Recent Advances in Congo Red Degradation by TiO2-Based Photocatalysts Under Visible Light
Abstract
1. Introduction
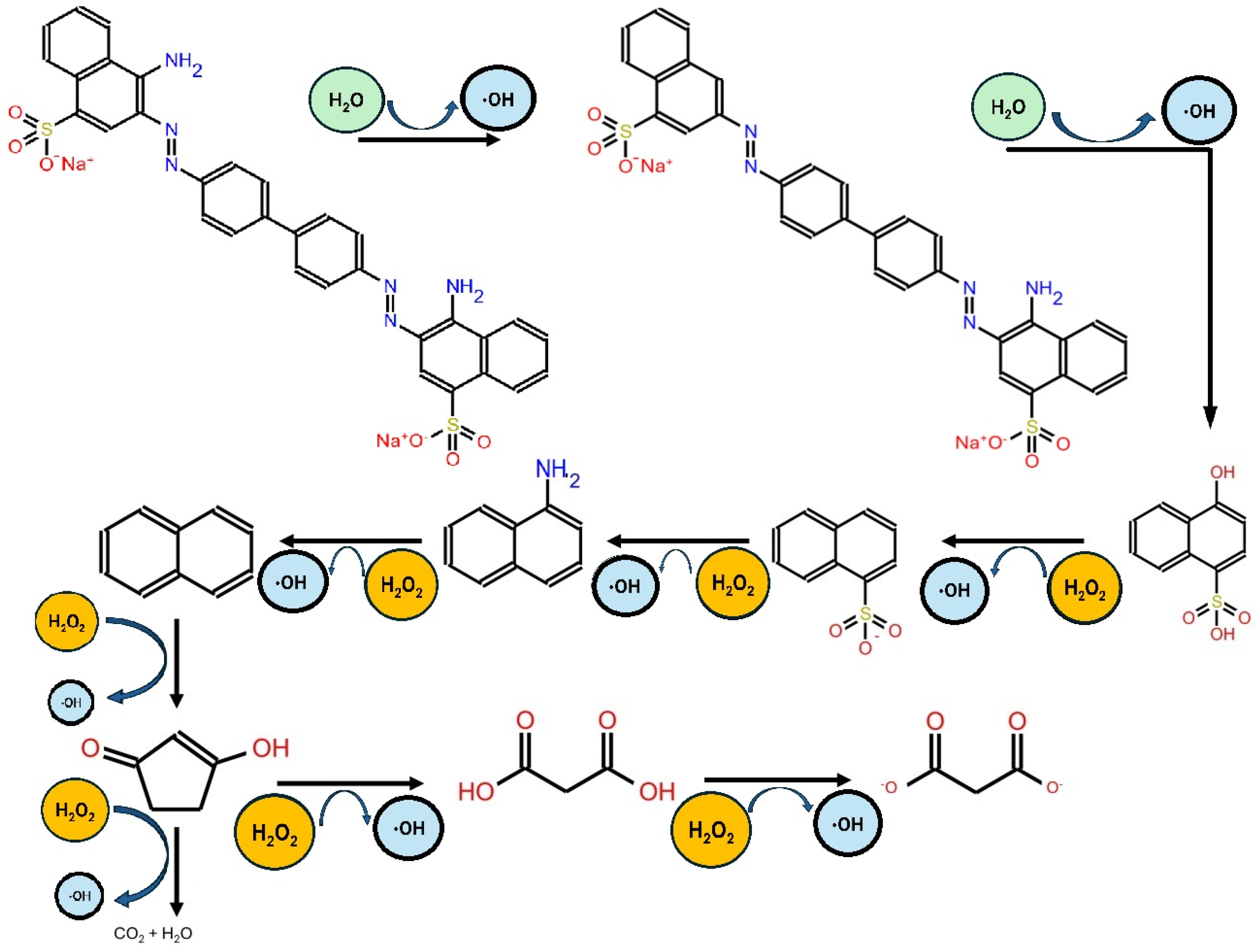
2. Degradation of Congo Red with TiO2-Based Photocatalysts: Advantages and Limitations
3. Degradation of Congo Red Using TiO2-Based Photocatalysts Under Visible Light
3.1. Modification of TiO2 Photocatalyst Using Metals
3.2. Modification of TiO2 Photocatalyst Using Non-Metals
3.3. Impact of the Synthesis Process on Degradation Ability
4. Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Oyekanmi, A.A.; Ahmad, A.; Mohd Setapar, S.H.; Alshammari, M.B.; Jawaid, M.; Hanafiah, M.M.; Abdul Khalil, H.P.S.; Vaseashta, A. Sustainable Durio zibethinus-derived biosorbents for congo red removal from aqueous solution: Statistical optimization, isotherms and mechanism studies. Sustainability 2021, 13, 13264. [Google Scholar] [CrossRef]
- Siddiqui, S.I.; Allehyani, E.S.; Al-Harbi, S.A.; Hasan, Z.; Abomuti, M.A.; Rajor, H.K.; Oh, S. Investigation of Congo Red Toxicity towards Different Living Organisms: A Review. Processes 2023, 11, 807. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, K.; Sun, F.; Liu, Y.; Chen, J. Enhanced Performance of Photocatalytic Treatment of Congo Red Wastewater by CNTs-Ag Modified TiO2 Under Visible Light. Environ. Sci. Pollut. Res. 2022, 29, 15516–15525. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, W.A.A.; El-Gawad, H.H.A.; Mousa, H.A.; Handal, H.T.; Galal, H.R.; Ibrahem, I.A.; El-Beih, A.A.; Fawzy, M.M.; Ahmed, M.A.M.; Mekkey, S.D.; et al. Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater. Nanotechnol. Rev. 2024, 13, 20240001. [Google Scholar] [CrossRef]
- NIH. Congo Red. Compound Summary. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Congo-red#section=Information-Sources (accessed on 25 November 2024).
