions;
- Umaximum—the maximum activity of the sample under the test conditions.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, S.; Sun, D.; Tian, H. Novel hydrophobic catalysts to promote hydration at the water-oil interface. RSC Adv. 2021, 11, 18299–18307. [Google Scholar] [CrossRef]
- Costa, M.; Paiva-Martins, F.; Losada-Barreiro, S.; Bravo-Díaz, C. Modeling Chemical Reactivity at the Interfaces of Emulsions: Effects of Partitioning and Temperature. Molecules 2021, 26, 4703. [Google Scholar] [CrossRef] [PubMed]
- El-Zohry, A.M.; Sølling, T. Mini-Review on Water-Oil Emulsions through Vibrational Sum Frequency Generation. Energy Fuels 2024, 38, 743–760. [Google Scholar] [CrossRef]
- Álvarez, M.S.; Deive, F.J.; Rodríguez, A.; Longo, M.A. Designing biodegradable aqueous biphasic systems for the selective separation of enzymes. Sep. Purif. Technol. 2025, 353, 128508. [Google Scholar] [CrossRef]
- Wysokowska, K.; Cupiał, Z.; Staszak, M.; Zgoła-Grześkowiak, A.; Koziołek, J.; Ławniczak, Ł.; Wysokowski, M.; Wyrwas, B. Photocatalytic degradation of non-ionic, anionic, and cationic surfactants: From batch experiments through equilibrium/kinetic study to ecotoxicology analysis. Chem. Pap. 2024, 78, 761–777. [Google Scholar] [CrossRef]
- Nagtode, V.S.; Cardoza, C.; Yasin, H.K.A.; Mali, S.N.; Tambe, S.M.; Roy, P.; Singh, K.; Goel, A.; Amin, P.D.; Thorat, B.R.; et al. Green Surfactants (Biosurfactants): A Petroleum-Free Substitute for Sustainability-Comparison, Applications, Market, and Future Prospects. ACS Omega 2023, 8, 11674–11699. [Google Scholar] [CrossRef]
- Andersen, A. Final Report on the Safety Assessment of Ceteareth-2, -3, -4, -5, -6, -7, -8, -9, -10, -11, -12, -13, -14, -15, -16, -17, -18, -20, -22, -23, -24, -25, -27, -28, -29, -30, -33, -34, -40, -50, -55, -60, -80, and -100. Int. J. Toxicol. 1999, 18, 41–49. [Google Scholar] [CrossRef]
- Alaayedi, M.H.; Maraie, N.K. Lomustine’s nanoemulsion as nose-to-brain drug delivery system for CNS tumor treatment. Saudi Pharm. J. 2023, 31, 101692. [Google Scholar] [CrossRef]
