Thiazolo[5,4-d]thiazole-Based Covalent Organic Frameworks for the Rapid Removal of RhB
Abstract
1. Introduction
2. Results and Discussion
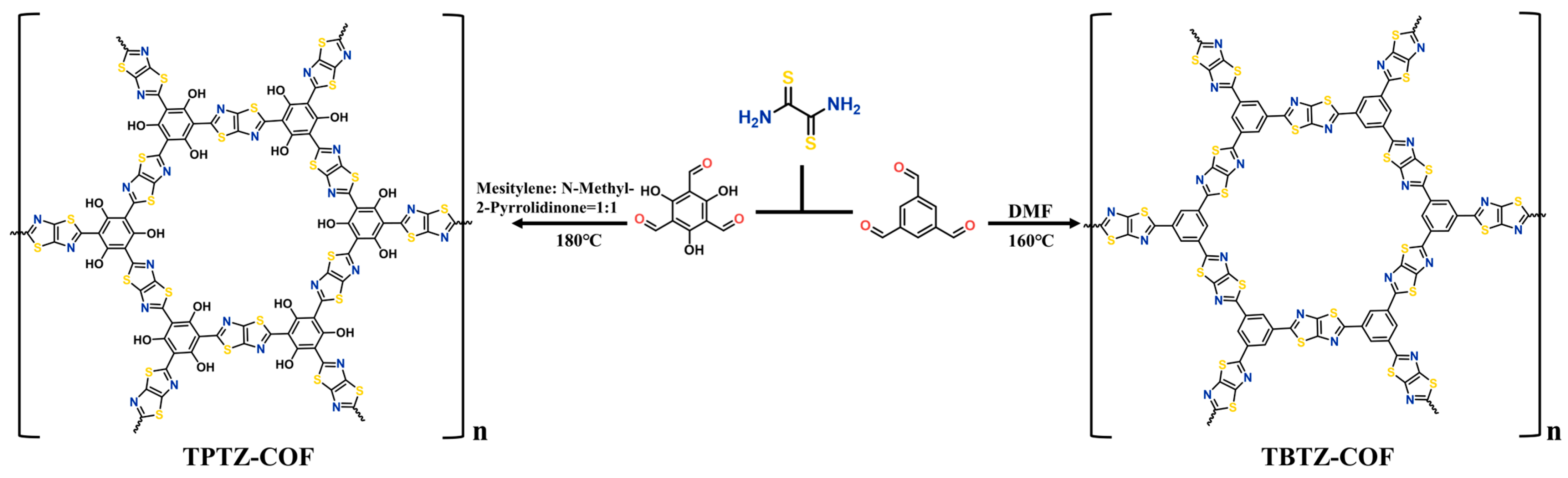
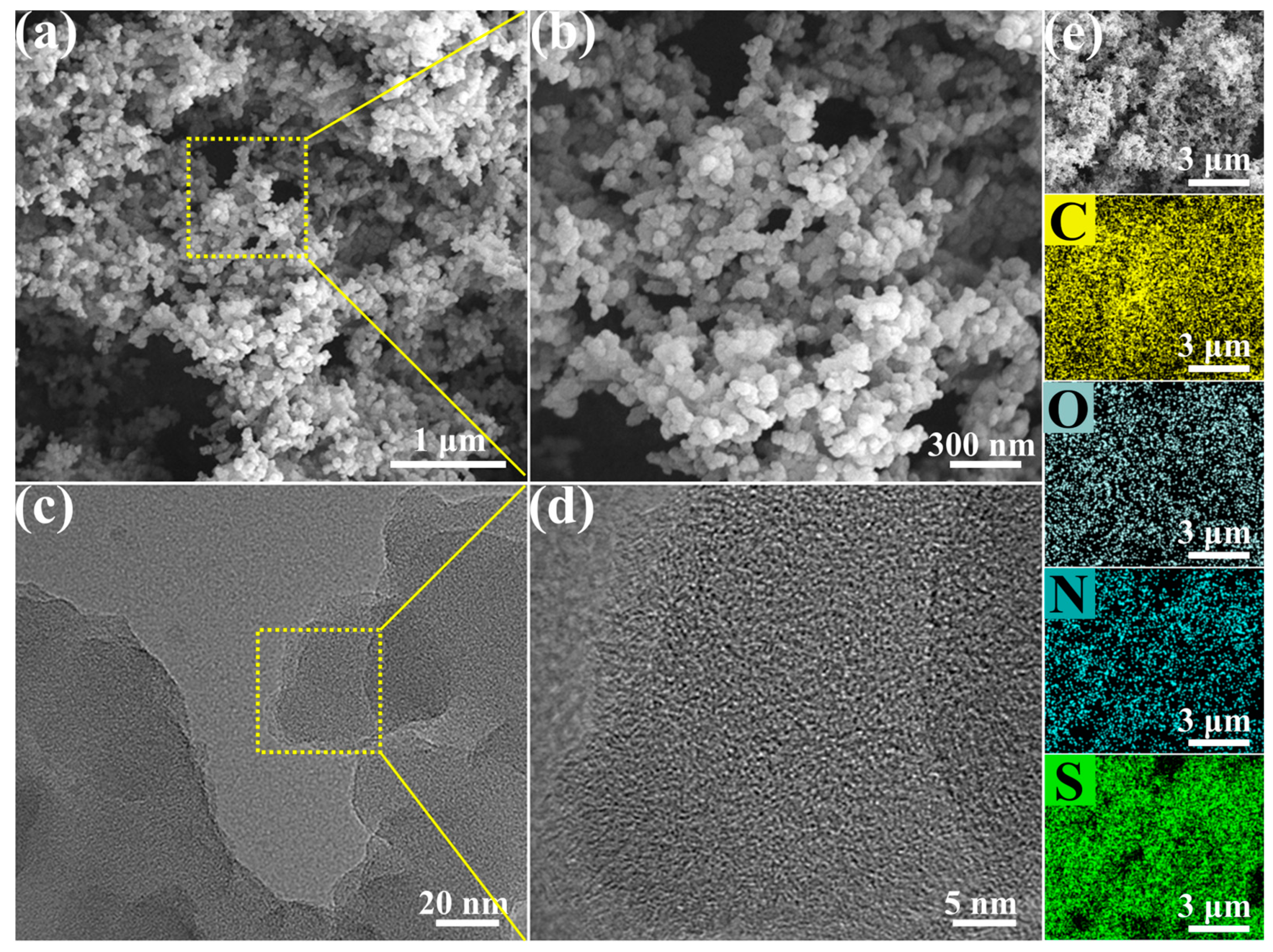
2.1. Synthesis and Characterization of TzTz-Linked COFs
2.2. Photoelectric Property Studies
2.3. Photodegradation of RhB
2.4. Degradation Mechanism
3. Experimental Section
3.1. Materials
3.2. Synthesis of TPTZ-COF
3.3. Synthesis of TBTZ-COF
3.4. Characterizations
3.5. Electrochemical Measurement
3.6. Photocatalytic Measurement
3.7. Theoretical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Naghdi, S.; Shahrestani, M.M.; Zendehbad, M.; Djahaniani, H.; Kazemian, H.; Eder, D. Recent Advances in Application of Metal-Organic Frameworks (MOFs) as Adsorbent and Catalyst in Removal of Persistent Organic Pollutants (POPs). J. Hazard. Mater. 2023, 442, 130127. [Google Scholar] [CrossRef] [PubMed]
- Ghahari, A.; Farzad, F.; Azadnejad, R. The Strategy of Three-Dimensional Covalent Organic Frameworks to Exclude Dye Contaminants in Aqueous Solutions. Npj Clean Water 2024, 7, 27. [Google Scholar] [CrossRef]
- Huang, L.; Huang, X.J.; Yan, J.; Liu, Y.H.; Jiang, H.; Zhang, H.G.; Tang, J.F.; Liu, Q. Research Progresses on the Application of Perovskite in Adsorption and Photocatalytic Removal of Water Pollutants. J. Hazard. Mater. 2023, 442, 130024. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.H.; Zhang, Z.T.; Tian, J.J.; Huang, G.C. Interface Engineering in a Nitrogen-Rich COF/BiOBr S-Scheme Heterojunction Triggering Efficient Photocatalytic Degradation of Tetracycline Antibiotics. J. Colloid Interface Sci. 2024, 661, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Pasini, S.M.; Valério, A.; Yin, G.L.; Wang, J.F.; De Souza, S.M.A.G.U.; Hotza, D.; De Souza, A.A.U. An Overview on Nanostructured TiO2—Containing Fibers for Photocatalytic Degradation of Organic Pollutants in Wastewater Treatment. J. Water Process Eng. 2021, 40, 101827. [Google Scholar] [CrossRef]
- Deng, M.J.; Wang, L.Y.; Wen, Z.L.; Chakraborty, J.; Sun, J.M.; Wang, G.Z.; Voort, P.V.D. Donor—Acceptor sp2 Covalent Organic Frameworks for Photocatalytic H2O2 Production and Tandem Bisphenol-A Degradation. Green Chem. 2024, 26, 3239–3248. [Google Scholar] [CrossRef]