- Asses, N.; Ayed, L.; Hkiri, N.; Hamdi, M. Congo Red Decolorization and Detoxification by Aspergillus niger: Removal Mechanisms and Dye Degradation Pathway. BioMed Res. Int. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Mota, T.R.; Kato, C.G.; Peralta, R.A.; Bracht, A.; de Morais, G.R.; Baesso, M.L.; de Souza, C.G.M. Decolourization of Congo Red by Ganoderma lucidum Laccase: Evaluation of Degradation Products and Toxicity. Water Air Soil Pollut. 2015, 226, 1–11. [Google Scholar] [CrossRef]
- Iark, D.; dos Reis Buzzo, A.J.; Garcia, J.A.A.; Côrrea, V.G.; Helm, C.V.; Corrêa, R.C.G.; Peralta, R.A.; Moreira, R.D.F.P.M.; Bracht, A.; Peralta, R.M. Enzymatic degradation and detoxification of azo dye Congo red by a new laccase from Oudemansiella canarii. Bioresour. Technol. 2019, 289, 121655. [Google Scholar] [CrossRef] [PubMed]
- Fowsiya, J.; Madhumitha, G.; Al-Dhabi, N.A.; Arasu, M.V. Photocatalytic degradation of Congo red using Carissa edulis extract capped zinc oxide nanoparticles. J. Photochem. Photobiol. B Biol. 2016, 162, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Said, A.; Ali, F.; Raziq, F.; Ali, Z.; Bilal, M.; Reinert, L.; Begum, T.; Iqbal, H.M.N. Photocatalytic Degradation of Congo Red Dye from Aqueous Environment Using Cobalt Ferrite Nanostructures: Development, Characterization, and Photocatalytic Performance. Water Air Soil Pollut. 2020, 231, 50. [Google Scholar] [CrossRef]
- Umamaheswari, C.; Lakshmanan, A.; Nagarajan, N. Green synthesis, characterization and catalytic degradation studies of gold nanoparticles against congo red and methyl orange. J. Photochem. Photobiol. B Biol. 2018, 178, 33–39. [Google Scholar] [CrossRef]
- Laouedj, N.; Ahmed, B. ZnO-Assisted Photocatalytic Degradation of Congo Red and Benzopurpurine 4B in Aqueous Solution. J. Chem. Eng. Process Technol. 2011, 2, 1–9. [Google Scholar] [CrossRef]
- Hitkari, G.; Chowdhary, P.; Kumar, V.; Singh, S.; Motghare, A. Potential of Copper-Zinc Oxide nanocomposite for photocatalytic degradation of congo red dye. Clean. Chem. Eng. 2022, 1, 100003. [Google Scholar] [CrossRef]
- Fardood, S.T.; Moradnia, F.; Ramazani, A. Green synthesis and characterisation of ZnMn2O4 nanoparticles for photocatalytic degradation of Congo red dye and kinetic study. Micro Nano Lett. 2019, 14, 986–991. [Google Scholar] [CrossRef]
- Wang, S.; Luo, C.; Tan, F.; Cheng, X.; Ma, Q.; Wu, D.; Li, P.; Zhang, F.; Ma, J. Degradation of Congo red by UV photolysis of nitrate: Kinetics and degradation mechanism. Sep. Purif. Technol. 2021, 262, 118276. [Google Scholar] [CrossRef]
- Güy, N.; Özacar, M. The influence of noble metals on photocatalytic activity of ZnO for Congo red degradation. Int. J. Hydrogen Energy 2016, 41, 20100–20112. [Google Scholar] [CrossRef]
- Solano, A.M.S.; Garcia-Segura, S.; Martínez-Huitle, C.A.; Brillas, E. Degradation of acidic aqueous solutions of the diazo dye Congo Red by photo-assisted electrochemical processes based on Fenton’s reaction chemistry. Appl. Catal. B Environ. 2015, 168–169, 559–571. [Google Scholar] [CrossRef]
- Muneer, M.; Saeed, M.; Bhatti, I.A.; Haq, A.-U.; Khosa, M.K.; Jamal, M.A.; Ali, S. Radiation induced degradation of Congo red dye: A mechanistic study. Nukleonika 2019, 64, 49–53. [Google Scholar] [CrossRef]
- Ma, H.; Wang, M.; Yang, R.; Wang, W.; Zhao, J.; Shen, Z.; Yao, S. Radiation degradation of Congo Red in aqueous solution. Chemosphere 2007, 68, 1098–1104. [Google Scholar] [CrossRef]
- Zhang, Z.; Shan, Y.; Wang, J.; Ling, H.; Zang, S.; Gao, W.; Zhao, Z.; Zhang, H. Investigation on the rapid degradation of congo red catalyzed by activated carbon powder under microwave irradiation. J. Hazard. Mater. 2007, 147, 325–333. [Google Scholar] [CrossRef]
- Feng, S.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Cheng, D.; Varjani, S.; Lei, Z.; Liu, Y. Roles and applications of enzymes for resistant pollutants removal in wastewater treatment. Bioresour. Technol. 2021, 335, 125278. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kermanshahi-Pour, A.; Brar, S.K.; He, Q.S.; Rainey, J.K. Influence of elevated pressure and pressurized fluids on microenvironment and activity of enzymes. Biotechnol. Adv. 2023, 68, 108219. [Google Scholar] [CrossRef]
- Zin, Z.M.; Yahaya, N.; Bashah, N.; Ibrahim, K.; Rusli, N.; Smedley, K.; Mohd, K.; Zainol, M. Effect of pH extraction buffer on antioxidant enzymes activities in water lily’s leaves and petioles. Food Res. 2022, 6, 34–44. [Google Scholar] [CrossRef]
- Braham, S.A.; Siar, E.-H.; Arana-Peña, S.; Carballares, D.; Morellon-Sterling, R.; Bavandi, H.; de Andrades, D.; Kornecki, J.F.; Fernandez-Lafuente, R. Effect of concentrated salts solutions on the stability of immobilized enzymes: Influence of inactivation conditions and immobilization protocol. Molecules 2021, 26, 968. [Google Scholar] [CrossRef]
- Diasanayake, M.A.K.L.; Senadeera, G.K.R.; Sarangika, H.N.M.; Ekanayake, P.M.P.C.; Thotawattage, C.A.; Divarathne, H.K.D.W.M.N.R.; Kumari, J.M.K.W. TiO2 as a Low Cost, Multi Functional Material. Mater. Today Proc. 2016, 3 (Suppl. S1), S40–S47. [Google Scholar] [CrossRef]
- Rahim, S.; Ghamsari, M.S.; Radiman, S.; Hamzah, A. Highly stable TiO2 Sol with High Photocatalytic Properties. In The Sol-Gel Process: Uniformity, Polymers and Applications; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011; pp. 773–785. [Google Scholar]
- Pan, D.; Zhang, C.; Wang, C.-S.; Zhang, P.; Jiao, X.-Y.; Ma, Q.-R.; Wang, L.-T.; Li, D.-J.; Li, L.-P. Unravelling hidden threats of water disinfection: Toxicity evaluation and toxic products identification during diclofenac degradation. Environ. Pollut. 2024, 345, 123424. [Google Scholar] [CrossRef]