- Goswami, D. Lipase Catalysis in Mixed Micelles. ChemBioEng. Rev. 2022, 9, 409–418. [Google Scholar] [CrossRef]
- Barreiro-Iglesias, R.; Alvarez-Lorenzo, C.; Concheiro, A. Incorporation of small quantities of surfactants as a way to improve the rheological and diffusional behavior of carbopol gels. J. Control. Release 2001, 77, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Milanović, M.; Krstonošić, V.; Dokić, L.; Hadnađev, M.; Dapčević Hadnađev, T. Insight into the Interaction Between Carbopol® 940 and Ionic/Nonionic Surfactant. J. Surfact. Deterg. 2015, 18, 505–516. [Google Scholar] [CrossRef]
- Dulęba, J.; Siódmiak, T.; Marszałł, M.P. Amano Lipase PS from Burkholderia cepacia—Evaluation of the Effect of Substrates and Reaction Media on the Catalytic Activity. Curr. Org. Chem. 2020, 24, 798–807. [Google Scholar] [CrossRef]
- Dulęba, J.; Siódmiak, T.; Marszałł, M.P. The influence of substrate systems on the enantioselective and lipolytic activity of immobilized Amano PS from Burkholderia cepacia lipase (APS-BCL). Process Biochem. 2022, 120, 126–137. [Google Scholar] [CrossRef]
- Siódmiak, J.; Dulęba, J.; Kocot, N.; Mastalerz, R.; Haraldsson, G.G.; Siódmiak, T. CALB Immobilized on Octyl-Agarose—An Efficient Pharmaceutical Biocatalyst for Transesterification in Organic Medium. Int. J. Mol. Sci. 2025, 26, 6961. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Q.; Yu, X.; Liang, J.; Zhang, Y.; Jiang, Y.; Su, W. Application of Lipase B from Candida antarctica in the Pharmaceutical Industry. Ind. Eng. Chem. Res. 2023, 62, 15733–15751. [Google Scholar] [CrossRef]
- Siódmiak, J.; Dulęba, J.; Kocot, N.; Mastalerz, R.; Haraldsson, G.G.; Marszałł, M.P.; Siódmiak, T. A New Approach in Lipase-Octyl-Agarose Biocatalysis of 2-Arylpropionic Acid Derivatives. Int. J. Mol. Sci. 2024, 25, 5084. [Google Scholar] [CrossRef]
