Eco-Friendly Biocatalysts: Laccase Applications, Innovations, and Future Directions in Environmental Remediation
Abstract
1. Introduction
2. Microbial Sources and Production of Laccase
| Organism | Origin | Application in Environmental Remediation | Reference |
|---|---|---|---|
| Trametes versicolor | White-rot fungus | Decolorization of azo and anthraquinone dyes, removal of phenols | [7] |
| Pleurotus ostreatus | White-rot fungus | Degradation of lignin, pesticides and pharmaceutical resides | [13] |
| Bacillus subtilis | Bacterium | Phenol and bisphenol A degradation at neutral pH | [14] |
| Azospirillum lipoferum | Bacterium | Dye degradation under alkaline conditions | [15] |
| Rhus vernicifera | Plant | Lignin biosynthesis, potential in phenol degradation | [12] |
| Bemisia tabaci | Insect | Cuticle sclerotization, potential for bioremediation | [20] |
| Production System | Optimal Conditions | Advantages | Limitations/Challenges | Industrial Relevance |
|---|---|---|---|---|
| Fungal Laccases (T. versicolor, P. ostreatus) | pH 3–6, 25–35 °C, copper/phenolic inducers | High catalytic efficiency, broad substrate range | Slow growth, complex regulation, sensitive to culture conditions | Widely used for dye decolorization, wastewater treatment, soil remediation |
| Bacterial Laccases (B. subtilis, P. putida) | pH 5–8, 30–60 °C | Better tolerance to alkaline pH, high thermal stability, easy genetic manipulation | Lower activity compared to fungal laccases, narrow substrate range | Ideal for alkaline effluents, high-temperature processes |
| Plant Laccases (e.g., Zea mays, Arabidopsis thaliana) | Plant growth-dependent | Naturally abundant in plant tissues | Difficult to extract/purify in large quantities, low yield | Potential use in lignin valorization and biomass conversion |
| Recombinant Expression in E. coli | Controlled fermentation, induction (IPTG) | Rapid growth, easy genetic engineering, scalable | Requires refolding steps for active enzyme, low secretion, yields still below industrial demand | Suitable for tailored enzyme design, but cost-intensive |
| Recombinant Expression in S. cerevisiae | pH 5–6, 28–30 °C | Produces active, glycosylated laccase similar to fungal form | Lower yield compared to native fungal hosts, requires optimization | Promising for large-scale production with process intensification |
| Other Heterologous Systems (Pichia pastoris, Aspergillus niger) | pH 4–6, optimized aeration | Higher expression than S. cerevisiae, scalable fermentation | Requires codon optimization and process control | Emerging platform for industrial-grade laccase production |
3. Biochemical Properties and Catalytic Mechanisms of Laccase
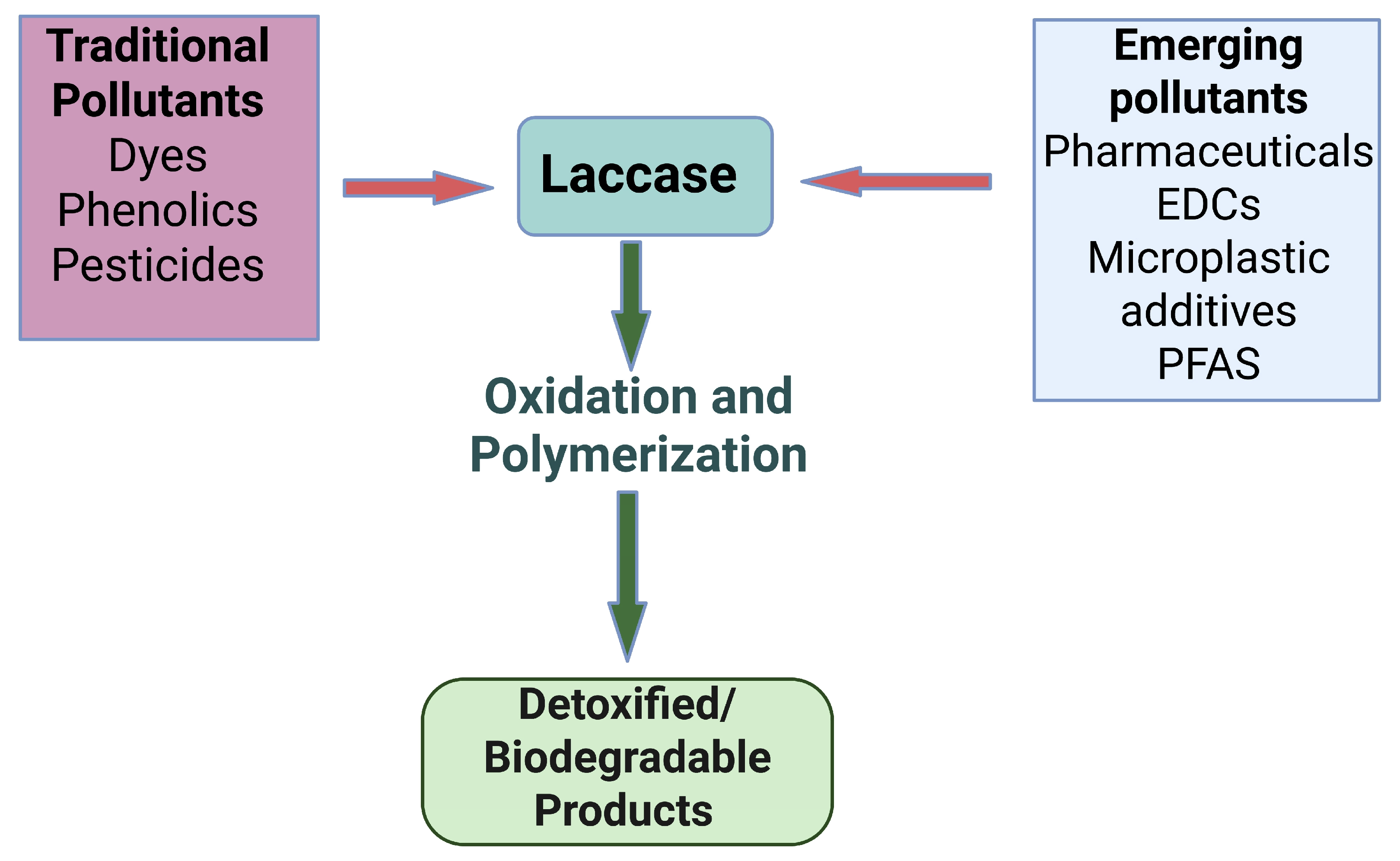
4. Laccase in Environmental Remediation: From Common Pollutants to Emerging Contaminants
4.1. Traditional Pollutants: Dyes, Phenolics, and Pesticides
4.2. Emerging Contaminants: Pharmaceuticals, EDCs, PCPs, Microplastics, and PFAS
4.3. Broader Environmental Applications
| Application Area | Pollutant Type | Laccase Source | Outcome | Reference |
|---|---|---|---|---|
| Wastewater Treatment—Dye Removal | Reactive black, reactive red, azo dyes | Trametes versicolor | >90% decolorization within hours | [50] |
| Wastewater Treatment—Phenolic Compounds | Phenol, chlorophenol | Pleurotus ostreatus | 85% phenol removal in pulp mill effluent | [51] |
| Pharmaceutical Residue Removal | Diclofenac, carbamazepine, ethinylestradiol | Ganoderma lucidum | >80% removal, reduced estrogenic activity | [52] |
| Soil Bioremediation—Pesticides | Chlorpyrifos, carbamate pesticides | Immobilized T. versicolor | Significant degradation, reduced toxicity | [53] |
| Soil Bioremediation—PAHs | Benzo[a]pyrene, anthracene | Bacterial laccase | Transformation to less toxic derivatives | [54] |
| Air Pollution Control—VOCs | Phenol vapors, styrene | Laccase biofilter | Reduced VOC concentration | [55] |
| Solid Waste Management—Composting | Lignin-rich residues | Laccase-producing consortia | Faster compost maturity, improved quality | [56] |
| EDC Removal | Bisphenol A | Immobilized fungal laccase | Continuous BPA removal | [57] |
| Heavy Metal–Organic Complex Breakdown | Dye-metal complexes | Laccase + mediators | Disruption of complexes, improved metal precipitation | [58] |
| Personal Care Products (PCPs) | Triclosan, parabens, and oxybenzone | Bacterial laccase Fungal laccase | Removal of PCPs | [59,60] |
| Per- and polyfluoroalkyl substances (PFAS) | PFAS compounds | Fungal laccase | Defluorination and degradation of PFAS | [61] |
| Biosensing | Phenols, pesticides | Laccase-based electrode | Detection at nanomolar concentrations | [62] |