- Petronella, F.; Truppi, A.; Dell’edera, M.; Agostiano, A.; Curri, M.L.; Comparelli, R. Scalable synthesis of mesoporous TiO2 for environmental photocatalytic applications. Materials 2019, 12, 1853. [Google Scholar] [CrossRef] [PubMed]
- Nurdin, M.; Widianingsih, E.; Azis, T.; Wibowo, D. Preparation of Visible Photocatalyst N-TiO2 and Its Activity on Congo Red Degradation. ARPN J. Eng. Appl. Sci. 2015, 10, 6250–6256. [Google Scholar]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Harun, N.H.; Rahman, M.A.; Kamarudin, W.W.; Irwan, Z.; Muhammud, A.; Akhir, N.E.F.M.; Yaafar, M.R. Photocatalytic Degradation of Congo Red Dye Based on Titanium Dioxide Using Solar and UV Lamp. J. Fundam. Appl. Sci. 2018, 10, 832–846. [Google Scholar] [CrossRef]
- Turcu, E.; Coromelci, C.G.; Harabagiu, V.; Ignat, M. Enhancing the Photocatalytic Activity of TiO2 for the Degradation of Congo Red Dye by Adjusting the Ultrasonication Regime Applied in Its Synthesis Procedure. Catalysts 2023, 13, 345. [Google Scholar] [CrossRef]
- Kong, L.; Tunku, U.; Rahman, A. Synthesis and Characterisations of Titanium Dioxide (TiO2)/Cellulose Biochar Composites for Photocatalytic Degradation of Congo Red. Doctoral Dissertation, Universiti Tunku Abdul Rahman, Jaya, Malaysia, 2020. [Google Scholar]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium dioxide nanoparticles: Prospects and applications in medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef]
- Mahdi, M.A.; Farhan, M.A.; Mahmoud, Z.H.; Rheima, A.M.; Abbas, Z.S.; Kadhim, M.M.; Al-Bayati, A.D.J.; Jaber, A.S.; Hachim, S.K.; Ismail, A.H. Direct sunlight photodegradation of congo red in aqueous solution by TiO2/rGO binary system: Experimental and DFT study. Arab. J. Chem. 2023, 16, 104992. [Google Scholar] [CrossRef]
- Magdalane, C.M.; Priyadharsini, G.M.A.; Kaviyarasu, K.; Jothi, A.I.; Simiyon, G.G. Synthesis and characterization of TiO2 doped cobalt ferrite nanoparticles via microwave method: Investigation of photocatalytic performance of congo red degradation dye. Surf. Interfaces 2021, 25, 101296. [Google Scholar] [CrossRef]
- Baruah, M.; Ezung, S.L.; Supong, A.; Bhomick, P.C.; Kumar, S.; Sinha, D. Synthesis, characterization of novel Fe-doped TiO2 activated carbon nanocomposite towards photocatalytic degradation of Congo red, E. coli, and S. aureus. Korean J. Chem. Eng. 2021, 38, 1277–1290. [Google Scholar] [CrossRef]
- Imran, M.; Saeed, Z.; Pervaiz, M.; Mehmood, K.; Ejaz, R.; Younas, U.; Nadeem, H.A.; Hussain, S. Enhanced visible light photocatalytic activity of TiO2 co-doped with Fe, Co, and S for degradation of Cango red. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 255, 119644. [Google Scholar] [CrossRef] [PubMed]
- Landge, V.K.; Huang, C.M.; Hakke, V.S.; Sonawane, S.H.; Manickam, S.; Hsieh, M.C. Solar-Energy-Driven Cu-ZnO/TiO2 Nanocomposite Photocatalyst for the Rapid Degradation of Congo Red Azo Dye. Catalysts 2022, 12, 605. [Google Scholar] [CrossRef]
- Aprilita, N.H.; Amalia, D.; Wahyuni, E.T. Removal of the Hazardous Congo Red Dye through Degradation under Visible Light Photocatalyzed by C,N Co-Doped TiO2 Prepared from Chicken Egg White. Sci. World J. 2022, 2022, 1–8. [Google Scholar] [CrossRef]
- Hammud, H.H.; Traboulsi, H.; Karnati, R.K.; Bakir, E.M. Photodegradation of Congo Red by Modified P25-Titanium Dioxide with Cobalt-Carbon Supported on SiO2 Matrix, DFT Studies of Chemical Reactivity. Catalysts 2022, 12, 248. [Google Scholar] [CrossRef]
- Prashanth, V.; Remya, N. Synthesis of TiO2 using Calotropis gigantea for visible light excitation and degradation of congo red dye. J. Hazard. Toxic Radioact. Waste 2021, 25, 04021026. [Google Scholar] [CrossRef]
- Sayqal, A.; Alfi, A.A.; Alatawi, N.M.; Al-Ghamdi, S.A.; Alatawi, I.S.; Alsharari, A.M.; Alessa, H.; El-Metwaly, N.M. Breakdown cost and recycling processes of Bentonite/TiO2 quantum dots of photo and solar degradation of Congo Red dye and industrial dyes wastes. Opt. Mater. 2024, 157, 116408. [Google Scholar] [CrossRef]
- Wahyuni, E.T.; Wahyuni, S.; Lestari, N.D.; Suherman, S. Utilization of tannery wastewater as a source of Cr doped into TiO2 for improving its activity under visible light in the Congo red degradation. React. Kinet. Catal. Lett. 2023, 136, 1067–1084. [Google Scholar] [CrossRef]
- Musmade, S.; Hase, D.P.; Waghmare, A.S.; Kadam, K.R.; Khedkar, J.; Gadhave, A.G.; Bhavsar, K.S.; Murade, V.D. Synthesis of shape controlled Cu2O and Cu2O/TiO2-QD composite for degradation of Congo red dye under visible-light irradiation. J. Water Environ. Nanotechnol. 2023, 8, 406–416. [Google Scholar] [CrossRef]
- Wahyuni, E.T.; Lestari, N.D.; Cinjana, I.R.; Annur, S.; Natsir, T.A.; Mudasir, M. Doping TiO2 with Fe from iron rusty waste for enhancing its activity under visible light in the Congo red dye photodegradation. J. Eng. Appl. Sci. 2023, 70, 9. [Google Scholar] [CrossRef]
- Rani, A.; Kumar, S.; Dhiman, L.; Singh, V. Photocatalytic Degradation of Congo Red and Methyl Orange Dye Under Visible Light Using Silver and Iron co-doped TiO2 Nanoparticles. Indian J. Pure Appl. Phys. 2022, 60, 325–334. [Google Scholar]
- Mousa, S.A.; Tareq, S.; Muhammed, E.A. Studying the Photodegradation of Congo Red Dye from Aqueous Solutions Using Bimetallic Au–Pd/TiO2 Photocatalyst. Baghdad Sci. J. 2021, 18, 1261–1268. [Google Scholar] [CrossRef]
- Nafis, N.; Sartika, H.; Rahmi, D.M. Photocatalytic Degradation of Congo Red with Sol-Gel Immobilized TiO2 Thin Layers on the Inner Surface of a Glass Tube Column. Chem Trendz J. 2024, 1, 1–23. [Google Scholar]