- Du, Y.; Gao, J.; Kong, W.; Zhou, L.; Ma, L.; He, Y.; Huang, Z.; Jiang, Y. Enzymatic Synthesis of Glycerol Carbonate Using a Lipase Immobilized on Magnetic Organosilica Nanoflowers as a Catalyst. ACS Omega 2018, 3, 6642–6650. [Google Scholar] [CrossRef]
- Yin, L.; Gao, K.; Mao, X.; Hu, Y. Lipase B from Candida antarctica immobilized on amphiphilic Janus halloysite nanosheet and application in biphasic interface conversion. Food Chem. 2024, 437, 137787. [Google Scholar] [CrossRef]
- Meng, Z.; Wu, J.; Li, Q.; Liu, Y.; Tang, A. Effective immobilization of Candida cylindracea lipase on surfactant-modified bentonite. Mol. Cryst. Liq. Cryst. 2023, 754, 84–97. [Google Scholar] [CrossRef]
- Rokhati, N.; Kusworo, T.D.; Prasetyaningrum, A.; Hamada, N.; Utomo, D.P.; Riyanto, T. Effect of Surfactant HLB Value on Enzymatic Hydrolysis of Chitosan. ChemEngineering 2022, 6, 17. [Google Scholar] [CrossRef]
- Siódmiak, T.; Haraldsson, G.G.; Dulęba, J.; Ziegler-Borowska, M.; Siódmiak, J.; Marszałł, M.P. Evaluation of Designed Immobilized Catalytic Systems: Activity Enhancement of Lipase B from Candida antarctica. Catalysts 2020, 10, 876. [Google Scholar] [CrossRef]
- Siódmiak, T.; Dulęba, J.; Kocot, N.; Wątróbska-Świetlikowska, D.; Marszałł, M.P. The High ‘Lipolytic Jump’ of Immobilized Amano A Lipase from Aspergillus niger in Developed ‘ESS Catalytic Triangles’ Containing Natural Origin Substrates. Catalysts 2022, 12, 853. [Google Scholar] [CrossRef]
- Goswami, D. Lipase Catalysis in Presence of Nonionic Surfactants. Appl. Biochem. Biotechnol. 2020, 191, 744–762. [Google Scholar] [CrossRef]
- Kunche, L.; Natarajan, U. Structure and dynamics of aqueous solutions containing poly-(acrylic acid) and non-ionic surfactant pentaethylene glycol n-octyl ether (C8E5): A molecular simulations study. Comput. Mater. Sci. 2021, 186, 110043. [Google Scholar] [CrossRef]