5. Immobilization and Stability Enhancement Techniques for Laccase
5.1. Immobilization Techniques
5.1.1. Physical Adsorption
5.1.2. Covalent Binding
5.1.3. Entrapment and Encapsulation
5.1.4. Cross-Linked Enzyme Aggregates (CLEAs)
5.2. Support Materials for Immobilization
5.3. Stability Enhancement Strategies
5.4. Advantages of Immobilized Laccase in Environmental Applications
6. Integration with Green Technologies
6.1. Coupling with Advanced Oxidation Processes (AOPs)
6.2. Integration with Nanotechnology
6.3. Bioelectrochemical Systems (BES)
6.4. Integration with Membrane Filtration Systems
6.5. Co-Immobilization with Other Enzymes
6.6. Integration with Renewable Energy Systems
6.7. Role in Circular Bioeconomy Models
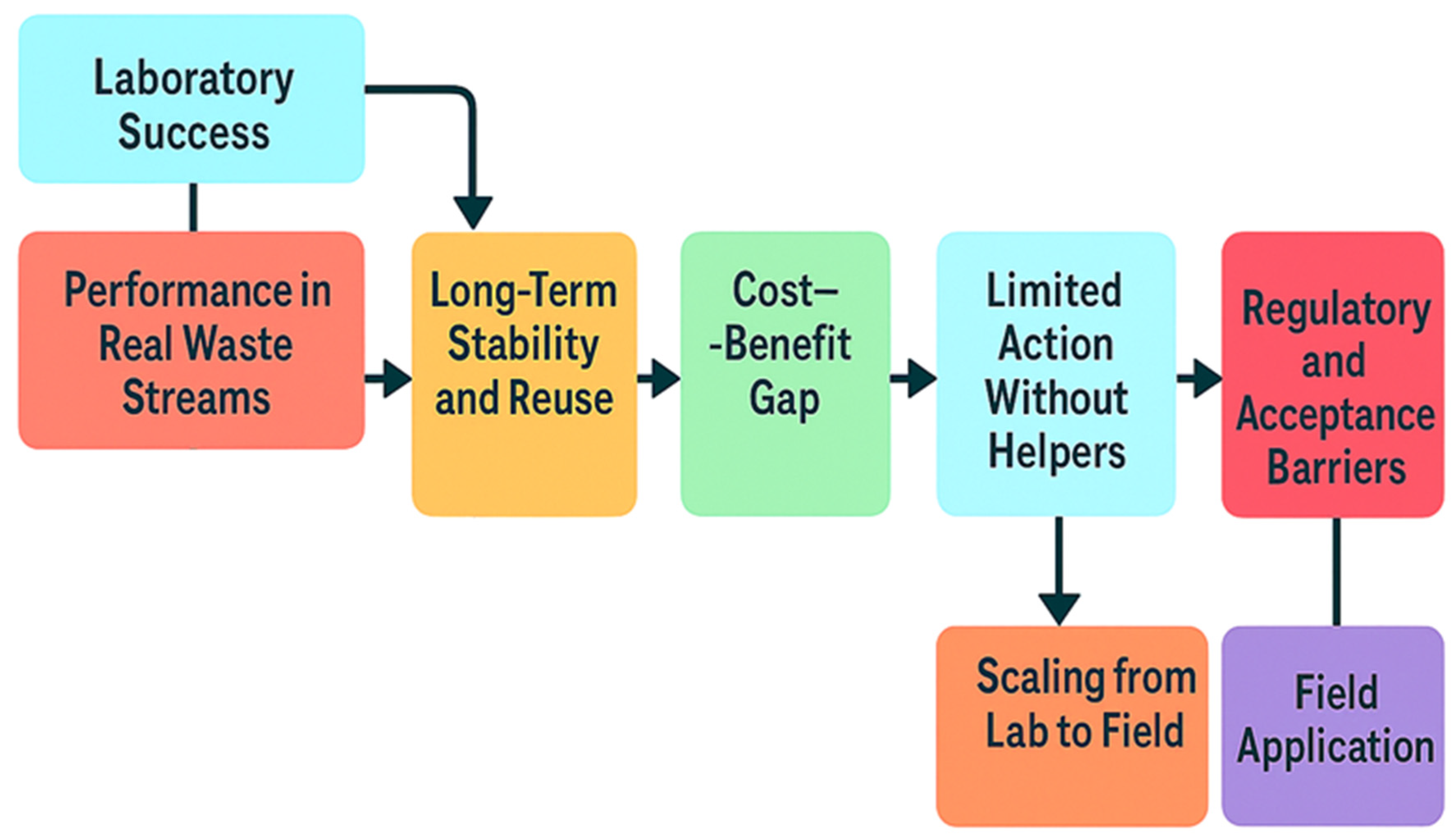
7. Translational Pathways of Laccase
7.1. From Laboratory Success to Real Waste Streams
7.2. Ensuring Long-Term Stability and Reuse
7.3. Addressing the Cost–Benefit Gap
7.4. Overcoming Limited Action Without Helpers
7.5. Scaling from Lab to Field
7.6. Regulatory and Acceptance Barriers
7.7. Field Application and Future Integration
8. Conclusions
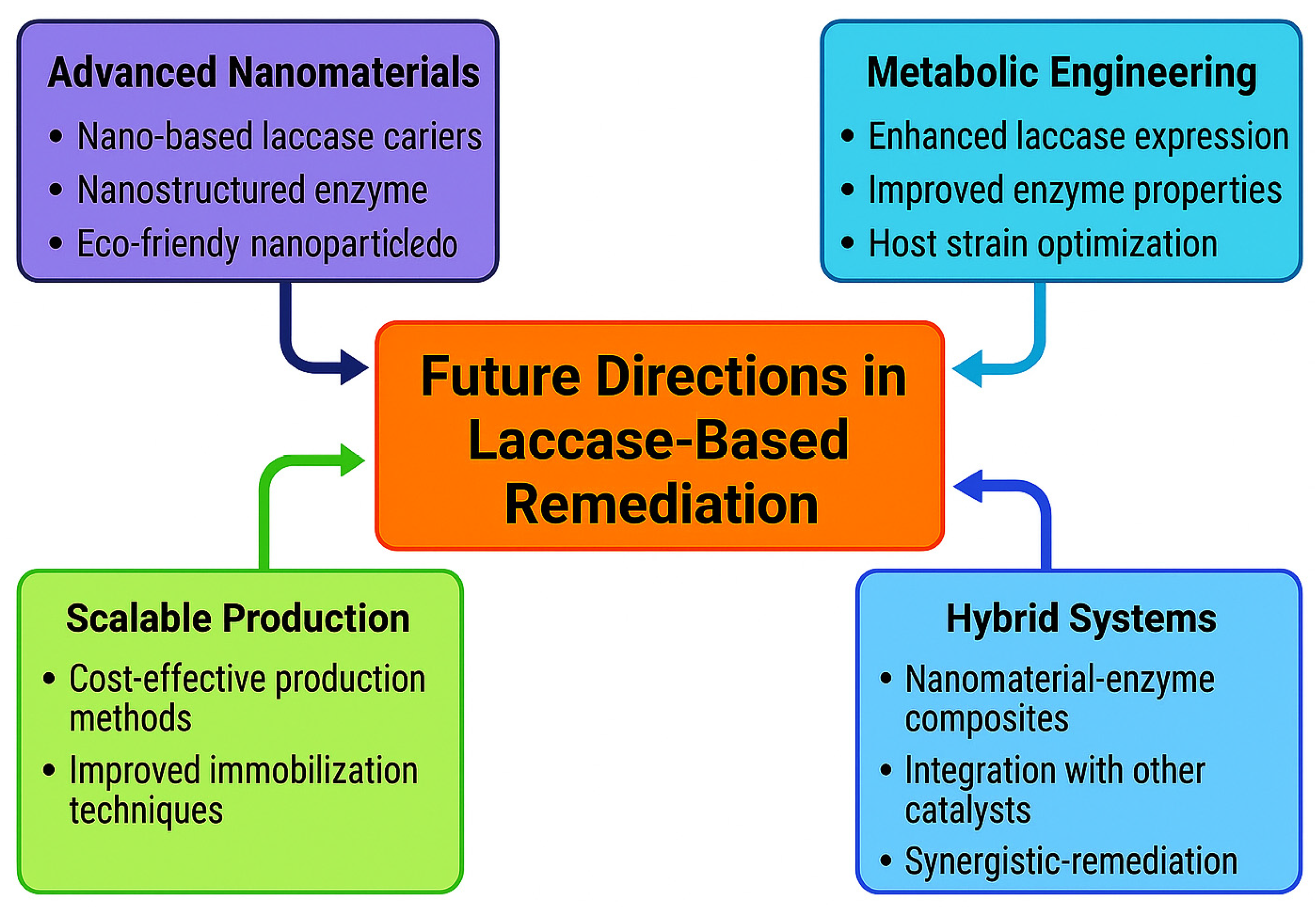
9. Future Directions in Laccase-Based Remediation
9.1. Advanced Nanomaterials
9.2. Metabolic Engineering
9.3. Scalable Production
9.4. Hybrid Systems
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orru, H.; Ebi, K.L.; Forsberg, B. The Interplay of Climate Change and Air Pollution on Health. Curr. Environ. Health Rep. 2017, 4, 504–513. [Google Scholar] [CrossRef]
- Satyam, S.; Patra, S. Innovations and challenges in adsorption-based wastewater remediation: A comprehensive review. Heliyon 2024, 10, e29573. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Ali, S.; Zaman, W. Innovative Adsorbents for Pollutant Removal: Exploring the Latest Research and Applications. Molecules 2024, 29, 4317. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Abellanas-Perez, P.; de Andrades, D.; Cornet, I.; Fernandez-Lafuente, R.; Bilal, M. Laccase-based biocatalytic systems application in sustainable degradation of pharmaceutically active contaminants. J. Hazard. Mater. 2025, 485, 136803. [Google Scholar] [CrossRef]
- Zofair, S.F.F.; Ahmad, S.; Hashmi, M.A.; Khan, S.H.; Khan, M.A.; Younus, H. Catalytic roles, immobilization and management of recalcitrant environmental pollutants by laccases: Significance in sustainable green chemistry. J. Environ. Manag. 2022, 309, 114676. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.C.; Martins, L.O.; Robalo, M.P. Laccases: Versatile Biocatalysts for the Synthesis of Heterocyclic Cores. Molecules 2021, 26, 3719. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, B.; Rajesh, B.; Janardhan, A.; Kumar, A.P.; Narasimha, G. Fungal laccases and their applications in bioremediation. Enzym. Res. 2014, 2014, 163242. [Google Scholar] [CrossRef]
- Mehta, P.K.; Peter, J.K.; Kumar, A.; Yadav, A.K.; Singh, R. From nature to applications: Laccase immobilization onto bio-based materials for eco-conscious environmental remediation. Int. J. Biol. Macromol. 2025, 307 Pt 3, 142157. [Google Scholar] [CrossRef]
- Aghaee, M.; Salehipour, M.; Rezaei, S.; Mogharabi-Manzari, M. Bioremediation of organic pollutants by laccase-metal-organic framework composites: A review of current knowledge and future perspective. Bioresour. Technol. 2024, 406, 131072. [Google Scholar] [CrossRef]
- Yang, J.; Li, W.; Ng, T.B.; Deng, X.; Lin, J.; Ye, X. Laccases: Production, Expression Regulation, and Applications in Pharmaceutical Biodegradation. Front. Microbiol. 2017, 8, 832. [Google Scholar] [CrossRef]
- Dong, C.D.; Tiwari, A.; Anisha, G.S.; Chen, C.W.; Singh, A.; Haldar, D.; Patel, A.K.; Singhania, R.R. Laccase: A potential biocatalyst for pollutant degradation. Environ. Pollut. 2023, 319, 120999. [Google Scholar] [CrossRef] [PubMed]
- Shraddha Shekher, R.; Sehgal, S.; Kamthania, M.; Kumar, A. Laccase: Microbial sources, production, purification, and potential biotechnological applications. Enzym. Res. 2011, 2011, 217861. [Google Scholar] [CrossRef]
- Wang, F.; Xu, L.; Zhao, L.; Ding, Z.; Ma, H.; Terry, N. Fungal Laccase Production from Lignocellulosic Agricultural Wastes by Solid-State Fermentation: A Review. Microorganisms 2019, 7, 665. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.; Solanki, V.S.; Gacem, A.; Hasan, M.A.; Pare, B.; Srivastava, A.; Singh, A.; Yadav, V.K.; Yadav, K.K.; Lee, C.; et al. Bacterial Laccases as Biocatalysts for the Remediation of Environmental Toxic Pollutants: A Green and Eco-Friendly Approach—A Review. Water 2022, 14, 4068. [Google Scholar] [CrossRef]