- Mohammed, A.M.; Mohtar, S.S.; Aziz, F.; Aziz, M.; Ul-Hamid, A.; Salleh, W.N.W.; Yusof, N.; Jaafar, J.; Ismail, A.F. Ultrafast degradation of Congo Red dye using a facile one-pot solvothermal synthesis of cuprous oxide/titanium dioxide and cuprous oxide/zinc oxide p-n heterojunction photocatalyst. Mater. Sci. Semicond. Process. 2021, 122, 105481. [Google Scholar] [CrossRef]
- Moulahi, A. New and effective photocatalysts for removal of mordant black 17 and Congo red pollutants: In3+ and Sb5+ doped and codoped TiO2 semiconductor. Opt. Mater. 2023, 137, 113573. [Google Scholar] [CrossRef]
- Abumousa, R.A.; Bououdina, M.; Aissa, M.A.B.; Khezami, L.; Modwi, A. Efficient photocatalytic degradation of Congo red and other dyes by ternary TiO2/Y2O3@ g-C3N4 nanohybrid. J. Mater. Sci. Mater. Electron. 2024, 35, 486. [Google Scholar] [CrossRef]
- Pouthika, K.; Madhumitha, G. Tailoring interfacial charge separation in Z-Scheme CuO@TiO2@ halloysite heterostructure for efficient photocatalytic removal of Congo red. J. Taiwan Inst. Chem. Eng. 2024, 166, 105752. [Google Scholar] [CrossRef]
- Bibi, S.; Shah, S.S.; Muhammad, F.; Siddiq, M.; Kiran, L.; Aldossari, S.A.; Mushab, M.S.S.; Sarwar, S. Cu-doped mesoporous TiO2 photocatalyst for efficient degradation of organic dye via visible light photocatalysis. Chemosphere 2023, 339, 139583. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, H.; Chen, P.; Song, Y.; Zhang, J. Magnetically Recyclable Wool/Fe3O4@TiO2/UiO-66 Core-Shell Structured Composite for Photocatalytic Removal of Methylene Blue, Congo Red, Tetracycline Hydrochloride and Cr (VI) Ions. Fibers Polym. 2022, 23, 2780–2797. [Google Scholar] [CrossRef]
- Ali, N.; Khan, A.; Riaz, A.; Asiri, A.M.; Kamal, T. Photocatalytic Performance Evaluation of Bismuth Doped Tin-Dioxide under UV and Direct Sunlight Irradiation for Congo Red Dye Degradation. J. Chem. Soc. Pak. 2020, 42, 687. [Google Scholar]
- Zaleska, A. Doped-TiO2: A Review. Recent Pat. Eng. 2008, 2, 157–164. [Google Scholar] [CrossRef]


| Method or Technology | Working Conditions | % of Degradation, Elimination, or Rate Constant/Toxicity Bioassays Performed | Reference |
|---|---|---|---|
| Biological/Aspergillus niger, producer of lignin peroxidase and manganese peroxidase | 2 g mycelia incubated at pH 5 in the presence of 200 mg L−1 of dye for 6 days at 28 °C and under 120 to 150 rpm. | 97% decolorization/Phytotoxicity bioassays with Zea mais and Solanum lycopersicum, and microtoxicity bioassays with Bacillus cereus ATCC 11,778 and Escherichia coli ATCC 10,536 strains found metabolites less toxic | [6] |
| Biological/native laccase enzyme from Ganoderma lucidum | 6 h of treatment at pH 4.0 and 40 °C, total volume of 50 mL containing 50 mg L−1 of Congo red and native laccase (5 U) | Decolorization of 80%/Toxicity study with lettuce seeds has shown Congo red inhibited the hypocotyl growth, with a significant reduction of toxicity (p ≤ 0.05) | [7] |
| Biological/laccase from Oudemansiella canarii | 5 U of the enzyme, 50 mg L−1 Congo red within 24 h at 30 °C and pH 5.5 | Decolorization of 80%/Microtox test determined a diminution of toxicity of 92.5% | [8] |
| Biological-Photocatalytic/Extract of Carissa edulis | Extract of Carissa edulis obtained by microwave-assisted extraction at 70 °C and 400 W, capped with ZnO nanoparticles (NPs) (1 mg) and mixed with 1 mM Congo red dye in water, for 130 min, at 365 nm in a photoreactor | 97% degradation/No toxicity bioassays were performed | [9] |
| Photocatalytic/Cobalt ferrite (CoFe2O4) nanostructures | Aqueous solution of dye (10 mg L−1), 10 mg of CoFe2O4 nanostructures were thoroughly mixed and kept at room temperature for 30 min in the dark and constant stirring was maintained; UV irradiation for 90 min, pH 9; centrifugation at 4500 rpm for 15 min | 91% degradation/No toxicity bioassays were performed | [10] |
| Physical-Chemical/Dalspinin Mediated gold nanoparticles (DLP-AuNPs) | Congo Red solution (1 × 10−5 M) in the presence of NaBH4 (1 × 10−3 M); 100 μL of DLP-AuNPs was added into a quartz tube containing dye solution (3.0 mL) and NaBH4, with a reaction time of 10 min | Degradation with a rate constant of 4.5 × 10−3 s−1/Toxic metabolites monitored by Fourier transform infrared (FTIR) spectroscopy/Toxicity not specified | [11] |
| Photocatalytic/ZnO | pH 8, 60 min of irradiation time, ZnO concentration of 0.5 g L−1, dye concentration of 20 mg L−1, temperature of 298 K, I = 90 j/cm2, λ = 365 nm | 95.02% degradation/No toxicity bioassays were performed | [12] |
| Photocatalytic/ZnO and ZnO/CuO composites | A 350 W Xe lamp was used, specific quartz reactors (100 mL), and 50 mg of each composite was added into 50 mL of dye aqueous solution (50 mg L−1); initial pH value 5.6, sonicated for 30 min in the dark | 25% and 95% degradation for ZnO and ZnO/CuO/No toxicity bioassays were performed | [13] |
| Physical-Chemical/ZnMn2O4 nanoparticles (NPs) and visible light | Room temperature, visible light (fluorescent lamp (λ > 400 nm, 90 W)), 15 min, dye concentration of 20 mg L−1, 30 mg of catalyst, natural pH | 96% degradation/No toxicity bioassays were performed | [14] |
| Physical–chemical/Ultraviolet photolysis of nitrate (UV/NO3−) | The photon flux of (I 253.7 nm) was calculated to be 1.23 × 10−7 Einstein⋅L−1⋅s−1; dye concentration of 20 μM and NO3− of 50 mM; sodium nitrate (50 mM) and Congo Red (20 μM) were added to deionized water (100 mL), 25 ± 1 °C, pH 7 ± 0.4 | 81.9% degradation/No toxicity bioassays were performed | [15] |
| Physical and chemical/Pd-doped ZnO catalyst and UV irradiation | UV irradiation (100 W, strongest emission at 365 nm) for 1 h, 50 mg of photocatalyst in 100 mL of dye solution (16 mg L−1), stirred for 30 min before irradiation | 98% degradation/No toxicity bioassays were performed | [16] |