- Yang, S.Z.; Hu, W.H.; Zhang, X.; He, P.L.; Pattengale, B.; Liu, C.M.; Cendejas, M.; Hermans, I.; Zhang, X.Y.; Zhang, J.; et al. 2D Covalent Organic Frameworks as Intrinsic Photocatalysts for Visible Light-Driven CO2 Reduction. J. Am. Chem. Soc. 2018, 140, 14614–14618. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.F.; Wang, Y.; Ma, Y.; Mal, A.; Gao, X.Y.; Gao, L.; Qiao, L.J.; Li, X.B.; Wu, L.Z.; Wang, C. Rational Design of Isostructural 2D Porphyrin-Based Covalent Organic Frameworks for Tunable Photocatalytic Hydrogen Evolution. Nat. Commun. 2021, 12, 1354. [Google Scholar] [CrossRef]
- Xu, N.; Wang, R.L.; Li, D.P.; Meng, X.; Mu, J.L.; Zhou, Z.Y.; Su, Z.M. A New Triazine-Based Covalent Organic Polymer for Efficient Photodegradation of Both Acidic and Basic Dyes under Visible Light. Dalton Trans. 2018, 47, 4191–4197. [Google Scholar] [CrossRef]
- Cai, L.J.; Li, Y.W.; Li, Y.H.; Wang, H.G.; Yu, Y.; Liu, Y.; Duan, Q. Synthesis of Zincphthalocyanine-Based Conjugated Microporous Polymers with Rigid-Linker as Novel and Green Heterogeneous Photocatalysts. J. Hazard. Mater. 2018, 348, 47–55. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, X.Y.; Chen, L.K.; Cui, Y.; Li, Q.Y.; Zhao, X.S.; Han, X.G.; Zheng, Y.C.; Wang, X.J. Rational Design of a Phenothiazine-Based Donor–Acceptor Covalent Organic Framework for Enhanced Photocatalytic Oxidative Coupling of Amines and Cyclization of Thioamides. J. Mater. Chem. A 2023, 11, 1208–1215. [Google Scholar] [CrossRef]
- Jin, S.B.; Supur, M.; Addicoat, M.; Furukawa, K.; Chen, L.; Nakamura, T.; Fukuzumi, S.; Irle, S.; Jiang, D.L. Creation of Superheterojunction Polymers via Direct Polycondensation: Segregated and Bicontinuous Donor–Acceptor π-Columnar Arrays in Covalent Organic Frameworks for Long-Lived Charge Separation. J. Am. Chem. Soc. 2015, 137, 7817–7827. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.L.; He, Y.Y.; Liu, X.P.; Wang, Z.; Liu, H.L.; Zhu, X.; Hou, C.C.; Weng, Y.X.; Zhang, Q.F.; Chen, Y. Regulating Excitonic Effects in Covalent Organic Frameworks to Promote Free Charge Carrier Generation. ACS Catal. 2022, 12, 9494–9502. [Google Scholar] [CrossRef]