- Panwar, V.; Dey, B.; Sheikh, J.N.; Dutta, T. Thermostable bacterial laccase for sustainable dyeing using plant phenols. RSC Adv. 2022, 12, 18168–18180. [Google Scholar] [CrossRef]
- Wan, Y.Y.; Lu, R.; Xiao, L.; Du, Y.M.; Miyakoshi, T.; Chen, C.L.; Knill, C.J.; Kennedy, J.F. Effects of organic solvents on the activity of free and immobilised laccase from Rhus vernicifera. Int. J. Biol. Macromol. 2010, 47, 488–495. [Google Scholar] [CrossRef]
- Téllez-Téllez, M.; Fernández, F.J.; Montiel-González, A.M.; Sánchez, C.; Díaz-Godínez, G. Growth and laccase production by Pleurotus ostreatus in submerged and solid-state fermentation. Appl. Microbiol. Biotechnol. 2008, 81, 675–679. [Google Scholar] [CrossRef]
- Dec, J.; Haider, K.; Bollag, J.M. Release of substituents from phenolic compounds during oxidative coupling reactions. Chemosphere 2003, 52, 549–556. [Google Scholar] [CrossRef]
- Sodhi, A.S.; Bhatia, S.; Batra, N. Laccase: Sustainable production strategies, heterologous expression and potential biotechnological applications. Int. J. Biol. Macromol. 2024, 280 Pt 1, 135745. [Google Scholar] [CrossRef]
- Yang, C.H.; Zhang, Q.; Zhu, W.Q.; Shi, Y.; Cao, H.H.; Guo, L.; Chu, D.; Lu, Z.; Liu, T.X. Involvement of Laccase2 in Cuticle Sclerotization of the Whitefly, Bemisia tabaci Middle East-Asia Minor 1. Insects 2022, 13, 471. [Google Scholar] [CrossRef]
- Jones, S.M.; Solomon, E.I. Electron transfer and reaction mechanism of laccases. Cell. Mol. Life Sci. 2015, 72, 869–883. [Google Scholar] [CrossRef]
- Ezike, T.C.; Udeh, J.O.; Joshua, P.E.; Ezugwu, A.L.; Isiwu, C.V.; Eze, S.O.O.; Chilaka, F.C. Substrate specificity of a new laccase from Trametes polyzona WRF03. Heliyon 2021, 7, e06080. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xu, F.; Eriksson, K.E. Comparison of fungal laccases and redox mediators in oxidation of a nonphenolic lignin model compound. Appl. Environ. Microbiol. 1999, 65, 2654–2660. [Google Scholar] [CrossRef] [PubMed]
- Mani, P.; Fidal Kumar, V.T.; Keshavarz, T.; Chandra, T.S.; Kyazze, G. The Role of Natural Laccase Redox Mediators in Simultaneous Dye Decolorization and Power Production in Microbial Fuel Cells. Energies 2018, 11, 3455. [Google Scholar] [CrossRef]
- Torres-Salas, P.; Mate, D.M.; Ghazi, I.; Plou, F.J.; Ballesteros, A.O.; Alcalde, M. Widening the pH activity profile of a fungal laccase by directed evolution. Chembiochem 2013, 14, 934–937. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, G.; Ngo, H.H.; Guo, W.; Zhang, S. Advances in thermostable laccase and its current application in lignin-first biorefinery: A review. Bioresour. Technol. 2020, 298, 122511. [Google Scholar] [CrossRef]
- Skoronski, E.; Fernandes, M.; Magalhães, M.D.L.B.; Da Silva, G.F.; João, J.J.; Soares, C.H.L.; Júnior, A.F. Substrate Specificity and Enzyme Recycling Using Chitosan Immobilized Laccase. Molecules 2014, 19, 16794–16809. [Google Scholar] [CrossRef]
- Abadulla, E.; Tzanov, T.; Costa, S.; Robra, K.H.; Cavaco-Paulo, A.; Gübitz, G.M. Decolorization and detoxification of textile dyes with a laccase from Trametes hirsuta. Appl. Environ. Microbiol. 2000, 66, 3357–3362. [Google Scholar] [CrossRef]
- Yang, S.O.; Sodaneath, H.; Lee, J.I.; Jung, H.; Choi, J.H.; Ryu, H.W.; Cho, K.S. Decolorization of acid, disperse and reactive dyes by Trametes versicolor CBR43. J. Environ. Sci. Health Part A 2017, 52, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, P.E.; Gkountela, C.I.; Patila, M.; Fotiadou, R.; Chatzikonstantinou, A.V.; Vouyiouka, S.N.; Stamatis, H. Laccase-Mediated Oxidation of Phenolic Compounds from Wine Lees Extract towards the Synthesis of Polymers with Potential Applications in Food Packaging. Biomolecules 2024, 14, 323. [Google Scholar] [CrossRef]
- Srinivasan, P.; Selvankumar, T.; Paray, B.A.; Rehman, M.U.; Kamala-Kannan, S.; Govarthanan, M.; Kim, W.; Selvam, K. Chlorpyrifos degradation efficiency of Bacillus sp. laccase immobilized on iron magnetic nanoparticles. 3 Biotech 2020, 10, 3660. [Google Scholar] [CrossRef]
- Li, X.; Shen, X.; Jiang, W.; Xi, Y.; Li, S. Comprehensive review of emerging contaminants: Detection technologies, environmental impact, and management strategies. Ecotoxicol. Environ. Saf. 2024, 278, 116420. [Google Scholar] [CrossRef] [PubMed]
- Boro, D.; Chirania, M.; Verma, A.K.; Chettri, D.; Verma, A.K. Comprehensive approaches to managing emerging contaminants in wastewater: Identification, sources, monitoring and remediation. Environ. Monit. Assess. 2025, 197, 456. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.C.; Zheng, Y.; Dzakpasu, M. Removal of pharmaceutical active compounds in wastewater by constructed wetlands: Performance and mechanisms. J. Environ. Manag. 2023, 325, 116478. [Google Scholar] [CrossRef] [PubMed]