| Physical-Chemical/Photoelectrochemical Fenton’s based | 0.260 mM of dye with 0.50 mM Fe2+ at 100 mAcm−2, current efficiency and 0.45 kWh (g DOC)−1 energy consumption, 240 min, pH 3, boron-doped diamond (BDD) air-stirred reactor | Mineralization current efficiency (MCE) of 49%/Liquid-Chromatography-Mass Spectrometry (LC-MS) used for monitoring metabolites/No toxicity bioassays were performed | [17] |
| Physical–Chemical/Co-60 gamma radiation | 5 kGy, 0.5 mL of H2O2 (37%) was added to the dye solution (50 mg L−1) | 100% decolorization/Radiolytic end products were monitored by FTIR and Gas-Chromatography-Mass Spectrometry (GC-MS)/No toxicity bioassays were performed | [18] |
| Physical–Chemical/gamma rays | A series of 1 × 10−4 M Congo Red aqueous solutions were bubbled with high-purity (99.99%) N2, N2O and O2, respectively, for 15 min in Pyrex glass tubes before gamma-ray irradiation at 11.9 kGy | Total Organic Carbon (TOC) decreased by 76% and 86% for solutions saturated with O2 and N2O, respectively/No toxicity bioassays were performed | [19] |
| Physical–Chemical/Activated carbon powder under microwave irradiation | A volume of reaction of 25 mL with a dye concentration of 50 mg L−1, 3.6 g L−1 of activated carbon powder, and 2.5 min of microwave irradiation at a microwave frequency of 2450 MHz and output power of 800 W | High-performance liquid chromatography (HPLC) monitored 96.49% degradation and the intermediate degradation products/No toxicity bioassays were performed | [20] |
| Photocatalyst | Catalyst Synthesis/Degradation Conditions | Band Gap and Absorption Wavelength/% of Degradation or Elimination/Toxicity Bioassays Perfomed | Reference |
|---|---|---|---|
| TiO2/reduced graphene oxide sheets (rGO/TiO2) | Synthesis: hydrothermal method, 10 mL of Titanium tetra isopropoxide (TTIP) stock was diluted with 40 mL of distilled water and irradiated using manual irradiation at 15 W in ice bath. Dried at 80 °C for 5 h and calcined at 500 °C for 2 h. The GO was synthesized by the Hemmer modified method. The rGO/TiO2 nanocomposite was carried out using the hydrothermal method. Degradation: 0.01 g of catalyst 5% in 100 mL of 10 mg L−1 dye solution, stirred in the dark for 30 min. Direct sunlight with an intensity of 1200 W·cm2, 100 min, pH = 7, and 50 mg L−1 of dye concentration. | Band gap of 2.7 eV, 330–500 nm/Up to 99% of degradation and mineralization/Monitoring of products by LC-MS/No toxicity bioassays were performed | [35] |
| TiO2-doped cobalt ferrite (CoFe2O4) nanoparticles | Synthesis: Microwave-assisted method, using a 2:1 molar ratio of ferric nitrate and cobalt nitrate solutions, in a domestic microwave oven for 15 min at 2:54 GHz frequency at 850 W power, ground in a mortar. TTIP (0.1 M) was added and irradiated at 2:54 GHz (850 W power) in a microwave oven for 15 min and washed with ethanol. CoFe2O4 (95%) and titania (5%) were mixed with 150 mL of tetrahydrofuran (THF) and stirred for 1 h. 1 g of ascorbic acid was added, filtered at 25 ◦C, and dried for 48 h. Finally, it was calcinated at 500 °C for 3 h. Degradation: 80 mg of TiO2-doped CoFe2O4 nanostructures were gagged for the degradation of 100 mL dye (10 mg L−1) under visible light irradiation (150 W metal Halide lamp; λ > 400 nm), and 120 min. Also, an assay was carried out with 5 mL of 10% of H2O2 to increase -OH radicals. | Band gap of 2.88 eV, 330–500 nm/85% degradation without H2O2 and 97% with H2O2/No toxicity bioassays were performed | [36] |
| Fe-doped TiO2 activated carbon (AC) nanocomposite | Synthesis: the ultrasonic–hydrothermal method was used, 0.15 g of TiO2 nanoparticles was added to 1 g of 100 mL ferric nitrate solution while stirred for 30 min. Then, 0.3 g activated carbon was poured into the mixture and ultra-sonicated for 1 h. Later, the mixture was shifted into a hydrothermal autoclave and kept at 150 °C inside an oven for 24 h. After the hydrothermal treatment, the solution was washed with double-refined water in a centrifuge machine to neutralize the solution pH. Finally, the solution was dried at 65 °C for 12 h. Degradation: 20 mg L−1 of dye solution for 60 min, pH 1, 0.06 g of catalyst, and irradiated at a maximum wavelength of 520 nm with a high-pressure mercury lamp, 350 W, located at the top of the reactor, 10 cm away from the reaction solution. | Band gap 2.3 eV, 468 nm/100% degradation/No toxicity bioassays were performed | [37] |
| TiO2 co-doped with Fe, Co, and S | Synthesis: TiO2 nanoparticles co-doped with Fe, Co, and S were prepared using the sol-gel method. TTIP solution was poured continuously and stirred for 1 h. The product was isolated using centrifugation. It was washed several times with C2H5OH and deionized water. After drying at 100 °C, this product was calcinated in a box furnace at 500 °C for 3 h. Degradation: under optimized conditions, 30 mg L−1 of dye was degraded at a slightly acidic pH (~6.5), with 0.14 g of photocatalyst within 70 min of irradiation time. | Band gap from 1.46–2.85 eV, maximum absorbance at 500 nm/99.3% degradation/No toxicity bioassays were performed | [38] |
| Cu-ZnO/TiO2 Nanocomposite | Synthesis: ultrasonic-assisted and solvothermal method, 100 mL TiO2 NPs suspension in distilled water was prepared using an ultrasound probe sonicator (20 kHz, 20 mm tip diameter, 220 W) operated with pulse mode (5 sec ON and 5 sec OFF) for 10 min. An appropriate quantity of CuSO4.5H2O, C4H6O4Zn·2H2O, and polyvinylpyrrolidone (PVP) was added as a stabilizer. The solution was dried at 80 °C for 6 h to provide the ternary Cu-ZnO/TiO2 nanocomposite photocatalyst. Degradation: direct sunlight, during 20 min, 0.025 g of catalyst, dye solution of 75 mg L−1. | Band gap of 2.68 eV, 500–650 nm/98% degradation/No toxicity bioassays were performed | [39] |