- Vyas, V.S.; Haase, F.; Stegbauer, L.; Savasci, G.; Podjaski, F.; Ochsenfeld, C.; Lotsch, B.V. A Tunable Azine Covalent Organic Framework Platform for Visible Light-Induced Hydrogen Generation. Nat. Commun. 2015, 6, 8508. [Google Scholar] [CrossRef]
- Li, L.Y.; Fang, W.; Zhang, P.; Bi, J.H.; He, Y.H.; Wang, J.Y.; Su, W.Y. Sulfur-Doped Covalent Triazine-Based Frameworks for Enhanced Photocatalytic Hydrogen Evolution from Water under Visible Light. J. Mater. Chem. A 2016, 4, 12402–12406. [Google Scholar] [CrossRef]
- Hou, Y.H.; Zhou, P.; Liu, F.Y.; Tong, K.; Lu, Y.Y.; Li, Z.M.; Liang, J.L.; Tong, M.P. Rigid Covalent Organic Frameworks with Thiazole Linkage to Boost Oxygen Activation for Photocatalytic Water Purification. Nat. Commun. 2024, 15, 7350. [Google Scholar] [CrossRef]
- Samal, M.; Valligatla, S.; Saad, N.A.; Rao, M.V.; Rao, D.N.; Sahu, R.; Biswal, B.P. A Thiazolo[5,4-d]Thiazole-Bridged Porphyrin Organic Framework as a Promising Nonlinear Optical Material. Chem. Commun. 2019, 55, 11025–11028. [Google Scholar] [CrossRef]
- Qu, Z.; Liu, S.; Wang, Y.F.; Yang, J.D.; Zhang, Y.; Zhang, J.Y.; Gao, Y.T.; Ma, H.X.; Guo, Z.Q. Enhanced Photocatalytic Activity of Calix [4] Arene-Based Donor–Acceptor Covalent Organic Frameworks by Dual Cocatalysts. ACS Appl. Polym. Mater. 2024, 6, 4756–4768. [Google Scholar] [CrossRef]
- Wang, Y.C.; Liu, H.; Pan, Q.Y.; Ding, N.X.; Yang, C.M.; Zhang, Z.H.; Jia, C.C.; Li, Z.B.; Liu, J.; Zhao, Y.J. Construction of Thiazolo[5,4-d]Thiazole-Based Two-Dimensional Network for Efficient Photocatalytic CO2 Reduction. ACS Appl. Mater. Interfaces 2020, 12, 46483–46489. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Q.; Yin, D.G.; Xiandi, G. Construction of a Novel 2D–2D Heterojunction by Coupling a Covalent Organic Framework and In2S3 for Photocatalytic Removal of Organic Pollutants with High Efficiency. New J. Chem. 2021, 45, 15789–15800. [Google Scholar] [CrossRef]
- Li, Z.; Li, L.; Wang, Y.; Yang, Y.W. Pillararene-Enriched Linear Conjugated Polymer Materials with Thiazolo[5,4-d]Thiazole Linkages for Photocatalysis. Chem. Commun. 2021, 57, 6546–6549. [Google Scholar] [CrossRef]