- Hahn, V.; Meister, M.; Hussy, S.; Cordes, A.; Enderle, G.; Saningong, A.; Schauer, F. Enhanced laccase-mediated transformation of diclofenac and flufenamic acid in the presence of bisphenol A and testing of an enzymatic membrane reactor. AMB Express 2018, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.; Herrmann, S.; Lucas, M. The role of endocrine-disrupting phthalates and bisphenols in cardiometabolic disease: The evidence is mounting. Curr. Opin. Endocrinol. Diabetes Obes. 2022, 29, 87–94. [Google Scholar] [CrossRef]
- Martínez-Ibarra, A.; Martínez-Razo, L.D.; MacDonald-Ramos, K.; Morales-Pacheco, M.; Vázquez-Martínez, E.R.; López-López, M.; Rodríguez Dorantes, M.; Cerbón, M. Multisystemic alterations in humans induced by bisphenol A and phthalates: Experimental, epidemiological and clinical studies reveal the need to change health policies. Environ. Pollut. 2021, 271, 116380. [Google Scholar] [CrossRef]
- Divya, L.M.; Prasanth, G.K.; Arun, K.G.; Sadasivan, C. Bisphenol-A carbonate dimer is a more preferred substrate for laccase mediated degradation than the Biphenol-A in its monomeric and dimeric forms. Int. Biodeterior. Biodegrad. 2018, 135, 19–23. [Google Scholar] [CrossRef]
- Brausch, J.M.; Rand, G.M. A review of personal care products in the aquatic environment: Environmental concentrations and toxicity. Chemosphere 2011, 82, 1518–1532. [Google Scholar] [CrossRef]
- Carey, D.E.; McNamara, P.J. The impact of triclosan on the spread of antibiotic resistance in the environment. Front. Microbiol. 2015, 5, 780. [Google Scholar] [CrossRef]
- Gałązka, A.; Jankiewicz, U.; Szczepkowski, A. Biochemical Characteristics of Laccases and Their Practical Application in the Removal of Xenobiotics from Water. Appl. Sci. 2023, 13, 4394. [Google Scholar] [CrossRef]
- Wu, B.; Yu, H.; Lei, P.; He, J.; Yi, J.; Wu, W.; Wang, H.; Yang, Q.; Zeng, G.; Sun, D. Microplastics in aquatic ecosystems: Detection, source tracing, and sustainable management strategies. Ecotoxicol. Environ. Saf. 2025, 291, 117883. [Google Scholar] [CrossRef]
- Rekik, H.; Arab, H.; Pichon, L.; El Khakani, M.A.; Drogui, P. Per-and polyfluoroalkyl (PFAS) eternal pollutants: Sources, environmental impacts and treatment processes. Chemosphere 2024, 358, 142044. [Google Scholar] [CrossRef]
- Zeng, J.; Li, Y.; Dai, Y.; Wu, Y.; Lin, X. Effects of polycyclic aromatic hydrocarbon structure on PAH mineralization and toxicity to soil microorganisms after oxidative bioremediation by laccase. Environ. Pollut. 2021, 287, 117581. [Google Scholar] [CrossRef]
- Paraschiv, G.; Ferdes, M.; Ionescu, M.; Moiceanu, G.; Zabava, B.S.; Dinca, M.N. Laccases Versatile Enzymes Used to Reduce Environmental Pollution. Energies 2022, 15, 1835. [Google Scholar] [CrossRef]
- Fernández-Sandoval, M.T.; García, A.; Teymennet-Ramírez, K.V.; Arenas-Olivares, D.Y.; Martínez-Morales, F.; Trejo-Hernández, M.R. Removal of phenolic inhibitors from lignocellulose hydrolysates using laccases for the production of fuels and chemicals. Biotechnol. Prog. 2024, 40, e3406. [Google Scholar] [CrossRef]
- Alvarado-Ramírez, L.; Machorro-García, G.; López-Legarrea, A.; Trejo-Ayala, D.; Rostro-Alanis, M.J.; Sánchez-Sánchez, M.; Blanco, R.M.; Rodríguez-Rodríguez, J.; Parra-Saldívar, R. Metal-organic frameworks for enzyme immobilization and nanozymes: A laccase-focused review. Biotechnol. Adv. 2024, 70, 108299. [Google Scholar] [CrossRef]
- Bravo, I.; Prata, M.; Torrinha, Á.; Delerue-Matos, C.; Lorenzo, E.; Morais, S. Laccase bioconjugate and multi-walled carbon nanotubes-based biosensor for bisphenol A analysis. Bioelectrochemistry 2022, 144, 108033. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Veksha, A.; Ge, L.; Lisak, G. Near real-time analysis of para-cresol in wastewater with a laccase-carbon nanotube-based biosensor. Chemosphere 2021, 269, 128699. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Gupta, R. Laccase: Exploring structural insights and functional versatility for applications. Biologia 2024, 79, 3381–3393. [Google Scholar] [CrossRef]
- Virk, A.P.; Sharma, P.; Capalash, N. Use of laccase in pulp and paper industry. Biotechnol. Prog. 2012, 28, 21–32. [Google Scholar] [CrossRef]
- Deng, W.; Zhao, W.; Yang, Y. Degradation and Detoxification of Chlorophenols with Different Structure by LAC-4 Laccase Purified from White-Rot Fungus Ganoderma lucidum. Int. J. Environ. Res. Public Health 2022, 19, 8150. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jiang, Y.; Jiao, J.; Liu, M.; Hu, F.; Griffiths, B.S.; Li, H. Adsorption of Trametes versicolor laccase to soil iron and aluminum minerals: Enzyme activity, kinetics and stability studies. Colloids Surf. B Biointerfaces 2014, 114, 342–348. [Google Scholar] [CrossRef]