| C,N Co-Doped TiO2 | Synthesis: hydrothermal method, 1 g of TiO2 suspended in water was mixed with chicken egg white (2 g) accompanied by constant stirring at 500 rpm for 2 h, and the mixture was transferred into an autoclave to be heated in the oven at 150 °C for 4 h. Afterward, the photocatalyst was collected, dried at 100 °C for 2 h, and calcined at 500 °C for 2 h. Degradation: 10 mg L−1 Congo Red dye in 100 mL of the solution under visible irradiation could be degraded by applying TiO2-C,N prepared from 2 g of the egg white within 45 min, at pH 7, and 50 mg of the photocatalyst mass. | Band gap from 2.69 to 3.04, absorbance from 415 to 430 nm/98% degradation/No toxicity bioassays were performed | [40] |
| Modified P25-TiO2 with Cobalt-Carbon Supported on SiO2 Matrix | Synthesis: solvothermal method, cobalt–carbon silica nanocomposite CoC@SiO2-bipy (s1) and CoC@SiO2-phen (s2) were prepared by ultrasonication of TiO2 (75%) and CoC@SiO2-bipy (s1) or CoC@SiO2-phen (s2) (25%) (weight ratio) for 30 min. The mixture was then filtered, dried at 60 °C, and milled Degradation: initial concentration of dye of 10µM; irradiation time of 60 min; solar power (UV index 5.0), volume of 2 mL; pH of 4.0, P25-TiO2 concentration of 56.25 mg L−1. | 92% degradation/No toxicity bioassays were performed | [41] |
| Green synthesized TiO2 (CG-TiO2) | Synthesis: CG-TiO2 was prepared from TTIP precursor using aqueous leaf extract of Calotropis gigantea (30 g leaves in 100 mL distilled water boiled for 2 h at 90 °C); TTIP:CG—1:3; mixing time and temperature—2 h and 27 °C, respectively; calcination temperature—300 °C. | 97% degradation/No toxicity bioassays were performed | [42] |
| Bentonite/TiO2 quantum dots (Bent/TiO2 QD) | Synthesis: co-precipitation method, using TTIP and calcination at 280 °C for 60 min in a muffle furnace. TiO2 was prepared via the sol-gel method using TTIP as the precursor. The resulting gel was aged at room temperature for 24 h, and after that was dried in an oven at 105 °C for 7 h and calcined at 280 °C for 4 h in a furnace. Degradation: 240 min of irradiation by xenon photoreactor, irradiance of 70 W/cm2, 500 mL of dye solution (4 × 10−5 M), pH 6.9, 0.5 g of catalyst, maintained in darkness for 20 min previous to irradiation. Then, it was centrifugated at 12,000 rpm/30 min to separate the catalyst from the dye solution. | Band gap of 3.15 eV/Removal efficiency of 84.5% and a Total Organic Carbon (TOC) reduction of 76.2%/No toxicity bioassays were performed | [43] |
| Cr doped into TiO2 | Synthesis: Sol–gel method. The doping was conducted by reacting TTIP as TiO2 precursor and Cr-containing tannery wastewater through the sol–gel method. Degradation: 10 mg L−1 of Congo red in 50 mL of reaction solution, 20 mg of TiO2-Cr (1:0.5) photocatalyst weight, solution pH at 5 in 60 min. | Band gaps from 2.21 to 2.59 eV/98% degradation/No toxicity bioassays were performed | [44] |
| Cu2O/TiO2-Quantum Dots (QD) | Synthesis: precipitation method. 10 mg copper (I) oxide nanoparticles in 10 mL ethanol were stirred for 30 min on a magnetic stirrer, and TiO2-QD sol was added. Then, the mixture was centrifuged at 8000 rpm, and the precipitate was filtered and washed several times using ethanol/water (1:1) solvent. To get Cu2O/TiO2-QD composite, the obtained precipitate was dried at room temperature. Degradation: 9 mg L−1 of dye, 150 mg L−1 of catalyst, 110 min, pH 6, visible light. | Band gap of 2.48 eV, 300–650 nm/89% degradation/No toxicity bioassays were performed | [45] |
| Doped TiO2 with Fe from rusty iron waste | Synthesis: sol–gel method, by interacting the TTIP solution with a solution containing Fe3+ dissolved from the rusty iron waste. The Fe3+ solution was prepared by dissolving 0.80 g of the rusty iron waste in 7.5 mL of HCl and HNO3 mix (3:1) then diluted up to 100 mL. The resulting gels were calcined at 500 °C for 3 h. Degradation: pH 5, in a closed box equipped with visible lamps, 60 mg of catalyst, irradiated with visible light accompanied by stirring magnetically for 60 min. | Band gap from 2.38 to 3.15 eV, 424, 554 nm/99% degradation/No toxicity bioassays were performed | [46] |
| Ag and Fe co-doped TiO2 Nanoparticles | Synthesis: The nanoparticles were synthesized by sol–gel method, using TTIP as precursor, AgNO3 and FeCl3. The obtained sol was kept for 24 h at room temperature to convert into gel, filtered, washed with methanol and distilled water several times, and then dried at 100 °C for 24 h in a hot oven. The dried material was ground with a mortar and pestle and annealed at 400 °C for 4 h. Similarly, AgxFe0.05Ti0.95-xO2; x = 0.08, 0.12 mol % were prepared by using 0.7 and 1.0 gm of AgNO3 in TTIP–methanol solution. Degradation: concentration of Ag (x = 0.12), custom-built reactor, 0.05 g of catalyst, 120 min, 100 mL of 10−5 M of dye, stirred 1 h in the dark, then illuminated with a phosphorous coated Hg vapor lamp with cutoff filters from 420–520 nm. | Band gap from 2.30 to 2.92, max absorbance with 0.12 mole% from 25′ to ~430 nm/82% degradation/No toxicity bioassays were performed | [47] |