- Vaidya, A.P.; Wigent, R.J.; Moore, J.C.; Schwartz, J.B. Protective Effect of Carbopol on Enzymatic Degradation of a Peptide-Like Substrate I: Effect of Various Concentrations and Grades of Carbopol and Other Reaction Variables on Trypsin Activity. Phar. Dev. Technol. 2007, 12, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Porfiri, M.C.; Melnichuk, N.; Braia, M.J.; Brinatti, C.; Loh, W.; Romanini, D. Analysis of the structure-function relationship of alpha amylase complexed with polyacrylic acid. Colloids Surf. B Biointerfaces 2020, 188, 110787. [Google Scholar] [CrossRef] [PubMed]
- Lindhoud, S.; Norde, W.; Cohen Stuart, M.A. Effects of polyelectrolyte complex micelles and their components on the enzymatic activity of lipase. Langmuir 2010, 26, 9802–9808. [Google Scholar] [CrossRef] [PubMed]
- Lupo, N.; Fidahic, U.; Hetényi, G.; Griesser, J.; Bernkop-Schnürch, A. Inhibitory effect of emulsifiers in sedds on protease activity: Just an illusion? Int. J. Pharm. 2017, 526, 23–30. [Google Scholar] [CrossRef]
- Shortall, K.; Otero, F.; Bendl, S.; Soulimane, T.; Magner, E. Enzyme Immobilization on Metal Organic Frameworks: The Effect of Buffer on the Stability of the Support. Langmuir 2022, 38, 13382–13391. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Abdel-Mageed, H.M.; Tayel, S.A.; El-Nabrawi, M.A.; Fahmy, A. Characterization of Mucor racemosus lipase with potential application for the treatment of cellulite. Process Biochem. 2011, 46, 642–648. [Google Scholar] [CrossRef]
- Thiele, M.; Davari, M.D.; Hofmann, I.; König, M.; Lopez, C.G.; Vojcic, L.; Richtering, W.; Schwaneberg, U.; Tsarkova, L.A. Enzyme-Compatible Dynamic Nanoreactors from Electrostatically Bridged Like-Charged Surfactants and Polyelectrolytes. Angew. Chem. Int. Ed. 2018, 57, 9402–9407. [Google Scholar] [CrossRef]
- Dulęba, J.; Siódmiak, T.; Górczak, M.; Gwiazda, D.; Oryl, K.; Pawłowska, W.; Zwiewka, H.; Marszałł, M.P. Surfactants—The application in pharmaceutical biocatalysis. Farm. Pol. 2023, 79, 735–745. [Google Scholar] [CrossRef]
- Holmberg, K. Interactions between surfactants and hydrolytic enzymes. Colloids Surf. B Biointerfaces 2018, 168, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Vishnyakov, A.; Mao, R.; Kam, K.; Potanin, A.; Neimark, A.V. Interactions of Crosslinked Polyacrylic Acid Polyelectrolyte Gels with Nonionic and Ionic Surfactants. J. Phys. Chem. B 2021, 125, 13817–13828. [Google Scholar] [CrossRef] [PubMed]