- Zeng, J.; Zhu, Q.; Wu, Y.; Shan, J.; Ji, R.; Lin, X. Oxidation of benzo[a]pyrene by laccase in soil enhances bound residue formation and reduces disturbance to soil bacterial community composition. Environ. Pollut. 2018, 242, 462–469. [Google Scholar] [CrossRef]
- Kennes, C.; Veiga, M.C. Fungal biocatalysts in the biofiltration of VOC-polluted air. J. Biotechnol. 2004, 113, 305–319. [Google Scholar] [CrossRef]
- Nadeem, A.; Baig, S.; Iqbal, K.; Sheikh, N. Impact of laccase enzyme inducers on solid waste compost maturity and stability. Environ. Technol. 2014, 35, 3130–3138. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Morales, R.; Rodríguez-Delgado, M.; Gomez-Mariscal, K.; Orona-Navar, C.; Hernandez-Luna, C.; Torres, E.; Parra, R.; Cárdenas-Chávez, D.; Mahlknecht, J.; Ornelas-Soto, N. Biotransformation of Endocrine-Disrupting Compounds in Groundwater: Bisphenol A, Nonylphenol, Ethynylestradiol and Triclosan by a Laccase Cocktail from Pycnoporus sanguineus CS43. Water Air Soil Pollut. 2015, 226, 251. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, K.; Kim, Y.M.; Jeon, J.R.; Chang, Y.S. Effect of metal ions on reactive dye decolorization by laccase from Ganoderma lucidum. J. Hazard. Mater. 2009, 168, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Tang, Z.; Liu, K.; Wang, S.; Li, Y.; Lv, J.; Yan, X.; Liu, D.; Bhople, P.; Cheng, K. A novel two-stage laccase application strategy to maintain enzyme activity and promote pharmaceuticals and personal care products degradation during sewage sludge composting. J. Environ. Chem. Eng. 2025, 13, 118877. [Google Scholar] [CrossRef]
- Kang, B.R.; Kim, S.Y.; Kang, M.; Lee, T.K. Removal of pharmaceuticals and personal care products using native fungal enzymes extracted during the ligninolytic process. Environ. Res. 2021, 195, 110878. [Google Scholar] [CrossRef]
- Mekureyaw, M.F.; Junker, A.L.; Bai, L.; Zhang, Y.; Wei, Z.; Guo, Z. Laccase based per- and polyfluoroalkyl substances degradation: Status and future perspectives. Water Res. 2025, 271, 122888. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.M.; Fátima Barroso, M.; Morais, S.; de Lima-Neto, P.; Correia, A.N.; Oliveira, M.B.; Delerue-Matos, C. Biosensor based on multi-walled carbon nanotubes paste electrode modified with laccase for pirimicarb pesticide quantification. Talanta 2013, 106, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Zofair, S.F.F.; Arsalan, A.; Khan, M.A.; Alhumaydhi, F.A.; Younus, H. Immobilization of laccase on Sepharose-linked anti-body support for decolourization of phenol red. Int. J. Biol. Macromol. 2020, 161, 78–87. [Google Scholar] [CrossRef]
- Tavares, A.P.; Silva, C.G.; Dražić, G.; Silva, A.M.; Loureiro, J.M.; Faria, J.L. Laccase immobilization over multi-walled carbon nanotubes: Kinetic, thermodynamic and stability studies. J. Colloid Interface Sci. 2015, 454, 52–600. [Google Scholar] [CrossRef]
- Al-sareji, O.J.; Abdulzahra, M.A.; Hussein, T.S.; Shlakaa, A.S.; Karhib, M.M.; Meiczinger, M.; Grmasha, R.A.; Al-Juboori, R.A.; Somogyi, V.; Domokos, E.; et al. Removal of Pharmaceuticals from Water Using Laccase Immobilized on Orange Peels Waste-Derived Activated Carbon. Water 2023, 15, 3437. [Google Scholar] [CrossRef]
- Jeyabalan, J.; Veluchamy, A.; Narayanasamy, S. Production optimization, characterization, and application of a novel thermo- and pH-stable laccase from Bacillus drentensis 2E for bioremediation of industrial dyes. Int. J. Biol. Macromol. 2025, 308 Pt 3, 142557. [Google Scholar] [CrossRef] [PubMed]
- Rani, M.; Shanker, U.; Chaurasia, A.K. Catalytic potential of laccase immobilized on transition metal oxides nanomaterials: Degradation of alizarin red S dye. J. Environ. Chem. Eng. 2017, 5, 2730–2739. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, J.; Li, G.; Zhang, J.; Huang, F.; Wei, Q. Laccase immobilization by chelated metal ion coordination chemistry. Polymers 2014, 6, 2357–2370. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Qu, Y. A strategy for efficient immobilization of laccase and horseradish peroxidase on single-walled carbon nanotubes. J. Chem. Technol. Biotechnol. 2013, 88, 2227–2232. [Google Scholar] [CrossRef]
- Habimana, P.; Gao, J.; Mwizerwa, J.P.; Ndayambaje, J.B.; Liu, H.; Luan, P.; Ma, L.; Jiang, Y. Improvement of Laccase Activity Via Covalent Immobilization over Mesoporous Silica Coated Magnetic Multiwalled Carbon Nanotubes for the Discoloration of Synthetic Dyes. ACS Omega 2021, 6, 2777–2789. [Google Scholar] [CrossRef]