| Bimetallic Au-Pd/TiO2 | Synthesis: synthesis of sol. Aqueous solutions of PdCl2 and HAuCl4 3H2O were prepared. PVA (1% weight solution) was supplied to the solution previously prepared. A 0.1 M NaBH4 solution was mixed to synthesize a dark brown sol. Titania and carbon and the H2SO4 were stirred and the catalyst produced was filtered before being thoroughly washed with 2 L of distilled water. Then, it was oven-dried at 120 °C for 16 h. Degradation: the best conditions were 28 mg L−1 of dye, 0.09 g. 75 mL−1 of dye solution of catalyst, 7 µL. 75 mL−1 of dye solution of H2O2, pH 5, 55 °C, 4 h. | 96% degradation/No toxicity bioassays were performed | [48] |
| Sol–Gel Immobilized TiO2 Thin Layers | Synthesis: TTIP was used as the precursor for TiO2. TiO2 thin films were synthesized using the sol–gel technique, involving hydrolysis and condensation of TTIP in a controlled solution. TiO2 thin films were synthesized using the sol–gel technique through hydrolysis and condensation in a controlled solution environment. The coated glass tube column underwent calcination at 400 °C to transform the deposited gel into crystalline TiO2 thin films. Degradation: 50 mg L−1 of dye, 240 min, batch reactor, 415 nm, a glass tube column with immobilized TiO2 thin film used as a catalyst. | Band gap of 3.2 eV, absorption started at 390 nm/99% degradation/No toxicity bioassays were performed | [49] |
| Cuprous oxide/titanium dioxide and cuprous oxide/zinc oxide p-n heterojunction photocatalyst (Cu2O/TiO2-Cu2O/ZnO | Synthesis: The nanoparticles were produced using a solvothermal one-pot process in a Teflon-coated stainless-steel autoclave under autogenous pressure. Cu(NO3)2⋅3H2O (1.5 M) was dissolved with absolute ethanol and autoclaved for 8 h at 170 °C. The final product (i.e., Cu2O) was obtained by filtration followed by washing thoroughly after the autoclave had cooled down. The product was then dried for 12 h at 60 °C. After that, 1.5 M Cu(NO3)2⋅3H2O and 1.5, 1.0, and 0.5 M Ti(OBu)4 were separately dissolved with 30 mL of absolute ethanol under stirring. The solutions were then combined, stirred, and transferred to the autoclave. The autoclave was operated at 170 °C for 8 h, cooled to room temperature, filtered, then washed and dried for 12 h at 60 °C. Degradation: 30 mg of photocatalysts into 100 mL dye solution (30 mg L−1), 10 min. Previously, the dye solution with the catalyst was stirred for 30 min in the dark. Next, a 300 W xenon lamp with a cut-off filter of 420 nm was switched on to begin photocatalytic degradation under visible light illumination. | Band gap of 2.61 eV, 500–570 nm/100% degradation/No toxicity bioassays were performed | [50] |
| In3+ and Sb5+ doped and co-doped TiO2 semiconductors | Synthesis: Chemical precipitation. The desired TiO2 compositions were synthesized by using titanium butoxide (Ti(OBu)4, 98%), indium chloride (InCl3, 99.9%), antimony acetate (Sb(CH3CO2)3, 99%). Pure TiO2 powder was synthesized by the addition of 30 mL n-butanol (C4H10O) to 10 titanium butoxide (Ti(C4H9O)4) solutions under constant stirring for 30 min. After that, the precipitate was produced by adding NH4OH solution at pH 8.5 under continuous stirring. The acquired precipitate was washed and dried under normal air using deionized water, followed by calcination at 700 °C for 4 h to form TiO2 powder. Degradation: 15 mg L−1 of dye, 100 mL of solution, 0.05 g of catalyst, stirred for 30 min before irradiation, then irradiated under natural sunlight for 80 min. | Band gap from 2.91 to 3.21, 500–1250 nm/81 and 86% for Ti0·97Sb0·03O2 and Ti0·94In0·03Sb0·03O2, respectively/No toxicity bioassays were performed | [51] |
| Ternary TiO2/Y2O3@ g-C3N4 nanohybrid | Synthesis: thermal and sonochemical method. The nanosheets of g-C3N4 were fabricated by thermally degrading urea. Ternary TiO2/Y2O3@g-C3N4 nanostructures were fabricated using a straightforward ultrasonication technique, including agitating 0.90 g of pure g-C3N4 in 0.150 L ethanol solvent. The ethanolic solution was combined with 0.2 g of TiO2 and Y2O3 (15% each) nanopowders to generate a suspension solution. The mixture was sonicated at 500 MHz for 80 min before being dried at 85 °C for 24 h, pulverized with a ceramic mortar, pestled, and heated at 150 °C for 60 min in a muffle furnace. The same protocol was adopted to fabricate TiO2@g-C3N4 and Y2O3@g-C3N4. Degradation: 0.05 g of catalyst, 100 mL of dye solution (30 mg L−1), stirred (400 rpm) in the darkness. Then, it was exposed for 60 min to visible light radiation (OSRAM lamp 58 IM/W). | Band gap from 2.47 to 2.55 eV, 440 nm/100% degradation/No toxicity assays. | [52] |
| Z-Scheme CuO@ TiO2@ halloysite heterostructure | Synthesis: two-step microwave and ultrasonic-assisted. Degradation: 50 mL of dye solution (10 mg L−1), 30 mg of catalyst, left in the dark for 30 min before irradiation, irradiated for 150 min with a Xenon lamp (300 W) with visible light (450 nm). | Band gap of 3.1 eV, max absorption at ~450 nm/82% degradation/No toxicity bioassays were performed | [53] |
| Carbon nanotubes - silver modified -titanium dioxide (CNTs-Ag-TiO2) | Synthesis: thermal and ultrasound-assisted method. 0.5 g of carbon nanotubes (CNTs), 10 mL concentrated nitric acid, and 30 mL concentrated sulfuric acid were stirred at 300 rpm for 30 min and heated to 80 °C; then, CNTs were washed with deionized water, taken out of the solid and dried at 70 °C, and stored in containers for subsequent use. The modified CNTs and nano silver powder (AgNPs) were added into 30 mL absolute ethanol at the same time, and the CNTs-Ag composite material was obtained by standing after ultrasonic treatment for 30 min, taking out the solid after centrifugal treatment for 5 min, and grinding. Degradation: 100 mL of dye (100 mg L−1), stirred in the dark for 60 min, then was irradiated with a 1000 W xenon lamp for 140 min. | Band gap and wavelength not specified/100% degradation/No toxicity bioassays were performed | [3] |