- Siódmiak, T.; Siódmiak, J.; Mastalerz, R.; Kocot, N.; Dulęba, J.; Haraldsson, G.G.; Wątróbska-Świetlikowska, D.; Marszałł, M.P. Climatic Chamber Stability Tests of Lipase-Catalytic Octyl-Sepharose Systems. Catalysts 2023, 13, 501. [Google Scholar] [CrossRef]
- Siódmiak, T.; Dulęba, J.; Haraldsson, G.G.; Siódmiak, J.; Marszałł, M.P. The Studies of Sepharose-Immobilized Lipases: Combining Techniques for the Enhancement of Activity and Thermal Stability. Catalysts 2023, 13, 887. [Google Scholar] [CrossRef]

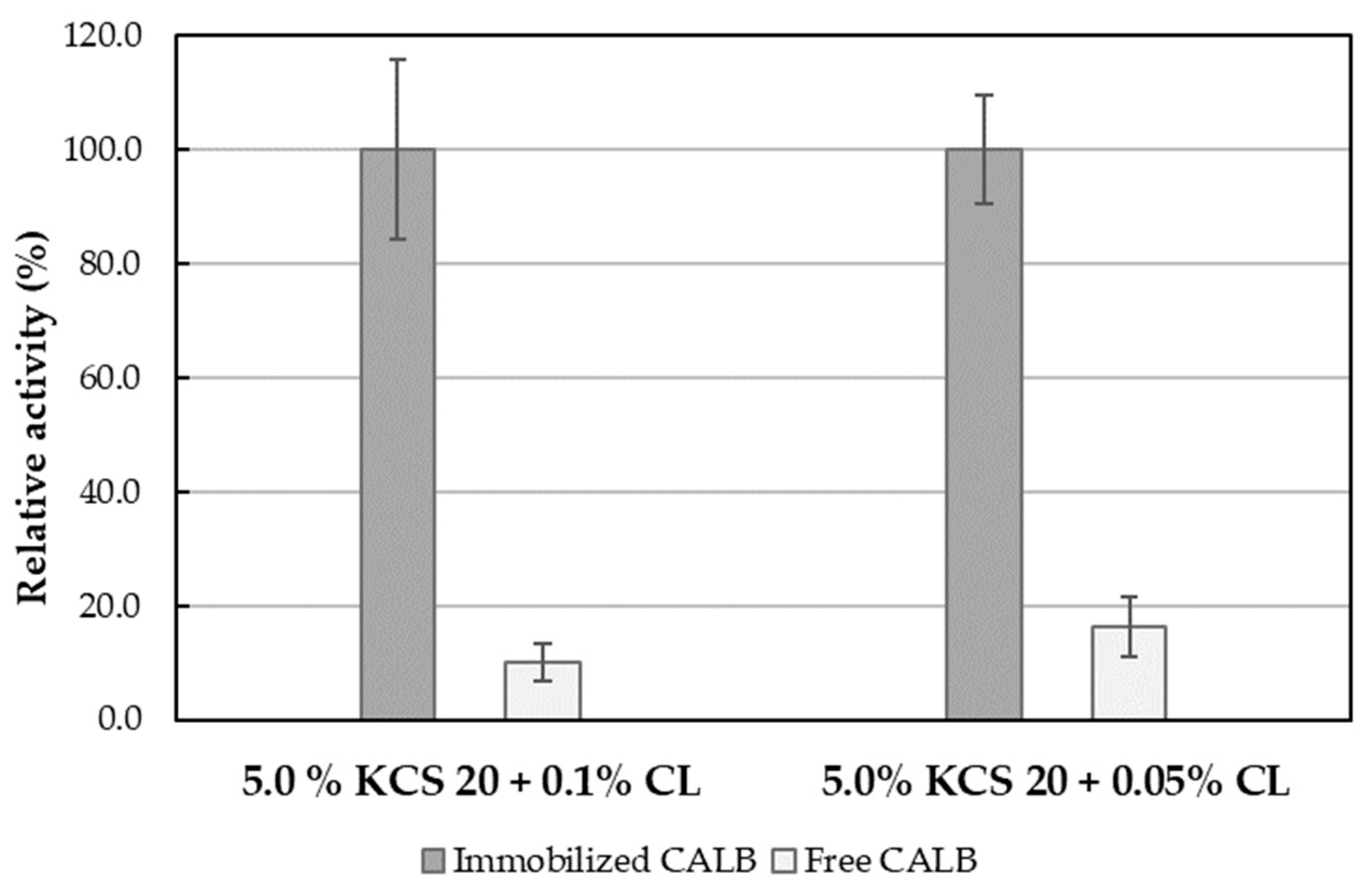

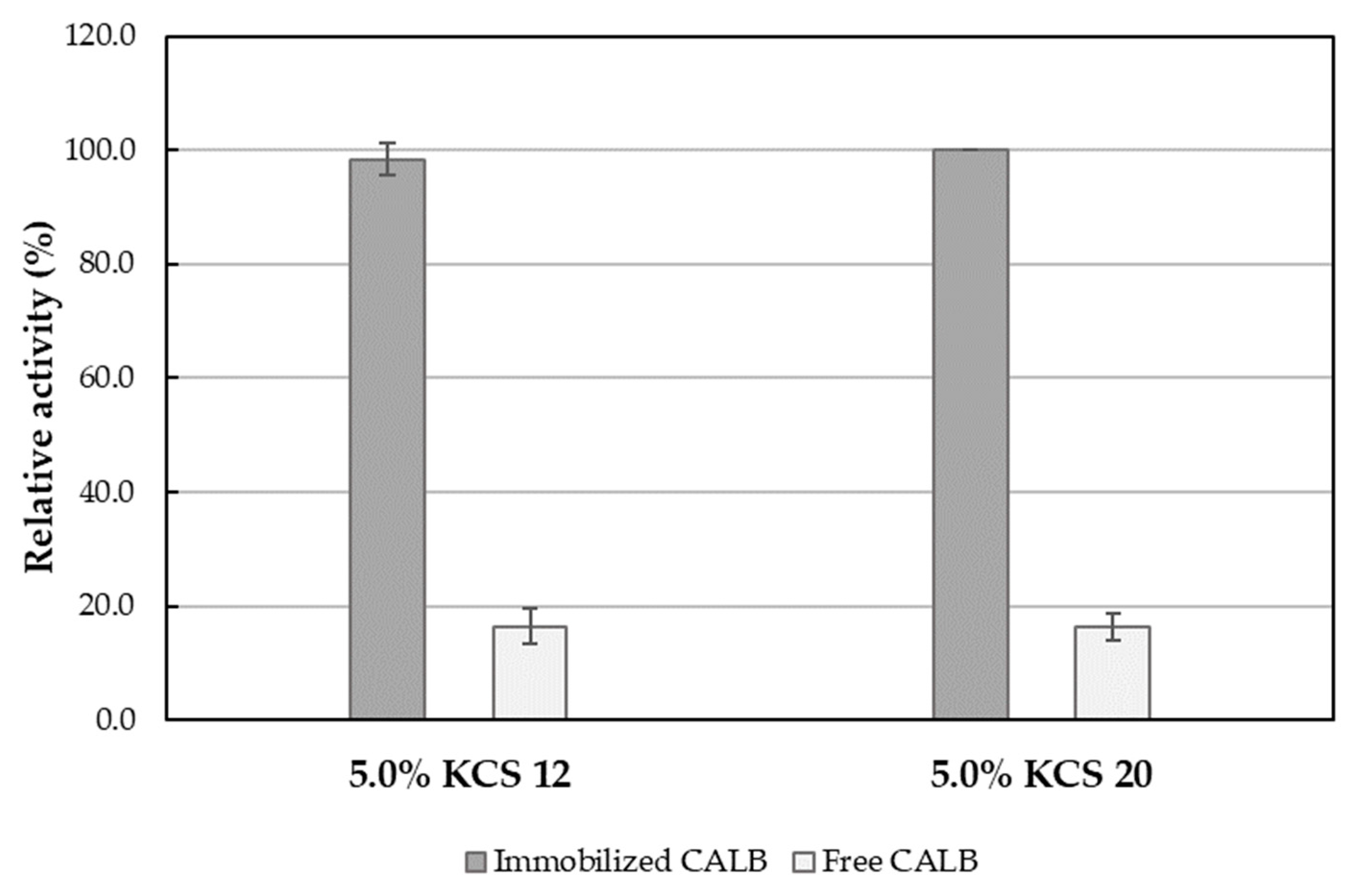
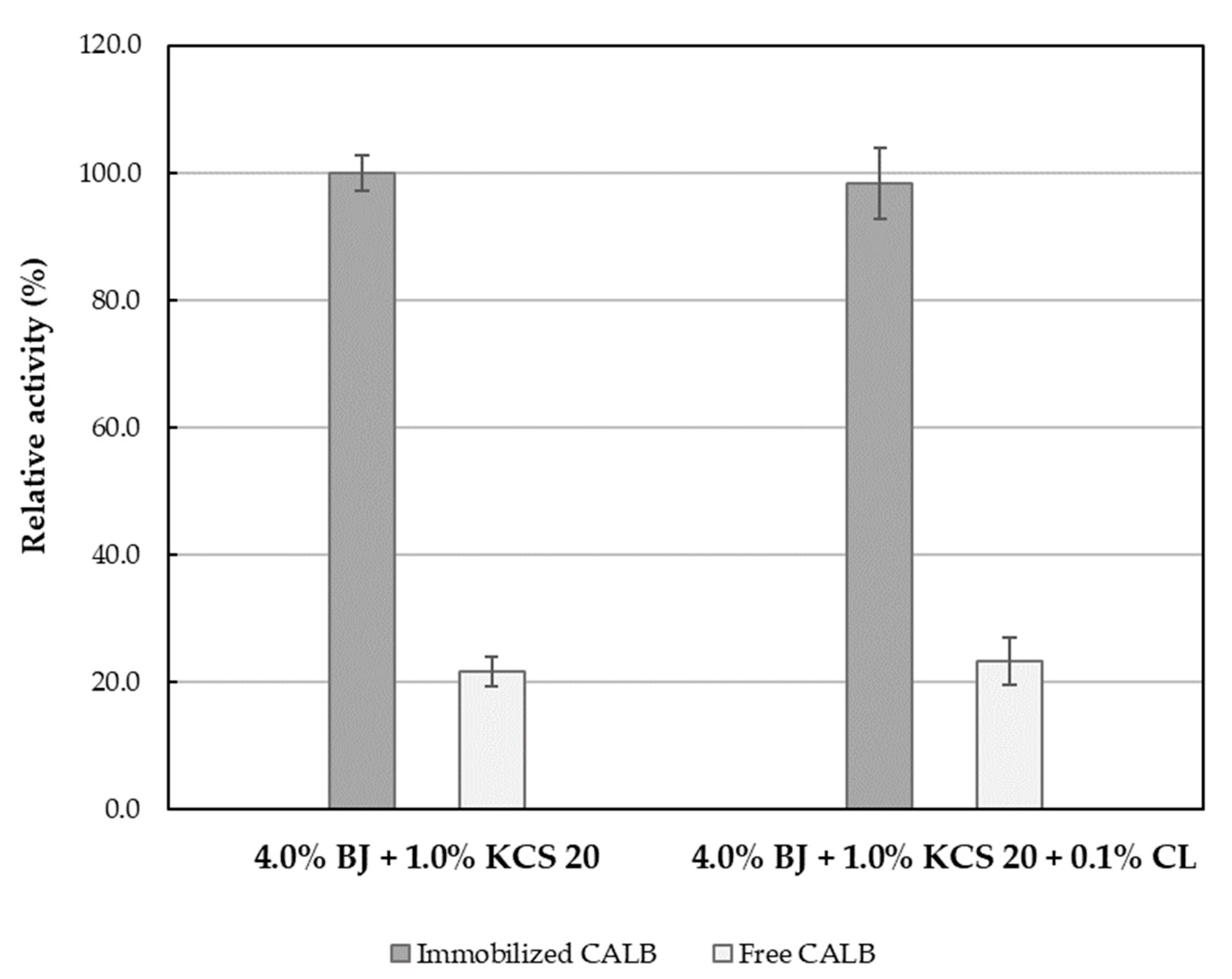
| Emulsion System | Activity [U/ml] | Specific Activity [U/mg Lipase] | Activity [U/g Support] | Residual Activity [%] * |
|---|---|---|---|---|
| 5.0% Kolliphor® CS 20 | 3.60 ± 0.23 | 2.86 ± 0.18 | 72.03 ± 4.63 | 46.1 ± 3.0 |
| 5.0% Kolliphor® CS 20 + 0.1% Carbopol® Ultrez 10 | 3.11 ± 0.19 | 2.47 ± 0.15 | 62.22 ± 3.85 | 39.8 ± 2.5 |
| 10.0% Kolliphor® CS 20 | 3.56 ± 0.19 | 2.82 ± 0.15 | 71.11 ± 3.86 | 45.5 ± 2.5 |
| 10.0% Kolliphor® CS 20 + 0.1% Carbopol® Ultrez 10 | 7.81 ± 0.20 | 6.20 ± 0.16 | 156.27 ± 3.91 | 100.0 ± 0.0 |
| 1.0% Kolliphor® CS 20 + 4.0% SP Brij® S2 MBAL | 3.33 ± 0.17 | 2.65 ± 0.13 | 66.67 ± 3.33 | 42.7 ± 2.1 |
| Emulsions with Kolliphor® CS 20 | ||
|---|---|---|
| The quantitative composition [g] | Type of stirrer |
| Olive oil | 25.0 | Eurostar Digital |
| Kolliphor® CS 20 | 1.25 | |
| Distilled water | ad 50.0 | |
| The quantitative composition [g] | Type of stirrer |
| Olive oil | 25.0 | Eurostar Digital |
| Kolliphor® CS 20 | 2.5 | |
| Distilled water | ad 50.0 | |
| The quantitative composition [g] | Type of stirrer |
| Olive oil | 25.0 | Eurostar Digital |
| Kolliphor® CS 20 | 3.75 | |
| Distilled water | ad 50.0 | |
| The quantitative composition [g] | Type of stirrer |