- Braham, S.A.; Siar, E.H.; Arana-Peña, S.; Carballares, D.; Morellon-Sterling, R.; Bavandi, H.; de Andrades, D.; Kornecki, J.F.; Fernandez-Lafuente, R. Effect of Concentrated Salts Solutions on the Stability of Immobilized Enzymes: Influence of Inactivation Conditions and Immobilization Protocol. Molecules 2021, 26, 968. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, X.; Zhou, Z.; Ye, G.; Wu, D. Covalent organic framework in-situ immobilized laccase for the covalent polymerization removal of sulfamethoxazole in the presence of natural phenols: Prominent enzyme stability and activity. J. Hazard. Mater. 2024, 462, 132714. [Google Scholar] [CrossRef]
- Wang, F.; Guo, C.; Yang, L.R.; Liu, C.Z. Magnetic mesoporous silica nanoparticles: Fabrication and their laccase immobilization performance. Bioresour. Technol. 2010, 101, 8931–8935. [Google Scholar] [CrossRef]
- Kuznetsov, B.A.; Shumakovich, G.P.; Koroleva, O.V.; Yaropolov, A.I. On applicability of laccase as label in the mediated and mediatorless electroimmunoassay: Effect of distance on the direct electron transfer between laccase and electrode. Biosens. Bioelectron. 2001, 16, 73–84. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Kalia, V.C.; Choi, J.-H.; Haw, J.-R.; Kim, I.-W.; Lee, J.K. Immobilization of laccase on SiO2 nanocarriers improves its stability and reusability. J. Microbiol. Biotechnol. 2014, 24, 639–647. [Google Scholar] [CrossRef]
- Fernández-Fernández, M.; Sanromán, M.Á.; Moldes, D. Recent developments and applications of immobilized laccase. Biotechnol. Adv. 2013, 31, 1808–1825. [Google Scholar] [CrossRef]
- Sá, H.; Silva, B.; Tavares, T.; Michelin, M. Entrapment of laccase-halloysite nanotubes in chitosan beads for pharmaceutical degradation in wastewater. Chem. Eng. J. 2025, 521, 167154. [Google Scholar] [CrossRef]
- Makas, Y.G.; Kalkan, N.A.; Aksoy, S.; Altinok, H.; Hasirci, N. Immobilization of laccase in κ-carrageenan based semi-interpenetrating polymer networks. J. Biotechnol. 2010, 148, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.N.; Balkus, K.J. Perspective of recent progress in immobilization of enzymes. ACS Catal. 2011, 1, 956–968. [Google Scholar] [CrossRef]
- Prévoteau, A.; Faure, C. Effect of onion-type multilamellar liposomes on Trametes versicolor laccase activity and stability. Biochimie 2012, 94, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Lloret, L.; Eibes, G.; Feijoo, G.; Moreira, M.T.; Lema, J.M.; Hollmann, F. Immobilization of laccase by encapsulation in a sol-gel matrix and its characterization and use for the removal of estrogens. Biotechnol. Prog. 2011, 27, 1570–1579. [Google Scholar] [CrossRef]
- Kyomuhimbo, H.D.; Brink, H.G. Applications and immobilization strategies of the copper-centred laccase enzyme: A review. Heliyon 2023, 9, e13156. [Google Scholar] [CrossRef]
- Ifko, D.; Vasić, K.; Knez, Ž.; Leitgeb, M. (Magnetic) Cross-Linked Enzyme Aggregates of Cellulase from T. reesei: A Stable and Efficient Biocatalyst. Molecules 2023, 28, 1305. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef]
- Cabana, H.; Jones, J.P.; Agathos, S.N. Preparation and characterization of cross-linked laccase aggregates and their application to the elimination of endocrine disrupting chemicals. J. Biotechnol. 2007, 132, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Vršanská, M.; Voběrková, S.; Jiménez Jiménez, A.M.; Strmiska, V.; Adam, V. Preparation and optimisation of cross-linked enzyme aggregates using native isolate white rot fungi Trametes versicolor and fomes fomentarius for the decolourisation of synthetic dyes. Int. J. Environ. Res. Public Health 2018, 15, 23. [Google Scholar] [CrossRef]
- Li, G.; Nandgaonkar, A.G.; Lu, K.; Krause, W.E.; Lucia, L.A.; Wei, Q. Laccase immobilized on PAN/O-MMT composite nanofibers support for substrate bioremediation: A de novo adsorption and biocatalytic synergy. RSC Adv. 2016, 6, 41420–41427. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A General Overview of Support Materials for Enzyme Immobilization: Characteristics, Properties, Practical Utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Zhao, Y.; Rasheed, T.; Iqbal, H.M.N. Magnetic nanoparticles as versatile carriers for enzymes immobilization: A review. Int. J. Biol. Macromol. 2018, 120, 2530–2544. [Google Scholar] [CrossRef]
- Ma, R.; Xue, Y.; Ma, Q.; Chen, Y.; Yuan, S.; Fan, J. Recent Advances in Carbon-Based Materials for Adsorptive and Photocatalytic Antibiotic Removal. Nanomaterials 2022, 12, 4045. [Google Scholar] [CrossRef]
- Alvarado-Ramírez, L.; Rostro-Alanis, M.; Rodríguez-Rodríguez, J.; Castillo-Zacarías, C.; Sosa-Hernández, J.E.; Barceló, D.; Iqbal, H.M.N.; Parra-Saldívar, R. Exploring current tendencies in techniques and materials for immobilization of laccases—A review. Int. J. Biol. Macromol. 2021, 181, 683–696. [Google Scholar] [CrossRef]