| TiO2 quantum dots (TDS) | Synthesis: synthesized via a low-temperature precipitation method using a syringe, 6.0 mL of TTIP was dispensed into a 250 mL beaker containing 180 mL of isopropyl alcohol, and the mixture was stirred continually at 0 °C for over 60 min to create solution A (pH 7). After 24 h at room temperature (25 °C), a white powder was produced by adding 0.03 mol of CTAB to solution (A). The dry white powder was removed from the crucible, placed in the mortar, coarsely crushed and deposited in an aluminum oxide crucible. Subsequently, the samples were subjected to the calcination process for 45 min at 280 °C. Degradation: Industrial textile effluent from a dying plant with a pH of 6.9 was treated immediately under direct sunlight. Throughout the experiment, the daily dosage of UV radiation was 4.7 mW/cm2, and the dose of visible light received during the middle of the day was 1635 mW/cm2, 100 min. | Band gap from 2.97–3.09 eV/Photodegradation rate of 22.49 × 10−3 S−1/No toxicity bioassays were performed | [4] |
| (TiO2)/Cellulose biochar | Synthesis: Sol–gel method. 5 mL of TTIP was dissolved in 90 mL of isopropanol and stirred for 5 min. 1 g of cellulose biochar was added, and continued stirring. After that, 9 mL of distilled water was added and stirred for 2 h to form a homogeneous gel. Then, the gel was dried at 100 °C for 5 h and ground using a mortar and pestle. Next, the solid materials were calcined at 500 °C for 2 h, washed with distilled water several times until the supernatants looked clear, and dried in an oven at 100 °C overnight. Degradation: The optimum conditions for the photocatalytic degradation of Congo Red were 0.5 g L−1 of cellulose biochar/TiO2−700 with 0.3 mM of potassium peroxymonosulfate (PMS) used to treat an initial dye concentration of 10 mg L−1 under 30 °C and solution pH 5, in 30 min. | Band gap and wavelength not specified/100% removal/No toxicity bioassays were performed | [33] |
| Cu-doped mesoporous TiO2 (mTiO2) | Synthesis: Sol–gel, ultrasonic, hydrothermal treatment, and calcination methods. 0.6 g of PVA was added in boiling water and stirred for 30 min to prepare solution A, and then 3 mL of TTIP were hydrolyzed in 1.5 mL of acetic acid to prepare solution B. Solution B was then mixed with solution A. An Ultrasonic Disruptor UD-21 was used to sonicate (sonication power: 70 W, frequency: 59 KHz, and speed: 24,000 rpm) the mixture for 60 min at room temperature. The intermediate products were placed inside a Teflon-sealed vessel for 17 h of hydrothermal treatment at 105 °C. The prepared material was then calcined for 5 h at 500 °C. The same technique was used to synthesize mesoporous TiO2 with 1, 2, and 3 w% Cu-doping. Degradation: 100 mL of dye (40 mg L−1), 45 mg of Cu-doped TiO2 (3 w%), pH 5, kept in the dark for 30 min, then irradiated with a Hg lamp with UV light output of 5.8 × 102 W/cm C/Co. | Band gap of 2.6 eV, wavelength not specified/99% degradation/No toxicity bioassays were performed | [54] |
| Magnetically Recyclable Wool/Fe3O4@ TiO2/UiO-66 Core-Shell Structured Composite | Synthesis: wool/Fe3O4@TiO2/UiO-66 composite was prepared by solvothermal and hydrothermal methods, using 0.2332 g of ZrCl4 and 0.1661 g of H2BDC, dissolved in 50 mL of N,N-dimethylformamide (DMF) solution, and 6 mL of glacial acetic acid was subsequently added to control the morphology of UiO-66. Then, 0.6 g of TBT was dissolved in 18 mL of absolute ethanol, and 12 mL of deionized water and 2 mL of PEG400 were added successively under vigorous stirring to obtain the TiO2 precursor solution B. After that, according to the mass ratios of TiO2 to UiO-66 1:2, 1:1, and 2:1 individually, a certain volume of B solution was added into a specific volume of A solution under continuous stirring and treated at 120 °C for 4 h in a 100 mL Teflon-lined stainless steel autoclave. In addition, the UiO-66 and TiO2 particles were prepared using the precursor solution A and B under solvothermal and hydrothermal conditions, respectively. Degradation: 0.01 g of catalyst, 30 mL of dye solution (40 mg L−1), and left in the darkness. Then, exposure to a white light diode lamp (LED, 25 W) with an optical power density of 155 Mw/cm2 or 1.3 W/cm2 under stirring. | Band gap from 2.49 to 3.03 eV, 450 nm/77.1% degradation/No toxicity bioassays were performed | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintanilla-Villanueva, G.E.; Sicardi-Segade, A.; Luna-Moreno, D.; Núñez-Salas, R.E.; Villarreal-Chiu, J.F.; Rodríguez-Delgado, M.M. Recent Advances in Congo Red Degradation by TiO2-Based Photocatalysts Under Visible Light. Catalysts 2025, 15, 84. https://doi.org/10.3390/catal15010084
Quintanilla-Villanueva GE, Sicardi-Segade A, Luna-Moreno D, Núñez-Salas RE, Villarreal-Chiu JF, Rodríguez-Delgado MM. Recent Advances in Congo Red Degradation by TiO2-Based Photocatalysts Under Visible Light. Catalysts. 2025; 15(1):84. https://doi.org/10.3390/catal15010084
Chicago/Turabian StyleQuintanilla-Villanueva, Gabriela Elizabeth, Analía Sicardi-Segade, Donato Luna-Moreno, Raisa Estefanía Núñez-Salas, Juan Francisco Villarreal-Chiu, and Melissa Marlene Rodríguez-Delgado. 2025. "Recent Advances in Congo Red Degradation by TiO2-Based Photocatalysts Under Visible Light" Catalysts 15, no. 1: 84. https://doi.org/10.3390/catal15010084
APA StyleQuintanilla-Villanueva, G. E., Sicardi-Segade, A., Luna-Moreno, D., Núñez-Salas, R. E., Villarreal-Chiu, J. F., & Rodríguez-Delgado, M. M. (2025). Recent Advances in Congo Red Degradation by TiO2-Based Photocatalysts Under Visible Light. Catalysts, 15(1), 84. https://doi.org/10.3390/catal15010084