| Olive oil | 25.0 | Eurostar Digital |
| Kolliphor® CS 20 | 5.0 | |
| Distilled water | ad 50.0 | |
| The quantitative composition [g] | Type of stirrer |
| Olive oil | 25.0 | Eurostar Digital + Ultra Turrax T 25 |
| Kolliphor® CS 20 | 2.5 | |
| Distilled water | ad 50.0 | |
| Emulsions Containing 5.0% Kolliphor® CS 20 and Carbopol® Ultrez 10 | ||
|---|---|---|
| The quantitative composition [g] | Type of stirrer |
| Olive oil | 25.0 | Eurostar Digital |
| Kolliphor® CS 20 | 2.5 | |
| Carbopol® Ultrez 10 | 0.05 | |
| Triethanolamine | q.s. | |
| Distilled water | ad 50.0 | |
| The quantitative composition [g] | Type of stirrer |
| Olive oil | 25.0 | Eurostar Digital |
| Kolliphor® CS 20 | 2.5 | |
| Carbopol® Ultrez 10 | 0.025 | |
| Triethanolamine | q.s. | |
| Distilled water | ad 50.0 | |
| Emulsions Containing Kolliphor® CS 20 with 0.1% Carbopol® Ultrez 10 | ||
|---|---|---|
| The quantitative composition [g] | Type of stirrer |
| Olive oil | 25.0 | Eurostar Digital |
| Kolliphor® CS 20 | 1.25 | |
| Carbopol® Ultrez 10 | 0.05 | |
| Triethanolamine | q.s. | |
| Distilled water | ad 50.0 | |
| The quantitative composition [g] | Type of stirrer |
| Olive oil | 25.0 | Eurostar Digital |
| Kolliphor® CS 20 | 2.5 | |
| Carbopol® Ultrez 10 | 0.05 | |
| Triethanolamine | q.s. | |
| Distilled water | ad 50.0 | |
| The quantitative composition [g] | Type of stirrer |
| Olive oil | 25.0 | Eurostar Digital |
| Kolliphor® CS 20 | 3.75 | |
| Carbopol® Ultrez 10 | 0.05 | |
| Triethanolamine | q.s. | |
| Distilled water | ad 50.0 | |
| The quantitative composition [g] | Type of stirrer |
| Olive oil | 25.0 | Eurostar Digital |
| Kolliphor® CS 20 | 5.0 | |
| Carbopol® Ultrez 10 | 0.05 | |
| Triethanolamine | q.s. | |
| Distilled water | ad 50.0 | |
| Emulsions Containing: Kolliphor® CS 12 or Kolliphor® CS 20; Mixtures of 1.0% Kolliphor® CS 20, 4.0% SP Brij® S2 MBAL and 0.1% Carbopol® Ultrez 10; Mixtures of 1.0% Kolliphor® CS 20 and 4.0% SP Brij® S2 MBAL | ||
|---|---|---|