- Qiu, L.; Huang, Z. The treatment of chlorophenols with laccase immobilized on sol–gel-derived silica. World J. Microbiol. Biotechnol. 2010, 26, 775–781. [Google Scholar] [CrossRef]
- Pang, R.; Li, M.; Zhang, C. Degradation of phenolic compounds by laccase immobilized on carbon nanomaterials: Diffusional limitation investigation. Talanta 2015, 131, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.M.; González-Domínguez, E.; Sanromán, Á.; Correa-Duarte, M.; Moldes, D. Immobilization of laccase on functionalized multiwalled carbon nanotube membranes and application for dye decolorization. RSC Adv. 2016, 6, 114690–114697. [Google Scholar] [CrossRef]
- Girelli, A.M.; Quattrocchi, L.; Scuto, F.R. Silica-chitosan hybrid support for laccase immobilization. J. Biotechnol. 2020, 318, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Arregui, L.; Ayala, M.; Gómez-Gil, X.; Gutiérrez-Soto, G.; Hernández-Luna, C.E.; Herrera De Los Santos, M.; Levin, L.; Rojo-Domínguez, A.; Romero-Martínez, D.; Saparrat, M.C.; et al. Laccases: Structure, function, and potential application in water bioremediation. Microb. Cell Factories 2019, 18, 200. [Google Scholar] [CrossRef]
- Feng, J.; Li, H.; Lu, Y.; Li, R.; Cavaco-Paulo, A.; Fu, J. Non-ionic surfactant PEG: Enhanced cutinase-catalyzed hydrolysis of polyethylene terephthalate. Int. J. Biol. Macromol. 2024, 273 Pt 1, 133049. [Google Scholar] [CrossRef]
- Özşölen, F.; Aytar, P.; Gedıklı, S.; Çelıkdemır, M.; Ardıç, M.; Çabuk, A. Enhanced production and stability of laccase using some fungi on different lignocellulosic materials. J. Appl. Biol. Sci. 2010, 1, 69–78. [Google Scholar]
- Ravalason, H.; Herpoël-Gimbert, I.; Record, E.; Bertaud, F.; Grisel, S.; de Weert, S.; van den Hondel, C.A.; Asther, M.; Petit-Conil, M.; Sigoillot, J.C. Fusion of a family 1 carbohydrate binding module of Aspergillus niger to the Pycnoporus cinnabarinus laccase for efficient softwood kraft pulp biobleaching. J. Biotechnol. 2009, 142, 220–226. [Google Scholar] [CrossRef]
- Li, N.; Xia, Q.; Li, Y.; Hou, X.; Niu, M.; Ping, Q.; Xiao, H. Immobilizing Laccase on Modified Cellulose/CF Beads to Degrade Chlorinated Biphenyl in Wastewater. Polymers 2018, 10, 798. [Google Scholar] [CrossRef] [PubMed]
- Muthuvelu, K.S.; Rajarathinam, R.; Selvaraj, R.N.; Rajendren, V.B. A novel method for improving laccase activity by immobilization onto copper ferrite nanoparticles for lignin degradation. Int. J. Biol. Macromol. 2020, 152, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Patila, M.; Kouloumpis, A.; Gournis, D.; Rudolf, P.; Stamatis, H. Laccase-Functionalized Graphene Oxide Assemblies as Efficient Nanobiocatalysts for Oxidation Reactions. Sensors 2016, 16, 287. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, J.; Li, G.; Zhang, J.; Li, D.; Huang, F.; Wei, Q. Laccase immobilized on a PAN/adsorbents composite nanofibrous membrane for catechol treatment by a biocatalysis/adsorption process. Molecules 2014, 19, 3376–3388. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Yong, Y.; Wang, C.; Zhang, Q. Immobilization of laccase on biochar for the remediation of organic pollutants: A comprehensive review. Int. J. Biol. Macromol. 2025, 322, 146778. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Z.; Ji, L.; Lu, Z.; Liu, R.; Nian, B.; Hu, Y. Laccase immobilized on nanocomposites for wastewater pollutants degradation: Current status and future prospects. Bioprocess. Biosyst. Eng. 2023, 46, 1513–1531. [Google Scholar] [CrossRef]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef]
- Tabrizi, G.B.; Mehrvar, M. Integration of advanced oxidation technologies and biological processes: Recent developments, trends, and advances. J. Environ. Sci. Health Part A 2004, 39, 3029–3081. [Google Scholar] [CrossRef]
- Pedroza, A.M.; Mosqueda, R.; Alonso-Vante, N.; Rodríguez-Vázquez, R. Sequential treatment via Trametes versicolor and UV/TiO2/RuxSey to reduce contaminants in waste water resulting from the bleaching process during paper production. Chemosphere 2007, 67, 793–801. [Google Scholar] [CrossRef]
- Chmelová, D.; Ondrejovič, M.; Miertuš, S. Laccases as Effective Tools in the Removal of Pharmaceutical Products from Aquatic Systems. Life 2024, 14, 230. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Kalia, V.C.; Lee, J.K. Laccase Immobilization on Copper-Magnetic Nanoparticles for Efficient Bisphenol Degradation. J. Microbiol. Biotechnol. 2023, 33, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Patila, M.; Athanasiou, P.E.; Kortessis, L.; Potsi, G.; Kouloumpis, A.; Gournis, D.; Stamatis, H. Immobilization of Laccase on Hybrid Super-Structured Nanomaterials for the Decolorization of Phenolic Dyes. Processes 2022, 10, 233. [Google Scholar] [CrossRef]