- Huang, Q.; Guo, L.P.; Wang, N.; Zhu, X.; Jin, S.B.; Tan, B. Layered Thiazolo[5,4-d] Thiazole-Linked Conjugated Microporous Polymers with Heteroatom Adoption for Efficient Photocatalysis Application. ACS Appl. Mater. Interfaces 2019, 11, 15861–15868. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guan, L.J.; Liu, J.W.; Lei, T.T.; Xue, Y.X.; Qu, Z.; Jin, S.B.; Ma, H.X.; Guo, Z.Q. A Thiazolo[5,4-d]Thiazole Functionalized Covalent Triazine Framework Showing Superior Photocatalytic Activity for Hydrogen Production and Dye Degradation. J. Mater. Chem. A 2022, 10, 16328–16336. [Google Scholar] [CrossRef]
- Huang, F.W.; Dong, X.Y.; Wang, Y.X.; Lang, X.J. Narrowing the Bandgaps of Thiazolo[5,4-d]Thiazole-Bridged Conjugated Microporous Polymers to Capture Green Light for Selective Oxidation of Amines. Appl. Catal. B Environ. 2023, 330, 122585. [Google Scholar] [CrossRef]
- Zhang, F.L.; Li, X.; Dong, X.Y.; Hao, H.M.; Lang, X.J. Thiazolo[5,4-d]Thiazole-Based Covalent Organic Framework Microspheres for Blue Light Photocatalytic Selective Oxidation of Amines with O2. Chin. J. Catal. 2022, 43, 2395–2404. [Google Scholar] [CrossRef]
- Meng, F.Y.; Wang, J.; Chen, M.H.; Wang, Z.G.; Bai, G.Y.; Lan, X.W. Extending the π-Conjugated System in Conjugated Microporous Polymers to Modulate Excitonic Effects for Metal-Free Selective CO2 Photoreduction to CH4. ACS Catal. 2023, 13, 12142–12152. [Google Scholar] [CrossRef]
- Tauc, J. Absorption Edge and Internal Electric Fields in Amorphous Semiconductors. Mater. Res. Bull. 1970, 5, 721–729. [Google Scholar] [CrossRef]
- Dong, P.Y.; Zhang, A.; Cheng, T.; Pan, J.K.; Song, J.; Zhang, L.; Guan, R.F.; Xi, X.G.; Zhang, J.L. 2D/2D S-Scheme Heterojunction with a Covalent Organic Framework and g-C3N4 Nanosheets for Highly Efficient Photocatalytic H2 Evolution. Chin. J. Catal. 2022, 43, 2592–2605. [Google Scholar] [CrossRef]
- Liao, L.; Zhang, Q.H.; Su, Z.H.; Zhao, Z.Z.; Wang, Y.N.; Li, Y.; Lu, X.X.; Wei, D.G.; Feng, G.Y.; Yu, Q.K.; et al. Efficient Solar Water-Splitting Using a Nanocrystalline CoO Photocatalyst. Nat. Nanotech. 2014, 9, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.Y.; Nie, C.Y.; Dong, Q.Q.; Ma, Z.Y.; Liu, W.; Tong, M.P. AgI Modified Covalent Organic Frameworks for Effective Bacterial Disinfection and Organic Pollutant Degradation under Visible Light Irradiation. J. Hazard. Mater. 2020, 398, 122865. [Google Scholar] [CrossRef]