| The quantitative composition [g] | Type of stirrer |
| Olive oil | 25.0 | Eurostar Digital |
| Kolliphor® CS 12 | 2.5 | |
| Distilled water | ad 50.0 | |
| The quantitative composition [g] | Type of stirrer |
| Olive oil | 25.0 | Eurostar Digital |
| Kolliphor® CS 20 | 2.5 | |
| Distilled water | ad 50.0 | |
| The quantitative composition [g] | Type of stirrer |
| Olive oil | 37.5 | Eurostar Digital |
| SP Brij® S2 MBAL | 3.0 | |
| Kolliphor® CS 20 | 0.75 | |
| Carbopol® Ultrez 10 | 0.075 | |
| Triethanolamine | q.s. | |
| Distilled water | ad 75.0 | |
| The quantitative composition [g] | Type of stirrer |
| Olive oil | 37.5 | Eurostar Digital |
| SP Brij® S2 MBAL | 3.0 | |
| Kolliphor® CS 20 | 0.75 | |
| Distilled water | ad 75.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siódmiak, J.; Dulęba, J.; Mieszkowski, D.; Bilski, P.; Siódmiak, T. Emulsion Systems Stabilized with Nonionic Emulsifier and Cross-Linked Polyacrylic Acid: A Promising Strategy to Enhance the Activity of Immobilized CALB. Catalysts 2025, 15, 916. https://doi.org/10.3390/catal15100916
Siódmiak J, Dulęba J, Mieszkowski D, Bilski P, Siódmiak T. Emulsion Systems Stabilized with Nonionic Emulsifier and Cross-Linked Polyacrylic Acid: A Promising Strategy to Enhance the Activity of Immobilized CALB. Catalysts. 2025; 15(10):916. https://doi.org/10.3390/catal15100916
Chicago/Turabian StyleSiódmiak, Joanna, Jacek Dulęba, Dominik Mieszkowski, Piotr Bilski, and Tomasz Siódmiak. 2025. "Emulsion Systems Stabilized with Nonionic Emulsifier and Cross-Linked Polyacrylic Acid: A Promising Strategy to Enhance the Activity of Immobilized CALB" Catalysts 15, no. 10: 916. https://doi.org/10.3390/catal15100916
APA StyleSiódmiak, J., Dulęba, J., Mieszkowski, D., Bilski, P., & Siódmiak, T. (2025). Emulsion Systems Stabilized with Nonionic Emulsifier and Cross-Linked Polyacrylic Acid: A Promising Strategy to Enhance the Activity of Immobilized CALB. Catalysts, 15(10), 916. https://doi.org/10.3390/catal15100916