- Pita, M.; Mate, D.M.; Gonzalez-Perez, D.; Shleev, S.; Fernandez, V.M.; Alcalde, M.; De Lacey, A.L. Bioelectrochemical oxidation of water. J. Am. Chem. Soc. 2014, 136, 5892–5895. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, B.; Li, J.; Chi, Y.; Zhai, H.; Liu, L.; Chi, Y.; Wang, R.; Yu, H.; Yuan, T.; et al. Laccase-loaded CaCO3 sustained-release microspheres modified SBES anode for enhance performance in the remediation of soil contaminated with phenanthrene and pyrene. J. Hazard. Mater. 2024, 480, 136106. [Google Scholar] [CrossRef]
- Rubenwolf, S.; Sané, S.; Hussein, L.; Kestel, J.; von Stetten, F.; Urban, G.; Krueger, M.; Zengerle, R.; Kerzenmacher, S. Prolongation of electrode lifetime in biofuel cells by periodic enzyme renewal. Appl. Microbiol. Biotechnol. 2012, 96, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Mani, P.; Fidal, V.T.; Keshavarz, T.; Chandra, T.S.; Kyazze, G. Laccase Immobilization Strategies for Application as a Cathode Catalyst in Microbial Fuel Cells for Azo Dye Decolourization. Front. Microbiol. 2021, 11, 620075. [Google Scholar] [CrossRef]
- Shahmansouri, A.; Bellona, C. Nanofiltration technology in water treatment and reuse: Applications and costs. Water Sci. Technol. 2015, 71, 309–319. [Google Scholar] [CrossRef]
- Singh, J.; Saharan, V.; Kumar, S.; Gulati, P.; Kapoor, R.K. Laccase grafted membranes for advanced water filtration systems: A green approach to water purification technology. Crit. Rev. Biotechnol. 2018, 38, 883–901. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, L.; Liu, G.; Yue, X.; Zheng, X.; Ma, L.; Liu, Y.; Chen, M.; Jiang, Y. Preparation of SCOF/UiO-66-NH2 immobilized laccase biocatalytic membrane for micropollutants removal from water. Chem. Eng. J. 2025, 509, 161310. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Q.; Yuan, J.; Fan, X.; Wang, P. Co-immobilization of cellulase and laccase onto the reversibly soluble polymers for decolorization of denim fabrics. Fibers Polym. 2017, 18, 993–999. [Google Scholar] [CrossRef]
- dos Santos, K.P.; Duarte, M.S.; Rios, N.S.; Brígida, A.I.S.; Gonçalves, L.R.B. A New Multi-Active Heterogeneous Biocatalyst Prepared Through a Layer-by-Layer Co-Immobilization Strategy of Lipase and Laccase on Nanocellulose-Based Materials. Catalysts 2025, 15, 99. [Google Scholar] [CrossRef]
- Saikia, K.; Vishnu, D.; Rathankumar, A.K.; Palanisamy Athiyaman, B.; Batista-García, R.A.; Folch-Mallol, J.L.; Cabana, H.; Kumar, V.V. Development of a magnetically separable co-immobilized laccase and versatile peroxidase system for the conversion of lignocellulosic biomass to vanillin. J. Air Waste Manag. Assoc. 2020, 70, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Cui, Y.; Jiang, Z.; Zhou, M.; Wang, P.; Yu, Y.; Wang, Q. Boosting laccase activity by synergistic drive of near infrared laser, surfactant, and sodium chloride for healthcare coatings with UV resistance, anti-oxidation, and photothermal conversion. Prog. Org. Coat. 2023, 184, 107838. [Google Scholar] [CrossRef]
- Xia, Y.; Deng, M.; Zhang, T.; Yuan, N.; Lin, X. Insights into a photo-driven laccase coupling system for enhanced photocatalytic oxidation and biodegradation. Chem. Eng. J. 2024, 501, 157658. [Google Scholar] [CrossRef]
- Tiwari, A.; Chen, C.-W.; Haldar, D.; Patel, A.K.; Dong, C.-D.; Singhania, R.R. Laccase in Biorefinery of Lignocellulosic Biomass. Appl. Sci. 2023, 13, 4673. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Younus, H.; Khan, M.A.; Khan, A.; Alhumaydhi, F.A. Eco-Friendly Biocatalysts: Laccase Applications, Innovations, and Future Directions in Environmental Remediation. Catalysts 2025, 15, 921. https://doi.org/10.3390/catal15100921
Younus H, Khan MA, Khan A, Alhumaydhi FA. Eco-Friendly Biocatalysts: Laccase Applications, Innovations, and Future Directions in Environmental Remediation. Catalysts. 2025; 15(10):921. https://doi.org/10.3390/catal15100921
Chicago/Turabian StyleYounus, Hina, Masood Alam Khan, Arif Khan, and Fahad A. Alhumaydhi. 2025. "Eco-Friendly Biocatalysts: Laccase Applications, Innovations, and Future Directions in Environmental Remediation" Catalysts 15, no. 10: 921. https://doi.org/10.3390/catal15100921
APA StyleYounus, H., Khan, M. A., Khan, A., & Alhumaydhi, F. A. (2025). Eco-Friendly Biocatalysts: Laccase Applications, Innovations, and Future Directions in Environmental Remediation. Catalysts, 15(10), 921. https://doi.org/10.3390/catal15100921