- Chen, J.J.; Li, H.; Ma, L.L.; Jiang, G.X.; Li, D.; Wu, Y.; Shi, X.W.; Li, D.; Wang, X.; Deng, H.B. Chitosan-Based Recyclable Composite Aerogels for the Photocatalytic Degradation of Rhodamine B. Carbohyd. Polym. 2021, 273, 118559. [Google Scholar] [CrossRef]
- Lin, D.Y.; Duan, P.; Yang, W.T.; Huang, X.J.; Zhao, Y.J.; Wang, C.T.; Pan, Q.H. Facile Fabrication of Melamine Sponge@covalent Organic Framework Composite for Enhanced Degradation of Tetracycline under Visible Light. Chem. Eng. J. 2022, 430, 132817. [Google Scholar] [CrossRef]
- Wang, J.H.; Hassan, A.E.; Elewa, A.M.; EL-Mahdy, A.F.M. Donor–Acceptor Hetero[6]radialene-Based Three-Dimensional Covalent Organic Frameworks for Organic Pollutant Adsorption, Photocatalytic Degradation, and Hydrogen Production Activity. J. Mater. Chem. A 2024, 12, 14005–14021. [Google Scholar] [CrossRef]
- Lee, J.J.; Noh, W.; Huh, T.H.; Kwark, Y.J.; Lee, T.S. Synthesis of Conjugated Microporous Polymer and Its Embedding in Porous Nanofibers for Visible-Light-Driven Photocatalysis with Reusability. Polymer 2020, 211, 123060. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Luo, Y.; Wang, L.Z.; Wang, Y.L.; Wang, Q.; Jiang, G.F.; Zhang, Q.F.; Cui, F.Z. Synthesis of Pyrene-Based Covalent Organic Frameworks for Photocatalytic Tetracycline Degradation. ACS Appl. Polym. Mater. 2023, 5, 9263–9273. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, J.; Ren, Y.; Sun, X.; Gao, Y.; Tian, Z.; Li, D. Thiazolo[5,4-d]thiazole-Based Covalent Organic Frameworks for the Rapid Removal of RhB. Catalysts 2025, 15, 42. https://doi.org/10.3390/catal15010042
Yin J, Ren Y, Sun X, Gao Y, Tian Z, Li D. Thiazolo[5,4-d]thiazole-Based Covalent Organic Frameworks for the Rapid Removal of RhB. Catalysts. 2025; 15(1):42. https://doi.org/10.3390/catal15010042
Chicago/Turabian StyleYin, Jinyue, Yuting Ren, Xuejiao Sun, Yu Gao, Zhongzhen Tian, and Dongmei Li. 2025. "Thiazolo[5,4-d]thiazole-Based Covalent Organic Frameworks for the Rapid Removal of RhB" Catalysts 15, no. 1: 42. https://doi.org/10.3390/catal15010042
APA StyleYin, J., Ren, Y., Sun, X., Gao, Y., Tian, Z., & Li, D. (2025). Thiazolo[5,4-d]thiazole-Based Covalent Organic Frameworks for the Rapid Removal of RhB. Catalysts, 15(1), 42. https://doi.org/10.3390/catal15010042






